Published online Jul 12, 2016. doi: 10.5499/wjr.v6.i2.23
Peer-review started: July 15, 2015
First decision: August 16, 2015
Revised: January 24, 2016
Accepted: March 7, 2016
Article in press: March 9, 2016
Published online: July 12, 2016
Processing time: 362 Days and 21.7 Hours
AIM: To determine whether evidential value exists that exercise reduces depression in adults with arthritis and other rheumatic conditions.
METHODS: Utilizing data derived from a prior meta-analysis of 29 randomized controlled trials comprising 2449 participants (1470 exercise, 979 control) with fibromyalgia, osteoarthritis, rheumatoid arthritis or systemic lupus erythematosus, a new method, P-curve, was utilized to assess for evidentiary worth as well as dismiss the possibility of discriminating reporting of statistically significant results regarding exercise and depression in adults with arthritis and other rheumatic conditions. Using the method of Stouffer, Z-scores were calculated to examine selective-reporting bias. An alpha (P) value < 0.05 was deemed statistically significant. In addition, average power of the tests included in P-curve, adjusted for publication bias, was calculated.
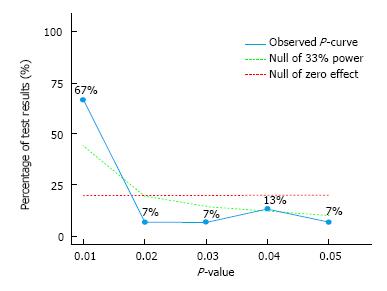
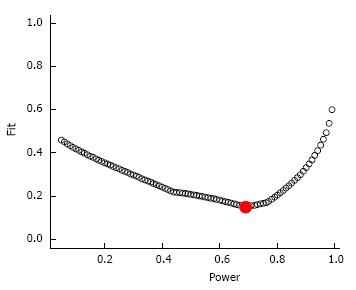
RESULTS: Fifteen of 29 studies (51.7%) with exercise and depression results were statistically significant (P < 0.05) while none of the results were statistically significant with respect to exercise increasing depression in adults with arthritis and other rheumatic conditions. Right-skew to dismiss selective reporting was identified (Z = -5.28, P < 0.0001). In addition, the included studies did not lack evidential value (Z = 2.39, P = 0.99), nor did they lack evidential value and were P-hacked (Z = 5.28, P > 0.99). The relative frequencies of P-values were 66.7% at 0.01, 6.7% each at 0.02 and 0.03, 13.3% at 0.04 and 6.7% at 0.05. The average power of the tests included in P-curve, corrected for publication bias, was 69%. Diagnostic plot results revealed that the observed power estimate was a better fit than the alternatives.
CONCLUSION: Evidential value results provide additional support that exercise reduces depression in adults with arthritis and other rheumatic conditions.
Core tip: The primary strength of this study was the use of a recent and novel approach to address the potential for selective reporting of statistically significant results, a common problem in the published literature, regarding the effects of exercise on depressive symptoms in adults with arthritis and other rheumatic diseases. The results revealed that selective reporting does not exist, thereby providing further support that exercise improves depressive symptoms in adults with arthritis and other rheumatic diseases.
- Citation: Kelley GA, Kelley KS. Exercise reduces depressive symptoms in adults with arthritis: Evidential value. World J Rheumatol 2016; 6(2): 23-29
- URL: https://www.wjgnet.com/2220-3214/full/v6/i2/23.htm
- DOI: https://dx.doi.org/10.5499/wjr.v6.i2.23
Arthritis and other rheumatic diseases are a major public health problem affecting more than 52 million adults in the United States[1]. By the year 2030, it is estimated that 67 million Americans 18 years of age and older will have doctor-diagnosed arthritis[2]. In terms of expenditures, the total costs associated with arthritis in the United States were estimated to be 128 billion dollars in 2003, an increase of 41.8 billion dollars compared to 1997[3].
One of the major psychological health problems associated with arthritis and other rheumatic diseases is depression[4]. To illustrate, recent estimates suggest that approximately 18% of United States adults with doctor-diagnosed arthritis have depression[4]. This is the result of people becoming depressed after developing arthritis vs the development of arthritis as a result of being depressed[4].
One potential lifestyle intervention for reducing the prevalence of depression in adults with arthritis and other rheumatic diseases is exercise[5]. For example, a recently completed meta-analysis of randomized controlled trials by the authors resulted in a statistically significant standardized mean difference effect size reduction in depressive symptoms equivalent to a percentile improvement of 16.4 as a result of exercise in adults with arthritis and other rheumatic diseases[5]. While encouraging, all investigations appeared in peer-reviewed academic journals, a potential problem given that publications in academic journals yield an overly excessive number of statistically significant results[6]. Consequently, such findings may not be representative of the truth. Factors associated with an excess of statistically significant outcomes include, but are not necessarily restricted to, selective reporting by researchers[7-13]. Across all levels of utilization, i.e., research, practice, and policy, it is crucial to recognize the genuine consequences of physical exercise on depression in adults with arthritis and other rheumatic conditions. While recommendations for the evaluation of selective reporting and associated biases in meta-analysis have been developed, all have noteworthy shortcomings. As a result, no correction techniques are currently endorsed[14]. However, since the time of publication of these recommendations[14], a new and novel approach known as P-curve has been developed for the purpose of determining whether selective reporting of studies exists and which does not require access to null results[15,16]. Therefore, given the importance of identifying the true effects of exercise on depression in adults with arthritis and other rheumatic conditions, the purpose of the current study was to determine whether there is evidential value that exercise improves depression in adults with arthritis and other rheumatic conditions.
The literature search for the present investigation originated from a previous and recent meta-analysis that has been explained thoroughly elsewhere[5]. In brief, research studies published between 1981 and January 2013 were retrieved by searching ten reference databases, the reference lists of included studies, and expert review.
The selection of studies has also been explained thoroughly elsewhere[5]. Succinctly, randomized controlled trials that investigated the effects of aerobic exercise, strength training, or a combination of aerobic and strength training exercise on depressive symptoms, as defined by the authors, in adults with arthritis and other rheumatic diseases (fibromyalgia, osteoarthritis, rheumatoid arthritis, or systemic lupus erythematosus), were included[17-45]. Studies in which exercise, defined as “physical activity that is planned, structured, and repetitive and purposive in the sense that the improvement or maintenance of one or more components of physical fitness is the objective”[46], were included.
The process for data extraction has been described in detail elsewhere[5]. Briefly, data were extracted by both authors, independent of each other. Disagreements were resolved by consensus.
Risk of bias, described in detail elsewhere, was accomplished using the Cochrane Risk of Bias Assessment Instrument and followed the same procedures as for data extraction[5].
The statistical methods of this study were reviewed and approved by a biostatistician, Dr. Matthew Gurka, Department of Biostatistics, West Virginia University.
Outcomes for depressive symptoms, as defined by the authors from each study, were computed using the standardized mean difference effect size. This was calculated by subtracting the change outcome difference in the exercise group from the change outcome difference in the control group, dividing by the pooled standard deviations of the outcomes for both groups, and then weighting them by the reciprocal of the combined variances. All effect sizes were corrected for small sample bias, i.e., Hedges et al[47]. Overall results were then combined using a random-effects model[48]. Heterogeneity and inconsistency were estimated using Cochran’s Q and I2 statistic, respectfully[48-50].
To identify whether evidential value exists in relation to exercise reducing depression in adults with arthritis and other rheumatic conditions, the primary purpose of the current study, a recent and novel method known as P-curve was utilized[15,16]. Briefly, the purpose of this approach is to determine whether selective reporting can be excluded as a cause of statistically significant results, thus providing greater confidence that the observed effect is true. It comprises a distribution of significant P-values (alpha level < 0.05) from the included studies. Studies with non-significant P-values (alpha level > 0.05), are excluded from the assessment. The focus of P-curve is on determining whether studies (1) contain evidential value (right skew); (2) lack evidential value, as indicated by a power < 33%; and (3) lack evidentiary importance, i.e., were P-hacked, as indicated by left skew, suggesting that researchers withheld non-significant results. P-results are suggestive of real effects, i.e., evidentiary worth, if the number of small P values (P = 0.01) are greater than the number of large P values (P = 0.04). Testing is twofold. Firstly, for every P-value < 0.05, the chance of detecting a significant P-value at least as excessive as if the null were correct is computed. This P value, i.e., P value of the P value, is computed by dividing each statistically significant probability value from every study by 0.05. With respect to the current investigation, probabilities were calculated using the Z-scores of the differences in depressive symptoms between the exercise and control groups from each included study. To maintain independence, studies that included multiple groups and/or multiple measures of depression using different instruments were combined so that only one probability value was included for that study. This approach was chosen because the focus of this study was on ruling out selective reporting of findings. In addition, P-curve has been found to perform better than previously existing tests to address publication bias[15,16]. Details regarding P-curve have been described in detail elsewhere[15,16].
The second step consists of aggregating PP values using Stouffer’s method[51]. This continuous test is accomplished by computing PP values for each test with a probability of < 0.05 and then converting them to Z-scores. The sum of the Z-scores is then divided by the square root of the number of tests with P-values < 0.05. A negative Z-score and overall P-value < 0.05 is indicative of right-skewed evidential value that results do not suffer from selective reporting bias in favor of statistically significant results. A nonexistent statistically significant right-skewed P-value implies either an absence of data to draw conclusions regarding evidentiary value or a dearth of evidentiary value. To assess for potential absence of data, i.e., power, the identical method as for right-skew is employed with the exception that PP values are recomputed for expected P-curves utilizing a power of 33% along with the sample size from each study, achieved by means of non-central distributions. To test for a lack of evidential value suggestive of the withholding of non-significant findings by investigators, i.e., left skewed P-hacking, the same approach is used as for right-skewed evidential value but the PP values for left skew are computed as 1 minus the right skew PP value. Probability values ≤ 0.05 were considered statistically significant.
In addition to testing for (1) right skew; (2) inadequate information; and (3) left skew, average power of the tests included in P-curve were calculated while correcting for publication bias. This was accomplished by comparing the expected P-curve for each possible value of power between 5% and 99% and then choosing the level of power that most closely matches the expected and observed P-curves.
All data were analyzed using version 3.0 of P-curve (http://www.p-curve.com/app3/), version 3.0 of Comprehensive Meta-Analysis (Englewood, New Jersey, 2015) and Microsoft Excel 2010.
Twenty-nine studies that included 2449 participants (1470 exercise, 979 control) with fibromyalgia, osteoarthritis, rheumatoid arthritis, or systemic lupus erythematosus met all eligibility criteria[17-45]. Exercise averaged 19 wk, 4 times per week for 34 min per session[5]. The within-study age of the participants ranged from 18 to 85 years. A detailed description of these studies can be found elsewhere[5].
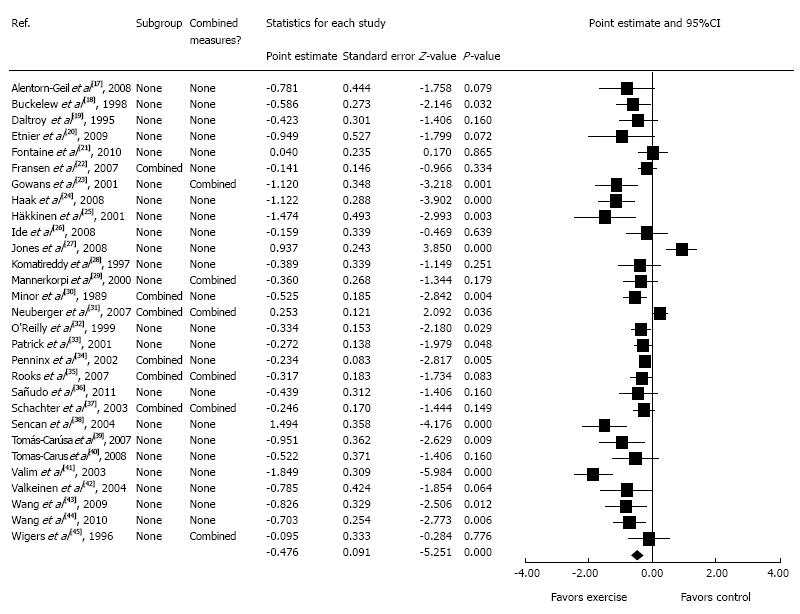
Figure 1 shows a forest plot of study-level as well as pooled results for changes in depressive symptoms. As shown, the overall results indicate a statistically significant decrease in depressive symptoms in support of exercise along with non-overlapping 95%CI (-0.643--0.298). Heterogeneity was statistically significant (Q = 122.8, P < 0.001) and a large amount of inconsistency was observed (I2 = 77.2%, 95%CI = 67.6%-84.0%). Standardized mean difference effect size changes ranged from -1.85 to 0.94. Fifteen of 29 (51.7%) results were statistically significant (P < 0.05) while none were statistically significant with respect to exercise increasing depression in adults with arthritis and other rheumatic conditions.
Evidential value results are displayed in Table 1 and Figure 2. As shown, there was statistically significant right-skew. This suggests that there is evidential value that exercise decreases depression in adults with arthritis and other rheumatic conditions. Consistent with this finding are the non-significant results for a lack of evidential value, including P-hacking. The average power of the tests included in P-curve, corrected for publication bias, was 69%. Interpretation of the diagnostic plot suggests that the observed power estimate was a better fit than the alternatives (Figure 3).
| Statistical inference | Z | P |
| Studies contain evidential value (right-skewed) | 5.28 | < 0.00011 |
| Studies lack evidential value (flatter than 33% power) | 2.39 | 0.99 |
| Studies lack evidential value and intensely P-hacked (left-skewed) | 5.28 | 0.99 |
The aim of the present investigation was to use a new approach, P-curve, to identify whether evidential value exists in support of exercise for reducing depression in adults with arthritis and other rheumatic conditions. The results suggest there is indeed evidential value in support of exercise aimed at reducing depression in adults with arthritis and other rheumatic conditions. These findings provide additional support to recently completed research on this issue[5]. These findings are noteworthy given: (1) the prevalence of depression in adults with arthritis and other rheumatic conditions[4]; (2) the potential benefits of exercise for improving depression in adults with arthritis and other rheumatic conditions[5]; and (3) the importance of determining if selective reporting bias exists in published exercise studies examining the effects of exercise on depression in adults with arthritis and other rheumatic conditions[7-13].
The findings of the present investigation provide further confirmation regarding the positive effects of exercise on depressive symptoms in adults with arthritis and other rheumatic diseases. However, while a random-effects model that incorporates heterogeneity was used, such models do not explain potential sources of heterogeneity, little of which could be identified in the primary meta-analysis on which the current investigation was based[5]. Given the former, it would appear plausible to suggest that a need exists for well-designed randomized controlled trials to determine what group of participants may benefit the most from exercise. Along those lines, the dose-response effects of exercise were not a purpose of the current study, and when studied previously, did not yield any significant results. Therefore, and as previously recommended[5], there is a need for additional randomized controlled trials in order to determine the dose-response effects of exercise in a representative sample of adults with arthritis and other rheumatic diseases. Until that time, it would appear feasible to recommend that adults with arthritis and other rheumatic diseases progress to achieving the general guidelines of: (1) 150 min per week of moderate-intensity aerobic activity (brisk walking, etc.), 75 min per week of vigorous-intensity aerobic activity (water aerobics, etc.), or some equivalent combination of the two; (2) muscle strengthening exercises at least 2 d per week; and (3) balance exercises at least 3 d per week[52].
The primary strength of the present investigation is the use of a new and innovative approach to deal with the issue of potential selective reporting of statistically significant results regarding the effects of exercise on depressive symptoms in adults with arthritis and other rheumatic diseases[15,16]. From the investigative team’s perspective, this is important given the potential for selective-reporting bias and resultant overestimates of beneficial effects found in peer-reviewed journals[7-13]. Alternatively, one possible limitation is that P-curve excludes P values > 0.05 as well as those near 0.05. Consequently, P-values indicative of no effect, while extremely rare when a genuine effect is present, are omitted[16].
The findings of the present investigation provide evidential value regarding the use of exercise for reducing depression in adults with arthritis and other rheumatic conditions. Given the deleterious consequences of depression, exercise should be recommended as a lifestyle intervention for improving depressive symptoms in adults with arthritis and other rheumatic diseases.
While previous meta-analytic work has demonstrated that exercise improves depressive symptoms in adults with arthritis, the potential for bias, i.e., tendency for statistically significant and positive results to be published, continues to exist.
There is currently an increased interest in understanding the true effects of exercise on depressive symptoms in adults.
Previous meta-analytic research has demonstrated that exercise improves depressive symptoms in adults with arthritis but the possibility of publication bias cannot be ruled out.
Using a novel and recently developed approach for assessing publication and other related biases, the results of this study provide additional confirmatory evidence that exercise improves depressive symptoms in adults, thereby providing greater confidence for practitioners when recommending exercise for improving depressive symptoms in adults.
Evidential value refers to a lack of publication bias, i.e., tendency for statistically significant and positive results to be published. P-curve refers to a statistical method that assesses whether or not publication and related biases can be ruled out.
In this study the authors introduce a new and novel approach known as P-curve to determine whether selective reporting of studies exists and which does not require access to null results. This is a well-written article with sufficient justification.
P- Reviewer: Paraskevas KI, Song J, Turiel M S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Centers for Disease Control and Prevention (CDC). Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation--United States, 2010-2012. MMWR Morb Mortal Wkly Rep. 2013;62:869-873. [PubMed] |
| 2. | Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 591] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 3. | Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56:1397-1407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Murphy LB, Sacks JJ, Brady TJ, Hootman JM, Chapman DP. Anxiety and depression among US adults with arthritis: prevalence and correlates. Arthritis Care Res (Hoboken). 2012;64:968-976. [PubMed] |
| 5. | Kelley GA, Kelley KS, Hootman JM. Effects of exercise on depression in adults with arthritis: a systematic review with meta-analysis of randomized controlled trials. Arthritis Res Ther. 2015;17:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Ioannidis JP. Why most discovered true associations are inflated. Epidemiology. 2008;19:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 852] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 7. | Chan AW, Krleza-Jerić K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ. 2004;171:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 324] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 8. | Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004;291:2457-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1229] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 9. | Kyzas PA, Loizou KT, Ioannidis JP. Selective reporting biases in cancer prognostic factor studies. J Natl Cancer Inst. 2005;97:1043-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Dwan K, Gamble C, Williamson PR, Kirkham JJ. Systematic review of the empirical evidence of study publication bias and outcome reporting bias - an updated review. PLoS One. 2013;8:e66844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 707] [Cited by in RCA: 670] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 11. | Dwan K, Kirkham JJ, Williamson PR, Gamble C. Selective reporting of outcomes in randomised controlled trials in systematic reviews of cystic fibrosis. BMJ Open. 2013;3:e002709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, Williamson PR. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ. 2010;340:c365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 800] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 13. | McGauran N, Wieseler B, Kreis J, Schüler YB, Kölsch H, Kaiser T. Reporting bias in medical research - a narrative review. Trials. 2010;11:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3796] [Cited by in RCA: 4931] [Article Influence: 352.2] [Reference Citation Analysis (0)] |
| 15. | Simonsohn U, Nelson LD, Simmons JP. p-Curve and Effect Size: Correcting for Publication Bias Using Only Significant Results. Perspect Psychol Sci. 2014;9:666-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 346] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 16. | Simonsohn U, Nelson LD, Simmons JP. P-curve: a key to the file-drawer. J Exp Psychol Gen. 2014;143:534-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 623] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 17. | Alentorn-Geli E, Padilla J, Moras G, Lázaro Haro C, Fernández-Solà J. Six weeks of whole-body vibration exercise improves pain and fatigue in women with fibromyalgia. J Altern Complement Med. 2008;14:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Buckelew SP, Conway R, Parker J, Deuser WE, Read J, Witty TE, Hewett JE, Minor M, Johnson JC, Van Male L. Biofeedback/relaxation training and exercise interventions for fibromyalgia: a prospective trial. Arthritis Care Res. 1998;11:196-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 149] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Daltroy LH, Robb-Nicholson C, Iversen MD, Wright EA, Liang MH. Effectiveness of minimally supervised home aerobic training in patients with systemic rheumatic disease. Br J Rheumatol. 1995;34:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Etnier JL, Karper WB, Gapin JI, Barella LA, Chang YK, Murphy KJ. Exercise, fibromyalgia, and fibrofog: a pilot study. J Phys Act Health. 2009;6:239-246. [PubMed] |
| 21. | Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity on perceived symptoms and physical function in adults with fibromyalgia: results of a randomized trial. Arthritis Res Ther. 2010;12:R55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Fransen M, Nairn L, Winstanley J, Lam P, Edmonds J. Physical activity for osteoarthritis management: a randomied controlled clinical trial evaluating hydrotherapy or Tai Chi classes. Arthritis Rheum. 2007;57:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 23. | Gowans SE, deHueck A, Voss S, Silaj A, Abbey SE, Reynolds WJ. Effect of a randomized, controlled trial of exercise on mood and physical function in individuals with fibromyalgia. Arthritis Rheum. 2001;45:519-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Haak T, Scott B. The effect of Qigong on fibromyalgia (FMS): a controlled randomized study. Disabil Rehabil. 2008;30:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Häkkinen A, Häkkinen K, Hannonen P, Alen M. Strength training induced adaptations in neuromuscular function of premenopausal women with fibromyalgia: comparison with healthy women. Ann Rheum Dis. 2001;60:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Ide MR, Laurindo IMM, Rodrigues-Júnior AL, Tanaka C. Effect of aquatic respiratory exercise-based program in patients with fibromyalgia. Int J Rhuem Dis. 2008;11:131-140. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Jones KD, Burckhardt CS, Deodhar AA, Perrin NA, Hanson GC, Bennett RM. A six-month randomized controlled trial of exercise and pyridostigmine in the treatment of fibromyalgia. Arthritis Rheum. 2008;58:612-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Komatireddy GR, Leitch RW, Cella K, Browning G, Minor M. Efficacy of low load resistive muscle training in patients with rheumatoid arthritis functional class II and III. J Rheumatol. 1997;24:1531-1539. [PubMed] |
| 29. | Mannerkorpi K, Nyberg B, Ahlmén M, Ekdahl C. Pool exercise combined with an education program for patients with fibromyalgia syndrome. A prospective, randomized study. J Rheumatol. 2000;27:2473-2481. [PubMed] |
| 30. | Minor MA, Hewett JE, Webel RR, Anderson SK, Kay DR. Efficacy of physical conditioning exercise in patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1989;32:1396-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 332] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 31. | Neuberger GB, Aaronson LS, Gajewski B, Embretson SE, Cagle PE, Loudon JK, Miller PA. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. Arthritis Rheum. 2007;57:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | O'Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis. 1999;58:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 275] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 33. | Patrick DL, Ramsey SD, Spencer AC, Kinne S, Belza B, Topolski TD. Economic evaluation of aquatic exercise for persons with osteoarthritis. Med Care. 2001;39:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Penninx BW, Rejeski WJ, Pandya J, Miller ME, Di Bari M, Applegate WB, Pahor M. Exercise and depressive symptoms: a comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol B Psychol Sci Soc Sci. 2002;57:P124-P132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Rooks DS, Gautam S, Romeling M, Cross ML, Stratigakis D, Evans B, Goldenberg DL, Iversen MD, Katz JN. Group exercise, education, and combination self-management in women with fibromyalgia: a randomized trial. Arch Intern Med. 2007;167:2192-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Sañudo B, Galiano D, Carrasco L, de Hoyo M, McVeigh JG. Effects of a prolonged exercise program on key health outcomes in women with fibromyalgia: a randomized controlled trial. J Rehabil Med. 2011;43:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Schachter CL, Busch AJ, Peloso PM, Sheppard MS. Effects of short versus long bouts of aerobic exercise in sedentary women with fibromyalgia: a randomized controlled trial. Phys Ther. 2003;83:340-358. [PubMed] |
| 38. | Sencan S, Ak S, Karan A, Muslumanoglu L, Ozcan E, Berker E. A study to compare the therapeutic efficacy of aerobic exercise and paroxetine in fibromyalgia syndrome. J Back Musculoskelet Rehabil. 2004;17:57-61. |
| 39. | Tomás-Carúsa P, Gusib N, Lealc A, Garcíab Y, Ortega-Alonso A. The fibromyalgia treatment with physical exercise in warm water reduces the impact of the disease on female patients' physical and mental health. Rheumatol Clin. 2007;3:33-37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Tomas-Carus P, Gusi N, Häkkinen A, Häkkinen K, Leal A, Ortega-Alonso A. Eight months of physical training in warm water improves physical and mental health in women with fibromyalgia: a randomized controlled trial. J Rehabil Med. 2008;40:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Valim V, Oliveira L, Suda A, Silva L, de Assis M, Barros Neto T, Feldman D, Natour J. Aerobic fitness effects in fibromyalgia. J Rheumatol. 2003;30:1060-1069. [PubMed] |
| 42. | Valkeinen H, Alen M, Hannonen P, Häkkinen A, Airaksinen O, Häkkinen K. Changes in knee extension and flexion force, EMG and functional capacity during strength training in older females with fibromyalgia and healthy controls. Rheumatology (Oxford). 2004;43:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Wang C, Schmid CH, Hibberd PL, Kalish R, Roubenoff R, Rones R, McAlindon T. Tai Chi is effective in treating knee osteoarthritis: a randomized controlled trial. Arthritis Rheum. 2009;61:1545-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 44. | Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, Lee Y, McAlindon T. A randomized trial of tai chi for fibromyalgia. N Engl J Med. 2010;363:743-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 275] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 45. | Wigers SH, Stiles TC, Vogel PA. Effects of aerobic exercise versus stress management treatment in fibromyalgia. A 4.5 year prospective study. Scand J Rheumatol. 1996;25:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 208] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | U.S. Department of Health and Human Services.. Physical Activity Guidelines Advisory Committee Report, 2008. Available from: http://health.gov/paguidelines/report/pdf/committeereport.pdf. |
| 47. | Hedges LV, Olkin I. Statistical methods for meta-analysis. San Diego, CA: Academic Press 1985; . |
| 48. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30416] [Article Influence: 779.9] [Reference Citation Analysis (0)] |
| 49. | Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3226] [Cited by in RCA: 3276] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 50. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46527] [Article Influence: 2114.9] [Reference Citation Analysis (3)] |
| 51. | Stouffer SA, Suchman EA, DeVinney LC, Star SA, Williams RM. The American soldier: Adjustment during army life. JAMA. 1949;140:1189. [DOI] [Full Text] |
| 52. | Centers for Disease Control and Prevention. Physical Activity for Arthritis Fact Sheet. 2014; Available from: http: //www.cdc.gov/arthritis/pa_factsheet.htm. |