Published online Nov 12, 2014. doi: 10.5499/wjr.v4.i3.44
Revised: September 1, 2014
Accepted: September 23, 2014
Published online: November 12, 2014
Processing time: 213 Days and 20.2 Hours
Rheumatoid arthritis (RA), the commonest inflammatory arthritis, is a debilitating disease leading to functional and social disability. In addition to the joints, RA affects several other tissues of the body including the muscle. RA patients have significantly less muscle mass compared to the general population. Several theories have been proposed to explain this. High grade inflammation, a central component in the pathophysiology of the disease, has long been proposed as the key driver of muscle wasting. More recent findings however, indicate that inflammation on its own cannot fully explain the high prevalence of muscle wasting in RA. Thus, the contribution of other potential confounders, such as nutrition and physical activity, has also been studied. Results indicate that they play a significant role in muscle wasting in RA, but again neither of these factors seems to be able to fully explain the condition. Oxidative stress is one of the major mechanisms thought to contribute to the development and progression of RA but its potential contribution to muscle wasting in these patients has received limited attention. Oxidative stress has been shown to promote muscle wasting in healthy populations and people with several chronic conditions. Moreover, all of the aforementioned potential contributors to muscle wasting in RA (i.e., inflammation, nutrition, and physical activity) may promote pro- or anti-oxidative mechanisms. This review aims to highlight the importance of oxidative stress as a driving mechanism for muscle wasting in RA and discusses potential interventions that may promote muscle regeneration via reduction in oxidative stress.
Core tip: Muscle wasting is common in rheumatoid arthritis (RA) and associates with significant health burden. To date several theories have been proposed to explain why RA patients loose muscle mass but the exact underlying mechanisms are not clear. Oxidative stress is a key driver of muscle wasting in the general population; however, its potential role in muscle wasting in RA has not been studied. As it arises from this review, oxidative stress seems to contribute to muscle wasting in RA. Further research on the subject is warranted, especially focusing on the underlying mechanisms and potential interventions.
- Citation: Stavropoulos-Kalinoglou A, Deli C, Kitas GD, Jamurtas AZ. Muscle wasting in rheumatoid arthritis: The role of oxidative stress. World J Rheumatol 2014; 4(3): 44-53
- URL: https://www.wjgnet.com/2220-3214/full/v4/i3/44.htm
- DOI: https://dx.doi.org/10.5499/wjr.v4.i3.44
Rheumatoid arthritis (RA) is the most common inflammatory arthritis, with a prevalence of approximately 1% in Europe and North America[1]. It is an autoimmune disease affecting mainly synovial joints[2] and associates with high-grade inflammation characterised by high levels of circulating pro-inflammatory cytokines, including tumour necrosis factor alpha (TNF-α), and the interleukins (IL) 1 and 6. These cytokines are produced in the inflamed synovium and are implicated in joint swelling, pain, and eventually destruction[3]. As the condition progresses, patients very frequently lose their jobs[4], functional ability (movement)[1] and eventually their independence[5]. Apart from this physical and psychological personal burden, RA has significant costs for the health and social care system[6]. Thus, the efforts of the scientific community have focused on the identification and elimination of the potential causes of RA as well as effective treatments. Despite significant therapeutic progress in recent years a cure remains elusive[1].
Apart from the joints, RA affects several other tissues of the body leading to systemic involvement and significant extra-articular manifestations[7]. It is these extra-articular manifestations, and not RA itself, that significantly shorten the life of RA patients and add extra layers of morbidity. Cardiac and vascular conditions are especially common among these patients. Heart disease in RA is both more prevalent and more likely to lead to death than in the general population[8]. The exact cause for this remains unknown, however genetic predisposition[9-12], classical cardiovascular disease risk factors[13,14] and the effects of systemic inflammation on the vasculature[15,16] are all thought to contribute. Other extra-articular manifestations are observed in the skin, eyes, lungs, renal, nervous and gastrointestinal systems[17].
The reasons for the development of such manifestations are not fully understood. It is believed that the endocrine functions of the pro-inflammatory cytokines (mainly TNF-α, IL-1 and IL-6) initiate a cascade of destructive processes in distant tissues, with reactive oxygen species playing a central role in cell membrane destruction and cellular death[18].
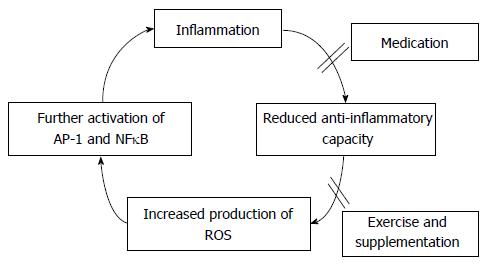
Free radicals, such as reactive oxygen species (ROS), have been proposed to play a significant role both in the development and progression of inflammation, as well as its deleterious effects on cell structure and function at the site of the inflammation and in distant tissues[19,20]. Free radicals, formed as by-products of normal biological processes - such as cellular metabolism in the mitochondrial electron transport chain and reperfusion injury - are highly reactive agents that can cause physiological damage[21]. Free radicals can damage all cellular components such as lipids, proteins and DNA. In the general population, they are counterbalanced by effective antioxidant defence mechanisms. However, in inflammatory conditions these defence mechanisms seem to be weakened[22]. It is not clear which is the sequence of events but it seems likely that inflammation reduces the anti-oxidant response, thereby increasing the accumulation of free radicals[19]. These further activate pro-inflammatory nuclear pathways (specifically Activator Protein one - AP-1 and nuclear factor kappa β - NFκB) that transcribe cytokines and adhesion molecules involved in the modulation of inflammation[23] resulting in further production of free radicals. Nitric oxide (NO) has a role in the regulation of vascular tone, superoxide free radical (O2·-) in fibroblast proliferation and hydrogen peroxide (H2O2) in the activation of pro-inflammatory transcription factors. Other control mechanisms which may be perturbed in inflammation include: the oxidative modification of low density lipoprotein, the oxidative inactivation of alpha-1-protease inhibitor, DNA damage, lipid peroxidation and heat shock protein formed with the activation of neutrophil, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and endothelial cell xanthine dehydrogenase, which associate with oxidative stress and contribute significantly to the inflammatory process[19,22,23].
Oxidative stress is frequently reported in patients with RA. Cells present in inflamed joints (e.g., macrophages, neutrophils and lymphocytes), have the ability to produce free radicals[24,25]. These liberate superoxide radical, hydrogen peroxide, elastase, hypochlorus acid and eicosanoids[26]. Another source of free radicals in RA is hypoxic reperfusion injury from elevated synovial cavity pressure during joint movement. A fivefold increase in mitochondrial ROS production in whole blood and monocytes of patients with RA compared with healthy subjects suggests that oxidative stress is a significant factor in RA[26].
Free radicals are implicated in joint damage both indirectly (via increasing inflammation as described above) and directly. They can degrade joint cartilage, by attacking proteoglycans (integral components of structural tissues) and inhibiting proteoglycan synthesis[27]. Indeed in patients with RA, serum and synovial fluid contain end products of lipid peroxidation which correlate with disease severity and activity[28]. In parallel, anti-oxidant capacity in patients with RA seems to be significantly reduced[29]. This low antioxidant status has been associated with low levels of tocopherols, beta-carotene and retinols and low activities of glutathione reductase and superoxide dismutase[26]. RA has also been linked to low levels of reduced glutathione- an intracellular antioxidant- in synovial fluid T cells[30]. Reduced glutathione, is among the most prominent defences against reactive oxygen species. It is a substrate for glutathione peroxidases, several transferases and several other enzymes and acts as a general radical quencher in cells by removing superoxide anions and hydrogen peroxide[26]. Serum concentrations of anti-oxidative vitamins A, C and E are also significantly reduced in RA[19,23,31].
A significant but little studied extra-articular manifestation of RA is rheumatoid cachexia (RC). RC is characterised by a high rate of muscle mass and strength loss, typically with preservation or slight increase in fat mass[32]. RC differs from other forms of cachexia such as those observed in cancer, chronic heart failure, kidney disease or chronic infection as it is rarely accompanied by a net weight loss[33]. RC also differs from sarcopenia (age-related reduction in muscle mass observed in the elderly) as it occurs at a younger age and muscle mass loss progresses at a substantially higher rate[34]. The prevalence of RA in the United Kingdom is 0.8%[5]. The exact prevalence of RC is not known today as there is no consensus on its definition and assessment. However, at least 10%-20% of patients with controlled RA[35,36] and > 40% of patients with active RA[37] suffer from muscle wasting. This makes it one of the most common complications of RA.
RC has been shown to associate with poorer disease outcomes including reduced quality of life, more fatigue and increased overall morbidity and mortality[34,38,39], although the independent nature and directionality of many of these associations remain uncertain and require further study. Low muscle mass also associates with dysmetabolic states such as insulin resistance and type II diabetes[40,41] and thus may be partly responsible for the increased cardiovascular risk observed in RA[33,35,42]. Inflammation associated with the disease is consistently identified as the potential cause of these manifestations. Indeed, inflammatory cytokines produced at the site of the disease (i.e., the joints) have endocrine functions and act on distant tissues such as the muscle[43].
Inflammation: High plasma concentrations of the inflammatory cytokines implicated in RA pathophysiology (TNF-α, IL-1 and IL-6) are thought to trigger muscle wasting[34,44]. TNF-α-induced activation of the classical NFκB pathway is now generally accepted to lead to inhibition of skeletal muscle differentiation and regeneration in a variety of muscle diseases, although this has not been confirmed yet as a mechanism of muscle wasting in RA patients. IL-1 among other cytokines has been shown to prevent the anabolic effect of insulin growth factor 1 (IGF-1) on myoblast differentiation, muscle protein synthesis, and myogenin expression[44,45], while intravenous infusion of IL-6 in healthy volunteers led to net muscle protein degradation in healthy individuals[46]. In RA patients, short term (3-6 mo) anti-TNF-α medication led to significant reduction of disease activity but did not improve body composition and had no effect of muscle mass[47,48] suggesting that cytokines might not be the most significant contributors to muscle wasting in RA.
Physical inactivity: Physical inactivity is the strongest predictor of fat mass in RA[49], while resistance exercise interventions may result in increased muscle mass and strength and partial reversal of muscle wasting in patients with RA[50,51]. Therefore muscle wasting in RA seems to be a consequence of a negative spiral between the metabolic and functional consequences of inflammation which enhance muscle catabolism and the premature adoption of an increasingly sedentary lifestyle in which the anabolic stimulus of regular exercise is missing[49] with consequent increase in fat mass. In line with this hypothesis is the observation that obesity is a common feature of RA and adds to the high risk for the metabolic syndrome and cardiovascular disease[34,52,53].
Adiposity: It is reasonable to assume that there are parallels between the mechanisms that lead to sarcopenia in healthy sedentary elderly individuals and the mechanisms that lead to muscle mass loss in RA patients. It is also reasonable to assume that there are parallels between the impact of obesity on the rate of sarcopenia and the potential role that obesity plays in the mechanisms that lead to muscle wasting in RA. An inherent consequence of the adoption of a sedentary life-style, without a reduction in energy intake, is a gradual increase of the subcutaneous and visceral adipose tissue mass[54]. Adipose tissue (especially visceral) is a well-known source of inflammation. In addition to adipocytes, pre-adipocytes and fibroblasts, up to 50% of the cell mass in adipose tissue of obese individuals are inflammatory cells such as monocytes and macrophages[55,56]. Adipocytes and macrophages both are a source of inflammatory cytokines[55,56]. In addition, the large adipose tissue stores in obese individuals are a constant source of lipolysis and lead to high circulatory concentrations of fatty acids and triglycerides. High plasma concentrations of inflammatory cytokines, FA and triglycerides contribute to the insulin resistance of skeletal muscle and its microvasculature[54-56] via mechanisms outlined below.
Insulin resistance: The most striking change in skeletal muscle through a sedentary lifestyle is a reduction of the mitochondrial density[54] and, therefore, of oxidative capacity of blood-borne fatty acids (NEFA’s). Sedentary muscles also have a reduced capacity to oxidize the lipid droplets that are stored in the muscle in the vicinity of the mitochondria[54,57,58]. This, combined with an increased supply of plasma fatty acids and triglycerides, leads to the accumulation of fatty acid metabolites (long-chain fatty acyl-coenzyme A, diacylglycerols and ceramides). Both these fatty acid metabolites and the exposure of the muscle to inflammatory cytokines activate serine kinases that lead to serine phosphorylation of insulin receptor substrate 1 (IRS-1) and prevent downstream activation of the insulin signalling cascade and, therefore, impair glucose transporter type 4 translocation and glucose uptake[58]. Insulin resistance (IR) also leads to an imbalance between protein synthesis and degradation[59] and is a major cause of the muscle mass loss in sedentary obese elderly individuals.
Endothelial dysfunction: The overload of the muscle with fatty acids, triglycerides and inflammatory cytokines also leads to major impairments in its associated vasculature. The endothelial cells that cover the luminal wall of feeding arteries, resistance arteries, and terminal arterioles (which control blood supply to the capillaries) normally dilate if they are exposed to meal-induced increases in insulin concentration[54,60]. Insulin in endothelial cells activates the enzyme eNOS [endothelial nitric oxide (NO) synthase] and the resultant NO leads to dilation of the smooth muscle layer in arteries and arterioles. This mechanism ensures that in the period after meal ingestion maximal amounts of glucose, amino acids and insulin are channelled to the muscle to maximize glucose uptake, increase protein synthesis and reduce protein degradation[54,60]. Vascular overload with lipids and inflammatory cytokines also leads to endothelial IR and reduces the supply of blood and nutrients to muscles of obese individuals[54,60].
To our knowledge, there is no study today investigating the associations of oxidative stress with muscle wasting in RA. However, there are numerous reports in the general population and several other conditions showing that oxidative stress may be a very important underlying mechanism that drives muscle wasting.
Experiments in obese Zucker rats and incubated endothelial cells have shown that high concentrations of long-chain fatty acylCoA and diacylglycerol activate protein kinase C (PKC)-β in aortic endothelium[61] and prevent insulin-induced activation of IRS-1, Akt, eNOS phosphorylation and increases in NO production. A high lipid and cytokine load (via PKC-activation) also leads to induction of NADPH oxidase in the vasculature of patients with the metabolic syndrome, hypertension or cardiovascular disease[54]. High NADPH oxidase activity will lead to excess production of superoxide anions (O2-) which will scavenge NO thereby reducing basal and insulin-induced NO-production. Superoxide anions react with NO resulting in the formation of peroxynitrite and reducing the amount of NO available for vasodilation[62].
Physical inactivity, in a population with constantly high grade inflammation, such as those with RA, may lead to significant intramuscular accumulation of ROS, as is the case for muscle disuse (e.g., due to limb immobilization or bed rest) in the general population[63]. Muscle atrophy from disuse is mainly attributed to oxidative stress, i.e., reduces anti-oxidant capacity and increased ROS production[64,65]. Mitochondria are the site for excessive ROS production[65,66]; and ROS production, such as H2O2, is increased by up to 100% following 14 d of limb immobilization[67]. Moreover, xanthine oxidase and NADPH oxidase contribute but to a lesser degree to ROS production in muscle disuse[68,69]. Similarly, lipid peroxidation has been shown to associate with muscle atrophy[70].
These affect the balance between protein synthesis and degradation[71,72]. Specifically, disturbed redox balance due to intramuscular ROS accumulation, such as that of H2O2[73,74], may activate transcriptional factors that increase expression of apoptotic pathways, such as the NF-κβ pathway and Foxo leading to severe protein degradation[68,75]. Moreover, oxidative stress may activate calpain and caspase-3, further increasing proteolytic processes[68,76]. Oxidation of muscle proteins themselves makes them susceptible to proteolytic damage[77].
ROS accumulation may also inhibit signalling pathways controlling protein synthesis[78,79]. It seems that ROS inhibit mRNA translation at an early stage; this reduces the ability of senescent satellite cells to become active and infiltrate the muscle cell[79,80]. However, these studies where performed in cell cultures, and it is not clear if these processes also occur in vivo.
Muscle wasting is commonly observed in the elderly, affecting their quality of life and independence[81]. Oxidative stress has long been associated with aging related processes[82]. The elderly exhibit increased concentrations of oxidative by-products compared to younger individuals[83,84]. In normal aging, ROS participate in a number of processes aiding the transmission of signals within the muscle and affect gene expression[85,86]. As is the case with disuse atrophy, in aging mitochondria are also the main site for ROS production. Aging mitochondria seem to produce larger amounts of ROS, and especially H2O2, compared to younger ones[87]. The wasting effect of H2O2 seems to be mediated by the presence of the Copper and Zinc containing superoxide dismutase (Cu,ZnSOD)[88,89]. Cu,ZnSOD has been shown to decrease with aging[90]. In animal studies, SOD1 (gene encoding Cu,ZnSOD) knockout mice exhibited a form of rapid muscle wasting with similar characteristics to that of aging, including oxidative stress and weakness[91].
The vast majority of studies in this field have focused on the use of exogenous anti-oxidative agents, such as the administration of vitamins (A, C, E) and omega-3 fatty acids. Vitamin E seems to uncouple joint inflammation and joint destruction in the transgenic KRN/NOD mouse model of RA, with a beneficial effect on joint destruction[92]. Some studies have even attributed therapeutic value to antioxidant supplementation as they reported better control[93] and improvement of RA-related symptoms[94]. Dietary interventions have been suggested to improve plasma levels of vitamin C, retinol and uric acid, which inversely correlate with variables related to disease activity[95]. Moreover, proper dietary antioxidant nutrient intake may reduce generation of free radicals and improve antioxidant status in RA patients[96]. Finally, intake of certain antioxidant micronutrients particularly beta-cryptoxanthine, supplemental zinc, and possibly diet in fruits and cruciferous vegetables have been suggested to protect against the development of RA[97].
Increasing anti-oxidant capacity in RA is a very attractive and potentially effective intervention. In current clinical practice, vitamin and micronutrient supplementation is frequently prescribed. However, we now know, that polypharmacy (prescription of a large number of medications) is one of the most significant reasons why patients forget to take their pills[98]. However, the most important question concerning use of antioxidants, is that of suicidal oxidative stress[19]. In certain conditions, such as presence of transition metals, antioxidants can act as pro-oxidants[99]. Similarly, high concentrations of anti-oxidants can cause the cell to undergo severe oxidative stress ultimately resulting in suicidal cell death[100].
In very recent years, the anti-oxidant potential of antiTNF therapy has also been investigated. Infliximab plays an essential role as an anti-oxidative agent against advanced glycation end-product formation, oxidative DNA damage and lipid peroxidation[101], whereas etanercept acts as a regulator against pentosidine formation, oxidative DNA damage, and lipid peroxidation in RA patients[102].
In the general population, exercise has been shown to increase anti-oxidant capacity. Working via the physiological concept of hormesis (an ancient practice where the induction of a sub-lethal dose of toxin was used to increase tolerance of the organism to withstand higher doses of toxins) acute exercise increases free radical production[103], in a dose-response fashion (i.e., increasing intensity, increases free radical production). This exercised-induced increase in free radicals is due to the increased electron leak from the mitochondria as well as the alterations in blood flow and oxygen supply that occur in response to exercise[104,105].
However, it has been consistently observed that trained individuals have high levels of antioxidant enzymes and certain nonenzymatic antioxidants in muscle[106] and demonstrate greater resistance to exercise-induced or -imposed oxidative stress[107,108]. Most likely, these adaptations result from cumulative effects of repeated exercise bouts on the gene expression of antioxidant enzymes. However, the attenuation of oxidative stress by exercise is reduced in the aging muscle, warranting concomitant nutritional supplementation with anti-oxidants to elicit the greatest potential benefits[109].
Exercise is a useful tool, with constantly increasing clinical relevance to several conditions. In recent years, a large number of studies have investigated the safety of different exercise modalities in RA. Despite the common misconception that it may increase joint pain and damage, all of the studies indicate that properly designed exercise interventions are safe and beneficial for RA patients[110]. de Jong et al[111-113] have investigated the safety of intensive aerobic exercise (in the form of cycling) in > 200 RA patients; they concluded that all patients were able to achieve the pre-determined intensity targets. However, they pointed out that patients with severe joint damage may need attention[114]. Along similar lines, resistance training improved body composition and muscle mass without any adverse effects on disease activity[50]. Finally, we have recently completed a randomised trial looking at the effects of intensive aerobic exercise on cardiovascular risk factors in RA patients[115]. From these and other studies[116-120], it is clear that exercise is a safe intervention for RA patients and its use in the clinical setting is gaining significant support. Moreover, it is evident that exercise is able to reverse muscle wasting and increase muscle mass in RA patients. Indeed, the regenerative capacity of the RA muscle seems to be unaffected as the number of satellite cells (muscular stem cells that are utilised for muscular regeneration) present in it are preserved[121] but the stimulus for their activation (i.e., exercise) is absent.
The role of oxidative stress in muscle wasting has been clearly demonstrated in several studies. However, to date there is no study looking at this in RA patients. We suggest that there is significant scope for such research in RA as the potential mechanisms by which oxidative stress drives muscle wasting have been already described in other populations. Identification of specific mechanisms induced by RA-associated inflammation could significantly aid towards improvement of pharmacological and non-pharmacological interventions aiming to counteract oxidative stress in RA. In addition to effective control of inflammation via medication, exercise and nutrition may prove significant aids towards the reduction of oxidative stress (Figure 1).
P- Reviewer: La Montagna G, Sakkas L S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 586] [Article Influence: 29.3] [Reference Citation Analysis (2)] |
| 2. | Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, Scott D, Silman A. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology (Oxford). 2002;41:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 416] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 3. | Buch M, Emery P. The aetiology and pathogenesis of rheumatiod arthritis. Hospital Pharmacist. 2002;9:5-10. |
| 4. | Callahan LF, Yelin EH. The social and economic consequences of rheumatic disease. Primer on the rheumatic diseases. 12th ed. Atlanta: Arthritis Foundation 2001; 1-4. |
| 5. | Westhoff G, Listing J, Zink A. Loss of physical independence in rheumatoid arthritis: interview data from a representative sample of patients in rheumatologic care. Arthritis Care Res. 2000;13:11-22. [PubMed] [DOI] [Full Text] |
| 6. | Pugner KM, Scott DI, Holmes JW, Hieke K. The costs of rheumatoid arthritis: an international long-term view. Semin Arthritis Rheum. 2000;29:305-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Turesson C, Jacobsson LT. Epidemiology of extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol. 2004;33:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 677] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 9. | Gonzalez-Gay MA, Gonzalez-Juanatey C, Lopez-Diaz MJ, Piñeiro A, Garcia-Porrua C, Miranda-Filloy JA, Ollier WE, Martin J, Llorca J. HLA-DRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2007;57:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 289] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 10. | Panoulas VF, Nikas SN, Smith JP, Douglas KM, Nightingale P, Milionis HJ, Treharne GJ, Toms TE, Kita MD, Kitas GD. Lymphotoxin 252A& gt; G polymorphism is common and associates with myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:1550-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Panoulas VF, Stavropoulos-Kalinoglou A, Metsios GS, Smith JP, Milionis HJ, Douglas KM, Nightingale P, Kitas GD. Association of interleukin-6 (IL-6)-174G/C gene polymorphism with cardiovascular disease in patients with rheumatoid arthritis: the role of obesity and smoking. Atherosclerosis. 2009;204:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Mattey DL, Dawes PT, Nixon NB, Goh L, Banks MJ, Kitas GD. Increased levels of antibodies to cytokeratin 18 in patients with rheumatoid arthritis and ischaemic heart disease. Ann Rheum Dis. 2004;63:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Panoulas VF, Douglas KM, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, Tselios AL, Metsios GS, Elisaf MS, Kitas GD. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Toms TE, Panoulas VF, Douglas KM, Griffiths HR, Kitas GD. Lack of association between glucocorticoid use and presence of the metabolic syndrome in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2008;10:R145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Stevens RJ, Douglas KM, Saratzis AN, Kitas GD. Inflammation and atherosclerosis in rheumatoid arthritis. Expert Rev Mol Med. 2005;7:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Gonzalez A, Maradit Kremers H, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ, O’Fallon WM, Gabriel SE. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis. 2008;67:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R. Extra-articular Manifestations in Rheumatoid Arthritis. Maedica (Buchar). 2010;5:286-291. [PubMed] |
| 18. | Prete M, Racanelli V, Digiglio L, Vacca A, Dammacco F, Perosa F. Extra-articular manifestations of rheumatoid arthritis: An update. Autoimmun Rev. 2011;11:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Mahajan A, Tandon VR. Antioxidants and Rheumatoid Arthritis. J Indian Rheumatol Assoc. 2004;12:139-142. |
| 20. | Winrow VR, Winyard PG, Morris CJ, Blake DR. Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull. 1993;49:506-522. [PubMed] |
| 21. | Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 564] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 22. | Vasanthi P, Nalini G, Rajasekhar G. Status of oxidative stress in rheumatoid arthritis. Int J Rheum Dis. 2009;12:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Sukkar SG, Rossi E. Oxidative stress and nutritional prevention in autoimmune rheumatic diseases. Autoimmun Rev. 2004;3:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Maly FE, Cross AR, Jones OT, Wolf-Vorbeck G, Walker C, Dahinden CA, De Weck AL. The superoxide generating system of B cell lines. Structural homology with the phagocytic oxidase and triggering via surface Ig. J Immunol. 1988;140:2334-2339. [PubMed] |
| 25. | Baskol G, Demir H, Baskol M, Kilic E, Ates F, Kocer D, Muhtaroglu S. Assessment of paraoxonase 1 activity and malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem. 2005;38:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Hassan MQ, Hadi RA, Al-Rawi ZS, Padron VA, Stohs SJ. The glutathione defense system in the pathogenesis of rheumatoid arthritis. J Appl Toxicol. 2001;21:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Hadjigogos K. The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med. 2003;45:7-13. [PubMed] |
| 28. | Rowley D, Gutteridge JM, Blake D, Farr M, Halliwell B. Lipid peroxidation in rheumatoid arthritis: thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci (Lond). 1984;66:691-695. [PubMed] |
| 29. | Kamanli A, Naziroğlu M, Aydilek N, Hacievliyagil C. Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem Funct. 2004;22:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Kalpakcioglu B, Senel K. The interrelation of glutathione reductase, catalase, glutathione peroxidase, superoxide dismutase, and glucose-6-phosphate in the pathogenesis of rheumatoid arthritis. Clin Rheumatol. 2008;27:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Henderson CJ, Panush RS. Diets, dietary supplements, and nutritional therapies in rheumatic diseases. Rheum Dis Clin North Am. 1999;25:937-968, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Dawson-Hughes B, Dinarello CA, Rosenberg IH. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest. 1994;93:2379-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 389] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 33. | Summers GD, Metsios GS, Stavropoulos-Kalinoglou A, Kitas GD. Rheumatoid cachexia and cardiovascular disease. Nat Rev Rheumatol. 2010;6:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Roubenoff R. Rheumatoid cachexia: a complication of rheumatoid arthritis moves into the 21st century. Arthritis Res Ther. 2009;11:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 35. | Metsios GS, Stavropoulos-Kalinoglou A, Panoulas VF, Sandoo A, Toms TE, Nevill AM, Koutedakis Y, Kitas GD. Rheumatoid cachexia and cardiovascular disease. Clin Exp Rheumatol. 2009;27:985-988. [PubMed] |
| 36. | Elkan AC, Håkansson N, Frostegård J, Cederholm T, Hafström I. Rheumatoid cachexia is associated with dyslipidemia and low levels of atheroprotective natural antibodies against phosphorylcholine but not with dietary fat in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2009;11:R37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Engvall IL, Elkan AC, Tengstrand B, Cederholm T, Brismar K, Hafstrom I. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scand J Rheumatol. 2008;37:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Kremers HM, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Giles JT, Bartlett SJ, Andersen RE, Fontaine KR, Bathon JM. Association of body composition with disability in rheumatoid arthritis: impact of appendicular fat and lean tissue mass. Arthritis Rheum. 2008;59:1407-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S-1003S. [PubMed] |
| 41. | Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953-963. [PubMed] |
| 42. | Kitas GD, Erb N. Tackling ischaemic heart disease in rheumatoid arthritis. Rheumatology (Oxford). 2003;42:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4404] [Cited by in RCA: 4605] [Article Influence: 177.1] [Reference Citation Analysis (0)] |
| 44. | Bakkar N, Guttridge DC. NF-kappaB signaling: a tale of two pathways in skeletal myogenesis. Physiol Rev. 2010;90:495-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, Dantzer R, Kelley KW. IL-1beta impairs insulin-like growth factor i-induced differentiation and downstream activation signals of the insulin-like growth factor i receptor in myoblasts. J Immunol. 2004;172:7713-7720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | van Hall G, Steensberg A, Fischer C, Keller C, Møller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab. 2008;93:2851-2858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Metsios GS, Stavropoulos-Kalinoglou A, Douglas KM, Koutedakis Y, Nevill AM, Panoulas VF, Kita M, Kitas GD. Blockade of tumour necrosis factor-alpha in rheumatoid arthritis: effects on components of rheumatoid cachexia. Rheumatology (Oxford). 2007;46:1824-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 48. | Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. Am J Clin Nutr. 2006;84:1463-1472. [PubMed] |
| 49. | Stavropoulos-Kalinoglou A, Metsios GS, Smith JP, Panoulas VF, Douglas KM, Jamurtas AZ, Koutedakis Y, Kitas GD. What predicts obesity in patients with rheumatoid arthritis? An investigation of the interactions between lifestyle and inflammation. Int J Obes (Lond). 2010;34:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Marcora SM, Lemmey AB, Maddison PJ. Can progressive resistance training reverse cachexia in patients with rheumatoid arthritis? Results of a pilot study. J Rheumatol. 2005;32:1031-1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Lemmey AB, Marcora SM, Chester K, Wilson S, Casanova F, Maddison PJ. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum. 2009;61:1726-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 52. | Metsios GS, Stavropoulos-Kalinoglou A, Panoulas VF, Wilson M, Nevill AM, Koutedakis Y, Kitas GD. Association of physical inactivity with increased cardiovascular risk in patients with rheumatoid arthritis. Eur J Cardiovasc Prev Rehabil. 2009;16:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Nevill AM, Douglas KM, Jamurtas A, van Zanten JJ, Labib M, Kitas GD. Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis. 2007;66:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Wagenmakers AJ, van Riel NA, Frenneaux MP, Stewart PM. Integration of the metabolic and cardiovascular effects of exercise. Essays Biochem. 2006;42:193-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1515] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 56. | Andersson CX, Gustafson B, Hammarstedt A, Hedjazifar S, Smith U. Inflamed adipose tissue, insulin resistance and vascular injury. Diabetes Metab Res Rev. 2008;24:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Shaw CS, Jones DA, Wagenmakers AJ. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem Cell Biol. 2008;129:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Shaw CS, Clark J, Wagenmakers AJ. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu Rev Nutr. 2010;30:13-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Wagenmakers AJ. Tracers to investigate protein and amino acid metabolism in human subjects. Proc Nutr Soc. 1999;58:987-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 61. | Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 62. | Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 63. | Powers SK, Smuder AJ, Judge AR. Oxidative stress and disuse muscle atrophy: cause or consequence? Curr Opin Clin Nutr Metab Care. 2012;15:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 64. | Falk DJ, Deruisseau KC, Van Gammeren DL, Deering MA, Kavazis AN, Powers SK. Mechanical ventilation promotes redox status alterations in the diaphragm. J Appl Physiol (1985). 2006;101:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med. 2009;46:842-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 66. | Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, Kavazis AN, Smuder AJ. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med. 2011;39:1749-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 67. | Min K, Smuder AJ, Kwon OS, Kavazis AN, Szeto HH, Powers SK. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol (1985). 2011;111:1459-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 68. | McClung JM, Judge AR, Talbert EE, Powers SK. Calpain-1 is required for hydrogen peroxide-induced myotube atrophy. Am J Physiol Cell Physiol. 2009;296:C363-C371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 69. | Whidden MA, McClung JM, Falk DJ, Hudson MB, Smuder AJ, Nelson WB, Powers SK. Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J Appl Physiol (1985). 2009;106:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Kondo H, Miura M, Itokawa Y. Oxidative stress in skeletal muscle atrophied by immobilization. Acta Physiol Scand. 1991;142:527-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337-R344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 263] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 72. | Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol (1985). 2007;102:2389-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 73. | Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol. 2003;285:C806-C812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 247] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 74. | McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, Gayan-Ramirez G, Decramer M, DeRuisseau KC, Powers SK. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med. 2009;37:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Dodd SL, Gagnon BJ, Senf SM, Hain BA, Judge AR. Ros-mediated activation of NF-kappaB and Foxo during muscle disuse. Muscle Nerve. 2010;41:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 76. | McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. 2010;298:C542-C549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 77. | Smuder AJ, Kavazis AN, Hudson MB, Nelson WB, Powers SK. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic Biol Med. 2010;49:1152-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 78. | O’Loghlen A, Pérez-Morgado MI, Salinas M, Martín ME. N-acetyl-cysteine abolishes hydrogen peroxide-induced modification of eukaryotic initiation factor 4F activity via distinct signalling pathways. Cell Signal. 2006;18:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Zhang L, Kimball SR, Jefferson LS, Shenberger JS. Hydrogen peroxide impairs insulin-stimulated assembly of mTORC1. Free Radic Biol Med. 2009;46:1500-1509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr. 2006;83:500S-507S. [PubMed] |
| 81. | Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911-M916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 354] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 82. | HARMAN D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5844] [Cited by in RCA: 5412] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 83. | Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic Biol Med. 1998;25:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 185] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 84. | Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. 2006;20:1549-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 85. | Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6483] [Cited by in RCA: 6350] [Article Influence: 276.1] [Reference Citation Analysis (0)] |
| 86. | Jackson MJ, Papa S, Bolaños J, Bruckdorfer R, Carlsen H, Elliott RM, Flier J, Griffiths HR, Heales S, Holst B. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002;23:209-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 87. | Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid Redox Signal. 2006;8:582-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 88. | Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064-49073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 731] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 89. | Iñarrea P. Purification and determination of activity of mitochondrial cyanide-sensitive superoxide dismutase in rat tissue extract. Methods Enzymol. 2002;349:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Jackson MJ. Skeletal muscle aging: role of reactive oxygen species. Crit Care Med. 2009;37:S368-S371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, Csete M. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 347] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 92. | De Bandt M, Grossin M, Driss F, Pincemail J, Babin-Chevaye C, Pasquier C. Vitamin E uncouples joint destruction and clinical inflammation in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum. 2002;46:522-532. [PubMed] |
| 93. | Helmy M, Shohayeb M, Helmy MH, el-Bassiouni EA. Antioxidants as adjuvant therapy in rheumatoid disease. A preliminary study. Arzneimittelforschung. 2001;51:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Jaswal S, Mehta HC, Sood AK, Kaur J. Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin Chim Acta. 2003;338:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 95. | Hagfors L, Leanderson P, Sköldstam L, Andersson J, Johansson G. Antioxidant intake, plasma antioxidants and oxidative stress in a randomized, controlled, parallel, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr J. 2003;2:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Bae SC, Kim SJ, Sung MK. Inadequate antioxidant nutrient intake and altered plasma antioxidant status of rheumatoid arthritis patients. J Am Coll Nutr. 2003;22:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 97. | Cerhan JR, Saag KG, Merlino LA, Mikuls TR, Criswell LA. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am J Epidemiol. 2003;157:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 98. | Treharne GJ, Douglas KM, Iwaszko J, Panoulas VF, Hale ED, Mitton DL, Piper H, Erb N, Kitas GD. Polypharmacy among people with rheumatoid arthritis: the role of age, disease duration and comorbidity. Musculoskeletal Care. 2007;5:175-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22:749-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1607] [Cited by in RCA: 1454] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 100. | Offer T, Russo A, Samuni A. The pro-oxidative activity of SOD and nitroxide SOD mimics. FASEB J. 2000;14:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 101. | Kageyama Y, Takahashi M, Ichikawa T, Torikai E, Nagano A. Reduction of oxidative stress marker levels by anti-TNF-alpha antibody, infliximab, in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2008;26:73-80. [PubMed] |
| 102. | Kageyama Y, Takahashi M, Nagafusa T, Torikai E, Nagano A. Etanercept reduces the oxidative stress marker levels in patients with rheumatoid arthritis. Rheumatol Int. 2008;28:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 103. | Davison GW, Morgan RM, Hiscock N, Garcia JM, Grace F, Boisseau N, Davies B, Castell L, McEneny J, Young IS. Manipulation of systemic oxygen flux by acute exercise and normobaric hypoxia: implications for reactive oxygen species generation. Clin Sci (Lond). 2006;110:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 619] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 105. | Nikolaidis MG, Jamurtas AZ. Blood as a reactive species generator and redox status regulator during exercise. Arch Biochem Biophys. 2009;490:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 106. | Kostaropoulos IA, Nikolaidis MG, Jamurtas AZ, Ikonomou GV, Makrygiannis V, Papadopoulos G, Kouretas D. Comparison of the blood redox status between long-distance and short-distance runners. Physiol Res. 2006;55:611-616. [PubMed] |
| 107. | Ji LL, Leeuwenburgh C, Leichtweis S, Gore M, Fiebig R, Hollander J, Bejma J. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann N Y Acad Sci. 1998;854:102-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 108. | Nikolaidis MG, Jamurtas AZ, Paschalis V, Fatouros IG, Koutedakis Y, Kouretas D. The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: magnitude and time-course considerations. Sports Med. 2008;38:579-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 109. | Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fanò G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 304] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 110. | Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen van Zanten JJ, Treharne GJ, Panoulas VF, Douglas KM, Koutedakis Y, Kitas GD. Rheumatoid arthritis, cardiovascular disease and physical exercise: a systematic review. Rheumatology (Oxford). 2008;47:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 111. | de Jong Z, Munneke M, Zwinderman AH, Kroon HM, Jansen A, Ronday KH, van Schaardenburg D, Dijkmans BA, Van den Ende CH, Breedveld FC. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? Results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 112. | de Jong Z, Munneke M, Kroon HM, van Schaardenburg D, Dijkmans BA, Hazes JM, Vliet Vlieland TP. Long-term follow-up of a high-intensity exercise program in patients with rheumatoid arthritis. Clin Rheumatol. 2009;28:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | de Jong Z, Vliet Vlieland TP. Safety of exercise in patients with rheumatoid arthritis. Curr Opin Rheumatol. 2005;17:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | de Jong Z, Munneke M, Zwinderman AH, Kroon HM, Ronday KH, Lems WF, Dijkmans BA, Breedveld FC, Vliet Vlieland TP, Hazes JM. Long term high intensity exercise and damage of small joints in rheumatoid arthritis. Ann Rheum Dis. 2004;63:1399-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 115. | Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 116. | Allen SH, Minor MA, Hillman LS, Kay DR. Effect of exercise on the bone mineral density and bone remodelling indices in women with rheumatoid arthritis: 2 case studies. J Rheumatol. 1993;20:1247-1249. [PubMed] |
| 117. | Anandarajah AP, Schwarz EM. Dynamic exercises in patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:1359-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 118. | Baslund B, Lyngberg K, Andersen V, Halkjaer Kristensen J, Hansen M, Klokker M, Pedersen BK. Effect of 8 wk of bicycle training on the immune system of patients with rheumatoid arthritis. J Appl Physiol (1985). 1993;75:1691-1695. [PubMed] |
| 119. | Bilberg A, Ahlmén M, Mannerkorpi K. Moderately intensive exercise in a temperate pool for patients with rheumatoid arthritis: a randomized controlled study. Rheumatology (Oxford). 2005;44:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 120. | de Jong Z, Munneke M, Lems WF, Zwinderman AH, Kroon HM, Pauwels EK, Jansen A, Ronday KH, Dijkmans BA, Breedveld FC. Slowing of bone loss in patients with rheumatoid arthritis by long-term high-intensity exercise: results of a randomized, controlled trial. Arthritis Rheum. 2004;50:1066-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Beenakker KG, Duijnisveld BJ, Van Der Linden HM, Visser CP, Westendorp RG, Butler-Brown G, Nelissen RG, Maier AB. Muscle characteristics in patients with chronic systemic inflammation. Muscle Nerve. 2012;46:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |