Published online Mar 22, 2016. doi: 10.5498/wjp.v6.i1.84
Peer-review started: September 4, 2015
First decision: September 28, 2015
Revised: December 24, 2015
Accepted: January 21, 2016
Article in press: January 22, 2016
Published online: March 22, 2016
Processing time: 200 Days and 17.3 Hours
Brain-derived neurotrophic factor (BDNF) plays an important role in central nervous system development, neurogenesis and neuronal plasticity. BDNF is also expressed in several non-neuronal tissues, and it could play an important role in other processes, such as cancer, angiogenesis, etc. Platelets are the major source of peripheral BDNF. However, platelets also contain high amounts of serotonin; they express specific surface receptors during activation, and a multitude of pro-inflammatory and immunomodulatory bioactive compounds are secreted from the granules. Until recently, there was insufficient knowledge regarding the relationship between BDNF and platelets. Recent studies showed that BDNF is present in two distinct pools in platelets, in α-granules and in the cytoplasm, and only the BDNF in the granules is secreted following stimulation, representing 30% of the total BDNF in platelets. BDNF has an important role in the pathophysiology of depression. Low levels of serum BDNF have been described in patients with major depressive disorder, and BDNF levels increased with chronic antidepressant treatment. Interestingly, there is an association between depression and platelet function. This review analyzed studies that evaluated the relationship between BDNF and platelet activation and the effect of treatments on both parameters. Only a few studies consider this possible confounding factor, and it could be very important in diseases such as depression, which show changes in both parameters.
Core tip: Brain-derived neurotrophic factor (BDNF) is expressed in neuronal and non- neuronal tissues and is stored peripherally in platelets. Platelet BDNF is present in α-granules and cytoplasm and only BDNF of granules is released by agonist stimulation. Little is known about mechanisms related to BDNF release in human platelets. Depressive disorders are associated with BDNF and platelet dysfunction. Low levels of serum BDNF have been described in major depression and they increased with antidepressant treatment. Only a few studies have evaluated the relationship between platelet activation and peripheral BDNF values. This review suggests that platelet reactivity may partly explain the alterations in BDNF.
- Citation: Serra-Millàs M. Are the changes in the peripheral brain-derived neurotrophic factor levels due to platelet activation? World J Psychiatr 2016; 6(1): 84-101
- URL: https://www.wjgnet.com/2220-3206/full/v6/i1/84.htm
- DOI: https://dx.doi.org/10.5498/wjp.v6.i1.84
Major depressive disorder (MDD) is a common and invalidating mental illness, with a global point prevalence of 4.7%[1]. Depressive disorder is one of the leading causes of disability, and it has been suggested to become the disease with the second highest burden[2]. The lack of diagnostic and treatment markers in depression is one of the most important problems in clinical practice. Researchers are deeply involved in the identification of validated markers, particularly biomarkers that could be useful in clinical practice. Evidence suggests that brain-derived neurotrophic factor (BDNF) is relevant in the pathophysiology of depression and that it may be more useful as a biomarker for diagnostic and prognostic purposes than the other potential biomarkers[3]. BDNF is expressed in the nervous system and in non-neuronal tissues. The relationship between both is inconsistent, and will be analyzed in this work. Another factor that it has not been studied extensively is the storage and release of BDNF from platelets. Furthermore, depressed patients exhibit enhanced platelet reactivity and increased expression of activation markers, which may influence the characterization of BDNF in depression. The following review will focus on the relationship between platelet activation and BDNF, particularly in depression. This review will examine current literature regarding this topic until August 2015.
BDNF, a member of the nerve growth factor family, is expressed in the central and peripheral nervous system and is widely distributed across subregions of the hippocampus and the frontal cortex, brain regions that are of crucial importance in the regulation of emotion, learning and memory[4-8] . BDNF-containing secretory vesicles are present in both the axon terminals and dendrites of neurons[9]. BDNF is also synthesized and released by astrocytes[10]. BDNF plays an important role in central nervous system development, neurogenesis, neuronal survival, migration, cell differentiation, growth of axons and dendrites and synapse formation[11-14], as well as in the synaptic process of hippocampal long-term potentiation (LTP) that play an important role in memory[4,15]. It also provides protection against learning and memory impairments under conditions of chronic stress[16].
The BDNF gene encodes a precursor protein (preproBDNF) that is cleaved into the smaller 35-kDa precursor, proBDNF, in the endoplasmic reticulum. ProBDNF need to be folded correctly, sorted and transported to the appropriate subcellular compartment. ProBDNF moves via the Golgi apparatus into the trans-Golgi network, where two kinds of secretory vesicles are generated: Those of the constitutive secretory pathway and those of the regulated pathway whose secretion is activity-dependent. ProBDNF can then be further cleaved into mature BDNF (mBDNF)[9]. In neurons, both proBDNF and mBDNF are preferentially sorted and packaged into vesicles in the activity-dependent secretory pathway. Activity-dependent secretion is believed to be an important feature because it may reflect the nature of the nervous system to respond to and form synaptic modulations based on experiences, and may be a cellular manifestation of memory and learning[17,18]. BDNF can also act via autocrine and paracrine mechanisms, depending on the site of the cell surface receptors through which it signals[19].
ProBDNF is either proteolytically cleaved by intracellular enzymes such as furin or pro-convertases and secreted as the 14 kDa mBDNF, or secreted as proBDNF and then cleaved by extracellular proteases[9]. The extent of the intracellular and extracellular processing of proBDNF is not exactly clear, but proBDNF is less efficiently processed by intracellular proteases compared to other neurotrophins, and the secretion of proBDNF seems to prevail over mBDNF[20-22]. It becomes important to identify the specific extracellular proteases that cleave proneurotrophins and understand their regulation. Several matrix metalloproteinases (MMP), including MMP3 and MMP7, have been shown to cleave pro nerve growth factor and proBDNF[23]. However, the most significant protease that cleaves proneurotrophins is the serine protease plasmin[23,24], which is generally expressed as an inactive plasminogen that must be activated by proteolytic cleavage by tissue plasminogen activator (tPA). In the brain, plasminogen is exclusively expressed in neurons and is present in the extracellular space, particularly at the synaptic cleft. tPA is secreted from axon terminals into the extracellular space, and this secretion depends on high-frequency neuronal activity[25]. Therefore, it is conceivable that tPA is the key trigger for the tPA-plasmin-proneurotrophin cascade. The regulation of MMP and plasmin expression or activation could regulate neurotrophin signaling in a spatially and temporally controlled manner. Other work has suggested that proBDNF (35 kDa) and tPA are secreted in an activity-dependent manner, and the extracellular conversion of proBDNF to mBDNF by the tPA/plasmin protease system is critical for late-phase LTP[24-26].
ProBDNF is not an inactive precursor and has been shown to have effects in the central nervous system that are independent of mature BDNF, as it acts at a separate receptor. Once released, proBDNF preferentially binds to the pan neurotrophin receptor p75 (p75NTR), and mBDNF preferentially binds to both pre- and post-synaptic tropomyosin-related kinase receptors (TrkB), activating different intracellular secondary messenger cascades and affecting distinct cellular responses[27]. The binding of BDNF with TrkB results in intracellular phosphorylation and the activation of intracellular signaling cascades that trigger the so-called pro-survival pathways, inactivate pro-apoptotic signaling and promote neurogenesis[8,28]. ProBDNF binds to p75NTR, which leads to apoptosis and initiates long-term depression of synaptic transmission[29], causing a reduction in the complexity and density of dendritic spines in hippocampal neurons. Proteolytic cleavage of proBDNF represents an important mechanism by which the opposing cellular actions of proBDNF and mBDNF may be regulated[25].
Platelets are the major source of peripheral BDNF[30,31], and they are important for storing the BDNF that is secreted from other tissues[32,33]. The BDNF and TrkB mRNAs are expressed in several non-neuronal tissues, including muscle, thymus, heart, liver, vascular smooth muscle cells, lung and spleen[34-38]. BDNF is also produced in monocytes, lymphocytes[39,40] and eosinophils. The latter cells produce BDNF via the autocrine system and utilize it to evoke and extend the allergic reaction[41,42]. BDNF has been shown to play a pivotal role in the growth, survival and chemoresistance of tumor cells in various types of cancers, including Hodgkin lymphoma, myeloma, hepatocellular carcinoma and neuroblastoma[43-47]. BDNF also mediates the survival and activation of endothelial cells through its interaction with TrkB[48-50], suggesting its potential role in angiogenesis. Many non-neuronal cells, such as smooth muscle cells, fibroblasts and astrocytes, may not express the molecular components of the regulated secretory pathway and therefore only secrete neurotrophins constitutively.
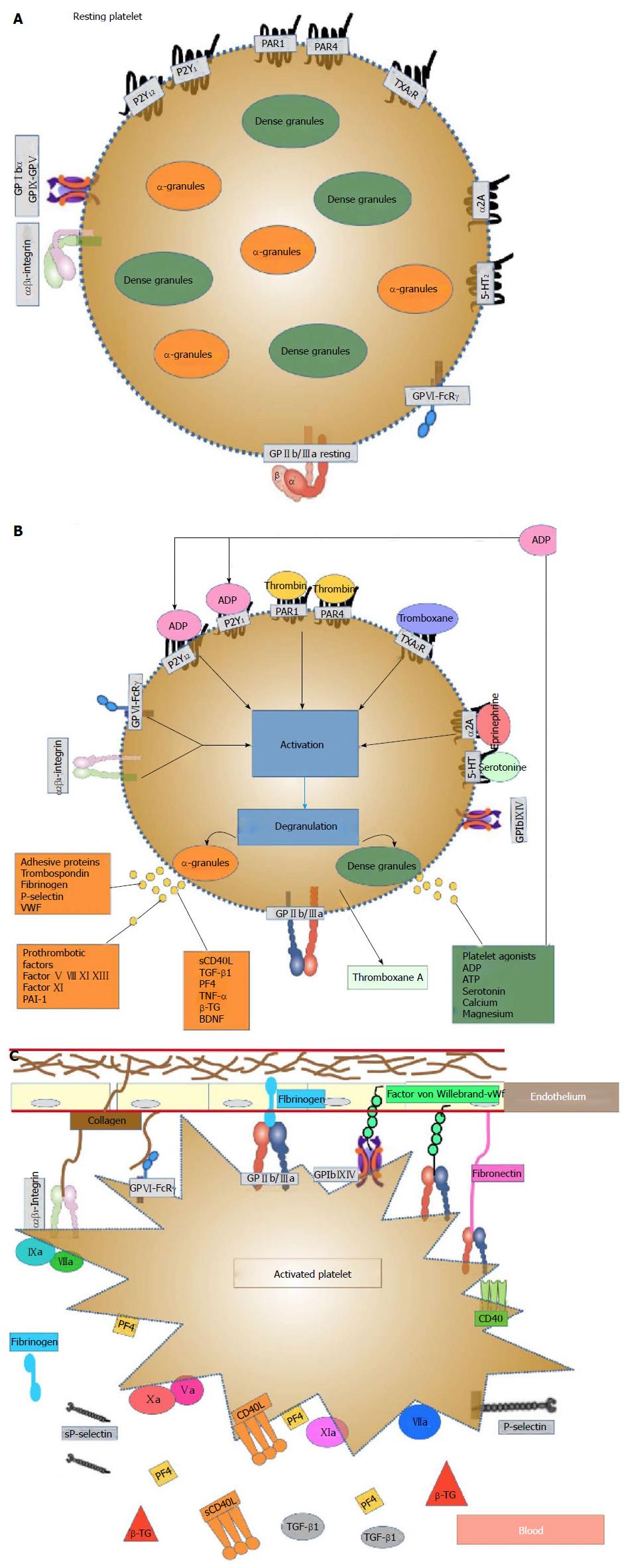
Platelets are small unnucleated blood cells with a size of approximately 3 μm that originate from megakaryocytes (MK) in the bone marrow, from which they are released into the blood system. They circulate for an average of seven to 10 d. Platelets contain many structures that are critical to stop bleeding. They contain proteins on their surface that allow them to adhere to breaks in the blood vessel wall and each other. They possess several important organelles: Three types of platelet secretory granules (α-granules - the most abundant, dense granules, and lysosomes), an open canalicular system and proteins similar to muscle proteins that allow them to change shape when they become sticky[51,52]. The platelets’ organelle content is primarily taken up from the plasma; however, platelets are also able to synthesize molecules, such as platelet-derived growth factor (PDGF), platelet factor 4 (PF4), β-thromboglobulin (β-TG) and thrombospondin[53]. Figure 1A shows a resting platelet.
α-granules are essential for normal platelet activity. In platelets, the α-granules fuse with the plasma membrane upon activation, releasing their cargo and increasing the platelet surface area. N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) and SNARE accessory proteins control α-granule secretion, and hundreds of bioactive proteins are released from α-granules. The presence of distinct subpopulations of α-granule suggests that platelets may regulate the differential release of distinct classes of α-granules. This breadth of proteins implies a versatile functionality, and the α-granules participate in thrombosis and hemostasis, inflammation, atherosclerosis, antimicrobial host defense, wound healing, angiogenesis, and malignancy. Many aspects of the formation, structure, content, protein sorting, transport and release of α-granules are poorly understood[54]. Figure 1B and C show stimulated platelets.
Platelets are an essential component of the hemostatic process. Platelets must have additional roles in several physiological and pathophysiological regulatory processes[55] . They express many immunomodulatory molecules and cytokines, and they have the ability to modulate the immune system through interactions with various cells[56]. The binding of biochemical agonists (thrombin, collagen, ADP, epinephrine, arachidonic acid) or mechanical disruption of blood vessels initiates localized hemostatic responses that involve interactions of the vascular endothelium, platelets, red blood cells, coagulation and fibrinolysis. Platelet thrombus formation involves three main steps: Platelet adhesion to the damaged endothelium, activation, and aggregation. Platelet activation involves the generation of intracellular chemical signals that are initiated by platelets through specific surface receptors (platelets express certain surface markers, such as the active form of the glycoprotein receptor GPIIb/IIIa, P-selectin and CD40 ligand). These signals cause dramatic morphological changes, such as the extension of pseudopodia, platelet-platelet aggregation, and granule secretion, resulting in the generation of a platelet thrombus.
Ninety percent or more of blood BDNF is stored in platelets[32]. A close relationship has been found between BDNF and platelets under physiological conditions, where platelets are an important source of BDNF storage. In addition, there is an approximately 100- to 200-fold difference between the plasma and serum levels of BDNF because platelets release BDNF during the clotting process[32,57]. It has been elegantly demonstrated that the amount of BDNF in serum is nearly identical to the amount of BDNF found in washed platelet lysates[32]. Thus, the difference between the serum and plasma BDNF levels seems to reflect the amount of BDNF stored in circulating platelets, and the BDNF in platelets might serve as a reservoir for circulating BDNF. The BDNF in platelets may play a role during tissue trauma or nerve injury, releasing their contents into the circulation at the site of the injury[58].
In the first studies, BDNF was not expressed in or produced from the megakaryocyte precursor cell of the mature platelets, in which protein synthesis is generally absent, but was sequestered from the circulation[32,34]. Recently, one study found that BDNF is present in the cytoplasm of platelets and in α-granules, suggesting that BDNF is either produced in platelets or passed down from MK[59]. A second study found that a megakaryocyte progenitor line (MEG-01) produces BDNF upon thrombopoietin stimulation, also expressed in the bone marrow from the liver and kidney[60] , and the levels of BDNF in MEG-01 cells increased in a time-dependent mannerly[61]. It was the first report of the production of BDNF in a megakaryocyte cell line and led to the hypothesis that BDNF potentiates the cell proliferation of the megakaryocyte lineage in vivo. It is likely that there is a receptor for BDNF on the MEG-01 cell surface, but the TrkB receptor was not detected in MEG-01 cells or human platelets[32,59,62]. Therefore, there should be an unidentified novel receptor in MKs or platelets.
Some agonists, such as thrombin, collagen, Ca2+ and shear stress, could induce a rapid release of BDNF from platelets. Even with agonist stimulation, only approximately half of the BDNF in platelets is secreted, which suggests that platelets maintain a stable pool of BDNF as a buffer system[30]. However, another study found that platelet-mediated BDNF release in depressed patients was independent of platelet reactivity[31]. There was little knowledge about the relationship between BDNF and platelets and only a few studies have assessed issues such as platelet activation mechanism that allows the release of BDNF and BDNF localization within platelets.
The rate of BDNF release paralleled the secretion of 5-HT from the dense granules and PF4 from the α-granules, although a greater proportion (90%) of the total 5-HT and PF4 were released compared to BDNF. Because only 40%-60% of the total content of platelet BDNF was released by maximal platelet activation, some authors postulated that platelets either have a non-releasable pool of BDNF, or that the released BDNF is sequestered by binding to a transporter or receptor on the platelet surface[32]. Such binding could promote the internalization of BDNF by the platelets, as has been reported for 5-HT[63,64] and for astrocyte recycling of BDNF[65,66]. The binding of BDNF to washed platelets was confirmed by microscopy and FACS analysis, as well as confocal microscopy, suggesting that platelets bind exogenous BDNF[32]. However, a recent study found two different locations where it is stored BDNF: In the α-granules and in the cytoplasm. Using immunoelectron microscopy, BDNF was clearly detected in the same fractions as P-selectin, an α-granule marker, and protein kinase C, a cytoplasmic marker[59].
BDNF is predominantly released from the platelets through protease-activated receptor 1 (PAR1) activation during thrombin stimulation, along with vascular endothelial growth factor but not endostatin stimulation. Platelets stimulated with concentrations of the PAR1-activator peptide showed a dose-response curve of BDNF release, exhibiting a two-phase pattern. The first phase is a drastic release phase at low level activation which is completely inhibited by Prostaglandin (PGE1) pretreatment suggesting that this phase depends on calcium mobilization. The second phase is a mild release phase at high level activation which is not affected to PGE1 pretreatment, suggesting another signal that is not affected by PGE1[59]. BDNF response curve was similar to that of PF4[59]. There was no significant difference in BDNF release between the non-stimulated and PAR4-AP-stimulated cells. Interestingly, PAR1 activation promotes the release of proangiogenic factors and these results support the action of BDNF as a proangiogenic factor[47-50,67,68].
Another important finding of this study is that the BDNF in the α-granules is released upon platelet activation, whereas the cytoplasmic BDNF is not[59]. The maximum BDNF release is approximately 30%-40% with stimulation and the remaining 70% of BDNF is equivalent to that found in the cytoplasm, which is not released, similar to study of Fujimura[32].
BDNF levels have been linked with various diseases, such as depression[69,70], schizophrenia[71,72], Alzheimer’s disease[73] and anorexia[74]. Some available non-invasive options for evaluating BDNF include measuring the BDNF concentrations in the whole blood, serum, plasma and platelets. The results of BDNF in one sample cannot be directly generalized to other peripheral BDNF parameters because there are only modest statistically significant associations among these peripheral measurements. Studies found that the correlations between the plasma and serum BDNF concentration are between r = 0.2 to r = 0.70[75-77]. The distinct biological significance of the serum and plasma BDNF levels has already been noted[78,79].
Human serum contains BDNF at far higher concentrations than human plasma. Because of the storage of BDNF in platelets, the concentrations of BDNF in serum and plasma differ by a factor of 200[58]. There are some confounding factors regarding the serum BDNF levels. Serum BDNF showed strong associations with race, platelet count, and depression after adjusting for other factors. Females, blacks, smokers, and those with high platelet counts had higher serum BDNF levels[80]. The platelet count is considered the factor with the strongest association with the serum BDNF concentrations[81,82] and this strong association is consistent with theory that the BDNF in serum is derived directly from the platelets[80]. The serum BDNF concentrations largely reflect the activation-dependent release of BDNF from platelets[32,57]. Interindividual differences in the serum BDNF concentrations are mediated by changes in the activity of the blood platelets caused by medications or pathological conditions[83].
The BDNF protein circulates in plasma for less than an hour[84,85]. The measures of BDNF in plasma samples are quite unstable, and previous studies reported a large degree of dispersion, which could reflect the different cellular sources of plasma BDNF (endothelia, central nervous system, etc.) and the influence of different factors on the expression and secretion of BDNF from different sources[57]. Lower levels in males was observed and age, body weight and cholesterol-LDL showed a negative correlation with the plasma BDNF levels[57]. This study showed that the BDNF plasma levels significantly decreased with increased age or weight, whereas the platelet counts did not. However, one study found that the platelet count is the most important predictor of plasma BDNF concentrations in children and adolescents, and the plasma BDNF concentrations in children may need to be interpreted with age-specific and platelet count-specific standards[86]. Another study found the plasma BDNF levels were positively associated with the platelet count and negatively associated with the fibrinogen level in patients with angina pectoris. Similar to BDNF, fibrinogen is a major storage protein of the platelet α-granules and is delivered to the α-granules by endocytosis. These associations may be associated with the level of BDNF release from platelets in inflammatory states, and the low plasma BDNF concentration in patients with angina pectoris may be primarily due to platelet release[87].
Another consideration is that the plasma BDNF levels have a very low retest stability when measured twice within one year, and there are substantial changes, even throughout one day. Recently, one study raised methodological concerns regarding the assessment of the BDNF levels in plasma and recommends measuring the BDNF levels in serum[88]. However, the plasma BDNF levels could represent the currently biologically active BDNF as a state-dependent marker, and it appeared to be influenced by inflammation mediators[89].
Platelets circulate for up to 11 d in the peripheral blood[84,85]. The platelet BDNF levels could represent a long-term marker of the varying plasma BDNF levels over a period of several days. The BDNF stored in platelets is likely obtained from both the circulating plasma pool, from cells in the brain or other organs and from megakaryocites. Variation in BDNF production in specific organs[57] may produce changes in platelet BDNF content and it measure could be a circulating indicator of altered production. Differences in platelet function and release of or sequestering BDNF from the blood may result in the differences between the serum and plasma BDNF concentrations[70]. The platelet and serum levels have been shown to strongly correlate, and it has been proposed that the BDNF released from platelets directly correlates to the serum BDNF levels; however, in recent studies, only 30% of the BDNF in platelets is secreted into the blood[59]. The platelet BDNF levels did not correlate with age, weight, cholesterol or long-term storage but changed with menstrual cycle[57,90].
Another limitation of measuring BDNF in the periphery is that the ELISA kits used in most studies quantified the total BDNF concentrations in serum and did not make the distinction between the pro- and the mBDNF variant, but the two BDNF variants are functionally different. Moreover, this raises the question of which parameter best serves as a mirror for the neurotrophic action in the brain. Some authors have suggested that the leukocyte BDNF mRNA content could more closely reflect central BDNF dynamics because of its short half-life[91] and therefore may be less subject to the peripheral confounding factors. In addition, it has been argued that a combination of peripheral BDNF indices may have advantages over a single index. Assessing both the platelet and serum BDNF concentrations could be particularly relevant[3].
Because the central BDNF levels are difficult to obtain for methodological and ethical reasons, there is a great interest in peripheral BDNF measures in relation to psychiatric illness. There are indications that the BDNF measured in peripheral tissues reflects BDNF activity in the brain. These indications include preclinical findings that BDNF crosses the blood-brain barrier[92] and positive correlations between the peripheral and central BDNF concentrations[57,93,94]. Referring to the blood-brain barrier, some rodent studies have shown that peripheral BDNF administration promotes the regeneration of spinal cord injury[95], has an effect on depressive-like behaviour[96] and could increase BDNF levels in the brain[97]. Other studies did not find these results but found that BDNF protein may have poor or null blood-brain barrier penetrability[98-100]. Systemically administered BDNF in rodents showed that BDNF signaling pathways were activated only in disrupted regions[101]. However, studies also point towards a role for vascular endothelial impairment in MDD[102]. A meta-analysis found an increased risk of MDD in those with major vascular diseases including diabetes, cardiovascular disease and stroke[103].
Evidences regarding the positive correlation between the peripheral and central BDNF concentrations[57,93,94] are as follows. Human post-mortem studies had indicated similar alterations in BDNF concentrations in the brain and periphery of persons who were depressed at the time of death[104]. In one human study, the BDNF levels were higher in blood that was derived from the internal jugular veins compared to the arterial blood[105], suggesting that the source of BDNF in the peripheral tissues can be found in the brain. These studies indicated that neurotrophic function can be estimated from the periphery in a rather non-invasive manner by taking advantage of this “window to the brain”. However, the specificity, extent and relationship between the peripheral BDNF levels to disease activity are not fully known. Due to the large variation in the amplitude between the serum and plasma BDNF levels, it is unlikely that it will translate as a useful biomarker of disease activity in clinical practice[106]. Future studies should determine the ratios of the BDNF concentrations in the cerebral spinal fluid (CSF), serum and plasma in acutely ill and remitted patients and controls.
Other studies report null findings with regard to an association between the peripheral BDNF concentrations and the more central parameters for BDNF activity, namely an absence of correlations between the plasma and CSF concentrations of BDNF[107]. In an older adult study, blood-based measures of BDNF are not representative of the CSF BDNF levels[80]. A study comparing the CSF and serum BDNF measurements in a sample of patients with Alzheimer’s disease found that they were not correlated[73]. Moreover, the concentrations of BDNF are much higher in the serum (> 1000 ×) or plasma (approximately 10 ×) than in cerebrospinal fluid, which may reflect peripheral synthesis[106-108]. One explanation is that tissues other than the brain, including the immune system, liver, smooth muscle and vascular endothelial cells serve as sources of BDNF[34,35]. Neurons and glia cells of the central nervous system might originate a substantial portion of the circulating BDNF, but our current knowledge does not allow us to distinguish whether the peripheral sources produce or release less BDNF or if decreased synthesis or release in the brain are responsible for the lower plasma and serum levels[92,106]. Another explanation is that these changes in the BDNF levels may represent a counter-regulatory response to other etiological factors of the illness, such as metabolic and redox factors[109]. Another reason to criticize this relationship between the brain and peripheral BDNF levels could be that the expression of BDNF is specific to a particular local and time[110], and animal studies have shown that the levels of BDNF are increased in some brain regions and decreased in others in some diseases[111].
The relationship between platelet activation and the peripheral BDNF level is poorly documented in humans. No evidence shows the interaction between BDNF and platelets under pathological conditions, such as tumor growth and metastasis[32]. Therefore, we will review the studies examining the relationship between BDNF and different markers of platelet activation.
Transforming grown factorβ1
Transforming grown factor β1 (TGF-β1) is abundant in platelets and is stored in the α-granules[112]. It plays an important role in regulating the immune response, cell proliferation and tissue fibrosis, and it recruits inflammatory cells to the wound area. Lommatzch et al[57] found a stronger correlation between the serum BDNF levels and the serum TGF-β1 levels than with the serum 5-HT levels and the first two could be anatomically and functionally related in the platelet both located in α-granule. The postoperative abdominal surgery changes in the serum BDNF levels virtually paralleled the changes in platelet number and the platelet mediator TGF-β1. The platelet numbers and TGF-β1 concentrations decreased in the immediate acute response and increased later. A positive relationship was observed between the serum BDNF and TGF-β1 values at all times after surgery[89].
Similarly to BDNF, fibrinogen is a major storage protein of platelet α-granules and is delivered to the α-granules by endocytosis[113]. Recent studies reported an association between elevated plasma fibrinogen levels and psychological distress and depression in individuals from the general population after adjusting for confounders[114], but the effect size of plasma fibrinogen could be small[115]. Another study found higher fibrinogen levels in non-responders than in responders in major depression patients, suggesting that baseline plasma fibrinogen levels can serve as a biomarker to gauge the success of antidepressant treatment response[116]. Hattori et al[115] found that a subpopulation of patients with MDD had high CSF fibrinogen levels compared with controls and those patients with a high fibrinogen level had white matter tract abnormalities. The increased CSF fibrinogen in patients could represent a trace of blood-brain barrier disruption induced by neuroinflammation, which is in accordance with the mild inflammation hypothesis in the aetiology of MDD[117,118]. The plasma BDNF levels were negatively associated with the fibrinogen levels in patients with angina pectoris[87]. The exact reason for the association between the decreased plasma BDNF levels and these factors is unclear, but it may be related to the level of BDNF release from platelets in inflammatory states.
P-selectin (CD 62-p) is primarily located in the α-granule membrane of resting platelets and is only found on the platelet surface after platelet activation. P-selectin is perhaps the most cited of the biologically active molecule that appears on the platelet surface after activation and secretion. P-selectin on platelets or endothelial cells has a key role in inflammation. The soluble P-selectin (sP-selectin) levels in blood represent a measure of platelet and/or endothelial cell activation. Elevated sP-selectin levels were associated with enhanced generation of tissue factor-expressing microparticles, leading to shorter plasma clotting time and a pro-coagulant phenotype, which facilitated fibrin generation and also altered the blood-brain barrier permeability and exacerbated stroke[112]. One study in cardiac patients showed that the median serum BDNF levels were higher in the myocardial infarction (MI) group than in the stable angina pectoris (SAP) group. In the MI patients, there was a significant correlation between the BDNF and sP-selectin levels. In contrast, no such correlation was observed in the SAP patients. The study suggested that the BDNF serum levels in MI patients could be related to platelet activation and the inflammatory response[119].
CD40L appears to be localized to the granule membranes. Upon platelet activation, it is translocated to the platelet surface, where it is cleaved and acts by associating with the αIIbβ3 integrin. This soluble form [soluble CD-40-ligand (sCD40L)] is predominantly derived from activated platelets and thus represents a circulating marker of platelet activation. Platelets are the main source of sCD40L and are responsible for > 95% of the circulating sCD40L levels. A study by Lorgis et al[119] in patients with coronary artery disease did not find a significant correlation between the serum BDNF levels and sCD40L in either the MI or SAP patients. The controversial results between the sCD40L and sP-selectin levels could be explained by methodological issues regarding the measurement of both markers[120,121].
PF4 is a platelet-specific protein that is stored in the α-granules and secreted into the plasma upon platelet activation[64]. PF4 is considered an index of platelet reactivity. Patients with depression showed increased PF4 plasma levels with respect to the controls, but there were no differences in the serum PF4 levels. The total PF4 levels obtained by completely clotting the platelets are the same in both groups. Alterations in the serum and plasma BDNF levels in depression are not related to the changes in either the whole blood BDNF levels or in the platelet release of the activation marker, PF4[31]. The plasma PF4 levels were elevated, indicating increased platelet reactivity without a change in the total PF4 levels in the serum. These results suggested that there are independent regulatory mechanisms for platelet BDNF and PF4 release. However, one question is whether it is possible for two proteins that are supposed to be stored in the same granules to be independently released or if there are different subcellular localizations for these proteins. Another study found that the rate of BDNF release paralleled the secretion of 5-HT from the dense granules and of PF4 from the α-granules, although a greater proportion (90%) of the total 5-HT and PF4 were released compared to BDNF[32].
The plasma β-TG levels were significantly decreased in patients with Alzheimer’s disease compared to the healthy controls. In Alzheimer’s disease patients, the serum BDNF concentrations were significantly correlated to the β-TG and plasma BDNF values. In contrast, the plasma BDNF and β-TG values were not significantly correlated. The levels of BDNF and β-TG in the blood of patients with Alzheimer’s disease are decreased compared to the controls. These results confirm an association between the serum BDNF concentration and the degree of platelet activation, as measured by the plasma β-TG levels[122].
The release of BDNF from platelets is a polemical issue. It seems likely that methodological differences may markedly affect the results, including the methodology for isolating the platelets (pH, use of diverse inhibitors, etc.); anticoagulation (using EDTA/citrate tubes, heparin a, etc.); buffers with or without calcium; acute/chronic treatment; receptor profile or mechanism of action of the drug; drug doses; animal or human studies; and direct study of platelet BDNF levels or calculating the difference between the serum and plasma BDNF levels. For example, an elevation in the calcium concentrations plays an important role in platelet activation and secretion[123]. High concentrations of calcium and thrombin lead to an immediate release of diverse growth factors, including PDGF and TGF-β1[124,125]. Under calcium-free conditions, only a low amount of 5-HT and BDNF (approximately 10%-16% of the total content) was released after 10 and 60 min; however, almost all of the nerve growth factor was released[126,127].
One study investigated the direct influence of antidepressants on BDNF release from platelets and their effects on the serum levels. Platelet BDNF release was studied using samples of washed platelets prepared from rat blood that they had incubated with sertraline, paroxetine, fluvoxamine and milnacipran, and the BDNF levels were determined at different time points. The changes in the serum BDNF concentrations were studied after single intravenous injection of antidepressants in rats at 1, 2 and 5 h following the injection. The BDNF from platelets was released by these antidepressants, and there were no differences between the effects of serotonin-norepinephrine reuptake inhibitors and selective serotonin re-uptake inhibitors (SSRIs). Antidepressants promoted BDNF release from platelets within 1 h, and the changes in BDNF release depended on the amount of the antidepressant and they were specific for each antidepressant. Sertraline was the most effective antidepressant in promoting platelet BDNF release[128], but other study with human controls did not found it[129]. The BDNF released represented approximately 20% of the total BDNF in platelets, similar to other agonists[32,59]. The serum BDNF concentration increased 1 h after sertraline injection; this change exhibited a significant difference at 5 h and was dose-dependent from 0.03 μmol/L to 0.3 μmol/L of sertraline in rat platelets. BDNF release from platelets is affected by antidepressants, which means that the administration of antidepressants might affect the changes in the BDNF in the peripheral tissues, such as platelets, and the serum BDNF concentrations. The authors suggested that the decreased serum BDNF concentrations in depressed patients may reflect reduced platelet BDNF levels[128].
Another study showed that treatment of rat platelets with sertraline, citalopram, paroxetine and indomethacin did not influence the release of BDNF after 10 and 60 min independently calcium conditions. BDNF release was significantly reduced by ibuprofen, an anti-inflammatory drug, after 10 and 60 min[127] but only when calcium was present, similarly with the work of Fujimura et al[32].
Glutamatergic modulator riluzole have been proposed as a strategy for the treatment of mood disorders. Riluzole could stimulate BDNF release, acting directly on these cells. When platelets of healthy controls were incubated with riluzole at low concentrations for 4 h, but not for 24 h, riluzole stimulated the release of BDNF. This acute effect, but not later, suggests that its effect derived from evoking neurotrophin release. Moreover, mean platelet volume and platelet distribution width did not change during study, so platelets used in this study were not activated at the time of exposure to riluzole[129].
One study evaluated the changes in the platelet BDNF levels in patients with major depression when they were treated with s-citalopram. The platelet BDNF levels of the untreated patients appeared significantly lower than those of the healthy subjects, and antidepressant treatment with an SSRI normalized the platelet BDNF levels. The platelet BDNF levels were normalized earlier (at eight weeks of treatment) than the plasma BDNF levels[70]. Another study evaluated the platelet BDNF levels in patients with MDD and childhood trauma. They were treated with antidepressant medications for three months, including escitalopram, mirtazapine, and duloxetine, without intensive psychotherapy. The platelet and serum BDNF levels showed a significant increase from baseline at the 3-mo follow-up in the patient group. Conversely, the plasma BDNF levels were not significantly different between the two time points in the patient group. There were no significant differences in BDNF levels between the different antidepressant treatment groups[76] in either of the peripheral samples analysed.
A study by Stoll et al[130] investigated the impact of common anti-platelet drugs on the BDNF concentrations in serum and plasma and on the release of BDNF from platelets in a group of healthy volunteers. They showed that a single oral dose of clopidogrel but not aspirin significantly reduced the release of BDNF from platelets in healthy volunteers. The platelet α-granule marker TGF-β1 was also significantly reduced in the serum after clopidogrel treatment but not after aspirin administration. In addition, the decrease in the serum TGF-β1 concentrations correlated with the decrease in the serum BDNF concentrations at 24 h after clopidogrel administration. Aspirin and clopidogrel had no significant effects on the plasma BDNF levels[130]. Another study found a reduction in the release of BDNF from platelets when treated with aspirin (a non-specific cyclooxygenase-inhibitor)[131]. These drugs act through different mechanisms; aspirin acts by inhibiting cyclooxygenases, and clopidogrel irreversibly binds to the membrane adenosine diphosphate receptor and impacts platelet α-granule degranulation[130]. The study by Stoll et al[130] is consistent with the decrease of the platelet α-granule marker TGF-β1 after clopidogrel treatment and the correlation between the effects of clopidogrel on the BDNF and TGF-β1 concentrations but not those after aspirin administration.
Many attempts have been made to generate reliable blood-derived candidate biomarkers based on the current models of disease pathogenesis. 5-HT and BDNF are known to modulate behavioral responses to stress and to mediate the therapeutic efficacy of antidepressant agents, and they interact at different levels[132]. BDNF has been implicated in the pathophysiology of depression[133], and it has been studied as biomarker of this disease. A large number of clinical studies have reported that the BDNF levels in serum[83,134-136] are significantly decreased in depressed patients and that this decrease is normalized by antidepressant treatments[135,137-140], which was confirmed by meta-analysis[141,142]. In some of these studies, the BDNF levels correlated with higher scores on scales for assessing depression[141], although there are studies that have found no such correlation[108,143]. A recent published meta-analysis[3] concluded that there are low concentrations of BDNF in the serum of patients with untreated depression, but the size of the effect becomes substantially smaller than in previous studies. However, no consistent associations were found between the serum concentrations of BDNF and the severity of the depressive symptoms. An aspect to consider is that the serum BDNF levels are dependent on the release of BDNF from platelets[32,57], which has not been evaluated in most studies. Another aspect to consider is that other diseases, such as schizophrenia, bipolar disorder, and anorexia, among others, have shown decreases in the serum BDNF levels; thus, this finding is not specific enough for a diagnostic marker.
With respect to the plasma levels, some studies showed lower plasma BDNF levels in depressive patients[78,144,145] or did not observe changes[146,147]. Our group reported that the plasma BDNF levels were significantly higher in the depressed patients compared to the healthy controls and that they were similar in both groups when the symptoms remitted[70]. One possible explanation could be that BDNF is released from platelets and increases the plasma BDNF levels. However, the BDNF in plasma has a short half-life, and platelets contain greater concentrations of BDNF than the plasma. The plasma BDNF levels also appeared to be influenced by inflammation mediators[89].
There are few studies that have evaluated the platelet BDNF levels in depression. Two studies that directly evaluated the platelet levels showed reductions in the platelet BDNF levels in depression, which may be associated with lower serum BDNF levels in patients with major depression, but these two studies did not evaluate the effects of treatment[148,149]. Another study showed that the platelet BDNF levels were significantly decreased with respect to the controls, but treatment with SSRIs normalized the levels to the levels of the controls[70]. These data are consistent with a preactivation state of platelets in major depression. Treatment with an SSRI would improve this preactivated state and explain the increase in the BDNF levels inside the platelets[70]. The remaining studies evaluated the platelet BDNF levels indirectly by the difference between the serum and plasma levels[76].
A disruption in serotonergic signaling in the brain is believed to be involved in the pathophysiology of depression. It is well known that 99% of the 5-HT found in the human body is stored in platelets and that 5-HT can induce downstream platelet aggregation and coronary vasoconstriction[150]. In contrast with arachidonic acid, 5-HT is a weak platelet agonist that requires co-stimulation with other agonists to induce full platelet activation[151]. Increasing evidence indicates that there is an association between depression and platelet function[152-154]. A relationship between depressive symptoms and increased platelet activity has been established in physically healthy depressed patients[155] and, in post-MI depressed patients[156]. Elevated platelet reactivity has been found in depressed patients, as indicated by the increased plasma levels of either PF4 or β-TG or the increased expression of procoagulant platelet surface receptors[157-159]. Our group demonstrated the existence of a prothrombotic endophenotype in the platelets of depressed patients before treatment that was characterized by a statistically significant increase in the average volume of platelets and high expression of glycoprotein GPIb and antigen markers. Compared to the controls, the patients’ platelets showed a significantly enhanced aggregation response to arachidonic acid. The clot firmness and procoagulant activity of platelet-associated tissue factor were also significantly elevated, which can contribute to the increased prothrombotic profile of platelets and precipitation events in ischemic vascular lesion sites[160]. Studies with circulating blood revealed increased fibrin formation and thrombin generation in depression compared to the blood of healthy donors, when exposed to a thrombogenic surface in flow conditions[161]. Other observed alterations in platelet parameters in patients with major depression included a reduction of 5-HT transporter [3H]-imipramine binding sites in platelets[162] and increased in 5-HT2 receptor binding sites on the platelet surface compared to the controls[163]. Platelet monoamine oxidase activity has been shown to be elevated in depressed patients[164]. Heightened membrane expression of glycoprotein IIb/IIIa and the P-selectin receptors has also been reported in depressed patients without heart disease[156]. These alterations have been proposed as a possible mechanism that contributes to the elevated cardiac risk associated with the diagnosis of major depression[165]. Several mechanisms could explain the platelet abnormalities observed in major depression[159]. Although many studies have shown exaggerated platelet activation in patients with depression, several have shown no such relationship[152].
During platelet activation, 5-HT stimulation also accelerates the exocytosis of the platelet α-granules, which secrete procoagulant molecules into the plasma. One of these molecules is plasminogen activator inhibitor-1 (PAI-1), which is released at the site of thrombus formation. The levels of PAI-1 in arterial clots are 2-3 times higher than those observed in venous clots, and the relative content of PAI-1 determines the resistance to thrombolysis. PAI-1 inhibits the bioavailability of tPA and plasmin, which are the proteases that cleave proBDNF to mBDNF; therefore, the elevated synthesis of PAI-1 reduces the production of mBDNF. Multiple lines of evidence have shown that split proBDNF is central to the pathophysiology of major depression and the mechanisms of action of antidepressants[166]. The inadequate split of proBDNF may increase the risk of mood disorders[167]. In fact, patients with major depression show increased levels of proBDNF and decreased levels of mBDNF[168]. Moreover, PAI-1 inhibits the production of plasmin, preventing the dissolution of blood clots in atherosclerotic plaques, and one would expect that patients with depressive disorders have a higher risk of cardiovascular events.
In platelets, SSRIs results in a decrease in the 5-HT storage in dense granules, and thus could affect platelet aggregation[169]. 5-HT can definitely potentiate platelet mediated thrombogenesis[151]. Continued treatment with the SSRI may modulate not only the circulating levels of 5-HT but also the presence and activity of the serotonergic mechanisms and inhibit the release of 5-HT during platelet aggregation[170,171]. Clinical data have also shown that antidepressant treatment influenced platelet activation by lowering the plasma PF4 and β-TG levels[158,159], but other studies did not find this association[172]. Citalopram inhibited human platelet aggregation in vitro in response to different agonists, with the strongest effect observed in response to ADP and ADP + 5-HT[151]. Ex vivo escitalopram treatment of blood resulted in a significant inhibition of ADP- or collagen-induced platelet aggregation[165,173,174]. In aggregation experiments with collagen or arachidonic acid, a significant decrease in the aggregation intensity was noted in the platelets of SSRI-treated patients[175]. The addition of ADP to platelets from patients treated with SSRIs induced an inhibition of ATP release and the secondary wave of platelet aggregation[176]. Furthermore, a significant decrease in the platelet activation markers CD62-P and annexin-V-binding was reported after preincubating platelets with citalopram, but the release of factor V/Va from the α-granules was not noticeably affected[151]. Treatment with SSRIs for 24 wk normalized the majority of the altered parameters in patients with depression, but it accentuated the expression of GPIIb/IIIa and the viscoelastic properties of the clots formed under low shear rate conditions[161]. SSRI treatment rapidly and effectively counteracted the enhanced fibrin or aggregate formation observed under flow conditions, confirming the previous in vitro results in the clinical setting[151]. However, the viscoelastic parameters showed a progressive acceleration of clotting time and enhanced clot strength during the treatment with escitalopram, but these results were obtained in almost static conditions[161]. Patients treated with SSRIs seem to have fibrinogen and PAI-1 levels in plasma that are similar to those of the healthy controls and lower than depressed patients who do not receive serotonergic antidepressants[177]. Overall, these results could explain why the patients treated with SSRIs show a reduction in cardiovascular disease risk when compared with patients not receiving antidepressants[178,179].
To the best of our knowledge, only a few studies have assessed both BDNF and platelet activation in depression[31]. BDNF has been implicated in the pathophysiology of depression[133], and it has been studied as a biomarker of this disease. A large number of clinical studies have reported that the BDNF levels in serum[83,134-136] are significantly decreased in depressed patients and that this decrease is normalized by antidepressant treatments[135,137-140], which was confirmed by meta-analysis[141,142]. Serum levels are influenced by platelets and plasma levels results are inconsistent. Ninety percent or more of blood BDNF is stored in platelets, but these studies did not consider the platelet alterations observed in depression. Increasing evidence indicates that there is an association between depression and platelet function[152-154], with an elevated platelet reactivity, a prothrombotic endophenotype and increased of plasma substance levels excreted from α-granules in depressed patients. Some authors propose that the lower peripheral BDNF concentrations in depression and their upregulation over the course of antidepressant treatment may be an epiphenomenon resulting from an altered BDNF metabolism or expression by these peripheral organs[3]. One possible explanation could be that alterations in peripheral BDNF levels in depression depend more on platelet reactivity that excretes more BDNF from α-granules than on alterations of central BDNF. A serious question remains to be answered of whether the relationships found between BDNF and depression may be mediated primarily by the relationship between depression and platelet activation. Further studies are required to evaluate the complexity of the relationship between BDNF and platelet reactivity and its possible influence on the peripheral levels in certain diseases, such as depression. Another group of studies is required to evaluate the implications of anti-platelet and antidepressant drugs in the relationship between BDNF and platelet activity.
P- Reviewer: Gonul AS, Kunugi H, Tovilla-Zarate CA S- Editor: Song XX L- Editor: A E- Editor: Jiao XK
| 1. | Ferrari AJ, Charlson FJ, Norman RE, Flaxman AD, Patten SB, Vos T, Whiteford HA. The epidemiological modelling of major depressive disorder: application for the Global Burden of Disease Study 2010. PLoS One. 2013;8:e69637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 2. | Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4227] [Cited by in RCA: 4049] [Article Influence: 144.6] [Reference Citation Analysis (2)] |
| 3. | Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry. 2014;19:791-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 510] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 4. | Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 882] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 5. | Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 430] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 6. | Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 578] [Cited by in RCA: 356] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 7. | Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994;17:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 587] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 8. | Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1473] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 9. | Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 467] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 10. | Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25:237-258. [PubMed] |
| 11. | Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3227] [Cited by in RCA: 3337] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 12. | Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1030] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 13. | Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 14. | Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25:77-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 591] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 16. | Radecki DT, Brown LM, Martinez J, Teyler TJ. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus. 2005;15:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 739] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 18. | Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2224] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 19. | Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol. 2001;63:71-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 649] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 20. | Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455-5463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 781] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 21. | Nagappan G, Zaitsev E, Senatorov VV, Yang J, Hempstead BL, Lu B. Control of extracellular cleavage of ProBDNF by high frequency neuronal activity. Proc Natl Acad Sci USA. 2009;106:1267-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Yang J, Siao CJ, Nagappan G, Marinic T, Jing D, McGrath K, Chen ZY, Mark W, Tessarollo L, Lee FS. Neuronal release of proBDNF. Nat Neurosci. 2009;12:113-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 23. | Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1244] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 24. | Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 865] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 25. | Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 983] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 26. | Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci. 2009;42:81-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 27. | Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1625] [Cited by in RCA: 1736] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 28. | Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Lu B, Chang JH. Regulation of neurogenesis by neurotrophins: implications in hippocampus-dependent memory. Neuron Glia Biol. 2004;1:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Bus BA, Molendijk ML, Penninx BJ, Buitelaar JK, Kenis G, Prickaerts J, Elzinga BM, Voshaar RC. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology. 2011;36:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 31. | Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728-734. [PubMed] |
| 33. | Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. J Neurosci. 1990;10:3469-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 343] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 35. | Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 655] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 36. | Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem. 1997;69:34-42. [PubMed] |
| 37. | Maisonpierre PC, Le Beau MM, Espinosa R, Ip NY, Belluscio L, de la Monte SM, Squinto S, Furth ME, Yancopoulos GD. Human and rat brain-derived neurotrophic factor and neurotrophin-3: gene structures, distributions, and chromosomal localizations. Genomics. 1991;10:558-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 396] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 38. | Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1008] [Cited by in RCA: 998] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 39. | Edling AE, Nanavati T, Johnson JM, Tuohy VK. Human and murine lymphocyte neurotrophin expression is confined to B cells. J Neurosci Res. 2004;77:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 757] [Cited by in RCA: 770] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 41. | Nockher WA, Renz H. Neurotrophins in clinical diagnostics: pathophysiology and laboratory investigation. Clin Chim Acta. 2005;352:49-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Raap U, Goltz C, Deneka N, Bruder M, Renz H, Kapp A, Wedi B. Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J Allergy Clin Immunol. 2005;115:1268-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Renné C, Willenbrock K, Küppers R, Hansmann ML, Bräuninger A. Autocrine- and paracrine-activated receptor tyrosine kinases in classic Hodgkin lymphoma. Blood. 2005;105:4051-4059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005;105:4429-4436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 46. | Ho R, Eggert A, Hishiki T, Minturn JE, Ikegaki N, Foster P, Camoratto AM, Evans AE, Brodeur GM. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462-6466. [PubMed] |
| 47. | Yang ZF, Ho DW, Lau CK, Tam KH, Lam CT, Poon RT, Fan ST. Platelet activation during tumor development, the potential role of BDNF-TrkB autocrine loop. Biochem Biophys Res Commun. 2006;346:981-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest. 2005;115:653-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 49. | Kim H, Li Q, Hempstead BL, Madri JA. Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J Biol Chem. 2004;279:33538-33546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibañez CF, Rafii S, Hempstead BL. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531-4540. [PubMed] |
| 51. | Frojmovic MM, Milton JG. Human platelet size, shape, and related functions in health and disease. Physiol Rev. 1982;62:185-261. [PubMed] |
| 52. | White JG, Clawson CC. The surface-connected canalicular system of blood platelets--a fenestrated membrane system. Am J Pathol. 1980;101:353-364. [PubMed] |
| 53. | Yoshioka A, Horiuchi H, Shirakawa R, Nishioka H, Tabuchi A, Higashi T, Yamamoto A, Kita T. Molecular dissection of alpha- and dense-core granule secretion of platelets. Ann N Y Acad Sci. 2001;947:403-406. [PubMed] |
| 54. | Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 879] [Cited by in RCA: 857] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 55. | Leslie M. Cell biology. Beyond clotting: the powers of platelets. Science. 2010;328:562-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 56. | Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1332] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 57. | Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 669] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 58. | Radka SF, Holst PA, Fritsche M, Altar CA. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996;709:122-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 276] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Tamura S, Suzuki H, Hirowatari Y, Hatase M, Nagasawa A, Matsuno K, Kobayashi S, Moriyama T. Release reaction of brain-derived neurotrophic factor (BDNF) through PAR1 activation and its two distinct pools in human platelets. Thromb Res. 2011;128:e55-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 320] [Article Influence: 16.8] [Reference Citation Analysis (2)] |
| 61. | Tamura S, Nagasawa A, Masuda Y, Tsunematsu T, Hayasaka K, Matsuno K, Shimizu C, Ozaki Y, Moriyama T. BDNF, produced by a TPO-stimulated megakaryocytic cell line, regulates autocrine proliferation. Biochem Biophys Res Commun. 2012;427:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Burnouf T, Kuo YP, Blum D, Burnouf S, Su CY. Human platelet concentrates: a source of solvent/detergent-treated highly enriched brain-derived neurotrophic factor. Transfusion. 2012;52:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Omenn GS, Smith LT. A common uptake system for serotonin and dopamine in human platelets. J Clin Invest. 1978;62:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets. 2001;12:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 369] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 65. | Alderson RF, Curtis R, Alterman AL, Lindsay RM, DiStefano PS. Truncated TrkB mediates the endocytosis and release of BDNF and neurotrophin-4/5 by rat astrocytes and schwann cells in vitro. Brain Res. 2000;871:210-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Rubio N. Mouse astrocytes store and deliver brain-derived neurotrophic factor using the non-catalytic gp95trkB receptor. Eur J Neurosci. 1997;9:1847-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Italiano JE, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Italiano JE, Battinelli EM. Selective sorting of alpha-granule proteins. J Thromb Haemost. 2009;7 Suppl 1:173-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Shimizu E, Hashimoto K, Watanabe H, Komatsu N, Okamura N, Koike K, Shinoda N, Nakazato M, Kumakiri C, Okada S. Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia are indistinguishable from controls. Neurosci Lett. 2003;351:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 70. | Serra-Millàs M, López-Vílchez I, Navarro V, Galán AM, Escolar G, Penadés R, Catalán R, Fañanás L, Arias B, Gastó C. Changes in plasma and platelet BDNF levels induced by S-citalopram in major depression. Psychopharmacology (Berl). 2011;216:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Toyooka K, Asama K, Watanabe Y, Muratake T, Takahashi M, Someya T, Nawa H. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Penadés R, Catalán R, López-Vílchez I. Brain-derived neurotrophic factor as a potential biomarker of cognitive recovery in schizophrenia. World J Psychiatry. 2013;3:93-102. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, Soekadar S, Richartz E, Koehler N, Bartels M. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 74. | Monteleone P, Fabrazzo M, Martiadis V, Serritella C, Pannuto M, Maj M. Circulating brain-derived neurotrophic factor is decreased in women with anorexia and bulimia nervosa but not in women with binge-eating disorder: relationships to co-morbid depression, psychopathology and hormonal variables. Psychol Med. 2005;35:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Terracciano A, Lobina M, Piras MG, Mulas A, Cannas A, Meirelles O, Sutin AR, Zonderman AB, Uda M, Crisponi L. Neuroticism, depressive symptoms, and serum BDNF. Psychosom Med. 2011;73:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 76. | Jeon HJ, Kang ES, Lee EH, Jeong EG, Jeon JR, Mischoulon D, Lee D. Childhood trauma and platelet brain-derived neurotrophic factor (BDNF) after a three month follow-up in patients with major depressive disorder. J Psychiatr Res. 2012;46:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Yoshimura R, Sugita-Ikenouchi A, Hori H, Umene-Nakano W, Hayashi K, Katsuki A, Ueda N, Nakamura J. A close correlation between plasma and serum levels of brain-derived neurotrophic factor (BDNF) in healthy volunteers. Int J Psychiatry Clin Pract. 2010;14:220-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Piccinni A, Marazziti D, Catena M, Domenici L, Del Debbio A, Bianchi C, Mannari C, Martini C, Da Pozzo E, Schiavi E. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord. 2008;105:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 79. | Krabbe KS, Mortensen EL, Avlund K, Pedersen AN, Pedersen BK, Jørgensen T, Bruunsgaard H. Brain-derived neurotrophic factor predicts mortality risk in older women. J Am Geriatr Soc. 2009;57:1447-1452. [PubMed] |
| 80. | Nettiksimmons J, Simonsick EM, Harris T, Satterfield S, Rosano C, Yaffe K. The associations between serum brain-derived neurotrophic factor, potential confounders, and cognitive decline: a longitudinal study. PLoS One. 2014;9:e91339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L, Pieri M, Genazzani AD, Luisi S, Genazzani AR. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod. 2007;22:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 82. | Saito S, Watanabe K, Hashimoto E, Saito T. Low serum BDNF and food intake regulation: a possible new explanation of the pathophysiology of eating disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 906] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 84. | Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res. 1996;36:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 465] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 85. | Kishino A, Katayama N, Ishige Y, Yamamoto Y, Ogo H, Tatsuno T, Mine T, Noguchi H, Nakayama C. Analysis of effects and pharmacokinetics of subcutaneously administered BDNF. Neuroreport. 2001;12:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Iughetti L, Casarosa E, Predieri B, Patianna V, Luisi S. Plasma brain-derived neurotrophic factor concentrations in children and adolescents. Neuropeptides. 2011;45:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Jiang H, Liu Y, Zhang Y, Chen ZY. Association of plasma brain-derived neurotrophic factor and cardiovascular risk factors and prognosis in angina pectoris. Biochem Biophys Res Commun. 2011;415:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 88. | Molendijk ML, van Tol MJ, Penninx BW, van der Wee NJ, Aleman A, Veltman DJ, Spinhoven P, Elzinga BM. BDNF val66met affects hippocampal volume and emotion-related hippocampal memory activity. Transl Psychiatry. 2012;2:e74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 89. | Chimienti G, Mezzapesa A, Rotelli MT, Lupo L, Pepe G. Plasma concentrations but not serum concentrations of brain-derived neurotrophic factor are related to pro-inflammatory cytokines in patients undergoing major abdominal surgery. Clin Biochem. 2012;45:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | Trajkovska V, Marcussen AB, Vinberg M, Hartvig P, Aznar S, Knudsen GM. Measurements of brain-derived neurotrophic factor: methodological aspects and demographical data. Brain Res Bull. 2007;73:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 91. | Gass P, Hellweg R. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker for affective disorders? Int J Neuropsychopharmacol. 2010;13:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 1018] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 93. | Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 534] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 94. | Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, Danker-Hopfe H, Gass P. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry. 2009;42:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 95. | Krishna V, Konakondla S, Nicholas J, Varma A, Kindy M, Wen X. Biomaterial-based interventions for neuronal regeneration and functional recovery in rodent model of spinal cord injury: a systematic review. J Spinal Cord Med. 2013;36:174-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 96. | Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 97. | Alcalá-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 2010;18:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 98. | Pardridge WM, Wu D, Sakane T. Combined use of carboxyl-directed protein pegylation and vector-mediated blood-brain barrier drug delivery system optimizes brain uptake of brain-derived neurotrophic factor following intravenous administration. Pharm Res. 1998;15:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 844] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 100. | Felts PA, Smith KJ, Gregson NA, Hughes RA. Brain-derived neurotrophic factor in experimental autoimmune neuritis. J Neuroimmunol. 2002;124:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 101. | Baseri B, Choi JJ, Deffieux T, Samiotaki G, Tung YS, Olumolade O, Small SA, Morrison B, Konofagou EE. Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood-brain barrier using focused ultrasound and microbubbles. Phys Med Biol. 2012;57:N65-N81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 102. | Lavoie KL, Pelletier R, Arsenault A, Dupuis J, Bacon SL. Association between clinical depression and endothelial function measured by forearm hyperemic reactivity. Psychosom Med. 2010;72:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 103. | Valkanova V, Ebmeier KP. Vascular risk factors and depression in later life: a systematic review and meta-analysis. Biol Psychiatry. 2013;73:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 104. | Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 105. | Dawood T, Anderson J, Barton D, Lambert E, Esler M, Hotchkin E, Haikerwal D, Kaye D, Lambert G. Reduced overflow of BDNF from the brain is linked with suicide risk in depressive illness. Mol Psychiatry. 2007;12:981-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 106. | Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, Mahadik SP. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 107. | Pillai A, Bruno D, Sarreal AS, Hernando RT, Saint-Louis LA, Nierenberg J, Ginsberg SD, Pomara N, Mehta PD, Zetterberg H. Plasma BDNF levels vary in relation to body weight in females. PLoS One. 2012;7:e39358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Fernandes BS, Berk M, Turck CW, Steiner J, Gonçalves CA. Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Mol Psychiatry. 2014;19:750-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 109. | Anderson G, Maes M, Berk M. Schizophrenia is primed for an increased expression of depression through activation of immuno-inflammatory, oxidative and nitrosative stress, and tryptophan catabolite pathways. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:101-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 110. | Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol. 2014;112:80-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 111. | Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1425] [Cited by in RCA: 1754] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 112. | Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105 Suppl 1:S13-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 469] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 113. | Sehgal S, Storrie B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J Thromb Haemost. 2007;5:2009-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 114. | Wium-Andersen MK, Orsted DD, Nordestgaard BG. Association between elevated plasma fibrinogen and psychological distress, and depression in 73,367 individuals from the general population. Mol Psychiatry. 2013;18:854-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 115. | Hattori K, Ota M, Sasayama D, YoshidaS , Matsumura R, Miyakawa T, Yokota Y, Yamaguchi S, Noda T, Teraishi T. Increased cerebrospinal fluid fibrinogen in major depressive disorder. Scientific Reports. 2015;5:11412. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 116. | Martins-de-Souza D, Maccarrone G, Ising M, Kloiber S, Lucae S, Holsboer F, Turck CW. (2014). Plasma fibrinogen: now also an antidepressant response marker? Transll Psychiatry. 2014;4:e352. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 117. | Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83:495-502. [RCA] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 118. | Rosenblat JD, Cha DS, Mansur RB, McIntyre RS. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 426] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 119. | Lorgis L, Amoureux S, de Maistre E, Sicard P, Bejot Y, Zeller M, Vergely C, Sequeira-Le Grand A, Lagrost AC, Berchoud J. Serum brain-derived neurotrophic factor and platelet activation evaluated by soluble P-selectin and soluble CD-40-ligand in patients with acute myocardial infarction. Fundam Clin Pharmacol. 2010;24:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 120. | Ivandic BT, Spanuth E, Haase D, Lestin HG, Katus HA. Increased plasma concentrations of soluble CD40 ligand in acute coronary syndrome depend on in vitro platelet activation. Clin Chem. 2007;53:1231-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Hartweg J, Gunter M, Perera R, Farmer A, Cull C, Schalkwijk C, Kok A, Twaalfhoven H, Holman R, Neil A. Stability of soluble adhesion molecules, selectins, and C-reactive protein at various temperatures: implications for epidemiological and large-scale clinical studies. Clin Chem. 2007;53:1858-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 122. | 122Laske C, Stransky E. Decreased brain-derived neurotrophic factor (BDNF)-and beta-thromboglobulin (beta-TG)-blood levels in Alzheimer’s disease. Thromb Haemost. 2006;96:102-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 123. | Varga-Szabo D, Braun A, Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 385] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 124. | Martineau I, Lacoste E, Gagnon G. Effects of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004;25:4489-4502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 125. | Roussy Y, Bertrand Duchesne MP, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res. 2007;18:639-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 126. | Jensen PN, Smith DF, Poulsen JH, Møller HJ, Rosenberg R. Effect of flunarizine and calcium on serotonin uptake in human and rat blood platelets and rat synaptosomes. Biol Psychiatry. 1994;36:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 127. | Hochstrasser T, Ehrlich D, Sperner-Unterweger B, Humpel C. Antidepressants and anti-inflammatory drugs differentially reduce the release of NGF and BDNF from rat platelets. Pharmacopsychiatry. 2013;46:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 128. | Watanabe K, Hashimoto E, Ukai W, Ishii T, Yoshinaga T, Ono T, Tateno M, Watanabe I, Shirasaka T, Saito S. Effect of antidepressants on brain-derived neurotrophic factor (BDNF) release from platelets in the rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1450-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 129. | Türck P, Frizzo ME. Riluzole stimulates BDNF release from human platelets. Biomed Res Int. 2015;2015:189307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 130. | Stoll P, Plessow A, Bratke K, Virchow JC, Lommatzsch M. Differential effect of clopidogrel and aspirin on the release of BDNF from platelets. J Neuroimmunol. 2011;238:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 131. | Storey RF. Biology and pharmacology of the platelet P2Y12 receptor. Curr Pharm Des. 2006;12:1255-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 132. | Homberg JR, Molteni R, Calabrese F, Riva MA. The serotonin-BDNF duo: developmental implications for the vulnerability to psychopathology. Neurosci Biobehav Rev. 2014;43:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 133. | Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2372] [Cited by in RCA: 2479] [Article Influence: 130.5] [Reference Citation Analysis (0)] |
| 134. | Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 810] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 135. | Gervasoni N, Aubry JM, Bondolfi G, Osiek C, Schwald M, Bertschy G, Karege F. Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology. 2005;51:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 136. | Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T, Goka E. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1256-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 137. | Aydemir O, Deveci A, Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 138. | Bocchio-Chiavetto L, Zanardini R, Bortolomasi M, Abate M, Segala M, Giacopuzzi M, Riva MA, Marchina E, Pasqualetti P, Perez J. Electroconvulsive Therapy (ECT) increases serum Brain Derived Neurotrophic Factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 139. | Huang TL, Lee CT, Liu YL. Serum brain-derived neurotrophic factor levels in patients with major depression: effects of antidepressants. J Psychiatr Res. 2008;42:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 140. | Yoshimura R, Mitoma M, Sugita A, Hori H, Okamoto T, Umene W, Ueda N, Nakamura J. Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1034-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 141. | Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11:1169-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 685] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 142. | Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 928] [Cited by in RCA: 892] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 143. | Fernandes B, Gama CS, Massuda R, Torres M, Camargo D, Kunz M, Belmonte-de-Abreu PS, Kapczinski F, de Almeida Fleck MP, Inês Lobato M. Serum brain-derived neurotrophic factor (BDNF) is not associated with response to electroconvulsive therapy (ECT): a pilot study in drug resistant depressed patients. Neurosci Lett. 2009;453:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 144. | Grassi-Oliveira R, Stein LM, Lopes RP, Teixeira AL, Bauer ME. Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression--a preliminary report. Biol Psychiatry. 2008;64:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 145. | Lee BH, Kim H, Park SH, Kim YK. Decreased plasma BDNF level in depressive patients. J Affect Disord. 2007;101:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 146. | Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen MG, Placentino A, Giovannini C, Rillosi L, Ventriglia M, Riva MA. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry. 2010;11:763-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 338] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 147. | Lee HY, Kim YK. Plasma brain-derived neurotrophic factor as a peripheral marker for the action mechanism of antidepressants. Neuropsychobiology. 2008;57:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 148. | Lee BH, Kim YK. Reduced platelet BDNF level in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:849-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 149. | Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Zhang H, Pavuluri MN. Brain-derived neurotrophic factor gene and protein expression in pediatric and adult depressed subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 150. | Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 984] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 151. | Galan AM, Lopez-Vilchez I, Diaz-Ricart M, Navalon F, Gomez E, Gasto C, Escolar G. Serotonergic mechanisms enhance platelet-mediated thrombogenicity. Thromb Haemost. 2009;102:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 152. | Parakh K, Sakhuja A, Bhat U, Ziegelstein RC. Platelet function in patients with depression. South Med J. 2008;101:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 153. | Ziegelstein RC, Parakh K, Sakhuja A, Bhat U. Platelet function in patients with major depression. Intern Med J. 2009;39:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 154. | Williams MS. Platelets and depression in cardiovascular disease: A brief review of the current literature. World J Psychiatry. 2012;2:114-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 155. | Piletz JE, Zhu H, Madakasira S, Pazzaglia P, Lindsay DeVane C, Goldman N, Halaris A. Elevated P-selectin on platelets in depression: response to bupropion. J Psychiatr Res. 2000;34:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 156. | Schins A, Hamulyák K, Scharpé S, Lousberg R, Van Melle J, Crijns H, Honig A. Whole blood serotonin and platelet activation in depressed post-myocardial infarction patients. Life Sci. 2004;76:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 157. | Kuijpers PM, Hamulyak K, Strik JJ, Wellens HJ, Honig A. Beta-thromboglobulin and platelet factor 4 levels in post-myocardial infarction patients with major depression. Psychiatry Res. 2002;109:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 158. | Markovitz JH, Shuster JL, Chitwood WS, May RS, Tolbert LC. Platelet activation in depression and effects of sertraline treatment: An open-label study. Am J Psychiatry. 2000;157:1006-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 159. | Nemeroff CB, Musselman DL. Are platelets the link between depression and ischemic heart disease? Am Heart J. 2000;140:57-62. [PubMed] |
| 160. | Lopez-Vilchez I, Galan AM, Hernandez MR, Caballo C, Roque M, Diaz-Ricart M, White JG, Escolar G. Platelet-associated tissue factor enhances platelet reactivity and thrombin generation in experimental studies in vitro. Thromb Res. 2012;130:e294-e300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 161. | Lopez-Vilchez I, Serra-Millas M, Navarro V, Rosa Hernandez M, Villalta J, Diaz-Ricart M, Gasto C, Escolar G, Galan AM. Prothrombotic platelet phenotype in major depression: downregulation by antidepressant treatment. J Affect Disord. 2014;159:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 162. | Nemeroff CB, Knight DL, Krishnan RR, Slotkin TA, Bissette G, Melville ML, Blazer DG. Marked reduction in the number of platelet-tritiated imipramine binding sites in geriatric depression. Arch Gen Psychiatry. 1988;45:919-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 163. | Hrdina PD, Bakish D, Ravindran A, Chudzik J, Cavazzoni P, Lapierre YD. Platelet serotonergic indices in major depression: up-regulation of 5-HT2A receptors unchanged by antidepressant treatment. Psychiatry Res. 1997;66:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 164. | Schneider LS, Severson JA, Pollock V, Cowan RP, Sloane RB. Platelet monoamine oxidase activity in elderly depressed outpatients. Biol Psychiatry. 1986;21:1360-1364. [PubMed] |
| 165. | Serebruany VL, Gurbel PA, O’Connor CM. Platelet inhibition by sertraline and N-desmethylsertraline: a possible missing link between depression, coronary events, and mortality benefits of selective serotonin reuptake inhibitors. Pharmacol Res. 2001;43:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 166. | Tsai SJ. The P11, tPA/plasminogen system and brain-derived neurotrophic factor: Implications for the pathogenesis of major depression and the therapeutic mechanism of antidepressants. Med Hypotheses. 2007;68:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 167. | Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 908] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 168. | Zhou L, Xiong J, Lim Y, Ruan Y, Huang C, Zhu Y, Zhong JH, Xiao Z, Zhou XF. Upregulation of blood proBDNF and its receptors in major depression. J Affect Disord. 2013;150:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 169. | Hergovich N, Aigner M, Eichler HG, Entlicher J, Drucker C, Jilma B. Paroxetine decreases platelet serotonin storage and platelet function in human beings. Clin Pharmacol Ther. 2000;68:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 170. | Günther L, Liebscher S, Jähkel M, Oehler J. Effects of chronic citalopram treatment on 5-HT1A and 5-HT2A receptors in group- and isolation-housed mice. Eur J Pharmacol. 2008;593:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 171. | Yamauchi M, Miyara T, Matsushima T, Imanishi T. Desensitization of 5-HT2A receptor function by chronic administration of selective serotonin reuptake inhibitors. Brain Res. 2006;1067:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 172. | van Zyl LT, Lespérance F, Frasure-Smith N, Malinin AI, Atar D, Laliberté MA, Serebruany VL. Platelet and endothelial activity in comorbid major depression and coronary artery disease patients treated with citalopram: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy Trial (CREATE) biomarker sub-study. J Thromb Thrombolysis. 2009;27:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 173. | Tseng YL, Chiang ML, Huang TF, Su KP, Lane HY, Lai YC. A selective serotonin reuptake inhibitor, citalopram, inhibits collagen-induced platelet aggregation and activation. Thromb Res. 2010;126:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 174. | Atar D, Malinin A, Pokov A, van Zyl L, Frasure-Smith N, Lesperance F, Serebruany VL. Antiplatelet properties of escitalopram in patients with the metabolic syndrome: a dose-ranging in vitro study. Neuropsychopharmacology. 2007;32:2369-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 175. | McCloskey DJ, Postolache TT, Vittone BJ, Nghiem KL, Monsale JL, Wesley RA, Rick ME. Selective serotonin reuptake inhibitors: measurement of effect on platelet function. Transl Res. 2008;151:168-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 176. | Maurer-Spurej E, Pittendreigh C, Solomons K. The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thromb Haemost. 2004;91:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 177. | Geiser F, Conrad R, Imbierowicz K, Meier C, Liedtke R, Klingmüller D, Oldenburg J, Harbrecht U. Coagulation activation and fibrinolysis impairment are reduced in patients with anxiety and depression when medicated with serotonergic antidepressants. Psychiatry Clin Neurosci. 2011;65:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 178. | Lespérance F, Frasure-Smith N, Koszycki D, Laliberté MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 454] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 179. | Taylor CB, Youngblood ME, Catellier D, Veith RC, Carney RM, Burg MM, Kaufmann PG, Shuster J, Mellman T, Blumenthal JA. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 387] [Article Influence: 19.4] [Reference Citation Analysis (0)] |