Published online Dec 22, 2015. doi: 10.5498/wjp.v5.i4.412
Peer-review started: April 24, 2015
First decision: June 3, 2015
Revised: September 2, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: December 22, 2015
Processing time: 248 Days and 10.3 Hours
AIM: To determine the prevalence of bipolar disorder (BD) and sub-threshold symptoms in children with attention deficit hyperactivity disorder (ADHD) through 14 years’ follow-up, when participants were between 21-24 years old.
METHODS: First, we examined rates of BD type I and II diagnoses in youth participating in the NIMH-funded Multimodal Treatment Study of ADHD (MTA). We used the diagnostic interview schedule for children (DISC), administered to both parents (DISC-P) and youth (DISCY). We compared the MTA study subjects with ADHD (n = 579) to a local normative comparison group (LNCG, n = 289) at 4 different assessment points: 6, 8, 12, and 14 years of follow-ups. To evaluate the bipolar variants, we compared total symptom counts (TSC) of DSM manic and hypomanic symptoms that were generated by DISC in ADHD and LNCG subjects. Then we sub-divided the TSC into pathognomonic manic (PM) and non-specific manic (NSM) symptoms. We compared the PM and NSM in ADHD and LNCG at each assessment point and over time. We also evaluated the irritability as category A2 manic symptom in both groups and over time. Finally, we studied the irritability symptom in correlation with PM and NSM in ADHD and LNCG subjects.
RESULTS: DISC-generated BD diagnosis did not differ significantly in rates between ADHD (1.89%) and LNCG 1.38%). Interestingly, no participant met BD diagnosis more than once in the 4 assessment points in 14 years. However, on the symptom level, ADHD subjects reported significantly higher mean TSC scores: ADHD 3.0; LNCG 1.7; P < 0.001. ADHD status was associated with higher mean NSM: ADHD 2.0 vs LNCG 1.1; P < 0.0001. Also, ADHD subjects had higher PM symptoms than LNCG, with PM means over all time points of 1.3 ADHD; 0.9 LNCG; P = 0.0001. Examining both NSM and PM, ADHD status associated with greater NSM than PM. However, Over 14 years, the NSM symptoms declined and changed to PM over time (df 3, 2523; F = 20.1; P < 0.0001). Finally, Irritability (BD DSM criterion-A2) rates were significantly higher in ADHD than LNCG (χ2 = 122.2, P < 0.0001), but irritability was associated more strongly with NSM than PM (df 3, 2538; F = 43.2; P < 0.0001).
CONCLUSION: Individuals with ADHD do not appear to be at significantly greater risk for developing BD, but do show higher rates of BD symptoms, especially NSM. The greater linkage of irritability to NSM than to PM suggests caution when making BD diagnoses based on irritability alone as one of 2 (A-level) symptoms for BD diagnosis, particularly in view of its frequent presentation with other psychopathologies.
Core tip: Despite its formal DSM delineation, alternative pediatric bipolar disorder (BD) definitions have been debated for decades. Some research suggests that attention deficit hyperactivity disorder (ADHD) poses a risk for BD and that pediatric BD presents differently as non-episodic, greater chronicity, and more frequent irritability. In our study, we found the ADHD status is not a risk factor for developing BD over 14 years of follow-ups. When we controlled for overlapping ADHD/BD, nonspecific symptoms showed decreasing rates of BD in ADHD-diagnosed children. Clinicians are encouraged to pay greater attention to specific symptoms of mania in order to establish an accurate BD diagnosis. Furthermore, irritability (DSM criteria A2), was a nonspecific symptom of mania and linked to common psychopathologies in the early development of these children.
- Citation: Elmaadawi AZ, Jensen PS, Arnold LE, Molina BS, Hechtman L, Abikoff HB, Hinshaw SP, Newcorn JH, Greenhill LL, Swanson JM, Galanter CA. Risk for emerging bipolar disorder, variants, and symptoms in children with attention deficit hyperactivity disorder, now grown up. World J Psychiatr 2015; 5(4): 412-424
- URL: https://www.wjgnet.com/2220-3206/full/v5/i4/412.htm
- DOI: https://dx.doi.org/10.5498/wjp.v5.i4.412
Bipolar disorder (BD) is a chronic mental illness affecting approximately 5.7 million United States children and adults, with adult prevalence rates about 2.6% of the United States population[1]. Pediatric bipolar disorder (PBD) has been surrounded by considerable debate[2] with experts divided about applying the adult -based DSM-IV criteria to children and adolescents[3]. Proponents for different criteria[4,5] argue that children are less likely to show clear episodes, more likely to demonstrate more chronic mania-like symptoms, and more often meet Criterion A principally via Irritability, not Euphoria[3]). If these arguments are correct then BD not otherwise specified (rather than BP type I with mania or BP type II with hypomania) might be the most accurate diagnostic “label” to characterize these children[5-7]. Yet many of these same children could also be appropriately diagnosed with attention deficit hyperactivity disorder (ADHD), comorbid with Oppositional Defiant Disorder (ODD), anxiety, depression, and/or intermittent explosive disorder. This lack of clarity and agreement among researchers and clinicians on the diagnostic criteria may have contributed to the forty-fold rise in reported prevalence of PBD over the last two decades[8].
Additional difficulties in PBD diagnostic clarity may be due to the overlap in some of the (mania) symptom criteria with ADHD criteria, such as “inattention” (ADHD) and “distractibility” (BD); “often talks excessively” (ADHD) and “more talkative than usual” (BD); and is often “on the go” or often acts as if “driven by a motor” (ADHD) and “increased goal directed activity or psychomotor agitation” (BD). In addition, many symptoms commonly occurring within children with ADHD (even though not currently part of the symptom criteria), but explicitly identified within BD (mania) criteria include “decreased need for sleep” and “irritability”. Because this latter symptom is common in ADHD and most other mental disorders, researchers have tried to distinguish whether irritability is (or should be) a major Criterion A for BD[9,10].
Although the co-occurrence of ADHD and BD is well documented, has a distinct phenotype, and evinces more unfavorable outcomes than either disorder alone[11,12], identifying each disorder in the context of the other remains a challenging task for clinicians. Though some research suggests that children with ADHD are at increased risk of developing BD[13], the actual proportion of ADHD children who eventually develop BD remains controversial[14-16]. The Longitudinal Assessment of Manic Symptoms (LAMS) study (n = 707, aged 6-12)[11] reported that ADHD and BD comorbidity was no greater than chance considering the rate of each disorder in the sample. Moreover, ADHD was not a significant risk for earlier BD; there was no difference in age of BD onset between the “BD alone” and “comorbid BD and ADHD” groups, and there was no cross-diagnosis familial loading[17]. In addition, Mannuzza et al[18] in a review of comorbidity in ADHD, identified both limitations and gaps in the current literature with regard to the estimates of comorbidity in both adult and childhood ADHD. Their 33-year follow-up study revealed no significantly greater risk for emergence of BD within ADHD than found in the general population.
Investigators attempting to understand the degree of risk conferred by the presence of one condition to develop the other have adopted several strategies, including analyses that attempt to control for, or remove, confounding (or overlapping) ADHD/BD symptoms[16]. Such analyses lead to substantial reductions (24%-53%) in the proportions of ADHD/BD-dually diagnosed children, who continue to meet BD diagnostic criteria[19,20]. Even when the issue of overlapping symptoms is accounted for, studies have generated different estimates concerning the likelihood that children with ADHD manifest BD, either cross-sectionally or longitudinally, with estimates ranging from 28%[21] to as low as 1.5%[18,22], leading critical reviewers to call for additional research, especially longitudinal studies that might clarify the exact nature of the relationship between ADHD and BD[23]. Another strategy Brotman et al[24] utilized to recognize bipolar variants which identified youths with chronic irritability phenotype that lacked the pathognomonic symptoms of euphoria and grandiosity, thereby creating the clinical syndrome called severe mood dysregulation or (SMD). Interestingly, in comparison to the episodic narrow band BD, youngsters with SMD have a higher rate of ADHD comorbidity. Such intermediate phenotypes’ of child bipolar diagnoses has been a significant advance for the field. Therefore, following Leibenluft’s research, investigators were interested to differentiate subtypes of BD and its relationship with ADHD.
Moreover, given the possible overlap of some ADHD and BD symptoms, including some clinically challenging symptoms that often complicate the course and management of both conditions (irritability, aggression)[25], as well as the genetic characteristics shared between them[26], researchers have increasingly called for a strategy of studying children with ADHD, in order to better understand the prevalence of bipolar symptomatology and the BD diagnosis[7].
For researchers striving to obtain unbiased estimates of the likelihood of a child diagnosed with ADHD concurrently meeting criteria for or eventually developing full symptoms of BD, epidemiologically ascertained, prospectively followed samples are required. In the absence of such data (usually the case within the continental United States), one viable strategy might be to study the emergence of BD within an ADHD sample, assuming that the ADHD sample has been identified early (before BD onset), rigorously-defined, longitudinally followed, and is generally representative of children with ADHD[6,27].
The longitudinal database now available from the NIMH Multimodal Treatment Study of Children with ADHD (MTA)[28-30] might be used to better understand the emergence of BD, given the early diagnosis of ADHD. Although the MTA’s principal aim was to evaluate different treatment approaches for ADHD, the study employed a rigorous assessment strategy, large sample size, geographic diversity and heterogeneity, and all of the diagnostic advantages afforded by following participants into young adulthood, when BD most often emerges[31]. We analyzed BD and ADHD at the symptom level from baseline through the 14-year follow-up data, in order to determine whether or not participants demonstrate BD and BP-NOS variants over follow-up.
At study outset, MTA investigators recruited 579 children (ages 7.0-9.9 years) with DSM-IV ADHD-Combined Type across 6 sites. Eighty percent of the sample was males and 20% were females (M = 465, F = 114). Sixty one percent of ADHD subjects were white Caucasian, 20% were African American and 8% were Hispanics. For comparison purposes, a “local normative comparison group” (LNCG, n = 289) was added to the study at 24 mo from the original baseline. LNCG children were matched with the initially selected children with ADHD, in order to reflect the same community, school, sex (Males = 235, Females = 54), and age composition as original participants. Children with presumed BD were meant to be excluded. Also, children on anti-psychotic agents or hospitalized within the last 6 mo were excluded. The institutional review board at the Mayo Clinic, Rochester, Minnesota approved the study, last approval date 10/31/2014; IRB number 12-00748. The study was determined as minimal risk retrospective chart review.
ADHD participants were evaluated at baseline, 14, 24, 36 mo, and then at 6, 8, 10, 12, and 14 years. LNCG participants completed the same assessments at 24 mo (LNCG baseline) and beyond.
The Diagnostic Interview Schedule for Children (DISC), developed by NIMH to assess more than 30 mental disorders, was administered to both parents (DISC-P) and youth (DISC)[32]. Through 3-year follow-up the DISC-P was used, followed by DISC Ver. 2.3/3.0 with both child and parent at the 6 years follow-up, then the DISC4 for parents at 8 years follow-up, and finally, a young adult self-report version of the DISC (DISC-YA) at the 12- and 14-year follow-ups. For this study, we focus on the DISC mania and hypomania assessments at 6, 8, 12, and 14 years. At those four follow-up assessments, retention rate of study participants with completed mania and hypomania assessments were: ADHD: 76%, 62%, 65%, and 73%; LNCG: 85%, 76%, 82%, and 84%, respectively.
Computer programs were used to generate the DSM diagnoses of Mania or hypomania within the last year’s timeframe covered within DISC interviews. Diagnoses were based on the DISC-YA (self-report), since parents rarely completed these diagnostic data at the final 2 time points, once youth reached ages > 18 years.
In addition to generating Mania or Hypomania diagnoses, the DISC assesses each of the individual symptoms that are required in order to meet BD diagnostic criteria. Thus, the DISC mania module inquires about 13 symptoms that are used to establish the presence or absence of the 9 DSM-IV A and B criteria of mania or hypomania. Thirteen questions are required because a) “inflated self-esteem or grandiosity” is broken into 2 questions; and ”increased goal directed activity (social, work or sexual) or agitation” is broken into 4 questions. Within the DISC, when a specific symptom is endorsed, additional questions were asked to determine if that symptom is truly positive, according to the DSM criteria (e.g., does that symptom meet the additional criteria for symptom duration, associated impairment, co-occurrence with other symptoms, etc.). Those symptoms that met these stringent criteria were counted in a “Total Symptom Count” (TSC).
Following previous investigators[16] we computed TSC-modified (TSC-M) scores, by subtracting 3 BD symptoms that could be considered to overlap with the ADHD symptoms of talkativeness, distractibility, and “on the go” (restlessness).
Besides these three symptoms, many others that occur in children with ADHD are also important for bipolar diagnosis, e.g., irritability. Therefore, we decided to examine BD symptomology by separating symptoms more specific to mania (pathognomonic) from non-specific symptoms. Pathognomonic Manic (PM) symptoms included elevated mood, grandiosity, inflated self-esteem, and increased goal directed activity (socially, sexually, and at work) (n = 6); and Non-Specific Manic (NSM) symptoms included irritability, decreased need for sleep, impulsive behavior, racing thoughts, pressured speech, distractibility, and restlessness (n = 7) (total = 13).
Irritability was evaluated at the symptom level to determine if it was more likely to correlate with elevated PM or NSM (excluding irritability) scores, and whether this differed between ADHD and LNCG.
Descriptive analyses were performed for all symptom and diagnosis frequency rates, comparing differences in frequency between MTA and LNCG subjects χ2 (and Fishers Exact Tests when appropriate). Subsequent analyses examined TSC across all subjects and compared group TSC means between ADHD and LNCG subjects. Second, given the longitudinal nature of the study and availability of multiple values for key outcomes (TSC, NSM, PM, etc.) within and across individuals over time, we used mixed-effects random regression methods (RRM) to examine the effects of group status (ADHD vs LNCG) or Irritability (Y/N), time, and group × time across all time points. The entire model tests the effect of the 3 variables interacting with each other. Now the preferred approach over traditional repeated measures ANOVAs for longitudinal studies, RRM allows all cases (even those with missing data) to contribute to the overall analysis, and may be less subject to selection/attrition biases.
No significant differences were found between ADHD and LNCG groups in the frequency of mania/hypomania clinical diagnoses at any assessment point of 6, 8, 12, or 14 years (χ2 = 0.024, P = 0.8). In fact, the prevalence rates, 0.24%-1.38% at different times for the ADHD subjects and 0%-0.93% at different times for the LNCG subjects, were very close to estimated bipolar prevalence rates in the general (adult) population. Fifteen subjects were diagnosed with DISC-Mania (n = 7) and Hypomania (n = 8). Their demographic differences from the whole sample were: 60% vs 80% males; 53% vs 62% Caucasian; one third vs 18% African-American (Table 1). Interestingly, the total of 15 participants (4 LNCG and 11 ADHD), met the DISC computed BD only once in all assessment points in 6-, 8-, 12- and 14-years (Table 2).
| ADHD | LNCG | |||||
| Mania | Hypomania | Prevalence (%) | Mania | Hypomania | Prevalence (%) | |
| 6 yr | 1 | 1 | 0.45 | 0 | 0 | 0 |
| 8 yr | 2 | 3 | 1.38 | 1 | 1 | 0.93 |
| 12 yr | 1 | 2 | 0.79 | 1 | 0 | 0.42 |
| 14 yr | 1 | 0 | 0.24 | 0 | 1 | 0.41 |
| Total | 5 | 6 | 1.89 | 2 | 2 | 1.38 |
| 6 yr | 8 yr | 12 yr | 14 yr | Total | ||
| LNCG | Manic | Missing | Yes | No | No | 2 |
| No | No | Yes | No | |||
| Hypomanic | No | Yes | No | No | 2 | |
| No | No | No | Yes | |||
| ADHD | Manic | Yes | No | No | No | |
| No | Yes | Missing | No | |||
| No | Yes | No | No | 5 | ||
| No | No | Yes | No | |||
| No | No | No | Yes | |||
| Hypomanic | Yes | No | No | No | ||
| No | Yes | No | No | |||
| No | Yes | No | No | 6 | ||
| No | Yes | No | No | |||
| No | Missing | Yes | No | |||
| No | No | Yes | No |
At all assessment points (6-, 8-, 12- and 14-years), ADHD subjects reported significantly more of the 13 symptoms of the DISC mania/hypomania module (TSC) with means of 3.0 (ADHD) and 1.7 (LNCG) symptoms (Table 3). RRM confirmed significant effects of the overall model (df 3, 2538; F = 63.9; P < 0.0001), as well as all specific factors within the model: group status (ADHD vs LNCG) (F = 177.1; P < 0.0001), time (F = 5.1; P < 0.02), and change of symptoms in groups over time (F = 5.4; P < 0.02). Thus, although ADHD subjects did not show higher rates of BD diagnoses, they did have almost twice the rates of bipolar-mania symptoms at the 4 assessment points, and over time. They did have almost twice the rates of bipolar-mania symptoms at the 4 assessment points, and over time.
| Assessment point | ADHD | LNCG | DF | F ratio | P values | ||
| n | Mean ± SD | n | Mean ± SD | ||||
| 6 yr | 441 | 3.38 ± 2.54 | 246 | 1.85 ± 1.93 | 1687 | 68.58 | < 0.0001 |
| 8 yr | 360 | 2.89 ± 2.3 | 219 | 1.45 ± 1.69 | 1578 | 63.70 | < 0.0001 |
| 12 yr | 378 | 3.05 ± 2.85 | 236 | 1.77 ± 2.07 | 1613 | 35.80 | < 0.0001 |
| 14 yr | 420 | 2.64 ± 2.59 | 242 | 1.72 ± 2.00 | 1661 | 22.51 | < 0.0001 |
| TSC Over Time | 579 | 3.0 ± 0 | 32538 | 63.9 | < 0.0001 | ||
In order to explore the possible ADHD - mania symptom confounds, as well as symptom specificity, we proceeded in the following three steps:
After applying the Wozniak adjustment to remove bipolar symptoms that overlapped with ADHD diagnostic criteria[16], we calculated modified TSC scores (TSC-M) for both groups. RRM analysis revealed that ADHD group subjects continued to endorse significantly more symptoms, with TSC-M means of 2.2 (ADHD) and 1.4 (LNCG) (df 3, 2538; F = 38.8; P < 0.0001). ADHD vs LNCG group status was the only factor linked to higher TSC-M (F = 114.8; P < 0.0001), and neither time nor group x time factors contributed significantly.
Likewise, overall RRM analyses of PM were significant (df 3, 2524; F = 30.5; P = 0.0001), with PM means over all time points of 1.3 (ADHD) and 0.9 (LNCG). Both ADHD vs LNCG group status and time were significant factors for PM decreasing with time (F = 71.2; P < 0.0001; and F = 17.6; P < 0.0001, respectively) but not group × time).
Finally, the RRM analyses of NSM comparing groups over time yielded significant overall model effects (df 3, 2537; F = 69.3; P < 0.0001); with means of 2.0 (ADHD) and 1.1 (LNCG), and with significant ADHD vs LNCG group status: F = 194.7; P < 0.0001 and group × time: F = 12.1; P = 0.0005. These NSM differences in means between the ADHD and LNCG groups appeared twice as large as differences found above in PM scores, raising the possibility of a group (ADHD vs LNCG) × symptom type (PM vs NSM) interaction.
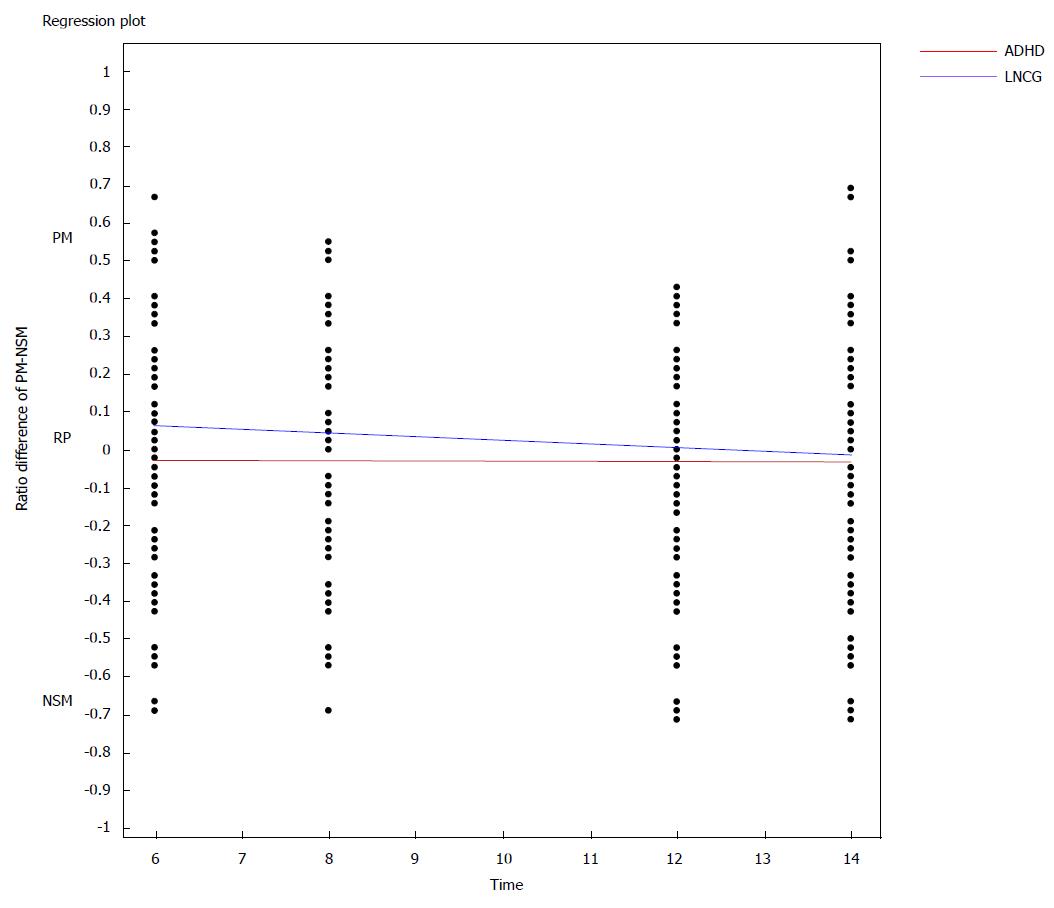
To examine this possible interaction, and to provide a sensitive test of the differential association of the relative proportions of PM vs NSM symptoms in ADHD vs LNCG subjects, we divided each subjects’ total PM by the total possible number of PM symptoms, thereby generating for each one a specific ratio (0.0 to 1.0) of PM symptoms; we also computed similar ratios for NSM for each subject (again, 0.0 to 1.0). Then, to test the interaction, i.e., differences in their relative proportions of PM vs NSM symptoms, we constructed a new variable by subtracting the NSM ratio from the PM ratio for each subject at each time point, thus generating rPM - rNSM difference scores, yielding a possible range of differences from -1.00 to +1.00 for each subject (positive score 0 to +1.0 is associated with PM and negative score -1.0 to 0 with NSM). Upon testing ADHD vs LNCG subjects on these difference scores, groups differed significantly, with disproportionately higher NSM ratios in subjects with ADHD, and significant change of ADHD group, shifting from NSM to PM over time. The entire RRM was significant (df 3, 2523; F = 20.1; P < 0.0001). All Variables were significantly linked to rPM - rNSM difference scores, including group status, time, and group × time (Figure 1).
Finally, we sought to examine the role of irritability across both ADHD and LNCG, and to assess its possible specificity/non-specificity as a key mania symptom criterion (Criteria A2), following 3 steps:
First, we calculated irritability criterion frequencies across the 2 groups. As expected, ADHD subjects reported significantly higher irritability compared to LNCG subjects, with relative risk of irritability 2.01 in ADHD subjects compared to the LNCG across the entire study (χ2 = 122.2, P < 0.0001).
We then assessed the relationship between irritability and the 2 mania symptom subscales (PM and NSM), after adjusting NSM values to remove irritability from its totals. The RRM model revealed that irritability was associated with both scales (PM: df 3, 2524; F = 86.5; P < 0.0001; NSM: df 3, 2524; F = 114.6; P < 0.0001).
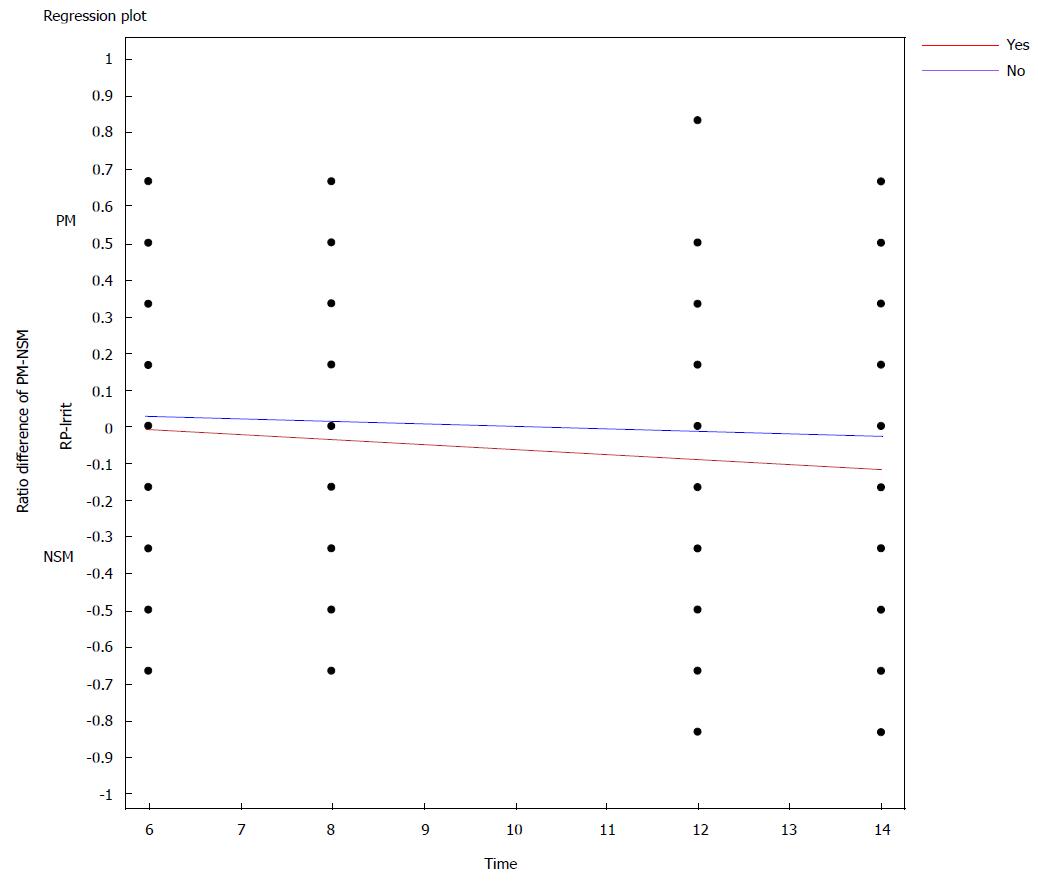
Because irritability was associated with both PM and NSM, we created difference score ratios as described in paragraph 3 above, allowing us to examine any differences in irritability associations between PM and NSM across LNCG and ADHD subjects. Findings revealed that compared to LNCG subjects, ADHD subjects’ likelihood of manifesting irritability significantly increased over time as the PM-NSM difference score decreased (became more negative) towards a greater preponderance of NSM symptoms (Overall Model RRM: df 3, 2538; F = 43.2 P≤ 0.0001). All variables were linked to PM-NSM difference scores (irritability status, time, and irritability × Time (see Figure 2).
Our prevalence analyses of PBD and adult BD from DISC-computed mania and hypomania diagnoses among subjects with and without ADHD (MTA vs LNCG subjects) revealed no significant differences in the small numbers and proportions of individuals meeting DSM BD criteria, paralleling the results of Mannuzza[33]. Interestingly, despite the fact that all subjects who were diagnosed with mania or hypomania by the DISC were evaluated at least 3 times, and 80% of them were evaluated in all 4 assessment points (6-14 years), none received mania or hypomania diagnoses more than once (Table 1). These findings raise questions about the stability of BD diagnoses over time, especially during early development - assuming reliability of the DISC, DIS, and DISC-YA[32]. After Shaffer et al[32] developed the DISC, many researchers widely evaluated the reliability and validity of the DISC, in comparison to other diagnostic tools. The results were consistent with high test-retest reliability across the study sample[34,35].
Various large epidemiological studies over the last 3 decades both in the United States and Europe have also noted that the diagnostic stability of all affective disorders (BP I, BP II, and major depressive disorder) varied depending on socioeconomic and even cultural factors[36,37]. Knowledgeable commentator-skeptics have raised concerns that both clinicians and clinical investigators alike are prone to succumb to the “Clinician’s Illusion” as observed in psychosis[38]. Almost 30% of individuals who suffered from a psychotic episode never re-experience further episodes after the first one - a finding that is likely only observable within community-wide, diagnostically rigorous longitudinal studies. Nonetheless, these concerns must be tempered by the realization that BD is by definition episodic, so that varying presence of diagnostic symptoms over time is to be expected.
Given the lack of significant differences found here between subjects with ADHD and local normative comparison subjects in BD diagnoses, we sought to determine if individuals from the ADHD group had more sub-threshold BD symptoms compared to the LNCG group. These analyses revealed that in fact, ADHD study subjects had higher BD symptoms (TSC scores) than LNCG subjects. Higher TSC in children with ADHD may count for the 40 folds increase in the BD diagnosis in the community. Although some authors have indicated that childhood ADHD may pose risks for developing BD over time; these 14-year longitudinal findings call this conclusion into question. We found an interesting decline of the TSC score over time in ADHD subjects. Eventually the TSC scores of young adults with ADHD were more similar to LNCG subjects by 14 years (though still significantly greater). Although childhood ADHD was unrelated to full BD diagnosis, it did pose a significant risk for BD symptoms (TSC) over time. However, the presence of an interaction effect (time × group) indicated that these symptom elevations (vs LNCG) decreased from 6 to 14 years’ follow-up. It is possible that these decreases in TSC counts might continue their declines over time.
These results are compatible with cross-sectional baseline analyses from the LAMS study[11,17]. This sample was clinically recruited to be enriched with high levels of manic symptoms, but more of the children (age 6-12) had ADHD than bipolar spectrum disorder, illustrating the overlap of NSM. Considering the possibility of Berkson bias (independent psychiatric disorders may associated in clinical sample due to the higher chance of seeking medical attention)[39], yet the overlap of diagnoses (comorbid ADHD and BD) was no greater than expected by chance from the prevalence of each disorder in the sample. The ADHD-alone children had fewer manic symptoms than those with BD alone but more than those with neither diagnosis (those with other psychiatric diagnoses), while the BD-alone children had more ADHD symptoms than those with neither diagnosis but less than those with ADHD alone. The ADHD-alone children had the same frequency of irritability as those with neither ADHD nor BD, which was half the rate in those with BD (with or without comorbid ADHD).
One possible interpretation is that the confounding of ADHD-associated symptoms with BD diagnoses may result in differences between ADHD subjects’vs normal comparison children’s TSC scores during earlier development, and over time these confounding symptoms dissipate. Thus, the application of developmental considerations within DSM-5 in the classification of mental illness may help clinicians and researchers to be more careful making the BD diagnosis in the earlier stages of development[40].
Seeking to understand these differences in TSC scores, we like previous investigators (15), evaluated BD at the symptom level, eliminating DSM ADHD/BD overlapping symptoms (distractibility, on the go-restlessness, and talkativeness). Taking further steps to “unpack” and better understand BD symptoms, we separated those BD symptoms that might be a part of a non-specific presentation with ADHD (NSM) from those that were more specific to BD (PM). We found that ADHD-diagnosed subjects who presented with one or more BD symptoms were more likely than LNCG subjects (with one or more BD symptoms) to show elevated NSM (vs PM) symptoms. Thus, the 6 PM symptoms as we defined them (expansive or euphoric mood, inflated self-esteem, grandiosity, and increased goal directed activity) were relatively less likely to be present among ADHD subjects with a BD symptom than comparison subjects with a BD symptom.
Although later follow-ups may raises the question of self-report bias in ADHD subjects that lean to under report, however our analyses of PM and NSM changes over time among ADHD vs control subjects showed a time × diagnostic status (ADHD vs LNCG) effect for NSM only, revealing a gradual greater diminution of non-specific mania symptoms among ADHD than among LNCG subjects. Moreover, children with ADHD had persistently stable PM differences from LNCG subjects. The gradual abatement of NSM symptoms may facilitate distinguishing between ADHD and BD in older adolescents and adults; conversely the distinction may be obfuscated at earlier ages. The clinical implication is to observe over time in doubtful cases, as recommended by the LAMS group[41].
Although the differences in PM and NSM counts between ADHD and LNCG subjects were small, they appeared twice as large for NSM as for PM (mean symptom difference between ADHD and LNCG was 0.4 for PM and 0.9 for NSM) with both counts higher in ADHD than LNCG subjects. ADHD subjects had proportionally more NSM and PM symptoms, compared to LNCG subjects, with the difference diminishing over time. One might speculate that ADHD subjects tend to be “messy”, i.e., have many associated non-specific symptoms) during earlier childhood years, and these non-specific symptoms (if they overlap with BD criteria) might result in ADHD subjects being misdiagnosed with Bipolar disorder (NOS or even I or II), despite their lacking most PM symptoms. It is unclear if such children/youth carry some bipolar genes or have a sub-threshold bipolar variant, or perhaps a different disorder altogether. However, the fact that the symptom counts decrease over time makes this unlikely. One way to guard against early over diagnosis is to require episodicity and at least 3 of the pathognomonic symptoms, as done in the COBY and LAMS longitudinal studies. We may also hypothesis association of the paradigm shift of BD symptoms and the decline in impulsivity-hyperactivity symptoms of ADHD over time.
To further understand these elevations in NSM symptomatology in ADHD vs LNCG subjects, we examined the irritability criterion, particularly in view of its inclusion as one of A criteria in DSM for BD, and given its common phenotypical presentation in child psychopathology, including ADHD. Our results were compatible with findings from other studies[42]: children with ADHD are more irritable than the normal population, and over time, fewer young adults with ADHD report the irritability symptom, in contrast to a more stable but lower-level presence in LNCG subjects (significant group × time effects). However, due to the increasing linkages over time of irritability with NSM symptoms (vs PM symptoms) in both ADHD and LNCG groups, our final PM-NSM difference score analysis vis-à-vis irritability suggests that irritability may be a non-specific component of chronic psychopathology, not only for BD, but also for unipolar depression, anxiety, ADHD and other forms of psychopathology. In their study of patients with a subtype of SMD, Stringaris et al[43] also reported such conclusions. If irritability is indeed increasingly linked to NSM symptoms over time, the question might be raised, should we reconsider irritability as an A criterion for the diagnosis of BD? It is possible that the new DSM-5 disruptive mood dysregulation disorder diagnosis may help clinicians find a more fitting diagnosis for children with chronic, severe irritability, although initial studies of its reliability raise concerns about its viability[44].
Future BD diagnostic criteria might need to eliminate symptom confounds and overlaps. For example an exclusionary clause might be created - i.e., in the presence of childhood ADHD, more symptoms might be required, or “irritability” might be excluded as an A criterion. More longitudinal research is required, both of patients first identified by manic symptoms (e.g., LAMS), and of those first identified with ADHD, if we are to fully understand what symptoms should characterize “true” BD or its variants across development, in view of longitudinal studies of high-risk children from parents with BD[45]. These studies indicate that emerging BD is not characterized by early onset irritability or ADHD, but instead by unfolding anxiety symptoms and sleep disturbances, before the first manic or hypomanic episode.
The limitation of our study involves the confining use of using a structure interview (DISC) that does not interpret invalid responses or atypical presentations, despite the fact that the assessment was conducted by experienced trained interviewers. There is also the possibility of positive illusionary bias in the ADHD group from self- reporting[46]. Moreover, like most retrospective studies, we were subject to recall bias, especially with relatively long follow-up over 14 years, and lengthy assessment intervals. The retention rate over 14 years was 75%, which also created a possible differential dropout in subjects with severe symptoms. With such limitations, we attempted to overcome the under-sample bias with RRP analysis.
Our findings suggest that individuals with childhood ADHD followed into early adulthood (ages 21-24) do not appear to be at a significantly greater risk for developing the full diagnostic picture of BD than comparison subjects. Although adolescents/young adults with ADHD do report modestly higher BD symptoms (e.g., TSC scores) over time than comparison subjects, one might expect higher symptoms of many different types among patients who have been ascertained on the basis of having at least one disorder (ADHD, in this case), compared to non-clinical community subjects. These BD symptom elevations tend to be non-specific rather than pathognomonic, and they decline over time in adolescence and young adulthood. Further, BD diagnosis was not persistent at early stages of development. Irritability, one of the A criteria for BD diagnosis, was more associated with NSM than PM symptoms. Our findings suggest caution when making BD diagnoses in youth and young adults with histories of childhood ADHD. Moreover, because the irritability criterion was linked to increases in the percentage of non-specific rather than pathognomonic bipolar symptoms, future BD classification attempts should consider whether its continued inclusion as one of 2 alternative essential (A-level) criteria for BD diagnosis is warranted in children. A possible solution is to require more PM symptoms when irritability is used for the A criterion, as was done for bipolar-NOS in the LAMS and COBY studies.
The Multimodal Treatment Study of Children with ADHD (MTA) was a National Institute of Mental Health (NIMH) cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and finally under a National Institute on Drug Abuse (NIDA) contract. Collaborators from NIMH: Benedetto Vitiello, M.D. (Child and Adolescent Treatment and Preventive Interventions Research Branch), Joanne B. Severe, M.S. (Clinical Trials Operations and Biostatistics Unit, Division of Services and Intervention Research), Peter S. Jensen, M.D. (currently at REACH Institute and Mayo Clinic), L. Eugene Arnold, M.D., M.Ed. (currently at Ohio State University), Kimberly Hoagwood, Ph.D. (currently at Columbia); previous contributors from NIMH to the early phases: John Richters, Ph.D. (currently at National Institute of Nursing Research); Donald Vereen, M.D. (currently at NIDA). Principal investigators and co-investigators from the sites are: University of California, Berkeley/San Francisco: Stephen P. Hinshaw, Ph.D. (Berkeley), Glen R. Elliott, Ph.D., M.D. (San Francisco); Duke University: Karen C. Wells, Ph.D., Jeffery N. Epstein, Ph.D. (currently at Cincinnati Children’s Hospital Medical Center), Desiree W. Murray, Ph.D.; previous Duke contributors to early phases: C. Keith Conners, Ph.D. (former PI); John March, M.D., M.P.H.; University of California, Irvine: James Swanson, Ph.D., Timothy Wigal, Ph.D.; previous contributor from UCLA to the early phases: Dennis P. Cantwell, M.D. (deceased); New York University: Howard B. Abikoff, Ph.D.; Montreal Children’s Hospital/ McGill University: Lily Hechtman, M.D.; New York State Psychiatric Institute/Columbia University/Mount Sinai Medical Center: Laurence L. Greenhill, M.D. (Columbia), Jeffrey H. Newcorn, M.D. (Mount Sinai School of Medicine). University of Pittsburgh: Brooke Molina, Ph.D., Betsy Hoza, Ph.D. (currently at University of Vermont), William E. Pelham, Ph.D. (PI for early phases, currently at Florida International University). Follow-up phase statistical collaborators: Robert D. Gibbons, Ph.D. (University of Illinois, Chicago); Sue Marcus, Ph.D. (Mt. Sinai College of Medicine); Kwan Hur, Ph.D. (University of Illinois, Chicago). Original study statistical and design consultant: Helena C. Kraemer, Ph.D. (Stanford University). Collaborator from the Office of Special Education Programs/US Department of Education: Thomas Hanley, Ed.D. Collaborator from Office of Juvenile Justice and Delinquency Prevention/Department of Justice: Karen Stern, Ph.D.
Pediatric bipolar disorder (PBD) is one of the most debatable mental illnesses of our time. Experts have been divided about whether the adult-based DSM criteria apply to children and adolescents. Interestingly, the diagnosis of PBD has risen 40-fold over the last two decades with bipolar-NOS being the most common diagnostic type. The prevalence of PBD ranges from 2.6% to a higher prevalence of 6.4%, which was reported when the subthreshold cases were included rather than classic Bipolar Disorder (BD). The differences in rates and difficulties of capturing PBD are related to many factors. First of all, BD presents in children and adolescents differently from adult BD, as children are less likely to show clear, (i.e., more chronic), episodes. Also, unlike adult bipolar, the most common Criteria A of mania in PBD is irritability, not euphoria. However, irritability is also a common symptom in many psychiatric disorders in children and adolescents. More importantly, there are notable overlaps in many of the specific symptom criteria for both PBD and more common disorders, such as attention deficit hyperactivity disorder (ADHD), thus making differentiating diagnosis challenging.
Misdiagnosing PBD would have detrimental consequences to children, if ADHD treatments for such children prove to be harmful, or would delay more appropriate PBD treatments. Hence, the relation between PBD comorbid phenotype, and ADHD, has continued to be the source of considerable study and debate for many years. Thus, the new DSM-5 disruptive mood dysregulation disorder, is an attempt to reduce over-diagnosing pediatric BD.
The auhors careful evaluation of bipolar symptoms suggests future revisions of the DSM may be necessary to re-evaluate the special status of irritability as one of two required (A criterion) symptoms for making a BD diagnosis.
Findings suggest that individuals with carefully diagnosed ADHD may not be at a significant risk for developing BD, vs controls. Given the lack of meaningful diagnostic differences and only modest differences in PBD symptom counts, the auhors were surprised to find that childhood ADHD diagnostic status predicted a greater likelihood of non-specific mania symptoms, rather than more pathognomonic symptoms. This suggests that such elevations might reflect general psychopathology rather than BD, per se. Even irritability, one of the 2 “A” criteria required for BD diagnosis, was more associated with non-specific mania than specific symptoms. Findings suggest that clinicians should be cautious when making a PBD diagnosis with individuals with histories of childhood ADHD. Such individuals with elevated non-specific BP symptoms, (sometimes called “messy” ADHD), may in fact be just that - “messy ADHD”. However, it is also possible that they may have a different disorder altogether, a question thus requiring more research and study.
MTA is the Multimodal Treatment Study of Children with ADHD (MTA). NIMH funded the study in the 90th of the last century. The main principal aim was to evaluate different treatment approaches for ADHD; the study employed a rigorous assessment strategy, large sample size, geographic diversity and heterogeneity of study subjects. DISC is a highly structured diagnostic interview assessment. NIMH to diagnose over 30 mental illnesses created it. TSC: Total symptoms count, which represents the total 13 questions that generated by the DISC to establish the diagnosis of Mania or Hypomania. PM: is the pathogmonic manic symptoms, included elevated mood, grandiosity, inflated self-esteem, and increased goal directed activity (socially, sexually, and at work), total of 6 DISC questions. NSM: Nonspecific manic symptoms included irritability, decreased need for sleep, impulsive behavior, racing thoughts, pressured speech, distractibility, and restlessness; total of 7 DISC questions.
The present findings raise questions about the stability of BD diagnoses over time, in particular during early development. However, it is also important to state that the authors adopted in their study a structure interview (DISC) that does not consider invalid responses or atypical presentations.
P- Reviewer: Hosak L, Serafini G, Yang YK S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7240] [Cited by in RCA: 7253] [Article Influence: 362.7] [Reference Citation Analysis (0)] |
| 2. | Biederman J, Klein RG, Pine DS, Klein DF. Resolved: mania is mistaken for ADHD in prepubertal children. J Am Acad Child Adolesc Psychiatry. 1998;37:1091-1096; discussion 1096-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 166] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Wozniak J, Biederman J, Kwon A, Mick E, Faraone S, Orlovsky K, Schnare L, Cargol C, van Grondelle A. How cardinal are cardinal symptoms in pediatric bipolar disorder? An examination of clinical correlates. Biol Psychiatry. 2005;58:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Geller B, Tillman R, Bolhofner K. Proposed definitions of bipolar I disorder episodes and daily rapid cycling phenomena in preschoolers, school-aged children, adolescents, and adults. J Child Adolesc Psychopharmacol. 2007;17:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | McClellan J, Kowatch R, Findling RL. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:107-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 280] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Geller B, Zimerman B, Williams M, Delbello MP, Bolhofner K, Craney JL, Frazier J, Beringer L, Nickelsburg MJ. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2002;12:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 208] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 441] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Case BG, Olfson M, Marcus SC, Siegel C. Trends in the inpatient mental health treatment of children and adolescents in US community hospitals between 1990 and 2000. Arch Gen Psychiatry. 2007;64:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Hunt J, Birmaher B, Leonard H, Strober M, Axelson D, Ryan N, Yang M, Gill M, Dyl J, Esposito-Smythers C. Irritability without elation in a large bipolar youth sample: frequency and clinical description. J Am Acad Child Adolesc Psychiatry. 2009;48:730-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Mick E, Spencer T, Wozniak J, Biederman J. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Arnold LE, Demeter C, Mount K, Frazier TW, Youngstrom EA, Fristad M, Birmaher B, Findling RL, Horwitz SM, Kowatch R. Pediatric bipolar spectrum disorder and ADHD: comparison and comorbidity in the LAMS clinical sample. Bipolar Disord. 2011;13:509-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J Clin Psychiatry. 2012;73:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Faraone SV, Biederman J, Wozniak J, Mundy E, Mennin D, O’Donnell D. Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry. 1997;36:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 155] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 340] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 15. | Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, Bowden CL, Sachs GS, Nierenberg AA. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry. 2004;55:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 592] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 16. | Wozniak J, Biederman J, Kiely K, Ablon JS, Faraone SV, Mundy E, Mennin D. Mania-like symptoms suggestive of childhood-onset bipolar disorder in clinically referred children. J Am Acad Child Adolesc Psychiatry. 1995;34:867-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 453] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 17. | Arnold LE, Mount K, Frazier T, Demeter C, Youngstrom EA, Fristad MA, Birmaher B, Horwitz S, Findling RL, Kowatch R. Pediatric bipolar disorder and ADHD: family history comparison in the LAMS clinical sample. J Affect Disord. 2012;141:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Mannuzza S, Castellanos FX, Roizen ER, Hutchison JA, Lashua EC, Klein RG. Impact of the impairment criterion in the diagnosis of adult ADHD: 33-year follow-up study of boys with ADHD. J Atten Disord. 2011;15:122-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Milberger S, Biederman J, Faraone SV, Murphy J, Tsuang MT. Attention deficit hyperactivity disorder and comorbid disorders: issues of overlapping symptoms. Am J Psychiatry. 1995;152:1793-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 173] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Kowatch RA, Youngstrom EA, Danielyan A, Findling RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disord. 2005;7:483-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Biederman J, Petty CR, Byrne D, Wong P, Wozniak J, Faraone SV. Risk for switch from unipolar to bipolar disorder in youth with ADHD: a long term prospective controlled study. J Affect Disord. 2009;119:16-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Beesdo K, Höfler M, Leibenluft E, Lieb R, Bauer M, Pfennig A. Mood episodes and mood disorders: patterns of incidence and conversion in the first three decades of life. Bipolar Disord. 2009;11:637-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Singh MK, DelBello MP, Kowatch RA, Strakowski SM. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8:710-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello EJ, Egger HL, Angold A, Pine DS, Leibenluft E. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 324] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 25. | Masi G, Perugi G, Toni C, Millepiedi S, Mucci M, Bertini N, Pfanner C. Attention-deficit hyperactivity disorder -- bipolar comorbidity in children and adolescents. Bipolar Disord. 2006;8:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Cross-Disorder Group of the Psychiatric Genomics C, Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, Mowry BJ, Thapar A, Goddard ME, Witte JS, Absher D, Agartz I, Akil H, Amin F, Andreassen OA, Anjorin A, Anney R, Anttila V, Arking DE, Asherson P, Azevedo MH, Backlund L, Badner JA, Bailey AJ, Banaschewski T, Barchas JD, Barnes MR, Barrett TB, Bass N, Battaglia A, Bauer M, Bayes M, Bellivier F, Bergen SE, Berrettini W, Betancur C, Bettecken T, Biederman J, Binder EB, Black DW, Blackwood DH, Bloss CS, Boehnke M, Boomsma DI, Breen G, Breuer R, Bruggeman R, Cormican P, Buccola NG, Buitelaar JK, Bunney WE, Buxbaum JD, Byerley WF, Byrne EM, Caesar S, Cahn W, Cantor RM, Casas M, Chakravarti A, Chambert K, Choudhury K, Cichon S, Cloninger CR, Collier DA, Cook EH, Coon H, Cormand B, Corvin A, Coryell WH, Craig DW, Craig IW, Crosbie J, Cuccaro ML, Curtis D, Czamara D, Datta S, Dawson G, Day R, De Geus EJ, Degenhardt F, Djurovic S, Donohoe GJ, Doyle AE, Duan J, Dudbridge F, Duketis E, Ebstein RP, Edenberg HJ, Elia J, Ennis S, Etain B, Fanous A, Farmer AE, Ferrier IN, Flickinger M, Fombonne E, Foroud T, Frank J, Franke B, Fraser C, Freedman R, Freimer NB, Freitag CM, Friedl M, Frisen L, Gallagher L, Gejman PV, Georgieva L, Gershon ES, Geschwind DH, Giegling I, Gill M, Gordon SD, Gordon-Smith K, Green EK, Greenwood TA, Grice DE, Gross M, Grozeva D, Guan W, Gurling H, De Haan L, Haines JL, Hakonarson H, Hallmayer J, Hamilton SP, Hamshere ML, Hansen TF, Hartmann AM, Hautzinger M, Heath AC, Henders AK, Herms S, Hickie IB, Hipolito M, Hoefels S, Holmans PA, Holsboer F, Hoogendijk WJ, Hottenga JJ, Hultman CM, Hus V, Ingason A, Ising M, Jamain S, Jones EG, Jones I, Jones L, Tzeng JY, Kahler AK, Kahn RS, Kandaswamy R, Keller MC, Kennedy JL, Kenny E, Kent L, Kim Y, Kirov GK, Klauck SM, Klei L, Knowles JA, Kohli MA, Koller DL, Konte B, Korszun A, Krabbendam L, Krasucki R, Kuntsi J, Kwan P, Landen M, Langstrom N, Lathrop M, Lawrence J, Lawson WB, Leboyer M, Ledbetter DH, Lee PH, Lencz T, Lesch KP, Levinson DF, Lewis CM, Li J, Lichtenstein P, Lieberman JA, Lin DY, Linszen DH, Liu C, Lohoff FW, Loo SK, Lord C, Lowe JK, Lucae S, MacIntyre DJ, Madden PA, Maestrini E, Magnusson PK, Mahon PB, Maier W, Malhotra AK, Mane SM, Martin CL, Martin NG, Mattheisen M, Matthews K, Mattingsdal M, McCarroll SA, McGhee KA, McGough JJ, McGrath PJ, McGuffin P, McInnis MG, McIntosh A, McKinney R, McLean AW, McMahon FJ, McMahon WM, McQuillin A, Medeiros H, Medland SE, Meier S, Melle I, Meng F, Meyer J, Middeldorp CM, Middleton L, Milanova V, Miranda A, Monaco AP, Montgomery GW, Moran JL, Moreno-De-Luca D, Morken G, Morris DW, Morrow EM, Moskvina V, Muglia P, Muhleisen TW, Muir WJ, Muller-Myhsok B, Murtha M, Myers RM, Myin-Germeys I, Neale MC, Nelson SF, Nievergelt CM, Nikolov I, Nimgaonkar V, Nolen WA, Nothen MM, Nurnberger JI, Nwulia EA, Nyholt DR, O’Dushlaine C, Oades RD, Olincy A, Oliveira G, Olsen L, Ophoff RA, Osby U, Owen MJ, Palotie A, Parr JR, Paterson AD, Pato CN, Pato MT, Penninx BW, Pergadia ML, Pericak-Vance MA, Pickard BS, Pimm J, Piven J, Posthuma D, Potash JB, Poustka F, Propping P, Puri V, Quested DJ, Quinn EM, Ramos-Quiroga JA, Rasmussen HB, Raychaudhuri S, Rehnstrom K, Reif A, Ribases M, Rice JP, Rietschel M, Roeder K, Roeyers H, Rossin L, Rothenberger A, Rouleau G, Ruderfer D, Rujescu D, Sanders AR, Sanders SJ, Santangelo SL, Sergeant JA, Schachar R, Schalling M, Schatzberg AF, Scheftner WA, Schellenberg GD, Scherer SW, Schork NJ, Schulze TG, Schumacher J, Schwarz M, Scolnick E, Scott LJ, Shi J, Shilling PD, Shyn SI, Silverman JM, Slager SL, Smalley SL, Smit JH, Smith EN, Sonuga-Barke EJ, St Clair D, State M, Steffens M, Steinhausen HC, Strauss JS, Strohmaier J, Stroup TS, Sutcliffe JS, Szatmari P, Szelinger S, Thirumalai S, Thompson RC, Todorov AA, Tozzi F, Treutlein J, Uhr M, van den Oord EJ, Van Grootheest G, Van Os J, Vicente AM, Vieland VJ, Vincent JB, Visscher PM, Walsh CA, Wassink TH, Watson SJ, Weissman MM, Werge T, Wienker TF, Wijsman EM, Willemsen G, Williams N, Willsey AJ, Witt SH, Xu W, Young AH, Yu TW, Zammit S, Zandi PP, Zhang P, Zitman FG, Zollner S, International Inflammatory Bowel Disease Genetics C, Devlin B, Kelsoe JR, Sklar P, Daly MJ, O’Donovan MC, Craddock N, Sullivan PF, Smoller JW, Kendler KS, Wray NR. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1903] [Cited by in RCA: 1637] [Article Influence: 136.4] [Reference Citation Analysis (1)] |
| 27. | Geller B, Tillman R, Bolhofner K, Zimerman B. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65:1125-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott G, Greenhill LL, Hechtman L, Hinshaw SP, Hoza B, Jensen PS. National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Arch Gen Psychiatry. 1997;54:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 155] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Jensen PS. Introduction--ADHD comorbidity and treatment outcomes in the MTA. J Am Acad Child Adolesc Psychiatry. 2001;40:134-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Epstein JN, Hoza B, Hechtman L, Abikoff HB. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48:484-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 873] [Cited by in RCA: 720] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 31. | Jensen PS, Arnold LE, Swanson JM, Vitiello B, Abikoff HB, Greenhill LL, Hechtman L, Hinshaw SP, Pelham WE, Wells KC. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 370] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 32. | Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2500] [Cited by in RCA: 2485] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 33. | Klein RG, Mannuzza S, Olazagasti MA, Roizen E, Hutchison JA, Lashua EC, Castellanos FX. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry. 2012;69:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 34. | Jensen P, Roper M, Fisher P, Piacentini J, Canino G, Richters J, Rubio-Stipec M, Dulcan M, Goodman S, Davies M. Test-retest reliability of the Diagnostic Interview Schedule for Children (DISC 2.1). Parent, child, and combined algorithms. Arch Gen Psychiatry. 1995;52:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 164] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Bravo M, Ribera J, Rubio-Stipec M, Canino G, Shrout P, Ramírez R, Fábregas L, Chavez L, Alegría M, Bauermeister JJ. Test-retest reliability of the Spanish version of the Diagnostic Interview Schedule for Children (DISC-IV). J Abnorm Child Psychol. 2001;29:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Overbeek G, Vollebergh W, Engels RC, Meeus W. Young adults’ relationship transitions and the incidence of mental disorders: a three-wave longitudinal study. Soc Psychiatry Psychiatr Epidemiol. 2003;38:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, Rojas M, Brook J, Streuning E. An epidemiological study of disorders in late childhood and adolescence: I. Age- and gender-specific prevalence. J Child Psychol Psychiatry. 1993;34:851-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 109] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Cohen P, Cohen J. The clinician’s illusion. Arch Gen Psychiatry. 1984;41:1178-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 303] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics. 1946;2:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 974] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 40. | Regier D. Interview with Darrel A. Regier. The developmental process for the diagnostic and statistical manual of mental disorders, fifth edition. Interview by Norman Sussman. CNS Spectr. 2008;13:120-124. [PubMed] |
| 41. | Arnold LE, Ganocy SJ, Mount K, Youngstrom EA, Frazier T, Fristad M, Horwitz SM, Birmaher B, Findling R, Kowatch RA. Three-year latent class trajectories of ADHD symptoms in a clinical sample not selected for ADHD. J Am Acad Child Adolesc Psychiatry. 2014;53:745-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1068] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 43. | Stringaris A, Zavos H, Leibenluft E, Maughan B, Eley TC. Adolescent irritability: phenotypic associations and genetic links with depressed mood. Am J Psychiatry. 2012;169:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 44. | Axelson D. Taking disruptive mood dysregulation disorder out for a test drive. Am J Psychiatry. 2013;170:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Duffy A. The early natural history of bipolar disorder: what we have learned from longitudinal high-risk research. Can J Psychiatry. 2010;55:477-485. [PubMed] |
| 46. | Evangelista NM, Owens JS, Golden CM, Pelham WE. The positive illusory bias: do inflated self-perceptions in children with ADHD generalize to perceptions of others? J Abnorm Child Psychol. 2008;36:779-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |