Published online Sep 22, 2015. doi: 10.5498/wjp.v5.i3.305
Peer-review started: March 7, 2015
First decision: April 27, 2015
Revised: June 9, 2015
Accepted: June 30, 2015
Article in press: July 2, 2015
Published online: September 22, 2015
Processing time: 205 Days and 21.9 Hours
AIM: To review the use of the Months Backwards Test (MBT) in clinical and research contexts.
METHODS: We conducted a systematic review of reports relating to the MBT based upon a search of PsychINFO and MEDLINE between January 1980 and December 2014. Only reports that specifically described findings pertaining to the MBT were included. Findings were considered in terms of rating procedures, testing performance, psychometric properties, neuropsychological studies and use in clinical populations.
RESULTS: We identified 22 data reports. The MBT is administered and rated in a variety of ways with very little consistency across studies. It has been used to assess various cognitive functions including focused and sustained attention as well as central processing speed. Performance can be assessed in terms of the ability to accurately complete the test without errors (“MB accuracy”), and time taken to complete the test (“MB duration”). Completion time in cognitively intact subjects is usually < 20 s with upper limits of 60-90 s typically applied in studies. The majority of cognitively intact adults can complete the test without error such that any errors of omission are strongly suggestive of cognitive dysfunction. Coverage of clinical populations, including those with significant cognitive difficulties is high with the majority of subjects able to engage with MBT procedures. Performance correlates highly with other cognitive tests, especially of attention, including the digit span backwards, trailmaking test B, serial threes and sevens, tests of simple and complex choice reaction time, delayed story recall and standardized list learning measures. Test-retest and inter-rater reliability are high (both > 0.90). Functional magnetic resonance imaging studies comparing the months forward test and MBT indicate greater involvement of more complex networks (bilateral middle and inferior frontal gyri, the posterior parietal cortex and the left anterior cingulate gyrus) for backwards cognitive processing. The MBT has been usefully applied to the study of a variety of clinical presentations, for both cognitive and functional assessment. In addition to the assessment of major neuropsychiatric conditions such as delirium, dementia and Mild Cognitive Impairment, the MBT has been used in the assessment of concussion, profiling of neurocognitive impairments in organic brain disorders and Parkinson’s disease, prediction of delirium risk in surgical patients and medication compliance in diabetes. The reported sensitivity for acute neurocognitive disturbance/delirium in hospitalised patients is estimated at 83%-93%. Repeated testing can be used to identify deteriorating cognitive function over time.
CONCLUSION: The MBT is a simple, versatile tool that is sensitive to significant cognitive impairment. Performance can be assessed according to accuracy and speed of performance. However, greater consistency in administration and rating is needed. We suggest two approaches to assessing performance - a simple (pass/fail) method as well as a ten point scale for rating test performance (467).
Core tip: The months backward test is a brief test of cognitive function that is commonly used in clinical practice. It provides a convenient test of central processing speed and both focused and sustained attention. This review of studies reporting its use in clinical populations identified many different approaches to administration and interpretation of the test. Overall, cognitively intact adults can complete the test within 60 s without omission errors, such that failure to achieve this is strongly suggestive of cognitive dysfunction. The sensitivity for neurocognitive disturbance in hospitalised patients is 83%-93% and repeated testing can identify deteriorating cognitive function over time.
- Citation: Meagher J, Leonard M, Donoghue L, O’Regan N, Timmons S, Exton C, Cullen W, Dunne C, Adamis D, Maclullich AJ, Meagher D. Months backward test: A review of its use in clinical studies. World J Psychiatr 2015; 5(3): 305-314
- URL: https://www.wjgnet.com/2220-3206/full/v5/i3/305.htm
- DOI: https://dx.doi.org/10.5498/wjp.v5.i3.305
The months backwards test (MBT), also known as the months of the year in reverse order (MOYR) test is a rapid (< 2 min) and simple to administer test of cognitive function that is widely used at the bedside. It has been described as primarily a test of attention[1-3], as well as a test of concentration[4], working memory, executive function[5,6], cognitive flexibility[7] and central processing speed[8]. The MBT has been applied to study cognitive function in Parkinson’s disease[9], to stage dementia[10-12], assess concussion in sports[13-15], predict medication adherence in patients with diabetes[6], and predict delirium risk[5]. The MBT has particular utility in screening for delirium and related disorders in hospitalised patients[16-18].
We conducted a review of the test to determine: (1) the ways in which is administered and rated; (2) its psychometric properties, including comparison with other simple bedside tests of cognition; and (3) findings from its use in clinical and research studies.
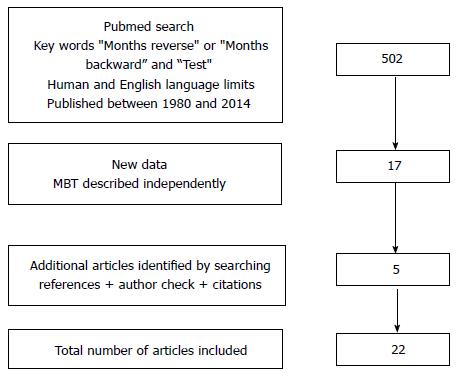
We searched PsychINFO and MEDLINE for papers reporting the use and characteristics of the MBT, searching from 1980 onwards using the search terms “Months reverse” or “months backward” and “test”, with human and English language limits. Because the MBT is a component of several composite test batteries, the review was limited to reports where the MBT was described separately, so that its application and findings could be individually reported.
“Months reverse” or “months backward” and “test” with English language limits identified 502 articles of which 17 were relevant to the review. Additional articles (n = 5) were identified by reviewing the references of these reports and checking for similar work by the authors and subsequent citations (Figure 1).
The 22 articles identified included descriptions of various ways that the test has been applied (including testing procedures and interpretation of test performance), psychometric properties, and findings in a variety of different populations.
The test requests the subject to recite the months of the year in reverse order starting with December, then November, then October and so on, until the subject reaches January or cannot continue. Some variants require the patient to reach a particular month (e.g., July) rather than recite all twelve. The test is often preceded by asking the patient to recite the months forwards, indicating their capacity to engage and understand simple commands (i.e., basic contextual awareness). In the reviewed papers, the MBT was mostly conducted at the bedside or in other routine clinical patient contacts (e.g., outpatient/memory clinic). It can be also be administered by telephone (Ball et al[8], 1999) and versions are available in several languages.
The test requires minimal formal training but must be explained clearly and logically to the subject (see Table 1 for an example of test introduction and procedures). Basic training for testers usually includes an explanation of procedures, followed by observing the test being performed, and then being observed conducting it, to ensure clarity of explanation and consistent administration and interpretation. However, training procedures are not addressed in most reports.
| "Hello Mr X, My name is Y. If it is convenient, I would like for you to help me with a simple test to assess your concentration |
| Can I ask you to say the months of the year from start to finish in their usual order, starting with January?..... |
| Now, can I get you to say the months again but this time in reverse order starting with December…." |
| Where a patient becomes “lost” they may require prompting to re- orientate them to the test and if this is required it should be noted, e.g., what were we doing again? How far did you get to? So before October would be? |
Where the test is timed, the patient is often asked to recite the months as quickly as possible[19]. The task commences after the participant has confirmed that they have understood the instructions and are ready to start. There is less consistency as to how to respond to the patient who is struggling with the test in respect of prompting or re-orientating them to the test. Similarly, the number of discrete trials allowable to assess (best) performance is not consistently defined. It is common for the rater to record the participant’s responses month by month to include omissions and commissions, as well as pauses (denoted by an underscore that corresponds with the duration of delay).
The test typically takes 1-2 min, with cognitively intact patients usually completing the test within 20 s[19]. Cut off times for completion of 60[20], 75[8], and 90 s[21] have been suggested to define an upper limit above which patients cannot successfully complete the test.
Table 2 details the approaches to MBT scoring adopted in the studies included in this review. Performance can be assessed in two ways: (1) ability to accurately complete the test without errors (“MB accuracy”); and (2) time taken to complete the test (“MB duration”). Most subjects under 65 years of age can readily complete the test without error[14,19,20], and therefore the duration to complete the test is more useful (measuring central processing speed) in younger populations.
| Ref. | Population and study purpose | Assessment of MBT performance |
| Halstead[10,28] (1944) | To develop a battery of tests for “senility” in 38 subjects, 20 with recognised dementia | Pass/fail according to capacity to recite months in reverse from December to January |
| Young et al[20] (1997) | To compare the performance of 522 uninjured high school athletes on three simple mental status tests that are commonly used on the sidelines for the evaluation of concussions | Participants were given 1-min time limits for each test, with passing defined as either 7 consecutive correct iterations or 11 correct with one mistake |
| Ball et al[8] (1999) | To evaluate the efficacy and reliability of the time to recite the months of the year in reverse order as a simple measure of central processing speed in 120 community-dwelling women, aged 67-94 | Timing of MBT performance commenced when the subject starts the sequence and continues regardless of omissions, juxtapositions, or corrections until the sequence is complete or 75 s has elapsed. Those unable to complete the sequence received a score of 75 s |
| Wildgruber et al[34] (1999) | Comparison of MBT vs MFT in respect of brain activation using fMRI in 18 neurologically normal subjects aged 19-36 | Subjects silently recited the MFT and subsequently the MBT as fast as possible and continuously across the whole length of each activation period |
| Ettlin et al[27] (2000) | Developing a scale (The FLS) to distinguish patients with various Ornagic brain difficulties | MBT rated from 0-2 in respect of both (1) accuracy (1 point for > 1 error of any kind) and (2) 1 point if completion time > 24 s |
| Lamar et al[22] (2002) | A comparison of the capacity to sustain mental set in participants with AD vs IVD vs a NC | An AcI was calculated using the following algorithm: AcI = {1 - [(false positive + misses)/number of possible correct]} × 100 This algorithm yielded a percentage score such that patients obtaining a score of 100% correctly identified all targets and made no false positive responses or misses |
| Alderson et al[29] (2003) | To develop a brief scale for assessing cognition in patients with TBI | A 4 point scale was applied where 0 = unable to complete of ≥ 2 errors; 1 = able to complete but with 2 errors; 2 = able to complete but with one error; 3 = completed without error |
| Marinus et al[9] (2003) | Development of a scale to assess cognition in PD. Eighty-five PD patients and 75 control subjects were assessed with a battery of tests of which the MBT was a significantly discriminating test of attention. Test–retest reliability was assessed in 30 patients after 6 wk | Performance rated on a scale of 0-2 but unclear how these scores were attributed |
| Rudolph et al[5] (2006) | To determine the extent to which preoperative performance on tests of executive function and memory was associated with delirium after coronary artery bypass graft surgery | Pass/fail according to ability to successfully recite from December back to January |
| Dubois et al[21] (2007) | To develop consensus amongst an expert group regarding diagnostic procedures for PD dementia | Pass/fail according to ability to successfully recite from December back to January. An unsuccessful performance equated with omission of two or more months, incorrect sequencing of the months, or failure to complete the test within 90 s |
| Ostberg et al[11] (2008) | To explore the utility of the MBT in diagnosis of cognitive impairment in N = 234 memory clinic attenders with subjective cognitive impairments, mild cognitive impairment and AD | Participants recited the months in reverse chronological order as quickly as possible. Ability to conduct the test accurately and duration to task completion were measured |
| Shehata et al[14] (2009) | To determine baseline symptom and neurocognitive norms in 260 university athletes with and without concussion histories using the SCAT | Months in reverse order was assessed on a pass/fail basis where a subject passed if they were able to recite the 12 mo in reverse order with no mistakes. The test was considered a fail if any months were in the wrong order or missed |
| Roca et al[24] (2010) | Comparison of neuropsychological performance in patients with documented frontal lobe lesions (n = 15) vs matched neurologically unimpaired controls | The patient was asked to list the months of the year backwards, starting with December. If subjects made no errors, the score was 2; for one error, the score was 1; otherwise the score was 0 |
| Schneider et al[15] (2010) | Assessing performance on tests of cognition in hockey players aged 9-17 with and without a history of concussion | The MBT was conducted as part of the SCAT: Months in reverse order was assessed on a pass/fail basis where a subject passed if they were able to recite the 12 mo in reverse order with no mistakes |
| Grober et al[6] (2011) | Investigating for predictors of diabetes control in 169 elderly diabetics | A composite score was constructed with 5 levels based upon animal naming and MBT performance as completed without errors, uncorrected errors or failed test |
| Östberg et al[19] (2012) | Identification of adult norms and test-retest reliability for durational and response accuracy on the MBT in 216 neurologically intact adults (aged 18-88). A retest was conducted with 40 participants after 3 wk | Participants were instructed to recite the months in backward order as quickly as possible without making any errors. The duration from commencement to cessation was defined as the MB duration score. Any uncorrected sequence error was noted down, and the total number of errors was defined as the MB response accuracy score. Errors were taken to include omissions and transpositions. Repetitions were not scored as errors |
| Jinguji et al[13] (2012) | To determine baseline scores in cognitive performance domains among 214 high school athletes with no prior history of a concussion | Ability to recite the months of the year in reverse order to January |
| Ryan et al[16] (2013) and O’Regan et al[17] (2014) | To screen for attention problems in 311 general hospital inpatients for further testing regarding possible delirium and dementia | Pass vs fail according to whether subjects could reach July without major error/with minimal prompting |
| Tardiff et al[12] (2013) | To identify predictors of cognitive decline in patients with Alzheimer’s dementia | Pass vs fail according to whether subjects could correctly order the months from December to January |
| Bellelli et al[18] (2014) | To evaluate a new screening tool for delirium (4AT) in 236 elderly medical patients | MBT performance rated as: Achieves 7 mo correctly = 0 Refuses or starts but scores < 7 mo = 1 Untestable = 2 |
“MB accuracy” assesses the capacity to complete the test, and how far subjects can reach without error and with/without prompting. Errors include: (1) omissions; (2) perseverations or stuttered responses (e.g., “September… September…August, July”); and (3) Commissions such as false positive errors (i.e., words that are relevant to the task but incorrect such as July where June should be) and intrusion errors involving words or phrases that are not related to the task[22].
In many cases, test performance is dichotimised into a positive or negative result according to specific criteria such as correctly reciting all months with minimal prompting (if under age 65 years) or reciting until July (if aged 65 or older)[16], completing either 7 consecutive correct iterations or 11 correct with no more than one mistake[20], omission of two or more months[21], or a total of two or more errors of any kind[7]. In other testing procedures, an ordinal scale of accuracy performance (ranging from 0-2) is used[9,23,24], while a continuous scale of accuracy performance can be established according to the total number of errors[19]. Lamar et al[25] used the Accuracy Index {= [1 - (false positive + misses)/(number of possible correct)] × 100}. This algorithm yields a percentage score whereby patients obtaining a score of 100% correctly identified all targets without commissions or omissions.
“MB duration” assessment varies considerably across studies and reflects the impact of factors such as age, gender and educational level on performance[13,19]. The Wechsler Memory Scale - third edition (WMS-III)[23] identifies four levels of performance - 0 (> 17 s), 1 (12-16 s), 2 (10-11 s), and 3 (1-9 s). Weintraub[26] suggests a performance cut off of 16 s while Ball et al[8] rated performance on a time continuum up to a predetermined limit of 75 s, with those unable to complete the sequence allocated a score of 75 s and no consideration given to errors. In other work, time limits of 60 s[20] and 90 s[21] have been applied.
Other work has combined accuracy (1 point for > 1 error of any kind) and speed of completion (1 point if completion time > 24 s) to rate performance on an ordinal scale (0, 1, 2)[27].
Studies have explored MBT performance in various populations, including examining the impact of age, gender and educational level upon results. Typically, the MBT takes twice as long as reciting the MF in cognitively intact persons, although this distinction is increased in subjects with mild cognitive impairment or established dementia[11]. Halstead[28] in a seminal paper cited the discrepancy between forwards and backwards test performance as evidence of “senile inelasticity” and a reduced capacity to inhibit the impulse to recite well established patterns in their (usual) forwards manner. In general, the MBT is a simple test that most participants can complete without error, so that being unable to perform the task is unusual in younger adults and in these populations is a sensitive indicator of impairment. Indeed, as response accuracy is so high, the MBT has primarily been used to test processing speed test in cognitively intact individuals.
Successful completion rates for the MBT have been reported for a range of populations. Young et al[20] found 89% of high school student athletes could successfully complete the test. Östberg et al[19] found 95% of neurologically intact adults completed the task without error, while the majority of the remaining participants made a single error, with more than one error occurring in less than 1% of those tested. Shehata et al[14] found that 92% of university-level athletes could complete the MBT without error. Ettlin et al[27] found that 8/70 patients with organic brain lesions made errors (of any type) while all 48 neurologically intact subjects completed the test without error. Conversely, Jinguji et al[13] reported that one third of high school athletes could not complete the test, with better completion rates in females and those aged 16-19 years compared with 13-15 years.
The completion time for the MBT differs across reports. In a community dwelling population aged over 65 years, Ball et al[8] found the MBT duration was 17 ± 10 s, increasing at four-year follow up to 25 ± 21 s. Ettlin et al[27] reported task completion times of 12.1 ± 11.4 in 48 neurologically intact individuals (mean age 40.4 ± 13.8) with much slower completion times for patients with frontal (33.1 ± 40.8) and non-frontal (21.6 ± 12.4) lesions. Ostberg et al[11] reported completion times of 9.6 ± 3.1 s in 66 elderly memory clinic attenders without identifiable objective cognitive impairment. Östberg et al[19] studied 216 neurologically intact adults and found a mean time for completion of 11.6 ± 5.6 s. Durations varied according to age and educational level from 9.0 ± 2.2 in the 30-60 age category with greater (third level) educational exposure to 16.3 ± 8.1 in over 60 years old who had lower educational attainments.
Numerous studies have identified how MBT performance varies with age. Östberg et al[19] found that peak performance (MB duration) occurred in 30-60 year olds compared with younger and older age bands. Ball et al[8] found an exponential relationship between advancing age and MB duration, both cross-sectionally and longitudinally. Jinguji et al[13] found greater completion rates amongst 16-19 years old compared to 13-15 years old. Ostberg et al[11] found a weak association between age and MBT but not MFT performance in elderly memory clinic attenders. Although some work suggests a trend towards better test performance in females[13,14], other studies suggest similar MB accuracy[20] and MB duration[11,19] for otherwise similar male and female subjects.
A key advantage of the MBT is that the universal nature of the content allows for excellent coverage, and performance can be studied in most patients from a variety of populations, including those with significant cognitive impairments[11,16]. Because most normal elderly (typically > 95% in studies) can complete the test without difficulty[19,16], poor performance is very suggestive of abnormality. Moreover, the lack of reliance on motor or visual abilities reduces the impact of disturbances to these functions on performance. Ostberg et al[11] found that only 6 of 234 memory clinic attendees could not be assessed with the MBT.
The MBT has high concordance with many other cognitive tests, including the digit span backwards[13,29], trailmaking test B[29], serial threes and sevens[20], delayed story recall (WMS-R) and standardized list learning measures[29]. In addition, concurrent validity with the MBT has been described for other cognitive tests including culture fair Intelligence Quotient (r = 0.31; P < 0.02)[24], short-blessed test (r = 0.5)[8], trailmaking test B (r = 0.45)[8], and tests of simple (r = 0.52) and complex choice (r = 0.51) reaction time[8].
Test-retest reliability has been examined for both “MB accuracy” and “MB duration”. Ball et al[8] found MB duration had excellent test-retest reliability (0.90) amongst 120 elderly community residents reassessed 1 wk to 10 d later, suggesting that it is an operationally stable measure. Of note, at four-year follow-up, the time taken for the MBT had increased considerably (as above) emphasising that it can also capture deteriorating cognitive function over time. Marinus et al[9] examined test-retest reliability (K = 0.44) for MB accuracy in 30 patients with Parkinson’s disease after a 6 wk interval. Östberg et al[19] found test-retest reliability of 0.82 for duration and 0.97 for accuracy in 40 neurologically intact adults aged 18-80 assessed by periods separated by three weeks. The inter-rater reliability of the MBT between a neuropsychologist and a postdoctoral research fellow in patients with traumatic brain injury was high (r = 0.95)[29].
The MBT engages a range of cognitive and perceptual faculties including hearing, speech, basic comprehension, the ability to focus and sustain attention, working memory and executive function. The information involved is an automatic word sequence that is non-novel and relatively universal - few patients have difficulties with the information content as it reflects culturally acquired factual knowledge. The basic ability to recite MF can be used to test the ability to generate automatic speech in patients with dysphasia[30], while the MBT requires mental control and sustained attention in order to inhibit the impulse to recite the months in a forwards direction.
The cognitive processes of attention and working memory are often conceptualised within a tripartite model[31] where a central executive component controls a phonological loop (allowing subvocal rehearsal) and a visuospatial sketchpad (facilitating mental visualisation). Forwards processing is linked to procedural memory, while backwards processing has greater engagement of working memory. In addition, backwards processing has greater involvement of visuospatial mechanisms[32] thought to reflect the role of visual imagery in transforming material into reverse order by, for example, visualising the calendar and reading from bottom to top. Neuroimaging studies of focal brain lesions and positron emission tomography studies of the neural elements that appear involved in forwards vs backwards processing (using the digit span tests) indicate that backwards processing is linked to greater activation of the dorsolateral prefrontal cortex bilaterally and recruitment of Broca’s area[33]. The degree of activation increases with increasing task difficulty. Increased activation of areas contributing to speech motor performance occurs during reverse rather than forwards recitation even though both tasks comprise the same words and behavioural data. Moreover, there is typically a much higher volume of word production under forward testing. This indicates that engagement of these areas depends on the degree of automatization rather than on production speed of verbal utterances, and demonstrates the contribution of Broca’s area to the silent rehearsal process under reverse testing[34]. However, other work[32] emphasises individual variability in the relative reliance upon visuospatial vs subvocal mechanisms in task performance, including backwards tests.
Wildgruber et al[34] compared the response of neural networks during MF vs MBT using functional magnetic resonance imaging. Both tests were associated with activation of the left motor cortex, supplementary motor area and temporo-parietal junction, while the MBT was associated with greater activation of the bilateral middle and inferior frontal gyri, the posterior parietal cortex and the left anterior cingulate gyrus. These studies indicating greater involvement of more complex networks for backwards cognitive processing are further supported by evidence that tasks involving backwards processing are more susceptible to the effects of ageing[11,25].
The simplicity and convenience of the MBT has allowed its use in a range of clinical scenarios, including some which require speed and portability of assessment procedures. The MBT has demonstrated utility as a single or independent measure and as part of composite testing tools. These studies include the use of the MBT in the assessment of neurocognitive functioning as relevant to the detection of major neuropsychiatric conditions such as delirium, dementia and MCI[10,11,12,17,18], assessment of concussion[13,14,20], profiling of neurocognitive impairments in organic brain disorders[24,35], assessment of Parkinson’s disease[9], prediction of delirium risk in surgical patients[5] and medication compliance in diabetes[6].
Early studies in elderly hospitalised patients with dementia[10,28] found that the MBT was one of seven tests (from a total of 25 tests) that discriminated well between different levels of cognitive impairment. Subsequent work has confirmed the capacity of the MBT to distinguish different levels of cognitive impairment. Ostberg et al[11], in a memory clinic population, found that MBT accuracy varied according to diagnostic category, with impaired performance in Alzheimer’s disease (AD) (37.7% correct), while most participants with mild cognitive impairment (MCI) or subjective cognitive impairment (SCI) performed accurately (86.9% and 93.9% correct, respectively). MB duration significantly differed for all three groups - the average in MCI (19.8 ± 18.2) was twice that of SCI participants (9.6 ± 3.1), and MB duration in mild AD (47.8 ± 32.1) was almost five-fold greater than in SCI. MB duration had equivalent discriminatory power to the Mini-Mental State Examination with superior specificity in the diagnosis of MCI. MB duration thus had a sensitivity of 95.0% in the diagnosis of AD vs SCI, and its specificity was 86.5%. Tardiff et al[12] found that MBT scores, rather than other tests of cognition (e.g., clock drawing) predicted conversion from mild to moderate AD and suggest that supplementing the mini mental state examination (MMSE) with the MBT can improve the predictive value of the MMSE for dementia.
The MBT is a widely-used test for inattention in delirium and is recommended for testing attention in the Revised Delirium Rating Scale administration manual[36] and the 4AT[18]. Rudolph et al[5] studied predictors of delirium in elderly patients undergoing cardiac surgery (n = 80; mean age 74.9 ± 6.2) and found that failing MBT pre-operatively was associated with a relative risk of 1.63 (1.07-2.49) of post-operative delirium. O’Regan et al[17] compared the diagnostic accuracy of the MBT with the Spatial Span Forwards, subjective “confusion” and objective “confusion’ in a general hospital population (n = 265) undergoing screening for inattention as part of a point prevalence study of delirium. Overall, ability to complete the MBT performed the best in terms of combined sensitivity (83.3%; 95%CI: 69.8-92.5) and specificity (90.8%; 95%CI: 86.1-94.3) for delirium, especially in older patients. Bellelli et al[18] studied 234 older inpatients and found the MTB demonstrated high sensitivity to delirium, at 93% for one error, and 86% for two or more errors. Interestingly, the specificity for one error was 50% and for two errors was 83%.
The MBT has also been studied in patients with organic brain syndrome (OBS). Wildgruber et al[35] found considerably slower completion times and more errors in patients with various organic brain lesions compared to neurologically intact controls. Also, within the OBS group, patients with frontal lobe lesions (determined through neuroimaging) had particularly severe impairments in performance compared to those without frontal involvement, thus highlighting the relevance of frontal lobe mechanisms in MBT performance. Similarly, Ettlin et al[27] found that MBT duration distinguished patients with organic brain lesions from those without, and also those with frontal lesions from those with lesions elsewhere. In contrast, Roca et al[24] found that performance on the MBT did not distinguish age-matched controls from patients with carefully documented chronic focal frontal lesions, but in contrast to other studies in OBS patients, performance rating used a simple 3 point scale which may have restricted the capacity of testing to distinguish these groups.
The MBT has also been used in studies of concussion, mainly as part of the standardised assessment of concussion (SAC)[37] and the Sports Concussion Assessment Tool[38], both of which include a variety of tests relevant to detecting concussion. The need for baseline values as a comparison point has stimulated the collection of data regarding MBT performance in younger populations at risk of concussion. The study findings contrast in respect of performance - Shehata et al[14] found completion (without error) rates of 90% or more for all groups studied. In contrast, Schneider et al[15] found much lower completion rates amongst youth hockey players (aged 9-17), especially in males where approximately 50% could complete the MBT at baseline. Similarly, Jinguji et al[13] reported completion rates of approximately two-thirds of college athletics scholarship students.
Performance on the MBT is significantly poorer in patients with Parkinson’s disease compared to controls without any history of neurological impairment. As a result the MBT is included as one of two tests of attention (along with serial threes) in the ten-item SCOPA-COG (SCales for Outcomes of PArkinson’s disease-COGnition), used to measure the cognitive impairments in Parkinson’s disease, including changes over time[9].
In other work, Grober et al[6] examined factors associated with glycaemic control in 169 diabetics (median age 74), finding that MBT performance independently predicted poor control (HbA1c levels ≥ 7). Moreover, the risk of poor control increased with greater impairment on MBT performance. They interpreted the relationship as bidirectional in causality - cognitive dysfunction interfered with diabetes management, and inadequate diabetic control contributed to cognitive dysfunction.
This paper focuses upon literature that relates specifically to MBT used as an independent measure of cognitive function. The MBT is also included in a variety of test batteries and, as such, we included findings where its use could be identified as separate from other tests. These batteries have used the MBT to measure attention, concentration, processing speed, automatic language generating capacity and mental control. These include the Weschler Memory Scale- Mental Control Subtest, Short orientation memory and concentration test[39], the SCOPA-COG[9], SAC[37], Scat-2[38], Boston Diagnostic Aphasia Score[40], Frontal Lobe Score[35], the 4AT[18] and the Bangor Dyslexia Test[30]. With the increasing interest in the MBT as an independent measure, it would be useful for reports of these tools to describe the findings in relation to individual elements, including the MBT.
The MBT has been used in various clinical settings for many decades. During this time, several approaches to delivery and interpretation have emerged which complicates comparisons across studies. The test has significant advantages in terms of brevity and the minimal resources required for its delivery, even for timed versions, and it is independent of motor and visual function. These together afford potential for the test to be used for widespread cognitive screening.
A further advantage relates to the complexity of “pitch” of the test such that performance on the MBT can be assessed for accuracy and duration in those with greater cognitive abilities, thus creating a useful range of performance for testing across populations with differing levels of cognitive abilities. Performance during different phases of the test can provide insights into the nature of cognitive deficits, such that errors at the beginning of the test suggest problems with focusing attention, while errors later in the test indicate problems with sustaining attention.
The MBT can be successfully completed by most cognitively intact adults such that difficulties in completing the test are a sensitive indicator of significant cognitive problems. In contrast, the majority of other attention tests have been developed for non-delirium purposes and are subject to a floor effect, especially in those with existing cognitive problems. A key challenge within healthcare is to identify tests that are attuned or calibrated to the functional range of the target population. In many cases (e.g., concussion, delirium) this requires so-called “simple-smart” tests, and MBT offers this flexibility.
However, despite its relative simplicity, there are considerable inconsistencies in how the MBT is conducted and interpreted. Uncertainties regarding how to deal with patient errors (e.g., interpretation of omissions, commissions, perserverations, stuttering, etc.) and the optimal use of verbal and body-language to prompt subjects are important potential sources of variability in test delivery. Increasing use of computer-assisted technology[41] and detailed protocolisation of the test and its interpretation can minimise these effects.
The choice of the most appropriate version of the MBT can be informed by factors such as the age and prior cognitive status of the population studied, desire to assess attention (or specific aspects of attention) vs other aspects of cognition such as central processing speed, and availability of a suitable timing device. Based upon this review, a suggested scheme for simple and more detailed hierarchical MBT performance assessment is shown in Table 3. This provides two levels of detail for assessing performance - a detailed ten point performance score as well as a simple 4 point scale where a binary (pass/fail) is distinguished by applying the cut off of a pass requiring that the subject completes the test without (omission) error within 30 s (if under 65 years old) or 60 s (if over 65 years old).
| Simple grading: |
| 0 = The subject cannot engage meaningfully with testing procedures |
| 1 = The subject engages but cannot complete the test, even with prompting |
| 2 = The subject completes the test, but with prompting and/or errors |
| 3 = The subject completes the test without error within 30 s (under 65 years old) or 60 s (over 65 years old) |
| Detailed grading (e.g., for research) |
| 0 The subject does not understand that they are being assessed? i.e., lacking basic awareness |
| 1 The subject understands that they are being assessed |
| 2 The subject understands the “rules” of the test (i.e., can grasp/ comprehend the test) |
| 3 The subject engages with testing procedures, e.g., recite months forward (i.e., focused attention) |
| 4 The subject engages in a sustained manner, i.e., sustained attention |
| 5 The subject successfully shifts to backwards testing, i.e., shift attention |
| 6 The subject can reach July |
| 7 The subject can reach January |
| 8 If the subject makes any errors, they recognise this and/or try to correct the errors |
| 9 The subject completes the test without errors |
| 10 Completion of the test takes less than 90 s/60 s/30 s |
The MBT is a versatile tool that can be applied across a variety of populations, including those with significant cognitive and other functional impairment. It is brief, easy to explain and administer, requires minimal resources, focuses upon automatic pre-existing knowledge, has good coverage of clinical populations, and can assess a range of functions from attention to central processing speed. Greater consistency in its application - both in respect of administration and evaluation can allow for more widespread use in a variety of clinical circumstances. The increasing application of computerised technologies can also improve the consistency of use. Greater work is needed to ascertain the extent to which the MBT can act as an independent tool and in which situations it should be combined with other assessments. The complexity of the test varies considerably according to whether it is evaluated in respect of ability to complete, accuracy or duration. The increasing use of routine and systematic cognitive testing in clinical environments creates a need for brief and efficient methods for formalised testing. The MBT has many characteristics that make it an attractive option in this regard, both for cognitive screening and for assessing the character of impairment where it is present.
In memory of our great friend and colleague in science Dr Maeve Leonard RIP 2015.
Efficient bedside testing of cognition is crucial to improving detection of a range of important and common neuropsychiatric conditions. A variety of tests are available but their actual utility and accuracy in real world practice is uncertain. The months backward test (MBT) is a simple and popular test of attention and other faculties. The authors reviewed the available evidence regarding its optimal use in research and real world clinical practice.
A key challenge in cognitive neuroscience is to identify a suitable test for use as a cognitive “vital sign”. However, there is a lack of consensus regarding the most versatile tests which is in part due to a lack of clear evidence regarding the optimal use of available tests in clinical practice. This work examines the characteristics of the MBT that inform its suitability for routine and systematic bedside cognitive screening in clinical settings.
This review explores evidence regarding a broad range of characteristics that are relevant to the use of the MBT as a cognitive test in everyday clinical practice with emphasis upon its suitability for rapid, routine and repeated use by a variety of healthcare professionals in a various clinical populations. The authors found that the MBT is a highly versatile test and have developed guidelines that can support greater consistency in its administration and interpretation.
The authors have distilled the findings into a set of guidelines that can allow for more consistent application of the MBT in clinical and research practice. This will allow for greater consistency across research studies.
Psychometric properties of a test relate to its ability to measure what is intended as well as the capacity to capture this consistently over time and by different assessors.
The manuscript entitled “Months backward test: A review of its use in clinical studies” is a straightforward and well-writtten review dealing with a highly intriguing cognitive test.
P- Reviewer: Contreras CM, Müller MJ S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Lezak MD. Neuropsychological assessment. 4th ed. Oxford: Oxford University Press 2004; . |
| 2. | Hodges JR. Cognitive assessment for clinicians. 2nd ed. Oxford: Oxford University Press 2007; . |
| 3. | Meagher D, Leonard M. The active management of delirium: improving detection and treatment. Adv Psychiatr Treat. 2008;14:292-301. |
| 4. | Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797-811. [PubMed] |
| 5. | Rudolph JL, Jones RN, Grande LJ, Milberg WP, King EG, Lipsitz LA, Levkoff SE, Marcantonio ER. Impaired executive function is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2006;54:937-941. [PubMed] |
| 6. | Grober E, Hall CB, Hahn SR, Lipton RB. Memory Impairment and Executive Dysfunction are Associated with Inadequately Controlled Diabetes in Older Adults. J Prim Care Community Health. 2011;2:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Hugo FJ. Bedside Cognitive Assessment. Oxford: Oxford University Press 2012; Available from: http://franshugo.files.wordpress.com/2012/07/bedside-cognitive-assessment.pdf. |
| 8. | Ball LJ, Bisher GB, Birge SJ. A simple test of central processing speed: an extension of the Short Blessed Test. J Am Geriatr Soc. 1999;47:1359-1363. [PubMed] |
| 9. | Marinus J, Visser M, Verwey NA, Verhey FR, Middelkoop HA, Stiggelbout AM, van Hilten JJ. Assessment of cognition in Parkinson’s disease. Neurology. 2003;61:1222-1228. [PubMed] |
| 10. | Halstead H. A Psychometric Study of Senility. Br J Psychiatry. 1943;89:363-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Ostberg P, Fernaeus SE, Bogdanovic N, Wahlund LO. Word sequence production in cognitive decline: forward ever, backward never. Logoped Phoniatr Vocol. 2008;33:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Tardiff M, Roy M, Remi B, Laforce R. Months backward test as a reliable predictor of cognitive decline in mild Alzheimer’s disease. Alzheimer’s Dementia. 2013;9:S741-742. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Jinguji TM, Bompadre V, Harmon KG, Satchell EK, Gilbert K, Wild J, Eary JF. Sport Concussion Assessment Tool-2: baseline values for high school athletes. Br J Sports Med. 2012;46:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Shehata N, Wiley JP, Richea S, Benson BW, Duits L, Meeuwisse WH. Sport concussion assessment tool: baseline values for varsity collision sport athletes. Br J Sports Med. 2009;43:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Schneider KJ, Emery CA, Kang J, Schneider GM, Meeuwisse WH. Examining Sport Concussion Assessment Tool ratings for male and female youth hockey players with and without a history of concussion. Br J Sports Med. 2010;44:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Ryan DJ, O’Regan NA, Caoimh RÓ, Clare J, O’Connor M, Leonard M, McFarland J, Tighe S, O’Sullivan K, Trzepacz PT. Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open. 2013;3:pii: e001772. [PubMed] |
| 17. | O’Regan NA, Ryan DJ, Boland E, Connolly W, McGlade C, Leonard M, Clare J, Eustace JA, Meagher D, Timmons S. Attention! A good bedside test for delirium? J Neurol Neurosurg Psychiatry. 2014;85:1122-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Bellelli G, Morandi A, Davis DH, Mazzola P, Turco R, Gentile S, Ryan T, Cash H, Guerini F, Torpilliesi T. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. 2014;43:496-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 498] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 19. | Östberg P, Hansson V, Häägg S. Adult norms and test-retest reliability for the Months Backward test: durational and response accuracy measures. Logoped Phoniatr Vocol. 2012;37:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Young CC, Jacobs BA, Clavette K, Mark DH, Guse CE. Serial sevens: not the most effective test of mental status in high school athletes. Clin J Sport Med. 1997;7:196-198. [PubMed] |
| 21. | Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314-2324. [PubMed] |
| 22. | Lamar M, Price CC, Davis KL, Kaplan E, Libon DJ. Capacity to maintain mental set in dementia. Neuropsychologia. 2002;40:435-445. [PubMed] |
| 23. | Wechsler D. Wechsler Memory Scale - third edition. Manual. Stockholm, Sweden: NCS Pearson Inc 2008; . |
| 24. | Roca M, Parr A, Thompson R, Woolgar A, Torralva T, Antoun N, Manes F, Duncan J. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133:234-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Lamar M, Resnick SM. Aging and prefrontal functions: dissociating orbitofrontal and dorsolateral abilities. Neurobiol Aging. 2004;25:553-558. [PubMed] |
| 26. | Weintraub S. Neuropsychological assessment of mental state. Principles of behavioral and cognitive neurology. 2nd ed. New York: Oxford University Press 2000; . |
| 27. | Ettlin TM, Kischka U, Beckson M, Gaggiotti M, Rauchfleisch U, Benson DF. The Frontal Lobe Score: part I: construction of a mental status of frontal systems. Clin Rehabil. 2000;14:260-271. [PubMed] |
| 28. | Halstead H. Mental tests in senile dementia. Brit J Psychiat. 1944;89:720-726. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Alderson AL, Novack TA. Reliable serial measurement of cognitive processes in rehabilitation: the Cognitive Log. Arch Phys Med Rehabil. 2003;84:668-672. [PubMed] |
| 30. | Miles TR. Dyslexia: the pattern of difficulties. 2nd ed. London: Whurr 1993; . |
| 31. | Baddeley A, Della Sala S. Working memory and executive control. Philos Trans R Soc Lond B Biol Sci. 1996;351:1397-1403; discussion 1403-1404. [PubMed] |
| 32. | St Clair-Thompson HL, Allen RJ. Are forward and backward recall the same? A dual-task study of digit recall. Mem Cognit. 2013;41:519-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Gerton BK, Brown TT, Meyer-Lindenberg A, Kohn P, Holt JL, Olsen RK, Berman KF. Shared and distinct neurophysiological components of the digits forward and backward tasks as revealed by functional neuroimaging. Neuropsychologia. 2004;42:1781-1787. [PubMed] |
| 34. | Wildgruber D, Kischka U, Ackermann H, Klose U, Grodd W. Dynamic pattern of brain activation during sequencing of word strings evaluated by fMRI. Brain Res Cogn Brain Res. 1999;7:285-294. [PubMed] |
| 35. | Wildgruber D, Kischka U, Fassbender K, Ettlin TM. The Frontal Lobe Score: part II: evaluation of its clinical validity. Clin Rehabil. 2000;14:272-278. [PubMed] |
| 36. | Trzepacz PT, Maldonado JR, Kean J, Abell M, Meagher DJ. The Delirium Rating Scale-Revised-98 (DRS-R98) Administration Manual. A guide to increase understanding of how to solicit delirium symptoms to administer the DRS-R98. Paula Trzepacz© {Trzepacz PT. London: Whurr 2009; #2331} Indianapolis, IN, USA. |
| 37. | McCrea M. Standardized Mental Status Testing on the Sideline After Sport-Related Concussion. J Athl Train. 2001;36:274-279. [PubMed] |
| 38. | McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, Cantu R. Consensus Statement on Concussion in Sport: the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Br J Sports Med. 2009;43 Suppl 1:i76-i90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 380] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 39. | Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734-739. [PubMed] |
| 40. | Goodglass H, Kaplan E, Barresi B. The Assessment of Aphasia and Related Disorders. 3rd Edition Lippincott. Philadelphia, PA: Williams and Wilkins 2001; . |
| 41. | King D, Brughelli M, Hume P, Gissane C. Concussions in amateur rugby union identified with the use of a rapid visual screening tool. J Neurol Sci. 2013;326:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |