Published online Dec 22, 2013. doi: 10.5498/wjp.v3.i4.93
Revised: October 6, 2013
Accepted: November 1, 2013
Published online: December 22, 2013
Processing time: 197 Days and 18.2 Hours
Brain-derived neurotrophic factor (BDNF) has been proposed as a biomarker of schizophrenia and, more specifically, as a biomarker of cognitive recovery. Evidence collected in this review indicates that BDNF is relevant in the pathophysiology of schizophrenia and could play a role as a marker of clinical response. BDNF has been shown to play a positive role as a marker in antipsychotic treatment, and it has been demonstrated that typical antipsychotics decrease BDNF levels while atypical antipsychotics maintain or increase serum BDNF levels. Furthermore, BDNF levels have been associated with severe cognitive impairments in patients with schizophrenia. Consequently, BDNF has been proposed as a candidate target of strategies to aid the cognitive recovery process. There is some evidence suggesting that BDNF could be mediating neurobiological processes underlying cognitive recovery. Thus, serum BDNF levels seem to be involved in some synaptic plasticity and neurotransmission processes. Additionally, serum BDNF levels significantly increased in schizophrenia subjects after neuroplasticity-based cognitive training. If positive replications of those findings are published in the future then serum BDNF levels could be definitely postulated as a peripheral biomarker for the effects of intensive cognitive training or any sort of cognitive recovery in schizophrenia. All in all, the current consideration of BDNF as a biomarker of cognitive recovery in schizophrenia is promising but still premature.
Core tip: The lack of diagnostic and treatment markers is one of the most important problems in clinical practice. Brain-derived neurotrophic factor (BDNF) has been proposed as a biomarker of schizophrenia and, more specifically, as a biomarker of cognitive recovery. Evidence collected in this review indicates that there is evidence suggesting that serum BDNF levels are involved in some synaptic plasticity processes. Additionally, serum BDNF levels significantly increased in schizophrenia subjects after neuroplasticity-based cognitive training. All in all, the current consideration of BDNF as a biomarker of cognitive recovery in schizophrenia is promising but still premature.
- Citation: Penadés R, Catalán R, López-Vílchez I, Arias B, González-Rodríguez A, Galán AM, Gastó C. Brain-derived neurotrophic factor as a potential biomarker of cognitive recovery in schizophrenia. World J Psychiatr 2013; 3(4): 93-102
- URL: https://www.wjgnet.com/2220-3206/full/v3/i4/93.htm
- DOI: https://dx.doi.org/10.5498/wjp.v3.i4.93
The lack of diagnostic and treatment markers is one of the most important problems in clinical practice. Researchers are deeply involved in the identification of validated markers, particularly biomarkers that could be useful in predicting treatment responses to different therapeutics. One of the most important challenges of schizophrenia research is to establish biological markers that can predict clinical outcome and identify clinical stages in these patients. Molecular genetics, analysis of serum and cerebrospinal fluid (CSF), and structural and functional neuroimaging have provided an attractive field of research for biomarkers[1]. For many reasons, such as small effect sizes and individual rarity, gene studies have traditionally shown that genetic markers are not suitable as diagnostic markers[2]. In this line, the study of CSF parameters has yielded a number of interesting candidate biomarkers, but this research has only recent begun[3]. In contrast, despite particularly promising research on neuroimaging, available techniques for evaluating structural and functional brain changes make them unsuitable as biomarkers in schizophrenia[4]. Further biomarker research is needed in schizophrenia. However, evidence suggests both that BDNF is relevant in the pathophysiology of schizophrenia and that BDNF is potentially more useful as a biomarker for diagnostic and prognostic purposes than are other potential biomarkers[5].
A biomarker has been defined by the United States Food and Drug Administration (FDA) as “A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention”. Biomarkers have been suggested to have the potential to augment the chances of successful drug and therapeutic development through better target validation, provision of surrogate end-points and patient stratification[6]. Recently, cognitive recovery has been considered among the most important targets in the treatment of patients with schizophrenia. Consequently, the identification of biomarkers of cognitive recovery is relevant not only for diagnostic purposes but also for the development of new approaches to treat cognitive impairment in schizophrenia[7]. Brain-derived neurotrophic factor (BDNF) has been proposed as one of the stronger biomarker candidates in schizophrenia and, more specifically, as a biomarker of cognitive recovery[5].
Neurotrophins are growth factors that play an important role in the survival, development, and functionalism of neurons. They prevent neurons from triggering programmed cell-death, which prolongs their survival. They are also involved in the formation of new neurons in certain areas of the brain. BDNF is one of the most studied neurotrophins. The properties of BDNF vary according to the brain region studied. BDNF has been described as a modulator of neuronal survival and differentiation, synaptic plasticity, and higher order cognitive functions such as learning and memory[8-10]. Moreover, there is also evidence that indicates a role for BDNF in the development of the cardiovascular system[11,12] and in the growth, survival, and chemoresistance of tumour cells in various types of cancer, including Hodgkin lymphoma, myeloma, and neuroblastoma[13-17].
BDNF is mainly synthesised in the brain and spinal cord by glial cells[18], but is also produced by Schwann cells associated with peripheral motor neurons[19]. BDNF is synthesised as a precursor (proBDNF) that is cleaved afterwards to generate the mature protein. Although it was believed that proBDNF had no function, work by Hempstead and collaborators reported that, by interacting with the p75 neurotrophin receptor, proBDNF could induce the opposite effect to that of mature BDNF, leading to cell death[20]. This work has opened a new line in the study of the mechanisms underlying BDNF because mature BDNF mainly acts through a different receptor, the tyrosine kinase receptor B (TrkB)[20]. In addition, it is known that BDNF can pass the blood-brain barrier, reaching non-neuronal tissues, such as the heart, lungs and platelets[21-24]. However, little is known about the function of non-neuronal BDNF. Moreover, BDNF mRNA has been found in several peripheral locations, such as activated human T cells, B cells, monocytes[25], the heart[26], the retina, smooth muscle[12], the lungs[27,28], endothelial cells[29] and platelets[24,29].
Given the difficulty of studying BDNF in situ in the brain, there has been growing interest in the accurate assessment of BDNF activity in the periphery. Studies in murine models have shown a good correlation between BDNF levels in the brain and circulating levels of the protein[30]. The amount of BDNF in serum, plasma and whole blood samples is commonly determined by using ELISA techniques with relatively high specificity and sensitivity. In spite of all the different sources of BDNF, it is believed that BDNF released from platelets is the major contributor to serum samples. BDNF stored in platelets is most likely derived from both the circulating plasma pool and from resident cells in the brain[30] and other organs[22,29,31,32].
Measured BDNF levels are highly dependent on the methodology used[33]. Karege et al[34] demonstrated that the stability of BDNF assessed in whole blood, serum, and plasma samples varied among different laboratories. Nonetheless, the accuracy and reproducibility of BDNF determination in serum has been validated[35]. However, there is still little consensus regarding standardised protocols for plasma collection and BDNF dosage. Interestingly, there have been reported changes in serum and blood BDNF levels in patients with neuropsychiatric disorders such as depression[21,36], schizophrenia[37], Alzheimer’s disease[38], multiple sclerosis[39], and anorexia[40] when compared to healthy individuals.
The BDNF gene (chromosome 11p13-14) encodes a precursor peptide (proBDNF) that is proteolytically cleaved to form the mature BDNF protein. This gene contains a functional polymorphism that has been widely studied in genetic association and gene-environment studies in psychiatry research[41,42]. This single nucleotide polymorphism (SNP) consists of a guanine substitution for an adenine in the position 196 of the gene (rs6265), provoking a change of a Valine (Val) to a Methionine (Met) in amino acid 66 of the protein. As a functional polymorphism, it has been claimed that the Val variant is associated with higher neuronal BDNF secretory activity than is the Met allele. Additionally, the co-expression of Val and Met alleles in heterozygotes results in less efficient intracellular trafficking and processing, leading to decreased BDNF secretion[43,44].
Genetic studies have revealed that the association between BDNF and schizophrenia has not been definitively established. The single nucleotide polymorphisms C270T (in the 5’ non-coding region) and Val66Met are two common functional genetic polymorphisms of the BDNF gene. A meta-analysis of case-control studies[42] stressed the association of this polymorphism with the risk of schizophrenia and other mental disorders, such as substance-related disorders and eating disorders. This study also showed that individuals with the Met/Met homozygous allele had 19% higher risk of developing schizophrenia and other psychotic disorders than did those with the Val/Met heterozygous alleles. However, another meta-analytic study of two of the most extensively studied BDNF polymorphisms, Val66Met and C270T, did not find an association of the Val66Met polymorphism with schizophrenia[45]. Nonetheless, several studies have shown positive associations between the BDNF Val66Met genetic variant and several aspects of the phenomenology of schizophrenia, such as age of onset, clinical symptoms, aggressive behaviour, suicide attempt, brain morphology, and cognitive function[41].
Schizophrenia has been conceptualised as being essentially a neurodevelopmental disorder[46,47]. It is well known that BDNF plays a key role in a number of processes that are thought to be impaired in schizophrenia, ranging from neuronal differentiation to neurite outgrowth and neuronal survival[48]. In addition, BDNF seems to be crucial to synaptic transmission and various cognitive processes that are severely impaired in schizophrenia. Currently, a considerable amount of data are available that highlight the role of BDNF in the pathophysiology of schizophrenia[49] in both chronic patients and first episodes. Commonly, it has been assumed that determination of BDNF levels in peripheral serum might be a useful measure. On one hand, levels of BDNF in peripheral serum seem to be correlated with BDNF concentrations in the central nervous system[30]. On the other hand, BDNF is able to cross the blood-brain barrier[32]. Unfortunately, the studies that measure serum BDNF concentrations in patients with schizophrenia are not conclusive and have even produced some conflicting results.
The majority of relevant studies report lower serum BDNF levels in schizophrenia patients compared to healthy controls[37,50-54]. However, other studies could not find any differences between schizophrenia patients and healthy controls[55,56]. Further, some studies have even found higher serum BDNF levels in patients with schizophrenia[57,58]. To clarify these controversial results, Green et al[59] assessed the published data in a meta-analysis. After a rigorous selection of the works with better methodology, the authors were able to demonstrate reduced serum BDNF levels in schizophrenia patients, not only for medicated patients but also for drug-naïve patients; no differences were shown between males and females. In addition, using meta-regression techniques Green et al[59] showed a significant association between reduced BDNF and increased age, but no association was found for medication dosage. In conclusion, after controlling for heterogeneity of samples and methodological aspects, these authors suggested that blood levels of BDNF are actually reduced in medicated and drug-naive patients with schizophrenia.
Although many studies about BDNF levels have been conducted in chronic schizophrenia patients, a few recent studies have examined BDNF profiles in first-episode patients. The earliest study to be conducted with first episodes and drug-naïve patients reported a significant decrease in plasma BDNF levels compared with controls[60]. The authors found a significant association between plasma BDNF levels and positive and negative syndrome scale scores. Since this study, a number of studies have replicated those findings suggesting differences in BDNF levels in first-episode patients. Jindal et al[61] showed a significant decrease in serum BDNF levels in patients with first episode schizophrenic psychosis but not in patients with non-schizophrenic psychosis. Unfortunately, they could not find significant correlations between BDNF levels and the severity of positive and negative symptoms or overall functioning. A different study tested the presence of cerebrospinal fluid (CSF) BDNF levels in drug-naïve first-episode patients[62]. Compared with controls, a significant decrease in CSF BDNF levels was found and they were significantly related with plasma levels. In addition, CSF and plasma BDNF levels also showed a significant negative correlation with baseline positive symptoms. Finally, a study conducted by the research team of Rizos[63] tried to determine the association between serum BDNF levels and hippocampal volumes in a sample of first psychotic episode drug-naïve schizophrenia patients. The authors found serum BDNF levels significantly reduced in the sample of first-episode patients when compared to levels of healthy subjects. Consequently, hippocampal volume was already decreased at the onset of schizophrenia in first-episode patients. Interestingly, BDNF levels and hippocampal volume reduction were significantly related, which suggests a putative relationship between lower serum BDNF levels and a reduction of hippocampal volume.
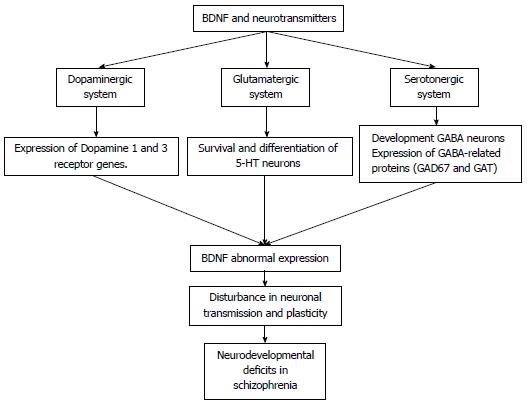
BDNF plays an important role as a regulator of synaptic transmission and has been associated with the pathophysiology of schizophrenia[64]. Furthermore, its relationship with dysfunctions in the dopaminergic, glutamatergic, and serotonergic neurotransmitter systems has been widely studied.
BDNF is a neurotrophic protein that is synthesised by dopamine cells and has been closely linked to the function of the dopaminergic system. It has been shown to be expressed throughout the cerebral cortex, hippocampus, basal forebrain, striatum, hypothalamus, and cerebellum neurons[65]. With regards to synaptic transmission, BDNF has also been shown to control the expression of D2-like receptors, Dopamine 1 and Dopamine 3 (D1, D3), in adults through the control of specific dopamine genes[66-68]. For this reason, BDNF is an important modulator of the dopaminergic system, and its changes can be observed in the brain and in the plasma of patients with schizophrenia[66].
Exposure to BDNF or a lack of this neurotrophin results in alterations to both excitatory and inhibitory synaptic systems[64]. The role of BDNF in the glutamatergic system has been well studied (Figure 1). BDNF promotes the development of GABA neurons and the expression of GABA-related proteins, such as GAD67 and GAT, in the cortex and other brain regions[65]. In rodents, BDNF regulates the GABAergic system in the hippocampus. In subjects with schizophrenia, altered GABA neurotransmission may contribute to prefrontal cortex dysfunction[69].
Some evidence suggests that BDNF has an influence on the development of the serotonergic system by promoting the survival and differentiation of 5-hydroxytryptamine (5-HT) neurons both in vivo and in vitro[70,71]. Furthermore, BDNF stimulates the expression of S100 beta in astrocytes and the production of myelin basic protein oligodendrocytes[70]. In mice, alterations in BDNF expression result in physiological disturbances in 5-HT neurons that have been shown to be deteriorated in advanced age[70]. In humans, high levels of central serotonergic activity are associated with high BDNF serum concentrations[72].
Although the aetiology of schizophrenia is still unknown, neuroimaging studies have consistently demonstrated brain abnormalities in patients with schizophrenia. These studies revealed significant reductions in gray matter volume in the cortex and hippocampus and decreases in neurons of the dorsal thalamus[73]. Within first-episode psychosis patients, a systematic review and meta-analysis has shown volumetric deficits in the hippocampus and in cortical grey matter, specifically in temporal grey matter[73]. Furthermore, histological studies have shown a significant reduction in synaptic and dendritic markers in the brains of schizophrenia patients[74].
Recent evidence suggests that BDNF has an important role in the growth and development of the central and peripheral nervous system and is associated with disruptions in the brain structure of patients with schizophrenia[75]. In previous studies, BDNF has been reported to regulate axonal and dendritic development and the differentiation and survival of new neurons by increasing the number and length of axons and their branches[64,70]. In mice, BDNF has demonstrated a specific role by promoting survival of embryonic retina ganglion cells and mesencephalic dopaminergic neurons in vitro. After administration of high BDNF concentrations in rodents, an extensive neuronal growth was observed[76].
BDNF has been shown to mediate some processes of cognition. It has demonstrated its role as a regulator of axonal and dendritic branching[77,78]. Thus, the process of hippocampal long-term potentiation implies a process of synaptic strengthening associated with learning and memory through its functional TrkB receptor[79,80]. In relation to schizophrenia, a study detected a significant positive correlation between serum BDNF levels and decreased cognitive functioning in 250 Chinese inpatients with schizophrenia[81]. In another study, serum truncated-BDNF abundance predicted a high presence of cognitive impairments, showing 67.5% of sensitivity and 97.5% of specificity[82] in the prediction. This result suggests that deficiency in pro-BDNF processing may be involved in the mechanism underlying the cognitive impairments observed in schizophrenia. In addition, impairment in spatial learning and memory has been found in BDNF-knockout mice[83]. Conversely, single intrahippocampal BDNF administration seems to affect the behavioural flexibility of rats in a Morris water-maze task[84]. Studies have also found impairments in long-term potentiation in BDNF gene-deleted mice[85]. These data support the role of BDNF in cognitive impairments observed in schizophrenia and suggest that BDNF could be a potential marker of cognition, as it is involved in learning and memory processes[5].
Currently, pharmacological response is mainly a process determined by a trial and error strategy. Identification of biomarkers in the near future could allow us to identify patients who are more likely to respond to a particular treatment and even determine their sensitivity to side effects. However, not only would this allow us to stratify patients according to their likely treatment response but it could also help clinicians and patients to partially avoid the uncertainty of the trial and error process. BDNF has been strongly proposed as a biomarker in schizophrenia and more specifically as a biomarker of cognitive recovery. But, is there now enough evidence to consider DBNF as a biomarker? As previously mentioned, a biomarker needs to have three core characteristics: (1) To be an indicator of normal biological processes; (2) To be an indicator of pathogenic processes; and (3) To be a marker of response to therapeutic interventions.
BDNF seems to play a crucial role in normal cognitive functions such as learning and memory. Its role as a regulator of axonal and dendritic branching has been shown in various studies[77,78]. Thus, the process of hippocampal long-term potentiation, which implies a process of synaptic strengthening associated with learning and memory through its functional TrkB receptor has also been found[79]. In addition to this, BDNF signalling has been implicated in the regulation of adult neurogenesis, suggesting its prominent role in synaptic plasticity and cognition[86]. On the other hand, the genetics of BDNF show that polymorphisms are relevant to understanding normal neurotrophic processes. Variation of BDNF polymorphisms includes a single-nucleotide polymorphism (SNP), rs6265, in the conserved, 5’-pro-protein-coding region; this entails a valine-to-methionine substitution (Val66Met). This last polymorphism has been suggested to cause inefficient BDNF trafficking and a reduced activity-dependent BDNF secretion.
Some studies have suggested that BDNF is strongly implicated in the pathophysiology of schizophrenia in both first-episode patients and chronic schizophrenia patients. Within first-episode schizophrenia patients, a number of studies have shown a significant decrease in plasma BDNF levels[87]. In addition, serum BDNF levels are lower in chronic schizophrenia patients compared to healthy controls[51]. In relation to reduced brain volumes in first-episode and chronic schizophrenia patients, recent studies have found a correlation to lower serum BDNF levels[63], specifically in reduced hippocampal volume[88]. BDNF levels in serum or CSF have been associated with the presence of schizophrenia in general and to other impairments in cognition. BDNF polymorphism is involved in less efficient intracellular trafficking and processing. This leads to decreased BDNF secretion and possibly to disturbances in neurotransmission processes, which may contribute to prefrontal cortex dysfunction. Neuroimaging studies have shown that reduced brain volumes in first-episode and chronic schizophrenia patients are related to lower serum BDNF levels[63] and, specifically, to reduced hippocampal volume[88]. Finally, using functional neuroimaging, Eisenberg and collaborators[89] have suggested that Val66Met polymorphism is significantly associated with hippocampal dysfunction.
Studies that aim to measure the effects of antipsychotics on BDNF have produced varying results depending on the type of antipsychotic used in the study[64]. Thus, some studies suggested that typical antipsychotics seem to reduce BDNF expression while atypical antipsychotics could increase BDNF expression, but these studies were carried out as animal experiments. Unfortunately, studies with clinical samples in humans are still scarce[90]. In the particular case of treatments that target cognition, studies are even scarcer. Nonetheless, BDNF has been shown to mediate some processes of cognitive change. There is some evidence about BDNF’s role as a regulator of axonal and dendritic branching[77,78]. The process of hippocampal long-term potentiation, which implies a process of synaptic strengthening, has been associated with learning and memory through its functional TrkB receptor[79,80]. Furthermore, a recent study conducted by Vinogradov et al[91] has directly tested whether neuroplasticity-based cognitive training is able to modify serum BDNF levels in schizophrenia patients. Samples consisted of 56 schizophrenia outpatients and 16 matched healthy comparison subjects. Both groups were assessed on baseline cognitive performance and serum BDNF levels. Schizophrenia subjects were randomly assigned to either 50 h of computerised auditory training or a computer-game control condition; this was followed by reassessment of cognition and serum BDNF levels. At baseline, schizophrenia participants had significantly lower serum BDNF levels than did healthy controls. Subjects who engaged in computerised cognitive training designed to improve auditory processing showed significant cognitive gains and a significant increase in serum BDNF when compared with subjects who played computer games (control condition). In sum, in a repeated-measures analyses of variance approach, subjects following cognitive training showed a statistically significant gain in global cognition (approximately 0.36 SD) from baseline to endpoint; subjects in the control group showed no change in global cognition (0.01 SD). After 10 wk, subjects following the neurocognitive training were able to increase their mean serum BDNF levels (mean ± SD, 25.27 ± 10.34) to the same level as healthy controls (mean ± SD, 31.30 ± 8.95); the control group showed no change. After the treatment, authors calculated the standardised mean difference (Cohen’s d) in BDNF levels between the control group and the therapeutic group and found a medium effect size of 0.67. Although this study has not been replicated, it opens a pathway in clinical research. It is probable that serum BDNF levels would be significantly increased after neuroplasticity-based cognitive training in schizophrenia subjects. If positive replications are published, then serum BDNF levels could be postulated as a peripheral biomarker for the effects of intensive cognitive training or any sort of cognitive recovery in schizophrenia.
Furthermore, pharmacogenetic studies have shown that the BDNF Val66 Met polymorphism could be helpful as an outcome predictor not only for cognitive recovery but also for drug response and adverse side effects. It has been suggested that BDNF polymorphism may be associated with antipsychotic therapeutic effects[41,92,93], treatment resistance[94] and adverse effects including weigh gain[95], tardive dyskinesis[96] and extrapyramidal syndrome[97]. Interestingly, Zhang et al[96] have indicated that BDNF genetic variants could be associated with antipsychotic treatment resistance.
Evidence collected in this review indicates that BDNF is relevant in the pathophysiology of schizophrenia and could play a role as a marker of clinical response. It has been confirmed that BDNF plays a crucial role as a regulator of synaptic transmission and seems to be related to dysfunctions in principal neurotransmitter systems, such as the dopaminergic, glutamatergic and serotonergic neurotransmitter systems. Particularly, BDNF has been associated with disruptions in brain structure and neurodevelopmental processes. Some studies suggest that BDNF levels are altered in schizophrenia patients. Consequently, the relationship between psychotic symptoms and alterations in the expression of BDNF has been well established. More specifically, BDNF might be playing a role as a marker of antipsychotic treatment because studies show that typical antipsychotics seem to decrease BDNF levels while atypical antipsychotics maintain or increase serum BDNF levels.
Regarding cognitive recovery, the evidence gathered in this review confirms the role of BDNF in brain plasticity and cognition. There is some evidence suggesting the role of BDNF as a regulator of axonal and dendritic branching. BDNF might also be involved in the process of hippocampal long-term potentiation through the process of synaptic strengthening. In patients with schizophrenia, BDNF levels have been related to more severe impairment in cognition. Consequently, BDNF might be proposed as a biomarker of the cognitive recovery process. It has been suggested that BDNF mediates some processes of cognitive change. Thus, serum BDNF levels seem to be significantly increased after neuroplasticity-based cognitive training in schizophrenia subjects. If positive replications are published then serum BDNF levels could be postulated as a peripheral biomarker for the effects of intensive cognitive training or any sort of cognitive recovery in schizophrenia.
Unfortunately, the effect of neuromodulation on BDNF is still far from being completely understood. Moreover, the specificity of BDNF as a biomarker for schizophrenia cannot be stated because reduction in BDNF has also been observed in patients with neurodegenerative disorders and other neuropsychiatric illnesses. Consequently, more studies are needed in order to establish BDNF as a marker of cognitive recovery in schizophrenia. Cognitive enhancing drugs have not been shown to be completely successful, and consequently, new therapeutic paradigms to improve cognition in schizophrenia need to be tested. The optimal approach may require a combination of specific drug treatment with cognitive training intervention. Finally, examining the prognostic correlation of baseline BDNF levels and the final outcome would be useful in establishing the status of BDNF as a marker of cognitive recovery in schizophrenia. For all these reasons, considering BDNF a biomarker of cognitive recovery in schizophrenia may be promising but still premature.
The authors would like also to thank the Miguel Servet Researcher’s stabilisation program of “Instituto de Salud Carlos III” from the Spanish government and “Direcció d’Estratègia i Coordinació del Departament de Salut” from the Generalitat de Catalunya where A.M.G belongs to. I.L-V. and A.M.G. belong also to the HERACLES RETIC, group RD06/0009/1003, supported by the Instituto de Salud Carlos III.
P- Reviewers: Guardia-Olmos J, Numakawa T S- Editor: Song XX L- Editor: A E- Editor: Liu XM
| 1. | Oertel-Knöchel V, Bittner RA, Knöchel C, Prvulovic D, Hampel H. Discovery and development of integrative biological markers for schizophrenia. Prog Neurobiol. 2011;95:686-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | Straub RE, Weinberger DR. Schizophrenia genes - famine to feast. Biol Psychiatry. 2006;60:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Schönknecht P, Hempel A, Hunt A, Seidl U, Volkmann M, Pantel J, Schröder J. Cerebrospinal fluid tau protein levels in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2003;253:100-102. [PubMed] |
| 4. | Koike S, Takano Y, Iwashiro N, Satomura Y, Suga M, Nagai T, Natsubori T, Tada M, Nishimura Y, Yamasaki S. A multimodal approach to investigate biomarkers for psychosis in a clinical setting: the integrative neuroimaging studies in schizophrenia targeting for early intervention and prevention (IN-STEP) project. Schizophr Res. 2013;143:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Nurjono M, Lee J, Chong SA. A Review of Brain-derived Neurotrophic Factor as a Candidate Biomarker in Schizophrenia. Clin Psychopharmacol Neurosci. 2012;10:61-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Breier A. Developing drugs for cognitive impairment in schizophrenia. Schizophr Bull. 2005;31:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res. 2010;122:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 435] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 9. | Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA. 2008;105:2711-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 508] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 10. | Nakajo Y, Miyamoto S, Nakano Y, Xue JH, Hori T, Yanamoto H. Genetic increase in brain-derived neurotrophic factor levels enhances learning and memory. Brain Res. 2008;1241:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Okada S, Yokoyama M, Toko H, Tateno K, Moriya J, Shimizu I, Nojima A, Ito T, Yoshida Y, Kobayashi Y. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arterioscler Thromb Vasc Biol. 2012;32:1902-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibañez CF, Rafii S, Hempstead BL. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531-4540. [PubMed] |
| 13. | Renné C, Willenbrock K, Küppers R, Hansmann ML, Bräuninger A. Autocrine- and paracrine-activated receptor tyrosine kinases in classic Hodgkin lymphoma. Blood. 2005;105:4051-4059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005;105:4429-4436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 447] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 16. | Ho R, Eggert A, Hishiki T, Minturn JE, Ikegaki N, Foster P, Camoratto AM, Evans AE, Brodeur GM. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462-6466. [PubMed] |
| 17. | Yang ZF, Ho DW, Lam CT, Luk JM, Lum CT, Yu WC, Poon RT, Fan ST. Identification of brain-derived neurotrophic factor as a novel functional protein in hepatocellular carcinoma. Cancer Res. 2005;65:219-225. [PubMed] |
| 18. | Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde YA. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1089] [Cited by in RCA: 1108] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 19. | Acheson A, Barker PA, Alderson RF, Miller FD, Murphy RA. Detection of brain-derived neurotrophic factor-like activity in fibroblasts and Schwann cells: inhibition by antibodies to NGF. Neuron. 1991;7:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 224] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1243] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 21. | Serra-Millàs M, López-Vílchez I, Navarro V, Galán AM, Escolar G, Penadés R, Catalán R, Fañanás L, Arias B, Gastó C. Changes in plasma and platelet BDNF levels induced by S-citalopram in major depression. Psychopharmacology (Berl). 2011;216:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87:728-734. [PubMed] |
| 23. | Pliego-Rivero FB, Bayatti N, Giannakoulopoulos X, Glover V, Bradford HF, Stern G, Sandler M. Brain-derived neurotrophic factor in human platelets. Biochem Pharmacol. 1997;54:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. J Neurosci. 1990;10:3469-3478. [PubMed] |
| 25. | Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 757] [Cited by in RCA: 770] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 26. | Hiltunen JO, Arumäe U, Moshnyakov M, Saarma M. Expression of mRNAs for neurotrophins and their receptors in developing rat heart. Circ Res. 1996;79:930-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990;247:1446-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 955] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 28. | Maisonpierre PC, Le Beau MM, Espinosa R, Ip NY, Belluscio L, de la Monte SM, Squinto S, Furth ME, Yancopoulos GD. Human and rat brain-derived neurotrophic factor and neurotrophin-3: gene structures, distributions, and chromosomal localizations. Genomics. 1991;10:558-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 396] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 343] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 30. | Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 650] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 31. | Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR, Renz H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. Am J Pathol. 1999;155:1183-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 216] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 1016] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 33. | Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11:1169-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 684] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 34. | Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 448] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 35. | Trajkovska V, Marcussen AB, Vinberg M, Hartvig P, Aznar S, Knudsen GM. Measurements of brain-derived neurotrophic factor: methodological aspects and demographical data. Brain Res Bull. 2007;73:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 810] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 37. | Toyooka K, Asama K, Watanabe Y, Muratake T, Takahashi M, Someya T, Nawa H. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 213] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 38. | Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, Soekadar S, Richartz E, Koehler N, Bartels M. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 39. | Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. J Neuroimmunol. 2005;167:215-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Nakazato M, Hashimoto K, Shimizu E, Kumakiri C, Koizumi H, Okamura N, Mitsumori M, Komatsu N, Iyo M. Decreased levels of serum brain-derived neurotrophic factor in female patients with eating disorders. Biol Psychiatry. 2003;54:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Hong CJ, Liou YJ, Tsai SJ. Effects of BDNF polymorphisms on brain function and behavior in health and disease. Brain Res Bull. 2011;86:287-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Gratacòs M, González JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry. 2007;61:911-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 43. | Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401-4411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 710] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 44. | Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2833] [Cited by in RCA: 2907] [Article Influence: 132.1] [Reference Citation Analysis (0)] |
| 45. | Kawashima K, Ikeda M, Kishi T, Kitajima T, Yamanouchi Y, Kinoshita Y, Okochi T, Aleksic B, Tomita M, Okada T. BDNF is not associated with schizophrenia: data from a Japanese population study and meta-analysis. Schizophr Res. 2009;112:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Weinberger DR, Marenco S. Schizophrenia as a neurodevelopmental disorder: A review of the concept. Schizophrenia. London: Blackwell 1995; 326. |
| 47. | Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed). 1987;295:681-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 684] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 48. | Tanaka S, Sekino Y, Shirao T. The effects of neurotrophin-3 and brain-derived neurotrophic factor on cerebellar granule cell movement and neurite extension in vitro. Neuroscience. 2000;97:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Pillai A. Brain-derived neurotropic factor/TrkB signaling in the pathogenesis and novel pharmacotherapy of schizophrenia. Neurosignals. 2008;16:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Grillo RW, Ottoni GL, Leke R, Souza DO, Portela LV, Lara DR. Reduced serum BDNF levels in schizophrenic patients on clozapine or typical antipsychotics. J Psychiatr Res. 2007;41:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 51. | Xiu MH, Hui L, Dang YF, Hou TD, Zhang CX, Zheng YL, Chen da C, Kosten TR, Zhang XY. Decreased serum BDNF levels in chronic institutionalized schizophrenia on long-term treatment with typical and atypical antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1508-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 52. | Tan YL, Zhou DF, Cao LY, Zou YZ, Zhang XY. Decreased BDNF in serum of patients with chronic schizophrenia on long-term treatment with antipsychotics. Neurosci Lett. 2005;382:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Zhang XY, Tan YL, Zhou DF, Cao LY, Wu GY, Xu Q, Shen Y, Haile CN, Kosten TA, Kosten TR. Serum BDNF levels and weight gain in schizophrenic patients on long-term treatment with antipsychotics. J Psychiatr Res. 2007;41:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Ikeda Y, Yahata N, Ito I, Nagano M, Toyota T, Yoshikawa T, Okubo Y, Suzuki H. Low serum levels of brain-derived neurotrophic factor and epidermal growth factor in patients with chronic schizophrenia. Schizophr Res. 2008;101:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 55. | Huang TL, Lee CT. Associations between serum brain-derived neurotrophic factor levels and clinical phenotypes in schizophrenia patients. J Psychiatr Res. 2006;40:664-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Shimizu E, Hashimoto K, Watanabe H, Komatsu N, Okamura N, Koike K, Shinoda N, Nakazato M, Kumakiri C, Okada S. Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia are indistinguishable from controls. Neurosci Lett. 2003;351:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Gama CS, Andreazza AC, Kunz M, Berk M, Belmonte-de-Abreu PS, Kapczinski F. Serum levels of brain-derived neurotrophic factor in patients with schizophrenia and bipolar disorder. Neurosci Lett. 2007;420:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Reis HJ, Nicolato R, Barbosa IG, Teixeira do Prado PH, Romano-Silva MA, Teixeira AL. Increased serum levels of brain-derived neurotrophic factor in chronic institutionalized patients with schizophrenia. Neurosci Lett. 2008;439:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 59. | Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 355] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 60. | Buckley PF, Pillai A, Evans D, Stirewalt E, Mahadik S. Brain derived neurotropic factor in first-episode psychosis. Schizophr Res. 2007;91:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 61. | Jindal RD, Pillai AK, Mahadik SP, Eklund K, Montrose DM, Keshavan MS. Decreased BDNF in patients with antipsychotic naïve first episode schizophrenia. Schizophr Res. 2010;119:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 62. | Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, Mahadik SP. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 63. | Rizos EN, Papathanasiou M, Michalopoulou PG, Mazioti A, Douzenis A, Kastania A, Nikolaidou P, Laskos E, Vasilopoulou K, Lykouras L. Association of serum BDNF levels with hippocampal volumes in first psychotic episode drug-naive schizophrenic patients. Schizophr Res. 2011;129:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Favalli G, Li J, Belmonte-de-Abreu P, Wong AH, Daskalakis ZJ. The role of BDNF in the pathophysiology and treatment of schizophrenia. J Psychiatr Res. 2012;46:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 65. | Buckley PF, Pillai A, Howell KR. Brain-derived neurotrophic factor: findings in schizophrenia. Curr Opin Psychiatry. 2011;24:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 66. | Guillin O, Demily C, Thibaut F. Brain-derived neurotrophic factor in schizophrenia and its relation with dopamine. Int Rev Neurobiol. 2007;78:377-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Guillin O, Griffon N, Diaz J, Le Foll B, Bezard E, Gross C, Lammers C, Stark H, Carroll P, Schwartz JC. Brain-derived neurotrophic factor and the plasticity of the mesolimbic dopamine pathway. Int Rev Neurobiol. 2004;59:425-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Sokoloff P, Guillin O, Diaz J, Carroll P, Griffon N. Brain-derived neurotrophic factor controls dopamine D3 receptor expression: implications for neurodevelopmental psychiatric disorders. Neurotox Res. 2002;4:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 472] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 70. | Djalali S, Höltje M, Grosse G, Rothe T, Stroh T, Grosse J, Deng DR, Hellweg R, Grantyn R, Hörtnagl H. Effects of brain-derived neurotrophic factor (BDNF) on glial cells and serotonergic neurones during development. J Neurochem. 2005;92:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Repérant C, Guilloux JP, Coudoré F, Hen R, Gardier AM. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology. 2008;55:1006-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Lang UE, Hellweg R, Gallinat J. Association of BDNF serum concentrations with central serotonergic activity: evidence from auditory signal processing. Neuropsychopharmacology. 2005;30:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 558] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 74. | Wong AH, Van Tol HH. Schizophrenia: from phenomenology to neurobiology. Neurosci Biobehav Rev. 2003;27:269-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 75. | Cahn W, Hulshoff Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, Schothorst PF, van Engeland H, Kahn RS. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 269] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 76. | Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 769] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 77. | Shimada A, Mason CA, Morrison ME. TrkB signaling modulates spine density and morphology independent of dendrite structure in cultured neonatal Purkinje cells. J Neurosci. 1998;18:8559-8570. [PubMed] |
| 78. | Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 222] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 79. | Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 632] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 80. | Gärtner A, Polnau DG, Staiger V, Sciarretta C, Minichiello L, Thoenen H, Bonhoeffer T, Korte M. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling. J Neurosci. 2006;26:3496-3504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Zhang XY, Liang J, Chen da C, Xiu MH, Yang FD, Kosten TA, Kosten TR. Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacology (Berl). 2012;222:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 82. | Niitsu T, Shirayama Y, Matsuzawa D, Hasegawa T, Kanahara N, Hashimoto T, Shiraishi T, Shiina A, Fukami G, Fujisaki M. Associations of serum brain-derived neurotrophic factor with cognitive impairments and negative symptoms in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1836-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 539] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 84. | Cirulli F, Berry A, Chiarotti F, Alleva E. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus. 2004;14:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 85. | Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 398] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 86. | Lu B, Chang JH. Regulation of neurogenesis by neurotrophins: implications in hippocampus-dependent memory. Neuron Glia Biol. 2004;1:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Chen da C, Wang J, Wang B, Yang SC, Zhang CX, Zheng YL, Li YL, Wang N, Yang KB, Xiu MH. Decreased levels of serum brain-derived neurotrophic factor in drug-naïve first-episode schizophrenia: relationship to clinical phenotypes. Psychopharmacology (Berl). 2009;207:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 88. | Smith GN, Thornton AE, Lang DJ, Macewan GW, Ehmann TS, Kopala LC, Tee K, Shiau G, Voineskos AN, Kennedy JL. Hippocampal volume and the brain-derived neurotrophic factor Val66Met polymorphism in first episode psychosis. Schizophr Res. 2012;134:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Eisenberg DP, Ianni AM, Wei SM, Kohn PD, Kolachana B, Apud J, Weinberger DR, Berman KF. Brain-derived neurotrophic factor (BDNF) Val(66)Met polymorphism differentially predicts hippocampal function in medication-free patients with schizophrenia. Mol Psychiatry. 2013;18:713-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Gama CS, Berk M, Andreazza AC, Kapczinski F, Belmonte-de-Abreu P. Serum levels of brain-derived neurotrophic factor and thiobarbituric acid reactive substances in chronically medicated schizophrenic patients: a positive correlation. Rev Bras Psiquiatr. 2008;30:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry. 2009;66:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 92. | Xu M, Li S, Xing Q, Gao R, Feng G, Lin Z, St Clair D, He L. Genetic variants in the BDNF gene and therapeutic response to risperidone in schizophrenia patients: a pharmacogenetic study. Eur J Hum Genet. 2010;18:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Zai GC, Zai CC, Chowdhury NI, Tiwari AK, Souza RP, Lieberman JA, Meltzer HY, Potkin SG, Müller DJ, Kennedy JL. The role of brain-derived neurotrophic factor (BDNF) gene variants in antipsychotic response and antipsychotic-induced weight gain. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 94. | Zhang JP, Lencz T, Geisler S, DeRosse P, Bromet EJ, Malhotra AK. Genetic variation in BDNF is associated with antipsychotic treatment resistance in patients with schizophrenia. Schizophr Res. 2013;146:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 95. | Tsai A, Liou YJ, Hong CJ, Wu CL, Tsai SJ, Bai YM. Association study of brain-derived neurotrophic factor gene polymorphisms and body weight change in schizophrenic patients under long-term atypical antipsychotic treatment. Neuromolecular Med. 2011;13:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 96. | Zhang XY, Zhang WF, Zhou DF, Chen da C, Xiu MH, Wu HR, Haile CN, Kosten TA, Kosten TR. Brain-derived neurotrophic factor levels and its Val66Met gene polymorphism predict tardive dyskinesia treatment response to Ginkgo biloba. Biol Psychiatry. 2012;72:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 97. | Xu MQ, St Clair D, Feng GY, Lin ZG, He G, Li X, He L. BDNF gene is a genetic risk factor for schizophrenia and is related to the chlorpromazine-induced extrapyramidal syndrome in the Chinese population. Pharmacogenet Genomics. 2008;18:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |