Published online Sep 19, 2025. doi: 10.5498/wjp.v15.i9.107828
Revised: April 28, 2025
Accepted: July 8, 2025
Published online: September 19, 2025
Processing time: 150 Days and 14 Hours
Depression is a common psychiatric condition with a considerable influence on global health. Although current pharmacotherapeutic choices are of indisputable relevance, there is sustained interest in natural medicines for treating depressive symptoms. Polyphenols, a class of plant-derived natural compounds, have recei
Core Tip: Dietary polyphenols are promising nutraceuticals for treating brain disorders. They have been elucidated as a non-invasive, natural, and cost-effective approach to alleviating depressive symptoms. Polyphenols may target a variety of pathophy
- Citation: Mijailović NR, Milojević-Rakić M, Mihajlović K. Polyphenols: A top-down approach to nutrition and depression. World J Psychiatry 2025; 15(9): 107828
- URL: https://www.wjgnet.com/2220-3206/full/v15/i9/107828.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i9.107828
Depression is the most common mental illness globally and represents the leading cause of disability worldwide[1]. According to the World Health Organization’s assessment study on global disease burden, depression was the second highest burden and disability-causing disease in 2020, and it is expected to become the world’s most significant disease burden by 2030[2]. Targeting molecular interactions and metabolites that are crucial for depression and cognitive changes is challenging and limits efficient therapeutic protocols[3-5]. Although pharmacotherapy and psychotherapy are essential and unavoidable components of overall treatment, a recent meta-analysis by Leichsenring et al[6] demonstrated the limited benefits of psychotherapy and pharmacotherapy, indicating that mental disorders are not sufficiently addressed by available treatments. Although antidepressant medications are efficient for some individuals, they are associated with multiple concerns, including delayed onset of therapeutic effects, incomplete or non-response in a considerable number of patients, high relapse rates, and a range of undesirable side effects, including weight gain, sexual dysfunction, gastrointestinal disturbances, insomnia, and increased risk of withdrawal symptoms upon discontinuation. These issues can compromise treatment adherence and overall quality of life. While these data should not be considered unfavorably, there is a clear necessity to investigate additional therapy alternatives and strategies that might improve the symptoms of this mental illness.
Investigations of nutritional status and its impact on mental health have opened up a new field of research into how the quality and type of food we consume can significantly affect our mental status. There has been sustained interest in natural substances within this context. Among the many investigated chemicals and formulations, polyphenols have attracted notable scientific interest in recent studies[7]. Natural chemicals created only by plants, polyphenols are defined by one or more phenyl rings and at least one or more hydroxyl groups. They represent a large class of molecules consisting of heterogeneous compounds. They are divided into two main classes: Flavonoids (e.g., anthocyanins, flavanols, flavanones, flavonols, flavonones, and isoflavones) and non-flavonoids (e.g., phenolic acids, stilbens, lignans, and tannins) polyphenols[8]. Polyphenols are found in fruits such as pears, cherries, oranges, and apples, and in vegetables such as cereals, pulses, dried legumes, spinach, tomatoes, and beans. They are also present in beverages like tea, coffee, wine, and fruit juices, but also in nuts, peppermint, cinnamon, and ginger[9]. Their protective effect has been explored in various disorders, including diabetes, obesity, osteoporosis, cardiovascular diseases, neurodegenerative diseases, and cancer[10,11]. These chemicals demonstrate neuroprotective, anti-inflammatory, and antioxidant properties that may affect mood regulation and neurotransmitter activity[12]. Polyphenols have gained recognition for their possible influence on mental health, especially in mitigating the risk of depression[13]. Their antidepressant and neuroprotective effects have been extensively studied[14,15]. Unlike conventional pharmacotherapy, polyphenol-rich diets may offer a holistic approach. Their multi-target mechanisms of action, favorable safety profile, along with their adjunctive potential with conventional antidepressant use[16], strengthen the rationale for investigating polyphenols not only as an alternative but also as adjunctive interventions in depression therapy.
In this mini-review, we discuss the current knowledge regarding the role of polyphenols in depression. The classification of polyphenols, their sources in various types of food, as well as dietary patterns based on polyphenol content, and their beneficial role in depression will be presented. We aim to summarize the role of polyphenols in modulating inflammatory mediators, neurogenesis, oxidative stress (OS), and gut microbiota from preclinical models. We also present the results of recent clinical studies on the influence of dietary polyphenols on depression symptoms. Future perspectives are also outlined.
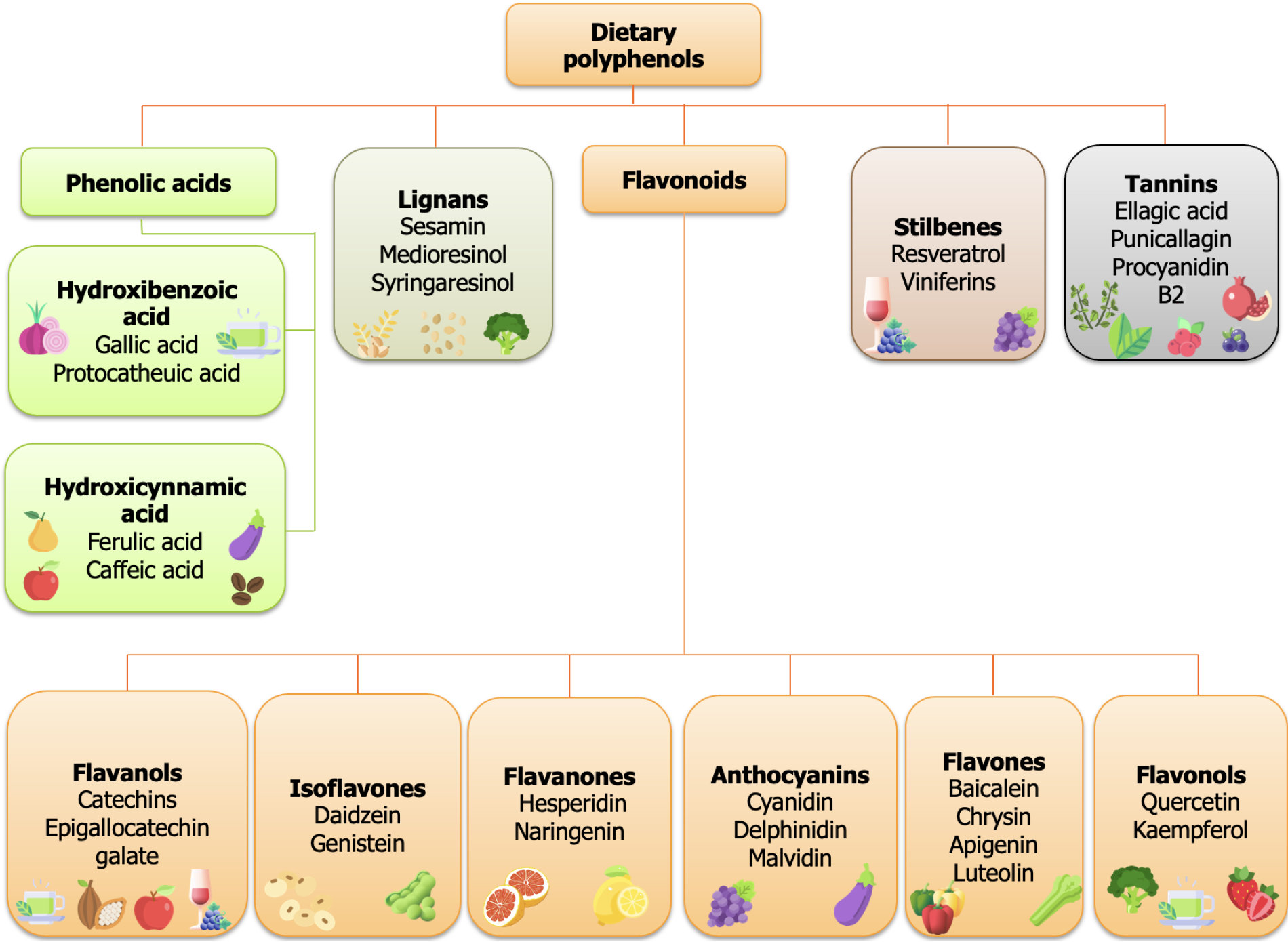
Polyphenols can be classified into five groups: Flavonoids, phenolic acids, lignans, stilbenes, and tannins. Flavonoids, the most common polyphenols in the human diet, are categorized as flavanols, isoflavones, flavanones, anthocyanins, flavones, and flavonols based on their molecular structure[17]. Rich in catechins (from the flavanol subgroup), chocolate and green tea decrease stress-related cortisol by hypothalamic-pituitary-adrenal axis modulation. Studies on humans and animals show their anxiety-reducing and antidepressant effects[18]. Isoflavones, with the main representatives being daidzein and genistein, are found in soy and legumes; they act as phytoestrogens and modify estrogen receptors, both of which influence brain activity[19]. In postmenopausal women, they have a strong connection to higher serotonin activity and reduced depressive symptoms[20]. Flavanone subgroup members hesperidin and naringenin are widely present in citrus fruits, grapefruits, and tomatoes. They exert strong antioxidant and free radical scavenging activities[21]. Anthocyanins, such as malvidin, cyaniding, and delphinidin, which give berries and red wine their pigmentation[22], activate serotonin and dopamine (DA) systems, thus enhancing mood and minimizing depression[23]. Flavones, such as baicalein, apigenin, chrysin, and luteolin are present in celery, parsley, peppers, chamomile, mint, ginkgo biloba, and red wine, and have been shown to have antioxidant, anti-inflammatory, antiallergic, neuroprotective, and cardioprotective effects[24]. Flavonols, such as quercetin and kaempferol, present in onions, apples, and tea, protect neurons from oxidative damage. Additionally, they may promote synaptic plasticity, which is essential for regulating emotions and cognitive processes[25]. Phenolic acids, such as hydroxybenzoic acids (gallic and protocatheutic acid) present in berries and whole grains, improve mitochondrial function and reduce neuroinflammation, hence providing antidepressant effects[26]. Also, phenolic acids, hydroxycinnamic acids (ferulic and caffeic acid), present in coffee and tomatoes, can control inflammatory cytokines and OS markers, therefore helping maintain neuronal integrity and neurotransmitter balance[27]. Lignans such as sesamin, medioresinol, syringaresinol are primarily found in flaxseeds, sesame seeds, whole grains, and vegetables. Their estrogenic and antioxidant effects have linked them to lower risks of cardiovascular disorders and hormone-related malignancies[28]. Stilbenes, such as resveratrol and its derivative viniferin, are found in grapes and red wine. Resveratrol activates sirtuins, proteins that regulate neuroprotection and cellular stress resilience. It can regulate inflammatory pathways and increase brain-derived neurotrophic factor (BDNF) levels to help achieve antidepressant-like effects[29]. Tannins (ellagic acid, punicalagin, procyanidin B2) can be found in fruits, cereals, cacao, peas, some leafy and green vegetables, coffee, tea, herbs, and spices. They possess antioxidant, anticancer, anti-allergic, and antimicrobial properties (Figure 1)[30].
Numerous pre-clinical studies have investigated the molecular mechanism of various compounds, diets[31], and polyphenols related to their antidepressant effects. These studies showed that polyphenols may alleviate depressive symptoms through various mechanisms.
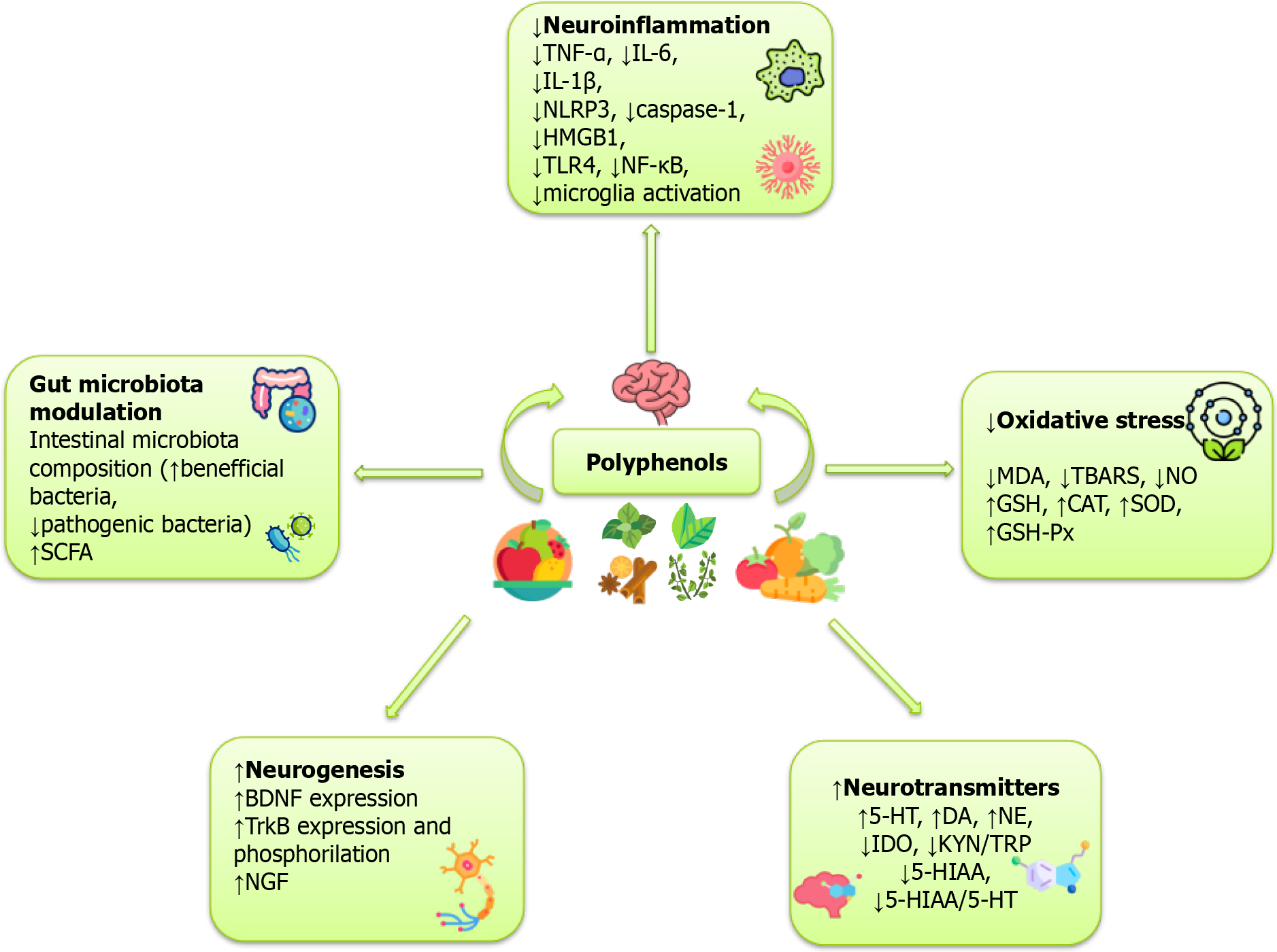
The immune system and inflammation are significant factors in the etiology of depression[32]. By modulating neurotransmission and neurocircuitry, inflammatory cytokines significantly impact central nervous system (CNS) homeostasis, neurogenesis, and mood regulation, influencing behavior[33]. Dysregulation of immune functions can lead to depression. The anti-inflammatory properties of various polyphenols have been investigated in different preclinical depression models. Baicalin is a flavone glycoside with various properties, including antibacterial, antiviral, anticancer, antioxidant, and neuroprotective effects[34]. Studies using the Chronic unpredictable mild stress (CUMS) model demonstrated its antidepressant effects through suppression of pro-inflammatory cytokines[35] and pathways like the glycogen synthase kinase/nuclear factor kappa B (NF-κB)/nucleotide-binding oligomerization domain-, leucine-rich repeat- and pyrin domain-containing protein 3 (NLRP3)[36] and high mobility group box 1 protein (HMGB1)/toll-like receptor 4 (TLR4)/NF-κB[37] and by inhibiting TLR4 expression[38]. Furthermore, baicalin decreased depression-related inflammation in the olfactory bulbectomy model (OBX) by modulating sirtuin-1 and NF-κB p65 acetylation[39]. Resveratrol, a polyphenolic compound found in grapes, peanuts, and berries, exerts strong anti-inflammatory properties[40]. Resveratrol reduced neuroinflammation by suppressing NF-κB and NLRP3 pathways[41,42] and by decreasing the levels of pro-inflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6)[41,43,44] and microglial activation[42,45]. Resveratrol modulated anti-depressive effects through protective mechanisms like sirtuin-1 activation[42,45] and BDNF upregulation[46]. Hesperidin is a flavanone glycoside present in citrus fruit with a variety of positive effects[47]. In the CUMS model, hesperidin decreased IL-1β, IL-6, TNF-α, NLRP3, and caspase-1 Levels in the prefrontal cortex and microglia[48]. In the same model, hesperidin reduced inflammatory cytokine levels by attenuating the HMGB1/receptor for advanced glycation end products (RAGE)/NF-κB signaling pathway and BDNF/tropomyosin-related kinase receptor type B (TrkB) pathway both in vivo and in vitro[49]. Hesperidin alleviated lipopolysaccharide (LPS)-induced neuroinflammation in mice by promoting the microRNA-132 pathway[50] and alleviated chronic restraint stress and LPS-induced damage in the hippocampus and frontal cortex by decreasing pro-inflammatory cytokine levels[51]. It also decreased depression-related symptoms in mice with mild traumatic brain injury[52]. Another extensively explored flavonoid polyphenol is curcumin, which originates from the rhizomes of the Curcuma longa plant. It possesses a variety of biological effects, including anti-inflammatory effects[53]. Curcumin application reversed CUMS-induced behavioral and neuroimmune abnormalities by decreasing the levels of IL-1β, IL-6, and TNF-α[54,55], and by suppressing the NLRP3 inflammasome and tryptophan-kynurenine (TRP-KYN) pathway[54] and microglial activation[55]. Quercetin, a flavonol found primarily in berries, apples, and onions, possesses beneficial effects on mental health[56]. Studies have shown that quercetin can reduce depressive-like behavior in models of chronic stress-induced depression by reducing levels of OS markers and inflammatory cytokines, including TNF-α, IL-6, and IL-1β (Figure 2)[57-60].
Chronic neuroinflammation affects many molecular and cellular processes in the CNS, thereby facilitating depression onset and development. Dietary polyphenols can reduce depression symptoms by blocking transcription factors, proinflammatory cytokines, and protein complexes that stimulate neuroinflammatory processes. Polyphenols are considered a potential alternative for the treatment of depression associated with inflammation due to their potent anti-neuroinflammatory effects.
The gut-brain axis is an essential factor influencing mental health. Interactions of brain and gut microbiota can be exerted through the neurological, endocrine, immunological, and metabolic pathways, which can result in disturbances in mood, sleep, and cognitive functions[61-63]. This bidirectional link can significantly influence psychiatric disorders including depression and anxiety[64]. The existence of a mutual and bidirectional interplay between polyphenols and the gut microbiota is well established[65]. Polyphenols modulate gut microbiota by preserving intestinal stability and regulating intestinal flora[66]. Polyphenol absorption and bioavailability are generally low but vary based on an individual’s gut microbiome[67]. Around 95% of polyphenols are not absorbed by the small intestine and reach the colon, where they interact with gut microbiota[68]. Dietary polyphenols act as prebiotics, enhancing the growth of specific beneficial bacterial species[69]. On the other hand, the gut microbiota can increase the production of bioactive phenolic acids derived from dietary polyphenols, increasing their beneficial biological activity[70-72]. Polyphenols can impact gut microbiome composition through the stimulation of probiotic bacteria populations, such as Lactobacillus and Bifidobacterium, and the suppression of pathogenic strains, including Clostridium perfringens and Clostridium histolyticum[73,74]. Microorganisms classified as psychobiotics (probiotics and prebiotics that provide mental health advantages via interactions with commensal gut bacteria) synthesize neurotransmitters and short-chain fatty acids (SCFAs), which directly influence the nervous system[75]. Tea polyphenols suppress the proliferation of Bacteroides-Prevotella and Clostridium histolyticum while promoting the growth of Bifidobacterium, Lactobacillus, and Enterococcus bacteria and enhanced SCFA synthesis[76,77]. Flavonoids, present in citrus fruits, onions, and green tea, improve gut microbiota diversity and produce metabolites that cross the blood-brain barrier, modulating neurotransmitters like serotonin and DA[78]. In an adrenocorticotropic hormone (ACTH)-induced rat model of depression, chlorogenic acid improved depression-like behavior, normalized serum levels of 5-hydroxytryptamine (5-HT), DA, IL-6, TNF-α, and modulated bacterial strains, including Desulfovibrio, Klebsiella, Burkholderiales, and Bifidobacterium[79]. Bifidobacteria-fermented red ginseng alleviated anxiety/depression in stress-induced gut dysbiosis and inflammation by ameliorating gut and hippocampal inflammation[80]. In a CUMS model of depression, soy isoflavones attenuated depressive behavior by increasing monoamine neurotransmitters and modulating gut microbiota composition[81]. In the same model, the phenolic acid compound coniferyl ferulat restructured gut microbiota and microbial metabolism to decrease depressive-like behavior[82]. Polyphenols can modulate intestinal barrier function and signaling pathways[83], while tea polyphenols affect the microbiome by preserving optimal redox status[84].
Taken together, polyphenols can interact with the gut flora by modulating its composition, promoting the proliferation of good strains and reducing that of pathogenic ones. Furthermore, the gut microbiota directly affects the immune system, which then affects nervous system function. This interaction implies a gut-brain-immune axis, which polyphenols might significantly control.
The process of generating new neurons from neural stem or progenitor cells primarily occurs during fetal development and is denoted as neurogenesis. It continues in adults, particularly in the hippocampus, and plays a key role in synaptic plasticity, cognition, and stress resilience[85]. Brain neurotrophic factors have an important role in the pathophysiology of depression. BDNF promotes neuronal growth, differentiation, and survival by activating the TrkB receptor[86]. It is a key regulator of neurogenesis and synaptic plasticity, fundamental to learning, memory, and behavior[87]. Inflammatory cytokines, including IL-1β, IL-6, and TNF-α, suppress the BDNF and TrkB expression while also inhibiting TrkB phosphorylation[88]. Plant-derived flavonoid baicalein showed antidepressant effects and increased the hippocampal level of BDNF[89]. Furthermore, the antidepressant effect was partially modulated through the extracellular signal-regulated kinase signaling pathway, which is associated with neurotropic action, cell survival, and proliferation[89]. The same flavonoid exerted antidepressant effects by regulating the Wnt/β-catenin signaling pathway and improved nerve cell survival in the hippocampal dentate gyrus[90]. Baicalin improved depressive-like behavior by promoting neuronal maturation, preventing apoptosis, and enhancing BDNF levels[36]. The up-regulation of 5-HT1A receptors and BDNF in chronically stressed mice verified the beneficial effects of curcumin[91]. Hesperidin, a flavonoid polyphenol from the flavanone glycoside subgroup highly present in citrus fruit[92], promotes neurogenesis by enhancing BDNF and nerve growth factor. Its antidepressant effects are mediated through the inhibition of neuroinflammation, modulation of OS, and regulation of important signaling pathways, including NF-κB, HMGB1/RAGE, and TLR4, which contribute to neuronal plasticity and survival[49,51,93,94]. Quercetin mitigated depressive-like behaviors by modulating the BDNF/TrkB/β-catenin axis in the prefrontal cortex[95]. Grape-derived polyphenols[96], chrysin from bee propolis, honey, and various plants[97], resveratrol[41,46], and salvianolic acid[98], also improved depressive-like behavior in animal models through modulation of BDNF, along with other mechanisms (Figure 2).
Dietary polyphenols show promising neuroprotective effects by increasing BDNF expression, suggesting they may play a role in preventing and treating depression symptoms. Future research should focus on identifying the specific molecular pathways through which polyphenols modulate BDNF signaling. This could lead to new nutraceutical interventions for depression treatment.
OS is considered to be in the background of depression[99]. Dietary intake of polyphenols has significant effects against many diseases that involve OS[100]. A diet rich in polyphenolic compounds, consisting of a variety of vegetables, especially yellow, orange, red, and green leafy vegetables, can help reduce depression symptoms[101]. Numerous studies have demonstrated an increase in antioxidant parameters and a decrease in OS parameters after the application of various polyphenol compounds[102]. Phenolic compounds exert antioxidant properties by scavenging reactive oxygen species (ROS), reducing ROS production through oxidative enzyme inhibition and trace element chelation, enhancing endogenous antioxidant activity and donating electrons or hydrogen atoms to neutralize singlet oxygen[103]. The application of quercetin mitigated OS by restoring antioxidant enzyme levels of superoxide dismutase (SOD), glutathione (GSH), catalase (CAT), and total thiol, and by reducing lipid peroxidation[57-60]. Curcumin ameliorated behavioral deficits and restored biochemical and molecular alterations by increasing GSH, SOD, and CAT activity[104]. Curcumin also effectively reduced OS in the CUMS model by decreasing lipid peroxidation and enhancing antioxidant enzyme activities[105]. Administration of baicalin decreased apoptosis and protected cells from OS by reducing malondialdehyde (MDA) levels and increasing SOD levels[36]. Enhanced oxido-nitrosative stress and lower antioxidants GSH, SOD, and CAT were restored by hesperidine application, alleviating anxiety and depressive-like behavior[51]. Ferulic acid and salvianolic acid B were also effective in counteracting OS by reducing lipid peroxidation and restoring the key antioxidant enzymes GSH, SOD, CAT, and GSH peroxidase (Figure 2)[106-108].
Based on the monoamine hypothesis of depression, modulation and restoration of the monoamine neurotransmitter system are crucial in treating depression. Numerous studies showed that increased 2,3-dioxygenase (IDO) activity correlates with depressive severity. This important enzyme is involved in the TRP/KYN metabolic pathway, as it decreases TRP and inhibits serotonin (5-HT) synthesis. The role of polyphenols within this concept has been widely investigated. Curcumin supplementation attenuated IDO activation and an increased KYN/TRP ratio[54], modulated DA and norepinephrine (NE) levels, downregulated monoamine oxidase B, and upregulated DA receptor mRNA in the limbic region[109]. These changes were followed by improvement in behavioral deficits. Curcumin dose-dependently ameliorated behavioral deficits by decreasing biogenic amine levels (DA, NE, 5-HT) in the cortex and hippocampus of reserpine-treated rats[104]. Similar effects were observed after chrysin administration[97,110]. Ferulic acid improves depression-like behaviors by regulating the monoaminergic system[106]. Deoilled sunflower seeds have been found to alleviate depressive-like behaviors in a CUMS model by increasing DA, 5-HT, and NE levels[111]. Similarly, Epigallocatechin Gallate (EGCG), catechin from apples, pears, tea, and cocoa, has been shown to improve depression-like behaviors[112], along with navel orange essential oil[113]. Nobiletin, a flavone from citrus peels, exhibits antidepressant effects by targeting 5-HT1A, 5-HT2, α1-adrenoceptors, and D1/D2 DA receptors, highlighting its therapeutic potential in depression[114]. Naringenin, a flavanone from grapefruit, improves serotonergic neurotransmission and 5-HT synthesis, contributing to its antidiabetic, antioxidant, and memory-improving effects[115]. The flavone apigenin mitigated stress-induced alterations of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA), DA levels, and the 5-HIAA/5-HT ratio in various brain regions of the CUMS rat model, confirming its role in mood regulation (Figure 2)[116].
Polyphenols exhibit neuroprotective properties by regulating neurotransmitter metabolism and modulating neurotransmitter receptor function, which poses them as potential adjunctive therapeutics.
Various animal models of depression have demonstrated that diverse phenolic compounds can alleviate depressive-like behavior.
Baicalin reduced depressive-like behaviors in CUMS rodent models of depression. Behavioral improvements were reflected through reduced anhedonia, decreased behavioral despair, and increased locomotor activity[35-38,117]. Baicalin enhanced neuronal survival and maturation and reduced apoptosis in the hippocampus[36]. In an LPS-induced depression model, baicalin reduced depressive-like behaviors by suppressing neuroinflammation via the peroxisome proliferator-activated receptor-γ coactivator-1α/NF-κB pathway[118], while in the OBX model of depression, it reversed depressive behaviors by normalizing corticosterone levels and reducing inflammatory cytokines in the hippocampus and hypothalamus[39]. In the CUMS and chronic unpredictable stress model, quercetin improved behavioral symptoms, including reduced depressive symptoms (anhedonia, despair-like behavior) and improved cognitive functions[57,58]. In the OBX model[60] and 1,2-dimethylhydrazine-induced depression model[95], quercetin improved anxiety, depressive-like behaviors, and cognitive dysfunction by decreasing oxidative-nitrosative stress and inflammation-related apoptosis in the brain[60], and by enhancing neuroprotective factors[95]. Curcumin is another widely investigated polyphenol. Its application for 4[54] or 5 weeks[119] in rats exposed to CUMS improved mood-related behavior, as measured by increased percent of sucrose consumption and increased mobility[119]. Similar results were obtained with curcumin in the chronic mild stress model[120]. Curcumin improved mood and cognitive function by restoring neurotransmitter balance and reducing OS and inflammation in ovariectomized-induced depression in female rats[109] and reversed pain-related depressive behaviors by modulating neuroinflammation-related pathways[104]. Treatment with curcumin reversed LPS-induced alterations in the forced swim test, tail suspension test, and sucrose preference test[121]. Resveratrol reversed depressive behaviors in CUMS[41,43,44], LPS-induced inflammation[45,46], ovariectomized[42], and social stress-induced depression models[122]. These effects were manifested as decreased anhedonia, decreased behavioral despair, and decreased anxiety. Using a model of agitated depression, Filho et al[97] found that the natural flavonoid chrysin alleviates depression-like behaviors in animal models by regulating the TRP-KYN pathway, reducing indoleamine IDO activity and restoring 5-HT levels. It also decreases ACTH and corticotropin-releasing hormone levels, suggesting stress modulation[97]. Antidepressive effects were also demonstrated in animal models after the application of epicatechin[123] and apigenin[124,125], and various polyphenolic acids like chlorogenic acid[126], dehydrozingerone[126], ellagic acid[127], ferulic acid[128,129], gallic acid[130], rosmarinic acid[131,132], and syringic acid[133,134].
Based on the presented data, polyphenols’ behavioral effects are strongly linked to their anti-inflammatory and neuroprotective properties, suggesting their potential as therapeutic agents for depression.
Despite the existence of many preclinical studies, research on the influence of individual phenols on depressive symptoms in humans is scarce. Observational studies have investigated the correlation between habitual polyphenol consumption and the prevalence of depressive symptoms. A study involving more than 1500 adults in southern Italy[135] suggested that higher total polyphenol intake was inversely related to depressive symptoms. Similarly, research focusing on older adults found that certain polyphenol-rich foods may contribute to a lower depression risk[101]. One meta-analysis of randomized controlled trials investigating the effectiveness of polyphenol supplementation in improving depression, anxiety, and quality of life revealed their beneficial effect on depressive symptoms[136]. Reduced depression scores in comparison to milk chocolate confirmed the positive effects of cocoa, which is high in polyphenols and has significant antioxidant properties[137]. A meta-analysis of dark chocolate/cocoa intake showed significant reductions in OS markers, including plasma MDA and higher nitric oxide levels, suggesting overall antioxidant advantages[138]. Consumption of grape seed proanthocyanidin extract (GCPE) decreased anxiety but with no significant impact on depression. GSPE’s anxiolytic effects are mostly based on its antioxidant activity and a potential interaction with Gamma-aminobutyric acid-A receptor[139].
A randomized, open-label intervention trial found that eating foods high in antioxidants (vitamin E, C, carotenoids, polyphenols), minerals (selenium, zinc), fiber, and polyunsaturated fatty acids improved quality of life and reduced anxiety and depression symptoms. A limitation of the study is the absence of a control group missing nutritional intervention for comparative analysis[140]. Walnuts are abundant in neuroprotective compounds, including vitamin E, folic acid, melatonin, antioxidant polyphenols, and omega-3 fatty acids. A clinical trial investigating the impact of > 8 weeks of walnut consumption on the mood of healthy individuals showed no effects on women, while men’s overall mood disorder scores significantly improved[141]. One placebo-controlled study examined the benefits of curcumin on people with major depressive disorder and demonstrated its significant antidepressant effects at 12 and 16 weeks after treatment initiation. The administration of curcumin showed safety and tolerability, even when used alongside antidepressants. The advantages of curcumin were more pronounced in males compared to females[142].
A study by Almudhi and Gabr[143] found that six cups of decaffeinated green tea daily over 6 weeks significantly reduced depression scores in the adolescent population. A trial from the United States exploring the effects of 8 weeks of 1500 mg chamomile daily also reported improvements in participants with comorbid depression[144]. Positive results were also reported for dietary flavonoids in postpartum women[145], flavonoid-rich orange juice[146], curcumin[147], wild blueberry supplementation in adolescents[148], and green tea extract in human immunodeficiency virus patients[149]. In another trial, Kontogianni et al[150] observed significantly lower Beck depression inventory (BDI)-II scores in a group consuming high amounts of fruits, vegetables, and dark chocolate compared to a low-consumption control group, with no effect on the depression, anxiety, and stress scale-21 depression subscale. A significant reduction in BDI scores among participants with multiple sclerosis was observed after the application of EGCG and coconut oil[151] or ellagic acid[152]. Parilli-Moser et al[153] found that peanut products reduced depression scores in healthy young adults. Meta-analyses have also confirmed antidepressant effects for curcumin[154], cocoa[155], and resveratrol[156].
Overall, the results reported from recent trials and meta-analyses suggest that certain dietary polyphenol interventions may reduce depressive symptoms across clinical and non-clinical populations. Yet, the limitations of these studies make it challenging to properly interpret the results. Polyphenol types, dosages, forms, and intervention durations significantly vary among these studies. Furthermore, various depression assessment tools make it difficult to compare the results. Future studies should focus on long-term, well-designed randomized controlled trials with diverse populations to better understand the potential role of polyphenols in managing depression.
The recognition of the role of diet in depression has led to the development of dietary interventions as adjunctive treatments for mental health disorders[157]. Among these, the Mediterranean diet (MD) and plant-based diets have gained attention for their potential protective effects against depression[135,158-160].
The MD is a nutritional pattern that has traditionally been followed by Mediterranean populations and has been associated with better health[161,162]. It emphasizes a diet rich in vegetables, fruits, legumes, whole grains, nuts, and olive oil, with modest amounts of fish, meat, and dairy. This eating pattern has been extensively studied for its role in preventing chronic diseases like cardiovascular disorders[163], metabolic syndrome[163], and neurodegenerative disorders[164]. Furthermore, accumulating evidence suggests its protective role in mental health, particularly in reducing the risk of depression[165-167]. The dietary components of the MD, including omega-3 fatty acids found in fish and olive oil, contribute to neuronal membrane function and neurotransmitter regulation. Polyphenols that are abundant in the MD, present in olive oil, nuts, and red wine, possess anti-inflammatory and neuroprotective properties[168,169], while whole grains and legumes provide B vitamins essential for neurotransmitter synthesis[170]. Additionally, minerals such as magnesium and zinc, found in nuts, seeds, and leafy greens, contribute to synaptic plasticity and neurogenesis[171].
The MD includes lots of fruits and vegetables including tomatoes, leafy greens, peppers, citrus fruits, and berries. These foods are high in fiber, vitamins, and polyphenols. It also contains whole grains, including bulgur, brown rice, oats, and whole wheat, which offer necessary vitamins, fiber, and complex carbs. Nuts and legumes include chickpeas, lentils, almonds, and walnuts, which provide plant-based protein, fiber, and good fats. Olive oil, which is high in monounsaturated fats and polyphenols with anti-inflammatory and cardiovascular benefits, is the main source of fat. Sardines, salmon, and mackerel, fish and shellfish have omega-3 fatty acids that help heart and brain function. While moderate consumption of red wine offers polyphenolic compounds like resveratrol, which have neuroprotective and anti-inflammatory properties, moderate consumption of dairy products, including yogurt and low-fat cheese, as well as eggs, provides protein and probiotics that support gut health important for modulating the gut-brain axis. While chicken and turkey are eaten in modest amounts, red meats such as beef and pork are eaten only occasionally. To reduce saturated fat intake, lean, unprocessed cuts are recommended[13,172].
Numerous studies have examined the impact of the MD on mental health. An inverse relationship between adherence to the MD and depression risk was outlined by a meta-analysis of cohort studies[173]. A systematic review by Bayes et al[159] linked higher polyphenol intake to a 32% lower depression risk but emphasized variability in study designs and the need for randomized trials. A meta-analysis of 14 observational studies (56043 participants) found no significant effect in cohort studies, while cross-sectional studies suggested a protective role between MD adherence and depression risk. Limitations of this meta-analysis were variations in dietary assessment and depression measurements[174]. Parletta et al[175] found a 45% reduction in depression scores with a combination of MD and fish oil, though these results are limited, constrained by a small sample size and a 3-month duration. Although these studies have their limitations, MD has confirmed its beneficial effect on mental health.
Plant-based diets, abundant in polyphenols and low in animal products, are associated with lower inflammation, improved gut microbiota, and better mental health[176,177]. However, strict vegan diets may lead to deficiencies in vitamin B12, iron, and omega-3 fatty acids, thus increasing depression risk[178]. Recent research has explored the potential impact of plant-based diets rich in polyphenols on depression risk[179,180]. These studies suggest an inverse relationship with depression. A study of 219 adults found that specific polyphenols, especially from citrus fruits and wine, were associated with reduced depressive symptoms, though total polyphenol intake showed no effect[181]. Another study showed that plant-based diets, particularly the MD, positively influenced mood in adults, while the Plant-based Diet Index was linked to positive mood in children[182]. Further research is needed to explore age-related effects.
Polyphenols function in concert with other nutrients to improve their absorption and efficacy[183,184]. A study by Li et al[185] found that ferulic acid combined with piperine significantly increased serotonin and noradrenaline levels in the frontal brain and hippocampus, thereby decreasing depressive symptoms. In addition to the interactions between polyphenols, their synergistic effects with other nutrients are essential to improving health effects[186]. Polyphenols enhance the absorption of critical minerals, including iron and zinc, by modifying the intestinal flora and preventing the formation of insoluble complexes[187]. Vitamin C increases the bioavailability of catechins and enhances their antioxidant activity[188]. Omega-3 fatty acids from fish or flaxseeds work with polyphenols to reduce neuroinflammation and OS, mechanisms linked to depressive disorders[189]. Additionally, dietary proteins such as casein and whey influence polyphenol stability and efficacy, forming complexes that enhance their bioactivity in the CNS[190].
Dietary interventions can be implemented through concentrated polyphenol-rich supplements (e.g., capsules containing curcumin, resveratrol, etc.) or through whole-food dietary patterns, such as the MD, which is naturally abundant in polyphenols. While supplements provide an exact dose and are convenient for use in clinical trials, whole-food diets contain many different nutrients and bioactive compounds, which can act synergistically and produce a stronger and more balanced effect. Whole-food diets can be integrated more easily into daily life and for longer time periods and have benefits beyond mental health (e.g., for heart disease, and diabetes). Methods for implementing diet commonly include structured meal plans, and dietitian-led counseling, while the evaluation of outcomes is based on standardized psychological scales, such as the Hamilton depression rating scale, BDI, etc. Studies have shown that personalized coaching, regular follow-ups, the use of dietary tracking tools (e.g., apps or food diaries), and integration with behavioral interventions (e.g., cognitive behavioral therapy) can significantly enhance adherence and outcomes of interventions applied[191].
While polyphenols are generally considered safe when consumed as part of a balanced diet rich in fruits, vegetables, herbs, and tea, their excessive intake, especially from supplements, may pose risks, particularly for individuals on polypharmacy or with chronic conditions. Overdosing on purified polyphenols (like aglycones) can potentially lead to prooxidant, mutagenic, or genotoxic effects[192]. These supplements can contain extremely high concentrations of pure polyphenols that are impossible to reach through natural foods. This discrepancy raises safety concerns, especially since such supplements are easily available, not expensive, and often taken without medical supervision.
It is important to note that substantial inter-individual differences may exist in response to polyphenol intake. These differences may arise from a combination of genetic, microbial, metabolic, dietary factors, age, and sex-related factors, but also from the presence of metabolic disorders such as diabetes, obesity, and liver or kidney impairment[192].
Polyphenol-drug interactions are based on their effects on drug-metabolizing enzymes and transporters and should be considered, especially in cases of polypharmacy. These interactions can lead to changes in drug efficacy or increase their toxicity, particularly for drugs with a narrow therapeutic index. Notably, polyphenols can interact with anticoagulants such as warfarin[193,194] and rivaroxaban[195], cardiac drugs such as digoxin[196], nadolol[197], and lisinopril[198], statins[199-201], and metformin[202].
It is difficult to distinguish the definitive interactions between polyphenols and other bioactive components. The expected dominant interaction of polyphenols is hydrogen bonding, along with other dipole-dipole, dipole-induced dipole, and hydrophobic interactions. Suggested sites for hydrogen bonding polyphenol to proteins include hydroxyl and amino groups of amino acid residues[203]. The range of possible synergistic or antagonistic effects of these interactions influences polyphenol bioavailability, stability, and activity.
The use of polyphenols as nutriceuticals is limited by their poor oral bioavailability, poor water solubility, and rapid metabolism. This can be overcome by optimized delivery systems such as nanoencapsulation, liposomes, or coadministration with enhancers.
The high rate of treatment resistance, significant suicide risk, and heavy financial burden emphasize the need for investigating new therapeutic approaches in treating depression. Yet, the path from discovering the mechanisms underlying depression and their translation into therapy is long and challenging. There is a need for larger and long-term human studies to determine the potential for using polyphenol-rich foods or supplements as adjunct therapies for depression. Furthermore, it is important to explore personalized nutrition approaches and consider individual variability in response to polyphenols, along with their potential for interactions with other medications.
The safety and tolerability of high doses of polyphenols should also be explored. Novel immune-based therapeutics could potentially address unmet clinical needs of patients resistant to conventional antidepressant medications. It is critical to investigate the potentially complex interactions between dietary polyphenols and numerous bioactive components, which may lead to beneficial complementary, additive, synergistic, as well as negative or neutralizing interactions. The diversity and richness of gut microbiota play a crucial role in determining dietary polyphenol bioavailability. Combining dietary polyphenols with an optimized probiotic formulation as a synbiotic could enhance the production of specific metabolites, whose neuroprotective mechanisms could be further elucidated and optimized for depression treatment.
The computational and semi-empirical methods can be effectively applied to investigate the molecular interactions between polyphenols and other bioactive molecules, such as neurotransmitters, receptor proteins, and cofactors involved in depression-related pathways. These computational methods can guide the design of novel polyphenol formulations with enhanced bioavailability and synergistic interactions, potentially leading to tailored treatments for different depression subtypes. Subsequently, in vitro and in vivo studies are necessary to distinguish whether targeted structures and supplements can support depression treatment. As methods for experimental testing evolve, this fundamental approach combining theory with experiment will be validated and yield novel polyphenol-based therapeutics.
Dietary polyphenols are promising nutraceuticals for treating brain diseases. Evidence from diverse animal and human studies discussed here supports that dietary sourced polyphenols play a potential protective role in modulating depressive symptoms. Although extensive results originate from preclinical studies, clinical evidence remains scarce. Furthermore, additional research is required to determine the pharmacokinetic properties, bioavailability, and safety of polyphenols to facilitate their clinical application in managing depressive symptoms. However, translating preclinical findings into clinical practice requires more rigorous human studies to unlock the full potential of these natural compounds.
| 1. | Moreno-Agostino D, Wu YT, Daskalopoulou C, Hasan MT, Huisman M, Prina M. Global trends in the prevalence and incidence of depression:a systematic review and meta-analysis. J Affect Disord. 2021;281:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 2. | Zhang Y, Jia X, Yang Y, Sun N, Shi S, Wang W. Change in the global burden of depression from 1990-2019 and its prediction for 2030. J Psychiatr Res. 2024;178:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 3. | Borovcanin MM, Vesić K, Arsenijević D, Milojević-Rakić M, Mijailović NR, Jovanovic IP. Targeting Underlying Inflammation in Carcinoma Is Essential for the Resolution of Depressiveness. Cells. 2023;12:710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Mijailovic NR, Vesic K, Borovcanin MM. The Influence of Serum Uric Acid on the Brain and Cognitive Dysfunction. Front Psychiatry. 2022;13:828476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 5. | Mijailović NR, Vesic K, Arsenijevic D, Milojević-Rakić M, Borovcanin MM. Galectin-3 Involvement in Cognitive Processes for New Therapeutic Considerations. Front Cell Neurosci. 2022;16:923811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Leichsenring F, Steinert C, Rabung S, Ioannidis JPA. The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta-analytic evaluation of recent meta-analyses. World Psychiatry. 2022;21:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 208] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 7. | Mijailović NR, Nedić Vasiljević B, Ranković M, Milanović V, Uskoković-Marković S. Environmental and Pharmacokinetic Aspects of Zeolite/Pharmaceuticals Systems—Two Facets of Adsorption Ability. Catalysts. 2022;12:837. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 8. | Singla RK, Dubey AK, Garg A, Sharma RK, Fiorino M, Ameen SM, Haddad MA, Al-Hiary M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J AOAC Int. 2019;102:1397-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 9. | Li AN, Li S, Zhang YJ, Xu XR, Chen YM, Li HB. Resources and biological activities of natural polyphenols. Nutrients. 2014;6:6020-6047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 555] [Cited by in RCA: 525] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 10. | Li W, Chen H, Xu B, Wang Y, Zhang C, Cao Y, Xing X. Research progress on classification, sources and functions of dietary polyphenols for prevention and treatment of chronic diseases. J Future Foods. 2023;3:289-305. [DOI] [Full Text] |
| 11. | Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, Novellino E, Santini A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33:2221-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 458] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 12. | Bakoyiannis I, Daskalopoulou A, Pergialiotis V, Perrea D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed Pharmacother. 2019;109:1488-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 13. | Zielińska M, Łuszczki E, Michońska I, Dereń K. The Mediterranean Diet and the Western Diet in Adolescent Depression-Current Reports. Nutrients. 2022;14:4390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 14. | Farhan M, Faisal M. The Potential Role of Polyphenol Supplementation in Preventing and Managing Depression: A Review of Current Research. Life (Basel). 2024;14:1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Annunziata G, Sureda A, Orhan IE, Battino M, Arnone A, Jiménez-García M, Capó X, Cabot J, Sanadgol N, Giampieri F, Tenore GC, Kashani HRK, Silva AS, Habtemariam S, Nabavi SF, Nabavi SM. The neuroprotective effects of polyphenols, their role in innate immunity and the interplay with the microbiota. Neurosci Biobehav Rev. 2021;128:437-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Travica N, Teasdale S, Marx W. Nutraceuticals in mood disorders: current knowledge and future directions. Curr Opin Psychiatry. 2023;36:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Ramos-Lopez O. Personalizing Dietary Polyphenols for Health Maintenance and Disease Management: A Nutrigenetic Approach. Curr Nutr Rep. 2025;14:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 18. | Han Z, Wang L, Zhu H, Tu Y, He P, Li B. Uncovering the effects and mechanisms of tea and its components on depression, anxiety, and sleep disorders: A comprehensive review. Food Res Int. 2024;197:115191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Sekikawa A, Ihara M, Lopez O, Kakuta C, Lopresti B, Higashiyama A, Aizenstein H, Chang YF, Mathis C, Miyamoto Y, Kuller L, Cui C. Effect of S-equol and Soy Isoflavones on Heart and Brain. Curr Cardiol Rev. 2019;15:114-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Chen LR, Chen KH. Utilization of Isoflavones in Soybeans for Women with Menopausal Syndrome: An Overview. Int J Mol Sci. 2021;22:3212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Rehman FU, Batool AI, Qadir R, Aslam M. Hesperidin and naringenin. In: Mushtaq M, Anwar F, editors. A Centum of Valuable Plant Bioactives. Academic Press, 2021: 403-429. [DOI] [Full Text] |
| 22. | Merecz-Sadowska A, Sitarek P, Kowalczyk T, Zajdel K, Jęcek M, Nowak P, Zajdel R. Food Anthocyanins: Malvidin and Its Glycosides as Promising Antioxidant and Anti-Inflammatory Agents with Potential Health Benefits. Nutrients. 2023;15:3016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 23. | Jennings A, Thompson AS, Tresserra-Rimbau A, O'Neill JK, Hill C, Bondonno NP, Kühn T, Cassidy A. Flavonoid-Rich Foods, Dementia Risk, and Interactions With Genetic Risk, Hypertension, and Depression. JAMA Netw Open. 2024;7:e2434136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Khan AU, Dagur HS, Khan M, Malik N, Alam M, Mushtaque M. Therapeutic role of flavonoids and flavones in cancer prevention: Current trends and future perspectives. Eur J Med Chem Rep. 2021;3:100010. [DOI] [Full Text] |
| 25. | Cui Z, Zhao X, Amevor FK, Du X, Wang Y, Li D, Shu G, Tian Y, Zhao X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front Immunol. 2022;13:943321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 204] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 26. | Cordeiro MLDS, Martins VGQA, Silva APD, Rocha HAO, Rachetti VPS, Scortecci KC. Phenolic Acids as Antidepressant Agents. Nutrients. 2022;14:4309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 27. | Dong X, Zhao D. Ferulic acid as a therapeutic agent in depression: Evidence from preclinical studies. CNS Neurosci Ther. 2023;29:2397-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Li Z, Zheng Y, Liu K, Liang Y, Lu J, Li Q, Zhao B, Liu X, Li X. Lignans as multi-targeted natural products in neurodegenerative diseases and depression: Recent perspectives. Phytother Res. 2023;37:5599-5621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Xu YY, Liang J, Xia QR. Novel insights into the pharmacological effects of resveratrol on the management of depression: a short review. Pharmazie. 2017;72:499-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Ozogul Y, Ucar Y, Tadesse EE, Rathod N, Kulawik P, Trif M, Esatbeyoglu T, Ozogul F. Tannins for food preservation and human health: A review of current knowledge. Appl Food Res. 2025;5:100738. [DOI] [Full Text] |
| 31. | Mijailovic N, Selakovic D, Joksimovic J, Mihailovic V, Katanic J, Jakovljevic V, Nikolic T, Bolevich S, Zivkovic V, Pantic M, Rosic G. The anxiolytic effects of atorvastatin and simvastatin on dietary-induced increase in homocysteine levels in rats. Mol Cell Biochem. 2019;452:199-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Poletti S, Mazza MG, Benedetti F. Inflammatory mediators in major depression and bipolar disorder. Transl Psychiatry. 2024;14:247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 33. | Yin Y, Ju T, Zeng D, Duan F, Zhu Y, Liu J, Li Y, Lu W. "Inflamed" depression: A review of the interactions between depression and inflammation and current anti-inflammatory strategies for depression. Pharmacol Res. 2024;207:107322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 34. | Hu Z, Guan Y, Hu W, Xu Z, Ishfaq M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran J Basic Med Sci. 2022;25:14-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 35. | Zhong J, Li G, Xu H, Wang Y, Shi M. Baicalin ameliorates chronic mild stress-induced depression-like behaviors in mice and attenuates inflammatory cytokines and oxidative stress. Braz J Med Biol Res. 2019;52:e8434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Zhang CY, Zeng MJ, Zhou LP, Li YQ, Zhao F, Shang ZY, Deng XY, Ma ZQ, Fu Q, Ma SP, Qu R. Baicalin exerts neuroprotective effects via inhibiting activation of GSK3β/NF-κB/NLRP3 signal pathway in a rat model of depression. Int Immunopharmacol. 2018;64:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Liu L, Dong Y, Shan X, Li L, Xia B, Wang H. Anti-Depressive Effectiveness of Baicalin In Vitro and In Vivo. Molecules. 2019;24:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 38. | Guo LT, Wang SQ, Su J, Xu LX, Ji ZY, Zhang RY, Zhao QW, Ma ZQ, Deng XY, Ma SP. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflammation. 2019;16:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 39. | Yu H, Zhang F, Guan X. Baicalin reverse depressive-like behaviors through regulation SIRT1-NF-kB signaling pathway in olfactory bulbectomized rats. Phytother Res. 2019;33:1480-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules. 2021;26:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 385] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 41. | Abd El-Fattah AA, Fahim AT, Sadik NAH, Ali BM. Resveratrol and dimethyl fumarate ameliorate depression-like behaviour in a rat model of chronic unpredictable mild stress. Brain Res. 2018;1701:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 42. | Liu T, Ma Y, Zhang R, Zhong H, Wang L, Zhao J, Yang L, Fan X. Resveratrol ameliorates estrogen deficiency-induced depression- and anxiety-like behaviors and hippocampal inflammation in mice. Psychopharmacology (Berl). 2019;236:1385-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Yang XH, Song SQ, Xu Y. Resveratrol ameliorates chronic unpredictable mild stress-induced depression-like behavior: involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatr Dis Treat. 2017;13:2727-2736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 44. | Shen J, Qu C, Xu L, Sun H, Zhang J. Resveratrol exerts a protective effect in chronic unpredictable mild stress-induced depressive-like behavior: involvement of the AKT/GSK3β signaling pathway in hippocampus. Psychopharmacology (Berl). 2019;236:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Liu L, Zhang Q, Cai Y, Sun D, He X, Wang L, Yu D, Li X, Xiong X, Xu H, Yang Q, Fan X. Resveratrol counteracts lipopolysaccharide-induced depressive-like behaviors via enhanced hippocampal neurogenesis. Oncotarget. 2016;7:56045-56059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 46. | Ge L, Liu L, Liu H, Liu S, Xue H, Wang X, Yuan L, Wang Z, Liu D. Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol. 2015;768:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Zhao C, Wang F, Lian Y, Xiao H, Zheng J. Biosynthesis of citrus flavonoids and their health effects. Crit Rev Food Sci Nutr. 2020;60:566-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 48. | Xie L, Gu Z, Liu H, Jia B, Wang Y, Cao M, Song R, Zhang Z, Bian Y. The Anti-Depressive Effects of Hesperidin and the Relative Mechanisms Based on the NLRP3 Inflammatory Signaling Pathway. Front Pharmacol. 2020;11:1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 49. | Fu H, Liu L, Tong Y, Li Y, Zhang X, Gao X, Yong J, Zhao J, Xiao D, Wen K, Wang H. The antidepressant effects of hesperidin on chronic unpredictable mild stress-induced mice. Eur J Pharmacol. 2019;853:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 50. | Li M, Shao H, Zhang X, Qin B. Hesperidin Alleviates Lipopolysaccharide-Induced Neuroinflammation in Mice by Promoting the miRNA-132 Pathway. Inflammation. 2016;39:1681-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Kwatra M, Ahmed S, Gawali B, Panda SR, Naidu V. Hesperidin alleviates chronic restraint stress and lipopolysaccharide-induced Hippocampus and Frontal cortex damage in mice: Role of TLR4/NF-κB, p38 MAPK/JNK, Nrf2/ARE signaling. Neurochem Int. 2020;140:104835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 52. | Kosari-Nasab M, Shokouhi G, Ghorbanihaghjo A, Abbasi MM, Salari AA. Hesperidin attenuates depression-related symptoms in mice with mild traumatic brain injury. Life Sci. 2018;213:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 53. | Mantzorou M, Pavlidou E, Vasios G, Tsagalioti E, Giaginis C. Effects of curcumin consumption on human chronic diseases: A narrative review of the most recent clinical data. Phytother Res. 2018;32:957-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 54. | Zhang WY, Guo YJ, Han WX, Yang MQ, Wen LP, Wang KY, Jiang P. Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress. Int Immunopharmacol. 2019;67:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 55. | Fan C, Song Q, Wang P, Li Y, Yang M, Liu B, Yu SY. Curcumin Protects Against Chronic Stress-induced Dysregulation of Neuroplasticity and Depression-like Behaviors via Suppressing IL-1β Pathway in Rats. Neuroscience. 2018;392:92-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 56. | Aghababaei F, Hadidi M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals (Basel). 2023;16:1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 185] [Reference Citation Analysis (0)] |
| 57. | Khan K, Najmi AK, Akhtar M. A Natural Phenolic Compound Quercetin Showed the Usefulness by Targeting Inflammatory, Oxidative Stress Markers and Augment 5-HT Levels in One of the Animal Models of Depression in Mice. Drug Res (Stuttg). 2019;69:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 58. | Mehta V, Parashar A, Udayabanu M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol Behav. 2017;171:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 59. | Guan T, Cao C, Hou Y, Li Y, Wei X, Li S, Jia S, Zhao X. Effects of quercetin on the alterations of serum elements in chronic unpredictable mild stress-induced depressed rats. Biometals. 2021;34:589-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Rinwa P, Kumar A. Quercetin suppress microglial neuroinflammatory response and induce antidepressent-like effect in olfactory bulbectomized rats. Neuroscience. 2013;255:86-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Appleton J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr Med (Encinitas). 2018;17:28-32. [PubMed] |
| 62. | Fakhfouri G, Mijailović NR, Rahimian R. Psychiatric Comorbidities of Inflammatory Bowel Disease: It Is a Matter of Microglia's Gut Feeling. Cells. 2024;13:177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 63. | Borovcanin MM, Vesic K, Petrovic I, Jovanovic IP, Mijailović NR. Diabetes mellitus type 2 as an underlying, comorbid or consequent state of mental disorders. World J Diabetes. 2023;14:481-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Andrioaie IM, Duhaniuc A, Nastase EV, Iancu LS, Luncă C, Trofin F, Anton-Păduraru DT, Dorneanu OS. The Role of the Gut Microbiome in Psychiatric Disorders. Microorganisms. 2022;10:2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 65. | Corrêa TAF, Rogero MM, Hassimotto NMA, Lajolo FM. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front Nutr. 2019;6:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 66. | Wang X, Yu J, Zhang X. Dietary Polyphenols as Prospective Natural-Compound Depression Treatment from the Perspective of Intestinal Microbiota Regulation. Molecules. 2022;27:7637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 67. | Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S-242S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2913] [Cited by in RCA: 2727] [Article Influence: 136.4] [Reference Citation Analysis (0)] |
| 68. | Braune A, Blaut M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes. 2016;7:216-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 319] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 69. | Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013;24:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1047] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 70. | Annunziata G, Maisto M, Schisano C, Ciampaglia R, Daliu P, Narciso V, Tenore GC, Novellino E. Colon Bioaccessibility and Antioxidant Activity of White, Green and Black Tea Polyphenols Extract after In Vitro Simulated Gastrointestinal Digestion. Nutrients. 2018;10:1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 71. | Filosa S, Di Meo F, Crispi S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen Res. 2018;13:2055-2059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 72. | Ozdal T, Sela DA, Xiao J, Boyacioglu D, Chen F, Capanoglu E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients. 2016;8:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 606] [Cited by in RCA: 527] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 73. | Wang X, Qi Y, Zheng H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants (Basel). 2022;11:1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 144] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 74. | Dueñas M, Muñoz-González I, Cueva C, Jiménez-Girón A, Sánchez-Patán F, Santos-Buelga C, Moreno-Arribas MV, Bartolomé B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int. 2015;2015:850902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 75. | Smith KS, Greene MW, Babu JR, Frugé AD. Psychobiotics as treatment for anxiety, depression, and related symptoms: a systematic review. Nutr Neurosci. 2021;24:963-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 76. | Sun H, Chen Y, Cheng M, Zhang X, Zheng X, Zhang Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J Food Sci Technol. 2018;55:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 77. | Guo T, Song D, Ho CT, Zhang X, Zhang C, Cao J, Wu Z. Omics Analyses of Gut Microbiota in a Circadian Rhythm Disorder Mouse Model Fed with Oolong Tea Polyphenols. J Agric Food Chem. 2019;67:8847-8854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 78. | Ortega MA, Fraile-Martínez Ó, García-Montero C, Alvarez-Mon MA, Lahera G, Monserrat J, Llavero-Valero M, Gutiérrez-Rojas L, Molina R, Rodríguez-Jimenez R, Quintero J, De Mon MA. Biological Role of Nutrients, Food and Dietary Patterns in the Prevention and Clinical Management of Major Depressive Disorder. Nutrients. 2022;14:3099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 79. | Song J, Zhou N, Ma W, Gu X, Chen B, Zeng Y, Yang L, Zhou M. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019;10:2947-2957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 80. | Han SK, Joo MK, Kim JK, Jeung W, Kang H, Kim DH. Bifidobacteria-Fermented Red Ginseng and Its Constituents Ginsenoside Rd and Protopanaxatriol Alleviate Anxiety/Depression in Mice by the Amelioration of Gut Dysbiosis. Nutrients. 2020;12:901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 81. | Wang L, Wu X, Ma Y, Li X, Zhang J, Zhao L. Supplementation with soy isoflavones alleviates depression-like behaviour via reshaping the gut microbiota structure. Food Funct. 2021;12:4995-5006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Hao WZ, Ma QY, Tao G, Huang JQ, Chen JX. Oral coniferyl ferulate attenuated depression symptoms in mice via reshaping gut microbiota and microbial metabolism. Food Funct. 2021;12:12550-12564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 83. | Bian Y, Lei J, Zhong J, Wang B, Wan Y, Li J, Liao C, He Y, Liu Z, Ito K, Zhang B. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J Nutr Biochem. 2022;99:108840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 84. | Ma H, Johnson SL, Liu W, DaSilva NA, Meschwitz S, Dain JA, Seeram NP. Evaluation of Polyphenol Anthocyanin-Enriched Extracts of Blackberry, Black Raspberry, Blueberry, Cranberry, Red Raspberry, and Strawberry for Free Radical Scavenging, Reactive Carbonyl Species Trapping, Anti-Glycation, Anti-β-Amyloid Aggregation, and Microglial Neuroprotective Effects. Int J Mol Sci. 2018;19:461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 85. | Tang MM, Lin WJ, Pan YQ, Guan XT, Li YC. Hippocampal neurogenesis dysfunction linked to depressive-like behaviors in a neuroinflammation induced model of depression. Physiol Behav. 2016;161:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 86. | Porter GA, O'Connor JC. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J Psychiatry. 2022;12:77-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 105] [Article Influence: 35.0] [Reference Citation Analysis (3)] |
| 87. | Carniel BP, da Rocha NS. Brain-derived neurotrophic factor (BDNF) and inflammatory markers: Perspectives for the management of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2021;108:110151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 88. | Jin Y, Sun LH, Yang W, Cui RJ, Xu SB. The Role of BDNF in the Neuroimmune Axis Regulation of Mood Disorders. Front Neurol. 2019;10:515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 89. | Xiong Z, Jiang B, Wu PF, Tian J, Shi LL, Gu J, Hu ZL, Fu H, Wang F, Chen JG. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol Pharm Bull. 2011;34:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 90. | Xiao Z, Cao Z, Yang J, Jia Z, Du Y, Sun G, Lu Y, Pei L. Baicalin promotes hippocampal neurogenesis via the Wnt/β-catenin pathway in a chronic unpredictable mild stress-induced mouse model of depression. Biochem Pharmacol. 2021;190:114594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 91. | Xu Y, Ku B, Cui L, Li X, Barish PA, Foster TC, Ogle WO. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007;1162:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 92. | Pyrzynska K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients. 2022;14:2387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 120] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 93. | Antunes MS, Jesse CR, Ruff JR, de Oliveira Espinosa D, Gomes NS, Altvater EET, Donato F, Giacomeli R, Boeira SP. Hesperidin reverses cognitive and depressive disturbances induced by olfactory bulbectomy in mice by modulating hippocampal neurotrophins and cytokine levels and acetylcholinesterase activity. Eur J Pharmacol. 2016;789:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 94. | Antunes MS, Cattelan Souza L, Ladd FVL, Ladd AABL, Moreira AL, Bortolotto VC, Silva MRP, Araújo SM, Prigol M, Nogueira CW, Boeira SP. Hesperidin Ameliorates Anxiety-Depressive-Like Behavior in 6-OHDA Model of Parkinson's Disease by Regulating Striatal Cytokine and Neurotrophic Factors Levels and Dopaminergic Innervation Loss in the Striatum of Mice. Mol Neurobiol. 2020;57:3027-3041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 95. | Sadighparvar S, Darband SG, Yousefi B, Kaviani M, Ghaderi-Pakdel F, Mihanfar A, Babaei G, Mobaraki K, Majidinia M. Combination of quercetin and exercise training attenuates depression in rats with 1,2-dimethylhydrazine-induced colorectal cancer: Possible involvement of inflammation and BDNF signalling. Exp Physiol. 2020;105:1598-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 96. | Jiang C, Sakakibara E, Lin WJ, Wang J, Pasinetti GM, Salton SR. Grape-derived polyphenols produce antidepressant effects via VGF- and BDNF-dependent mechanisms. Ann N Y Acad Sci. 2019;1455:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Filho CB, Jesse CR, Donato F, Del Fabbro L, Gomes de Gomes M, Rossito Goes AT, Souza LC, Boeira SP. Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem Biol Interact. 2016;260:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 98. | Zhang J, Xie X, Tang M, Zhang J, Zhang B, Zhao Q, Han Y, Yan W, Peng C, You Z. Salvianolic acid B promotes microglial M2-polarization and rescues neurogenesis in stress-exposed mice. Brain Behav Immun. 2017;66:111-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 99. | Correia AS, Cardoso A, Vale N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants (Basel). 2023;12:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 135] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 100. | Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, Alshabrmi FM, Palai S, Deb PK, Devi R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front Pharmacol. 2022;13:806470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 363] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 101. | Gamage E, Orr R, Travica N, Lane MM, Dissanayaka T, Kim JH, Grosso G, Godos J, Marx W. Polyphenols as novel interventions for depression: Exploring the efficacy, mechanisms of action, and implications for future research. Neurosci Biobehav Rev. 2023;151:105225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 102. | Nedić Vasiljević B, Takić M, Mijailović NR, Janošević Ležaić A, Jevremović A, Uskoković-Marković S, Milojević-Rakić M, Bajuk-Bogdanović D. Phenolics over Zeolites and Related Materials-Biomedical and Environmental Applications. Antioxidants (Basel). 2024;13:1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 103. | Winiarska-Mieczan A, Baranowska-Wójcik E, Kwiecień M, Grela ER, Szwajgier D, Kwiatkowska K, Kiczorowska B. The Role of Dietary Antioxidants in the Pathogenesis of Neurodegenerative Diseases and Their Impact on Cerebral Oxidoreductive Balance. Nutrients. 2020;12:435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 104. | Arora V, Kuhad A, Tiwari V, Chopra K. Curcumin ameliorates reserpine-induced pain-depression dyad: behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology. 2011;36:1570-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 105. | Naqvi F, Saleem S, Naqvi F, Batool Z, Sadir S, Tabassum S, Ahmed S, Liaquat L, Haider S. Curcumin lessens unpredictable chronic mild stress-induced depression and memory deficits by modulating oxidative stress and cholinergic activity. Pak J Pharm Sci. 2019;32:1893-1900. [PubMed] |
| 106. | Xu Y, Zhang L, Shao T, Ruan L, Wang L, Sun J, Li J, Zhu X, O'Donnell JM, Pan J. Ferulic acid increases pain threshold and ameliorates depression-like behaviors in reserpine-treated mice: behavioral and neurobiological analyses. Metab Brain Dis. 2013;28:571-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 107. | Huang Q, Ye X, Wang L, Pan J. Salvianolic acid B abolished chronic mild stress-induced depression through suppressing oxidative stress and neuro-inflammation via regulating NLRP3 inflammasome activation. J Food Biochem. 2019;43:e12742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Liao D, Chen Y, Guo Y, Wang C, Liu N, Gong Q, Fu Y, Fu Y, Cao L, Yao D, Jiang P. Salvianolic Acid B Improves Chronic Mild Stress-Induced Depressive Behaviors in Rats: Involvement of AMPK/SIRT1 Signaling Pathway. J Inflamm Res. 2020;13:195-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 109. | Saied NM, Georgy GS, Hussien RM, Hassan WA. Neuromodulatory effect of curcumin on catecholamine systems and inflammatory cytokines in ovariectomized female rats. Clin Exp Pharmacol Physiol. 2021;48:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 110. | Filho CB, Jesse CR, Donato F, Del Fabbro L, de Gomes MG, Goes ATR, Souza LC, Giacomeli R, Antunes M, Luchese C, Roman SS, Boeira SP. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur J Pharmacol. 2016;791:284-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 111. | Lu X, Ce Q, Jin L, Zheng J, Sun M, Tang X, Li D, Sun J. Deoiled sunflower seeds ameliorate depression by promoting the production of monoamine neurotransmitters and inhibiting oxidative stress. Food Funct. 2021;12:573-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 112. | Li G, Yang J, Wang X, Zhou C, Zheng X, Lin W. Effects of EGCG on depression-related behavior and serotonin concentration in a rat model of chronic unpredictable mild stress. Food Funct. 2020;11:8780-8787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 113. | Zhang L, Yang Z, Ren J, Fan G, Pan S. Dietary essential oil from navel orange alleviates depression in reserpine‐treated mice by monoamine neurotransmitters. Flavour Frag J. 2019;34:252-259. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 114. | Yi LT, Xu HL, Feng J, Zhan X, Zhou LP, Cui CC. Involvement of monoaminergic systems in the antidepressant-like effect of nobiletin. Physiol Behav. 2011;102:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 115. | Nouri Z, Fakhri S, El-Senduny FF, Sanadgol N, Abd-ElGhani GE, Farzaei MH, Chen JT. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules. 2019;9:690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 116. | Yi LT, Li JM, Li YC, Pan Y, Xu Q, Kong LD. Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin. Life Sci. 2008;82:741-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 117. | Liu X, Liu C. Baicalin ameliorates chronic unpredictable mild stress-induced depressive behavior: Involving the inhibition of NLRP3 inflammasome activation in rat prefrontal cortex. Int Immunopharmacol. 2017;48:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 118. | Fu X, Jiao J, Qin T, Yu J, Fu Q, Deng X, Ma S, Ma Z. A New Perspective on Ameliorating Depression-Like Behaviors: Suppressing Neuroinflammation by Upregulating PGC-1α. Neurotox Res. 2021;39:872-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 119. | Fan C, Song Q, Wang P, Li Y, Yang M, Yu SY. Neuroprotective Effects of Curcumin on IL-1β-Induced Neuronal Apoptosis and Depression-Like Behaviors Caused by Chronic Stress in Rats. Front Cell Neurosci. 2018;12:516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 120. | Jiang H, Wang Z, Wang Y, Xie K, Zhang Q, Luan Q, Chen W, Liu D. Antidepressant-like effects of curcumin in chronic mild stress of rats: involvement of its anti-inflammatory action. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 121. | Wang Z, Zhang Q, Yuan L, Wang S, Liu L, Yang X, Li G, Liu D. The effects of curcumin on depressive-like behavior in mice after lipopolysaccharide administration. Behav Brain Res. 2014;274:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 122. | Finnell JE, Lombard CM, Melson MN, Singh NP, Nagarkatti M, Nagarkatti P, Fadel JR, Wood CS, Wood SK. The protective effects of resveratrol on social stress-induced cytokine release and depressive-like behavior. Brain Behav Immun. 2017;59:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 123. | Martínez-Damas MG, Genis-Mendoza AD, Pérez-de la Cruz V, Canela-Tellez GD, Jiménez-Estrada I, Nicolini-Sanchez JH, Ramos-Chávez LA, García S, Ramírez-Ramírez M, Coral-Vázquez RM. Epicatechin treatment generates resilience to chronic mild stress-induced depression in a murine model. Physiol Behav. 2021;238:113466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 124. | Sharma P, Sharma S, Singh D. Apigenin reverses behavioural impairments and cognitive decline in kindled mice via CREB-BDNF upregulation in the hippocampus. Nutr Neurosci. 2020;23:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 125. | Bijani S, Dizaji R, Sharafi A, Hosseini MJ. Neuroprotective Effect of Apigenin on Depressive-Like Behavior: Mechanistic Approach. Neurochem Res. 2022;47:644-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 126. | Lim DW, Han T, Jung J, Song Y, Um MY, Yoon M, Kim YT, Cho S, Kim IH, Han D, Lee C, Lee J. Chlorogenic Acid from Hawthorn Berry (Crataegus pinnatifida Fruit) Prevents Stress Hormone-Induced Depressive Behavior, through Monoamine Oxidase B-Reactive Oxygen Species Signaling in Hippocampal Astrocytes of Mice. Mol Nutr Food Res. 2018;62:e1800029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 127. | Huang X, Li W, You B, Tang W, Gan T, Feng C, Li C, Yang R. Serum Metabonomic Study on the Antidepressant-like Effects of Ellagic Acid in a Chronic Unpredictable Mild Stress-Induced Mouse Model. J Agric Food Chem. 2020;68:9546-9556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 128. | Zeni ALB, Camargo A, Dalmagro AP. Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids. 2017;125:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 129. | Zheng X, Cheng Y, Chen Y, Yue Y, Li Y, Xia S, Li Y, Deng H, Zhang J, Cao Y. Ferulic Acid Improves Depressive-Like Behavior in Prenatally-Stressed Offspring Rats via Anti-Inflammatory Activity and HPA Axis. Int J Mol Sci. 2019;20:493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 130. | Can ÖD, Turan N, Demir Özkay Ü, Öztürk Y. Antidepressant-like effect of gallic acid in mice: Dual involvement of serotonergic and catecholaminergic systems. Life Sci. 2017;190:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 131. | Lataliza AAB, de Assis PM, da Rocha Laurindo L, Gonçalves ECD, Raposo NRB, Dutra RC. Antidepressant-like effect of rosmarinic acid during LPS-induced neuroinflammatory model: The potential role of cannabinoid receptors/PPAR-γ signaling pathway. Phytother Res. 2021;35:6974-6989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 132. | Wang J, Wang S, Guo H, Li Y, Jiang Z, Gu T, Su B, Hou W, Zhong H, Cheng D, Zhang X, Fang Z. Rosmarinic acid protects rats against post-stroke depression after transient focal cerebral ischemic injury through enhancing antioxidant response. Brain Res. 2021;1757:147336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 133. | Dalmagro AP, Camargo A, Zeni ALB. Morus nigra and its major phenolic, syringic acid, have antidepressant-like and neuroprotective effects in mice. Metab Brain Dis. 2017;32:1963-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 134. | Dalmagro AP, Camargo A, Severo Rodrigues AL, Zeni ALB. Involvement of PI3K/Akt/GSK-3β signaling pathway in the antidepressant-like and neuroprotective effects of Morus nigra and its major phenolic, syringic acid. Chem Biol Interact. 2019;314:108843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 135. | Godos J, Castellano S, Ray S, Grosso G, Galvano F. Dietary Polyphenol Intake and Depression: Results from the Mediterranean Healthy Eating, Lifestyle and Aging (MEAL) Study. Molecules. 2018;23:999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 136. | Lin K, Li Y, Toit ED, Wendt L, Sun J. Effects of Polyphenol Supplementations on Improving Depression, Anxiety, and Quality of Life in Patients With Depression. Front Psychiatry. 2021;12:765485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 137. | Abdoli E, Rezaie E, Mirghafourvand M, Payahoo L, Naseri E, Ghanbari-Homaie S. A clinical trial of the effects of cocoa rich chocolate on depression and sleep quality in menopausal women. Sci Rep. 2024;14:23971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 138. | Behzadi M, Bideshki MV, Ahmadi-Khorram M, Zarezadeh M, Hatami A. Effect of dark chocolate/ cocoa consumption on oxidative stress and inflammation in adults: A GRADE-assessed systematic review and dose-response meta-analysis of controlled trials. Complement Ther Med. 2024;84:103061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 139. | Terauchi M, Horiguchi N, Kajiyama A, Akiyoshi M, Owa Y, Kato K, Kubota T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: a randomized, double-blind, placebo-controlled pilot study. Menopause. 2014;21:990-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 140. | Bourdel-Marchasson I, Ostan R, Regueme SC, Pinto A, Pryen F, Charrouf Z, d'Alessio P, Baudron CR, Guerville F, Durrieu J, Donini LM, Franceschi C, Valentini L. Quality of Life: Psychological Symptoms-Effects of a 2-Month Healthy Diet and Nutraceutical Intervention; A Randomized, Open-Label Intervention Trial (RISTOMED). Nutrients. 2020;12:800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 141. | Pribis P. Effects of Walnut Consumption on Mood in Young Adults-A Randomized Controlled Trial. Nutrients. 2016;8:668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 142. | Kanchanatawan B, Tangwongchai S, Sughondhabhirom A, Suppapitiporn S, Hemrunrojn S, Carvalho AF, Maes M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox Res. 2018;33:621-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 143. | Almudhi A, Gabr SA. Green tea consumption and the management of adrenal stress hormones in adolescents who stutter. Biomed Rep. 2022;16:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 144. | Amsterdam JD, Li QS, Xie SX, Mao JJ. Putative Antidepressant Effect of Chamomile (Matricaria chamomilla L.) Oral Extract in Subjects with Comorbid Generalized Anxiety Disorder and Depression. J Altern Complement Med. 2020;26:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 145. | Barfoot KL, Forster R, Lamport DJ. Mental Health in New Mothers: A Randomised Controlled Study into the Effects of Dietary Flavonoids on Mood and Perceived Quality of Life. Nutrients. 2021;13:2383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 146. | Park M, Choi J, Lee HJ. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients. 2020;12:1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 147. | Soltani M, Hosseinzadeh-Attar MJ, Rezaei M, Alipoor E, Vasheghani-Farahani A, Yaseri M, Rezayat SM. Effect of nano-curcumin supplementation on cardiometabolic risk factors, physical and psychological quality of life, and depression in patients with coronary slow flow phenomenon: a randomized double-blind clinical trial. Trials. 2024;25:515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 148. | Fisk J, Khalid S, Reynolds SA, Williams CM. Effect of 4 weeks daily wild blueberry supplementation on symptoms of depression in adolescents. Br J Nutr. 2020;124:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 149. | Manshadi Seyed Ali D, Seyed Alireza M, Mohammad Reza S, Jayran Z, SeyedAhmad S, Shams Ali R, Seyed Saeid M, Ali AA. Effect of green tea consumption in treatment of mild to moderate depression in Iranian patients living with HIV: A double-blind randomized clinical trial. Chin Herb Med. 2021;13:136-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 150. | Kontogianni MD, Vijayakumar A, Rooney C, Noad RL, Appleton KM, McCarthy D, Donnelly M, Young IS, McKinley MC, McKeown PP, Woodside JV. A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients. 2020;12:2445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 151. | Platero JL, Cuerda-Ballester M, Sancho-Cantus D, Benlloch M, Ceron JJ, Peres Rubio C, García-Pardo MP, López-Rodríguez MM, de la Rubia Ortí JE. The Impact of Epigallocatechin Gallate and Coconut Oil Treatment on Cortisol Activity and Depression in Multiple Sclerosis Patients. Life (Basel). 2021;11:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 152. | Hajiluian G, Karegar SJ, Shidfar F, Aryaeian N, Salehi M, Lotfi T, Farhangnia P, Heshmati J, Delbandi AA. The effects of Ellagic acid supplementation on neurotrophic, inflammation, and oxidative stress factors, and indoleamine 2, 3-dioxygenase gene expression in multiple sclerosis patients with mild to moderate depressive symptoms: A randomized, triple-blind, placebo-controlled trial. Phytomedicine. 2023;121:155094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 153. | Parilli-Moser I, Domínguez-López I, Trius-Soler M, Castellví M, Bosch B, Castro-Barquero S, Estruch R, Hurtado-Barroso S, Lamuela-Raventós RM. Consumption of peanut products improves memory and stress response in healthy adults from the ARISTOTLE study: A 6-month randomized controlled trial. Clin Nutr. 2021;40:5556-5567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 154. | Fusar-Poli L, Vozza L, Gabbiadini A, Vanella A, Concas I, Tinacci S, Petralia A, Signorelli MS, Aguglia E. Curcumin for depression: a meta-analysis. Crit Rev Food Sci Nutr. 2020;60:2643-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 155. | Fusar-Poli L, Gabbiadini A, Ciancio A, Vozza L, Signorelli MS, Aguglia E. The effect of cocoa-rich products on depression, anxiety, and mood: A systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;62:7905-7916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 156. | Marx W, Kelly JT, Marshall S, Cutajar J, Annois B, Pipingas A, Tierney A, Itsiopoulos C. Effect of resveratrol supplementation on cognitive performance and mood in adults: a systematic literature review and meta-analysis of randomized controlled trials. Nutr Rev. 2018;76:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 157. | Cabrera-Suárez BM, Lahortiga-Ramos F, Sayon-Orea C, Hernández-Fleta JL, González-Pinto A, Molero P, Vega-Pérez R, Sánchez-Villegas A; PREDI-DEP investigators. Effect of a dietary intervention based on the Mediterranean diet on the quality of life of patients recovered from depression: Analysis of the PREDIDEP randomized trial. Exp Gerontol. 2023;175:112149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 158. | Hu FB. Diet strategies for promoting healthy aging and longevity: An epidemiological perspective. J Intern Med. 2024;295:508-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 159. | Bayes J, Schloss J, Sibbritt D. Effects of Polyphenols in a Mediterranean Diet on Symptoms of Depression: A Systematic Literature Review. Adv Nutr. 2020;11:602-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 160. | Kiani AK, Medori MC, Bonetti G, Aquilanti B, Velluti V, Matera G, Iaconelli A, Stuppia L, Connelly ST, Herbst KL, Bertelli M. Modern vision of the Mediterranean diet. J Prev Med Hyg. 2022;63:E36-E43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 161. | Kushkestani M, Moghadassi M, Sidossis L. Mediterranean Lifestyle: More Than a Diet, A Way of Living (and Thriving). Endocr Metab Immune Disord Drug Targets. 2024;24:1785-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 162. | Sofi F, Martini D, Angelino D, Cairella G, Campanozzi A, Danesi F, Dinu M, Erba D, Iacoviello L, Pellegrini N, Rossi L, Vaccaro S, Tagliabue A, Strazzullo P. Mediterranean diet: Why a new pyramid? An updated representation of the traditional Mediterranean diet by the Italian Society of Human Nutrition (SINU). Nutr Metab Cardiovasc Dis. 2025;35:103919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 163. | Pagidipati NJ, Taub PR, Ostfeld RJ, Kirkpatrick CF. Dietary patterns to promote cardiometabolic health. Nat Rev Cardiol. 2025;22:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 164. | Menichetti F, Battezzati A, Bertoli S, De Amicis R, Foppiani A, Sileo F, Leone A. Adherence to the Mediterranean diet and risk of anxiety and depression in people with obesity: a cross-sectional analysis. Eur J Clin Nutr. 2025;79:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 165. | Bizzozero-Peroni B, Martínez-Vizcaíno V, Fernández-Rodríguez R, Jiménez-López E, Núñez de Arenas-Arroyo S, Saz-Lara A, Díaz-Goñi V, Mesas AE. The impact of the Mediterranean diet on alleviating depressive symptoms in adults: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2025;83:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 166. | Ünal G, Özenoğlu A. Association of Mediterranean diet with sleep quality, depression, anxiety, stress, and body mass index in university students: A cross-sectional study. Nutr Health. 2025;31:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 167. | Camprodon-Boadas P, Gil-Dominguez A, De la Serna E, Sugranyes G, Lázaro I, Baeza I. Mediterranean Diet and Mental Health in Children and Adolescents: A Systematic Review. Nutr Rev. 2025;83:e343-e355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 168. | Kurowska A, Ziemichód W, Herbet M, Piątkowska-Chmiel I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients. 2023;15:1436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 169. | Ross FC, Mayer DE, Horn J, Cryan JF, Del Rio D, Randolph E, Gill CIR, Gupta A, Ross RP, Stanton C, Mayer EA. Potential of dietary polyphenols for protection from age-related decline and neurodegeneration: a role for gut microbiota? Nutr Neurosci. 2024;27:1058-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 170. | Ventriglio A, Sancassiani F, Contu MP, Latorre M, Di Salvatore M, Fornaro M, Bhugra D. Mediterranean Diet and its Benefits on Health and Mental Health: A Literature Review. Clin Pract Epidemiol Ment Health. 2020;16:156-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 171. | Solch RJ, Aigbogun JO, Voyiadjis AG, Talkington GM, Darensbourg RM, O'Connell S, Pickett KM, Perez SR, Maraganore DM. Mediterranean diet adherence, gut microbiota, and Alzheimer's or Parkinson's disease risk: A systematic review. J Neurol Sci. 2022;434:120166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 172. | Ahmad S, Moorthy MV, Lee IM, Ridker PM, Manson JE, Buring JE, Demler OV, Mora S. Mediterranean Diet Adherence and Risk of All-Cause Mortality in Women. JAMA Netw Open. 2024;7:e2414322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 173. | Molendijk M, Molero P, Ortuño Sánchez-Pedreño F, Van der Does W, Angel Martínez-González M. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J Affect Disord. 2018;226:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 369] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 174. | Shafiei F, Salari-Moghaddam A, Larijani B, Esmaillzadeh A. Adherence to the Mediterranean diet and risk of depression: a systematic review and updated meta-analysis of observational studies. Nutr Rev. 2019;77:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 175. | Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani A, Itsiopoulos C, Niyonsenga T, Blunden S, Meyer B, Segal L, Baune BT, O'Dea K. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr Neurosci. 2019;22:474-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 176. | Jain R, Larsuphrom P, Degremont A, Latunde-Dada GO, Philippou E. Association between vegetarian and vegan diets and depression: A systematic review. Nutr Bull. 2022;47:27-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 177. | Vega-Cabello V, Al Hinai M, Yévenes-Briones H, Caballero FF, Lopez-García E, Baylin A. Plant-Based Diets and Risk of Multimorbidity: The Health and Retirement Study. J Nutr. 2024;154:2264-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 178. | Wu H, Gu Y, Meng G, Wu H, Zhang S, Wang X, Zhang J, Huang T, Niu K. Quality of plant-based diet and the risk of dementia and depression among middle-aged and older population. Age Ageing. 2023;52:afad070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 179. | Carcelén-Fraile MDC, Déniz-Ramírez NDP, Sabina-Campos J, Aibar-Almazán A, Rivas-Campo Y, González-Martín AM, Castellote-Caballero Y. Exercise and Nutrition in the Mental Health of the Older Adult Population: A Randomized Controlled Clinical Trial. Nutrients. 2024;16:1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 180. | Cabrera-Suárez B, Pla J, González-Pinto A, Hernández J, Chiclana-Actis C, Ortuño F, Florido-Rodriguez M, Sanchez-Villegas A; Investigators PD. 'Effectiveness of a remote nutritional intervention to increase the adherence to the Mediterranean diet among recovered depression patients'. Nutr Neurosci. 2023;26:696-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 181. | Lee MF, Eather R, Best T. Plant-based dietary quality and depressive symptoms in Australian vegans and vegetarians: a cross-sectional study. BMJ Nutr Prev Health. 2021;4:479-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 182. | Ma X, Li Y, Xu Y, Gibson R, Williams C, Lawrence AJ, Nosarti C, Dazzan P, Rodriguez-Mateos A. Plant-based dietary patterns and their association with mood in healthy individuals. Food Funct. 2023;14:2326-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 183. | Meng W, Chao W, Kaiwei Z, Sijia M, Jiajia S, Shijie X. Bioactive compounds from Chinese herbal plants for neurological health: mechanisms, pathways, and functional food applications. Front Nutr. 2025;12:1537363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 184. | Businaro R, Corsi M, Asprino R, Di Lorenzo C, Laskin D, Corbo RM, Ricci S, Pinto A. Modulation of Inflammation as a Way of Delaying Alzheimer's Disease Progression: The Diet's Role. Curr Alzheimer Res. 2018;15:363-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 185. | Li G, Ruan L, Chen R, Wang R, Xie X, Zhang M, Chen L, Yan Q, Reed M, Chen J, Xu Y, Pan J, Huang W. Synergistic antidepressant-like effect of ferulic acid in combination with piperine: involvement of monoaminergic system. Metab Brain Dis. 2015;30:1505-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 186. | Awika JM, Rose DJ, Simsek S. Complementary effects of cereal and pulse polyphenols and dietary fiber on chronic inflammation and gut health. Food Funct. 2018;9:1389-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 187. | Wei X, Wang J, Wang Y, Zhao Y, Long Y, Tan B, Li QX, Dong Z, Wan X. Dietary fiber and polyphenols from whole grains: effects on the gut and health improvements. Food Funct. 2024;15:4682-4702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 188. | Vetvicka V, Vetvickova J. Anti-stress action of an orally-given combination of resveratrol, β-glucan, and vitamin C. Molecules. 2014;19:13724-13734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 189. | Schwager J, Richard N, Riegger C, Salem N Jr. ω-3 PUFAs and Resveratrol Differently Modulate Acute and Chronic Inflammatory Processes. Biomed Res Int. 2015;2015:535189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 190. | Moran C, Scotto di Palumbo A, Bramham J, Moran A, Rooney B, De Vito G, Egan B. Effects of a Six-Month Multi-Ingredient Nutrition Supplement Intervention of Omega-3 Polyunsaturated Fatty Acids, vitamin D, Resveratrol, and Whey Protein on Cognitive Function in Older Adults: A Randomised, Double-Blind, Controlled Trial. J Prev Alzheimers Dis. 2018;5:175-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 191. | Lucassen DA, Lasschuijt MP, Camps G, Van Loo EJ, Fischer ARH, de Vries RAJ, Haarman JAM, Simons M, de Vet E, Bos-de Vos M, Pan S, Ren X, de Graaf K, Lu Y, Feskens EJM, Brouwer-Brolsma EM. Short and Long-Term Innovations on Dietary Behavior Assessment and Coaching: Present Efforts and Vision of the Pride and Prejudice Consortium. Int J Environ Res Public Health. 2021;18:7877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 192. | Duda-Chodak A, Tarko T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules. 2023;28:2536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 94] [Reference Citation Analysis (0)] |
| 193. | Huang TY, Yu CP, Hsieh YW, Lin SP, Hou YC. Resveratrol stereoselectively affected (±)warfarin pharmacokinetics and enhanced the anticoagulation effect. Sci Rep. 2020;10:15910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 194. | Yu CP, Yang MS, Hsu PW, Lin SP, Hou YC. Bidirectional Influences of Cranberry on the Pharmacokinetics and Pharmacodynamics of Warfarin with Mechanism Elucidation. Nutrients. 2021;13:3219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 195. | Shi H, Zhao F, Chen H, Zhou Q, Geng P, Zhou Y, Wu H, Chong J, Wang F, Dai D, Yang J, Wang S. Naringenin has an inhibitory effect on rivaroxaban in rats both in vitro and in vivo. Pharmacol Res Perspec. 2021;9:e00782. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 196. | Kim TE, Shin KH, Park JE, Kim MG, Yun YM, Choi DH, Kwon KJ, Lee J. Effect of green tea catechins on the pharmacokinetics of digoxin in humans. Drug Des Devel Ther. 2018;12:2139-2147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 197. | Misaka S, Abe O, Ono T, Ono Y, Ogata H, Miura I, Shikama Y, Fromm MF, Yabe H, Shimomura K. Effects of single green tea ingestion on pharmacokinetics of nadolol in healthy volunteers. Br J Clin Pharmacol. 2020;86:2314-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 198. | Misaka S, Ono Y, Uchida A, Ono T, Abe O, Ogata H, Sato H, Suzuki M, Onoue S, Shikama Y, Shimomura K. Impact of Green Tea Catechin Ingestion on the Pharmacokinetics of Lisinopril in Healthy Volunteers. Clin Transl Sci. 2021;14:476-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 199. | Zeng W, Hu M, Lee HK, Wat E, Lau CBS, Ho CS, Wong CK, Tomlinson B. Effects of Soy Isoflavones and Green Tea Extract on Simvastatin Pharmacokinetics and Influence of the SLCO1B1 521T > C Polymorphism. Front Nutr. 2022;9:868126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 200. | Kim TE, Ha N, Kim Y, Kim H, Lee JW, Jeon JY, Kim MG. Effect of epigallocatechin-3-gallate, major ingredient of green tea, on the pharmacokinetics of rosuvastatin in healthy volunteers. Drug Des Devel Ther. 2017;11:1409-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 201. | Huang S, Xu Q, Liu L, Bian Y, Zhang S, Huang C, Miao L. Effect of Green Tea and (-)-Epigallocatechin Gallate on the Pharmacokinetics of Rosuvastatin. Curr Drug Metab. 2020;21:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 202. | Knop J, Misaka S, Singer K, Hoier E, Müller F, Glaeser H, König J, Fromm MF. Inhibitory Effects of Green Tea and (-)-Epigallocatechin Gallate on Transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-Glycoprotein. PLoS One. 2015;10:e0139370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 203. | Feng Y, Jin C, Lv S, Zhang H, Ren F, Wang J. Molecular Mechanisms and Applications of Polyphenol-Protein Complexes with Antioxidant Properties: A Review. Antioxidants (Basel). 2023;12:1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |