Published online Aug 19, 2025. doi: 10.5498/wjp.v15.i8.106125
Revised: April 12, 2025
Accepted: June 6, 2025
Published online: August 19, 2025
Processing time: 173 Days and 0.4 Hours
Bipolar disorder (BD) is a severe mood disorder characterized by recurrent episodes of mania and depression, and it is prone to delayed diagnosis, which can lead to worsened outcomes, including more frequent mood episodes, greater functional impairment, and comorbidities. Early diagnosis of BD remains a significant challenge, although recent advances offer promising insights, such as research in molecular biomarkers, neuroimaging, exosomes, genetics, and epigenetics. This mini-review highlights their potential for providing earlier, more accurate identification of BD and discusses the underlying reasons why current research has not yet succeeded. For instance, the high heterogeneity of sym
Core Tip: Bipolar disorder (BD) is a severe mood disorder with delayed diagnosis, leading to worsened outcomes such as frequent mood episodes and comorbidities. Early diagnosis remains challenging, despite advances in biomarkers, neuroi
- Citation: Cui P, Han DY, Yang CH. Early diagnosis of bipolar disorder. World J Psychiatry 2025; 15(8): 106125
- URL: https://www.wjgnet.com/2220-3206/full/v15/i8/106125.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i8.106125
Bipolar disorder (BD) is a multifaceted psychiatric condition characterized by alternating episodes of depression and mania/hypomania, with a global incidence rate of 2%[1]. The first onset of BD most commonly occurs around the age of 15 years, with the median age of onset being ~20 years[2]; both of which are crucial stages for individual learning and development. The chronic course of BD and its high comorbidity rate are linked to increased morbidity and mortality, making it one of the leading causes of disability among young and working-age individuals[3]. Early diagnosis and optimal intervention are critical, as the response to treatment is more effective in the early stages[4]. However, the average delay in clinical diagnosis of BD is 9 years[5]. Approximately 50% of BD patients initially present with depressive symptoms, making the clinical manifestations closely resemble those of major depressive disorder (MDD)[6]. In addition, BD can vary greatly in terms of intensity and duration among infected individuals. This nature of BD contributes to the complexity of its clinical presentation and poses challenge for its early diagnosis. Despite advances in understanding the early diagnosis of BD, significant limitations remain. This article reviews the progress in research on the early diagnosis of BD and its limitations.
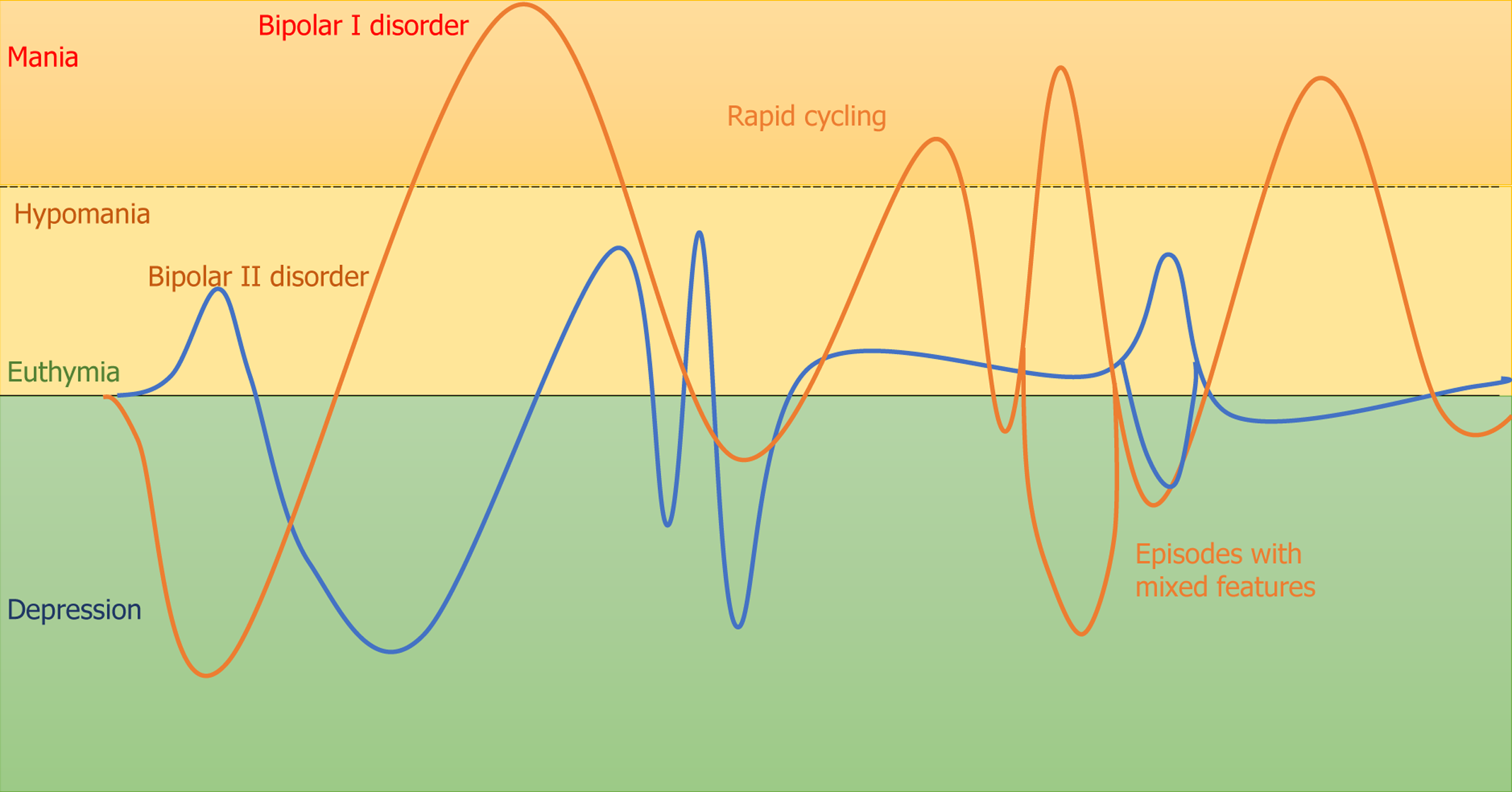
The concept of BD has evolved over time, beginning with Aretaeus of Cappadocia's early suggestion of the homogeneity between depression and mania[7], followed by the introduction of the concept of manic–depressive psychosis by Kraepelin[7], and later updates in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) and the International Classification of Diseases, 11th Revision (ICD-11). According to the DSM-5-TR, the criteria for BD require at least one episode of mania or hypomania. Mania is characterized by an expansive, elevated, or irritable mood lasting at least 1 wk, with significant impairment that often necessitates hospitalization (bipolar I disorder). Hypomania, which defines bipolar II disorder when paired with a previous depressive episode, is a milder form of mania, requiring at least 4 d of elevated mood and increased energy. The ICD-11 offers more flexibility in defining hypomania, no longer requiring it to last several days. A key distinction between the ICD-11 and DSM-5-TR lies in their approach to mixed symptoms. In DSM-5-TR, the criteria are expanded to include subsyndromal mixed symptoms – defined as at least three counterpolar symptoms –during manic, hypomanic, or depressive episodes. In contrast, ICD-11 maintains that mixed symptoms represent an episode, requiring several prominent symptoms from the opposing mood state; a less stringent criterion that aligns more closely with Kraepelin's broader view of mixed states, as shown in Figure 1 for symptomatic expressions of BD subtypes. However, traditional diagnostic tools largely rely on clinical assessments and patient history, which can be subjective and affected by recall bias. Therefore, objective, reliable biomarkers are urgently needed to help differentiate BD from other mental conditions at an early stage.
Peripheral biomarkers, which can be detected in blood, saliva, or other bodily fluids, offer a noninvasive means of identifying BD. One promising area of research focuses on inflammatory biomarkers. Studies have shown that proinflammatory cytokines are elevated in BD patients, particularly during manic and depressive phases[8]. These findings suggest that inflammation may be associated with the acute phases of BD, and monitoring these markers could aid in early diagnosis and management[9]. For instance, a systematic review and meta-analysis found that levels of interleukin (IL)-7 were significantly decreased in BD, while IL-9, C-C motif chemokine ligand (CCL)3, CCL4, CCL5, and CCL11 were significantly increased in BD compared to MDD, suggesting a role of inflammation and T-cell network dysregulation in these disorders[10]. Another study highlighted the differential expression of cytokines such as IL-4, which was found to have diagnostic value in distinguishing between active depressive episodes in MDD and BD[11]. A study on peripheral mitochondrial DNA suggested its potential as a neuroinflammatory biomarker for MDD, which could help distinguish it from BD[12]. Finally, a real-world retrospective study analyzed various hormones and inflammatory markers, finding that MDD patients had higher levels of IgA and IgM, while BD patients had elevated neutrophil and monocyte counts, providing a basis for differentiation[13]. Metabolic profiling also offers insights into the differentiation of BD from MDD. A study utilizing metabolomics-based urinary biomarker models identified distinct metabolic signatures for MDD and BD, with specific metabolites showing potential as diagnostic markers[14]. Similarly, serum metabolic profiles assessed through nuclear magnetic resonance spectroscopy revealed differences in metabolites such as pyruvate and pantothenic acid, which could serve as potential biomarkers for distinguishing BD from MDD[15]. Changes in insulin-signaling-related proteins have been observed in both manic and mixed mood states, suggesting their potential as diagnostic markers for distinguishing BD from MDD[16]. The exploration of homocysteine levels as a potential biomarker for BD has also been a focus of research. Although some studies suggest that high homocysteine levels may be a risk biomarker for BD, the association between homocysteine and MDD reduces the value of homocysteine as a diagnostic marker for BD. Further well-powered studies are needed to clarify its utility as a biomarker for early diagnosis[17]. Additionally, machine learning (ML) approaches have also been used to integrate peripheral biomarkers with clinical data, enhancing diagnostic accuracy. For example, an AI algorithm combined with RNA editing-based blood biomarkers demonstrated high accuracy in discriminating MDD from depressive BD in an external cohort, underscoring the potential of integrating biomarker data with advanced computational techniques for differential diagnosis[18].
Exosomes, small extracellular vesicles released by cells, carry molecular signatures reflective of their cell of origin, including proteins, lipids, and RNAs. The advantages of exosomes include their stability in bodily fluids, ability to cross the blood–brain barrier, and ease of isolation from blood samples[19]. For instance, brain-derived exosomal miRNA profiles have been analyzed to understand their correlation with peripheral exosomal miRNA, offering insights into the pathological processes of BD and the potential for early diagnostic assays[20]. Similarly, research into plasma extracellular vesicles enriched for neuronal origin has demonstrated their utility in providing a sensitive and accurate basis for biomarker discovery in neurological disorders, further supporting the concept of exosomes as a window into brain pathological processes[21]. Moreover, the exploration of noncoding RNAs, such as miRNAs and long noncoding RNAs (lncRNAs), within exosomes has gained attention. These molecules have regulatory roles in gene expression and are implicated in the pathophysiology of mood disorders. Exosomal miRNAs and lncRNAs could provide insights into the molecular mechanisms underlying BD and MDD, offering potential biomarkers for differential diagnosis[22]. In addition, the exploration of brain insulin resistance in adults with bipolar depression using extracellular vesicles of neuronal origin has provided evidence that these vesicles can reflect changes in insulin signaling pathways, which are implicated in BD pathogenesis. This underscores the potential of exosomal content to reveal underlying neurobiological mechanisms and guide therapeutic interventions[23], although this area of research remains underexplored. Collectively, these studies illustrate the promising role of exosomal content in capturing neurobiological changes associated with BD, offering a valuable tool for advancing our understanding and management of the disorder.
A large-scale meta-analysis of functional neuroimaging studies has identified condition-dependent differences in brain activation and connectivity in individuals with BD[24]. These studies have highlighted functional differences in specific brain regions, such as the prefrontal, parietal, and limbic areas, which are crucial for cognitive and emotional processing[24], providing a framework for identifying reproducible neural biomarkers that can guide diagnosis and treatment. The ENIGMA Bipolar Disorder Working Group has been instrumental in conducting large-scale neuroimaging studies that reveal widespread patterns of lower cortical thickness, subcortical volume, and disrupted white matter integrity associated with BD. These studies have provided further insights into the pathophysiological mechanisms of BD and thus help advance early diagnosis of the disorder[25]. Additionally, systematic reviews of neuroimaging studies have explored brain gyrification patterns in BD, revealing altered patterns that may reflect early neurodevelopmental processes and suggesting that brain gyrification could serve as a potential biomarker for optimizing the prediction and diagnosis of BD, particularly in its early stages[26]. Precision neuroimaging biomarkers are also being developed to offer personalized insights into brain alterations associated with BD. Techniques such as ML applied to neuroimaging data have been used to differentiate patients with BD from healthy individuals, providing mechanistic insights into the pathophysiology of the disorder. These advances underscore the potential of neuroimaging to contribute to precision medicine approaches in BD[27]. Endophenotypic studies that directly compare imaging differences in key brain regions across BD, MDD, and healthy populations could play a crucial role in identifying BD-specific alterations, thus contributing to early and accurate diagnosis. One of the key developments in this area is the integration of multimodal neuroimaging techniques, such as combining behavioral assessments or genomic approaches with magnetic resonance imaging (MRI) features[28,29], which has been shown to enhance diagnostic accuracy in adolescents at risk for BD.
The advancement of genetic testing technology has significantly promoted the genetic research of mental disorders. The identification of genetic markers and the elucidation of the genetic architecture of BD have provided fresh insights into the disorder. Genome-wide association studies have been instrumental in uncovering numerous genetic loci associated with BD, which are crucial for understanding its pathogenesis and for developing early diagnostic tools[30,31]. These studies have revealed that BD is highly polygenic, with a substantial overlap in genetic risk factors with other psychiatric disorders, emphasizing the complexity of its genetic underpinnings[32]. For instance, research into the genetic association between personality traits and mood disorders has revealed that polygenic scores for neuroticism are significantly associated with MDD, while extraversion scores are associated with BD. This suggests shared genetic risk factors for neuroticism and MDD, and for extraversion and BD, providing another layer of genetic differentiation between these disorders[33]. The application of polygenic risk scores (PRSs) has also been investigated as a means to differentiate BD from MDD. A study found that PRSs for BD and MDD were associated with cortical alterations in different brain areas, suggesting that genetic risk for these disorders might affect neurodevelopmental processes. These findings highlight the potential of PRSs as a tool for distinguishing between BD and MDD based on genetic susceptibility[34]. BDNF gene expression has been highlighted for its capacity to distinguish between the two disorders, as it plays a fundamental role in brain function and mood regulation[35]. Another study explored the association of ANK3 variants with BD in the Korean population, suggesting that certain haplotypes may confer susceptibility to BD. This genetic association un
Behavioral and clinical data analysis using advanced computational techniques has shown promise in diagnosis of BD. ML approaches, including support vector machines and random forests, have been increasingly applied to differentiate between psychiatric disorders and identify predictors for early onset of BD[38,39]. For instance, engagement of ML techniques in analyzing electroencephalogram data helps to differentiate between BD and MDD, as well as other psychiatric conditions[38]. The integration of passive sensing and deep anomaly detection in a contactless cohort study aimed to understand individual illness trajectories and predict relapses in BD. This approach involves continuous monitoring of physiological and behavioral data, which can provide insights into the dynamic changes associated with episode generation in BD[40]. Use of smartphone data to predict and monitor symptoms in patients with depression, including those with comorbid BD, has been explored. Behavioral markers derived from smartphone usage, such as screen-off events and communication patterns, have been correlated with changes in depressive states. This method of utilizing digital behavioral markers offers a supplementary tool for clinical evaluations, potentially aiding in the detection and monitoring of mood disorders[41]. These behavioral and psychological parameters associated with manic or hypomanic episode could aid in early diagnosis of BD. Lastly, the integration of clinical, cognitive, and MRI data has been explored to predict the transition to psychosis in individuals at ultra-high risk. Although the addition of neuroimaging and cognitive data provided marginal predictive value, these studies highlight the potential of multimodal modeling in improving the accuracy of clinical risk assessments and early diagnosis[42].
The early diagnosis of BD remains a significant challenge, despite advances in the field of psychiatry[43]. First, one of the primary reasons is the inherent heterogeneity of BD. BD is a complex illness with variability at the level of symptom presentation, severity, clinical course, cognitive capacity, and everyday function, which complicates the identification of reliable diagnostic markers[44]. Second, genetic studies have identified several potential candidate genes associated with an increased risk for developing BD, but these findings have not yet translated into practical diagnostic tools. The genetic underpinnings of BD are complex and involve multiple genes, each contributing a small effect, which makes it chal
Although significant efforts have been made, limited progress has been achieved in the early diagnosis of BD. The clinical diagnosis of BD mainly relies on the presentation of signs and symptoms, which can lead to misdiagnosis due to recall bias from patients and physicians' varying experiences. Future research should focus on integrating multiple diagnostic approaches, including genetic, biomarker, neuroimaging, and clinical assessments, with more homogeneous participant groups to validate their effectiveness, which could improve the accuracy and timeliness of BD diagnosis in clinical practice.
| 1. | Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1693] [Cited by in RCA: 1756] [Article Influence: 125.4] [Reference Citation Analysis (0)] |
| 2. | McGrath JJ, Al-Hamzawi A, Alonso J, Altwaijri Y, Andrade LH, Bromet EJ, Bruffaerts R, de Almeida JMC, Chardoul S, Chiu WT, Degenhardt L, Demler OV, Ferry F, Gureje O, Haro JM, Karam EG, Karam G, Khaled SM, Kovess-Masfety V, Magno M, Medina-Mora ME, Moskalewicz J, Navarro-Mateu F, Nishi D, Plana-Ripoll O, Posada-Villa J, Rapsey C, Sampson NA, Stagnaro JC, Stein DJ, Ten Have M, Torres Y, Vladescu C, Woodruff PW, Zarkov Z, Kessler RC; WHO World Mental Health Survey Collaborators. Age of onset and cumulative risk of mental disorders: a cross-national analysis of population surveys from 29 countries. Lancet Psychiatry. 2023;10:668-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 211] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 3. | Mesman E, Nolen WA, Reichart CG, Wals M, Hillegers MH. The Dutch bipolar offspring study: 12-year follow-up. Am J Psychiatry. 2013;170:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Vieta E, Salagre E, Grande I, Carvalho AF, Fernandes BS, Berk M, Birmaher B, Tohen M, Suppes T. Early Intervention in Bipolar Disorder. Am J Psychiatry. 2018;175:411-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 214] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 5. | Fritz K, Russell AMT, Allwang C, Kuiper S, Lampe L, Malhi GS. Is a delay in the diagnosis of bipolar disorder inevitable? Bipolar Disord. 2017;19:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Drancourt N, Etain B, Lajnef M, Henry C, Raust A, Cochet B, Mathieu F, Gard S, Mbailara K, Zanouy L, Kahn JP, Cohen RF, Wajsbrot-Elgrabli O, Leboyer M, Scott J, Bellivier F. Duration of untreated bipolar disorder: missed opportunities on the long road to optimal treatment. Acta Psychiatr Scand. 2013;127:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Marneros A. Expanding the group of bipolar disorders. J Affect Disord. 2001;62:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Becking K, Haarman BC, van der Lek RF, Grosse L, Nolen WA, Claes S, Drexhage HA, Schoevers RA. Inflammatory monocyte gene expression: trait or state marker in bipolar disorder? Int J Bipolar Disord. 2015;3:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Sayana P, Colpo GD, Simões LR, Giridharan VV, Teixeira AL, Quevedo J, Barichello T. A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J Psychiatr Res. 2017;92:160-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Gopalakrishnan R, Wang Y, Kapczinski F, Frey BN, Wollenhaupt-Aguiar B. Peripheral protein inflammatory biomarkers in bipolar disorder and major depressive disorder: A systematic review and meta-analysis. J Affect Disord. 2025;376:149-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Lu L, Hu X, Jin X. IL-4 as a potential biomarker for differentiating major depressive disorder from bipolar depression. Medicine (Baltimore). 2023;102:e33439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Ye J, Duan C, Han J, Chen J, Sun N, Li Y, Yuan T, Peng D. Peripheral mitochondrial DNA as a neuroinflammatory biomarker for major depressive disorder. Neural Regen Res. 2025;20:1541-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 13. | Lyu N, Wang H, Zhao Q, Fu B, Li J, Yue Z, Huang J, Yang F, Liu H, Zhang L, Li R. Peripheral biomarkers to differentiate bipolar depression from major depressive disorder: a real-world retrospective study. BMC Psychiatry. 2024;24:543. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Wang T, Yang J, Zhu Y, Niu N, Ding B, Wang P, Zhao H, Li N, Chao Y, Gao S, Dong X, Wang Z. Evaluation of metabolomics-based urinary biomarker models for recognizing major depression disorder and bipolar disorder. J Affect Disord. 2024;356:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 15. | Sun XL, Ma LN, Chen ZZ, Xiong YB, Jia J, Wang Y, Ren Y. Search for serum biomarkers in patients with bipolar disorder and major depressive disorder using metabolome analysis. Front Psychiatry. 2023;14:1251955. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Haenisch F, Alsaif M, Guest PC, Rahmoune H, Yolken RH, Dickerson F, Bahn S. Multiplex immunoassay analysis of plasma shows differences in biomarkers related to manic or mixed mood states in bipolar disorder patients. J Affect Disord. 2015;185:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Ghanizadeh A, Singh AB, Berk M, Torabi-Nami M. Homocysteine as a potential biomarker in bipolar disorders: a critical review and suggestions for improved studies. Expert Opin Ther Targets. 2015;19:927-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Salvetat N, Checa-Robles FJ, Delacrétaz A, Cayzac C, Dubuc B, Vetter D, Dainat J, Lang JP, Gamma F, Weissmann D. AI algorithm combined with RNA editing-based blood biomarkers to discriminate bipolar from major depressive disorders in an external validation multicentric cohort. J Affect Disord. 2024;356:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 19. | Wu Y, Li Y, An X, Li J, Yang C, Wang Y. Study on exosomes for identifying bipolar disorder in early stage: A cross-sectional and validation study protocol. Brain Behav. 2024;14:e3494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Xiao Q, Yan X, Sun Y, Tang Y, Hou R, Pan X, Zhu X. Brain-Derived Exosomal miRNA Profiles upon Experimental SAE Rats and Their Comparison with Peripheral Exosomes. Mol Neurobiol. 2024;61:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Mustapic M, Eitan E, Werner JK Jr, Berkowitz ST, Lazaropoulos MP, Tran J, Goetzl EJ, Kapogiannis D. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front Neurosci. 2017;11:278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 335] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 22. | Todeva-Radneva A, Aryutova K, Kandilarova S, Paunova R, Stoyanov D. The Translational Potential of Non-coding RNAs and Multimodal MRI Data Sets as Diagnostic and Differential Diagnostic Biomarkers for Mood Disorders. Curr Top Med Chem. 2021;21:949-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Mansur RB, Delgado-Peraza F, Subramaniapillai M, Lee Y, Iacobucci M, Nasri F, Rodrigues N, Rosenblat JD, Brietzke E, Cosgrove VE, Kramer NE, Suppes T, Raison CL, Fagiolini A, Rasgon N, Chawla S, Nogueras-Ortiz C, Kapogiannis D, McIntyre RS. Exploring brain insulin resistance in adults with bipolar depression using extracellular vesicles of neuronal origin. J Psychiatr Res. 2021;133:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Schumer MC, Chase HW, Rozovsky R, Eickhoff SB, Phillips ML. Prefrontal, parietal, and limbic condition-dependent differences in bipolar disorder: a large-scale meta-analysis of functional neuroimaging studies. Mol Psychiatry. 2023;28:2826-2838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Ching CRK, Hibar DP, Gurholt TP, Nunes A, Thomopoulos SI, Abé C, Agartz I, Brouwer RM, Cannon DM, de Zwarte SMC, Eyler LT, Favre P, Hajek T, Haukvik UK, Houenou J, Landén M, Lett TA, McDonald C, Nabulsi L, Patel Y, Pauling ME, Paus T, Radua J, Soeiro-de-Souza MG, Tronchin G, van Haren NEM, Vieta E, Walter H, Zeng LL, Alda M, Almeida J, Alnaes D, Alonso-Lana S, Altimus C, Bauer M, Baune BT, Bearden CE, Bellani M, Benedetti F, Berk M, Bilderbeck AC, Blumberg HP, Bøen E, Bollettini I, Del Mar Bonnin C, Brambilla P, Canales-Rodríguez EJ, Caseras X, Dandash O, Dannlowski U, Delvecchio G, Díaz-Zuluaga AM, Dima D, Duchesnay É, Elvsåshagen T, Fears SC, Frangou S, Fullerton JM, Glahn DC, Goikolea JM, Green MJ, Grotegerd D, Gruber O, Haarman BCM, Henry C, Howells FM, Ives-Deliperi V, Jansen A, Kircher TTJ, Knöchel C, Kramer B, Lafer B, López-Jaramillo C, Machado-Vieira R, MacIntosh BJ, Melloni EMT, Mitchell PB, Nenadic I, Nery F, Nugent AC, Oertel V, Ophoff RA, Ota M, Overs BJ, Pham DL, Phillips ML, Pineda-Zapata JA, Poletti S, Polosan M, Pomarol-Clotet E, Pouchon A, Quidé Y, Rive MM, Roberts G, Ruhe HG, Salvador R, Sarró S, Satterthwaite TD, Schene AH, Sim K, Soares JC, Stäblein M, Stein DJ, Tamnes CK, Thomaidis GV, Upegui CV, Veltman DJ, Wessa M, Westlye LT, Whalley HC, Wolf DH, Wu MJ, Yatham LN, Zarate CA, Thompson PM, Andreassen OA; ENIGMA Bipolar Disorder Working Group. What we learn about bipolar disorder from large-scale neuroimaging: Findings and future directions from the ENIGMA Bipolar Disorder Working Group. Hum Brain Mapp. 2022;43:56-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 26. | Miola A, Cattarinussi G, Loré ML, Ghiotto N, Collantoni E, Sambataro F. Brain gyrification in bipolar disorder: a systematic review of neuroimaging studies. Brain Imaging Behav. 2022;16:2768-2784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Janiri D, Frangou S. Precision neuroimaging biomarkers for bipolar disorder. Int Rev Psychiatry. 2022;34:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Wu J, Lin K, Lu W, Zou W, Li X, Tan Y, Yang J, Zheng D, Liu X, Lam BY, Xu G, Wang K, McIntyre RS, Wang F, So KF, Wang J. Enhancing Early Diagnosis of Bipolar Disorder in Adolescents Through Multimodal Neuroimaging. Biol Psychiatry. 2025;97:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Oraki Kohshour M, Papiol S, Ching CRK, Schulze TG. Genomic and neuroimaging approaches to bipolar disorder. BJPsych Open. 2022;8:e36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Mullins N, Forstner AJ, O'Connell KS, Coombes B, Coleman JRI, Qiao Z, Als TD, Bigdeli TB, Børte S, Bryois J, Charney AW, Drange OK, Gandal MJ, Hagenaars SP, Ikeda M, Kamitaki N, Kim M, Krebs K, Panagiotaropoulou G, Schilder BM, Sloofman LG, Steinberg S, Trubetskoy V, Winsvold BS, Won HH, Abramova L, Adorjan K, Agerbo E, Al Eissa M, Albani D, Alliey-Rodriguez N, Anjorin A, Antilla V, Antoniou A, Awasthi S, Baek JH, Bækvad-Hansen M, Bass N, Bauer M, Beins EC, Bergen SE, Birner A, Bøcker Pedersen C, Bøen E, Boks MP, Bosch R, Brum M, Brumpton BM, Brunkhorst-Kanaan N, Budde M, Bybjerg-Grauholm J, Byerley W, Cairns M, Casas M, Cervantes P, Clarke TK, Cruceanu C, Cuellar-Barboza A, Cunningham J, Curtis D, Czerski PM, Dale AM, Dalkner N, David FS, Degenhardt F, Djurovic S, Dobbyn AL, Douzenis A, Elvsåshagen T, Escott-Price V, Ferrier IN, Fiorentino A, Foroud TM, Forty L, Frank J, Frei O, Freimer NB, Frisén L, Gade K, Garnham J, Gelernter J, Giørtz Pedersen M, Gizer IR, Gordon SD, Gordon-Smith K, Greenwood TA, Grove J, Guzman-Parra J, Ha K, Haraldsson M, Hautzinger M, Heilbronner U, Hellgren D, Herms S, Hoffmann P, Holmans PA, Huckins L, Jamain S, Johnson JS, Kalman JL, Kamatani Y, Kennedy JL, Kittel-Schneider S, Knowles JA, Kogevinas M, Koromina M, Kranz TM, Kranzler HR, Kubo M, Kupka R, Kushner SA, Lavebratt C, Lawrence J, Leber M, Lee HJ, Lee PH, Levy SE, Lewis C, Liao C, Lucae S, Lundberg M, MacIntyre DJ, Magnusson SH, Maier W, Maihofer A, Malaspina D, Maratou E, Martinsson L, Mattheisen M, McCarroll SA, McGregor NW, McGuffin P, McKay JD, Medeiros H, Medland SE, Millischer V, Montgomery GW, Moran JL, Morris DW, Mühleisen TW, O'Brien N, O'Donovan C, Olde Loohuis LM, Oruc L, Papiol S, Pardiñas AF, Perry A, Pfennig A, Porichi E, Potash JB, Quested D, Raj T, Rapaport MH, DePaulo JR, Regeer EJ, Rice JP, Rivas F, Rivera M, Roth J, Roussos P, Ruderfer DM, Sánchez-Mora C, Schulte EC, Senner F, Sharp S, Shilling PD, Sigurdsson E, Sirignano L, Slaney C, Smeland OB, Smith DJ, Sobell JL, Søholm Hansen C, Soler Artigas M, Spijker AT, Stein DJ, Strauss JS, Świątkowska B, Terao C, Thorgeirsson TE, Toma C, Tooney P, Tsermpini EE, Vawter MP, Vedder H, Walters JTR, Witt SH, Xi S, Xu W, Yang JMK, Young AH, Young H, Zandi PP, Zhou H, Zillich L; HUNT All-In Psychiatry, Adolfsson R, Agartz I, Alda M, Alfredsson L, Babadjanova G, Backlund L, Baune BT, Bellivier F, Bengesser S, Berrettini WH, Blackwood DHR, Boehnke M, Børglum AD, Breen G, Carr VJ, Catts S, Corvin A, Craddock N, Dannlowski U, Dikeos D, Esko T, Etain B, Ferentinos P, Frye M, Fullerton JM, Gawlik M, Gershon ES, Goes FS, Green MJ, Grigoroiu-Serbanescu M, Hauser J, Henskens F, Hillert J, Hong KS, Hougaard DM, Hultman CM, Hveem K, Iwata N, Jablensky AV, Jones I, Jones LA, Kahn RS, Kelsoe JR, Kirov G, Landén M, Leboyer M, Lewis CM, Li QS, Lissowska J, Lochner C, Loughland C, Martin NG, Mathews CA, Mayoral F, McElroy SL, McIntosh AM, McMahon FJ, Melle I, Michie P, Milani L, Mitchell PB, Morken G, Mors O, Mortensen PB, Mowry B, Müller-Myhsok B, Myers RM, Neale BM, Nievergelt CM, Nordentoft M, Nöthen MM, O'Donovan MC, Oedegaard KJ, Olsson T, Owen MJ, Paciga SA, Pantelis C, Pato C, Pato MT, Patrinos GP, Perlis RH, Posthuma D, Ramos-Quiroga JA, Reif A, Reininghaus EZ, Ribasés M, Rietschel M, Ripke S, Rouleau GA, Saito T, Schall U, Schalling M, Schofield PR, Schulze TG, Scott LJ, Scott RJ, Serretti A, Shannon Weickert C, Smoller JW, Stefansson H, Stefansson K, Stordal E, Streit F, Sullivan PF, Turecki G, Vaaler AE, Vieta E, Vincent JB, Waldman ID, Weickert TW, Werge T, Wray NR, Zwart JA, Biernacka JM, Nurnberger JI, Cichon S, Edenberg HJ, Stahl EA, McQuillin A, Di Florio A, Ophoff RA, Andreassen OA. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 609] [Cited by in RCA: 869] [Article Influence: 217.3] [Reference Citation Analysis (0)] |
| 31. | Li M, Li T, Xiao X, Chen J, Hu Z, Fang Y. Phenotypes, mechanisms and therapeutics: insights from bipolar disorder GWAS findings. Mol Psychiatry. 2022;27:2927-2939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | O'Connell KS, Coombes BJ. Genetic contributions to bipolar disorder: current status and future directions. Psychol Med. 2021;51:2156-2167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 33. | Middeldorp CM, de Moor MH, McGrath LM, Gordon SD, Blackwood DH, Costa PT, Terracciano A, Krueger RF, de Geus EJ, Nyholt DR, Tanaka T, Esko T, Madden PA, Derringer J, Amin N, Willemsen G, Hottenga JJ, Distel MA, Uda M, Sanna S, Spinhoven P, Hartman CA, Ripke S, Sullivan PF, Realo A, Allik J, Heath AC, Pergadia ML, Agrawal A, Lin P, Grucza RA, Widen E, Cousminer DL, Eriksson JG, Palotie A, Barnett JH, Lee PH, Luciano M, Tenesa A, Davies G, Lopez LM, Hansell NK, Medland SE, Ferrucci L, Schlessinger D, Montgomery GW, Wright MJ, Aulchenko YS, Janssens AC, Oostra BA, Metspalu A, Abecasis GR, Deary IJ, Räikkönen K, Bierut LJ, Martin NG, Wray NR, van Duijn CM, Smoller JW, Penninx BW, Boomsma DI. The genetic association between personality and major depression or bipolar disorder. A polygenic score analysis using genome-wide association data. Transl Psychiatry. 2011;1:e50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Cattarinussi G, Delvecchio G, Sambataro F, Brambilla P. The effect of polygenic risk scores for major depressive disorder, bipolar disorder and schizophrenia on morphological brain measures: A systematic review of the evidence. J Affect Disord. 2022;310:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Menezes IC, von Werne Baes C, Lacchini R, Juruena MF. Genetic biomarkers for differential diagnosis of major depressive disorder and bipolar disorder: A systematic and critical review. Behav Brain Res. 2019;357-358:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Cho CH, Kim S, Geum D, Lee HJ. Association analysis of ANK3 variants with bipolar disorder in the Korean population. Nord J Psychiatry. 2017;71:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Guglielmo R, Miskowiak KW, Hasler G. Evaluating endophenotypes for bipolar disorder. Int J Bipolar Disord. 2021;9:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Song YW, Lee HS, Kim S, Kim K, Kim BN, Kim JS. How to Solve Clinical Challenges in Mood Disorders; Machine Learning Approaches Using Electrophysiological Markers. Clin Psychopharmacol Neurosci. 2024;22:416-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Amanollahi M, Jameie M, Looha MA, A Basti F, Cattarinussi G, Moghaddam HS, Di Camillo F, Akhondzadeh S, Pigoni A, Sambataro F, Brambilla P, Delvecchio G. Machine learning applied to the prediction of relapse, hospitalization, and suicide in bipolar disorder using neuroimaging and clinical data: A systematic review. J Affect Disord. 2024;361:778-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 40. | Ortiz A, Hintze A, Burnett R, Gonzalez-Torres C, Unger S, Yang D, Miao J, Alda M, Mulsant BH. Identifying patient-specific behaviors to understand illness trajectories and predict relapses in bipolar disorder using passive sensing and deep anomaly detection: protocol for a contactless cohort study. BMC Psychiatry. 2022;22:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Ikäheimonen A, Luong N, Baryshnikov I, Darst R, Heikkilä R, Holmen J, Martikkala A, Riihimäki K, Saleva O, Isometsä E, Aledavood T. Predicting and Monitoring Symptoms in Patients Diagnosed With Depression Using Smartphone Data: Observational Study. J Med Internet Res. 2024;26:e56874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 42. | Hartmann S, Cearns M, Pantelis C, Dwyer D, Cavve B, Byrne E, Scott I, Yuen HP, Gao C, Allott K, Lin A, Wood SJ, Wigman JTW, Amminger GP, McGorry PD, Yung AR, Nelson B, Clark SR. Combining Clinical With Cognitive or Magnetic Resonance Imaging Data for Predicting Transition to Psychosis in Ultra High-Risk Patients: Data From the PACE 400 Cohort. Biol Psychiatry Cogn Neurosci Neuroimaging. 2024;9:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Ketter TA. Nosology, diagnostic challenges, and unmet needs in managing bipolar disorder. J Clin Psychiatry. 2010;71:e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Burdick KE, Millett CE. Cognitive heterogeneity is a key predictor of differential functional outcome in patients with bipolar disorder. Eur Neuropsychopharmacol. 2021;53:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Frey BN, Andreazza AC, Houenou J, Jamain S, Goldstein BI, Frye MA, Leboyer M, Berk M, Malhi GS, Lopez-Jaramillo C, Taylor VH, Dodd S, Frangou S, Hall GB, Fernandes BS, Kauer-Sant'Anna M, Yatham LN, Kapczinski F, Young LT. Biomarkers in bipolar disorder: a positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust N Z J Psychiatry. 2013;47:321-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 46. | Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1263-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 47. | Liu X; Bipolar Genome Study (BiGS), Kelsoe JR, Greenwood TA. A genome-wide association study of bipolar disorder with comorbid eating disorder replicates the SOX2-OT region. J Affect Disord. 2016;189:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Birur B, Kraguljac NV, Shelton RC, Lahti AC. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder-a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophr. 2017;3:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |