Published online Aug 19, 2025. doi: 10.5498/wjp.v15.i8.103735
Revised: March 21, 2025
Accepted: May 28, 2025
Published online: August 19, 2025
Processing time: 163 Days and 3.5 Hours
Some studies have demonstrated that combination treatment with anlotinib and albumin-bound paclitaxel has superior efficacy in stage IV non-small cell lung cancer (NSCLC). Howbeit, there is limited research on the effects of combination therapy.
To determine the efficacy of anlotinib plus albumin-paclitaxel in stage IV NSCLC.
Forty-two patients diagnosed with stage IV NSCLC who were treated at our hospital from January 2022 to February 2023 were selected as study subjects. According to the research protocol, the patients were divided into two groups: conventional therapy (albumin paclitaxel, n = 20) and combination therapy (anlotinib plus albumin paclitaxel, n = 22). The clinical effect, serum tumor markers, progression-free survival, overall survival, immune function, quality of life, mental state, and toxic side effects were compared between the two groups.
The disease remission rate, disease control rate, CD3+, CD4+, CD4+/CD8+ and Karnofsky Performance Scale (KPS) score in combined therapy were higher than conventional therapy. After treatment, levels of carcinoembryonic antigen, cytokeratin 19 fragment antigen 21-1, and vascular endothelial growth factor, self-rating anxiety scale, and self-rating depression scale score were all lower in combination therapy compared to conventional therapy. In addition, there was no remarkable difference in adverse reactions between the two groups.
Anlotinib combined with albumin-paclitaxel demonstrated therapeutic efficacy in stage IV NSCLC, reducing depression, anxiety, and tumor biomarker levels, while enhancing immune function, prolonging survival, and improving quality of life.
Core Tip: In this study, we evaluated the efficacy of anlotinib combined with albumin-paclitaxel in stage IV non-small cell lung cancer (NSCLC). Forty-two patients were split into two groups: one group received albumin-paclitaxel (n = 20), while the other group received anlotinib plus albumin-paclitaxel (n = 22). The study compared clinical outcomes, tumor markers, progression-free survival, overall survival, immune function, quality of life, mental health, and side effects. The combination therapy group showed higher disease remission and control rates, better immune markers and quality of life scores, and lower tumor biomarkers and mental health scores. Adverse reactions were similar in both groups. The combination of anlotinib plus albumin-paclitaxel was effective in treating stage IV NSCLC, reducing depression and anxiety, lowering tumor biomarkers, enhancing immune function, prolonging survival, and improving quality of life.
- Citation: Zhang W, Zhao YF, Liang FF, Liu XX, Liu JH, Hao JD, Cheng SQ, Wu YF. Role of anlotinib plus albumin paclitaxel regimen in stage IV non-small cell lung cancer and mental state. World J Psychiatry 2025; 15(8): 103735
- URL: https://www.wjgnet.com/2220-3206/full/v15/i8/103735.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i8.103735
In recent years, environmental and lifestyle changes have contributed to the rise of lung cancer as one of the most threatening malignant, affecting nearly 260 million[1,2]. Non-small cell lung cancer (NSCLC) accounts for 80%-85% of all lung cancer cases[3]. Due to its nonspecific early symptoms, NSCLC can be confused with respiratory diseases, delaying diagnosis[4]. Therefore, most patients have already progressed to the late stage when diagnosed when chemotherapy is applied to prevent disease progression and prolong survival time[5,6]. In the clinic, paclitaxel and platinum are common NSCLC treatments[7]. Patients may experience severe negative emotions due to the serious gastrointestinal reactions caused by chemotherapy and some patients may find it difficult to persist through completion of chemotherapy, which limits its clinical application[8-10]. Long-term use of paclitaxel and platinum-based chemotherapy can lead to chemo
Anlotinib, a novel tyrosine kinase inhibitor, exerts dual effects by inhibiting both tumor growth and angiogenesis[14,15]. It has been shown to improve progression-free survival (PFS) and overall survival (OS) for patients with NSCLC by blocking vascular endothelial growth factor (VEGF)-mediated signaling[16,17]. Furthermore, anlotinib has shown advantages as a multi-target TKI in inhibiting tumor progression in patients with NSCLC[18-20]. Studies on anlotinib and chemotherapy in NSCLC have been relatively limited. To fill the gap in the current research, our study explored anlotinib plus albumin-paclitaxel in stage IV NSCLC and its impact on immune function, quality of life, and mental state, supplying a prospective treatment strategy for NSCLC.
From January 2022 to February 2023, 42 patients with stage IV NSCLC in our hospital were selected for this study. Patients were divided into conventional therapy (n = 20) and combination therapy (n = 22) according to a random number table. In the conventional therapy group, there were 11 males and 9 females, aged 45-80 years, with an average age of 70.12 ± 4.75 years. There were eight cases of squamous cell carcinoma and 12 cases of adenocarcinoma. In the combination therapy group, there were 12 males and 10 females, aged 46-79 years with an average age of 71.24 ± 4.81 years. There were nine cases of squamous cell carcinoma and 13 cases of adenocarcinoma. There was no statistically significant difference between the two groups in terms of sex, age, and other basic data (P > 0.05). All patients signed the informed consent form.
Inclusion criteria: (1) Met the diagnostic criteria for NSCLC[21] and confirmed via pathological and imaging diagnosis; and (2) PS score ≤ 2.
Exclusion criteria: (1) Liver or kidney dysfunction; (2) Presence of other malignant tumors; and (3) Chemotherapy intolerance.
Both groups were given basic treatment. The conventional therapy group received albumin paclitaxel (130 mg/m2, d1, d8, 4-6 cycles), while the combination therapy was treated with anlotinib plus albumin paclitaxel (12 mg/day for 2 consecutive weeks, stopping for 1 week, with 21 days as one treatment cycle +130 mg/m2, d1, d8, 4-6 cycles). Both groups received 2 treatment cycles.
Clinical effect: Complete response (CR): All target lesions disappear and persist for ≥ 4 weeks; Partial response (PR): Compared to baseline levels, the sum of the two diameters of all target lesions decreased by more than 30%; Progress (PD): Baseline lesion length increases by ≥ 20% or new lesions appear; Stable (SD): Baseline lesion length and diameter did not reach PR or increased without reaching PD.
Disease control rate (DCR) rate = (CR + PR + SD)/total cases × 100%.
Objective response rate (ORR) rate = (CR + PR)/total cases × 100%.
Serum tumor marker: A morning venous blood sample was acquired from the patient's elbow before and after two cycles of treatment. Serum was collected to determine levels of carcinoembryonic antigen (CEA), cytokeratin 19 fragment antigen 21-1 (Cyfra21-1), and VEGF levels. CEA, Cyfra21-1, and VEGF were measured through electrochemiluminescence. All test kits were purchased from Shanghai Kameishu Biotechnology Co., Ltd.
PFS and OS: Patients were monitored through follow-up appointments for 12 months after treatment, using outpatient follow-up and telephone follow-up to clarify disease progression and calculate the PFS (from the end of treatment to tumor progression) and OS (from the start of treatment to death) for the two groups.
Immune function: Before and after treatment, computed tomography (CT) was applied to scan the patient's chest, and a high-resolution CT semi-quantitative score was obtained for each patient. Pulmonary fibrosis was estimated by recording the imaging characteristics of pulmonary fibrosis (ground glass shadow, grid shadow, honeycomb lung) scores.
Quality of life: The experiment used the KPS score to evaluate patients’ quality of life. The total score was based on a 100-point scale, with a score of 60 or above indicating self-care ability, and a higher score indicating a better quality of life.
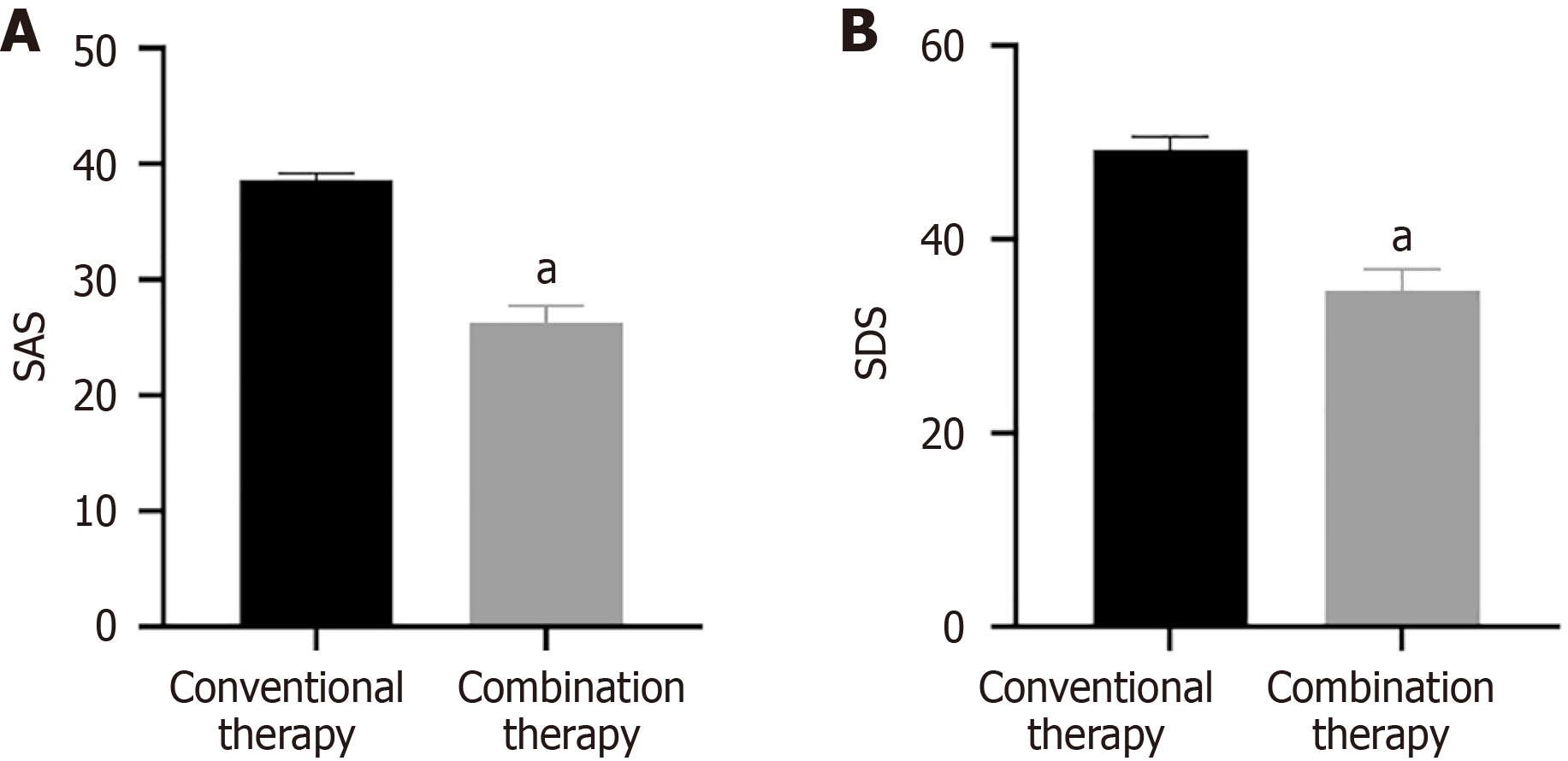
Mental state: The Self Rating Anxiety Scale (SAS) and Self Rating Depression Scale (SDS) were applied to evaluate the mental state of patients, with total scores ranging from 20 to 80. Higher scores represented more severe anxiety/depression.
Toxic side effects: The occurrence of adverse drug reactions was documented and compared between the two groups. Reactions included renal and liver dysfunction, decreased white blood cell count, gastrointestinal reactions, and hair loss.
Statistical analysis was performed using SPSS 23.0 for data processing. Experimental data are shown as mean ± SD. The differences between correlation and causation were analyzed using one-way analysis of variance (ANOVA) and t-test. P < 0.05 was considered statistically significant.
The DCR and ORR of combination therapy were higher than conventional therapy after two treatment cycles (Table 1, P < 0.05).
| Groups | Number of patients | CR | PR | SD | PD | ORR, n (%) | DCR, n (%) |
| Conventional therapy | 20 | 1 | 1 | 9 | 9 | 2 (10.00) | 11 (55.00) |
| Combination therapy | 22 | 3 | 6 | 10 | 3 | 9 (40.91) | 19 (86.36) |
| χ2 | 5.177 | 5.05 | |||||
| P value | 0.023 | 0.025 |
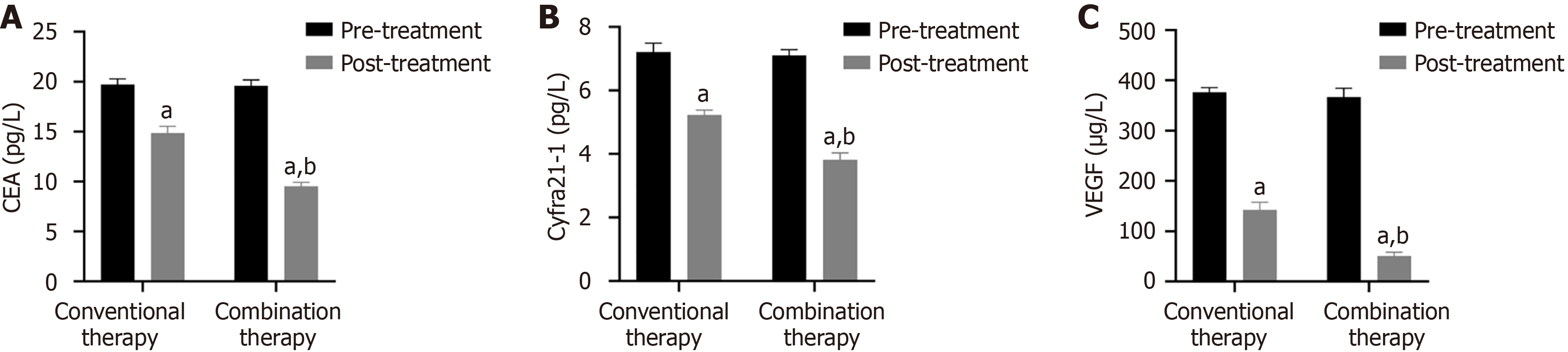
Before treatment, the CEA, Cyfra21-1 as well as VEGF levels were similar between conventional therapy and combination therapy (P > 0.05). In contrast, serum levels for all three were lower in combination therapy compared to conventional therapy after two treatment cycles (Figure 1, P < 0.05).
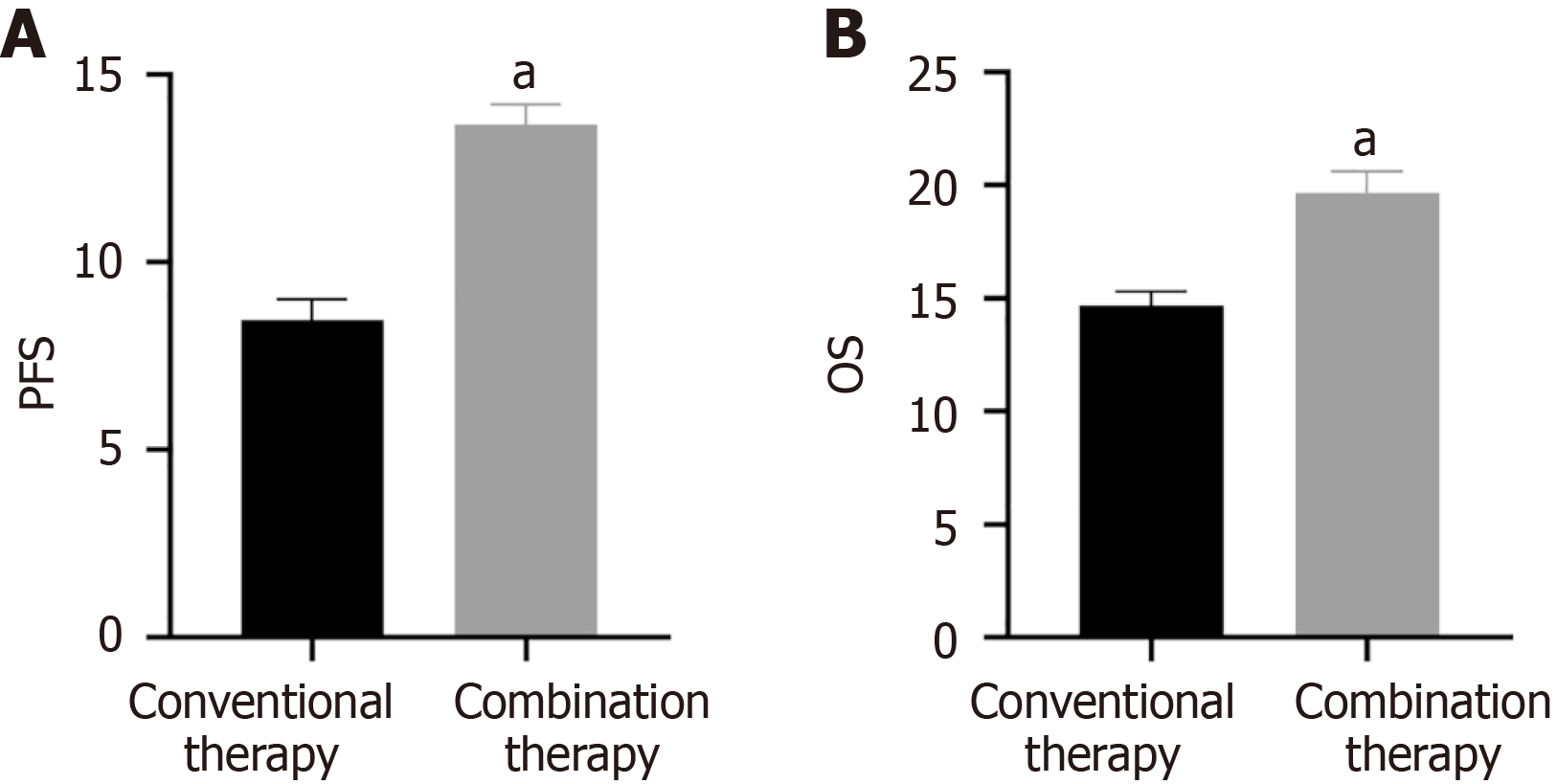
There was no notable difference in PFS or OS between the two groups before treatment (P > 0.05). However, after treatment, the PFS and OS were higher in combination therapy compared to conventional therapy (Figure 2, P < 0.05).
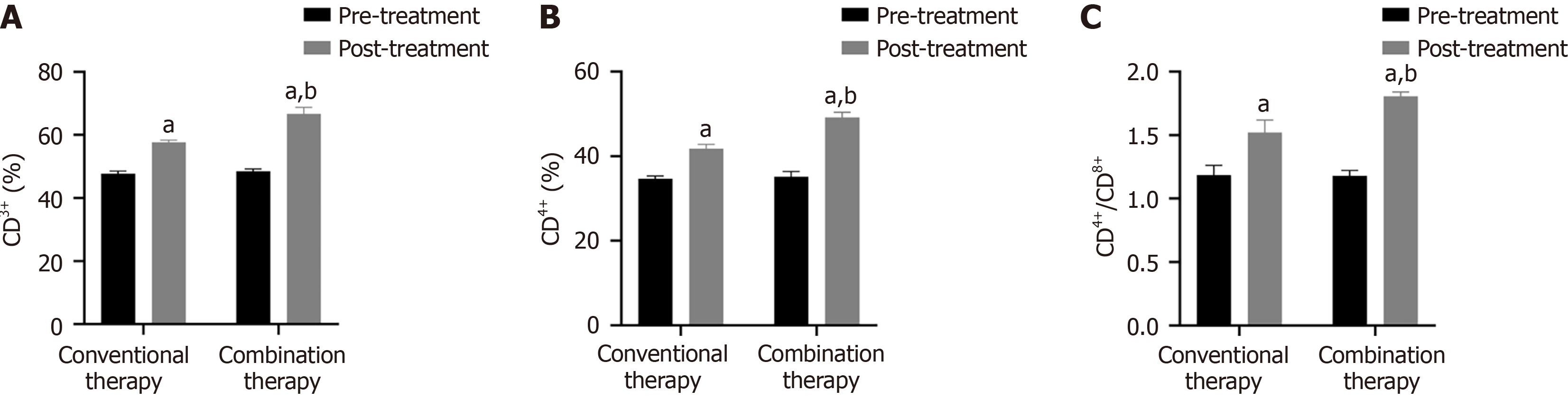
CD3+, CD4+, and CD4+/CD8+ levels were markedly higher in the combination therapy compared to the conventional group after two treatment cycles (Figure 3, P < 0.05).
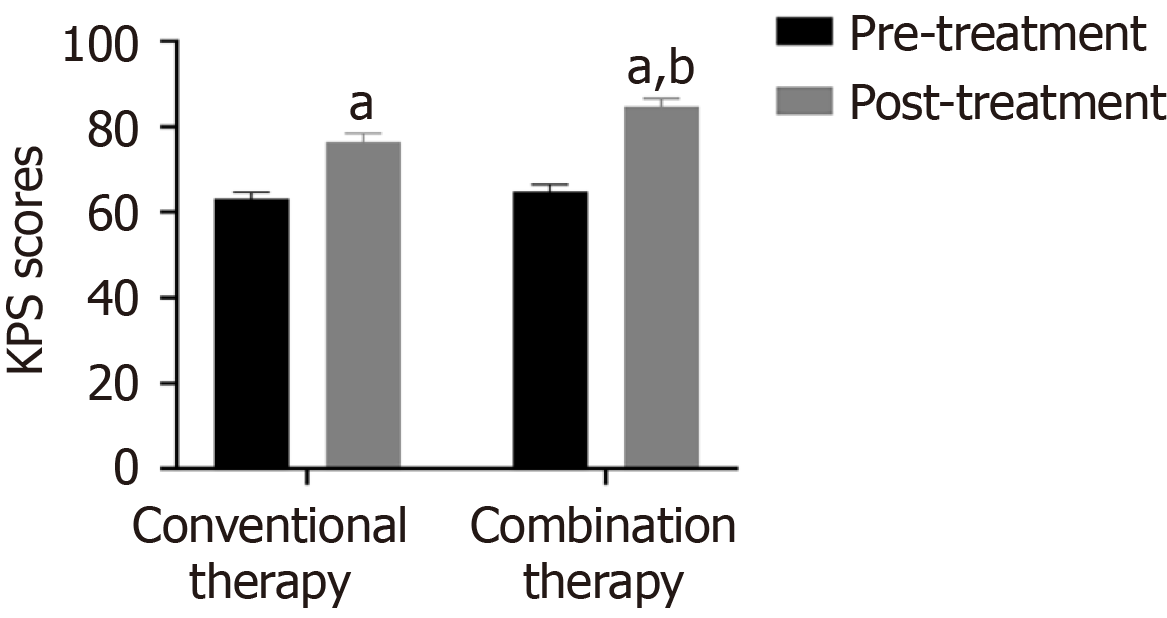
The KPS scores of the two groups were similar before therapy (P > 0.05). As shown in Figure 4, KPS scores were higher after combination therapy compared to conventional therapy (P < 0.05).
As shown in Figure 5, the SAS and SDS were lower in combination therapy, and its effect was superior to conventional therapy (P < 0.05).
There was no obvious difference in the incidence of adverse side effects between the two groups, including gas
Statistics indicate that NSCLC is a common malignant tumor in clinical practice, characterized by rapid onset, rapid progression, and a high tendency for metastasis[22]. The 2-year survival rate for stage IV NSCLC is approximately 55%, and the 5-year survival rate is approximately 36%[23]. Therefore, reducing the incidence of NSCLC, improving early diagnosis rate, and prolonging patient survival are currently key focuses of cancer prevention and treatment efforts. The current standard clinical treatment for NSCLC is drug chemotherapy. Albumin-paclitaxel promotes microtubule polymerization, inhibits depolymerization, maintains microtubule stability and inhibits cell mitosis to kill tumor cells[24]. Albumin-paclitaxel has a broad anti-tumor spectrum, demonstrates efficacy against hypoxic tumor cells, and exerts strong therapeutic effects. It is frequently used in combination with other chemotherapy agents. Research has shown that albumin paclitaxel can damage cell membranes, alter their antigenicity, stimulate the body to exert immune effects, and inhibit tumor cell proliferation and metastasis. However, some patients exhibit low tolerance to paclitaxel, and tumor cells may show limited sensitivity to the drug, which can compromise the effectiveness of clinical treatment[25]. As a novel anti-angiogenic drug, anlotinib can suppress tumor angiogenesis and prevent tumor cell proliferation to exert anti-tumor effects. As a multi-target tyrosine kinase inhibitor, anlotinib restrained tumor angiogenesis via inhibition of VEGFR, PDGFR, and FGFR. A retrospective analysis initiated by Cheng et al[26] included 118 patients with advanced NSCLC receiving anlotinib therapy. Furthermore, another multicenter study found that anlotinib possesses higher safety and efficacy in stage III-IV NSCLC[27]. However, studies on anlotinib and chemotherapy combination therapy is relatively limited for NSCLC. Therefore, we explored the effect of anlotinib plus albumin-paclitaxel in stage IV NSCLC.
Our findings demonstrated that combination therapy achieved significantly higher DCR and ORR compared to conventional therapy, suggesting that anlotinib combined with albumin-paclitaxel has a noticeable therapeutic effect on stage IV NSCLC.
Malignant tumor markers exert a certain auxiliary role in tumor diagnosis. CEA is an acidic glycoprotein distributed on the tumor surface. Abnormal high-expressed serum CEA reflects tumor metastasis and recurrence[28]. Among 925 patients, Fang et al[29] identified a positive correlation between CEA and NSCLC staging and malignancy. Cyfra21-1 is a fragment of cytokeratin 19, and its overexpression is related to tumor metastasis[30]. Wang et al[31] demonstrated that the sensitivity of Cyfra21-1 in NSCLC increased to 90.3%, and the specificity increased to 88.2%. VEGF is positively correlated with clinical stage and can serve as a biomarker for disease monitoring and treatment efficacy evaluation in NSCLC[32]. In CT perfusion imaging of 67 NSCLC patients, Li et al[33] found that plasma VEGF levels were positively correlated with blood flow. Interestingly, increasing VEGF levels correlated with lymphatic metastasis. Our findings demonstrated that VEGF serum levels significantly decreased after two treatment cycles of anlotinib plus albumin-paclitaxel, suggesting that combination therapy was more effective in decreasing VEGF serum levels. The PFS and OS of 22 patients receiving anlotinib plus albumin-paclitaxel therapy were 13.7 months and 19.6 months respectively, similar to previous studies[34]. Subsequently, our results demonstrated that combination therapy increased CD3+, CD4+, CD4+/CD8+, and KPS scores, suggesting that anlotinib plus-albumin paclitaxel enhances the patient's immune function, further controlling lesion development, improving the patient's quality of life, and improving prognosis. Simultaneously, SDS and SAS were decreased in combination therapy, suggesting that anlotinib and albumin-paclitaxel alleviated the patient's negative emotions. Interestingly, there was no difference in adverse reactions between the two groups, likely because the adverse reactions of anlotinib are primarily mild hypertension, diarrhea, etc[35].
Although the combination of anlotinib and albumin-paclitaxel is promising in treating stage IV NSCLC, our study has some limitations. First, the limited sample size may affect the accuracy of the experimental results. The study did not account for potential confounding factors such as variations in NSCLC severity, genetic predispositions, and lifestyle differences among patients, which could influence treatment outcomes. In addition, this study is a retrospective study that relied on existing medical records, which may lack comprehensive data that could affect the accuracy of the research results.
Future studies should include more stage IV NSCLC patients to validate the accuracy of data results. It is also necessary to track the impact of anlotinib plus albumin-paclitaxel on KPS scores to assess whether the patient's condition is acceptable for later treatment. The Cox proportional hazards regression model to analyze factors affecting the prognosis of stage IV NSCLC patients. By pursuing these directions, anlotinib plus albumin-paclitaxel can be effectively integrated into mainstream cancer treatments, utilizing their synergistic effects to improve overall treatment outcomes for stage IV NSCLC patients.
In this study, we examined the effects of anlotinib and albumin-paclitaxel on stage IV NSCLC. The combination of anlotinib and albumin-paclitaxel had a positive therapeutic effect on patients with advanced NSCLC. It reduced depression, anxiety, and tumor biomarker levels, enhanced immune function, prolonged patient survival, and improved quality of life. Furthermore, it did not increase adverse drug reactions, suggesting it may be a novel effective strategy in treating stage IV NSCLC treatment.
| 1. | Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27:1345-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 576] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 2. | Tian Y, Xu L, Li X, Li H, Zhao M. SMARCA4: Current status and future perspectives in non-small-cell lung cancer. Cancer Lett. 2023;554:216022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 3. | Meyer ML, Fitzgerald BG, Paz-Ares L, Cappuzzo F, Jänne PA, Peters S, Hirsch FR. New promises and challenges in the treatment of advanced non-small-cell lung cancer. Lancet. 2024;404:803-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 4. | Girard N, Basse C. EGFR-mutant NSCLC: monitoring the molecular evolution of tumors in 2022. Expert Rev Anticancer Ther. 2022;22:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Casaluce F, Sgambato A, Sacco PC, Palazzolo G, Maione P, Rossi A, Ciardiello F, Gridelli C. Resistance to Crizotinib in Advanced Non-Small Cell Lung Cancer (NSCLC) with ALK Rearrangement: Mechanisms, Treatment Strategies and New Targeted Therapies. Curr Clin Pharmacol. 2016;11:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Sartori F, Bortolotti L, Marulli G, Rizzardi G, Favaretto A, Zuin A, Breda C, Rea F. The surgeon and the oncologist in non-small cell lung cancer (NSCLC). Ann Oncol. 2006;17 Suppl 5:v94-v98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Akman M, Monteleone C, Doronzo G, Godel M, Napoli F, Merlini A, Campani V, Nele V, Balmas E, Chontorotzea T, Fontana S, Digiovanni S, Barbu FA, Astanina E, Jafari N, Salaroglio IC, Kopecka J, De Rosa G, Mohr T, Bertero A, Righi L, Novello S, Scagliotti GV, Bussolino F, Riganti C. TFEB controls sensitivity to chemotherapy and immuno-killing in non-small cell lung cancer. J Exp Clin Cancer Res. 2024;43:219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Shyam Sunder S, Sharma UC, Pokharel S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Signal Transduct Target Ther. 2023;8:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 181] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 9. | Luo Y, Zhang Z, Guo X, Tang X, Li S, Gong G, Gao S, Zhang Y, Lin S. Comparative safety of anaplastic lymphoma kinase tyrosine kinase inhibitors in advanced anaplastic lymphoma kinase-mutated non-small cell lung cancer: Systematic review and network meta-analysis. Lung Cancer. 2023;184:107319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Bauer TM, Felip E, Solomon BJ, Thurm H, Peltz G, Chioda MD, Shaw AT. Clinical Management of Adverse Events Associated with Lorlatinib. Oncologist. 2019;24:1103-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Pfisterer J, Shannon CM, Baumann K, Rau J, Harter P, Joly F, Sehouli J, Canzler U, Schmalfeldt B, Dean AP, Hein A, Zeimet AG, Hanker LC, Petit T, Marmé F, El-Balat A, Glasspool R, de Gregorio N, Mahner S, Meniawy TM, Park-Simon TW, Mouret-Reynier MA, Costan C, Meier W, Reinthaller A, Goh JC, L'Haridon T, Baron Hay S, Kommoss S, du Bois A, Kurtz JE; AGO-OVAR 2. 21/ENGOT-ov 18 Investigators. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Wang M, Yin Z, Miao J, Wu Y. The fetal outcomes after neoadjuvant platinum and paclitaxel chemotherapy during pregnancy: analysis of three cases and review of the literature. Arch Gynecol Obstet. 2022;305:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Tossetta G. Metformin Improves Ovarian Cancer Sensitivity to Paclitaxel and Platinum-Based Drugs: A Review of In Vitro Findings. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | Lei T, Xu T, Zhang N, Zou X, Kong Z, Wei C, Wang Z. Anlotinib combined with osimertinib reverses acquired osimertinib resistance in NSCLC by targeting the c-MET/MYC/AXL axis. Pharmacol Res. 2023;188:106668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 15. | Wu J, Liang J, Liu R, Lv T, Fu K, Jiang L, Ma W, Pan Y, Tan Z, Liu Q, Qiu W, Ge M, Wang J. Autophagic blockade potentiates anlotinib-mediated ferroptosis in anaplastic thyroid cancer. Endocr Relat Cancer. 2023;30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Zhang C, Wang J, Wang X, Meng Z, Cheng Y, Li K. Peripheral blood indices to predict PFS/OS with anlotinib as a subsequent treatment in advanced small-cell lung cancer. Cancer Biol Med. 2021;19:1249-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Li J, Han B, Liu H. Outcomes of Anlotinib Maintenance Therapy in Patients With Advanced NSCLC in a Real-World Setting. Front Oncol. 2022;12:785865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Chen Y, Jiang N, Liang X, Chen N, Chen Y, Zhang C, Shi J, Guo R. Lowdose anlotinib combined with EGFRTKI can be used as an alternative for EGFRTKIresistant nonsmall cell lung cancer in elderly patients. Oncol Lett. 2023;26:323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Zhang C, Cao H, Cui Y, Jin S, Gao W, Huang C, Guo R. Concurrent use of anlotinib overcomes acquired resistance to EGFR-TKI in patients with advanced EGFR-mutant non-small cell lung cancer. Thorac Cancer. 2021;12:2574-2584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 20. | Ye H, Li Z, Liu K, Zhang F, Cheng Z. Anlotinib, a novel TKI, as a third-line or further-line treatment in patients with advanced non-small cell lung cancer in China: A systemic review and meta-analysis of its efficacy and safety. Medicine (Baltimore). 2021;100:e25709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, Escriu C, Peters S; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1-iv21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1352] [Article Influence: 169.0] [Reference Citation Analysis (0)] |
| 22. | Esfahanian N, Chan SWS, Zhan LJ, Brown MC, Khan K, Lee J, Balaratnam K, Yan E, Parker J, Garcia-Pardo M, Barghout SH, Eng L, Bradbury PA, Shepherd FA, Leighl NB, Sacher AG, Snow S, Juergens R, Liu G. Presentation and outcomes of KRAS(G12C) mutant non-small cell lung cancer patients with stage IV disease at diagnosis (de novo) versus at recurrence. Cancer Treat Res Commun. 2023;37:100774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Suda K, Mitsudomi T. [Current Status and Future Perspectives of Molecular Targeted Therapies for Lung Cancer]. Kyobu Geka. 2022;75:53-66. [PubMed] |
| 24. | West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, Sadiq A, Sandler A, Lin W, Ochi Lohmann T, Archer V, Wang L, Kowanetz M, Cappuzzo F. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 1193] [Article Influence: 198.8] [Reference Citation Analysis (0)] |
| 25. | Oi H, Matsuda T, Kimura T, Morise M, Yamano Y, Yokoyama T, Kataoka K, Kondoh Y. Weekly nanoparticle albumin-bound paclitaxel and paclitaxel for relapsed small cell lung cancer: A retrospective observational study. Medicine (Baltimore). 2022;101:e28863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Cheng JD, Chai LX, Zhao ZP, Hao YY, Li S. Efficacy and Safety of Anlotinib for Patients with Advanced NSCLC Who Progressed After Standard Regimens and the Preliminary Analysis of an Efficacy Predictor. Cancer Manag Res. 2020;12:5641-5650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Wang M, Mao M, Yang Y, Cai Z, Li Y, Chen Y, Cai J, Ye Q. Safety and efficacy of anlotinib hydrochloride capsules in advanced non-small-cell lung cancer: a multicenter, real-world study. Future Oncol. 2023;19:1729-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Rajkumar V, Goh V, Siddique M, Robson M, Boxer G, Pedley RB, Cook GJ. Texture analysis of (125)I-A5B7 anti-CEA antibody SPECT differentiates metastatic colorectal cancer model phenotypes and anti-vascular therapy response. Br J Cancer. 2015;112:1882-1887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Fang R, Zhu Y, Khadka VS, Zhang F, Jiang B, Deng Y. The Evaluation of Serum Biomarkers for Non-small Cell Lung Cancer (NSCLC) Diagnosis. Front Physiol. 2018;9:1710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Hou M, Ma L, Yang H, Si F, Liu Y. Background-free and signal-amplified upconversion fluorescent biosensing platform for sensitive detection of CYFRA21-1. Talanta. 2023;262:124659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 31. | Wang J, Yi Y, Li B, Wang Z, Sun H, Zhang P, Huang W. CYFRA21-1 can predict the sensitivity to chemoradiotherapy of non-small-cell lung carcinoma. Biomarkers. 2010;15:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Zhao Y, Guo S, Deng J, Shen J, Du F, Wu X, Chen Y, Li M, Chen M, Li X, Li W, Gu L, Sun Y, Wen Q, Li J, Xiao Z. VEGF/VEGFR-Targeted Therapy and Immunotherapy in Non-small Cell Lung Cancer: Targeting the Tumor Microenvironment. Int J Biol Sci. 2022;18:3845-3858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 121] [Reference Citation Analysis (0)] |
| 33. | Li DW, Wu BZ, Shi YS, Li ZQ, Liu XD, Li XH. Association of CT perfusion imaging with plasma levels of TGF-β1 and VEGF in patients with NSCLC. Asian Pac J Trop Med. 2016;9:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Shao Y, Luo Z, Yu Y, He Y, Liu C, Chen Q, Zhu F, Nie B, Liu H. A real-world study of anlotinib as third-line or above therapy in patients with her-2 negative metastatic breast cancer. Front Oncol. 2022;12:939343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 35. | Tan T, Han G, Cheng Z, Jiang J, Zhang L, Xia Z, Wang X, Xia Q. Genetic Polymorphisms in CYP2C19 Cause Changes in Plasma Levels and Adverse Reactions to Anlotinib in Chinese Patients With Lung Cancer. Front Pharmacol. 2022;13:918219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |