Published online May 19, 2025. doi: 10.5498/wjp.v15.i5.102552
Revised: December 24, 2024
Accepted: March 4, 2025
Published online: May 19, 2025
Processing time: 190 Days and 19.7 Hours
Exploring hypnotherapy's potential to modulate attention bias offers promising avenues for treating social anxiety disorder (SAD).
To determine if hypnotherapy can alleviate social anxiety by influencing attention bias, specifically identifying the aspects of attention processes affected by hypnosis.
In this study, 69 SAD participants were divided into three groups based on their Liebowitz Social Anxiety Scale scores: Experimental group, control group, and baseline group. The experimental group (n = 23) underwent six weekly hypnosis sessions, while the control (n = 23) and baseline groups (n = 23) received no treatment. To evaluate alterations in SAD severity and attention bias towards threatening stimuli following hypnotherapy, we employed a combination of self-report questionnaires, an odd-one-out task, and electroencephalography recordings.
The experimental group showed significant reductions in P1, N170, N2pc, and late positive potential (LPP) brain wave activities during attention sensitivity and disengagement conditions. This indicates that hypnotherapy modulates early-stage face processing and later-stage emotional evaluation of threat-related stimuli in SAD patients.
Our findings highlight P1, N170, N2pc, and LPP as key neural markers in SAD treatment. By identifying these neural markers influenced by hypnotherapy, we offer insight into the mechanisms by which this treatment modality impacts attentional processes, potentially easing SAD symptoms.
Core Tip: Our study rigorously investigates the utility of hypnotherapy in ameliorating the symptoms of social anxiety disorder (SAD) by focusing on the modulation of attention bias. Utilizing a rigorous methodological design, including a sample of 69 participants diagnosed with SAD, and employing electroencephalography measurements, we provide compelling evidence that hypnotherapy can significantly alter attentional processes. Specifically, we observed marked reductions in P1, N170, N2pc, and late positive potential components, which were positively correlated with symptom improvement. To the best of our knowledge, this is the first event-related potential study to investigate the effects of hyp
- Citation: Zhang H, Zhang M, Li N, Wei WZ, Yang LX, Li YY, Zu ZY, Ma LJ, Wang HX, Wang K, Li XM. Event-related potentials reveal hypnotherapy's impact on attention bias in social anxiety disorder. World J Psychiatry 2025; 15(5): 102552
- URL: https://www.wjgnet.com/2220-3206/full/v15/i5/102552.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i5.102552
Social anxiety disorder (SAD), a common form of anxiety, is marked by enduring fear of social interactions, public performances, or being watched and judged, as noted by researchers[1,2]. Recent research highlights the crucial influence of cognitive biases in SAD's development[3,4]. Notably, a focus on attentional bias, which is strongly linked to SAD in various studies, has been identified as a key area[5,6]. Researchers also suggest that those with SAD tend to process negative emotional information more readily, indicating that this bias in attention could be fundamental in the emergence and persistence of anxiety-related symptoms[7,8].
Hypnosis, an advanced method in psychology, has been shown to significantly alter states of consciousness and cognitive processes[9,10]. The effectiveness of hypnosis in enhancing imaginative engagement is further strengthened by its ability to provoke strong mental imagery and foster relaxation, potentially increasing patient endurance in exposure therapy[11]. Our previous research found that hypnosis reduces examination-related stress by influencing students' focus of attention through hypnotic cues[12]. This suggests that hypnosis not only mitigates social anxiety symptoms but also impacts attentional bias. However, the exact brain processes by which hypnosis reduces social anxiety are not fully understood, and there is a lack of neuroimaging research focusing on this impact [13]. To more clearly comprehend the specific mechanisms responsible for the changes in attentional bias caused by hypnosis, employing event-related potentials (ERPs) is seen as a valuable method for investigation.
People with SAD often show a heightened tendency to focus on threat-related signals and struggle to shift their attention away from these perceived threats[14-16]. The "odd-one-out" task[17] was used to precisely assess the increased attentional sensitivity and slower disengagement from threatening stimuli in anxious individuals. In this task, participants determine if facial expressions in a grid are matching or not. Studies have noted that those with increased anxiety levels quickly notice threatening faces but have a delayed response in shifting their focus away from them.
In our recent research, which sought to clarify changes in social anxiety symptoms and attentional bias before and after hypnosis, we recorded electroencephalography (EEG) signals as participants performed the odd-one-out task. Building on earlier studies[18], we concentrated on particular EEG components—specifically P1, N2pc, N170, and late positive potential (LPP)—to reveal how hypnosis might influence the timing and dynamics of attentional processing.
P1 exhibits heightened response to threat-related facial expressions like fear, disgust, and anger[19], indicating the automatic nature of rapid threat detection. It reflects early resource allocation and automatic visual stimulus processing[20], with increased amplitude signifying greater attentional focus on the stimulus.
The N2pc component, evident during the N2 latency phase at posterior electrodes opposite to the target location, signifies visuospatial selective attention. It highlights focused processing of relevant tasks or distractor stimuli due to their novelty[21]. Past studies have shown a more distinct N2pc response to angry faces over happy ones in visual search activities[22]. Furthermore, an investigation using sketchy faces as stimuli revealed an N2pc pattern akin to that produced by real faces[23]. These insights imply that individuals with heightened social anxiety may exhibit a more pronounced N2pc component due to intense focus on threats.
The N170 component, a face-sensitive ERP marker, is characterized by temporal-parietal negativity linked to the perceptual encoding of faces[24]. Its amplitude increases with emotional intensity, particularly for negative emotions[25,26], and is larger in individuals with high social anxiety when viewing threatening faces[18]. This indicates early reactivity to facial emotions in SAD but requires further investigation to understand the mechanisms and potential modulatory effects of therapeutic interventions.
LPP, another ERP component, emerges during emotional stimulus processing and represents prolonged focus on emotional stimuli[27,28]. Studies show that emotional stimuli generally demand more attention, with higher LPP amplitude in response to threatening expressions compared to positive ones in individuals with high anxiety, indicating intensive perceptual interaction with negative expressions. Thus, LPP may be an effective indicator for measuring emotional responses in SAD[29].
In this research, we set out to evaluate the effects of a six-week hypnotherapy program on social anxiety, attentional bias (gauged via the odd-one-out visual search task), and EEG markers linked to how participants process different emotional facial expressions and their attentional biases. Drawing from previous studies, we hypothesized that hypnotherapy would not only decrease social anxiety but also modify attentional bias, as evident in the outcomes of the odd-one-out task. Moreover, our aim was to clarify the neurocognitive processes affected by hypnosis on attentional bias through the analysis of ERPs. In line with our initial predictions, we anticipated that post-treatment, individuals with SAD would show decreased P1, N2pc, N170, and LPP amplitudes in the specified task.
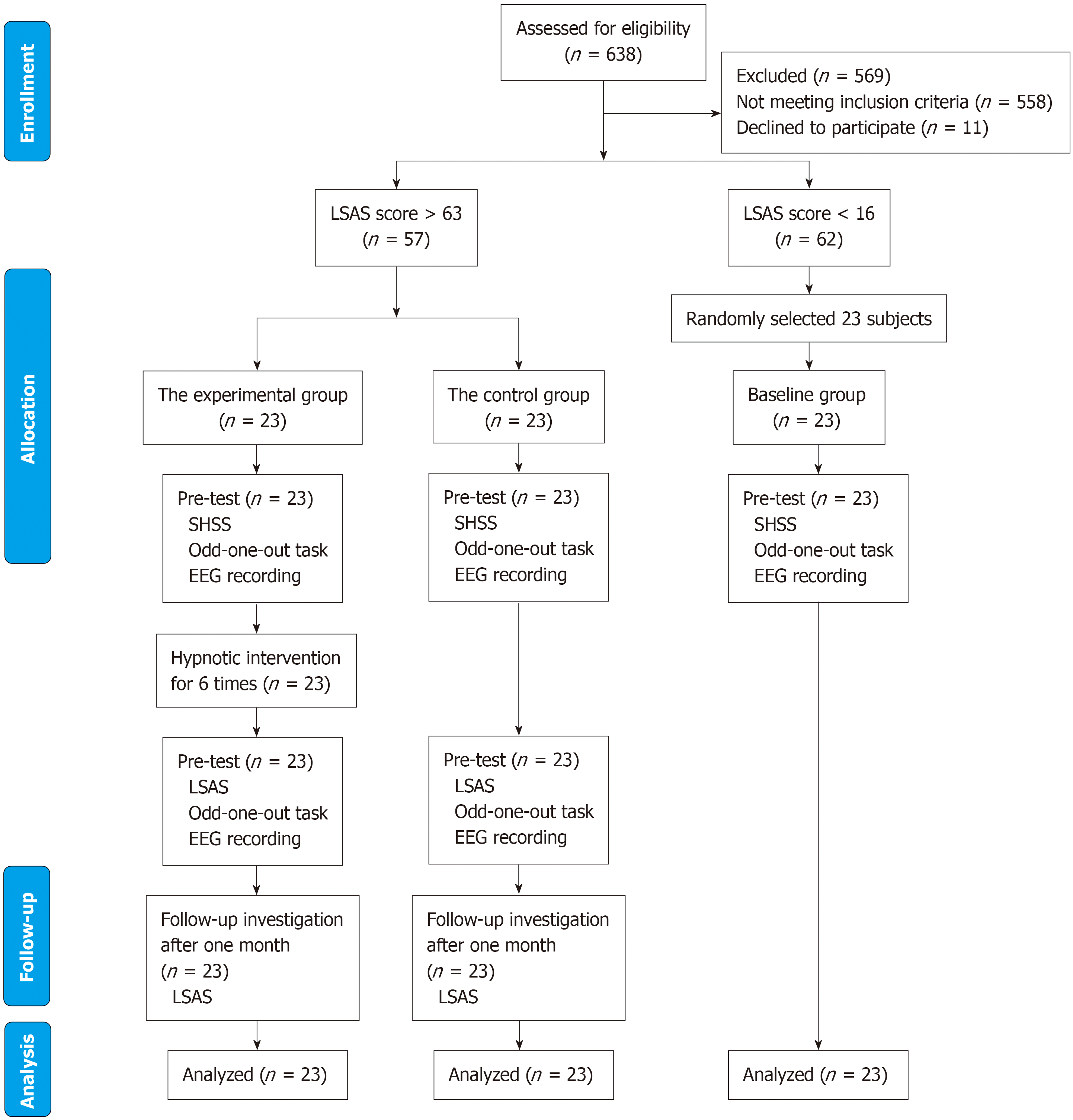
Participants were drawn from a pool of 638 undergraduate students based on their performance on the Chinese version of the Liebowitz Social Anxiety Scale (LSAS)[30]. Additionally, diagnoses were made by two psychiatrists from Anhui Medical University. Each psychiatrist independently evaluated the participants using standardized diagnostic criteria. To ensure reliability, a consensus was required for the final diagnosis. If there was any disagreement, it was resolved through discussion until a mutual agreement was reached. This approach aimed to minimize individual bias and enhance the accuracy of the diagnostic outcomes. Participants with an LSAS score exceeding 63 were randomly assigned to either the experimental group (n = 23, comprising 11 males and 12 females, with an average age of 19.57 years and SD = 0.72 years) or the control group (n = 23, consisting of 14 males and 9 females, with an average age of 19.48 years and SD = 0.90 years). Individuals scoring below 16 points were allocated to the baseline group (n = 23, including 8 males and 15 females, with an average age of 19.57 years and SD = 0.51 years). The threshold of 63 was chosen to minimize the risk of false positives among individuals without SAD[31]. Due to the nature of the intervention, it was not feasible to blind therapists or participants to the assignment of hypnosis and control conditions. All participants had normal or corrected-to-normal vision. During the participant screening process, we excluded individuals with severe physical illnesses, neurological or nervous system diseases, depression, severe medical conditions, or other health issues that could potentially affect the experimental results. Additionally, those currently undergoing psychological treatment or taking psychotropic medi
Prior to the pre-test, participants were asked to complete two questionnaires: The LSAS and the Stanford Hypnotic Susceptibility Scale. Following this initial assessment, all participants took part in an odd-one-out task while their EEG data was recorded, enabling the collection of ERP data. After completing the pre-test, which spanned one or two days, the experimental group underwent a hypnosis intervention. This intervention was administered weekly across a six-week period, comprising 30-minute sessions per intervention cycle. No interventions were administered to the control and baseline groups. Within a 48-hour window following hypnosis intervention completion, both experimental and control participants underwent post-intervention assessments mirroring the initial evaluation procedures. One month following the post-test, both groups were asked to complete the LSAS questionnaire. A schematic representation of the study's procedures is provided in Figure 1.
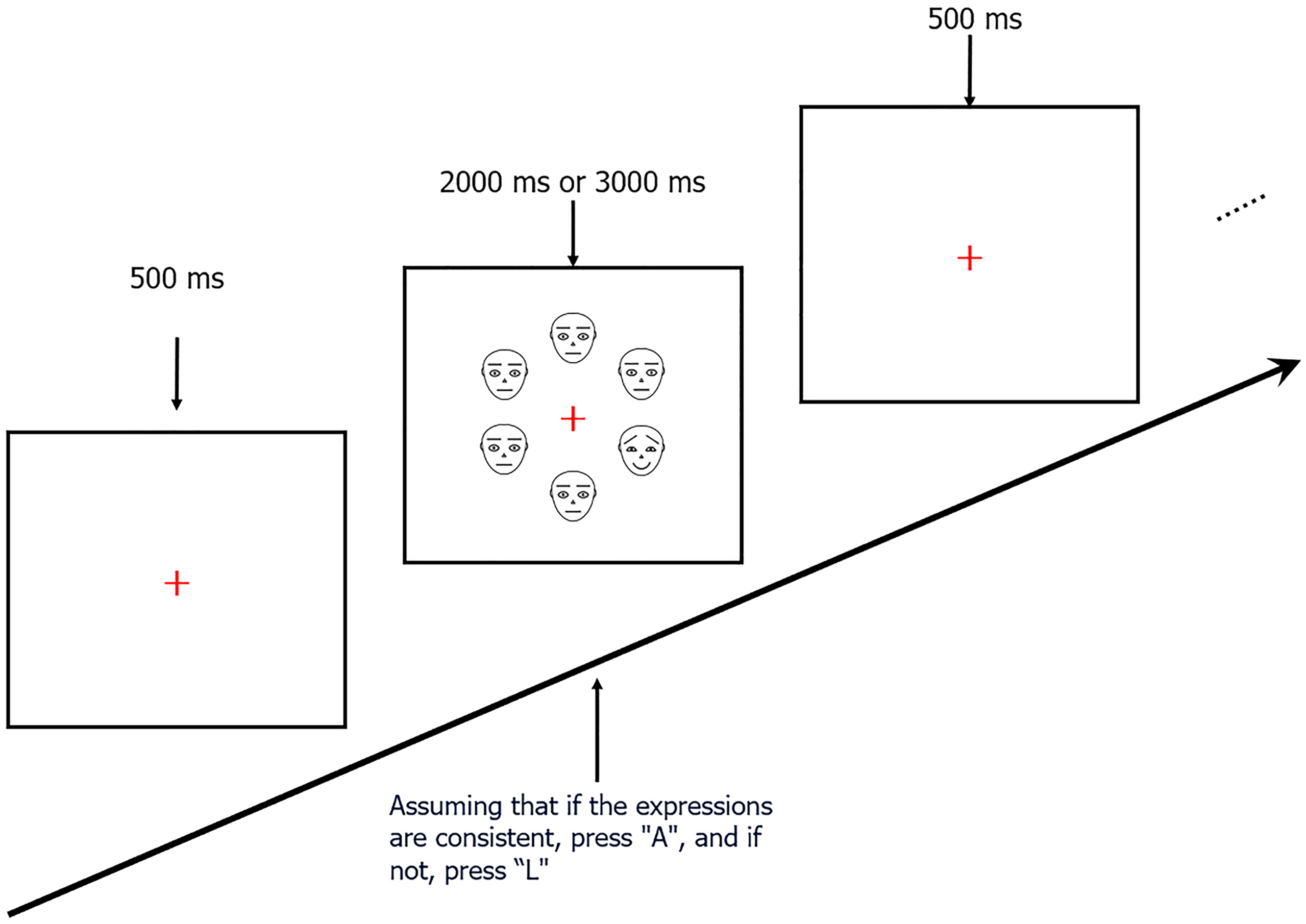
The E-prime 2.0 was used for both presenting experimental stimuli and recording behavioral responses. Participants were shown a sequence of facial images arranged in a circle. Initially, a fixation point appeared on the computer screen for 500 milliseconds (ms), followed by a set of facial images. These sets varied, including either one emotional and five neutral faces, all neutral faces, one neutral and five emotional faces, or all emotional faces. The images were displayed for a duration of either 2000 ms or 3000 ms. Participants had to decide if all six facial images were the same, pressing "A" for yes or "L" for no (Figure 2). In the task measuring attention sensitivity, emotional faces were intermittently shown among neutral faces. In contrast, for the attention disengagement task, neutral faces appeared amidst emotional faces. A 36-trial practice session was conducted before the main experiment to acclimate participants to the procedure. The actual experiment comprised two tasks, one to assess attention sensitivity and another to evaluate difficulty in disengaging attention, each containing 360 trials. The order of these tasks was randomized to avoid sequence bias, and a 3-minute intermission was given between tasks for participant comfort and to maintain concentration.
The study's experimental group underwent a series of six hypnosis sessions, each lasting 30 minutes, scheduled weekly over a span of around one and a half months. These sessions were led by a skilled hypnotherapist who utilized a standardized procedure for inducing hypnosis[32]. In contrast, the control group and the baseline group did not undergo any intervention. Hypnotic suggestion text can be found in the Supplementary material.
EEG recordings were made using a 64-channel NeuroScan system (NeuroScan Inc., Herndon, VA). Reference points for the recordings included the nose tip and the left and right mastoids. Vertical EEG data were gathered from above and below the left eye, and horizontal EEG data from the outer edges of both eyes. To guarantee the quality of the data, the impedance of all channels was kept under 5 kΩ before starting ERP recordings. The EEG was continuously sampled at a rate ranging from 0.05 to 100 Hz for later offline analysis. The EEG data were then segmented into 1000 ms periods for offline examination, starting 100 ms before and extending to 900 ms after the stimulus onset. Artifacts caused by eye movements, such as blinks or shifts, were carefully removed during the data processing stage. The average amplitudes for different facial expression categories (like angry vs happy) and types of target stimuli (such as attention sensitivity vs attention disengagement) were calculated separately.
The P1 component was quantified by calculating the average activity between 80 ms and 130 ms post-stimulus at electrode sites O1 and O2. Extending upon prior investigations, the N170 component was evaluated at electrode positions P7 and P8. This assessment involved computing the average activity within the time window spanning from 130 ms to 190 ms after stimulus onset. The time frame for computing the average amplitude of the N2pc component, evoked by facial targets, was set at 200-300 ms following stimulus presentation. The analysis primarily focused on electrode sites Po7 and Po8. The LPP component was quantified by calculating the mean activity from 400 ms to 600 ms after the stimulus at electrode positions P1, Pz, and P2[33,34].
In the pre-test stage, we observed that there was no significant difference in the reaction time of the experimental group and the control group to emotional facial expressions under the attention sensitivity and attention disengagement conditions. However, the reaction time of the two groups to emotional facial expressions was significantly different from that of the baseline group. These two groups were able to more sensitively detect emotional facial expressions, as evidenced by their shorter reaction time when presented against a neutral background. In terms of attention disengagement, they found it more challenging to disengage from emotional facial expressions, resulting in longer reaction times when judging the consistency of the facial expressions when presented against an emotional background. In the post-test stage after hypnotic intervention, we found that under the attention sensitivity conditions, the reaction time of the experimental group to angry and happy facial expressions was significantly longer than that in the pre-test. Under the attention disengagement conditions, the reaction time of the experimental group to emotional facial expre
There was no significant difference in LSAS scores between the experimental group and the control group at the pre-test stage. However, as the experiment progressed, only the experimental group exhibited a significant reduction in LSAS scores, which persisted through both the post-test and one-month follow-up stages. More detailed behavioral results will be presented in another article.
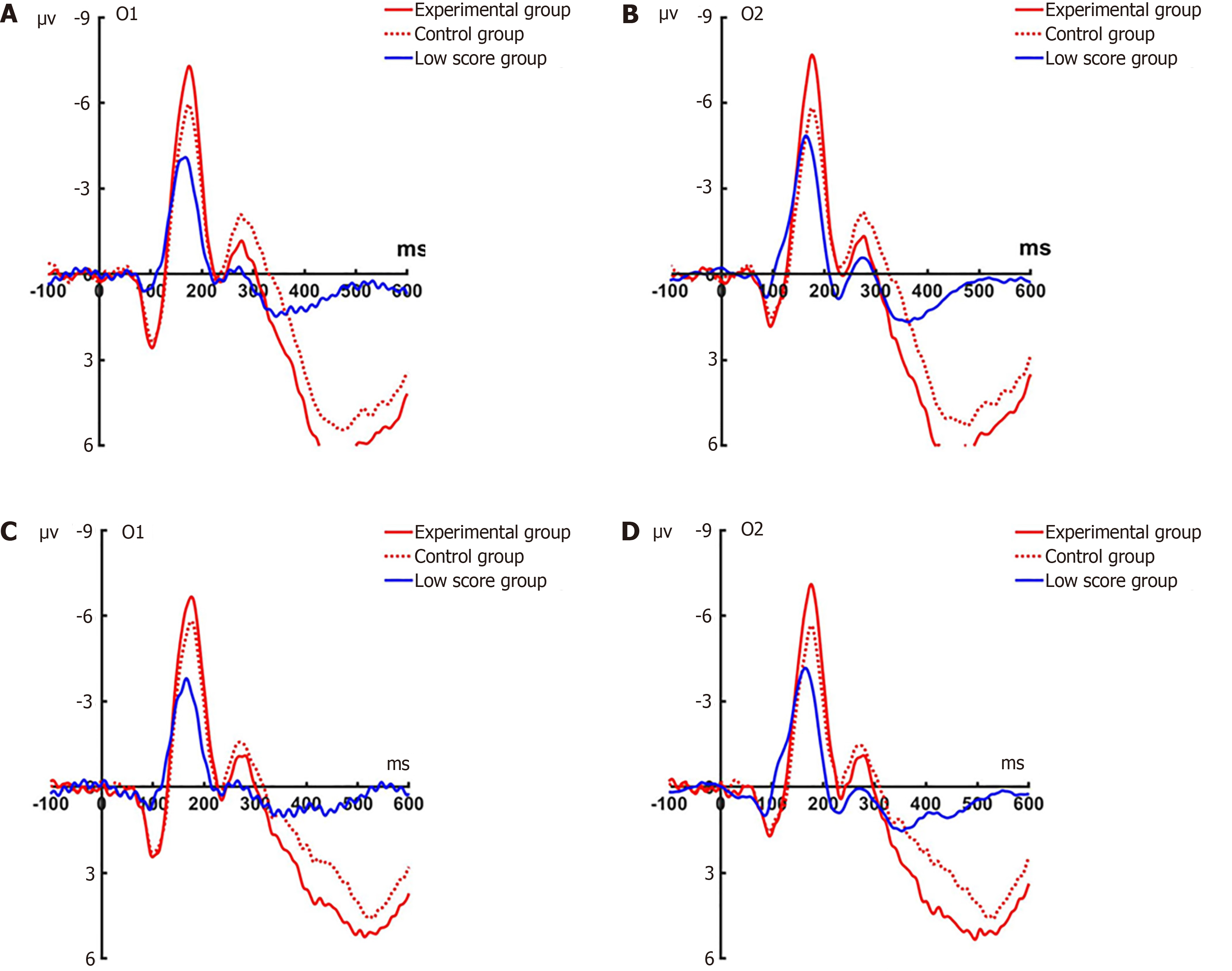
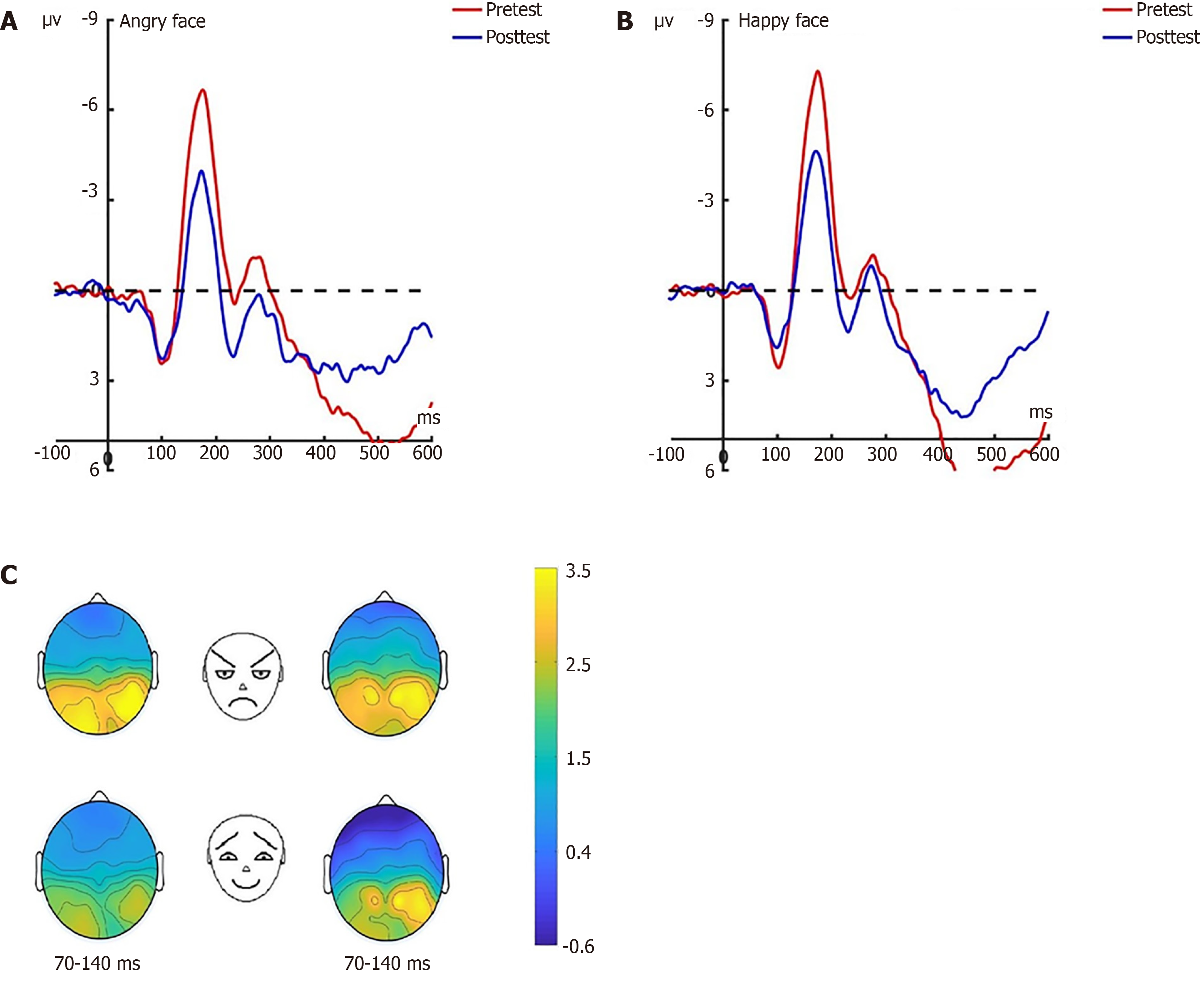
P1 pre-test: Under the attention sensitivity conditions, a repeated-measures analysis of variance (ANOVA) was conducted on the amplitude of P1 with factors of 2 (electrode: O1, O2) × 2 (faces: Angry, happy) × 3 (groups: Experimental, control, baseline). Significant main effects emerged for group (F(2, 66) = 17.417, P < 0.001) and electrode site (F(1, 66) = 7.421, P = 0.012). The main effect of face was not significant (F(2, 66) = 0.139, P = 0.712). A significant interaction effect between time and face was observed (F(1, 44) = 5.461, P = 0.012). Follow-up simple effects analyses demonstrated marked between-group disparities in responses to angry faces at both occipital sites (O1: F(2, 66) = 17.689, O2: P < 0.001; F(2, 66) = 7.613, P = 0.001), with comparable patterns observed for happy facial processing (O1: F(2, 66) = 16.207, P < 0.001; O2: F(2, 66) = 6.231, P = 0.003). Post-hoc test revealed no significant differences between the experimental group and the control group in response to angry faces at O1 and O2 (O1: P = 0.424; O2: P = 0.281), contrasted with substantial experimental-baseline (both P < 0.001) and control-baseline differences (P < 0.001; P = 0.009). There was analogous non-significance between the experimental and control groups (O1: P = 0.418; O2: P = 0.501), yet pronounced experimental/control vs baseline differences (all P ≤ 0.010; Figure 3).
A mixed-model ANOVA under the disengagement conditions revealed significant main effects for both group (F(2, 66) = 28.241, P < 0.001) and facial valence (face: F(1, 66) = 4.775, P = 0.040), while electrode demonstrated no measurable influence (F(1, 66) = 0.004, P = 0.949; Figure 4).
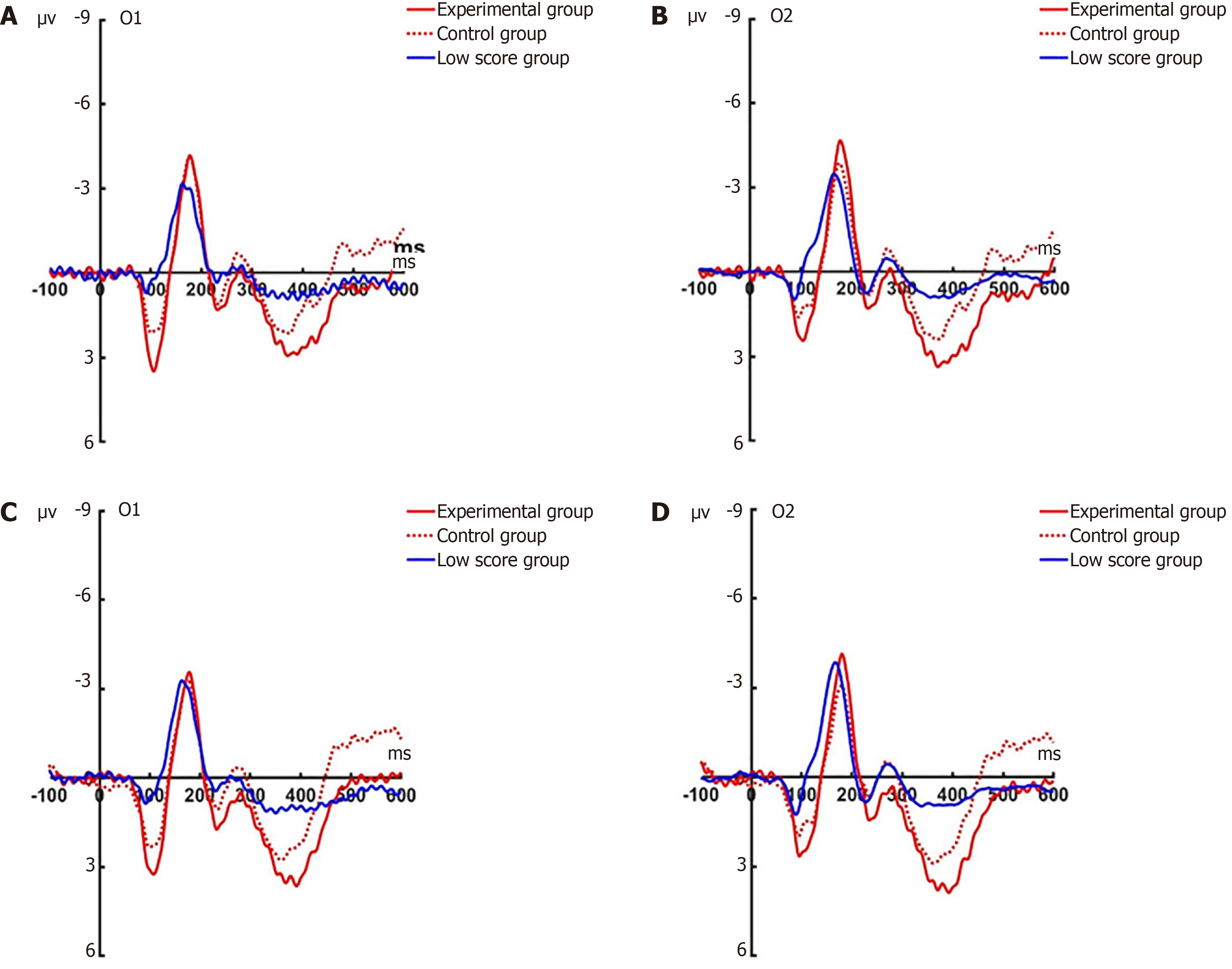
P1 post-test: Under the attention sensitivity conditions, a repeated-measures ANOVA was conducted on the amplitude of P1 with factors of 2 (electrodes: O1, O2) × 2 (faces: Angry, happy) × 2 (groups: Experimental, control) × 2 (time: Pre-test, post-test). The results showed that there were no significant main effects of time (F(1, 44) = 0.322, P = 0.576), face (F(1, 44) = 0.004, P = 0.949), or electrode site (F(1, 44) = 0.077, P = 0.784). The main effect of group was significant (F(1, 44) = 19.185, P < 0.001; Figure 5 for O1 electrode; Figure 6 for O2 electrode).
Under the attention disengagement conditions, a mixed-design ANOVA was performed incorporating four experimental variables: Scalp electrode (O1/O2), facial affect (angry/happy), group (experimental/control), and temporal phase (pre-test/post-test). Statistical evaluation demonstrated a significant main effect of facial expression type (F(1, 44) = 7.288, P = 0.013; Figure 7 for O1 electrode; Figure 8 for O2 electrode).
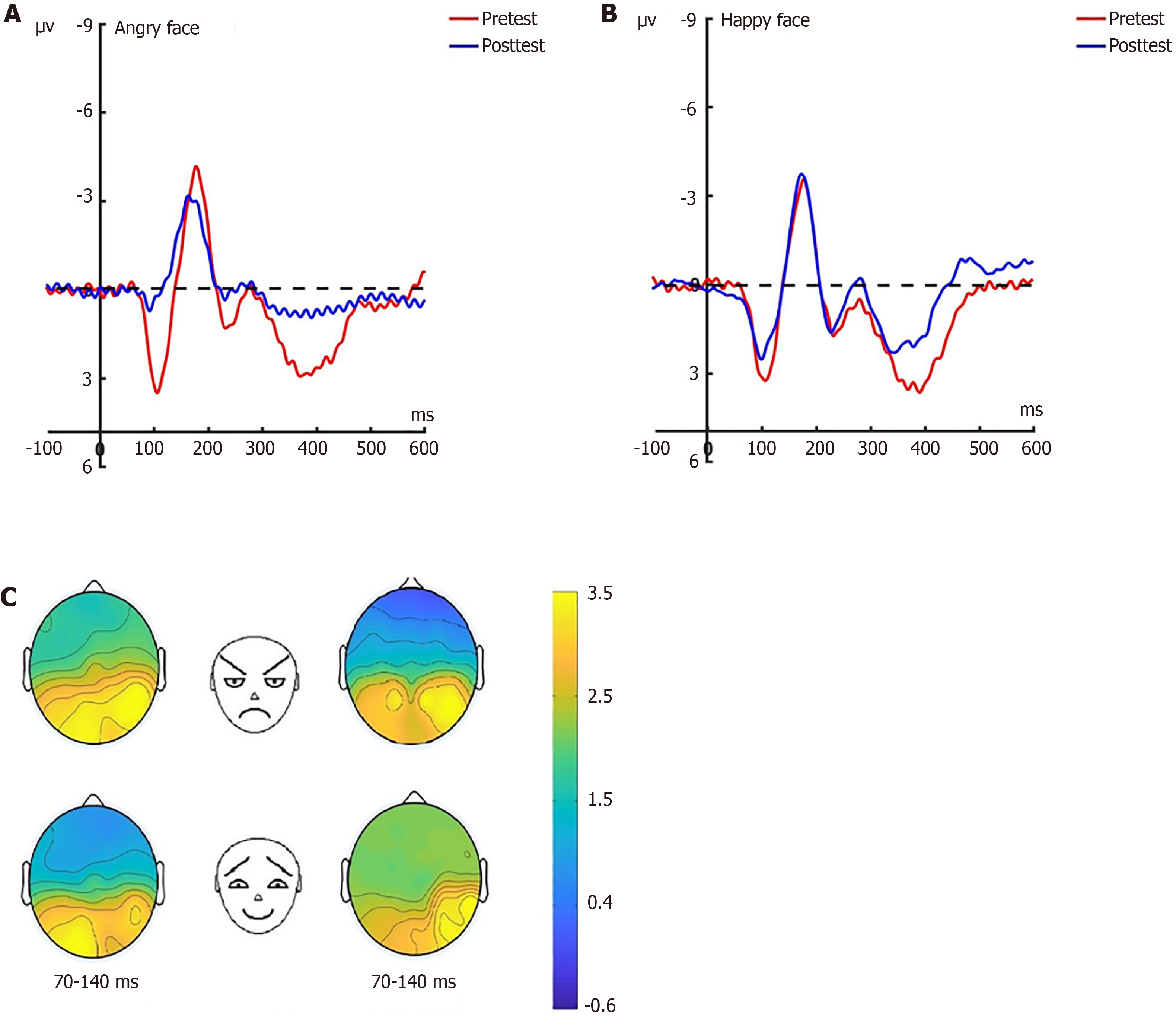
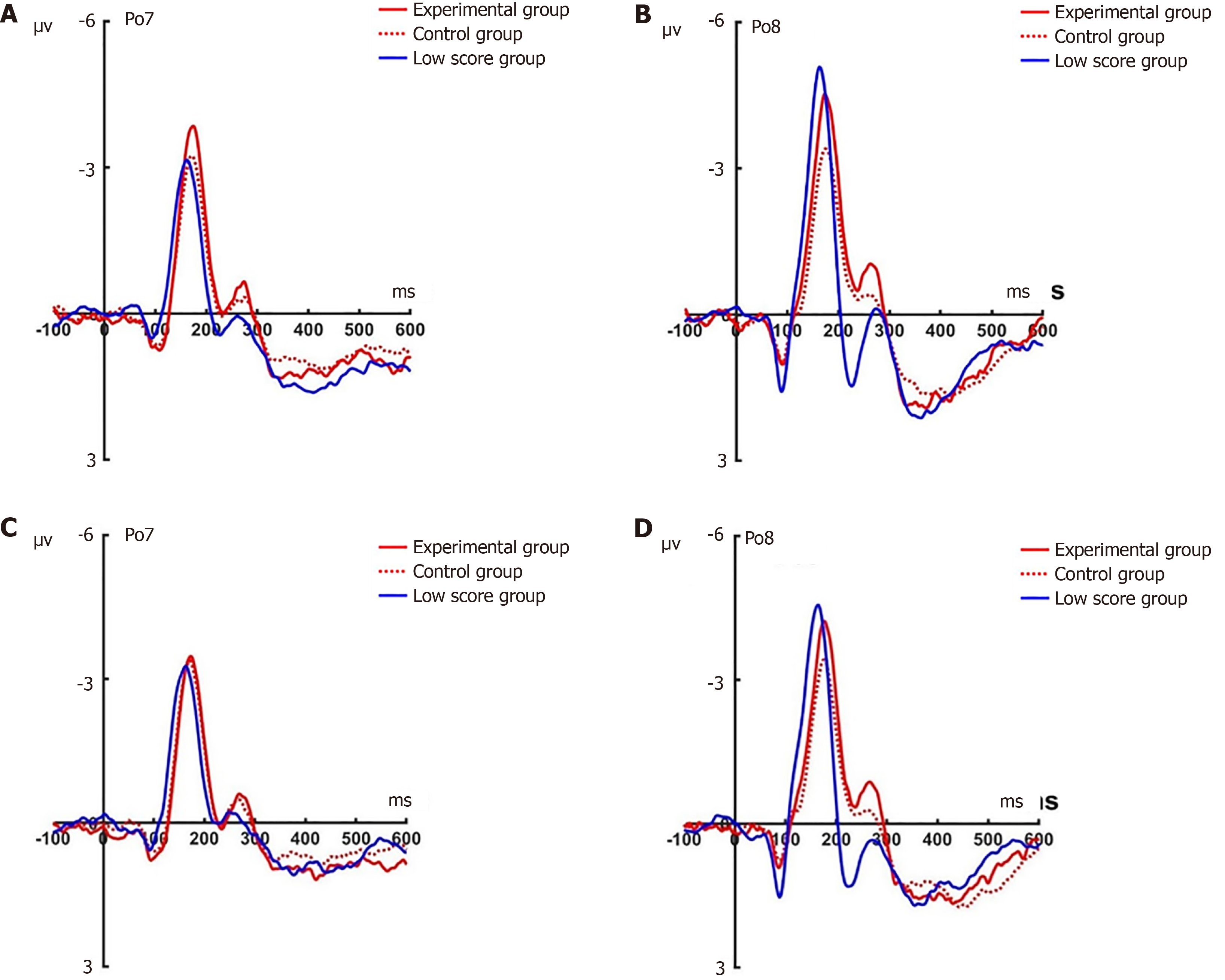
N2pc pre-test: Under the attention sensitivity conditions, a 2 × 2 × 3 factorial repeated-measures ANOVA examining N2pc amplitude was performed, incorporating electrode (Po7/Po8), face (angry/happy), and experimental grouping (experimental/control/baseline). The statistical model identified significant main effects for group (F(2, 66) = 63.87, P < 0.001) and electrode (F(1, 66) = 5.29, P < 0.05), whereas facial stimulus type showed nonsignificant effects (F(2, 66) = 1.87, P = 0.176). Critically, a significant face × electrode interaction emerged (F(1, 66) = 10.71, P < 0.01). Subsequent simple effects analysis confirmed consistent between-group disparities in both angry face processing (Po7: F(2, 66) = 52.66, P < 0.001; Po8: F(2, 66) = 48.10, P < 0.001) and happy face responses (Po7: F(2, 66) = 42.14, P < 0.001; Po8: F(2, 66) = 41.23, P < 0.001). Post-hoc evaluations revealed nonsignificant experimental-control differences for angry faces at Po7 and Po8 (Po7: P = 0.126; Po8: P = 0.007), contrasting with marked experimental-baseline (both P < 0.001) and control-baseline distinctions (both P < 0.001). This pattern persisted for happy face processing, where experimental-control comparisons at Po7 (P = 0.166) and Po8 (P = 0.063) remained nonsignificant, while both the experimental and control groups exhibited pronounced differentiation from baseline across electrodes (all P < 0.001; Figure 9).
Under the attention disengagement conditions, a mixed-model ANOVA yielded a significant main effect of group
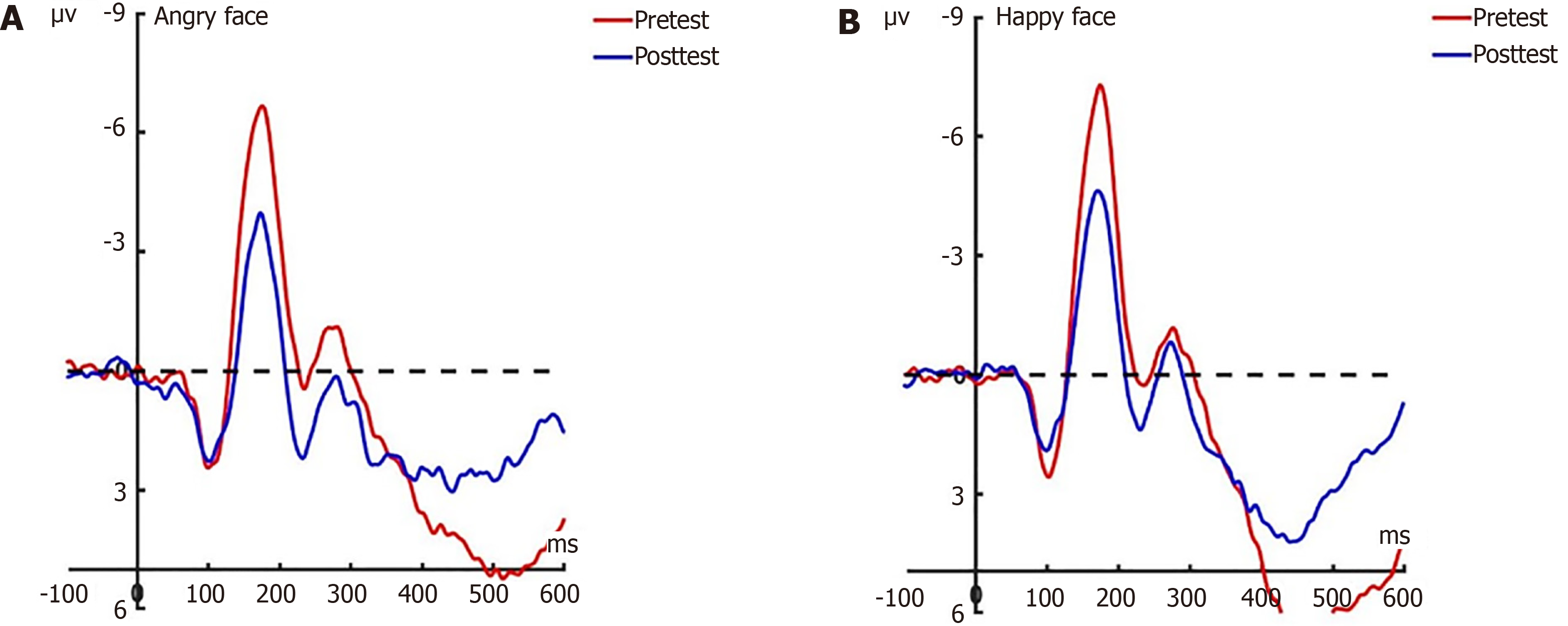
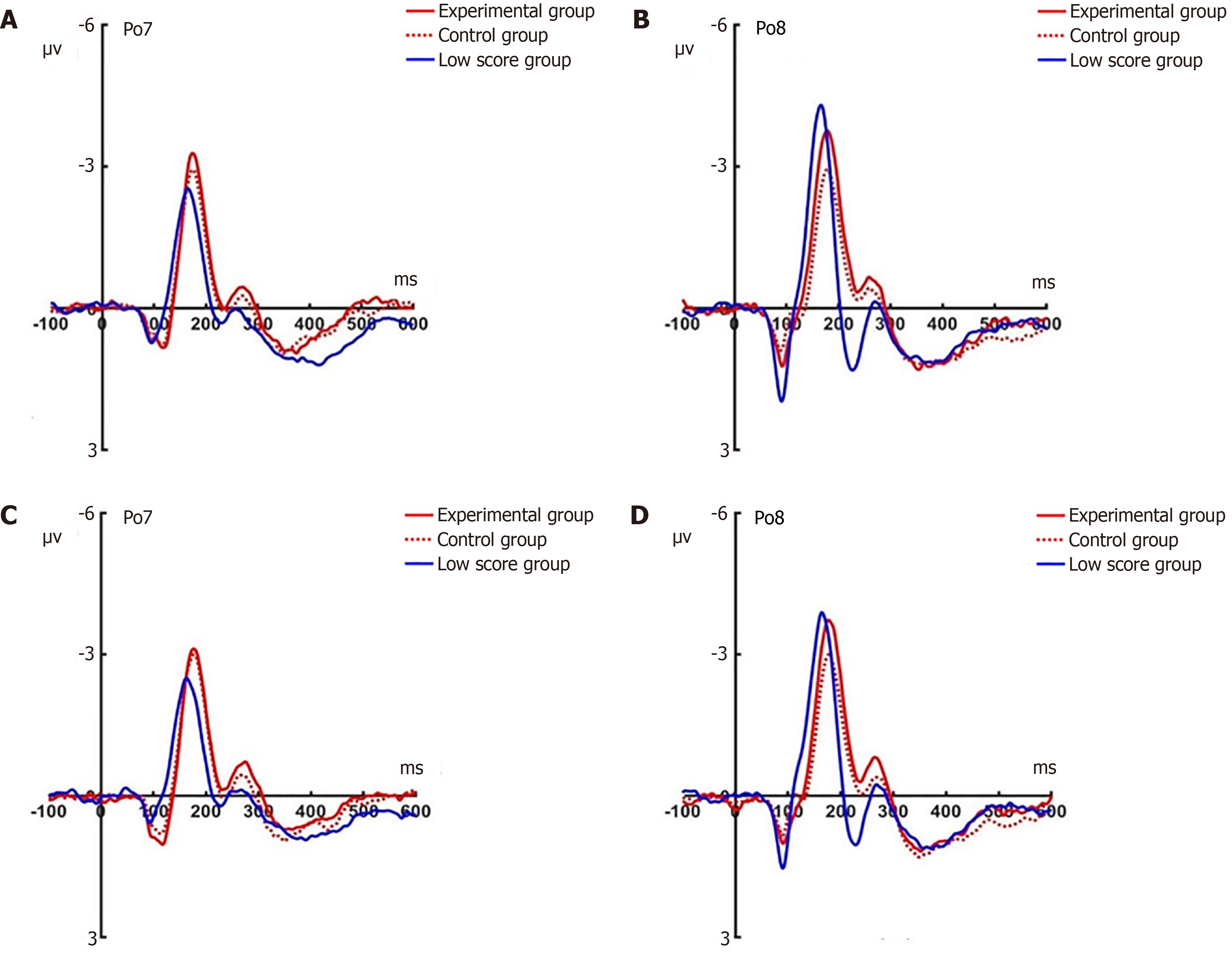
N2pc post-test: Under the attention sensitivity conditions, a 2 × 2 × 2 × 2 factorial repeated-measures ANOVA examining N2pc amplitude variations was performed, incorporating electrode (Po7/Po7), face (angry/happy), experimental grouping (experimental/control), and temporal phase (pre-/post-test). Statistical evaluation revealed a significant temporal main effect (F(1, 44) = 4.74, P < 0.05) alongside robust facial stimulus effects (F(1, 44) = 20.58, P < 0.001), whereas group (F(1, 44) = 3.42, P = 0.071) and electrode localization (F(1, 44) = 0.37, P = 0.546) demonstrated nonsignificant influences. Notably, a face × time interaction emerged (F(1, 44) = 6.79, P < 0.05) , with follow-up analyses indicating amplitude redu
Under the attention disengagement conditions, a repeated measures ANOVA detected significant temporal modulation (F(1, 44) = 11.884, P < 0.05) and face × time interaction (F(1, 44) = 5.31, P < 0.05). Simple effects analysis revealed selective N2pc attenuation for angry faces at both occipital sites in the experimental group (Po7: t(22) = -3.596, P = 0.002; Po8: t(22) = -2.82, P = 0.01; Figure 12). Control group comparisons revealed stable amplitudes across temporal phases for all stimuli at Po7 (happy: t(22) = 0.203, P = 0.841; angry: t(22) = 0.461, P = 0.649) and Po8 (happy: t(22) = 0.263, P = 0.795; angry: t(22) = 0.539, P = 0.595).
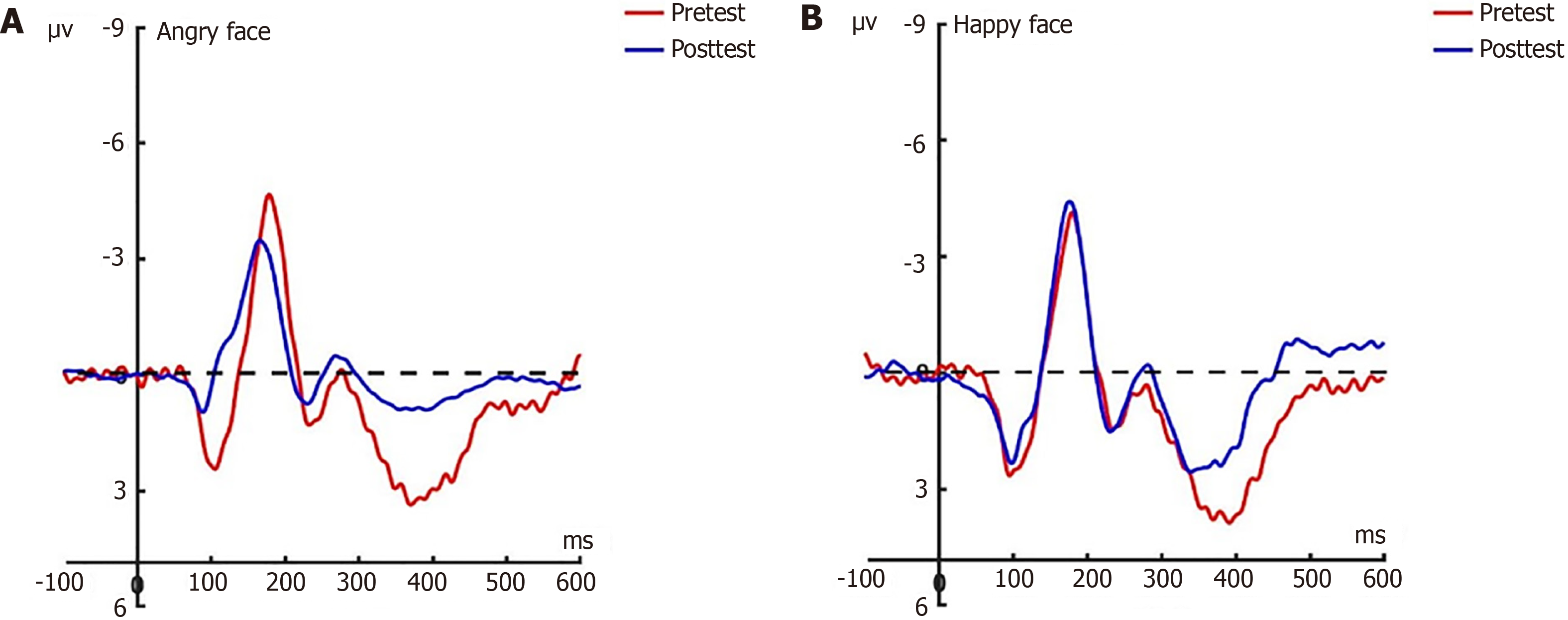
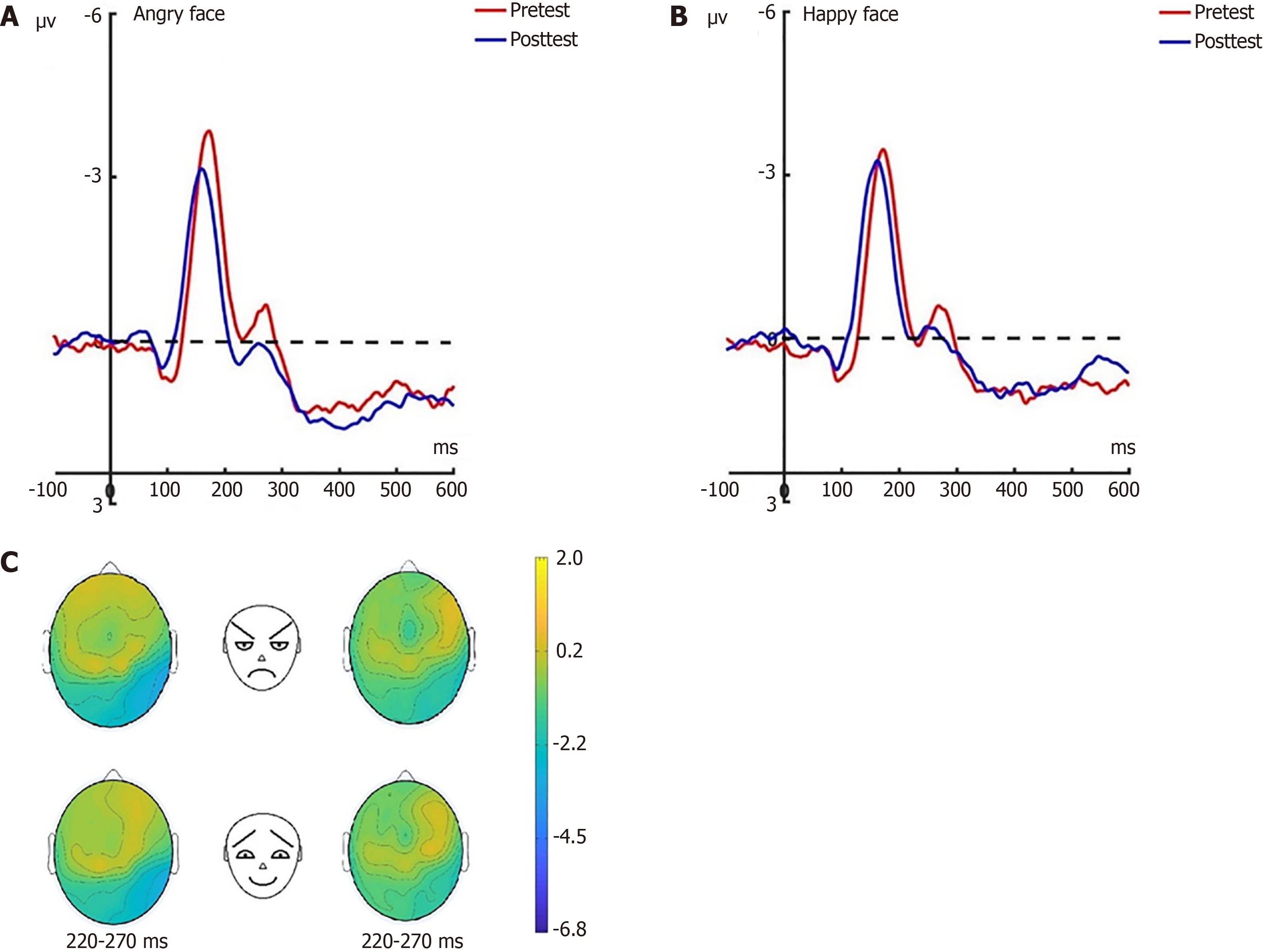
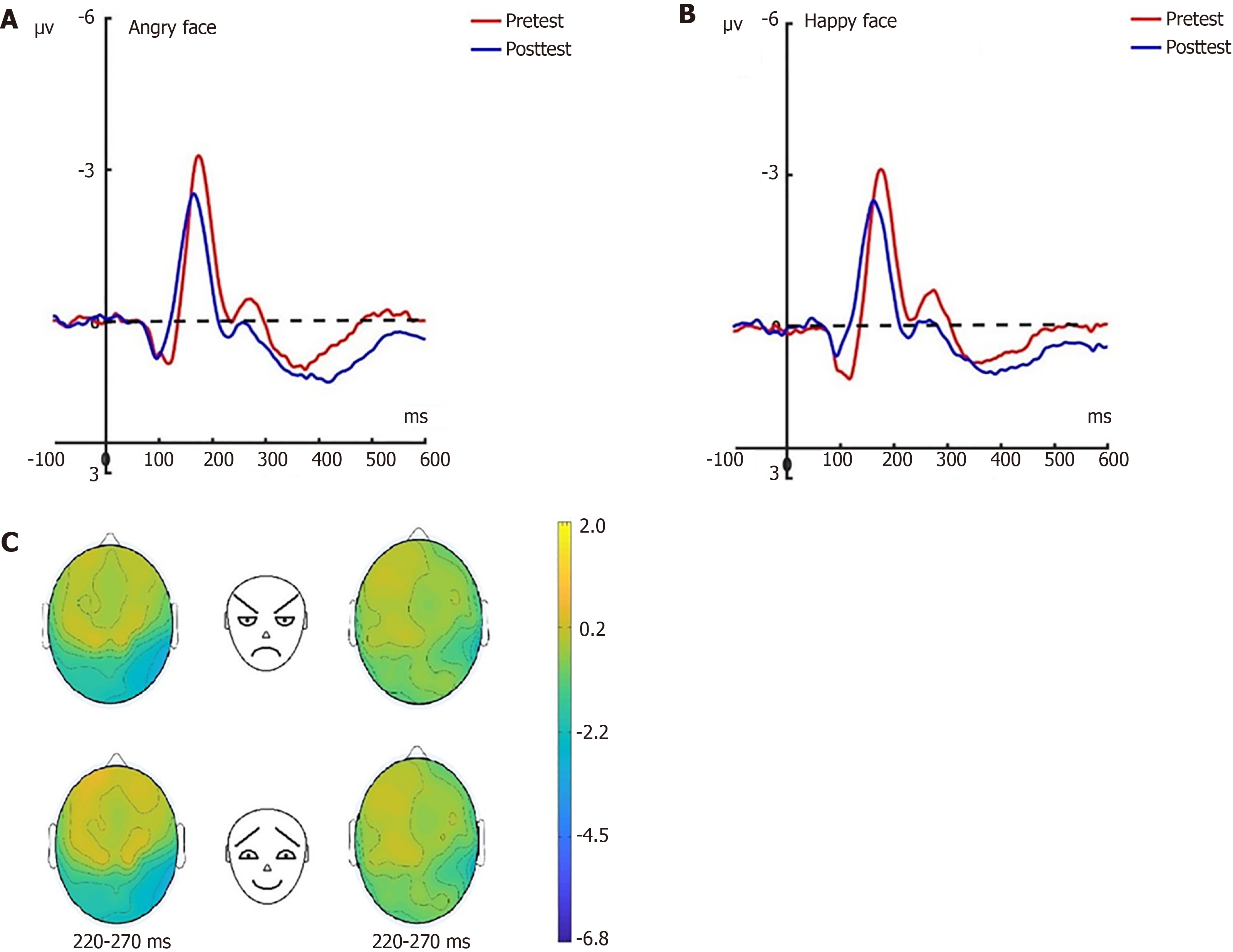
N170: Prior to the hypnotherapy intervention, a repeated measures ANOVA conducted on the N170 amplitude across the three groups revealed significant effects related to group dynamics, electrode placement, and facial expressions under the attention sensitivity conditions, along with a notable interaction effect between facial expressions and electrode placement. Similarly, under the attention disengagement conditions, significant group effects were observed. Post-hypnosis intervention showed that in the experimental group, the N170 amplitude significantly decreased under both attention sensitivity and disengagement conditions, particularly in response to angry facial expressions. Conversely, no significant changes in the N170 amplitude were noted in the control group between pre-test and post-test assessments. Further N170 findings will be detailed in a separate research paper.
LPP: Before the hypnotherapy intervention, a significant impact on the LPP amplitude was observed across the three groups, with main effects attributed to group dynamics, electrode placement, and facial expressions under the attention sensitivity conditions. This was accompanied by significant interaction effects. Similarly, under the attention disen
This pioneering ERP study delves into the impact of hypnosis on social anxiety, specifically examining its influence on attention bias modulation in SAD. Our findings, grounded in behavioral and electrophysiological measures, reveal that hypnotherapy significantly reduces attention bias in individuals with SAD. This is evidenced by decreased sensitivity to threatening stimuli, enhanced ability to shift attention away from these stimuli, and notable improvements in SAD symptoms. Electrophysiologically, we observed marked differences in ERP components between the groups post-hypnosis. Notably, the experimental group showed a significant reduction in P1 amplitude after the intervention, sugge
Attentional biases significantly contribute to the development and maintenance of SAD by reducing exposure to positive social experiences and feedback[35,36]. Individuals with SAD often avoid anxiety-inducing social situations, thereby missing crucial opportunities to enhance their social skills and gain confidence in interpersonal interactions. This avoidance can create a detrimental cycle of social withdrawal and increased anxiety, adversely affecting their ability to forge meaningful relationships. Our study demonstrates that hypnotherapy effectively modifies attentional biases in individuals with SAD. The intervention led to marked improvements in attentional sensitivity and reduced difficulty in disengaging from negative stimuli, particularly angry faces. Additionally, the positive outcomes of the hypnotherapy were sustained over time, as evidenced by the significant improvements still present in the one-month follow-up assessment. This indicates that hypnotherapy offers a durable therapeutic approach for addressing the core attentional issues underlying SAD, potentially disrupting the cycle of avoidance and fostering better social engagement.
Research exploring the neural underpinnings of attentional biases in social anxiety, particularly through ERPs, has gained momentum. For instance, individuals with SAD display increased P1 amplitudes in response to faces, indicating an early-phase attentional bias in face perception[37]. Our study contributes to this body of knowledge by showing that hypnosis interventions can significantly alter P1 amplitude patterns in participants with SAD, differing notably from those in the control group. This finding, aligning with previous research, highlights the potential of P1 as a crucial neural marker for understanding attentional processes in SAD, specifically those related to social anxiety and its associated biases. The alteration of P1 amplitude through hypnotherapy suggests that this therapeutic approach may influence early stages of face processing. This insight offers a new perspective on therapeutic strategies for modifying attentional biases in SAD, emphasizing the importance of early intervention. For example, the research by Rossignol indicates the role of ERPs in detecting the neuropsychological implications of SAD[38]. Additionally, the work of Eldar and Bar-Haim further supports the concept of attentional bias modifications as a treatment for anxiety disorders[39]. Overall, our results suggest that hypnotherapy may offer a novel and effective approach to addressing the entrenched biases in SAD, potentially leading to more effective management of the disorder through targeted early-stage interventions.
N170, a critical neurophysiological marker in human face processing, significantly influences the cognitive mechanisms underlying facial expression recognition[40,41]. Studies have shown that individuals with heightened social anxiety exhibit larger N170 amplitudes in response to faces, highlighting an increased sensitivity to facial expressions in these individuals[42]. Our study contributes to this understanding by demonstrating that hypnotherapy can effectively reduce N170 amplitude in patients with SAD, indicating an improvement in their facial recognition abilities. This reduction in N170 amplitude post-hypnosis suggests that hypnotherapy may attenuate the heightened vigilance towards facial expressions typically observed in SAD patients. This finding is particularly relevant given the role of face perception in social interactions and the distress that misinterpreting facial expressions can cause in individuals with SAD. The research by Rossignol supports this, highlighting the significance of facial expression processing in social anxiety and the potential of ERPs in exploring these mechanisms[38]. Moreover, this study's results align with recent advancements in understanding the neural basis of SAD and the therapeutic potential of interventions targeting these neurophysiological markers. For example, a study by Mühlberger investigated the use of ERPs to understand the neural mechanisms involved in emotional processing in individuals with SAD, offering insights into potential ERP markers for therapeutic interventions[43]. Our findings suggest that hypnotherapy may offer a novel and effective approach for modifying the neural processing of facial expressions in SAD, contributing to a reduction in social anxiety symptoms.
The N2pc component reflects the spatial selective attention processing of relevant visual stimuli[35]. The N2pc elicited by angry faces in the pre-test phase indicates that selective attention was allocated to angry faces, which is consistent with previous research[44]. The amplitude of the N2pc component in the high social anxiety group was significantly higher than that in the baseline group, and its latency was slower compared to that of the baseline group. In previous studies, individuals with SAD have an attentional bias towards threatening information, which is somewhat related to the “anger superiority effect” of the N2pc component[45]. After hypnosis intervention, the amplitude of the N2pc component elicited by angry faces in the experimental group decreased, and its latency shortened. However, there was no significant change in the N2pc component elicited by happy faces. It can be speculated that the N2pc component is more strongly associated with angry faces and threatening information in the environment.
LPP, recognized as a key neural marker for attentional engagement with emotional stimuli, plays a crucial role in emo
This study implemented a novel hypnotherapy approach, blending systematic desensitization therapy with progressive relaxation techniques. In a hypnotic state, participants received tailored suggestions aimed at reducing anxiety symptoms and gradually desensitizing them to anxiety-provoking situations. These suggestions encompassed relaxation, positive affirmations, step-by-step desensitization, and cognitive reframing. The efficacy of this hypnotherapy extends beyond alleviating social anxiety symptoms to modifying attentional biases, thereby establishing it as a potent intervention for social anxiety and related disorders. Its broader application in psychotherapy merits further exploration. Utilizing ERPs to probe the neural underpinnings of hypnosis, our research unveiled significant changes in P1, N170, N2pc, and LPP amplitudes concurrent with social anxiety modification. This indicates that alterations in attention bias towards angry faces, as denoted by these ERP components, might be central to hypnotherapy's therapeutic impact. Our findings align with prior research by our team[12], underscoring attentional bias as a key treatment target in social anxiety. Moreover, P1, N170, N2pc, and LPP emerge as crucial neural indicators of attentional bias in SAD, further enriching our understanding of the neurophysiological mechanisms at play in hypnotherapy's effectiveness.
While this study marks a significant advancement in the application of hypnotherapy for SAD, it is important to acknowledge its limitations and the need for further research. First, objective physiological markers of social anxiety, such as heart rate, respiration, blood pressure, and blood oxygen levels, were not evaluated. Future studies should include these measurements to better understand the physiological changes accompanying hypnosis interventions. The second limitation of this study is its small sample size, which may limit the generalizability of the findings. Future research with larger sample sizes is recommended to strengthen the external validity and potentially yield more broadly applicable results. As a third methodological approach, neurophysiological indices of attentional processing were evaluated using P1, N2pc, N170, and LPP waveforms derived from ERPs. Future research should consider employing additional neuroimaging techniques like functional magnetic resonance imaging to gain deeper insights into the neural mechanisms of hypnosis in treating social anxiety. This will also aid in identifying the specific mechanisms through which hypno
This pioneering ERP study explores the efficacy of hypnotherapy in treating SAD, marking a significant advancement in understanding the neurological impacts of hypnosis on social anxiety. The results demonstrate that hypnotherapy can effectively alleviate symptoms of SAD, substantiated by notable reductions in the amplitudes of the P1, N170, N2pc, and LPP components. These neurophysiological changes indicate a diminished sensitivity to threatening stimuli among individuals with SAD after undergoing hypnosis. The study's findings are pivotal, suggesting that hypnotherapy not only improves SAD symptoms but also modifies key neural markers, including P1, N170, N2pc, and LPP, which emerge as crucial biomarkers in the treatment of social anxiety. This research thus provides a foundation for further exploration into hypnotherapy as a viable and effective treatment option for SAD.
We are very grateful to all participants in this study.
| 1. | Wiegand A, Munk MHJ, Drohm S, Fallgatter AJ, MacIsaac JL, Kobor MS, Nieratschker V, Kreifelts B. Neural correlates of attentional control in social anxiety disorder: the impact of early-life adversity and DNA methylation. J Psychiatry Neurosci. 2021;46:E663-E674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Winton EC, Clark DM, Edelmann RJ. Social anxiety, fear of negative evaluation and the detection of negative emotion in others. Behav Res Ther. 1995;33:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 152] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Peschard V, Philippot P. Social anxiety and information processing biases: An integrated theoretical perspective. Cogn Emot. 2016;30:762-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Spokas ME, Rodebaugh TL, Heimberg RG. Cognitive biases in social phobia. Psychiatry. 2004;3:51-55. [DOI] [Full Text] |
| 5. | Abend R, de Voogd L, Salemink E, Wiers RW, Pérez-Edgar K, Fitzgerald A, White LK, Salum GA, He J, Silverman WK, Pettit JW, Pine DS, Bar-Haim Y. Association between attention bias to threat and anxiety symptoms in children and adolescents. Depress Anxiety. 2018;35:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Schultz LT, Heimberg RG. Attentional focus in social anxiety disorder: potential for interactive processes. Clin Psychol Rev. 2008;28:1206-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Heinrichs N, Hofmann SG. Information processing in social phobia: a critical review. Clin Psychol Rev. 2001;21:751-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 230] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Kolassa IT, Miltner WH. Psychophysiological correlates of face processing in social phobia. Brain Res. 2006;1118:130-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Kihlstrom JF. The fox, the hedgehog, and hypnosis. Int J Clin Exp Hypn. 2003;51:166-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Raz A. Hypnosis: a twilight zone of the top-down variety Few have never heard of hypnosis but most know little about the potential of this mind-body regulation technique for advancing science. Trends Cogn Sci. 2011;15:555-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Lipsitz JD, Marshall RD. Alternative psychotherapy approaches for social anxiety disorder. Psychiatr Clin North Am. 2001;24:817-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Yang XX, Luo JY, Liang M, Li N, Tao Q, Ma LJ, Li XM. Randomized trial estimating effects of hypnosis versus progressive muscle relaxation on medical students' test anxiety and attentional bias. World J Psychiatry. 2022;12:801-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Wolf TG, Faerber KA, Rummel C, Halsband U, Campus G. Functional Changes in Brain Activity Using Hypnosis: A Systematic Review. Brain Sci. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Bantin T, Stevens S, Gerlach AL, Hermann C. What does the facial dot-probe task tell us about attentional processes in social anxiety? A systematic review. J Behav Ther Exp Psychiatry. 2016;50:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Fox E, Russo R, Dutton K. Attentional Bias for Threat: Evidence for Delayed Disengagement from Emotional Faces. Cogn Emot. 2002;16:355-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 628] [Cited by in RCA: 570] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 16. | Hope DA, Rapee RM, Heimberg RG, Dombeck MJ. Representations of the self in social phobia: Vulnerability to social threat. Cogn Ther Res. 1990;14:177-189. [RCA] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 222] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Rinck M, Becker ES. A comparison of attentional biases and memory biases in women with social phobia and major depression. J Abnorm Psychol. 2005;114:62-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Cui L, Dong X, Zhang S. ERP evidence for emotional sensitivity in social anxiety. J Affect Disord. 2021;279:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Williams LM, Liddell BJ, Rathjen J, Brown KJ, Gray J, Phillips M, Young A, Gordon E. Mapping the time course of nonconscious and conscious perception of fear: an integration of central and peripheral measures. Hum Brain Mapp. 2004;21:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 180] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 1006] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 21. | Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalogr Clin Neurophysiol. 1996;99:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 707] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 22. | Holmes A, Bradley BP, Kragh Nielsen M, Mogg K. Attentional selectivity for emotional faces: evidence from human electrophysiology. Psychophysiology. 2009;46:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Smith NK, Cacioppo JT, Larsen JT, Chartrand TL. May I have your attention, please: electrocortical responses to positive and negative stimuli. Neuropsychologia. 2003;41:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 24. | Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Brain Res Cogn Brain Res. 2003;17:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 689] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 25. | Luo W, Feng W, He W, Wang NY, Luo YJ. Three stages of facial expression processing: ERP study with rapid serial visual presentation. Neuroimage. 2010;49:1857-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 383] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 26. | Zhang D, Luo W, Luo Y. Single-trial ERP analysis reveals facial expression category in a three-stage scheme. Brain Res. 2013;1512:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Langeslag SJE, Van Strien JW. Comparable Modulation of the Late Positive Potential by Emotion Regulation in Younger and Older Adults. J Psychophys. 2010;24:186-197. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Hajcak G, Weinberg A, Macnamara A, Foti D. ERPs and the Study of Emotion. Oxford Handbooks Online. 2011. [DOI] [Full Text] |
| 29. | Moser JS, Huppert JD, Duval E, Simons RF. Face processing biases in social anxiety: an electrophysiological study. Biol Psychol. 2008;78:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Heimberg RG, Horner KJ, Juster HR, Safren SA, Brown EJ, Schneier FR, Liebowitz MR. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol Med. 1999;29:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 574] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 31. | Lazarov A, Abend R, Bar-Haim Y. Social anxiety is related to increased dwell time on socially threatening faces. J Affect Disord. 2016;193:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | WEITZENHOFFER AM. The significance of hypnotic depth in therapy. Int J Clin Exp Hypn. 1962;10:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: an ERP analysis. Psychophysiology. 2004;41:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 493] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 34. | Schupp HT, Ohman A, Junghöfer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004;4:189-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 613] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 35. | Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behav Res Ther. 2003;41:1325-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 251] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Mathews A, Macleod C. Induced processing biases have causal effects on anxiety. Cognition Emotion. 2002;16:331-354. [RCA] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 348] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 37. | Pei G, Xiao Q, Pan Y, Li T, Jin J. Neural evidence of face processing in social anxiety disorder: A systematic review with meta-analysis. Neurosci Biobehav Rev. 2023;152:105283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Rossignol M, Campanella S, Bissot C, Philippot P. Fear of negative evaluation and attentional bias for facial expressions: an event-related study. Brain Cogn. 2013;82:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Eldar S, Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychol Med. 2010;40:667-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 40. | Hinojosa JA, Mercado F, Carretié L. N170 sensitivity to facial expression: A meta-analysis. Neurosci Biobehav Rev. 2015;55:498-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 41. | Osborne KJ, Kraus B, Curran T, Earls H, Mittal VA. An Event-Related Potential Investigation of Early Visual Processing Deficits During Face Perception in Youth at Clinical High Risk for Psychosis. Schizophr Bull. 2022;48:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Ran G, Chen X. The Impact of Top-Down Prediction on Emotional Face Processing in Social Anxiety. Front Psychol. 2017;8:1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Mühlberger A, Wieser MJ, Herrmann MJ, Weyers P, Tröger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. J Neural Transm (Vienna). 2009;116:735-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 44. | Weymar M, Löw A, Ohman A, Hamm AO. The face is more than its parts--brain dynamics of enhanced spatial attention to schematic threat. Neuroimage. 2011;58:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2300] [Cited by in RCA: 2406] [Article Influence: 133.7] [Reference Citation Analysis (0)] |
| 46. | Brown KW, Goodman RJ, Inzlicht M. Dispositional mindfulness and the attenuation of neural responses to emotional stimuli. Soc Cogn Affect Neurosci. 2013;8:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 47. | Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1295] [Cited by in RCA: 1298] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 48. | Dunning JP, Hajcak G. See no evil: directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology. 2009;46:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |