Published online Sep 19, 2024. doi: 10.5498/wjp.v14.i9.1404
Revised: August 22, 2024
Accepted: August 30, 2024
Published online: September 19, 2024
Processing time: 134 Days and 0 Hours
Perception is frequently impaired in patients with Alzheimer’s disease (AD). Several patients exhibit visual or haptic hallucinations.
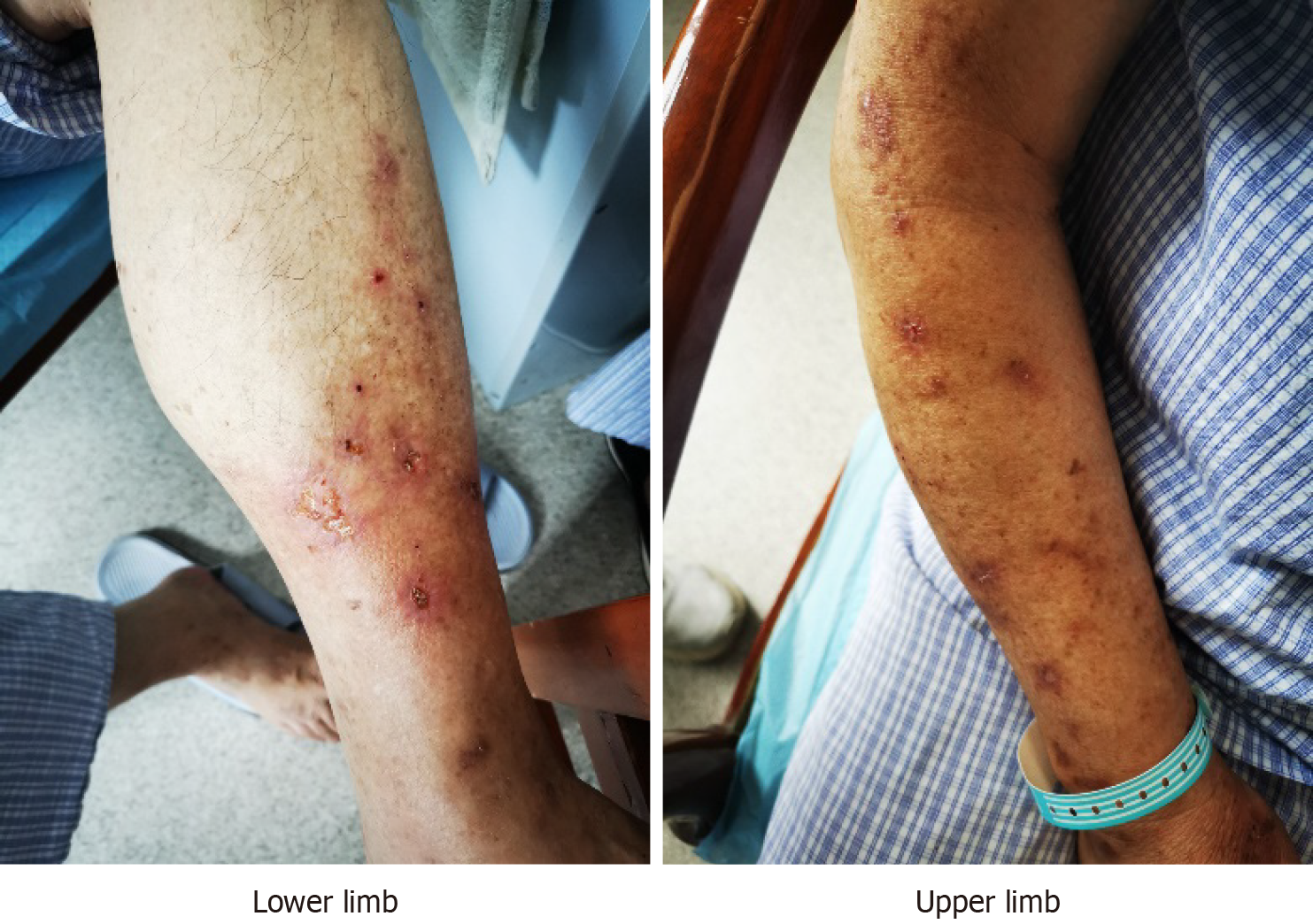
A 71-year-old Chinese man presented with visual and haptic hallucinations he had been experiencing for 2 weeks. The clinical manifestations were the feeling of insects crawling and biting the limbs and geison. He looked for the insects while itching and scratching, which led to skin breakage on the limbs. He was treated with topical and anti-allergic drugs in several dermatology departments without any significant improvement. After admission, the patient was administered risperidone (0.5 mg) and duloxetine (2 mg/day). One week later, the dose of risperidone was increased to 2 mg/day, and that of duloxetine was increased to 60 mg/day. After 2 weeks of treatment, the patient’s sensation of insects crawling and biting disappeared, and his mood stabilized.
This patient manifested psychiatric behavioral symptoms caused by AD brain atrophy. It was important to re-evaluate the patient’s cognitive-psychological status when the patient repeatedly went to the hospital for treatment. Follow-up attention to cognitive function and the consideration of perceptual deficits as early manifestations of AD should be considered.
Core Tip: Topical and anti-allergic drugs did not provide improvement. After admission the patient was diagnosed with psychiatric behavioral symptoms of brain atrophy due to Alzheimer’s disease. The patient was administered risperidone (0.5 mg) and duloxetine (2 mg/day). One week later, the dose of risperidone was increased to 2 mg/day, and that of duloxetine was increased to 60 mg/day. After 2 weeks of treatment the patient no longer had the sensation of insects biting a crawling and his mood stabilized.
- Citation: Xu T, Mei X, Zhao Z, Liu YH, Zheng CY. Effectiveness of anti-psychiatric treatment on visual and haptic perceptual disorder for a patient with Alzheimer’s disease: A case report. World J Psychiatry 2024; 14(9): 1404-1410
- URL: https://www.wjgnet.com/2220-3206/full/v14/i9/1404.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i9.1404
Perception includes visual, auditory, touch, and other perceptions[1]. Perception is frequently impaired in patients with Alzheimer’s disease (AD), which reduces their quality of life and complicates the evaluation of other cognitive deficits[2,3]. Changes in visuospatial perception can be detected in the early stages of AD[4]. Altered haptic/tactile perception is also observed in patients with mild cognitive impairment[5,6]. AD, accompanied by behavioral and psychological symptoms, can manifest with visual and haptic hallucinations[7]. The symptoms of visual and haptic hallucinations were as follows: Visual hallucination referred to the experience of visual images appearing in the absence of visual stimuli. The visual hallucinations are varied and the patients have fearful and disturbed emotions and avoidance behaviors. Haptic hallucinations were also known as cutaneous hallucinations. The patient feels some kind of abnormal sensation on the skin. Such as insects crawling, electric current passing, wind blowing, etc. Visual and haptic hallucinations are more common in schizophrenia and organic mental disorders. Patients with other types of dementia, including Lewy body dementia, also have hallucinations[8].
Patients with a visual perception dysfunction see things that are not present. These things are most commonly human figure or faces, although animals, objects, and meaningful patterns are also frequently observed[9], they generally move, and are perceptually convincing. They occur episodically at specific times and locations. In this patient, visual perceptual disturbance was accompanied by haptic/tactile perceptual deficits. Clinical manifestations of retinal- and hand-based visual and haptic perceptual disturbances in patients with AD can be induced by atrophy of the cortical region for multisensory integration[10]. Regarding to epidemiologic data, although there are currently no statistics on the prevalence of visual and haptic hallucinations in patients with AD, retrospective studies will further examine the prevalence in the future. Meanwhile, many studies provide useful pointers to an understanding of the mechanisms in visual and haptic hallucinations in AD[11,12]. Treatment with nootropics is an effective method for suppressing the psychiatric behavioral symptoms caused by AD brain atrophy[13]. Antipsychotic drugs suppress the neuropsychiatric symptoms in patients with AD[14,15].
A 71-year-old Chinese man presented to the psychiatric clinic complaining of physical discomfort for 2 weeks. He stated that he felt insects crawling and biting the limbs and geison every day severely.
The patient experienced dizziness, unsteady gait, walking like stepping on cotton, the feeling of insects crawling and biting on the limbs, and the feeling of insects crawling on the geison. He always looked for insects, and itched, and scratched the perceived affected areas, which led to skin breakage on the limbs. He was treated with topical and anti-allergic drugs in several dermatology departments without any significant improvement; therefore, he was disturbed, unhappy, and sad and was then transferred to the psychiatry department.
The patient had a history of hypertension and hyperlipidemia. He had herniated lumbar intervertebral discs due to a fall more than 10 years ago. His lumbar pain reappeared 4 months prior, and was treated with acupuncture. He denied a history of tuberculosis, hepatitis, typhoid fever, vaccinations, major organ diseases such as cardiovascular, cere
The patient was the second of five compatriots. He denied a family history of psychiatric disorders.
On physical examination, the vital signs were: Body temperature, 36.4 °C; Blood pressure, 134/73 mmHg; Heart rate, 74 beats per min; Respiratory rate, 17 breaths per min; Body weight, 76.9 kg; Body height, 176.5 cm. The skin of the limbs was damaged (Figure 1).
Blood counts, ultrasensitive C-reactive protein, and biochemistry were unremarkable.
Cranial computed tomography (CT) revealed enlargement of the cisterna magna and the left lateral fissure cistern, a rounded hypodense lesion at the anterior border of the left lateral fissure cistern, and lacunar lesions in the bilateral basal ganglia and bilateral lateral ventricles.
The patient initially presented with gait abnormalities and a sensation of stepping on cotton. Cranial CT suggested that there were hypodense lesions at the anterior border of the left lateral fissure cistern and lacunar lesions in the bilateral basal ganglia and bilateral lateral ventricles. Psychiatric symptoms manifest as sensory and perceptual disturbances, including insect crawling and biting. There were strong clinical manifestations of tactile hallucinations. Organic hallucinosis was considered based on the diagnostic criteria of the International Classification of diseases, Tenth Edition.
Based on the patient’s medical history, the final diagnosis was organic hallucination disorder.
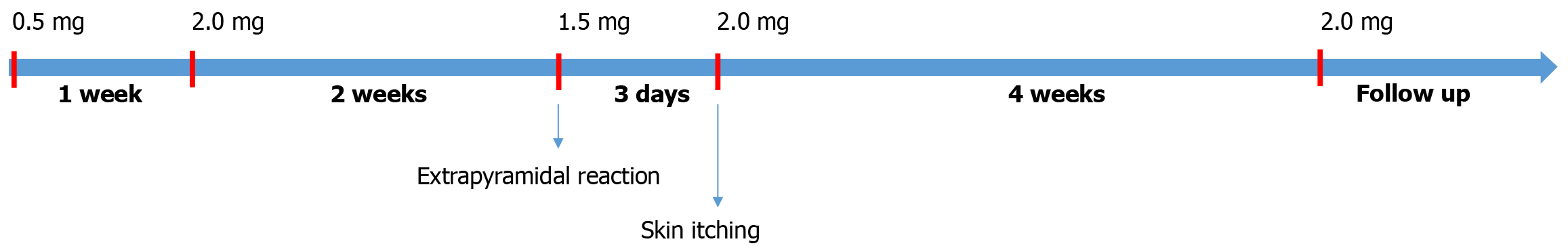
After admission, the patient was administered risperidone (0.5 mg) and duloxetine (20 mg/day) as shown in Figure 2. One week later, the dose of risperidone was increased to 2 mg/day, and that of duloxetine was increased to 60 mg/day. After 2 weeks of treatment, the patient’s sensation of insect crawling and biting disappeared, and his mood stabilized. During hospitalization, the patient was slightly sluggish, which was considered to be caused by an extrapyramidal reaction to risperidone; therefore, the dose of risperidone was reduced to 1.5 mg/day. The patient complained of physical discomfort, and the sensation of insects crawling and biting recurred. He began looking for worms in his body. Risperidone (2 mg/day) treatment was immediately resumed, and the symptoms were relieved.
Outpatient follow-up was conducted for 6 months, and the psychiatric symptoms of the patient stabilized. Patients’ cognitive and self-care abilities decreased compared with those in the previous period.
Currently, many older patients visit the dermatology departments. Families and physicians of older patients need to be reminded that when patients present with itchy skin that does not improve with repeated treatments in the dermatology department, it is important to reevaluate the patient’s physical disease condition and cognitive psychological status. Consideration should be given to whether the condition is a psychiatric-behavioral symptom, as well as follow-up attention to cognitive function and possible perceptual deficits as early manifestation of dementia, including AD[16].
In the present case, the patient had two manifestations of perceptual abnormalities: Visual and haptic hallucinations. The patient saw insects crawling and simultaneously felt itching on the skin, causing haptic hallucinations. The family first saw the patient’s broken skin, which was considered to be from a skin disease. Regarding the patient’s expression of crawling insects, family members, who were without general medical knowledge mistakenly believed that it was a misinterpretation of old age and blurred vision. Actually, they were mental and behavioral symptoms caused by brain atrophy in AD. Since AD has an insidious onset, it is difficult to detect and is often mistaken for a physical disease[17]. Most patients with AD repeatedly visit internal medicine outpatient clinics, such as the dermatology department, however when treatment does not provide a significant effect, then these patients are suspected of having neuropsychiatric disorders, including AD or other types of dementias[18]. This patient subsequently presented to the psychiatric department for antipsychotic treatment and received remarkable improvement.
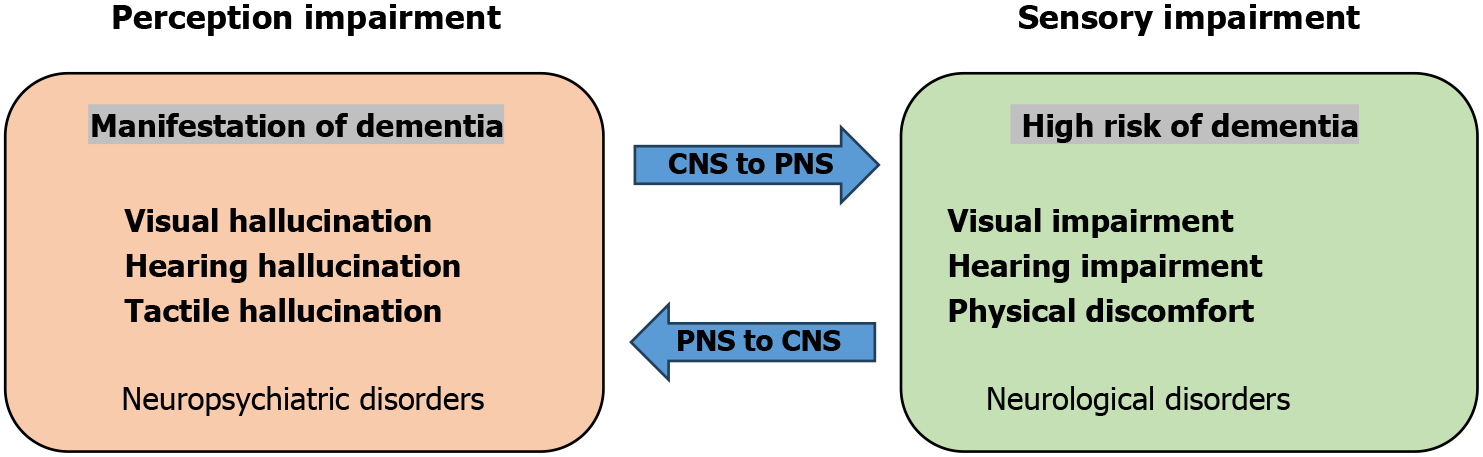
The patient in this case had brain imaging abnormalities and was considered to have had a cerebrovascular infarction with brain function affected, secondary to the development of sensory-perceptual deficits manifestations. The manifestations of sensory-perceptual deficits in this case were visual hallucination with itchy skin accompanied by haptic hallucination. This perceptual disorder is a type of psychiatric disorder caused by degeneration of brain function. As shown in Figure 3, the condition of sensory impairment, a risk factor for cognitive decline, in patients with hearing impairment is a significantly greater risk for dementia than for those with intact hearing[19]. Moreover, sensory impairments, including vision impairment, hearing impairment, and dual sensory impairment, usually occur in an older population, causing an increased risk of dementia and leading to decreased functional capacity and quality of life[20]. Previous studies have found that impairments in sensory function are associated with cognitive decline and an increased risk of premature mortality[21,22]. Perception disturbances may also happen after stroke[23].
Regarding differential diagnosis, first, in considering an organic mental disorder, the patient did not have fever, headache, or other discomforts; there was no history of severe craniocerebral and somatic diseases; there was no obvious consciousness, memory, or intelligence disorder; no symptoms of multiple organic mental disorder characteristics; the patient’s cranial CT suggested that the occipital and left lateral fissure cistern were enlarged, and the anterior edge of the left lateral fissure cistern seemed to be seen as rounded hypodense foci, the two sides of the basal ganglia area lateral paraventricular lacunar foci; and disease onset was sudden, which manifested as a vivid image of visual hallucination symptoms, thus organic hallucination disorder could not be excluded. Second, in considering psychoactive substances and non-addictive substances caused by depression: The patient did not have a history of psychoactive and non-addictive substances. Third, in considering bipolar affective disorder: The patient developed the disease at an older age; In the history of the disease, there was no mood elevation, accelerated thoughts, feeling very good, or other symptoms; Therefore, bipolar affective disorder could be excluded. In diagnosis of AD, the cerebrospinal fluid examination can improve AD diagnostic accuracy according to large numbers of biomarkers. But in this case, the cerebrospinal fluid examination was not performed as consent was not obtained from the patient and family.
The protocol for treatment with risperidone is that in elderly patients with organic psychiatric disorders including dementia, the typical dose is 1/3 to 1/2 comparing to that of an adult. Start with a small dose and slowly increase the dosage. After one to two weeks, the dose is increased to a therapeutic dose. After the psychotic symptoms have disappeared, treatment is maintained for 2 weeks. The patient is then evaluated in order to achieve a balance between efficacy and side effects. Usually, mild side effects are present but efficacy is maximized. The dose reduced to avoid extrapyramidal symptoms, but the reduction of dose may lead to relapse of psychotic symptoms. Overall, it is a matter of using the smallest dose to achieve maximum benefit.
Different brain regions have different functions and are connected to each other. Therefore, damage to one brain region can have a range of consequences[24,25]. AD brain atrophy is a serious neurodegenerative disorder and the leading cause of dementia. Aside from cognitive impairment, ophthalmologic abnormalities such as visual acuity, color recognition, and motion perception were reported in patients with AD[26,27]. Similar findings have also been reported in studies of other neurodegenerative conditions that are associated with cognitive decline, including multiple sclerosis[28], Down’s syndrome[29], Parkinson disease[30] and Lewy body dementia[31]. Accompanied with neurodegenerative manifestations, the psychiatric-behavioral symptom are clearly present in patients. Furthermore, cognitive decline exacerbates psychiatric symptoms[32].
Itchy skin is commonly observed in older adults[33]. Many factors are associated with itchy skin such as dry skin (desiccation), eczema (dermatitis), and physical diseases[34]. Generalized itching can be a symptom of an underlying condition, such as liver disease, kidney disease, anemia, diabetes, and thyroid problems, and mental disorders including anxiety, obsessive-compulsive disorder, and depression[35].
Perceptual deficits as early manifestations of AD needed to be pay more attention in clinical. Regarding to early clinical intervention, cognition improvement or suppressing the psychiatric-behavioral symptom is significantly helpful in relieving skin itching.
| 1. | Wang M, Arteaga D, He BJ. Brain mechanisms for simple perception and bistable perception. Proc Natl Acad Sci U S A. 2013;110:E3350-E3359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Day BL, Ocal D, Peters A, Bancroft MJ, Cash D, Kaski D, Crutch SJ, Yong KXX. Altered visual and haptic verticality perception in posterior cortical atrophy and Alzheimer's disease. J Physiol. 2022;600:373-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Marine N, Boriana A. Olfactory markers of depression and Alzheimer's disease. Neurosci Biobehav Rev. 2014;45:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Mandal PK, Joshi J, Saharan S. Visuospatial perception: an emerging biomarker for Alzheimer's disease. J Alzheimers Dis. 2012;31 Suppl 3:S117-S135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Grunwald M, Busse F, Hensel A, Riedel-Heller S, Kruggel F, Arendt T, Wolf H, Gertz HJ. Theta-power differences in patients with mild cognitive impairment under rest condition and during haptic tasks. Alzheimer Dis Assoc Disord. 2002;16:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Collerton D, Barnes J, Diederich NJ, Dudley R, Ffytche D, Friston K, Goetz CG, Goldman JG, Jardri R, Kulisevsky J, Lewis SJG, Nara S, O'Callaghan C, Onofrj M, Pagonabarraga J, Parr T, Shine JM, Stebbins G, Taylor JP, Tsuda I, Weil RS. Understanding visual hallucinations: A new synthesis. Neurosci Biobehav Rev. 2023;150:105208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 7. | El Haj M, Roche J, Jardri R, Kapogiannis D, Gallouj K, Antoine P. Clinical and neurocognitive aspects of hallucinations in Alzheimer's disease. Neurosci Biobehav Rev. 2017;83:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Jurek L, Herrmann M, Bonze M, Brunet S, Padovan C, Dorey JM. Behavioral and psychological symptoms in Lewy body disease: a review. Geriatr Psychol Neuropsychiatr Vieil. 2018;16:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Naasan G, Shdo SM, Rodriguez EM, Spina S, Grinberg L, Lopez L, Karydas A, Seeley WW, Miller BL, Rankin KP. Psychosis in neurodegenerative disease: differential patterns of hallucination and delusion symptoms. Brain. 2021;144:999-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Donato L, Mordà D, Scimone C, Alibrandi S, D'Angelo R, Sidoti A. Bridging Retinal and Cerebral Neurodegeneration: A Focus on Crosslinks between Alzheimer-Perusini's Disease and Retinal Dystrophies. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Milner AD, Cavina-Pratesi C. Perceptual deficits of object identification: apperceptive agnosia. Handb Clin Neurol. 2018;151:269-286. [PubMed] [DOI] [Full Text] |
| 12. | Uhlhaas PJ, Pantel J, Lanfermann H, Prvulovic D, Haenschel C, Maurer K, Linden DE. Visual perceptual organization deficits in Alzheimer's dementia. Dement Geriatr Cogn Disord. 2008;25:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Andrade C. Cholinesterase Inhibitors for Delusions and Hallucinations in Alzheimer Disease and Parkinson Disease: Questionably Significant Benefits. J Clin Psychiatry. 2023;84. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Lavretsky H. Hallucinations Predict Relapse After Discontinuation of Risperidone in Patients With Alzheimer's Disease and Psychosis or Agitation. Am J Psychiatry. 2017;174:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Devanand DP, Mintzer J, Schultz SK, Andrews HF, Sultzer DL, de la Pena D, Gupta S, Colon S, Schimming C, Pelton GH, Levin B. Relapse risk after discontinuation of risperidone in Alzheimer's disease. N Engl J Med. 2012;367:1497-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Arora S, Jolly AJ, Suhas S, Arasappa R, Kamble N, Pal PK, Varambally S. Subacute Sclerosing Panencephalitis Masquerading as Schizophrenia: An Example of Diagnostic Overshadowing in Neuropsychiatry. Prim Care Companion CNS Disord. 2022;24. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer's disease. Lancet. 2021;397:1577-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 2696] [Article Influence: 674.0] [Reference Citation Analysis (0)] |
| 18. | Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, Cedarbaum J, Brashear R, Miller DS. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dement. 2011;7:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 714] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 19. | Luo Y, He P, Guo C, Chen G, Li N, Zheng X. Association Between Sensory Impairment and Dementia in Older Adults: Evidence from China. J Am Geriatr Soc. 2018;66:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Kuo PL, Huang AR, Ehrlich JR, Kasper J, Lin FR, McKee MM, Reed NS, Swenor BK, Deal JA. Prevalence of Concurrent Functional Vision and Hearing Impairment and Association With Dementia in Community-Dwelling Medicare Beneficiaries. JAMA Netw Open. 2021;4:e211558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 21. | Yesantharao L, Cai Y, Schrack JA, Gross AL, Wang H, Bilgel M, Dougherty R, Simonsick EM, Ferrucci L, Resnick SM, Agrawal Y. Sensory impairment and beta-amyloid deposition in the Baltimore longitudinal study of aging. Alzheimers Dement (Amst). 2023;15:e12407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Fuller-Thomson E, Nowaczynski A, MacNeil A. The Association Between Hearing Impairment, Vision Impairment, Dual Sensory Impairment, and Serious Cognitive Impairment: Findings from a Population-Based Study of 5.4 million Older Adults. J Alzheimers Dis Rep. 2022;6:211-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 23. | Hazelton C, McGill K, Campbell P, Todhunter-Brown A, Thomson K, Nicolson DJ, Cheyne JD, Chung C, Dorris L, Gillespie DC, Hunter SM, Brady MC. Perceptual Disorders After Stroke: A Scoping Review of Interventions. Stroke. 2022;53:1772-1787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Wu Z, Feng K, Huang J, Ye X, Yang R, Huang Q, Jiang Q. Brain region changes following a spinal cord injury. Neurochem Int. 2024;174:105696. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Xu J, Wu Z, Nürnberger A, Sabel BA. Reorganization of Brain Functional Connectivity Network and Vision Restoration Following Combined tACS-tDCS Treatment After Occipital Stroke. Front Neurol. 2021;12:729703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Almario G, Piñero DP. Impact of Alzheimer's Disease in Ocular Motility and Visual Perception: A Narrative Review. Semin Ophthalmol. 2022;37:436-446. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Tzekov R, Mullan M. Vision function abnormalities in Alzheimer disease. Surv Ophthalmol. 2014;59:414-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Frau J, Fenu G, Signori A, Coghe G, Lorefice L, Barracciu MA, Sechi V, Cabras F, Badas M, Marrosu MG, Cocco E. A cross-sectional and longitudinal study evaluating brain volumes, RNFL, and cognitive functions in MS patients and healthy controls. BMC Neurol. 2018;18:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Walpert MJ, Normando EM, Annus T, Jennings SR, Wilson LR, Watson P, Zaman SH, Cordeiro MF, Holland AJ. Age-related retinal thickness in Down's syndrome: A high-risk population for dementia. Alzheimers Dement (Amst). 2019;11:744-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Nieto-Escamez F, Obrero-Gaitán E, Cortés-Pérez I. Visual Dysfunction in Parkinson's Disease. Brain Sci. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Moreno-Ramos T, Benito-León J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson's disease, dementia with Lewy bodies, and Alzheimer's disease. J Alzheimers Dis. 2013;34:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Brewster KK, Rutherford BR. Hearing Loss, Psychiatric Symptoms, and Cognitive Decline: An Increasingly Important Triad in Older Adults. Am J Geriatr Psychiatry. 2021;29:554-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Chung BY, Um JY, Kim JC, Kang SY, Park CW, Kim HO. Pathophysiology and Treatment of Pruritus in Elderly. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Cevikbas F, Lerner EA. Physiology and Pathophysiology of Itch. Physiol Rev. 2020;100:945-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 35. | Roh YS, Choi J, Sutaria N, Kwatra SG. Itch: Epidemiology, clinical presentation, and diagnostic workup. J Am Acad Dermatol. 2022;86:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |