INTRODUCTION
Neuropathic pain (NP) arises from structural lesions that induce functional abnormalities in the central and peripheral nervous systems. This condition is prevalent in an estimated 10% of the general population[1] and constitutes a frequent disorder. Patients with NP often experience mood disorders, such as depression and anxiety. Chronic pain can lead to depressive symptoms, such as low mood, loss of interest, sleep disturbances, and reduced energy. The association between NP and depression has been confirmed in multiple studies. For instance, a review by Meda et al[2] indicated a high prevalence of depression among patients with NP, and antidepressants were effective in treating chronic pain. Additionally, structural and functional changes in the brain regions associated with emotion regulation, such as the prefrontal cortex, amygdala, and hippocampus, have been observed in patients with NP, similar to those observed in patients with depression[3]. Furthermore, anxiety is a common comorbidity among patients with NP. Chronic pain may lead to a persistent state of anxiety, which could be associated with adaptive changes in the neural circuits involved in the stress response[4]. Notably, NP is the primary symptom of various neurological conditions, including post-herpetic and trigeminal neuralgia, encompassing these painful states[1]. Effective management of NP poses a significant therapeutic challenge for clinicians. Various pharmacological and non-pharmacological interventions have been suggested to yield varying degrees of benefit[5]. Pharmacological approaches include anticonvulsants, nonsteroidal anti-inflammatory drugs, antidepressants, and opioids despite the potential limitations arising from their side effects[6]. Surprisingly, < 60% of patients experience partial relief from recently approved agents targeting NP[7]. Therefore, the search for novel treatments and therapeutic targets commonly used for various indications persists.
Microglia play a dual role in preventing tissue damage or responding to stimuli caused by pathogen infections[8,9]. Alterations in microglial signaling pathways associated with NP involve inflammatory reactions[10]. Excessive inflammatory responses, especially the secretion of abundant pro-inflammatory cytokines, ultimately lead to neural inflammation[11,12]. Microglial pyroptosis, a newly discovered form of inflammatory cell death, is associated with immune responses and inflammation-related disorders of the central nervous system, including spinal cord injury and depression[13,14].
Initially limited to specific neurological disturbances, botulinum toxin type A (BTX-A) has been extensively used in various medical domains, including neurological, urological, gastroenterological, surgical, dermatological, and cosmetic applications. Within neurorehabilitation, BTX serves as a valuable adjunct to other interventions, particularly in aiding the treatment of individuals with neurological disability by primarily targeting spasticity reduction[15]. While historically associated with motor neurons, research indicates the potential entry of BTX into diverse neuron types, prompting studies exploring the efficacy of BTX-A in treating neurological disorders not solely associated with muscle hyperactivity[16-18]. Furthermore, BTX-A has been used to manage several painful conditions, including NP[19-22]. Notably, although both BTX-A and BTX-B are commercially available, clinical investigations have predominantly focused on the use of BTX-A for pain relief. In this study, we determined the effects of BTX-A on microglial growth and pyroptosis and investigated their potential mechanisms.
MATERIALS AND METHODS
Cell culture
Mouse glial cells BV2 (adherent cells) were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 1% penicillin/streptomycin. The cells were cultured in a 37 °C incubator with 5% CO2. For lipopolysaccharide (LPS) induction, BV2 cells were seeded in six-well plates and cultured to 70 % confluence. Subsequently, LPS (5 mg/mL) was added to the culture medium and incubated for 8 hours. For BTX-A treatment, BTX-A (0.1 U/mL) was added to the culture medium for 24 hours.
Cell transfection
Lipofectamine 2000 reagent was used to conduct RNA-silencing experiments on microglia, according to the manufacturer’s protocol. Briefly, cells were seeded in a six-well plate and incubated overnight at 37 °C in a cell culture incubator until they reached 70% confluence. The culture medium was replaced with serum-free medium for 2 hours to induce cell starvation. Subsequently, 200 pmol of short hairpin (sh)RNA was mixed with lipofectamine 2000 in 50 μL of Opti-MEM medium. The mixture was incubated at room temperature for 15 minutes and then added to a cell culture medium. Cells were incubated at 37 °C for 48 hours and collected for further experimentation.
RNA extraction and polymerase chain reaction assay
RNA was extracted using TRIzol reagent (Invitrogen, Waltham, MA, United States). RNA was reverse transcribed to cDNA using a first-strand synthesis kit (TransGen, China). Subsequently, RNA was quantified using the SYBR Green kit (Takara, China) and calculated using the 2-∆∆Ct method. The primers used in this study were as follows: Tumor necrosis factor (TNF)-α sense: 5’-CAGGCGGTGCCTATGTCTC-3’; TNF-α anti-sense: 5’-CGATCACCCCGAAGTTCAGTAG-3’. Tnterleukin (IL)-6 sense: 5’-CTGCAAGAGACTTCCATCCAG-3’; IL-6 anti-sense: 5’-AGTGGTATAGACAGGTCTGTTGG-3’. IL-1β sense: 5’-GAAATGCCACCTTTTGACAGTG-3’; IL-1β anti-sense: 5’-TGGATGCTCTCATCAGGACAG-3’. Spp1 sense: 5’-AGAGCGGTGAGTCTAAGGAGT-3’; Spp1 anti-sense: 5’-TGCCCTTTCCGTTGTTGTCC-3’. GAPDH sense: 5’-GGAGCGAGATCCCTCCAAAAT-3’; GAPDH anti-sense: 5’-GGCTGTTGTCATACTTCTCATGG-3’.
Cell culture
Cells of different treatments were collected and suspended in complete media, and 100 μL cell suspension buffer was added to each well of the 96-well plate so that the number of cells per well was approximately 104, with five replicates in each group. The 96-well plate was incubated at 37 °C for 24 hours, and 10 μL of cell counting kit-8 reagent (Beyotime, China) was added to each well and cultured for 2 hours. The absorbance of each well was measured at 450 nm using a microplate reader (Thermo, Waltham, MA, United States).
Cell apoptosis
Apoptosis was detected using an Annexin V/propidium iodide apoptosis detection kit (Beyotime, China). Cells were collected and re-suspended in 1 × binding buffer at a density of approximately 1 × 106 cells/mL. Subsequently, 5 μL each of FITC-Annexin V and propidium iodide staining reagent were added into each well and incubated for 30 minutes in the dark. The cells were then centrifuged at 800 rpm for 5 minutes, re-suspended with 500 μL phosphate-buffered saline (PBS), and examined using a flow cytometer (BD Biosciences, United States).
Spared nerve injury surgery
The spared nerve injury (SNI) model was constructed as previously described[23]. Sprague-Dawley rats were purchased from the Beijing Vital River Laboratory. Animals were anesthetized with 100 mg/kg ketamine, and an incision was made on the thigh skin, reaching through the biceps femoris muscle to expose the three terminal branches of the sciatic nerve: The sural, common peroneal, and tibial nerves. The common peroneal and tibial nerves were securely ligated using 6.0 silk and then sectioned distal to the ligation, removing 2-4 mm of the distal nerve stump to separate the layers of the muscle and skin. Subsequently, BoNT/A (10 U/kg/day) was injected into the cheek for three consecutive days for treatment. Behavioral assessments commenced the day after surgery and continued for 14 days after surgery.
Thermal stimulation
The thermal withdrawal thresholds were determined following established protocols[19]. Animals were given 10 to 15 minutes to acclimate to the apparatus (Ugo Basile, Varese, Italy) before testing commenced. The rats were housed in a clear plastic chamber (18 cm × 29 cm × 12.5 cm) with a glass floor for 5 minutes to familiarize themselves with the environment. Initially, the rats displayed exploratory behavior; however, they later settled and stood quietly with occasional bouts of grooming. After acclimation, a radiant heat source was placed directly beneath the hind paw through a glass floor. Each trial began when the switch activated the radiant heat source, and an electronic timer was initiated. This assay measured the latency in seconds until hind paw withdrawal. The heat intensity was calibrated to provide an average latency of 8-10 seconds in naive, untreated animals; a maximum cut-off value of 20 seconds was established to prevent tissue injury. For each animal, the withdrawal latency was calculated as the average of three separate determinations, with at least 2 minutes intervals between trials.
Tissue collection
The rats were anesthetized with ketamine and transcardially perfused. Brief perfusion was conducted using a saline solution containing 5000 IU/mL heparin, followed by perfusion with a 4 % PFA solution. The glabrous skin of the hind paw and spinal cord were then dissected, fixed in PFA solution for 2 hours, and subsequently transferred into 30% sucrose solution for at least 24 hours. Subsequently, tissues were sectioned into 40 μm thick slices.
Enzyme-linked immunosorbent assay
The levels of inflammatory cytokines (TNF-α, IL-6, and IL-1β) were detected by enzyme-linked immunosorbent assay kits (Elabscience, China) in accordance with the manufacturer’s protocol.
Immunofluorescence staining
The cells and tissue samples were incubated with 4% paraformaldehyde for 10 minutes and then with 0.1% Triton X-100 in PBS solution for 10 minutes. The cells were incubated with PBS containing 1% bovine serum albumin for 30 minutes to block nonspecific proteins. The samples were incubated with anti-NLRP3 and anti-ASC specific antibodies overnight in a refrigerator at 4 °C. Fluorescent secondary antibodies were then incubated for 1 hours. Nuclei were stained with a 4’,6-diamidino-2-phenylindole staining solution for 10 minutes. The images were captured using a confocal microscope (Nikon, Tokyo, Japan).
Statistical analyses
Data presented in this study are the averages of three independent experiments. Statistical analyses were conducted using SPSS 20.0 and GraphPad Prism 7.0 software. Statistical significance was defined based on standard deviation. Student’s t-test or one-way analysis of variance were used for statistical analyses between two or multiple groups. Statistical significance was set at P < 0.05.
RESULTS
SPP1 and pyroptosis are activated in LPS-induced microglia
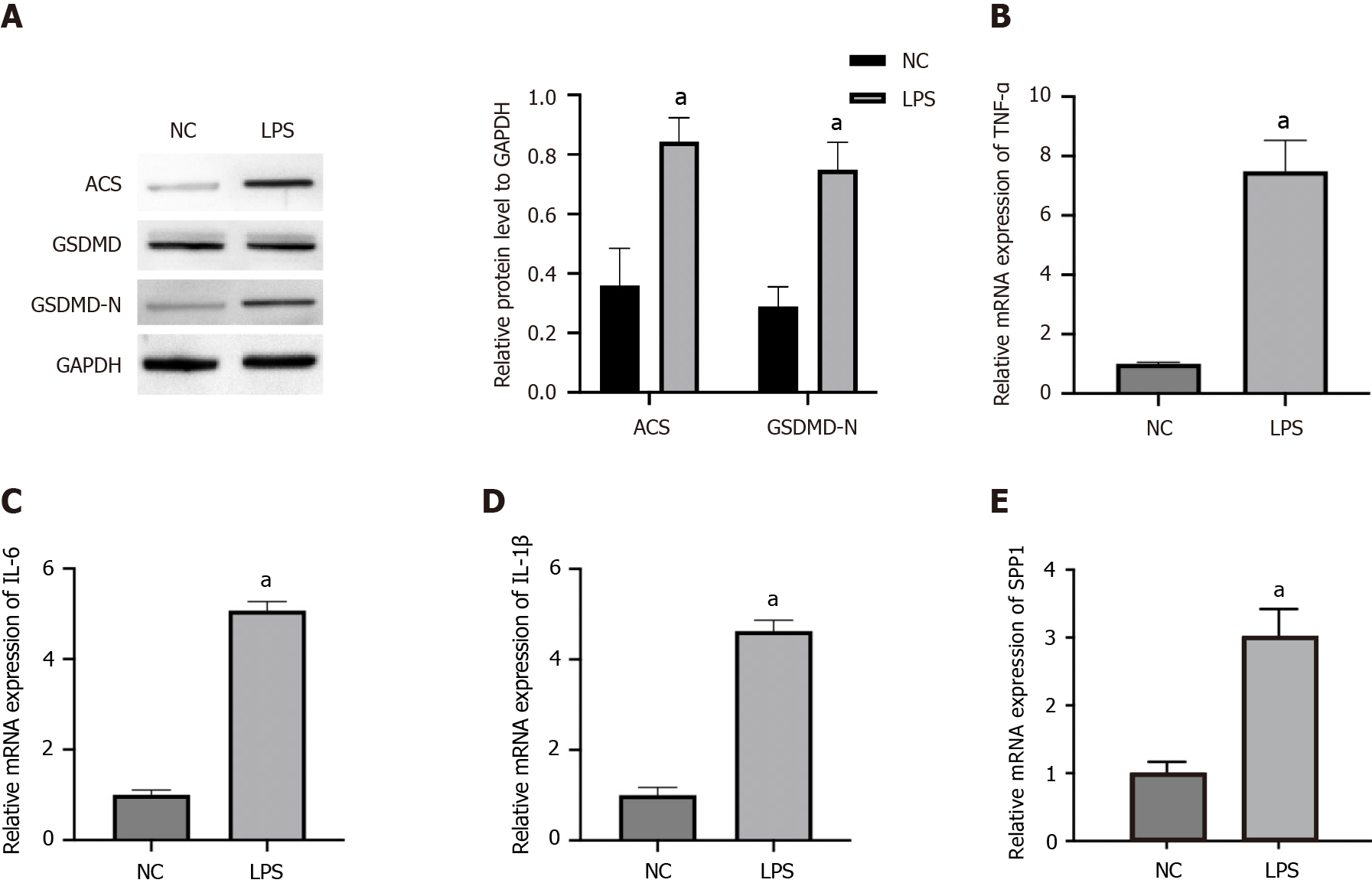
To investigate the correlation of SPP1 with microglia pyroptosis, the levels of pyroptosis markers, inflammatory factors, and SPP1 were analyzed in LPS-treated microglia. The expression levels of ACS and GSDMD-N were enhanced in LPS-treated microglia (Figure 1A). Consistently, the mRNA expression of TNF-α, IL-6, and IL-1β was upregulated in LPS-treated microglia (Figure 1B-D). Importantly, SPP1 expression was induced in LPS-treated microglia (Figure 1E), indicating that SPP1 expression may positively correlate with microglial pyroptosis and inflammation.
Figure 1 SPP1 and pyroptosis are activated in lipopolysaccharide-induced microglia.
A-E: The microglia were treated with lipopolysaccharide. The expression of ACS, GSDMD, and GSDMD-N was measured by western blot (A). The levels of tumor necrosis factor-α, interleukin (IL)-6, and IL-1β were detected by quantitative real-time polymerase chain reaction (B-D). The expression of SPP1 was analyzed by quantitative real-time polymerase chain reaction (E). aP < 0.001. LPS: Lipopolysaccharide; TNF: Tumor necrosis factor; IL: Interleukin; NC: Negative control.
SPP1 targeted by BTX-A promotes proliferation and represses apoptosis of LPS-induced microglia
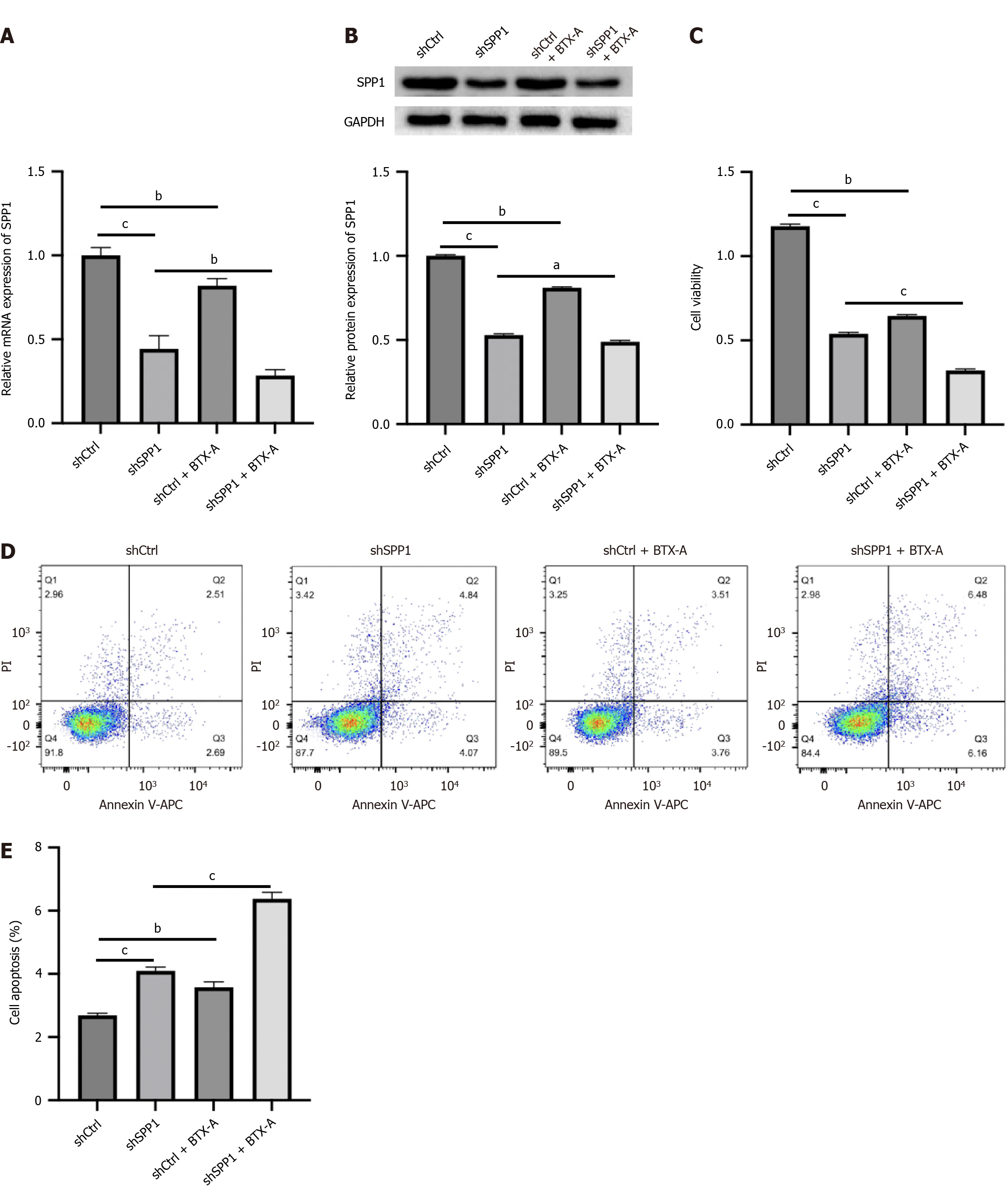
BTX-A is a gram-positive anaerobic Clostridium botulinum exotoxin that is widely used clinically for aesthetics and dystonia. Some clinical studies have provided evidence of the effect of BTX-A on the attenuation of NP. Our data showed that BTX-A inhibited SPP1 mRNA and protein expression in LPS-treated microglia (Figure 2A and B). In addition, the effectiveness of SPP1 depletion by shRNA was validated in microglia (Figure 2A and B). Functionally, the depletion of SPP1 or BTX-A inhibited cell viability and induced apoptosis of LPS-treated microglia, and co-treatment with BTX-A enhanced the effect of SPP1 shRNA on LPS-treated microglia (Figure 2C-E), indicating that SPP1 targeted by BTX-A promotes proliferation and represses apoptosis in LPS-induced microglia.
Figure 2 SPP1 targeted by botulinum toxin type A promotes proliferation and represses apoptosis of lipopolysaccharide-induced microglia.
A-E: Lipopolysaccharide-treated microglia were treated with control short hairpin RNA (shRNA) or SPP1 shRNA, or co-treated with botulinum toxin type A. The expression of SPP1 was analyzed by quantitative real-time polymerase chain reaction (A). The expression of SPP1 was measured by western blot (B). Cell viability was detected by cell counting kit-8 assay (C). Cell apoptosis was examined by flow cytometry (D and E). aP < 0.05, bP < 0.01, cP < 0.001. BTX-A: Botulinum toxin type A.
SPP1 targeted by BTX-A contributes to pyroptosis of LPS-induced microglia
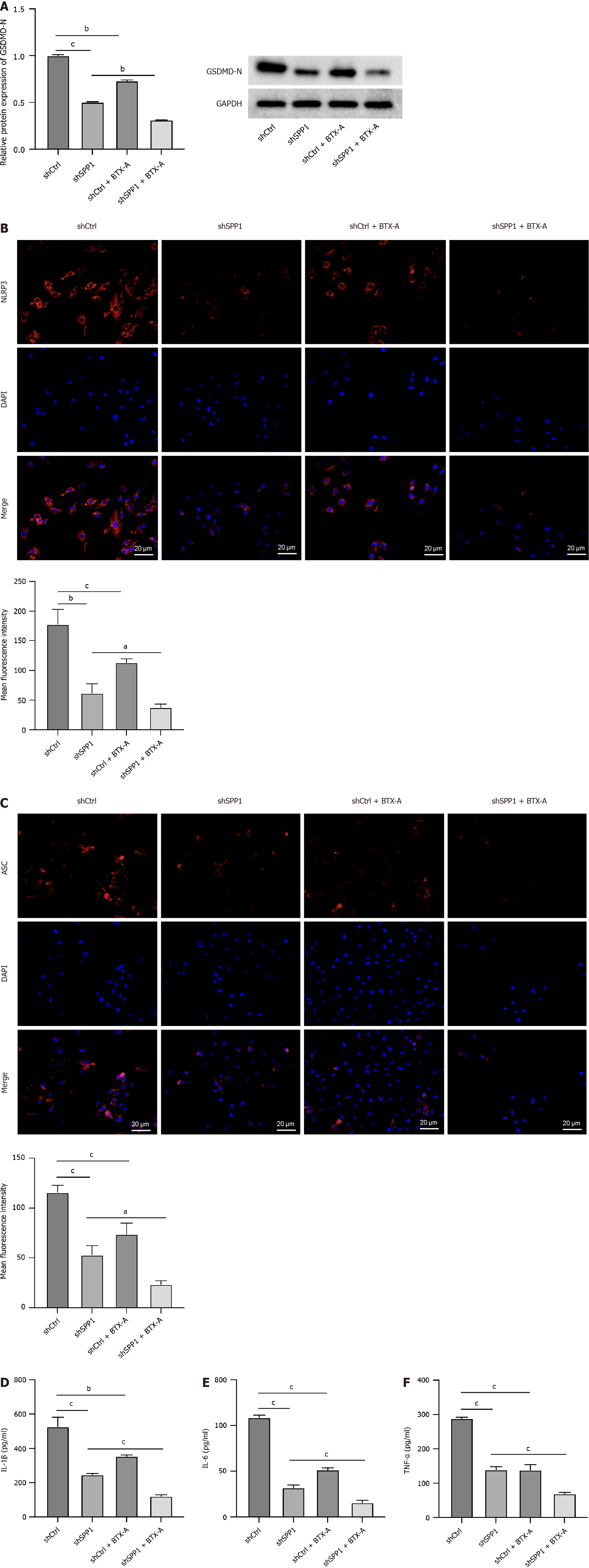
We assessed the effect of SPP1 targeted by BTX-A on microglial pyroptosis. We observed that the levels of GSDMD-N, NLPRP3, and ASC were repressed by SPP1 knockdown or BTX-A treatment in LPS-treated microglia and that co-treatment with BTX-A enhanced the effect of SPP1 shRNA on pyroptosis in LPS-treated microglia (Figure 3A-C). Consistently, SPP1 depletion or BTX-A treatment was able to inhibit the levels of TNF-α, IL-6, and IL-1β in LPS-treated microglia, and SPP1 shRNA and BTX-A co-treatment presented a more observable effect on the phenotype (Figure 3D-F), indicating that SPP1 targeted by BTX-A contributes to pyroptosis of LPS-induced microglia.
Figure 3 SPP1 targeted by botulinum toxin type A contributes to pyroptosis of lipopolysaccharide-induced microglia.
A-F: Lipopolysaccharide-treated rat microglia were treated with control short hairpin RNA (shRNA) or SPP1 shRNA, or co-treated with botulinum toxin type A. The expression of GSDMD-N was measured by western blot (A). The levels of NLRP3 and ASC were detected by immunofluorescence (B and C). The levels of tumor necrosis factor-α, interleukin (IL)-6, and IL-1β were analyzed by enzyme-linked immunosorbent assay (D-F). aP < 0.05, bP < 0.01, cP < 0.001. TNF: Tumor necrosis factor; IL: Interleukin; BTX-A: Botulinum toxin type A.
SPP1 targeted by BTX-A enhances microglial pyroptosis during NP induced by SNI in rat
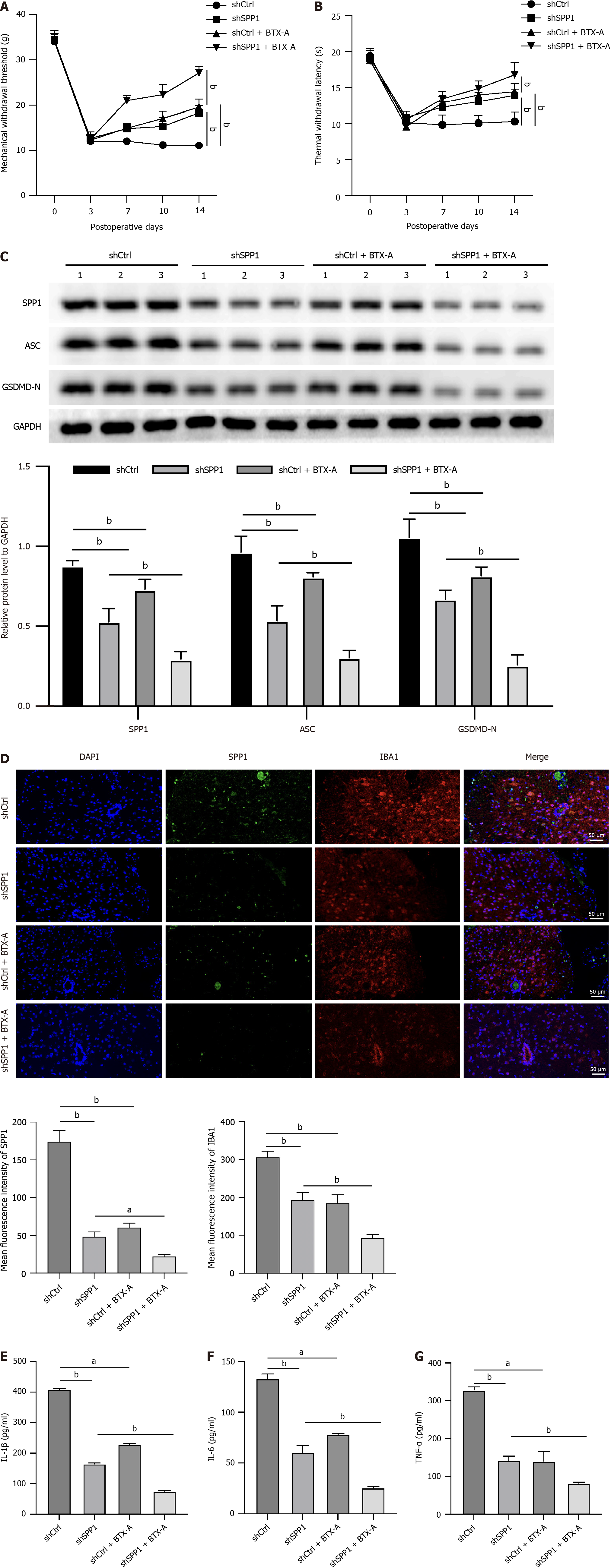
Next, we evaluated the effect of SPP1 targeting by BTX-A on NP in a rat model of SNI. We observed that the mechanical withdrawal threshold and thermal withdrawal latency were promoted by SPP1 knockdown or BTX-A in rats with SNI, whereas co-treatment with BTX-A enhanced the effect of SPP1 shRNA (Figure 4A and B). The levels of SPP1, IBA1, ACS, and GSDMD-N were inhibited by SPP1 depletion or BTX-A in rats with SNI, and co-treatment with BTX-A and SPP1 depletion had a more observable effect (Figure 4C and D). Consistently, the levels of TNF-α, IL-6, and IL-1β were repressed by BTX-A or SPP1 depletion in rats with SNI, and co-treatment with BTX-A could enhance the effect of SPP1 depletion (Figure 4E-G).
Figure 4 SPP1 targeted by botulinum toxin type A enhances microglia pyroptosis during neuropathic pain induced by spared nerve injury in rat.
A-G: The spared nerve injury model was established in SD rats and the rats were treated with control short hairpin RNA (shRNA) or SPP1 shRNA, or co-treated with botulinum toxin type A. The mechanical withdrawal threshold and thermal withdrawal latency were analyzed in the rats (A and B). The expression of SPP1, ACS, and GSDMD-N was measured by western blot in spinal cord of the rats (C). The levels of IBA1 and SPP1 were detected by immunofluorescence in spinal cord of the rats (D). The levels of tumor necrosis factor-α, interleukin (IL)-6, and IL-1β were analyzed by enzyme-linked immunosorbent assay (E-G). aP < 0.05, bP < 0.01, cP < 0.001. TNF: Tumor necrosis factor; IL: Interleukin; BTX-A: Botulinum toxin type A.
DISCUSSION
In the present study, we investigated the potential mechanisms by which BTX-A alleviates PN. By establishing models of LPS-induced microglial pyroptosis and SNI, we demonstrated a significant elevation in SPP1 within microglial cells. Moreover, SPP1 knockdown inhibited microglial pyroptosis and alleviated pain in rats with nerve injury. Microglial pyroptosis is a novel form of cell death mediated by inflammasomes and is associated with immune and inflammatory diseases of the central nervous system, including depression, radiation-induced brain injury, NP, and spinal cord injury[24-27]. Notably, NLRP3 inflammasomes play a crucial role in microglial activation, are predominantly expressed in microglial cells, and have been extensively studied in the context of NP[28-31]. Recent studies have indicated the involvement of NLRP3 and pyroptosis in depression and anxiety, two primary symptoms of NP[32]. For example, Li et al[33] reported that isoliquiritin protects primary microglia by suppressing NLRP3-mediated pyroptosis and possesses potent antidepressant properties[33]. The primary components of the NLRP3 inflammasome include the pattern recognition receptor NLRP3, adaptor protein ASC, and pro-caspase-1 enzyme[34]. Activation of NLRP3 by various stimuli and ligands triggers the assembly of the NLRP3-ASC inflammasome complex, activating caspase-1. Activated caspase-1 cleaves IL-1β and IL-18, increasing the secretion of mature IL-1β and IL-18[34]. Additionally, activated caspase-1 cleaves GSDMD to produce an N-terminal fragment, which forms pores in the cell membrane. This leads to the secretion of IL-1β and IL-18 and an influx of water molecules, ultimately resulting in excessive inflammatory responses and cell pyroptosis[35]. In this study, we observed that BTX-A treatment alleviated the production of pro-inflammatory cytokines and repressed the level of GSDMD-N in LPS-induced microglia, suggesting that BTX-A affects NP by modulating microglial pyroptosis.
Aberrantly activated microglia and innate immune cells exacerbate inflammatory pain by upregulating inflammatory factors[36,37]. A wealth of research indicates that NLRP3 inflammasome activation triggers functional alterations in microglia, contributing to the onset and progression of chronic pain[24,38]. Previous studies have suggested that silicate agents modulate microglial pyroptosis by inhibiting the activation of the NLRP3-caspase-1-GSDMD pathway, thereby regulating chronic neuroinflammation and NP[24]. Moreover, both acute and chronic pain have associations with pyroptosis in inflammatory cells[39,40]. Consequently, targeting microglial pyroptosis holds promise in alleviating inflammatory damage and mitigating NP. Accumulating evidence suggests a role for the NLRP3 inflammasome in inflammatory pain[41,42]. For example, NLRP3 knockout male mice were protected from surgery-induced postoperative inflammation and neuron-sensitized postoperative inflammatory pain[43]. Furthermore, complete Freund’s adjuvant injection induced the activation of the NLRP3 inflammasome in claw skin macrophages and promoted the maturation of inflammatory cytokine IL-1β through the cleavage of caspase-1[44]. Therefore, NLRP3-mediated pyroptosis may mediate the therapeutic effects of BTX-A in NP.
Notably, BTX-A has been reported to modulate pain. Currently, the only approved use of BTX-A in relation to pain is for the treatment of chronic migraine. However, controlled clinical studies have shown promising results for neuropathic and other chronic pain disorders[45,46]. Several studies have revealed potential mechanisms underlying BTX-A treatment. For example, in the sensory ganglia of injured nerves, BoNT/A reduces pain-evoked upregulated protein expression of nociception-related ion channels such as TRPV1 and purinoceptor P2X3, and reduces the mRNA expression of pronociceptive peptides such as preprodynorphin[47]. Intraplantar injection of BTX-A in neuropathic mice improved the sciatic index and weight bearing, along with increased cell division cycle 2 protein expression and Schwann cell proliferation and maturation[48]. In this study, we found that the RNA and protein levels of SPP1 were significantly increased during LPS-induced pyroptosis of microglia. Knockdown of SPP1 synergistically downregulated the expression of inflammatory factors and pyroptosis signaling with BTX-A treatment to inhibit microglial viability and improve pain in rats with nerve injury. Notably, SPP1, also known as osteopontin, has been widely studied in several diseases, and is a multifunctional glycoprotein that was originally thought to be a pro-inflammatory cytokine secreted by T cells. It was later found to be expressed in macrophages in different tissues and is associated with the active clearance of apoptotic cells, chemotaxis, and macrophage migration[49,50]. In the brain, SPP1 expression is highly regulated in a spatiotemporal and cell type-specific manner, depending on the environment, age, and brain region. In the perinatal and prenatal brain, SPP1 is expressed by microglia. It is associated with the axon tract of the corpus callosum. In contrast, in the adult posterior brain, SPP1 expression is limited to glutaminergic and γ-aminobutyric acid neurons[51-55]. Consistent with previous reports, we elucidated the important role of SPP1 in BTX-A-induced NP relief.