Published online May 19, 2024. doi: 10.5498/wjp.v14.i5.695
Revised: April 9, 2024
Accepted: April 22, 2024
Published online: May 19, 2024
Processing time: 145 Days and 17 Hours
Cognitive reserve (CR) and the catechol-O-methyltransferase (COMT) Val/Met polymorphism are reportedly linked to negative symptoms in schizophrenia. However, the regulatory effect of the COMT genotype on the relationship bet
To investigate whether the relationship between CR and negative symptoms could be regulated by the COMT Val/Met polymorphism.
In a cross-sectional study, 54 clinically stable patients with schizophrenia underwent assessments for the COMT genotype, CR, and negative symptoms. CR was estimated using scores in the information and similarities subtests of a short form of the Chinese version of the Wechsler Adult Intelligence Scale.
COMT Met-carriers exhibited fewer negative symptoms than Val homozygotes. In the total sample, significant negative correlations were found between negative symptoms and information, similarities. Associations between information, similarities and negative symptoms were observed in Val homozygotes only, with information and similarities showing interaction effects with the COMT genotype in relation to negative symptoms (information, β = -0.282, 95%CI: -0.552 to -0.011, P = 0.042; similarities, β = -0.250, 95%CI: -0.495 to -0.004, P = 0.046).
This study provides initial evidence that the association between negative symptoms and CR is under the regulation of the COMT genotype in schizophrenia.
Core Tip: Cognitive reserve (CR) and the catechol-O-methyltransferase (COMT) Val/Met polymorphism are reportedly linked to negative symptoms, which are a core clinical manifestation of schizophrenia. However, the regulatory effect of the COMT genotype on the relationship between CR and negative symptoms is unclear. In this study, COMT Met-carriers exhibited fewer negative symptoms than Val homozygotes. Information and similarities showed interaction effects with the COMT genotype in terms of negative symptoms. This preliminary study shows that the association between negative symptoms and CR may be under the regulation of the COMT genotype in schizophrenia.
- Citation: Hou WP, Qin XQ, Hou WW, Han YY, Bo QJ, Dong F, Zhou FC, Li XB, Wang CY. Interaction between catechol-O-methyltransferase Val/Met polymorphism and cognitive reserve for negative symptoms in schizophrenia. World J Psychiatry 2024; 14(5): 695-703
- URL: https://www.wjgnet.com/2220-3206/full/v14/i5/695.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i5.695
Negative symptoms are a core clinical manifestation of schizophrenia, encompassing blunted affect, alogia, apathy, anhedonia, and avolition[1]. Negative symptoms and cognitive deficits are the most critical determinants in the functional outcome and overall quality of life in schizophrenia[2,3]. Furthermore, negative symptoms have been documented as mediators in the influence of cognitive impairments on functional outcomes[4]. Currently, both pharmacological and non-pharmacological interventions show limited efficacy in treating negative symptoms[5-7]. It is essential to identify the influential factors of negative symptoms to develop individualized and comprehensive intervention strategies for patients with schizophrenia.
Cognitive reserve (CR) is frequently reported as a predictor of negative symptoms in cross-sectional and longitudinal studies[8-11]. CR refers to the ability to buffer the effects of illness through pre-existing and compensatory cognitive processes[12]. It is typically assessed using socio-behavioral measures, including intelligence quotient (IQ), educational level, occupational attainment, and leisure activity participation[13,14]. Apart from affecting negative symptoms, CR can also mitigate the adverse effects of exaggerated structural brain deterioration and disease relapse on neurocognitive function while concurrently enhancing psychosocial functioning[15-20]. The relationship between CR and negative symptoms may be even more pronounced in more severe pathological states[21,22].
Standardized and unified assessment tools are lacking in terms of assessing CR. Although educational level is the most commonly used indicator[12], it is a static measure that reflects learning and cognitive activities during a specific period in early life. By contrast, crystallized intelligence refers to the breadth and depth of knowledge and information that a person acquires over their lifetime[23]. Thus, measures of crystallized intelligence are dynamic and can capture the effects of continuous learning, thereby making them more suitable as alternative indicators of CR[24,25].
Dopamine activity in the prefrontal cortex plays a vital role in regulating negative symptoms in schizophrenia[26]. The catechol-O-methyltransferase (COMT) enzyme deactivates dopamine[27]. The COMT Val/Met polymorphism is a key genetic factor affecting the activity variation of the eponymous enzyme. Val homozygotes have approximately 40% higher enzyme activity in the prefrontal cortex than Met/Met carriers, potentially leading to lower levels of prefrontal dopamine signaling[28]. Therefore, the COMT genotype may be a remarkable factor influencing negative symptoms[29-31], as evidenced by studies that reported increased levels of negative symptoms in Val homozygotes[29].
To date, no study has been conducted on the interaction effects of the COMT genotype with CR in terms of negative symptoms. We hypothesized that the association between CR and negative symptoms would be regulated by the COMT genotype. Specifically, the correlation between CR and negative symptoms could be stronger in Val homozygotes with relatively more severe pathological features. A cross-sectional study was conducted among stable patients with schizophrenia to test this hypothesis. In this study, CR was primarily estimated by crystallized intelligence.
All data were derived from the baseline dataset of a randomized, controlled, double-blind clinical trial. The trial was conducted from September 2021 to May 2023 at Beijing Anding Hospital in China. The trial was approved by the Ethics Committee of Beijing Anding Hospital (No. 2020-70), and it was registered in the Chinese Clinical Trial Registry (ChiCTR2000038961). The participants in the trial were clinically stable outpatients or community-dwelling individuals with schizophrenia, aged between 18 and 50 years, with an IQ score above 70, and a minimum of 8 years of education. The primary exclusion criteria included a history of severe physical illness such as craniocerebral trauma or infection, brain tumor, cerebrovascular disease, and epilepsy according to patient self-description; as well as a history of alcohol or substance use disorders during the past 6 months. Sixty participants were enrolled at baseline, of which 54 completed testing for single-nucleotide polymorphisms for the cross-sectional analysis of this study.
The diagnoses of schizophrenia and alcohol or substance use disorders were based on the DSM-5 criteria utilizing the Mini International Neuropsychiatric Interview (version 7.0.2). Clinical symptoms were assessed using the Positive and Negative Syndrome Scale, which includes a positive subscale for positive symptoms, a negative subscale for negative symptoms, and the general psychopathology subscale for general symptoms[32]. IQ was evaluated using a short form of the Chinese version of the Wechsler Adult Intelligence Scale (WAIS-RC), consisting of four subtests[33]. The information and similarities subtests primarily reflect crystallized intelligence, whereas the picture completion and block design subtests are designed to measure fluid intelligence which is the capacity to think logically and solve problems in novel situations. Together, these four subtests represent a rough measure of general ability[33,34].
Whole blood was utilized for DNA extraction. The rs4680 locus sequence was probed for the COMT Val/Met polymorphism. Genotypes were identified by polymerase chain reaction amplification, incorporation of terminator nucleotides and subsequent agarose gel electrophoresis. Among the 54 samples analyzed, 27 were Val/Val carriers, 22 were Val/Met carriers, and five were Met/Met carriers. The Val/Met and Met/Met carriers were combined as Met-carriers for subsequent analysis.
Chi square test was utilized to examine whether the distribution of the COMT genotype deviated from the Hardy-Weinberg equilibrium. Chi square tests, Fisher’s exact tests, and t-tests were applied to compare differences in demographics, negative symptoms, other clinical symptoms, and IQ between the two genotype groups (COMT Met-carriers and Val homozygotes). Where applicable, multiple linear regression analysis was conducted to control for potential confounding variables. Pearson’s correlation analyses and t-tests were used to identify potential indicators associated with negative symptoms in the total sample, including scores from the four WAIS-RC subtests and the total score, educational level, age, chlorpromazine equivalents, duration of illness, gender, employment and marriage.
Pearson’s correlation analyses were used to assess the correlation of negative symptoms with information, similarities in COMT Met-carriers and Val homozygotes. Following Fisher’s r to z transformation, Z-tests were employed to compare the correlation coefficients between the two genotype groups[35]. Additionally, multiple linear regression analyses were conducted to investigate the interaction effects of information, similarities and the genotype, and other influential factors in relation to negative symptoms. The variables were standardized to reduce collinearity in the multiple linear regression. All the above analyses were conducted in SPSS 20.0 (SPSS Inc., Chicago, IL, United States), and the results were visualized with the ggplot2 package in R version 4.2.3. Statistical significance was established at P < 0.05.
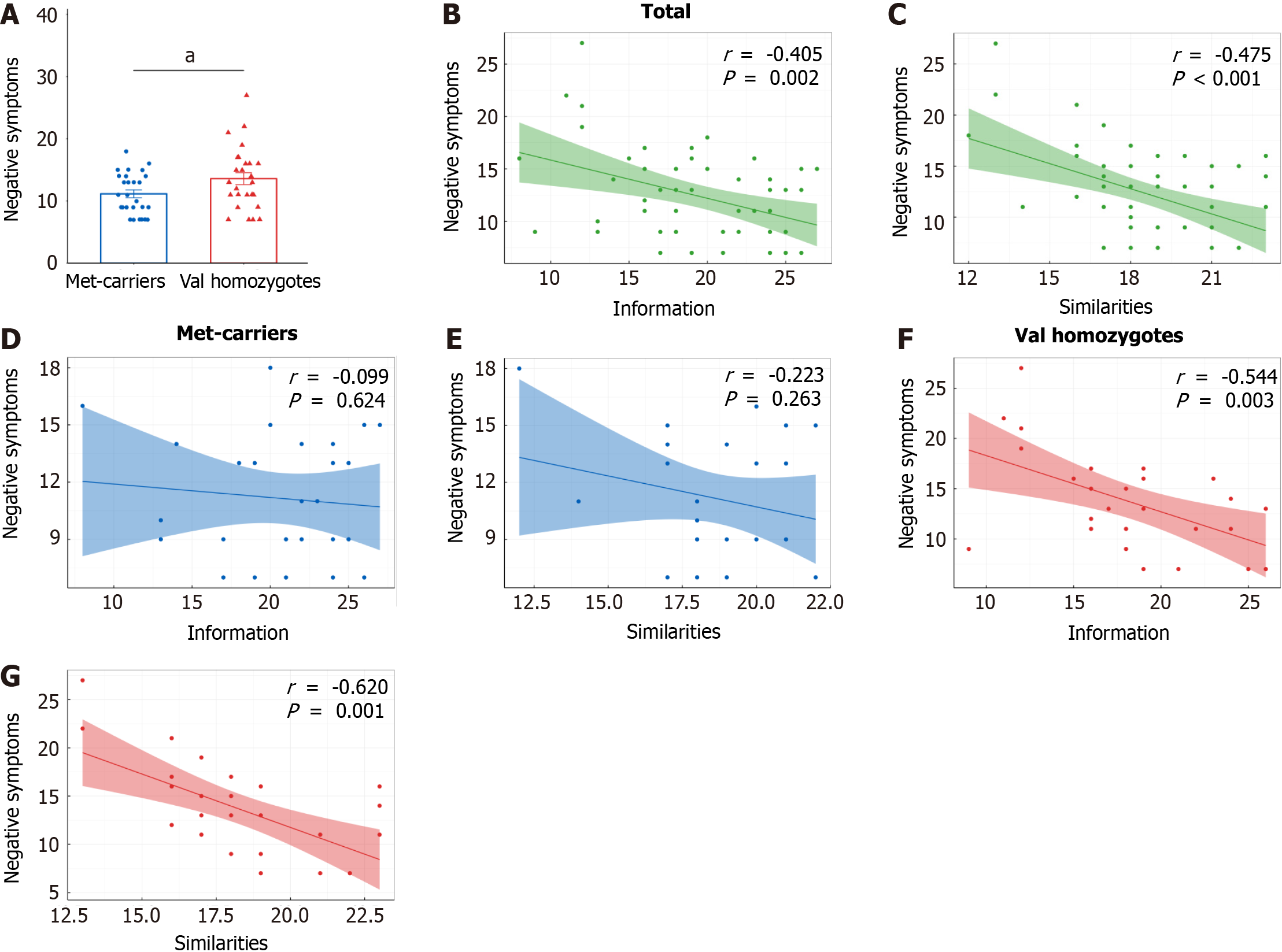
The distribution of the COMT genotype showed no deviation from the Hardy-Weinberg equilibrium (χ² = 0.013, P = 0.993). Met-carriers showed significantly lower scores in negative symptoms than Val homozygotes (t52 = -2.138, P = 0.037) (Table 1) (Figure 1A). The inclusion of employment as a covariate did not significantly alter the results.
| Met-carriers, n = 27 | Val homozygotes, n = 27 | Statistic | u value | P value | |||||
| Mean | SD | Mean | SD | ||||||
| Age | 34.59 | 7.66 | 33.15 | 7.23 | t = 0.713 | 52 | 0.479 | ||
| Educational level | 14.37 | 3.52 | 14.98 | 3.90 | t = -0.604 | 52 | 0.548 | ||
| Female/male | 16/11 | 14/13 | χ² = 0.300 | 1 | 0.584 | ||||
| Han nationality/non | 24/3 | 25/2 | NA | NA | 1.000 | ||||
| Employment/non | 8/19 | 15/12 | χ² = 3.711 | 1 | 0.054 | ||||
| Marriage/non | 12/15 | 10/17 | χ² = 0.307 | 1 | 0.580 | ||||
| Current smoker/non | 3/24 | 3/24 | NA | NA | 1.000 | ||||
| Clozapine user/non | 5/22 | 4/23 | NA | NA | 1.000 | ||||
| Chlorpromazine equivalents | 474.96 | 258.23 | 426.50 | 323.60 | t = 0.608 | 52 | 0.546 | ||
| Duration of illness | 11.73 | 8.56 | 11.24 | 7.51 | t = 0.223 | 52 | 0.825 | ||
| PANSS positive | 9.48 | 3.89 | 8.56 | 2.90 | t = 0.992 | 52 | 0.326 | ||
| PANSS negative | 11.15 | 3.31 | 13.59 | 4.93 | t = -2.138 | 52 | 0.037 | ||
| PANSS general | 21.48 | 5.15 | 20.59 | 3.86 | t = 0.718 | 52 | 0.476 | ||
| PANSS total | 42.11 | 10.02 | 42.74 | 7.89 | t = -0.257 | 52 | 0.799 | ||
| WAIS-RC information | 20.67 | 4.66 | 18.41 | 4.80 | t = 1.756 | 52 | 0.085 | ||
| WAIS-RC similarities | 18.67 | 2.27 | 18.33 | 2.76 | t = 0.485 | 52 | 0.630 | ||
| WAIS-RC picture completion | 11.41 | 3.12 | 10.15 | 2.80 | t = 1.563 | 52 | 0.124 | ||
| WAIS-RC block design | 36.19 | 7.19 | 36.44 | 8.44 | t = -0.122 | 52 | 0.904 | ||
| WAIS-RC total | 106.61 | 9.75 | 103.00 | 10.54 | t = 1.306 | 52 | 0.197 | ||
In the total sample, significant negative correlations were observed between negative symptoms and information (r = -0.405, P = 0.002), similarities (r = -0.475, P < 0.001) (Figure 1B and C). In addition, negative symptoms also correlated with picture completion, block design, WAIS-RC total scores and educational level (all P < 0.05) (Table 2). However, t-test did not reveal any significant effects of gender, employment, and marital status on negative symptoms (Table 3).
| Information | Similarities | Picture completion | Block design | WAIS-RC total | Education | Age | CE | Duration of illness | ||
| PANSS negative | r value | -0.405 | -0.475 | -0.332 | -0.385 | -0.527 | -0.384 | 0.019 | -0.243 | -0.119 |
| P value | 0.002 | < 0.001 | 0.014 | 0.004 | < 0.001 | 0.004 | 0.894 | 0.076 | 0.391 |
| Gender/employment/marriage | n | Mean | SD | t value | u value | P value | |
| PANSS negative | Male | 24 | 13.63 | 4.79 | 1.949 | 52 | 0.057 |
| Female | 30 | 11.37 | 3.73 | ||||
| Employed | 23 | 11.91 | 3.85 | -0.663 | 52 | 0.510 | |
| Non | 31 | 12.71 | 4.71 | ||||
| Married | 32 | 12.09 | 4.71 | -0.561 | 52 | 0.577 | |
| Non | 22 | 12.77 | 3.80 |
Negative symptoms were associated with information (r = -0.544, P = 0.003) and similarities (r = -0.620, P = 0.001) in Val homozygotes only (Figure 1D-G). Moreover, the correlation coefficients of information and similarities with negative symptoms showed significant differences between the two genotype groups (information, Z = 1.768, P = 0.038; similarities, Z = 1.726, P = 0.042).
Given the marginally significant effects of chlorpromazine equivalents (P = 0.076) and gender (P = 0.057) on negative symptoms in the univariate analyses, these variables were subsequently included in the multiple linear regression analyses. In these analyses, negative symptoms were treated as the dependent variable, with COMT genotype, information or similarities, the product of COMT genotype and information or similarities, gender, and chlorpromazine equivalents serving as independent variables. Multiple linear regression analyses further revealed the interaction effects of information, similarities with the genotype in terms of negative symptoms (information, β = -0.282, 95%CI: -0.552 to -0.011, P = 0.042; similarities, β = -0.250, 95%CI: -0.495 to -0.004, P = 0.046; Table 4). However, the effects of gender and chlorpromazine equivalents on negative symptoms were not found to be significant.
| Information | Similarities | |||
| PANSS negative | Met-carriers (n = 27) | r value | -0.099 | -0.223 |
| P value | 0.624 | 0.263 | ||
| Val/Val (n = 27) | r value | -0.544 | -0.62 | |
| P value | 0.003 | 0.001 | ||
| Correlation coefficient comparison | Z value | 1.768 | 1.726 | |
| P value | 0.038 | 0.042 | ||
| Interaction test1 | β value | -0.282 | -0.250 | |
| 95%CI | -0.552 to -0.011 | -0.495 to -0.004 | ||
| P value | 0.042 | 0.046 |
This study is the first to examine the regulatory role of the COMT Val/Met polymorphism in the relationship between negative symptoms and CR in schizophrenia. The results showed that COMT Met-carriers exhibited milder negative symptoms than Val homozygotes. In the total sample, negative symptoms were associated with CR reflected by information and similarities. Furthermore, the correlation of negative symptoms with CR was regulated by the COMT genotype.
COMT Met-carriers showed fewer negative symptoms than Val homozygotes. This observation aligns with the findings of Wang et al[29], who reported milder negative symptoms in Met-carriers among older patients with schizophrenia. Bosia et al[36] documented that the COMT genotype influenced the improvement of negative symptoms in patients with schizophrenia after taking clozapine. These findings support the statement that prefrontal dopamine levels mediated by the COMT genotype contribute to inter-individual variability of negative symptoms[1].
Negative symptoms were negatively related to CR and IQ. Similarly, Bucci et al[37] and Chang et al[9] reported that patients with poor CR exhibited more severe primary negative symptoms and worse working memory in cross-sectional studies. Furthermore, prospective studies found CR to be predictive of improvements in the negative symptoms or persistent negative symptoms within 1-10 years after first-episode psychosis[8,11,38]. CR-related factors may mitigate the effect of the disease pathology on the clinical phenotype through fostering new connections between neurons or different brain regions, activating compensatory neural networks, or enhancing the efficiency of existing neural network connections[39,40]. Nevertheless, the detailed mechanisms are yet to be elucidated, and relevant research remains limited in schizophrenia.
Our study did not observe significant effects of demographic or clinical characteristics on negative symptoms. How
The relationship of negative symptoms with CR was regulated by the COMT genotype. This study is a novel report in the context of schizophrenia. Similarly, research demonstrated that CR interacted with the apolipoprotein ε4 (APOE-ε4) genotype, a genetic risk factor for dementia, in relation to cognitive function in healthy elders[44]. CR may have a stronger protective effect against the risk of dementia in APOE-ε4 carriers[45]. These findings may suggest that in increased pathological states, there is greater room for clinical phenotype improvement, thereby leading to more pronounced protective effects of CR.
Considering that the protective effect of CR is evident only in the early stages of dementia[46,47], further investigation is warranted to determine whether a similar threshold phenomenon exists in the interaction effects of certain factors with CR in terms of the clinical phenotype of schizophrenia. Nonetheless, the current findings hold implications for the individualized and comprehensive intervention of negative symptoms in clinically stable patients with schizophrenia, suggesting that the intervention for negative symptoms in COMT Val homozygotes may require the application of more CR strategies. Additionally, our findings indicate that the effect of COMT on negative symptoms may be influenced by complex factors. This complexity might result in negative outcomes in clinical trials of COMT inhibitors for negative symptoms[48], despite the effect of COMT genotype on dopamine activity suggesting the potential efficacy of COMT inhibitors[49].
One of the strengths of this study is that it is the first report on the regulatory effect of the COMT genotype on the relationship between CR and negative symptoms. Additionally, all participants were clinically stable patients, thereby reducing potential confounding effects of fluctuating medications and clinical symptoms on the findings. Despite this, this study has some limitations that need to be acknowledged. Firstly, as a cross-sectional survey, it could not establish the dynamic and causal relationships among CR, COMT genotype and negative symptoms, necessitating further cohort studies and intervention trials. Secondly, the relatively small sample size limited the generalizability of the findings, thus requiring validation through larger-scale studies. Thirdly, due to the limited sample size, we combined the Val/Met and Met/Met carriers into a single group identified as Met-carriers. Although this grouping strategy is a common analysis approach[50-52], it may overlook valuable information that warrants further investigation in studies with larger sample sizes. Fourthly, this study did not comprehensively collect potential factors influencing negative symptoms and faced limitations in detecting weakly associated factors due to the small sample size. Lastly, due to relatively mild negative symptoms of the participants, it remains unknown whether the interaction effects of CR and the COMT genotype are influenced by negative symptom severity.
In summary, our study showed that the correlation between negative symptoms and CR was regulated by the COMT Val/Met polymorphism. The findings enhance the understanding of the mechanisms underlying individual differences in negative symptoms and provide insightful evidence for the individualized and comprehensive intervention of negative symptoms in schizophrenia.
The authors would like to thank all participants in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade A
Creativity or Innovation: Grade A
Scientific Significance: Grade B
P-Reviewer: Ng IK, Singapore S-Editor: Chen YL L-Editor: A P-Editor: Zhao S
| 1. | Galderisi S, Mucci A, Buchanan RW, Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5:664-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 2. | Mucci A, Galderisi S, Gibertoni D, Rossi A, Rocca P, Bertolino A, Aguglia E, Amore M, Bellomo A, Biondi M, Blasi G, Brasso C, Bucci P, Carpiniello B, Cuomo A, Dell'Osso L, Giordano GM, Marchesi C, Monteleone P, Niolu C, Oldani L, Pettorruso M, Pompili M, Roncone R, Rossi R, Tenconi E, Vita A, Zeppegno P, Maj M; Italian Network for Research on Psychoses. Factors Associated With Real-Life Functioning in Persons With Schizophrenia in a 4-Year Follow-up Study of the Italian Network for Research on Psychoses. JAMA Psychiatry. 2021;78:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 3. | Galderisi S, Rossi A, Rocca P, Bertolino A, Mucci A, Bucci P, Rucci P, Gibertoni D, Aguglia E, Amore M, Bellomo A, Biondi M, Brugnoli R, Dell'Osso L, De Ronchi D, Di Emidio G, Di Giannantonio M, Fagiolini A, Marchesi C, Monteleone P, Oldani L, Pinna F, Roncone R, Sacchetti E, Santonastaso P, Siracusano A, Vita A, Zeppegno P, Maj M; Italian Network For Research on Psychoses. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry. 2014;13:275-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 359] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 4. | Koshiyama D, Thomas ML, Miyakoshi M, Joshi YB, Molina JL, Tanaka-Koshiyama K, Sprock J, Braff DL, Swerdlow NR, Light GA. Hierarchical Pathways from Sensory Processing to Cognitive, Clinical, and Functional Impairments in Schizophrenia. Schizophr Bull. 2021;47:373-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr Bull. 2015;41:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 478] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 6. | Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, Arndt T, Bäckers L, Rothe P, Cipriani A, Davis J, Salanti G, Leucht S. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394:939-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1211] [Cited by in RCA: 988] [Article Influence: 164.7] [Reference Citation Analysis (0)] |
| 7. | Cella M, Preti A, Edwards C, Dow T, Wykes T. Cognitive remediation for negative symptoms of schizophrenia: A network meta-analysis. Clin Psychol Rev. 2017;52:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Üçok A, Ergül C. Persistent negative symptoms after first episode schizophrenia: A 2-year follow-up study. Schizophr Res. 2014;158:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Chang WC, Lau CFC, Chan SSI, Hui CLM, Chan SKW, Lee EHM, Lin J, Chen EYH. Premorbid, clinical and cognitive correlates of primary negative symptoms in first-episode psychosis. Psychiatry Res. 2016;242:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Mezquida G, Amoretti S, Bioque M, García-Rizo C, Sánchez-Torres AM, Pina-Camacho L, Lopez-Pena P, Mané A, Rodriguez-Jimenez R, Corripio I, Sarró S, Ibañez A, Usall J, García-Portilla MP, Vieta E, Mas S, Cuesta MJ, Parellada M, González-Pinto A, Berrocoso E, Bernardo M; 2EPs Group. Identifying risk factors for predominant negative symptoms from early stages in schizophrenia: A longitudinal and sex-specific study in first-episode schizophrenia patients. Span J Psychiatry Ment Health. 2023;16:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Mezquida G, Cabrera B, Bioque M, Amoretti S, Lobo A, González-Pinto A, Espliego A, Corripio I, Vieta E, Castro-Fornieles J, Bergé D, Escartí MJ, Ibañez Á, Penadés R, Sánchez-Torres AM, Bernardo M; PEPs Group. The course of negative symptoms in first-episode schizophrenia and its predictors: A prospective two-year follow-up study. Schizophr Res. 2017;189:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Nogueira J, Gerardo B, Santana I, Simões MR, Freitas S. The Assessment of Cognitive Reserve: A Systematic Review of the Most Used Quantitative Measurement Methods of Cognitive Reserve for Aging. Front Psychol. 2022;13:847186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Magdaleno Herrero R, Murillo-García N, Yorca-Ruiz Á, Neergaard K, Crespo-Facorro B, Ayesa-Arriola R; PAFIP Group. Biomarkers as proxies for cognitive reserve: The role of high density lipoprotein cholesterol in first episode of psychosis. Rev Psiquiatr Salud Ment. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Herrero P, Contador I, Stern Y, Fernández-Calvo B, Sánchez A, Ramos F. Influence of cognitive reserve in schizophrenia: A systematic review. Neurosci Biobehav Rev. 2020;108:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Sánchez-Torres AM, Amoretti S, Enguita-Germán M, Mezquida G, Moreno-Izco L, Panadero-Gómez R, Rementería L, Toll A, Rodriguez-Jimenez R, Roldán A, Pomarol-Clotet E, Ibáñez Á, Usall J, Contreras F, Vieta E, López-Ilundain JM, Merchán-Naranjo J, González-Pinto A, Berrocoso E, Bernardo M, Cuesta MJ; 2EPs group. Relapse, cognitive reserve, and their relationship with cognition in first episode schizophrenia: a 3-year follow-up study. Eur Neuropsychopharmacol. 2023;67:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 16. | Amoretti S, Rosa AR, Mezquida G, Cabrera B, Ribeiro M, Molina M, Bioque M, Lobo A, González-Pinto A, Fraguas D, Corripio I, Vieta E, de la Serna E, Morro L, Garriga M, Torrent C, Cuesta MJ, Bernardo M; PEPs Group. The impact of cognitive reserve, cognition and clinical symptoms on psychosocial functioning in first-episode psychoses. Psychol Med. 2022;52:526-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | González-Ortega I, González-Pinto A, Alberich S, Echeburúa E, Bernardo M, Cabrera B, Amoretti S, Lobo A, Arango C, Corripio I, Vieta E, de la Serna E, Rodriguez-Jimenez R, Segarra R, López-Ilundain JM, Sánchez-Torres AM, Cuesta MJ; PEPs Group:, Zorrilla I, López P, Bioque M, Mezquida G, Barcones F, De-la-Cámara C, Parellada M, Espliego A, Alonso-Solís A, Grasa EM, Varo C, Montejo L, Castro-Fornieles J, Baeza I, Dompablo M, Torio I, Zabala A, Eguiluz JI, Moreno-Izco L, Sanjuan J, Guirado R, Cáceres I, Garnier P, Contreras F, Bobes J, Al-Halabí S, Usall J, Butjosa A, Sarró S, Landin-Romero R, Ibáñez A, Selva G. Influence of social cognition as a mediator between cognitive reserve and psychosocial functioning in patients with first episode psychosis. Psychol Med. 2020;50:2702-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Buonocore M, Inguscio E, Bechi M, Cuoco F, Martini F, Agostoni G, Spangaro M, Cocchi F, Terragni R, Diddi O, Terreni S, Cavallaro R, Bosia M. Cognitive reserve profiles are associated with outcome in schizophrenia. J Neurol Sci. 2022;443:120496. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Sharip S, Zairani MI, Ing LP, Baharom NN, Asarapoo NS, Rahman NA, Saini SM, Abdul Rahman AH. Association between cognitive reserve with cognitive impairment, social and occupational functioning, and quality of life in schizophrenia: A preliminary study in the Malaysian population. Asian J Psychiatr. 2020;53:102230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Van Rheenen TE, Cropley V, Fagerlund B, Wannan C, Bruggemann J, Lenroot RK, Sundram S, Weickert CS, Weickert TW, Zalesky A, Bousman CA, Pantelis C. Cognitive reserve attenuates age-related cognitive decline in the context of putatively accelerated brain ageing in schizophrenia-spectrum disorders. Psychol Med. 2020;50:1475-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Ohi K, Takai K, Sugiyama S, Kitagawa H, Kataoka Y, Soda M, Kitaichi K, Kawasaki Y, Ito M, Shioiri T. Intelligence decline across major depressive disorder, bipolar disorder, and schizophrenia. CNS Spectr. 2021;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Rodriguez M, Knížková K, Keřková B, Siroňová A, Šustová P, Jonáš J, Španiel F. The relationships between cognitive reserve, cognitive functioning and quality of life in first-episode schizophrenia spectrum disorders. Psychiatry Res. 2022;310:114479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Ziegler M, Danay E, Heene M, Asendorpf J, Bühner M. Openness, fluid intelligence, and crystallized intelligence: Toward an integrative model. J Res Pers. 2012;46:173-183. [RCA] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Malek-Ahmadi M, Lu S, Chan Y, Perez SE, Chen K, Mufson EJ. Static and Dynamic Cognitive Reserve Proxy Measures: Interactions with Alzheimer's Disease Neuropathology and Cognition. J Alzheimers Dis Parkinsonism. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Thow ME, Summers MJ, Saunders NL, Summers JJ, Ritchie K, Vickers JC. Further education improves cognitive reserve and triggers improvement in selective cognitive functions in older adults: The Tasmanian Healthy Brain Project. Alzheimers Dement (Amst). 2018;10:22-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Wu Q, Wang X, Wang Y, Long YJ, Zhao JP, Wu RR. Developments in Biological Mechanisms and Treatments for Negative Symptoms and Cognitive Dysfunction of Schizophrenia. Neurosci Bull. 2021;37:1609-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1272] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 29. | Wang Y, Fang Y, Shen Y, Xu Q. Analysis of association between the catechol-O-methyltransferase (COMT) gene and negative symptoms in chronic schizophrenia. Psychiatry Res. 2010;179:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Clelland CL, Drouet V, Rilett KC, Smeed JA, Nadrich RH, Rajparia A, Read LL, Clelland JD. Evidence that COMT genotype and proline interact on negative-symptom outcomes in schizophrenia and bipolar disorder. Transl Psychiatry. 2016;6:e891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Sun Z, Zhang Z, Mao P, Ma Y, Li W, Li J, Yang X, Ling S, Tang Y. Association between COMT gene polymorphisms, clinical symptoms, and cognitive functions in Han Chinese patients with schizophrenia. Psychiatr Genet. 2018;28:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13937] [Cited by in RCA: 15624] [Article Influence: 411.2] [Reference Citation Analysis (0)] |
| 33. | Pang YX, Zhang J, Yang CL, Cang Y, Wang XL. [Application of WAIS-RC short forms and adult intelligence disability scale in mental impairment assessment]. Fa Yi Xue Za Zhi. 2011;27:189-192. [PubMed] [DOI] [Full Text] |
| 34. | Benson N, Hulac DM, Kranzler JH. Independent examination of the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV): what does the WAIS-IV measure? Psychol Assess. 2010;22:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 35. | Lenhard W, Lenhard A. Hypothesis tests for comparing correlations. Psychometrica 2014. [cited 9 April 2024]. Available from: https://www.psychometrica.de/correlation.html. |
| 36. | Bosia M, Lorenzi C, Pirovano A, Guglielmino C, Cocchi F, Spangaro M, Bramanti P, Smeraldi E, Cavallaro R. COMT Val158Met and 5-HT1A-R -1019 C/G polymorphisms: effects on the negative symptom response to clozapine. Pharmacogenomics. 2015;16:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Bucci P, Galderisi S, Mucci A, Rossi A, Rocca P, Bertolino A, Aguglia E, Amore M, Andriola I, Bellomo A, Biondi M, Cuomo A, dell'Osso L, Favaro A, Gambi F, Giordano GM, Girardi P, Marchesi C, Monteleone P, Montemagni C, Niolu C, Oldani L, Pacitti F, Pinna F, Roncone R, Vita A, Zeppegno P, Maj M; Italian Network for Research on Psychoses. Premorbid academic and social functioning in patients with schizophrenia and its associations with negative symptoms and cognition. Acta Psychiatr Scand. 2018;138:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Mäkinen J, Miettunen J, Jääskeläinen E, Veijola J, Isohanni M, Koponen H. Negative symptoms and their predictors in schizophrenia within the Northern Finland 1966 Birth Cohort. Psychiatry Res. 2010;178:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, Rugg MD, Steffener J, Rajah MN. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 689] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 40. | Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1680] [Cited by in RCA: 2173] [Article Influence: 167.2] [Reference Citation Analysis (0)] |
| 41. | Carter B, Wootten J, Archie S, Terry AL, Anderson KK. Sex and gender differences in symptoms of early psychosis: a systematic review and meta-analysis. Arch Womens Ment Health. 2022;25:679-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Correll CU, Schooler NR. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr Dis Treat. 2020;16:519-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 466] [Article Influence: 93.2] [Reference Citation Analysis (0)] |
| 43. | Fervaha G, Takeuchi H, Lee J, Foussias G, Fletcher PJ, Agid O, Remington G. Antipsychotics and amotivation. Neuropsychopharmacology. 2015;40:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | López ME, Turrero A, Delgado ML, Rodríguez-Rojo IC, Arrazola J, Barabash A, Maestú F, Fernández A. APOE ε4 Genotype and Cognitive Reserve Effects on the Cognitive Functioning of Healthy Elders. Dement Geriatr Cogn Disord. 2017;44:328-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Dekhtyar S, Marseglia A, Xu W, Darin-Mattsson A, Wang HX, Fratiglioni L. Genetic risk of dementia mitigated by cognitive reserve: A cohort study. Ann Neurol. 2019;86:68-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Bialystok E. Bilingualism: Pathway to Cognitive Reserve. Trends Cogn Sci. 2021;25:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 47. | Mazzeo S, Padiglioni S, Bagnoli S, Bracco L, Nacmias B, Sorbi S, Bessi V. The dual role of cognitive reserve in subjective cognitive decline and mild cognitive impairment: a 7-year follow-up study. J Neurol. 2019;266:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Kaphzan H, Ben-Shachar D, Klein E. Entacapone augmentation of antipsychotic treatment in schizophrenic patients with negative symptoms; a double-blind placebo-controlled study. Int J Neuropsychopharmacol. 2014;17:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Apud JA, Weinberger DR. Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors. CNS Drugs. 2007;21:535-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Xu H, Zhou Y, Xiu M, Chen D, Wang W, Wang L, Zhang X. The inconsistent mediating effect of catechol O methyl transferase Val(158)Met polymorphism on the sex difference of cognitive impairment in schizophrenia patients. Front Psychiatry. 2022;13:993859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 51. | Wang J, Xu H, Wang D, Wei G, Zhou H, Wang L, Zhou Y, Zhang X. The interactive effect of genetic polymorphisms of IL-10 and COMT on cognitive function in schizophrenia. J Psychiatr Res. 2021;136:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Nieratschker V, Kiefer C, Giel K, Krüger R, Plewnia C. The COMT Val/Met polymorphism modulates effects of tDCS on response inhibition. Brain Stimul. 2015;8:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |