Published online Mar 19, 2024. doi: 10.5498/wjp.v14.i3.456
Peer-review started: November 28, 2023
First decision: January 25, 2024
Revised: February 4, 2024
Accepted: March 6, 2024
Article in press: March 6, 2024
Published online: March 19, 2024
Processing time: 111 Days and 21.4 Hours
Adolescent major depressive disorder (MDD) is a significant mental health concern that often leads to recurrent depression in adulthood. Resting-state functional magnetic resonance imaging (rs-fMRI) offers unique insights into the neural mechanisms underlying this condition. However, despite previous research, the specific vulnerable brain regions affected in adolescent MDD patients have not been fully elucidated.
To identify consistent vulnerable brain regions in adolescent MDD patients using rs-fMRI and activation likelihood estimation (ALE) meta-analysis.
We performed a comprehensive literature search through July 12, 2023, for studies investigating brain functional changes in adolescent MDD patients. We utilized regional homogeneity (ReHo), amplitude of low-frequency fluctuations (ALFF) and fractional ALFF (fALFF) analyses. We compared the regions of aberrant spontaneous neural activity in adolescents with MDD vs healthy controls (HCs) using ALE.
Ten studies (369 adolescent MDD patients and 313 HCs) were included. Combining the ReHo and ALFF/fALFF data, the results revealed that the activity in the right cuneus and left precuneus was lower in the adolescent MDD patients than in the HCs (voxel size: 648 mm3, P < 0.05), and no brain region exhibited increased activity. Based on the ALFF data, we found decreased activity in the right cuneus and left precuneus in adolescent MDD patients (voxel size: 736 mm3, P < 0.05), with no regions exhibiting increased activity.
Through ALE meta-analysis, we consistently identified the right cuneus and left precuneus as vulnerable brain regions in adolescent MDD patients, increasing our understanding of the neuropathology of affected adolescents.
Core Tip: Utilizing activation likelihood estimation meta-analysis, this study identified consistently vulnerable brain regions in adolescent major depressive disorder (MDD) patients. The findings of this study revealed distinct neural alterations, specifically decreased activity in the precuneus and cuneus areas, indicating the potential neurobiological underpinnings specific to adolescent MDD. This study offers crucial insights into the unique neural signatures of depression in adolescents, paving the way for targeted interventions and advancing our understanding of adolescent mental health.
- Citation: Ding H, Zhang Q, Shu YP, Tian B, Peng J, Hou YZ, Wu G, Lin LY, Li JL. Vulnerable brain regions in adolescent major depressive disorder: A resting-state functional magnetic resonance imaging activation likelihood estimation meta-analysis. World J Psychiatry 2024; 14(3): 456-466
- URL: https://www.wjgnet.com/2220-3206/full/v14/i3/456.htm
- DOI: https://dx.doi.org/10.5498/wjp.v14.i3.456
Major depressive disorder (MDD) is a prevailing mental health challenge that disproportionately affects adolescents and has profound clinical and societal implications[1]. MDD typically originates during adolescence with a marked increase in incidence, particularly among females, and a male-to-female ratio of approximately 1:2[2]. In addition, the recurrence rate of adolescent depression is substantial, constituting a pivotal risk factor for suicide and giving rise to severe social consequences[3]. Understanding the intricate neural underpinnings of MDD during this critical developmental phase is imperative for advancing effective therapeutic interventions.
Resting-state functional magnetic resonance imaging (rs-fMRI) has emerged as an indispensable tool in neuroimaging research, offering unparalleled insights into the intrinsic functional architecture of the brain[4]. By assessing spontaneous fluctuations in blood oxygen level-dependent signals during rest, rs-fMRI can reveal intricate patterns of connectivity and activity across distinct brain regions, providing a unique perspective for comprehending the aberrant neurocircuitry implicated in MDD. The analytical techniques for localized spontaneous brain activity in rs-fMRI include regional homogeneity (ReHo), amplitude of low-frequency fluctuations (ALFF), and fractional ALFF (fALFF)[5]. These methods are frequently employed to characterize intrinsic brain activity during rest. ReHo can be used to evaluate the local coherence of rs-fMRI signals, aiding in identifying neural synchronization anomalies; ALFF can directly reflect the changes in the functional activities of the corresponding local brain regions by calculating the ALFF value of each voxel; and fALFF can be used to measure the relative contribution of low-frequency signal power, highlighting aberrant neural activity patterns in MDD[6]. Accordingly, the combination of ReHo, ALFF, and fALFF can more comprehensively reflect the pattern of changes in spontaneous local brain activity in adolescent depression patients. Functional connectivity (FC) indicates the functional correlation between seed sites and surrounding brain regions, which is distinct from the spontaneous neurobrain functional activity reflected by ReHo, ALFF, and fALFF. FC is not a suitable candidate for meta-analysis unless all studies are the same kind of network study[7]. To our knowledge, although many previous studies have used ReHo, ALFF, and fALFF methods and rs-fMRI to explore the changes in spontaneous brain activity in adolescent depression patients[8-17], the results of these studies are inconsistent and are still controversial.
We used neuroimaging activation likelihood estimation (ALE) to analyze the pattern of changes in spontaneous brain activity in adolescents with MDD. ALE aggregates the peak activation coordinates across neuroimaging studies to create spatial probability maps highlighting consistent brain region involvement in specific tasks[18]. Previously, Yuan et al[19] used the ALE method to conduct a meta-analysis of MDD patients; however, they did not distinguish age ranges specific to adolescents and may not have captured the consistently vulnerable brain regions in the resting state that may differ between adolescent depression patients and adults. In this study, we employed ALE analysis to focus exclusively on the integration and assessment of data from abnormal active brain regions reported in prior studies using ReHo and ALFF/fALFF approaches. This analysis enables us to further explore the more consistently impaired brain regions involved in the spontaneous activity of the local brain in adolescent depression patients, with the aim of uncovering the potential neural mechanisms underlying brain injury in these patients.
Study selection was conducted in accordance with the PRISMA 2020 guidelines. This review was registered with PROSPERO (ID: CRD42023371521). A comprehensive literature search in PubMed, Google Scholar, Embase, Web of Science, and CNKI was conducted to identify all fMRI studies published before June 13, 2022. The keywords used for the search included "depression", "major depressive disorder", "adolescent", "regional homogeneity", "amplitude of low-frequency fluctuation", "fractional amplitude of low-frequency fluctuation", "resting", "functional magnetic resonance" and "fMRI". Moreover, we searched the references of several reviews and imported them into the EndNote 20.2 document management tool for filtering.
The studies that met the following inclusion criteria were considered for subsequent analysis: (1) MDD diagnosed according to the DSM-5 criteria; (2) inclusion of adolescent participants; (3) whole-brain analysis of differences in brain functional activity between adolescents with MDD and healthy controls (HCs) via rs-fMRI; (4) ReHo or ALFF/fALFF analysis methods; and (5) brain regions with differences between adolescents with MDD and HCs presented as Montreal Neurological Institute (MNI) or Talairach three-dimensional peak coordinates (x, y, z).
The studies were excluded if they met at least one of the following criteria: (1) Studies using rs-fMRI methods to assess FC, independent component analysis (ICA), degree centrality, default mode network (DMN), or other networks; (2) studies using voxel-based morphometry (VBM), task-state fMRI (t-fMRI) or cerebral perfusion; (3) meta-analyses, reviews, or case reports; (4) studies with incomplete three-dimensional coordinates (x, y, z); and (5) studies involving subjects other than adolescents with MDD.
The quality of the included studies was assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS)[20]. The NOS has 3 levels and a total of 8 items: (1) 4 items for subject selection; (2) 1 item for comparability between groups; and (3) 3 items for outcome measurement. The total possible score is 9 points. Studies with a score ≥ 5 points were included in the data analysis.
Two independent reviewers systematically compiled pertinent details from the selected studies. These data included study particulars, such as authors, publication year, and design; participant characteristics, such as sample size, age, and sex; rs-fMRI details, such as the MRI scanner model, field strength, and analysis software/methods used; and differential brain regions between adolescents with MDD and HCs, including quantities and central coordinates of reported discrepancies.
ALE meta-analysis was performed using GingerALE 3.0.2 software (www.brainmap.org/ale)[21]. For the ALE meta-analysis, our study was conducted in the MNI standard space. Hence, we utilized the Lancaster transformation in GingerALE 3.0.2 to convert the three-dimensional coordinates of brain regions in the Talairach space to MNI space.
Subsequently, Gaussian function smoothing with a full width at half maximum (FWHM) was performed based on the sample size of each test group. Using the FWHM values, Gaussian functions were simulated on the three-dimensional brain mask of coordinates for a set of aberrantly activated brain regions reported in the study group. This process yielded three-dimensional modeling activation (MA) maps for each study group.
Then, based on the 3D-MA maps, a 3D ALE map was generated from the Gaussian probability distribution of the activated brain regions between different study groups, and the p value of the activation probability of the brain regions was calculated according to the Gaussian model to construct a 3D-P value distribution map. Moreover, the statistical test threshold was set by a 3D-P value distribution plot. The main parameters were as follows: The cluster-level familywise error correction was set at P < 0.05, the threshold permutations were set at P < 0.001 with 1000 permutations, and a threshold map (ALE image) was obtained[18]. Finally, Mango software (http://rii.uthscsa.edu/mango/) was used to analyze the resulting ALE images.
The jackknife sensitivity analysis method was used to assess the reproducibility of the meta-analysis outcomes. In this approach, a single study was systematically excluded from the dataset, and the remaining study data were subjected to ALE meta-analysis using GingerALE 3.0.2 software. This procedure was repeated 7 times, removing one study each time, to verify the consistency of the results after the exclusion of a study and to compare these results with the original analysis.
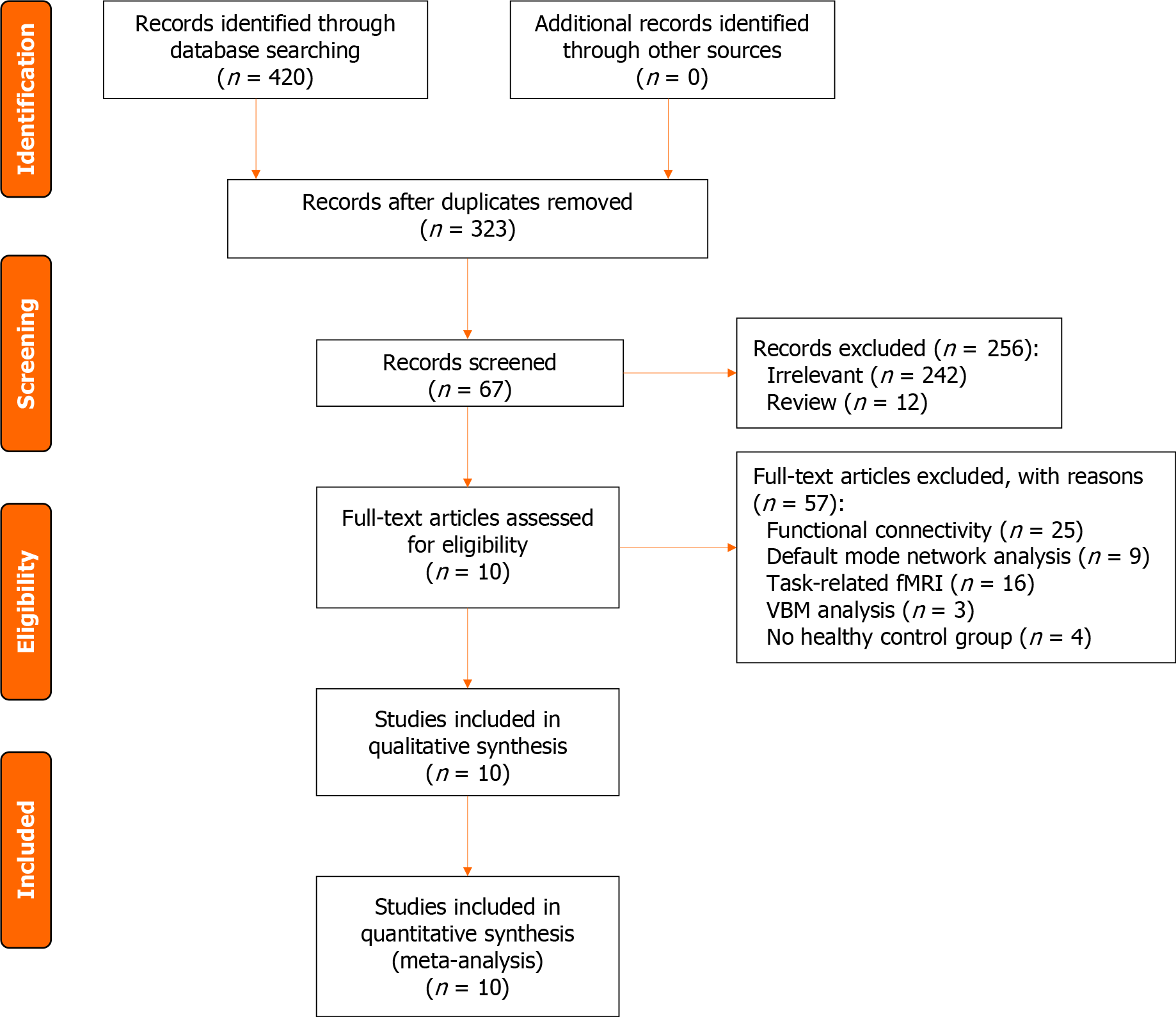
Based on the aforementioned inclusion and exclusion criteria, a total of 420 retrieved articles were screened. There were 97 duplicates, 242 irrelevant studies, 12 reviews, 25 FC studies, 9 DMN studies, 16 t-fMRI studies, 3 VBM studies, and 4 studies without HC groups. Ultimately, 10 studies were included (Figure 1), including 2 ReHo studies, 7 ALFF studies, and 1 fALFF study.
Finally, a total of 369 adolescent depression patients and 313 HCs were retained for the ALE meta-analysis. There were 38 distinct brain areas in total, including 28 ALFF, 7 ReHo, and 3 fALFF regions (Table 1).
| Ref. | Sample size | Age (mean ± SD) | Field strength | Method | Differential brain region | Coordinate | Quality | |||
| Adolescent MDD | HC | Patients | HC | |||||||
| 1 | Jiao et al[8], 2011 | 18 | 18 | 15.78 ± 1.20 | 16.20 ± 0.90 | 3.0T | ALFF | 9 | MNI | 4/1/1 |
| 2 | Gong et al[9], 2014 | 15 | 16 | 15.00 ± 2.00 | 15.00 ± 2.00 | 3.0T | ALFF | 10 | MNI | 4/1/1 |
| 3 | Jiang et al[10], 2016 | 19 | 24 | 15.58 ± 1.47 | 15.71 ± 1.55 | 3.0T | ReHo | 2 | MNI | 4/1/1 |
| 4 | Zhu et al[11], 2016 | 27 | 28 | 21.67 ± 3.39 | 21.33 ± 2.4 | 3.0T | ALFF | 2 | MNI | 4/1/1 |
| 5 | Hu et al[12], 2019 | 76 | 44 | 20.40 ± 3.50 | 20.30 ± 2.10 | 3.0T | ALFF | 3 | MNI | 4/1/1 |
| 6 | Mao et al[13], 2020 | 24 | 23 | 17.31 ± 1.34 | 18.21 ± 1.29 | 3.0T | ReHo | 5 | MNI | 4/1/1 |
| 7 | Kang et al[14], 2020 | 30 | 28 | 15.00 ± 1.66 | 15.18 ± 2.04 | 3.0T | ALFF | 1 | MNI | 4/1/1 |
| 8 | Yang et al[15], 2021 | 39 | 39 | ≤ 21 | ≤ 21 | N/A | fALFF | 3 | MNI | 4/1/1 |
| 9 | Zhang et al[16], 2023 | 50 | 39 | 15.80 ± 1.43 | 15.82 ± 1.89 | 3.0T | ALFF | 1 | MNI | 4/1/1 |
| 10 | Zhou et al[17], 2023 | 71 | 54 | 13.97 ± 1.51 | 14.17 ± 1.48 | 3.0T | ALFF | 2 | MNI | 4/1/1 |
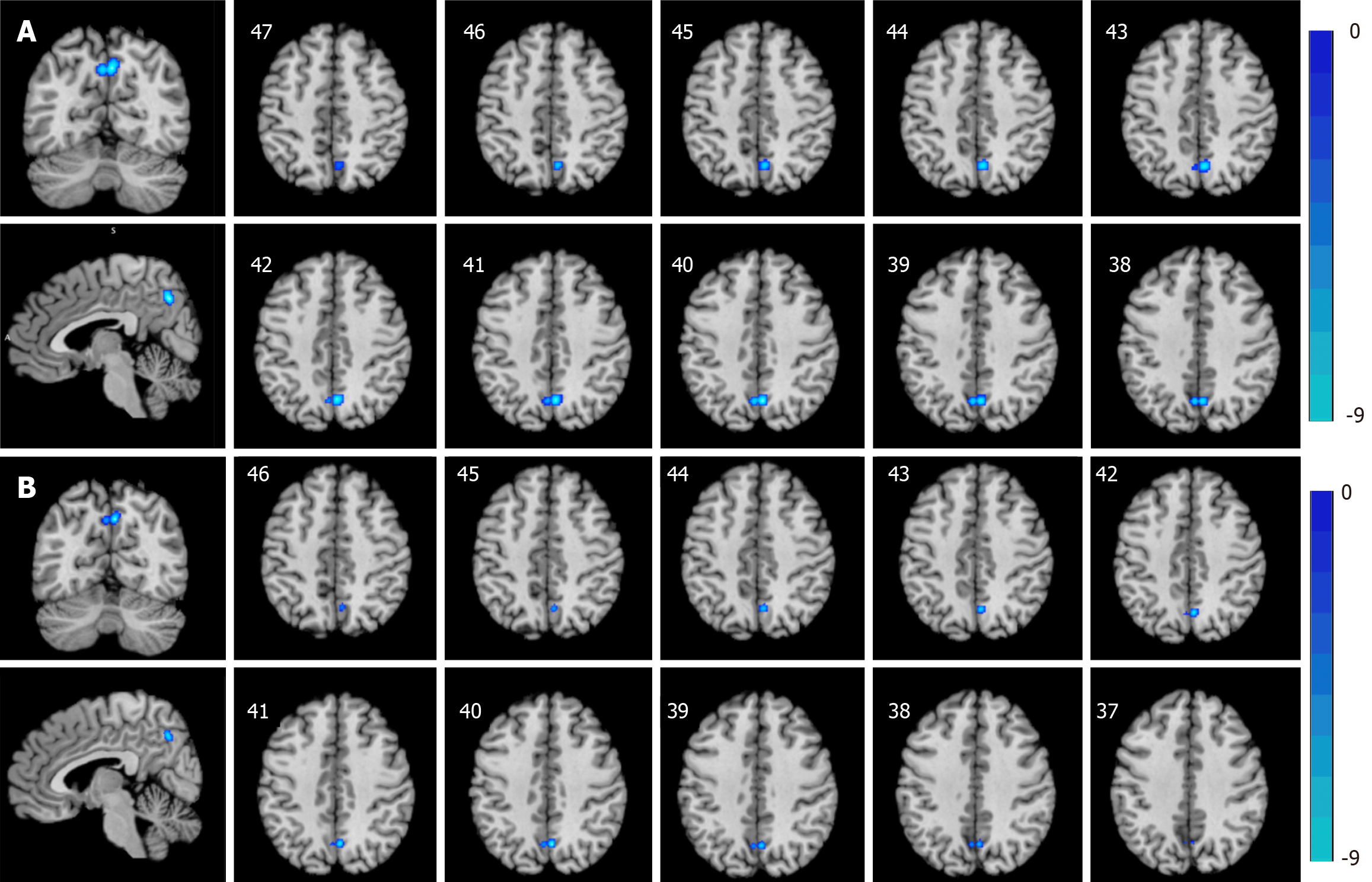
ALE meta-analysis results: Incorporating the results of both the ReHo and ALFF/fALFF data analyses, adolescents with depressive disorder exhibited reduced activity in the right cuneus and left precuneus regions compared to HCs (Table 2, Figure 2A). Then, ReHo and ALFF/fALFF ALE meta-analyses were carried out. The ALFF method ALE meta-analysis revealed that adolescent depression patients exhibited decreased activity in the right cuneus and left precuneus regions compared to HCs (Table 2, Figure 2B), but no brain regions with increased activity were found. However, the ALE meta-analyses for the ReHo and fALFF methods indicated no discernible increase or decrease in brain activity in adolescents with depressive disorder compared to HCs.
| Research methods | Anatomical label BA | Peak MNI coordinate | ALE value | Cluster | Volume (mm3) | ||
| X | Y | Z | |||||
| ReHo and ALFF/fALFF decrease | Right cuneus BA 7 | 4 | -66 | 40 | 0.011956828 | 1 | 648 |
| Left precuneus BA 7 | -2 | -66 | 40 | 0.0098253535 | 1 | 648 | |
| ALFF decrease | Right cuneus BA 7 | 4 | -66 | 40 | 0.011956828 | 1 | 736 |
| Left precuneus BA 7 | -2 | -66 | 40 | 0.0098253535 | 1 | 736 | |
Sensitivity analysis results: In the sensitivity analysis for decreased activity, the jackknife method indicated that the cuneus and precuneus consistently appeared in 5 out of the 7 dataset combinations (Table 3).
In this study, we used an ALE meta-analysis method with rs-fMRI data to explore the brain regions associated with changes in brain activity between adolescents with MDD and HCs. By integrating the findings of previous studies, this ALE meta-analysis revealed brain regions with relatively consistent changes in brain function and activity in adolescents with depression. The results showed that the vulnerable brain regions in adolescent patients with depressive disorder were mainly distributed in the right cuneus and left precuneus regions and revealed the possible neuroimaging mechanism of brain injury in adolescent patients with depression. This convergence of evidence underscores the robustness of our findings. The subsequent jackknife sensitivity and heterogeneity analyses affirmed the reproducibility and reliability of our results, further confirming the validity of the observed differences. Thus, these results could lead to the identification of a potential therapeutic target for the treatment of brain injury in adolescents with MDD.
The DMN is a network of interconnected brain regions that are active when an individual is at rest[22], notably including both the precuneus and cuneus[23]. The precuneus plays a key role in executive functions related to visuospatial imagery, episodic memory retrieval, and self-processing operations[24]. However, the cuneus is primarily responsible for processing visual information[25]. Like in other regions within the occipital lobe, the cuneus is essential for the perception and interpretation of visual stimuli, underpinning our ability to recognize and interact with our environment. Abnormal functioning of both the precuneus and cuneus can indicate compromised integrity of the DMN, a phenomenon that is frequently observed in depression[26]. The cuneus, which is a crucial part of the visual recognition network and is situated in the occipital lobe of the brain, has the primary functions of processing visual data, facial perception, emotion, and working memory[27]. Gong et al[9] showed that, compared to control individuals, adolescents with MDD had lower ALFF values in the bilateral cuneus. An fMRI reward processing task study demonstrated that adolescents with unremitting depression exhibited less activation in the cuneus than adolescents with remitting depression[28]. Hence, in adolescent MDD patients, interruptions in spontaneous brain activity associated with visual processing could lead to depressive symptoms. This finding is consistent with the abnormal spontaneous neuronal activity discovered in our ALE analysis, namely, a decrease in the spontaneous activity of the right cuneus in adolescents with depression. Additionally, in their fALFF study of sleep disorder depression, Zhu et al[29] reported that, compared to those in the normal sleep efficiency depression group, patients in the low sleep efficiency group exhibited a decrease in the fALFF in the right cuneus. Therefore, we speculated that right cuneus dysfunction in the DMN of adolescents with MDD may be related to a decrease in visual-associated brain activity. Recently, Yan et al[30] reported that the ReHo values of the bilateral cuneus were lower in MDD patients with functional gastroenterological diseases than in HCs. This finding suggested that gastrointestinal symptoms in MDD patients might be associated with the information analyzing and interpreting functions of the occipital gyrus. Yao et al[31] reported ReHo changes in the cuneus in both patients with bipolar depression and patients with unipolar depression. Moreover, Sun et al[32] reported that, compared to those in the nontreatment resident depression group, the treatment resident depression group exhibited a decrease in ALFF in the left cuneus. These findings provide a neuroimaging perspective that might help elucidate the consistently vulnerable brain regions in adolescent MDD patients according to local spontaneous brain activity. Moreover, this study could further elucidate the pathophysiological mechanisms behind depressive symptoms in adolescents with MDD. The precuneus is located within the medial aspect of the parietal lobe, serving as a pivotal nexus in the DMN and playing an indispensable role in various cognitive processes. Functionally, it is closely linked with memory, emotion, and visuospatial executive functions[33]. A task-based fMRI study of adolescents with depression revealed a correlation between activity in the precuneus and the severity of depression, where greater activity in the precuneus was associated with more severe depression. This discovery may be attributed to the rapid neural development period in adolescents with depression, which makes them more sensitive to negative features and thus allows them to access more attentional resources in the precuneus[34]. In addition, Cullen et al[35] reported that in adolescent depression patients treated with medication, treatment response was linked to increased amygdala connectivity with the right frontal cortex but reduced amygdala connectivity with the right precuneus and posterior cingulate cortex. Adolescent MDD can be simplistically regarded as an early-onset subtype of the adult disease, given its close association with later recurrences. However, the vulnerable brain regions involved in MDD among adolescents differ from those involved in adults[36]. A study on adolescent depression showed that both anhedonia and depression severity were related to decreased dorsal medial prefrontal cortex resting-state FC with the precuneus[37]. This finding suggested that decreased activity in the precuneus may be associated with adolescent MDD. A previous study compared resting-state FC (rsFC) in the precuneus subregions between adult patients with MDD and HCs and revealed that patients with MDD exhibited increased rsFC between the left precuneus and several brain regions[38]. Our study confirms reduced precuneus activity, a pivotal element in cognitive function, among adolescents with MDD, indicating a potential link to compromised cognitive functions in comparison to their healthy counterparts. These findings may improve our understanding of functional dysconnectivity in adolescents with MDD.
In this study, we observed a decrease in spontaneous neural activity in the brain regions of adolescents with MDD through integrated ALE meta-analysis or meta-analysis of ALFF alone, and no increased spontaneous neural activity was found. There have been studies reporting elevated spontaneous neural activity, such as those conducted by Kang and Kong[14], Jiao et al[8], and Zhang et al[16], which revealed that certain brain regions in adolescents with MDD exhibited increased ALFF values in comparison to those in the control group during the resting state. Jiang et al[10] and Mao et al[13] also discovered that ReHo values were greater in adolescent depression patients than in the control group. However, during the ALE meta-analysis process, a limited number of coordinates might preclude reaching the significance threshold. Consequently, although our ALE meta-analysis included 4 ALFF analyses, 2 ReHo analyses, and 1 fALFF analysis with coordinates for enhanced brain regions, comprising 21 peak coordinates of activated brain regions (foci), these activated regions are too scattered to yield results in relatively fixed brain regions. It is important to note that rs-fMRI studies have identified abnormal spontaneous low-frequency brain activity in individuals with various conditions, including adolescents with MDD[39]. However, these studies often reported inconsistent results, which may be related to the small sample sizes and different study methods. Other studies have reported structural and functional abnormalities in the anterior cingulate cortex and other brain regions in adolescents with MDD[40]. Another study revealed shared reductions in FC among the sensorimotor, visual, and auditory networks in adolescents with MDD, as well as increased sensorimotor-subcortical FC[41]. However, these findings were not found in our meta-analysis, possibly due to limitations inherent to the ALE meta-analysis method[42]. ALE meta-analysis, a probabilistic analytical approach, is effective at reducing false positives but may still encounter false negatives, particularly when dealing with a limited number of coordinates or excessively dispersed coordinates. Peak-based meta-analyses in neuroimaging studies, such as those involving adolescents with MDD, rely on summing coordinates from previously published studies rather than original statistical brain maps[43]. This approach may produce less accurate results due to potential confounding factors, such as sex distribution, mean age, symptom severity, illness duration, and scanner field strength. In this study, we concentrated on analyzing ReHo and ALFF/fALFF in adolescent MDD patients, excluding other neuroimaging methods, such as FC, ICA, and DMN, to avoid potential confusion arising from combining different rs-fMRI analysis methods. Considering the inconsistency in rs-fMRI studies and the complexity of potential neurobiological mechanisms in adolescents with MDD, further research in larger sample sizes and using more advanced imaging techniques may help to better understand the changes in spontaneous neural activity in this population.
Although this ALE meta-analysis can properly reflect the changes in spontaneous neural activity in the brains of adolescent patients with MDD, our study has several limitations. First, the ALE meta-analysis does not account for variation between studies or activation intensity, potentially omitting brain regions with low activation intensity[44]. Second, as our analysis exclusively included studies conducted in Asian countries, caution should be exercised in extending these findings to other populations, particularly Caucasians, given the potential cultural and genetic variations that can impact neural patterns. Third, inadequate data in the included studies prevented us from analyzing adolescents' educational backgrounds and current statuses, potentially overlooking the correlation between educational factors and changes in brain regions linked to depression. Fourth, neuroimaging data can be significantly affected by common artifacts, including respiratory effects and head movements, which can potentially impact outcomes. Finally, given the cross-sectional design of these studies, our meta-analysis could not elucidate any causal association between adolescent MDD and spontaneous brain function alterations, highlighting the need for essential longitudinal research.
In conclusion, our ALE meta-analysis revealed consistent vulnerability in the right cuneus and left precuneus among adolescents with MDD in the resting state compared to HCs. These findings may help further the understanding of the neurophysiological mechanisms underlying adolescent MDD and contribute to the development of more targeted interventions.
Major depressive disorder (MDD) significantly impacts adolescents, leading to recurrent depression in adulthood. Despite previous research, the specific vulnerable brain regions affected in adolescent MDD patients have not been fully elucidated. Resting-state functional magnetic resonance imaging (rs-fMRI) offers a unique opportunity to understand the neural mechanisms underlying this condition, focusing on spontaneous brain activity patterns.
Adolescent MDD poses a serious threat to the recurrence of depression in adulthood. By exploring the spontaneous neural activity in the brains of adolescents with MDD, this study not only contributes to a deeper understanding of the neurobiological mechanisms behind adolescent depression but also aims to pave the way for more targeted intervention measures and broader advancements in the field of mental health research.
To address the inconsistencies in existing neuroimaging studies on adolescent MDD, this research aims to identify consistent vulnerable brain regions through an activation likelihood estimation (ALE) meta-analysis of rs-fMRI data. The realized objectives include the integration of diverse studies to unveil specific brain regions with decreased activity in adolescents with MDD. Through the exploration of spontaneous neural activity, this research contributes to establishing critical knowledge for improving mental health outcomes in adolescents.
A comprehensive literature search was conducted, encompassing studies up to July 12, 2023, employing regional homogeneity, amplitude of low-frequency fluctuations (ALFF), and fractional ALFF (fALFF) analyses. Ten studies involving 369 adolescent MDD patients and 313 healthy controls (HCs) were included in the meta-analysis. The ALE method was utilized to aggregate peak activation coordinates, creating spatial probability maps and highlighting consistent brain regions with abnormal spontaneous activity.
The ALE meta-analysis revealed consistently decreased activity in the right cuneus and left precuneus in adolescents with MDD compared to HCs. No brain region exhibited increased activity. This consistent vulnerability in specific brain regions, particularly within the default mode network, sheds light on potential neurobiological mechanisms associated with adolescent MDD.
This study consistently identifies the right cuneus and left precuneus as vulnerable brain regions in adolescent MDD. The findings contribute to the comprehension of the neurophysiological mechanisms associated with depression in this demographic. By delineating specific brain regions with altered activity, this research lays a foundation for targeted interventions in adolescent MDD. The implications extend to future investigations, offering a nuanced understanding of the neuropathology that can inform advancements in therapeutic approaches and contribute to the broader discourse in mental health research.
While the study provides crucial insights into the unique neural signatures of depression in adolescents, future research with larger sample sizes and advanced imaging techniques is warranted. Longitudinal studies could help establish causal associations between adolescent MDD and spontaneous brain function alterations, addressing current limitations and informing more targeted interventions.
We would like to express our sincere gratitude to Guizhou Second People's Hospital for their generous support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosak L, Czech Republic S-Editor: Lin C L-Editor: A P-Editor: Zhao S
| 1. | Marwaha S, Palmer E, Suppes T, Cons E, Young AH, Upthegrove R. Novel and emerging treatments for major depression. Lancet. 2023;401:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 276] [Article Influence: 138.0] [Reference Citation Analysis (0)] |
| 2. | Thapar A, Eyre O, Patel V, Brent D. Depression in young people. Lancet. 2022;400:617-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 367] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 3. | Grossberg A, Rice T. Depression and Suicidal Behavior in Adolescents. Med Clin North Am. 2023;107:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 4. | Massalha Y, Maggioni E, Callari A, Brambilla P, Delvecchio G. A review of resting-state fMRI correlations with executive functions and social cognition in bipolar disorder. J Affect Disord. 2023;334:337-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Salvia E, Tissier C, Charron S, Herent P, Vidal J, Lion S, Cassotti M, Oppenheim C, Houdé O, Borst G, Cachia A. The local properties of bold signal fluctuations at rest monitor inhibitory control training in adolescents. Dev Cogn Neurosci. 2019;38:100664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Chen S, Yin Y, Yue Y, Li Y, Zhang Y, Jiang W, Hou Z, Yuan Y. Integrating functional neuroimaging and serum proteins improves the diagnosis of major depressive disorder. J Affect Disord. 2023;325:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Zang YF, Zuo XN, Milham M, Hallett M. Toward a Meta-Analytic Synthesis of the Resting-State fMRI Literature for Clinical Populations. Biomed Res Int. 2015;2015:435265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Jiao Q, Ding J, Lu G, Su L, Zhang Z, Wang Z, Zhong Y, Li K, Ding M, Liu Y. Increased activity imbalance in fronto-subcortical circuits in adolescents with major depression. PLoS One. 2011;6:e25159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Gong Y, Hao L, Zhang X, Zhou Y, Li J, Zhao Z, Jiang W, DU Y. Case-control resting-state fMRI study of brain functioning among adolescents with first-episode major depressive disorder. Shanghai Arch Psychiatry. 2014;26:207-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Jiang XW, Zhou Q, Kong LD, Wu F, Wang F, Tang YQ, Fan DA. A resting brain functional magnetic resonance imaging study on first-episode untreated adolescent depression patients. Chin J Nerv Ment Dis. 2016;1:56-59. |
| 11. | Zhu XL, Chen L, Yuan FL. Amplitude of Low Frequency-fluctuation in Young MDD Patients: A Resting-state fMRI Study. Chin J Clin Psychol. 2016;5:805-807. |
| 12. | Hu L, Xiao M, Ai M, Wang W, Chen J, Tan Z, Cao J, Kuang L. Disruption of resting-state functional connectivity of right posterior insula in adolescents and young adults with major depressive disorder. J Affect Disord. 2019;257:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Mao N, Che K, Chu T, Li Y, Wang Q, Liu M, Ma H, Wang Z, Lin F, Wang B, Ji H. Aberrant Resting-State Brain Function in Adolescent Depression. Front Psychol. 2020;11:1784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Kang JH, Kong LD. Amplitude of low-frequency fluctuation (ALFF) in adolescent and adult major depression: a resting-state functional MRI study. Chin J Gen Pract. 2020;2:269-272. |
| 15. | Yang L, Wei AH, Ouyang TT, Cao ZZ, Duan AW, Zhang HH. Functional plasticity abnormalities over the lifespan of first-episode patients with major depressive disorder: a resting state fMRI study. Ann Transl Med. 2021;9:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Zhang X, Cao J, Huang Q, Hong S, Dai L, Chen X, Chen J, Ai M, Gan Y, He J, Kuang L. Severity related neuroanatomical and spontaneous functional activity alteration in adolescents with major depressive disorder. Front Psychiatry. 2023;14:1157587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | Zhou Y, Song Y, Chen C, Yan S, Chen M, Liu T. Abnormal amplitude of low-frequency fluctuation values as a neuroimaging biomarker for major depressive disorder with suicidal attempts in adolescents: A resting-state fMRI and support vector machine analysis. Front Psychol. 2023;14:1146944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 1095] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 19. | Yuan J, Yu H, Yu M, Liang X, Huang C, He R, Lei W, Chen J, Tan Y, Liu K, Zhang T, Luo H, Xiang B. Altered spontaneous brain activity in major depressive disorder: An activation likelihood estimation meta-analysis. J Affect Disord. 2022;314:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12615] [Article Influence: 841.0] [Reference Citation Analysis (0)] |
| 21. | Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907-2926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1655] [Cited by in RCA: 1477] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 22. | Afzali MH, Dagher A, Bourque J, Spinney S, Conrod P. Cross-lagged Relationships Between Depressive Symptoms and Altered Default Mode Network Connectivity Over the Course of Adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Zhou J, Ma X, Li C, Liao A, Yang Z, Ren H, Tang J, Li J, Li Z, He Y, Chen X. Frequency-Specific Changes in the Fractional Amplitude of the Low-Frequency Fluctuations in the Default Mode Network in Medication-Free Patients With Bipolar II Depression: A Longitudinal Functional MRI Study. Front Psychiatry. 2020;11:574819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Messina A, Cuccì G, Crescimanno C, Signorelli MS. Clinical anatomy of the precuneus and pathogenesis of the schizophrenia. Anat Sci Int. 2023;98:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 25. | Dadario NB, Sughrue ME. The functional role of the precuneus. Brain. 2023;146:3598-3607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 113] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 26. | Kim JH, Suh SI, Lee HJ, Lee JH, Lee MS. Cortical and subcortical gray matter alterations in first-episode drug-naïve adolescents with major depressive disorder. Neuroreport. 2019;30:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Wei L, Li X, Huang L, Liu Y, Hu L, Shen W, Ding Q, Liang P. An fMRI study of visual geometric shapes processing. Front Neurosci. 2023;17:1087488. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Fischer AS, Ellwood-Lowe ME, Colich NL, Cichocki A, Ho TC, Gotlib IH. Reward-circuit biomarkers of risk and resilience in adolescent depression. J Affect Disord. 2019;246:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Zhu DM, Zhang C, Yang Y, Zhang Y, Zhao W, Zhang B, Zhu J, Yu Y. The relationship between sleep efficiency and clinical symptoms is mediated by brain function in major depressive disorder. J Affect Disord. 2020;266:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Yan M, Chen J, Liu F, Li H, Huang R, Tang Y, Zhao J, Guo W. Disrupted Regional Homogeneity in Major Depressive Disorder With Gastrointestinal Symptoms at Rest. Front Psychiatry. 2021;12:636820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Yao X, Yin Z, Liu F, Wei S, Zhou Y, Jiang X, Wei Y, Xu K, Wang F, Tang Y. Shared and distinct regional homogeneity changes in bipolar and unipolar depression. Neurosci Lett. 2018;673:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Sun J, Ma Y, Chen L, Wang Z, Guo C, Luo Y, Gao D, Li X, Xu K, Hong Y, Hou X, Tian J, Yu X, Wang H, Fang J, Xiao X. Altered Brain Function in Treatment-Resistant and Non-treatment-resistant Depression Patients: A Resting-State Functional Magnetic Resonance Imaging Study. Front Psychiatry. 2022;13:904139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Liu C, Pan W, Zhu D, Mao P, Ren Y, Ma X. Altered Intrinsic Brain Activity in Patients With Late-Life Depression: A Resting-State Functional MRI Study. Front Psychiatry. 2022;13:894646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Bradley KA, Colcombe S, Henderson SE, Alonso CM, Milham MP, Gabbay V. Neural correlates of self-perceptions in adolescents with major depressive disorder. Dev Cogn Neurosci. 2016;19:87-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Cullen KR, Klimes-Dougan B, Vu DP, Westlund Schreiner M, Mueller BA, Eberly LE, Camchong J, Westervelt A, Lim KO. Neural Correlates of Antidepressant Treatment Response in Adolescents with Major Depressive Disorder. J Child Adolesc Psychopharmacol. 2016;26:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet. 2012;379:1056-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1412] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 37. | Rzepa E, McCabe C. Anhedonia and depression severity dissociated by dmPFC resting-state functional connectivity in adolescents. J Psychopharmacol. 2018;32:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Zhu J, Lin X, Lin C, Zhuo C, Yu Y. Selective functional dysconnectivity of the dorsal-anterior subregion of the precuneus in drug-naive major depressive disorder. J Affect Disord. 2018;225:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Zhang B, Qi S, Liu S, Liu X, Wei X, Ming D. Altered spontaneous neural activity in the precuneus, middle and superior frontal gyri, and hippocampus in college students with subclinical depression. BMC Psychiatry. 2021;21:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 40. | MacMaster FP, Carrey N, Langevin LM, Jaworska N, Crawford S. Disorder-specific volumetric brain difference in adolescent major depressive disorder and bipolar depression. Brain Imaging Behav. 2014;8:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Long Y, Li X, Cao H, Zhang M, Lu B, Huang Y, Liu M, Xu M, Liu Z, Yan C, Sui J, Ouyang X, Zhou X. Common and distinct functional brain network abnormalities in adolescent, early-middle adult, and late adult major depressive disorders. Psychol Med. 2024;54:582-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 42. | Zhukovsky P, Anderson JAE, Coughlan G, Mulsant BH, Cipriani A, Voineskos AN. Coordinate-Based Network Mapping of Brain Structure in Major Depressive Disorder in Younger and Older Adults: A Systematic Review and Meta-Analysis. Am J Psychiatry. 2021;178:1119-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 43. | Arnone D, Job D, Selvaraj S, Abe O, Amico F, Cheng Y, Colloby SJ, O'Brien JT, Frodl T, Gotlib IH, Ham BJ, Kim MJ, Koolschijn PC, Périco CA, Salvadore G, Thomas AJ, Van Tol MJ, van der Wee NJ, Veltman DJ, Wagner G, McIntosh AM. Computational meta-analysis of statistical parametric maps in major depression. Hum Brain Mapp. 2016;37:1393-1404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 44. | Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus DM, Cardoner N, Surguladze S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 589] [Article Influence: 42.1] [Reference Citation Analysis (0)] |