Published online Jul 19, 2023. doi: 10.5498/wjp.v13.i7.453
Peer-review started: May 4, 2023
First decision: May 15, 2023
Revised: May 31, 2023
Accepted: June 21, 2023
Article in press: June 21, 2023
Published online: July 19, 2023
Processing time: 75 Days and 4.6 Hours
Treatment-refractory schizophrenia (TRS), accounting for approximately 30% of all schizophrenia cases, has poor treatment response and prognosis despite treatment with antipsychotic drugs.
To analyze the therapeutic effectiveness of repetitive transcranial magnetic stimulation (rTMS) combined with olanzapine (OLZ) and amisulpride (AMI) for TRS and its influence on the patient’s cognitive function.
This study enrolled 114 TRS patients who received treatment at the First Affiliated Hospital of Zhengzhou University between July 2019 and July 2022. In addition to the basic OLZ + AMI therapy, 54 cases of the control group (Con group) received modified electroconvulsive therapy, while 60 cases of the research group (Res group) received rTMS. Data on therapeutic effectiveness, safety (incidence of drowsiness, headache, nausea, vomiting, or memory impairment), Positive and Negative Symptom Scale, Montreal Cognitive Assessment Scale, and Schizophrenia Quality of Life Scale were collected from both cohorts for comparative analyses.
The Res group elicited a higher overall response rate and better safety profile when compared with the Con group. Additionally, a significant reduction was observed in the post-treatment Positive and Negative Symptom Scale and Schizophrenia Quality of Life Scale scores of the Res group, presenting lower scores than those of the Con group. Furthermore, a significant increase in the Montreal Cognitive Assessment Scale score was reported in the Res group, with higher scores than those of the Con group.
The treatment of TRS with rTMS and OLZ + AMI is effective and safe. Moreover, it can alleviate the patients’ mental symptoms, improve their cognitive function and quality of life, and has a high clinical application value.
Core Tip: Patients with treatment-refractory schizophrenia always have an unsatisfactory treatment response and prognosis despite antipsychotic therapy, which poses significant challenges to clinical management. Therefore, it is necessary to continuously explore and validate effective treatments for treatment-refractory schizophrenia.
- Citation: Liu JL, Tan ZM, Jiao SJ. Repetitive transcranial magnetic stimulation combined with olanzapine and amisulpride for treatment-refractory schizophrenia. World J Psychiatry 2023; 13(7): 453-460
- URL: https://www.wjgnet.com/2220-3206/full/v13/i7/453.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i7.453
Schizophrenia, a heterogeneous progressive mental illness that may lead to cognitive impairment in patients, has a great negative impact on the patient’s social interaction and work[1]. The etiology of the disease is complicated and has been linked to environmental factors, hereditary factors, cortical excitation-to-inhibition imbalance, and subcortical dopamine dysfunction[2]. Schizophrenia is prone to occur in early adulthood, and its symptoms are classified as positive symptoms, such as hallucinations, delusions, and speech disorders, and negative symptoms, such as emotional retardation, abulia, and social barriers[3]. The overall prevalence of schizophrenia is approximately 0.4%, and the mortality rate of schizophrenia patients is 2-4 times that of the general population[4,5]. Treatment-refractory schizophrenia (TRS), accounting for approximately 30% of the total schizophrenia cases, has a poor treatment response and prognosis despite treatment with antipsychotic drugs, which also poses great clinical challenges[6,7]. Nevertheless, it is a compelling responsibility of physicians to explore effective treatment options for TRS.
Repetitive transcranial magnetic stimulation (rTMS), a non-invasive brain stimulation method, induces local neuronal activation in the brain regions via electromagnetic induction of electric fields, thus ameliorating the abnormal connections between the brain regions to a certain extent[8,9]. According to the research by Zhu et al[10], rTMS has a significant long-term ameliorating effect on working memory defects in schizophrenia patients. Olanzapine (OLZ) and amisulpride (AMI) are both first-line antipsychotic drugs, which are highly effective in reducing the Positive and Negative Syndrome Scale (PANSS) scores and alleviating the mental symptoms of the patients[11]. OLZ is a 5-hydroxytryptamine 2A/2C antagonist affecting the glucolipid metabolism[12] and an efficient AMP-activated protein kinase activator that enhances the AMP-activated protein kinase activity in the hypothalamus, thus mediating energy homeostasis and metabolic modulation during neuronal activity[13,14]. As for AMI, it is beneficial in relieving depressive symptoms and major negative symptoms as well as enhancing the patient’s quality of life (QOL)[15].
We proposed that the combination of rTMS and OLZ + AMI has certain beneficial clinical effects in TRS patients.
This study selected 114 TRS patients who received treatment at the First Affiliated Hospital of Zhengzhou University between July 2019 and July 2022. Among them, 54 cases were included in the control group (Con group) treated with modified electroconvulsive therapy (mECT) and 60 cases in the research group (Res group) treated with rTMS; the patients in both groups received OLZ + AMI therapy. The patients in the Res and Con groups presented similar demographic data (P > 0.05), suggesting clinical comparability and feasibility during follow-up research.
Inclusion criteria: The inclusion criteria for this study were that patients of both groups fulfill the TRS diagnostic criteria and present complete medical records with no treatment-associated contraindications.
Exclusion criteria: Patients with a history of allergy to the study medications, serious diseases such as heart, lung, or kidney dysfunction, and mental retardation that hindered compliance and cooperation were excluded. Furthermore, pregnant and lactating women and drug abusers were excluded from this study.
The Con group patients were treated with mECT and OLZ + AMI, as described here. The patients received mECT 2-3 times a week using a Thymatron ECT therapeutic apparatus. The treatment frequency could be adjusted to once weekly according to the treatment effect up to a maximum of 8 times during the treatment course. The initial oral dose of OLZ was 5 mg once daily, and the drug dose could be gradually increased to 10-15 mg/d, depending on patient tolerance, for a total period of 8 wk. AMI was administered orally at an initial dose of 200 mg/d and increased according to patient tolerance and efficacy but not beyond a maximum daily dose of 1200 mg for a total period of 8 wk.
The Res group received rTMS combined with OLZ + AMI. An rTMS therapeutic instrument was used for treatment. The coil of the instrument was placed on the patient’s forehead on the left, tangential to the scalp. The dorsolateral left frontal lobe was stimulated according to the threshold stimulation intensity of 80%-110% of the motor threshold, and the frequency was set at 20 Hz. The treatment time was 20 min, once a day, 5 times a week for 8 wk. OLZ and AMI were administered in the same way as that in the Con group.
Clinical effectiveness: The PANSS scores were compared before and after treatment between the Res and Con groups. A decrease of more than 80% in the PANSS score was considered a “marked response,” a decrease of 50%-79% was considered a “response,” and failure to meet the above reduction criteria was considered as “non-response.” The overall response rate (ORR) was the percentage of the sum of the number of “marked response” and “response” patients among the total number of cases.
Safety: We observed and recorded the number of cases of drowsiness, headache, nausea, vomiting, and memory impairment and calculated the overall incidence.
Severity of schizophrenia: All patients were assessed for schizophrenia severity using the PANSS (total score: 98) with positive and negative subscales. The higher the score, the more serious the symptoms of schizophrenia.
Cognitive function: The cognitive function (CF) of patients was evaluated using the Montreal Cognitive Assessment (MoCA), comprising eight items such as visuospatial/executive ability, memory, naming, and attention. On the 30-point scale, lower scores suggested worse CF.
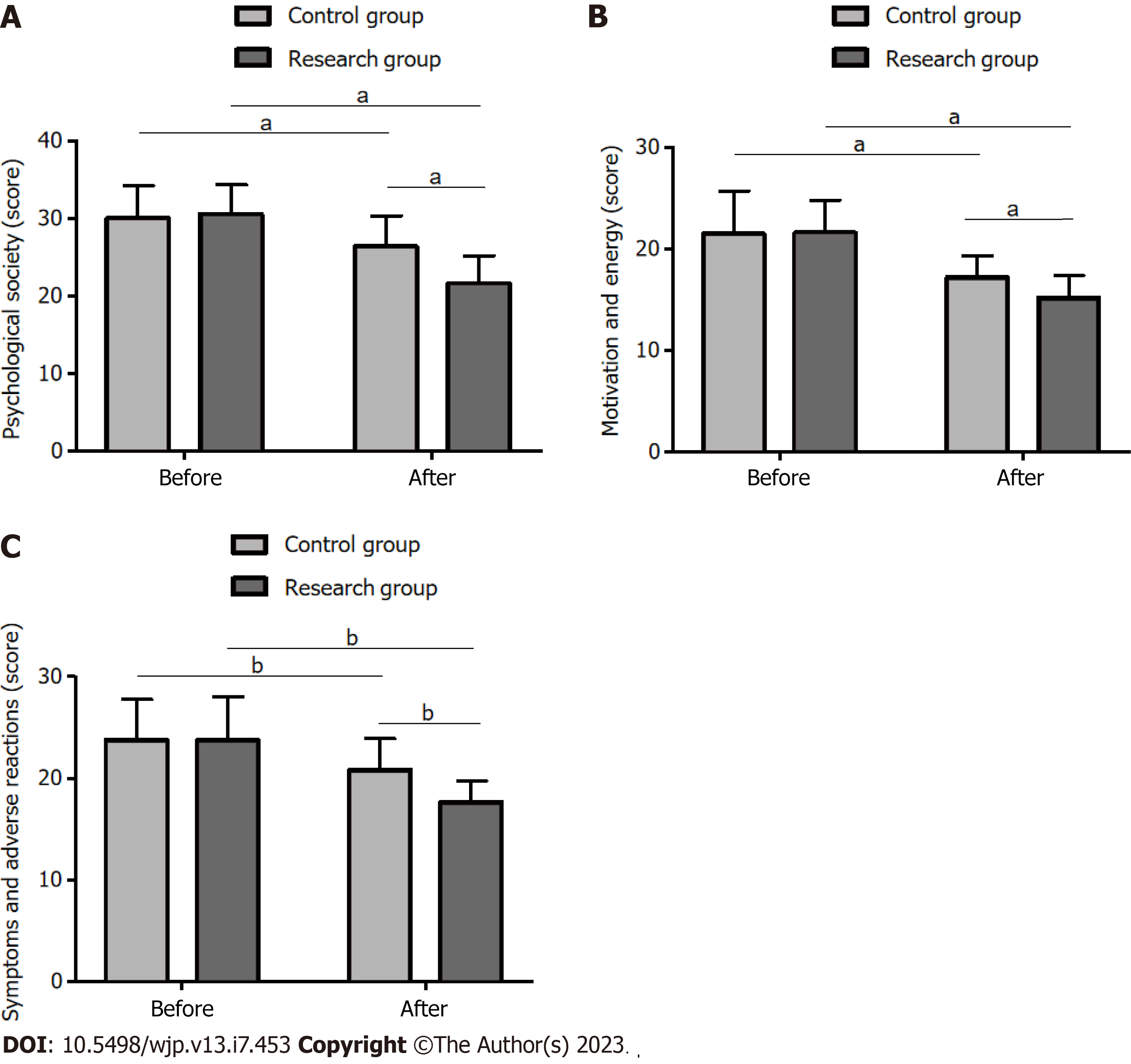
QOL: The QOL of schizophrenia patients was evaluated using the Schizophrenia Quality of Life Scale (SQLS), comprising psychosocial factors (15 items, total score: 60), motivation and energy (7 items, total score: 28), and symptoms and adverse reactions (8 items, total score: 32). The score was inversely associated with the patient’s QOL.
This study used Statistical Product and Service Solutions version 19.0 for data analysis; P-values < 0.05 were considered statistically significant. Sex and other categorical variables, expressed as the number of cases/percentages (n/%), were compared between the groups using the χ2 test. Continuous variables, such as the PANSS scores, expressed as means ± standard error, were compared between the groups using the t-test.
Sex, age, course of the disease, family history, marital status, and educational level did not differ significantly between the Res and Con groups, indicating possible comparability between them (P > 0.05) (Table 1).
| Categories | Control group, n = 54 | Research group, n = 60 | χ2/t value | P value |
| Sex | 0.069 | 0.793 | ||
| Male | 32 (59.26) | 37 (61.67) | ||
| Female | 22 (40.74) | 23 (38.33) | ||
| Age in yr | 40.07 ± 7.01 | 41.28 ± 8.74 | 0.810 | 0.420 |
| Course of the disease in yr | 19.56 ± 7.55 | 18.93 ± 5.83 | 0.501 | 0.617 |
| Family medical history | 0.252 | 0.616 | ||
| Yes | 6 (11.11) | 5 (8.33) | ||
| No | 48 (88.89) | 55 (91.67) | ||
| Marital status | 0.381 | 0.537 | ||
| Married | 33 (61.11) | 40 (66.67) | ||
| Single | 21 (38.89) | 20 (33.33) | ||
| Educational level | 1.481 | 0.224 | ||
| Below high school | 30 (55.56) | 40 (66.67) | ||
| High school and above | 24 (44.44) | 20 (33.33) |
The ORRs of the Con and Res groups were 72.22% and 93.33%, respectively, demonstrating significantly higher efficacy of rTMS and OLZ + AMI than that of mECT and OLZ + AMI (P < 0.05) (Table 2).
| Indicators | Control group, n = 54 | Research group, n = 60 | χ2 value | P value |
| Marked response | 19 (35.19) | 36 (60.00) | - | - |
| Response | 20 (37.04) | 20 (33.33) | - | - |
| Non-response | 15 (27.78) | 4 (6.67) | - | - |
| Overall response | 39 (72.22) | 56 (93.33) | 9.120 | 0.003 |
Observation and records of the occurrence of drowsiness, headache, nausea, vomiting, and memory impairment in both cohorts showed that the incidence of adverse events was statistically higher in the Con group than in the Res group (25.93% vs 8.33%, P < 0.05) (Table 3).
| Indicators | Control group, n = 54 | Research group, n = 60 | χ2 value | P value |
| Drowsiness | 4 (7.41) | 1 (1.67) | - | - |
| Headache | 4 (7.41) | 1 (1.67) | - | - |
| Nausea | 3 (5.56) | 2 (3.33) | - | - |
| Vomiting | 2 (3.70) | 1 (1.67) | - | - |
| Memory impairment | 1 (1.85) | 0 (0.00) | - | - |
| Total | 14 (25.93) | 5 (8.33) | 6.333 | 0.012 |
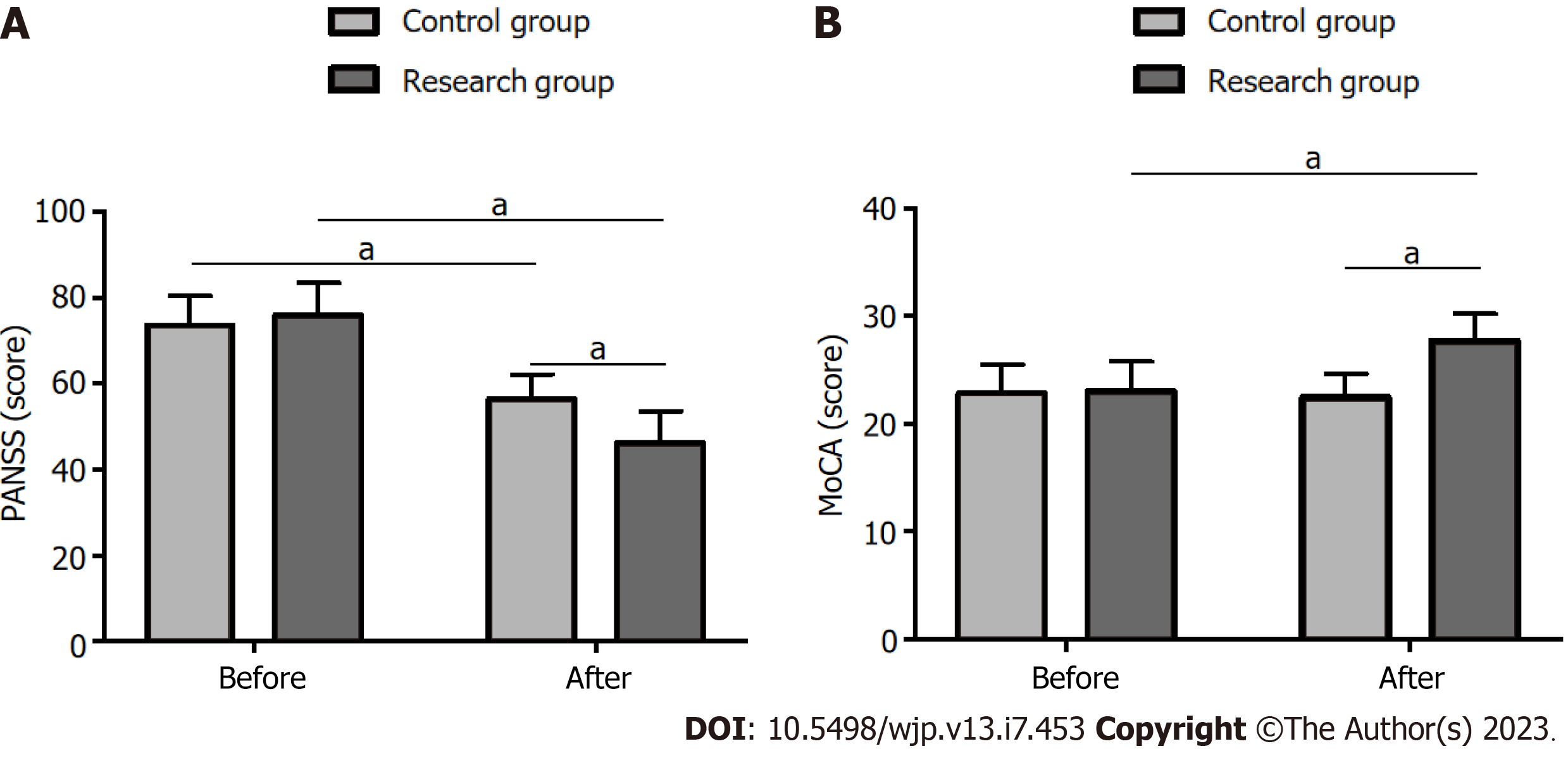
Analysis of the mental symptoms using the PANSS revealed that the scores did not differ significantly between the groups before treatment (P > 0.05). However, the scores reduced significantly after treatment in both groups (P < 0.05), with the Res group exhibiting lower scores than those of the Con group (P < 0.05) (Figure 1A).
CF, analyzed using the MoCA, did not differ significantly between the groups before treatment (P > 0.05). However, the MoCA scores increased significantly in both cohorts after treatment (P < 0.05), with higher scores in the Res group than in the Con group (P < 0.05) (Figure 1B).
The QOL assessment using the SQLS showed that the two groups did not differ statistically in the QOL before treatment (P > 0.05). However, the post-treatment QOL improved significantly, manifesting as significantly reduced SQLS scores in various dimensions (P < 0.05), with the Res group exhibiting better QOL (lower SQLS scores) than that of the Con group (P < 0.05) (Figure 2).
TRS, a chronic mental disorder, is associated with an increased risk of metabolic syndrome, including hypertension and diabetes, as well as cardiovascular diseases and death in patients[16]. The difficulty in treating this disease lies in the fact that a significant proportion of patients do not respond well to non-clozapine antipsychotic drugs, ECT, or other enhancement strategies, imposing an economic burden on families and the healthcare system[17,18].
This study comparatively analyzed the effectiveness and safety of two treatment modalities for TRS, mECT and OLZ + AMI (Con group) vs rTMS and OLZ + AMI (Res group), to provide clinical support and references for the formulation of effective treatment strategies for TRS patients to improve their clinical outcomes and reduce medical costs. Our research results identified a significantly higher ORR in the Res group than in the Con group (93.33% vs 72.22%), indicating the superior therapeutic effectiveness of rTMS and OLZ + AMI than that of mECT and OLZ + AMI for TRS. Kahn et al[19] reported that 45% of the 93 patients receiving AMI and 44% of those receiving OLZ achieved “response” in the first stage of treatment, indicating that AMI or OLZ monotherapy induced less than satisfactory effects in TRS patients.
Currently, there are limited studies on AMI + OLZ combination therapy, most of which focus on the comparison of AMI or OLZ monotherapy. For instance, Men et al[20] demonstrated equivalent clinical efficacy and safety of AMI and OLZ in the treatment of schizophrenia. In our study, the total incidence of drowsiness, headache, nausea, vomiting, and memory impairment was significantly lower in the Res group than in the Con group (8.33% vs 25.93%), suggesting that rTMS contributes to fewer adverse events and is more cost-effective when compared with mECT in the treatment of TRS patients.
Additionally, we analyzed and compared the mental symptoms, CF, and QOL of the cohorts before and after treatment using the PANSS, MoCA, and SQLS, respectively. The Res group showed significantly reduced PANSS and SQLS scores after treatment. Moreover, the post-treatment scores in the Res group were significantly lower than those before treatment and those in the Con group. On the other hand, the MoCA scores increased significantly in the Res group, and the post-treatment scores were higher than those before treatment and those in the Con group. This suggests that the combination of rTMS and OLZ + AMI has a significant effect on the improvement of mental symptoms, CF, and QOL in TRS patients.
An open-label clinical study indicated that OLZ and AMI have positive and equivalent effects on ameliorating the negative symptoms and cognitive impairment in schizophrenia patients[21]. Currently, antipsychotics alone cannot fully relieve social cognitive impairment and enhance functional outcomes in patients with mental illnesses, while rTMS is highly effective in improving their CF and mental symptoms[22]. Li et al[23] reported that a combination of rTMS and family intervention plays a synergistic role in schizophrenia patients, which is conducive to ameliorate the patients’ negative symptoms and CF.
Additionally, an animal study confirmed that rTMS elicits an antidepressant effect by enhancing the endogenous cannabinoid signaling transduction and upregulating the endogenous cannabinoid 1 receptor and diacylglycerol lipase-α in the hippocampal astrocytes and neurons in rats under chronic and unpredictable stress[24]. It has also been noted that rTMS may modulate the cortical plasticity by affecting the permanent changes in the excitability of the cerebellar-thalamic-cortical pathway and that its mechanism of action in TRS could be related to its promotion of interconnection of the remote areas in the neural network system[25].
This study had several limitations that require further consideration. First, this was a single-center retrospective study; hence, the inclusion of more cases from multiple centers would be beneficial to improve the accuracy of the research results. Second, basic experiments should be supplemented to explore the underlying mechanism of the combination of rTMS and OLZ + AMI in treating TRS to understand this therapy and TRS better. Finally, the supplement of multivariate analyses influencing the efficacy of rTMS and OLZ + AMI in the treatment of TRS will help gain deeper insights regarding the pathways to enhance treatment efficacy. Future studies improving the aforementioned limitations are warranted.
rTMS in combination with OLZ + AMI may be preferred over the combination of mECT and OLZ + AMI for treating TRS, as the former has a clinical ORR as high as 93.33% and an adverse event rate as low as 8.33%. Moreover, this therapy has outstanding effects in relieving mental symptoms and improving CF and QOL; hence, it should be considered widely in clinical practice.
Treatment-refractory schizophrenia (TRS) accounts for approximately 30% of all patients with schizophrenia, with unsatisfactory treatment response and poor patient prognosis despite antipsychotic therapy.
The treatment of TRS is difficult and challenging, but it is still the responsibility of doctors to explore effective treatment options for the disease.
To analyze the therapeutic effectiveness of repetitive transcranial magnetic stimulation (rTMS) combined with olanzapine (OLZ) and amisulpride (AMI) for TRS and its influence on the patients’ cognitive function (CF).
First, 114 TRS patients who received treatment between July 2019 and July 2022 were selected. On the basis of OLZ + AMI therapy, 54 cases in the control group (Con group) received modified electroconvulsive therapy, and 60 cases in the research group (Res group) were given rTMS. Information on the therapeutic effectiveness, safety (drowsiness, headache, nausea, vomiting, and memory impairment), Positive and Negative Symptom Scale, Montreal Cognitive Assessment Scale, and Schizophrenia Quality of Life Scale were collected from both patient cohorts for competitive analyses.
A higher overall response rate and a better safety profile of treatment were determined in the Res group compared with the Con group. In addition, marked reductions in the Positive and Negative Symptom Scale and Schizophrenia Quality of Life Scale scores were found in the Res group after treatment, which were lower compared with the Con group. A significant increase in the Montreal Cognitive Assessment Scale score was observed in the Res group, with higher scores than the Con group.
rTMS plus OLZ + AMI was effective and safe in the treatment of TRS, which can alleviate the patients’ mental symptoms and improve their CF and quality of life, with clinical promotion value.
rTMS plus OLZ + AMI, with both clinical efficacy and safety, may be more suitable for TRS patients than modified electroconvulsive therapy plus OLZ + AMI. This therapy has significant advantages in relieving psychiatric symptoms and improving CF and quality of life, which is worth promoting clinically.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nyashanu M, United Kingdom; Shook NJ, United States S-Editor: Wang JL L-Editor: Filipodia P-Editor: Xu ZH
| 1. | Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 496] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 2. | McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-An Overview. JAMA Psychiatry. 2020;77:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 731] [Article Influence: 146.2] [Reference Citation Analysis (0)] |
| 3. | Girdler SJ, Confino JE, Woesner ME. Exercise as a Treatment for Schizophrenia: A Review. Psychopharmacol Bull. 2019;49:56-69. [PubMed] |
| 4. | Winship IR, Dursun SM, Baker GB, Balista PA, Kandratavicius L, Maia-de-Oliveira JP, Hallak J, Howland JG. An Overview of Animal Models Related to Schizophrenia. Can J Psychiatry. 2019;64:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Crawford P, Go KV. Schizophrenia. Am Fam Physician. 2022;106:388-396. [PubMed] |
| 6. | Kane JM, Agid O, Baldwin ML, Howes O, Lindenmayer JP, Marder S, Olfson M, Potkin SG, Correll CU. Clinical Guidance on the Identification and Management of Treatment-Resistant Schizophrenia. J Clin Psychiatry. 2019;80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 7. | Correll CU, Howes OD. Treatment-Resistant Schizophrenia: Definition, Predictors, and Therapy Options. J Clin Psychiatry. 2021;82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Walton D, Spencer DC, Nevitt SJ, Michael BD. Transcranial magnetic stimulation for the treatment of epilepsy. Cochrane Database Syst Rev. 2021;4:CD011025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Huang H, Zhang B, Mi L, Liu M, Chang X, Luo Y, Li C, He H, Zhou J, Yang R, Li H, Jiang S, Yao D, Li Q, Duan M, Luo C. Reconfiguration of Functional Dynamics in Cortico-Thalamo-Cerebellar Circuit in Schizophrenia Following High-Frequency Repeated Transcranial Magnetic Stimulation. Front Hum Neurosci. 2022;16:928315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Zhu X, Huang C, Fan H, Fan F, Zhao Y, Xiu M, Wang Y, Li Y, Tan Y, Wang Z, Tan S. The effect of transcranial direct current stimulation combined with working memory training on working memory deficits in schizophrenic patients: study protocol for a randomized controlled trial. Trials. 2022;23:826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Johnsen E, Kroken RA, Løberg EM, Rettenbacher M, Joa I, Larsen TK, Reitan SK, Walla B, Alisauskiene R, Anda LG, Bartz-Johannessen C, Berle JØ, Bjarke J, Fathian F, Hugdahl K, Kjelby E, Sinkeviciute I, Skrede S, Stabell L, Steen VM, Fleischhacker WW. Amisulpride, aripiprazole, and olanzapine in patients with schizophrenia-spectrum disorders (BeSt InTro): a pragmatic, rater-blind, semi-randomised trial. Lancet Psychiatry. 2020;7:945-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Carli M, Kolachalam S, Longoni B, Pintaudi A, Baldini M, Aringhieri S, Fasciani I, Annibale P, Maggio R, Scarselli M. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 13. | Lian J, Huang XF, Pai N, Deng C. Betahistine ameliorates olanzapine-induced weight gain through modulation of histaminergic, NPY and AMPK pathways. Psychoneuroendocrinology. 2014;48:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. From the Cover: Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci U S A. 2007;104:3456-3459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Hadryś T, Rymaszewska J. Amisulpride - is it as all other medicines or is it different? An update. Psychiatr Pol. 2020;54:977-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Ying J, Wan J, Sim K, Seah ED, Subramaniam M. Perceived knowledge of psychiatry and family medicine residents regarding medical management of schizophrenia, hypertension, diabetes mellitus, and dyslipidemia: opportunities to refine the residency training. BMC Med Educ. 2021;21:232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Wada M, Noda Y, Iwata Y, Tsugawa S, Yoshida K, Tani H, Hirano Y, Koike S, Sasabayashi D, Katayama H, Plitman E, Ohi K, Ueno F, Caravaggio F, Koizumi T, Gerretsen P, Suzuki T, Uchida H, Müller DJ, Mimura M, Remington G, Grace AA, Graff-Guerrero A, Nakajima S. Dopaminergic dysfunction and excitatory/inhibitory imbalance in treatment-resistant schizophrenia and novel neuromodulatory treatment. Mol Psychiatry. 2022;27:2950-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 18. | Stewart AJ, Patten S, Fiest KM, Williamson TS, Wick JP, Ronksley PE. Factors associated with high health care spending among patients with schizophrenia. Health Promot Chronic Dis Prev Can. 2022;42:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Kahn RS, Winter van Rossum I, Leucht S, McGuire P, Lewis SW, Leboyer M, Arango C, Dazzan P, Drake R, Heres S, Díaz-Caneja CM, Rujescu D, Weiser M, Galderisi S, Glenthøj B, Eijkemans MJC, Fleischhacker WW, Kapur S, Sommer IE; OPTiMiSE study group. Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study. Lancet Psychiatry. 2018;5:797-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 20. | Men P, Yi Z, Li C, Qu S, Xiong T, Yu X, Zhai S. Comparative efficacy and safety between amisulpride and olanzapine in schizophrenia treatment and a cost analysis in China: a systematic review, meta-analysis, and cost-minimization analysis. BMC Psychiatry. 2018;18:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Kumar S, Chaudhury S. Efficacy of amisulpride and olanzapine for negative symptoms and cognitive impairments: An open-label clinical study. Ind Psychiatry J. 2014;23:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Wölwer W, Lowe A, Brinkmeyer J, Streit M, Habakuck M, Agelink MW, Mobascher A, Gaebel W, Cordes J. Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain Stimul. 2014;7:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Li X, Yuan X, Kang Y, Pang L, Liu Y, Zhu Q, Lv L, Huang XF, Song X. A synergistic effect between family intervention and rTMS improves cognitive and negative symptoms in schizophrenia: A randomized controlled trial. J Psychiatr Res. 2020;126:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Xue SS, Xue F, Ma QR, Wang SQ, Wang Y, Tan QR, Wang HN, Zhou CH, Peng ZW. Repetitive high-frequency transcranial magnetic stimulation reverses depressive-like behaviors and protein expression at hippocampal synapses in chronic unpredictable stress-treated rats by enhancing endocannabinoid signaling. Pharmacol Biochem Behav. 2019;184:172738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Koch G. Repetitive transcranial magnetic stimulation: a tool for human cerebellar plasticity. Funct Neurol. 2010;25:159-163. [PubMed] |