Published online Apr 19, 2023. doi: 10.5498/wjp.v13.i4.161
Peer-review started: December 27, 2022
First decision: January 9, 2023
Revised: February 16, 2023
Accepted: March 23, 2023
Article in press: March 23, 2023
Published online: April 19, 2023
Processing time: 111 Days and 12.3 Hours
In recent years, epidemiological and genetic studies have shown an association between autoimmune diseases and psychosis. The question arises whether patients with schizophrenia are more likely to develop multiple sclerosis (MS) later in life. It is well known that the immune system plays an important role in the etiopathogenesis of both disorders. Immune disturbances may be similar or very different in terms of different types of immune responses, disturbed myelination, and/or immunogenetic predispositions. A psychotic symptom may be a consequence of the MS diagnosis itself or a separate entity. In this review article, we discussed the timing of onset of psychotic symptoms and MS and whether the use of corticosteroids as therapy for acute relapses in MS is unfairly neglected in patients with psychiatric comorbidities. In addition, we discussed that the anti-inflammatory potential of antipsychotics could be useful and should be considered, especially in the treatment of psychosis that coexists with MS. Autoimmune disorders could precipitate psychotic symptoms, and in this context, autoimmune psychosis must be considered as a persistent symptomatology that requires continuous and specific treatment.
Core Tip: Immunological perturbations in multiple sclerosis (MS) can lead to the development of psychotic symptoms. The use of corticosteroids and antipsychotics might prove beneficial and in patients with MS-related psychosis and outweigh their adverse effects.
- Citation: Vesic K, Gavrilovic A, Mijailović NR, Borovcanin MM. Neuroimmune, clinical and treatment challenges in multiple sclerosis-related psychoses. World J Psychiatry 2023; 13(4): 161-170
- URL: https://www.wjgnet.com/2220-3206/full/v13/i4/161.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i4.161
Multiple sclerosis (MS) is an autoimmune disorder of the central nervous system (CNS) that is characterized by neuroinflammation, demyelination, axonal loss and neurodegeneration[1]. It is one of the most common neurologic disorders and the most common cause of acquired neurological disability in young adults, that has an effect on 2.5 million people worldwide[2]. MS patients are two to three times more likely than the general population to suffer from mood and mental disorders[3,4].
Neurological and psychiatric diseases often overlap and co-occur[5,6]. Psychiatric symptoms may occur at any time during a neurological autoimmune disease, but they may also be an initial clinical manifestation and precede the onset of typical neurological symptoms[7]. The initial or early presence of these symptoms may complicate the establishment of a correct diagnosis, hinder the early recognition of the underlying brain disorder, and lead to inadequate treatment and a poorer prognosis[8,9]. Furthermore, the presence of various psychotic symptoms in neurological diseases additionally compromises these conditions and has an impact on individual functioning and overall disease burden[10]. The prevalence of psychosis/psychotic disorders is approximately 4.3%[11].
Mental disorders in neurological diseases may have different underlying causes[12]. In MS patients, the onset of psychiatric symptoms may be a consequence of the MS diagnosis itself[13]. Brain lesions, physical disabilities, symptoms of MS and pharmacological treatments may cause psychopathological manifestations[14].
There is limited scientific evidence that could be useful for clinical decision-making in the resolution of psychotic symptoms in patients with MS. Recent data by Sabe and Sentissi have confirmed our clinical observations[15]. There is a lack of data considering MS and psychosis, especially schizophrenia (SCH), simultaneously. We might think that this is due to a lack of interest in the field, but it is also possible that these disorders do not exist at their full capacity simultaneously. In this mini-review, we aim to resolve some clinical dilemmas. First, we discuss the timing of the onset of psychotic symptoms and MS. Second, whether the use of corticosteroids is unfairly neglected and whether a more aggressive treatment approach is warranted. And third, whether antipsychotics may be of benefit as adjunctive therapy is discussed. Immune disturbances may be similar or very different in terms of different types of immune responses, disturbed myelination, and/ or immunogenetic predispositions.
This mini-review was conducted through a systematic electronic search of the PubMed, Cochrane, and Web of Science databases to identify cases of MS with psychotic symptoms. The terms used were “multiple sclerosis”; “psychosis”; “schizophrenia“; “neurodegeneration”; “neuroinflammation”; “corticosteroids”; and “antipsychotics.” We searched for studies published in English, with no restriction on year of publication. Experimental studies, randomized or nonrandomized clinical trials, cohort studies, and case-control studies were included. We have assessed the abstracts of potentially relevant titles and reviewed the full text of potentially eligible studies. In this mini-review, we attempted to select and focus on only up-to-date and clinically relevant data.
Genetic predisposition, individual and environmental factors, and specific immune responses had a significant impact on disease onset and clinical presentation. This suggests that the same etiological factors and pathophysiological pathways may influence the association between MS and psychosis. It raises the question of whether patients with SCH might also suffer from MS later in life. The genetic basis of MS is supported by the fact that the risk of disease is higher in MS family members than in the general population, that certain racial and ethnic groups have a lower illness tendency, and that the concordance rate is higher in monozygotic twins[16]. Previous genetic studies have shown that the predisposition to develop MS depends on several independent polymorphic genes and their interaction. No single gene variant is necessary or sufficient to cause MS, but each one increases the overall risk of the disease in an additive manner[17]. Genome-wide association studies (GWAS), which examine gene polymorphisms in the whole genome, suggest that over 200 gene loci that have different immune functions are responsible for the development of MS[18,19]. The risk genes control the differentiation and state of these cells through their function in specific cellular processes in immune cells. In addition, recent evidence indicates that many immune cell populations are highly heritable, raising the possibility that MS risk genes define differences of immune cell populations that could also be involved in the pathogenesis of illness[18].
It is well known that the immune system plays an important role in the etiopathogenesis of MS and SCH[20,21]. Some authors assign them to the same group of neuroinflammatory and neurodegenerative properties, but with marked differences in the immune response. From the clinician’s point of view, this fact could mean that these disorders are mutually exclusive. We have already considered confronting results of the prevalent immune responses in MS and SCH as prototypes of disorders of type 1 vs type 2 immune responses, respectively[22,23]. In recent years, growing epidemiological evidence has suggested a bidirectional association between autoimmune diseases and increased risks with SCH[24]. A family history of autoimmune disease has been shown to be related to an increased risk of psychotic disorders and vice versa[25]. In addition, having a first-degree relative with SCH increases the risk of autoimmune diseases by 6%[26]. Previous studies have found that patients with MS are at an increased risk of developing SCH[27]. SCH and other psychotic disorders have been associated with genetic markers of immune activation, suggesting a possible etiological link between MS and psychosis[28]. GWAS emphasized the significant overlapping of genes, noted the involvement of similar HLA alleles, and identified 21 independent loci associated with SCH and also associated with MS. In these diseases it seems that the major histocompatibility complex is responsible for the genetic overlap[29,30]. A possible additional role of genetics in this association could be an inherited susceptibility to common risk factors for infections or a predisposition to abnormal immune responses that further increases the risk of autoimmune reactions[31]. Despite the contrasting nature of these diseases, some common features have been identified. Risk factors for both MS and SCH include an interaction between genetic and environmental factors[32,33]. Infection is one of the most important triggers for the development of both diseases[34,35]. In the last decade, considerable attention has been paid to the gut microbiome as a possible etiological factor in the pathophysiology of MS and SCH[36]. It has been suggested that the composition of the intestinal flora plays an important role in CNS and immune system development[37]. Dysbiosis of the microbiome has been shown to affect the Th1/Th2 balance and the ratio of regulatory T cells to Th17 cells, which affects the immune response to pathogens[38,39]. Dysbiosis has been found to affect T cell-mediated inflammation in MS and SCH patients[40,41].
In SCH, it has been previously hypothesized that inflammation in the microvasculature persists as chronic, low-grade inflammation and does not disseminate in whole brain parenchyma as in acute encephalitis[21]. This organic substrate could be related to the soft neurological signs, observed in patients with SCH[42]. Similar to SCH, the clinical manifestations in MS may be the result of acute or chronic neuroinflammation. In the acute stage, the peripheral immune system is involved, and T cells, B cells, and macrophages enter into CNS and cause acute inflammation in the brain[21]. In addition, these mechanisms have no influence on the chronic phase. Recently, a concept of “compartmentalization” of the inflammatory process in the brain has been postulated, in which a local immune response in the CNS occurs separately from the peripheral immune system[43]. This hypothesis is supported by the fact that disseminated activation of microglial cells is the primary characteristic of the chronic phase of the illness[44]. There is ample evidence of biomarkers indicating a link between immunological processes, psychotic disorders and MS. Elevated levels of different inflammatory markers have been found in the blood and cerebrospinal fluid (CSF) of patients with psychosis and MS, with particularly high levels in patients with first-episode psychosis or acute relapse[45,46]. Dysregulation of regulatory T cells and Th17 cells may be essential for immunological homeostasis and play a role in the development of both diseases[47,48]. In recent years, much attention has been paid to B cells and their role in the autoimmune pathology of MS and psychosis[49,50]. Oligoclonal bands (OCBs) in CSF have been detected in approximately 90% of patients with MS[51]. In up 12.5% of patients with SCH were found OCBs[45]. It is important to keep in mind that there are other possible triggering and contributing factors besides the specifics of the immune response in MS and psychosis. Within the new concept of nomothetic network psychiatry and causal models, perhaps the identification of these transdiagnostic classes could even be presented as new nosological categories[52].
In clinical practice, it is important to distinguish whether psychosis precedes the onset of MS, coincides with MS, or is observed in the later stages of this somatic disease. We remind that these psychotic symptoms could be integral to SCH, a schizoaffective disorder, affective disorders, or even delirium. Also, we must point out that psychosis should be considered as a much broader concept than SCH. This information is important for the choice of future MS treatment.
Symptoms and signs of MS include ataxia, loss of coordination, hyperreflexia, spasticity, loss of visual acuity, sphincter incontinence, fatigue, anxiety, depression, and cognitive deficits[53]. Most patients have a relapsing-remitting form of the disease which is characterized by progression of symptoms in relapse and possible further deterioration over time[53].
Neuropsychiatric symptoms were previously reported as a rare manifestation of MS. Recently, the most commonly observed behavioural disturbances preceding the onset of MS have been summarized. Symptoms described include lack of insight, delusions, auditory and visual hallucinations, mood disturbances, behaviour disorder, and confusion[15]. Psychotic symptoms reported in MS patients also include irritability/agitation, sleep disturbances, grandiosity, blunted affect, and rare symptoms such as catatonia and transient catalepsy[4,14].
In a recent systematic review of case reports and case series, the authors pointed out that psychotic symptoms preceded or accompanied the MS diagnosis in half of the cases, with a mean time to MS diagnosis of 0.8 ± 1.4 years, whereas 15.1% of MS diagnoses were discovered after isolated psychiatric symptoms. They observed a significant delay considering MS treatment initiation between initial psychotic symptoms and MS diagnosis (2.73 ± 3 years), and in patients with the first episode of psychos and MS diagnosis (0.8 ± 1.2 years)[15]. Another important observation in their analysis was that typical MS white matter lesions were found in a small group of patients with psychiatric disorders[54]. Finally, they pointed out that resistance or poor response to antipsychotics was associated with excellent improvement of both psychiatric and neurological symptoms with corticosteroids in most cases[15]. Autoimmune psychosis must be considered as a persistent symptomatology that requires continuous treatment[55].
Pathological changes in white and grey matter structures may play an important role in the pathogenesis of MS-related psychosis. Neuroimaging studies revealed that MS patients with psychotic symptoms have a higher total lesion score, especially in periventricular areas[56]. Psychotic symptoms in MS are correlated with a higher number or volume of lesions in the temporal or frontal lobes, cerebellum, and corpus callosum[57,58]. In addition, the presence of soft neurological signs in patients with sudden or acute psychotic symptoms without a prior personal or family history of psychosis is an indication for neurological exploration[15,59].
Immunomodulatory medications for the treatment of MS could be useful to achieve remission of the disease, but in some cases may have a direct effect on the development of psychotic symptoms, although the mechanisms are poorly understood. This effect also occurs after initiation of corticosteroids or could be related with interferon beta (IFN-β) treatment. The study of 11 cases confirmed severe depression with suicidality in patients with MS, who were taking IFN-β and had no psychiatric history[60]. These patients also had phobic, aggressive, behavioural, psychotic, and manic symptoms, indicating the presence of a complex mood-behaviour disorder associated with IFN-β use. Complete remission of psychiatric complications was observed after discontinuation of IFN-β. Glatiramer acetate was generally not associated with deterioration of mood[61]. Gasim et al[62] conducted a systematic review to investigate whether the use of disease-modifying therapies is associated with adverse psychiatric effects in MS patients. This study included natalizumab, fingolimod, dimethyl fumarate, teriflunomide, and alemtuzumab and showed that their use do not increase risk of adverse psychiatric effects in MS, and some of them, such as fingolimod, even reduced the incidence of depressive symptoms. In addition, Krivinko et al[63] recently demonstrated that fingolimod treatment attenuated psychosis-associated behavioural deficits in rodents.
The standard MS treatment for acute exacerbations is pulse therapy with systemic glucocorticoids. Intravenous methylprednisolone at a dose of 1000 mg/d for 3 or 5 days or oral prednisone with or without tapering dose is usually used[64]. Administration of high-dose corticosteroids (HDC) may be repeated, depending on the MS course and disease activity[65]. Pulsed regimens of corticosteroid administration in the short term in MS have been reported to be well tolerated and safe, with only minor transient and dose- dependent side effects such as palpitations, hot flashes, dyspepsia, insomnia, and virulent taste[65].
An important clinical question is whether the use of corticosteroids, which are regularly used to treat MS relapses, can induce psychiatric symptoms. Several reports suggest that psychiatric side effects may occur with HDC use, including hypomania/mania, depression, psychosis, and suicidal ideation[66-71]. Mood changes, particularly (hypo)mania and depressive symptoms could be potentiated by HDC treatment[72]. In patients with MS, depression and mood disorders have been associated with pulse steroid therapy, suggesting that the risk may be related to the patient's psychiatric history[73].
Steroid-induced psychosis is a well-documented phenomenon[74]. In clinical practice, there are different approaches to corticosteroid treatment for MS-related psychosis. The literature on the treatment of corticosteroid-induced psychosis is very scarce and limited to MS reports and small-sample size studies. In our country, the prevailing opinion is that patients with psychosis should not be treated with corticosteroids because they may exacerbate psychosis. Moreover, nearly 30% of patients in the acute phase of their psychotic symptoms were successfully treated with corticosteroid therapies[75]. The question arises whether it is more beneficial for the patient to keep the psychosis under control or to treat the acute relapse, act on the inflammation, and prevent further disease progression and neurological disability. The Maudsley guideline indicates that the benefits of corticosteroid therapy may outweigh its adverse effects[76]. To date, however, few studies have explored the impact of corticosteroid therapy on psychosis in people affected by MS, and the specific risk factors predictive of psychiatric changes remain unclear[72,77]. Chronic consumption of corticosteroids and increase in their dose and duration of treatment are associated with these adverse effects[78]. As frequently mentioned, a general therapeutic strategy for corticosteroid-induced psychiatric symptoms should begin with dose reduction or discontinuation of the drug whenever possible[69,79].
Corticosteroid-induced hypomania, mania, and mixed mania showed to be successfully treated with a typical antipsychotic or mood stabilizer, most commonly haloperidol, haloperidol plus lithium, quetiapine, risperidone, olanzapine, olanzapine with valproate, carbamazepine, lithium, lamotrigine plus clonazepam, or clonazepam alone when lithium was ineffective[68]. In some cases, a combination of an antipsychotic and a benzodiazepine was needed[68]. In the case of medication-induced psychosis, adjustment of the MS drug should be considered and treatment with an antipsychotic should be initiated. There are few practical guidelines for the choice of antipsychotic drug and dosage, but there is evidence of good results with the use of clozapine, risperidone, ziprasidone, low-dose chlorpromazine, or the prophylactic use of lithium along with corticosteroid therapy[80]. In patients with steroid-induced psychosis, use of the antipsychotic quetiapine resulted in decreased irritability, reduced psychological distress, and improved sleep[81]. Atypical antipsychotics remain the treatment of choice in these patients because they have a more favourable tolerability profile, as they are less likely to affect the extrapyramidal system and reduce the risk of developing pseudoparkinsonism and catalepsy[82,83]. Researchers also presumed that patients with MS had an unexpected sensitivity to antipsychotic drugs[84]. MS is closely related to various movement disorders[85]. However, clinicians should be aware that movement disorders can also represent adverse drug reactions caused by chronic antipsychotic therapy[86], especially in cases of polypharmacy.
The anti-inflammatory potential of antipsychotics could be useful and should be considered especially in the treatment of psychosis that coexists with MS. Patients with SCH have been found to have decreased intracortical myelination, whereas certain antipsychotic agents may restore this defect. Patergnani et al[87] showed that human and experimental MS induce a mitochondrial deficit leading to activation of autophagy and mycophagy. These phenomena play a causal role in MS as their inhibition by antipsychotic drugs such as haloperidol and clozapine may prevent demyelination, induce remyelination, and reverse MS behavioural deficits[87]. In addition, Stamoula et al[88] argued that the atypical antipsychotics clozapine, risperidone, quetiapine, and olanzapine dramatically reduce the severity of experimental autoimmune encephalomyelitis and delay its onset by downregulating the production of pro-inflammatory cytokines and chemokines and attenuating T-cell infiltration, myeloid cell activation, and upregulation of T regulatory cells.
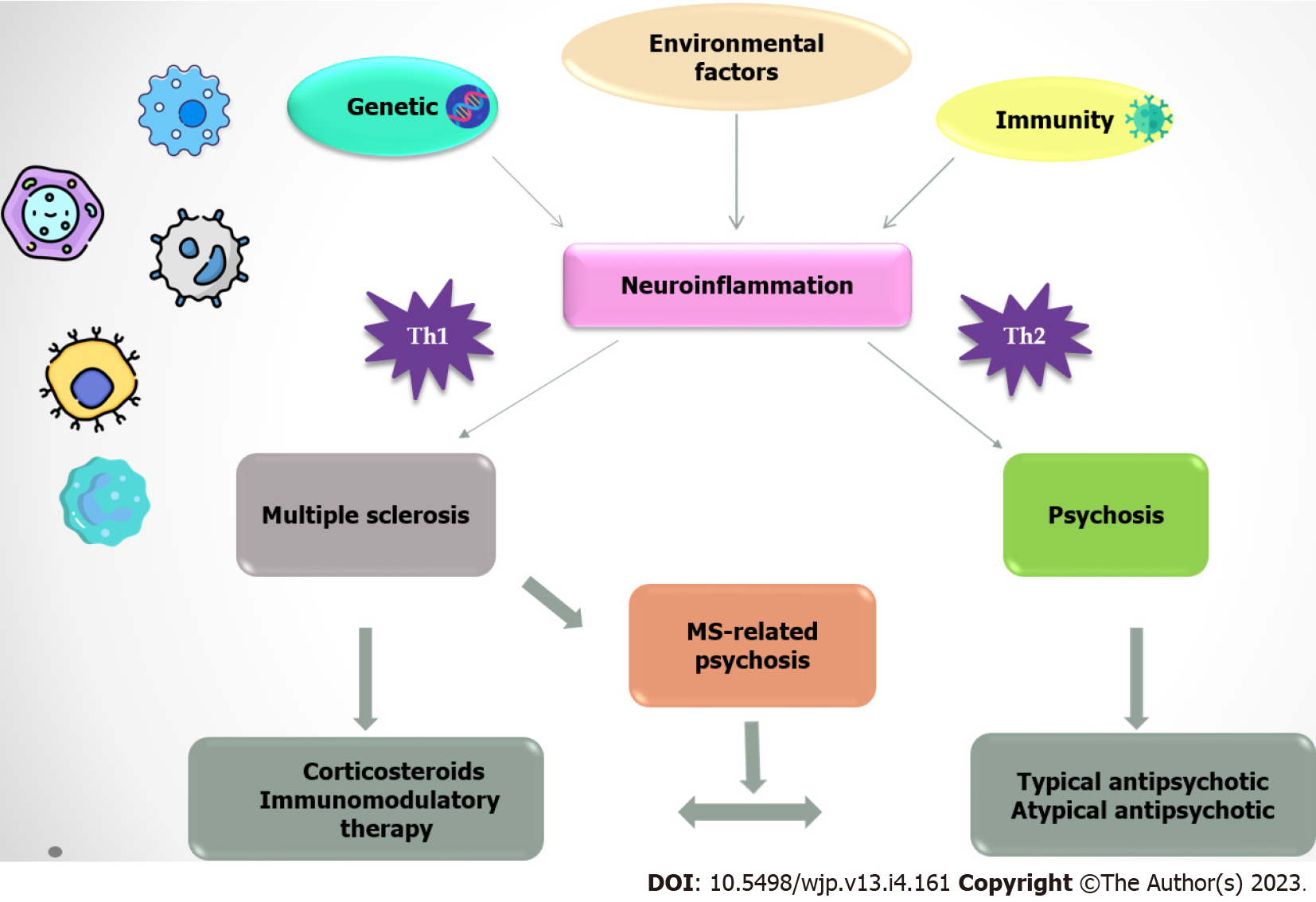
Psychotic symptoms could represent an acutisation of MS, considering the specific localization of the lesions. In this context, it can be assumed that causal therapy of MS also leads to resolution of psychosis. It is also important to exclude somatic comorbidities to make the diagnosis of SCH. Autoimmune disorders could precipitate psychotic symptoms, and in this context, special attention should be paid to patients with the first psychotic episode and soft neurological signs, sudden cognitive decline, and unsatisfactory response to antipsychotic treatment. Only when all of the previously mentioned facts have been ruled out can one conclude that psychosis is a separate entity (Figure 1). Physicians treating patients with MS and psychosis should assume, first and foremost, that the psychotic syndromes are related to MS and not triggered by medication. Based on the literature data, it is extremely important for clinicians to perform accurate screening of psychiatric status in patients with MS before initiating HDC treatment and to note that HDC should be used with caution in patients with an acute MS exacerbation. The properties and indications of available immunomodulatory drugs need to be better understood, and the therapeutic approach should be adjusted with careful consideration of the individual's unique constellation of symptoms. The mechanism of action and pharmacokinetics of the antipsychotic drug, the safety and efficacy profile from clinical trials, and knowledge of potential side effects should also be incorporated into the therapeutic strategy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kar SK, India; Stoyanov D, Bulgaria S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Ciccarelli O, Barkhof F, Bodini B, De Stefano N, Golay X, Nicolay K, Pelletier D, Pouwels PJ, Smith SA, Wheeler-Kingshott CA, Stankoff B, Yousry T, Miller DH. Pathogenesis of multiple sclerosis: insights from molecular and metabolic imaging. Lancet Neurol. 2014;13:807-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 2. | Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, Thompson AJ. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83:1022-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 821] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 3. | Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis: diagnosis, prevalence and treatment. Ther Adv Neurol Disord. 2009;2:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Kosmidis MH, Giannakou M, Messinis L, Papathanasopoulos P. Psychotic features associated with multiple sclerosis. Int Rev Psychiatry. 2010;22:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Gentile A, D'Acquisto F, Leposavić G. Editorial: The Bidirectional Communication Between Neurons and Immune Cells in the Development of Psychiatric, Neurological and Immune-Mediated Disorders. Front Immunol. 2021;12:781151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Hesdorffer DC. Comorbidity between neurological illness and psychiatric disorders. CNS Spectr. 2016;21:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Sparaco M, Lavorgna L, Bonavita S. Psychiatric disorders in multiple sclerosis. J Neurol. 2021;268:45-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Paoli RA, Botturi A, Ciammola A, Silani V, Prunas C, Lucchiari C, Zugno E, Caletti E. Neuropsychiatric Burden in Huntington's Disease. Brain Sci. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Menculini G, Chipi E, Paolini Paoletti F, Gaetani L, Nigro P, Simoni S, Mancini A, Tambasco N, Di Filippo M, Tortorella A, Parnetti L. Insights into the Pathophysiology of Psychiatric Symptoms in Central Nervous System Disorders: Implications for Early and Differential Diagnosis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Orr J, Bernstein CN, Graff LA, Patten SB, Bolton JM, Sareen J, Marriott JJ, Fisk JD, Marrie RA; CIHR Team in Defining the Burden and Managing the Effects of Immune-mediated Inflammatory Disease. Factors associated with perceived need for mental health care in multiple sclerosis. Mult Scler Relat Disord. 2018;25:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Davis BE, Lakin L, Binns CC, Currie KM, Rensel MR. Patient and Provider Insights into the Impact of Multiple Sclerosis on Mental Health: A Narrative Review. Neurol Ther. 2021;10:99-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Husain M. Transdiagnostic neurology: neuropsychiatric symptoms in neurodegenerative diseases. Brain. 2017;140:1535-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Solaro C, Gamberini G, Masuccio FG. Depression in Multiple Sclerosis: Epidemiology, Aetiology, Diagnosis and Treatment. CNS Drugs. 2018;32:117-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 14. | Silveira C, Guedes R, Maia D, Curral R, Coelho R. Neuropsychiatric Symptoms of Multiple Sclerosis: State of the Art. Psychiatry Investig. 2019;16:877-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 15. | Sabe M, Sentissi O. Psychotic symptoms prior or concomitant to diagnosis of multiple sclerosis: a systematic review of case reports and case series. Int J Psychiatry Clin Pract. 2022;26:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Didonna A, Oksenberg JR. The Genetics of Multiple Sclerosis. In: Multiple Sclerosis: Perspectives in Treatment and Pathogenesis [Internet]. Brisbane (AU): Codon Publications; 2017-Nov-27. [PubMed] |
| 17. | Goris A, Vandebergh M, McCauley JL, Saarela J, Cotsapas C. Genetics of multiple sclerosis: lessons from polygenicity. Lancet Neurol. 2022;21:830-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | Parnell GP, Booth DR. The Multiple Sclerosis (MS) Genetic Risk Factors Indicate both Acquired and Innate Immune Cell Subsets Contribute to MS Pathogenesis and Identify Novel Therapeutic Opportunities. Front Immunol. 2017;8:425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Patsopoulos NA. Genetics of Multiple Sclerosis: An Overview and New Directions. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | van Langelaar J, Rijvers L, Smolders J, van Luijn MM. B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front Immunol. 2020;11:760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 189] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 21. | Müller N. Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations. Schizophr Bull. 2018;44:973-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 22. | Hernández-Pedro NY, Espinosa-Ramirez G, de la Cruz VP, Pineda B, Sotelo J. Initial immunopathogenesis of multiple sclerosis: innate immune response. Clin Dev Immunol. 2013;2013:413465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Borovcanin M, Jovanovic I, Radosavljevic G, Djukic Dejanovic S, Bankovic D, Arsenijevic N, Lukic ML. Elevated serum level of type-2 cytokine and low IL-17 in first episode psychosis and schizophrenia in relapse. J Psychiatr Res. 2012;46:1421-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Chen SF, Wang LY, Chiang JH, Hsu CY, Shen YC. Assessing whether the association between rheumatoid arthritis and schizophrenia is bidirectional: A nationwide population-based cohort study. Sci Rep. 2019;9:4493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Jeppesen R, Benros ME. Autoimmune Diseases and Psychotic Disorders. Front Psychiatry. 2019;10:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Benros ME, Pedersen MG, Rasmussen H, Eaton WW, Nordentoft M, Mortensen PB. A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. Am J Psychiatry. 2014;171:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 27. | Arneth BM. Multiple Sclerosis and Schizophrenia. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Andreassen OA, Harbo HF, Wang Y, Thompson WK, Schork AJ, Mattingsdal M, Zuber V, Bettella F, Ripke S, Kelsoe JR, Kendler KS, O'Donovan MC, Sklar P; Psychiatric Genomics Consortium (PGC) Bipolar Disorder and Schizophrenia Work Groups; International Multiple Sclerosis Genetics Consortium (IMSGC), McEvoy LK, Desikan RS, Lie BA, Djurovic S, Dale AM. Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol Psychiatry. 2015;20:207-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 29. | Bush WS, Moore JH. Chapter 11: Genome-wide association studies. PLoS Comput Biol. 2012;8:e1002822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 738] [Cited by in RCA: 666] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 30. | Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1476] [Cited by in RCA: 1505] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 31. | Cooper GS, Miller FW, Pandey JP. The role of genetic factors in autoimmune disease: implications for environmental research. Environ Health Perspect. 1999;107 Suppl 5:693-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 737] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 33. | Robinson N, Bergen SE. Environmental Risk Factors for Schizophrenia and Bipolar Disorder and Their Relationship to Genetic Risk: Current Knowledge and Future Directions. Front Genet. 2021;12:686666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 34. | Alfredsson L, Olsson T. Lifestyle and Environmental Factors in Multiple Sclerosis. Cold Spring Harb Perspect Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 35. | Kneeland RE, Fatemi SH. Viral infection, inflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Rutsch A, Kantsjö JB, Ronchi F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front Immunol. 2020;11:604179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 469] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 37. | Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation. 2019;16:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 466] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 38. | Chen P, Tang X. Gut Microbiota as Regulators of Th17/Treg Balance in Patients With Myasthenia Gravis. Front Immunol. 2021;12:803101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 39. | Zhao Q, Elson CO. Adaptive immune education by gut microbiota antigens. Immunology. 2018;154:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 40. | Ochoa-Repáraz J, Kirby TO, Kasper LH. The Gut Microbiome and Multiple Sclerosis. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 41. | Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry. 2021;78:1343-1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 429] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 42. | Rathod B, Kaur A, Basavanagowda DM, Mohan D, Mishra N, Fuad S, Nosher S, Alrashid ZA, Heindl SE. Neurological Soft Signs and Brain Abnormalities in Schizophrenia: A Literature Review. Cureus. 2020;12:e11050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 43. | Meinl E, Krumbholz M, Derfuss T, Junker A, Hohlfeld R. Compartmentalization of inflammation in the CNS: a major mechanism driving progressive multiple sclerosis. J Neurol Sci. 2008;274:42-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Kerschensteiner M, Meinl E, Hohlfeld R. Neuro-immune crosstalk in CNS diseases. Neuroscience. 2009;158:1122-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 45. | Rattay TW, Martin P, Vittore D, Hengel H, Cebi I, Tünnerhoff J, Stefanou MI, Hoffmann JF, von der Ehe K, Klaus J, Vonderschmitt J, Herrmann ML, Bombach P, Al Barazi H, Zeltner L, Richter J, Hesse K, Eckstein KN, Klingberg S, Wildgruber D. Cerebrospinal fluid findings in patients with psychotic symptoms-a retrospective analysis. Sci Rep. 2021;11:7169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Borovcanin MM, Janicijevic SM, Mijailovic NR, Jovanovic IP, Arsenijevic NN, Vesic K. Uric Acid Potential Role in Systemic Inflammation and Negative Symptoms After Acute Antipsychotic Treatment in Schizophrenia. Front Psychiatry. 2021;12:822579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Haque R, Kim Y, Park K, Jang H, Kim SY, Lee H, Kim HJ. Altered distributions in circulating follicular helper and follicular regulatory T cells accountable for imbalanced cytokine production in multiple sclerosis. Clin Exp Immunol. 2021;205:75-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Kelly DL, Li X, Kilday C, Feldman S, Clark S, Liu F, Buchanan RW, Tonelli LH. Increased circulating regulatory T cells in medicated people with schizophrenia. Psychiatry Res. 2018;269:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Comi G, Bar-Or A, Lassmann H, Uccelli A, Hartung HP, Montalban X, Sørensen PS, Hohlfeld R, Hauser SL; Expert Panel of the 27th Annual Meeting of the European Charcot Foundation. Role of B Cells in Multiple Sclerosis and Related Disorders. Ann Neurol. 2021;89:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 50. | Fernandez-Egea E, Vértes PE, Flint SM, Turner L, Mustafa S, Hatton A, Smith KG, Lyons PA, Bullmore ET. Peripheral Immune Cell Populations Associated with Cognitive Deficits and Negative Symptoms of Treatment-Resistant Schizophrenia. PLoS One. 2016;11:e0155631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 51. | Deisenhammer F, Zetterberg H, Fitzner B, Zettl UK. The Cerebrospinal Fluid in Multiple Sclerosis. Front Immunol. 2019;10:726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 52. | Stoyanov D, Maes MH. How to construct neuroscience-informed psychiatric classification? World J Psychiatry. 2021;11:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (2)] |
| 53. | Dutta R, Trapp BD. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr Opin Neurol. 2014;27:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 54. | Lyoo IK, Seol HY, Byun HS, Renshaw PF. Unsuspected multiple sclerosis in patients with psychiatric disorders: a magnetic resonance imaging study. J Neuropsychiatry Clin Neurosci. 1996;8:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Borovcanin MM, Muric NN, Milovanovic M, Milovanovic J, Arsenijevic AN, Arsenijevic NN, Vesic K. Autoimmunity and psychosis. In: Translational Autoimmunity: Autoimmune Disease Associated with Different Clinical Features. Elsevier, 2022: 343–365. [DOI] [Full Text] |
| 56. | Feinstein A, du Boulay G, Ron MA. Psychotic illness in multiple sclerosis. A clinical and magnetic resonance imaging study. Br J Psychiatry. 1992;161:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 86] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Yadav R, Zigmond AS. Temporal lobe lesions and psychosis in multiple sclerosis. BMJ Case Rep. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Solana E, Martinez-Heras E, Montal V, Vilaplana E, Lopez-Soley E, Radua J, Sola-Valls N, Montejo C, Blanco Y, Pulido-Valdeolivas I, Sepúlveda M, Andorra M, Berenguer J, Villoslada P, Martinez-Lapiscina EH, Prados F, Saiz A, Fortea J, Llufriu S. Regional grey matter microstructural changes and volume loss according to disease duration in multiple sclerosis patients. Sci Rep. 2021;11:16805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Varambally S, Venkatasubramanian G, Gangadhar BN. Neurological soft signs in schizophrenia - The past, the present and the future. Indian J Psychiatry. 2012;54:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Fragoso YD, Frota ER, Lopes JS, Noal JS, Giacomo MC, Gomes S, Gonçalves MV, da Gama PD, Finkelsztejn A. Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin Neuropharmacol. 2010;33:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Feinstein A. Multiple sclerosis, disease modifying treatments and depression: a critical methodological review. Mult Scler. 2000;6:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Gasim M, Bernstein CN, Graff LA, Patten SB, El-Gabalawy R, Sareen J, Bolton JM, Marriott JJ, Fisk JD, Marrie RA; CIHR team “Defining the burden and managing the effects of psychiatric comorbidity in chronic inflammatory disease”. Adverse psychiatric effects of disease-modifying therapies in multiple Sclerosis: A systematic review. Mult Scler Relat Disord. 2018;26:124-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 63. | Krivinko JM, Erickson SL, MacDonald ML, Garver ME, Sweet RA. Fingolimod mitigates synaptic deficits and psychosis-like behavior in APP/PSEN1 mice. Alzheimers Dement (N Y). 2022;8:e12324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Burton JM, O’Connor PW, Hohol M, Beyene J. Oral vs intravenous steroids for treatment of relapses in multiple sclerosis. In: Burton JM, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd, 2009. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Shaygannejad V, Ashtari F, Alinaghian M, Norouzi R, Salari M, Fatehi F. Short-term safety of pulse steroid therapy in multiple sclerosis relapses. Clin Neuropharmacol. 2013;36:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Brown ES, Chandler PA. Mood and Cognitive Changes During Systemic Corticosteroid Therapy. Prim Care Companion J Clin Psychiatry. 2001;3:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days' corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 68. | Kenna HA, Poon AW, de los Angeles CP, Koran LM. Psychiatric complications of treatment with corticosteroids: review with case report. Psychiatry Clin Neurosci. 2011;65:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 69. | Lewis DA, Smith RE. Steroid-induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5:319-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 341] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 70. | Wada K, Yamada N, Suzuki H, Lee Y, Kuroda S. Recurrent cases of corticosteroid-induced mood disorder: clinical characteristics and treatment. J Clin Psychiatry. 2000;61:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Chwastiak LA, Ehde DM. Psychiatric issues in multiple sclerosis. Psychiatr Clin North Am. 2007;30:803-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 72. | Morrow SA, Barr J, Rosehart H, Ulch S. Depression and hypomania symptoms are associated with high dose corticosteroids treatment for MS relapses. J Affect Disord. 2015;187:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Lorefice L, Fenu G, Fois M, Frau J, Coghe G, Marrosu MG, Cocco E. Pulse steroid therapy in multiple sclerosis and mood changes: An exploratory prospective study. Mult Scler Relat Disord. 2018;20:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 74. | Janes M, Kuster S, Goldson TM, Forjuoh SN. Steroid-induced psychosis. Proc (Bayl Univ Med Cent). 2019;32:614-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Camara-Lemarroy CR, Ibarra-Yruegas BE, Rodriguez-Gutierrez R, Berrios-Morales I, Ionete C, Riskind P. The varieties of psychosis in multiple sclerosis: A systematic review of cases. Mult Scler Relat Disord. 2017;12:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Taylor DM, Barnes TRE, Young AH. Drug treatment of psychiatric symptoms occuring in the context of other disorders. In: The Maudsley practice guidelines for physical health conditions in psychiatry. John Wiley & Sons, 2018: 699–712. [DOI] [Full Text] |
| 77. | Wada K, Yamada N, Sato T, Suzuki H, Miki M, Lee Y, Akiyama K, Kuroda S. Corticosteroid-induced psychotic and mood disorders: diagnosis defined by DSM-IV and clinical pictures. Psychosomatics. 2001;42:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 78. | Thrower BW. Relapse management in multiple sclerosis. Neurologist. 2009;15:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 80. | Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:743-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Hrebicek K, Olinka MIN. Identifying and treating steroid-induced psychosis in MS patients using quetiapine. In: Betoux F, editor. Annual Meeting of the Consortium of Multiple Sclerosis Centers. Delaware Media Group, Glen Rock, USA, 2021: 15–16. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 82. | Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2013;16:1205-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 83. | Murray R, Correll CU, Reynolds GP, Taylor D. Atypical antipsychotics: recent research findings and applications to clinical practice: Proceedings of a symposium presented at the 29th Annual European College of Neuropsychopharmacology Congress, 19 September 2016, Vienna, Austria. Ther Adv Psychopharmacol. 2017;7:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | La Flamme AC, Abernethy D, Sim D, Goode L, Lockhart M, Bourke D, Milner I, Garrill TM, Joshi P, Watson E, Smyth D, Lance S, Connor B. Safety and acceptability of clozapine and risperidone in progressive multiple sclerosis: a phase I, randomised, blinded, placebo-controlled trial. BMJ Neurol Open. 2020;2:e000060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Ghosh R, Roy D, Dubey S, Das S, Benito-León J. Movement Disorders in Multiple Sclerosis: An Update. Tremor Other Hyperkinet Mov (N Y). 2022;12:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 86. | Iuppa CA, Diefenderfer LA. Risperidone-induced Pisa syndrome in MS: resolution with lurasidone and recurrence with Chlorpromazine. Ann Pharmacother. 2013;47:1223-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Patergnani S, Bonora M, Ingusci S, Previati M, Marchi S, Zucchini S, Perrone M, Wieckowski MR, Castellazzi M, Pugliatti M, Giorgi C, Simonato M, Pinton P. Antipsychotic drugs counteract autophagy and mitophagy in multiple sclerosis. Proc Natl Acad Sci USA. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 88. | Stamoula Ε, Ainatzoglou A, Stamatellos VP, Dardalas I, Siafis S, Matsas A, Stamoulas K, Papazisis G. Atypical antipsychotics in multiple sclerosis: A review of their in vivo immunomodulatory effects. Mult Scler Relat Disord. 2022;58:103522. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |