Published online Jul 19, 2022. doi: 10.5498/wjp.v12.i7.874
Peer-review started: January 20, 2022
First decision: April 18, 2022
Revised: May 3, 2022
Accepted: June 16, 2022
Article in press: June 16, 2022
Published online: July 19, 2022
Processing time: 179 Days and 12.6 Hours
The coronavirus disease 2019 (COVID-19) pandemic has been linked to an increased prevalence of mental health disorders, particularly anxiety and depression. Moreover, the COVID-19 pandemic has caused stress in people worldwide due to several factors, including fear of infection; social isolation; difficulty in adapting to new routines; lack of coping methods; high exposure to social media, misinformation, and fake reports; economic impact of the measures implemented to slow the contagion and concerns regarding the disease pathogenesis. COVID-19 patients have elevated levels of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α, and other inflammation-related factors. Furthermore, invasion of the central nervous system by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may potentially contribute to neuroinflammatory alterations in infected individuals. Neuroinflammation, a consequence of psychological stress due to the COVID-19 pandemic, may also play a role in the development of anxiety and depressive symptoms in the general population. Considering that neuroinflammation plays a significant role in the pathophysiology of depression and anxiety, this study investigated the effects of SARS-CoV-2 on mental health and focused on the impact of the COVID-19 pandemic on the neuroinflammatory pathways.
Core Tip: The coronavirus disease 2019 pandemic has impacted the mental health of the population worldwide. This review summarizes the evidence of the role of neuroinflammation, either as a result of chronic stress caused by the pandemic or severe acute respiratory syndrome coronavirus 2 infection, in the development of anxiety and depressive disorders.
- Citation: de Mello AJ, Moretti M, Rodrigues ALS. SARS-CoV-2 consequences for mental health: Neuroinflammatory pathways linking COVID-19 to anxiety and depression. World J Psychiatry 2022; 12(7): 874-883
- URL: https://www.wjgnet.com/2220-3206/full/v12/i7/874.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i7.874
On March 11, 2020, the World Health Organization (WHO) declared the outbreak of the coronavirus disease 2019 (COVID-19) as a pandemic[1]. More than two years have passed since the emergence of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and its ramifications have changed human lives worldwide. In response to the COVID-19 outbreak, the scientific community has collaborated to provide information on all aspects of the disease, including the devastating sequelae in survivors. The pandemic has directly affected people through infections and resulted in increased psychological stress in the general population.
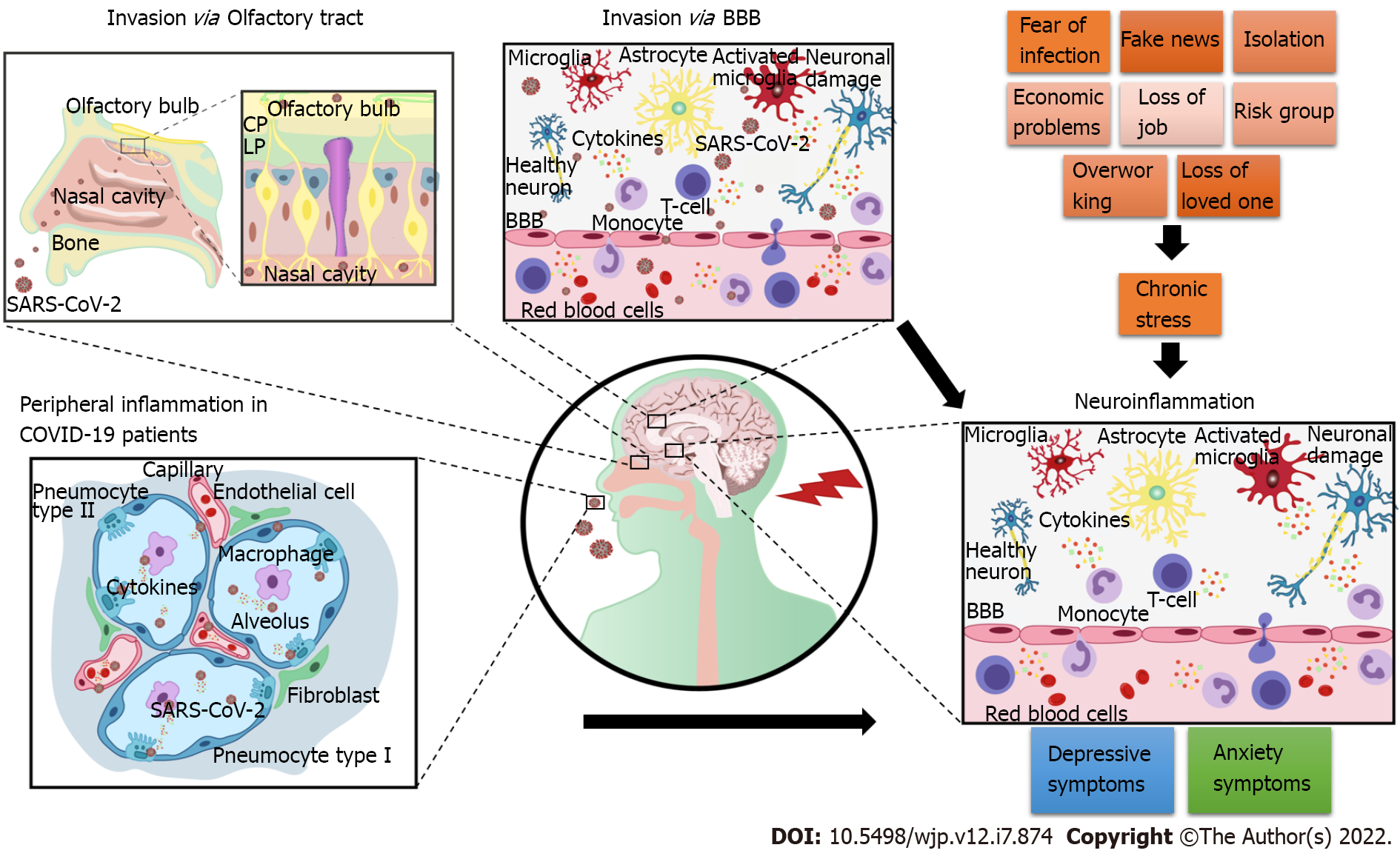
Several factors contributed to the psychological consequences of the pandemic in the affected population, such as poor knowledge about the disease, previously undiagnosed mental health disorders, lack of a healthy lifestyle, no prior mental health assessment, economic problems, changes in eating and sleeping habits, difficulty in adapting to new routines, lack of coping methods, high exposure to social media, misinformation and fake reports, and social isolation during quarantine[2,3]. Quarantine and lockdowns have severely impacted everyday life worldwide, ranging from student education to the immense workload on health professionals[4]. Social distancing has isolated people inside their houses and significantly impacted the economy[5-7]. People with an infected household or a close contact with COVID-19 patients or those with a history of chronic illnesses have been shown to a higher risk of developing psychiatric distress[8,9].
Another concern is related to patients hospitalized due to COVID-19. Hospitalized patients are at risk of experiencing depression, anxiety, insomnia, and delirium[10]. Among all sequelae resulting from the disease, those of psychopathological nature can be induced either directly through the invasion of the virus in the central nervous system (CNS) or indirectly as a consequence of systemic inflammation and immune response[11]. Neuroinflammatory alterations have been postulated to cause depression and anxiety[12]. Although there are several comprehensive literature reviews on the impact of SARS-CoV-2 on human health, in this minireview, we have discussed how neuroinflammation caused by chronic stress or SARS-CoV-2 infection can lead to anxiety and depression. We hypothesized that the neuroinvasion of SARS-CoV-2 in the brain, peripheral pro-inflammatory cytokines that may enter the brain after SARS-CoV-2 infection, and psychological stress associated with the pandemic, alone or in combination, could cause neuroinflammation and contribute to the development of anxiety and depression disorders.
The increase in depression and anxiety during the COVID-19 pandemic has become a major health concern[2-4]. Depression and anxiety frequently co-occur and are prevalent and burdensome psychiatric disorders[13]. Depression was the second largest cause of disease burden in 2020[14-16] and has been projected to take precedence by 2030[17]. The most recent Atlas of Mental Health published by the WHO in 2020 revealed the indicators of mental health and Comprehensive Mental Health Action Plan, which has been extended till 2030 to assist individuals whose mental health has been affected by the COVID-19 pandemic[17]. Generally, anxiety disorders have a high annual prevalence at approximately 14%, with the United States and Europe presenting a higher rate than other areas[18,19]. One in four individuals is likely to develop or has already developed anxiety disorders[20]. Of note, the risk of developing anxiety and depression has been closely associated with exposure to chronic stress[21] such as that in the COVID-19 pandemic[22].
Coronaphobia, or excess anxiety about COVID-19, is strongly associated with elevated reports of depression, general anxiety, a lack of hope, and suicidal ideation[14,15,23]. A systematic review and meta-analysis of 13 studies with a total of 33062 participants indicated a 23.2% and 22.8% prevalence of anxiety and depression, respectively, in healthcare workers in China during the beginning of the pandemic, with a higher prevalence in female nurses[4]. In addition, the prevalence of depression and anxiety has increased in the general population, especially in young adults. During the initial stages of the COVID-19 pandemic in the United States, at least one-third of participants in a cross-sectional study reported high levels of depression (43.3%), anxiety (45.4%), and post-traumatic stress (31.8%)[24]. These rates were higher than those found in a previous study conducted in 2009 using the same assessment tools, showing a prevalence rate of 6.2% among young adults aged 18–24 years and 13.1% among those aged 25–34 years[25]. These symptoms were also associated with loneliness and low resilience to stress, whereas a higher tolerance to stress was associated with lower anxiety. Family support has been previously associated with lower levels of depression and post-traumatic stress disorder[24].
Another Chinese study conducted in 2020 reported a four times higher prevalence of depression, anxiety, or both, than a study published in 2019 (20.4% in 2020 vs 4% in 2019)[26,27]. This study associated the development of depressive and anxiety symptoms with some common pandemic stressors, including worrying about oneself or loved ones being infected; concerns about income, jobs, school, and ability to pay loans; and hardships involving home quarantine in everyday life[26]. Depression and anxiety reported by Bangladeshi University students during the pandemic were associated with uncertainty about their academic or professional future and financial instability[28]. Early reports between mid-February and mid-March 2020 showed an increase of 34.1% in the demand for anxiolytic drugs, followed by 18.6% for antidepressants and 14.8% for sleep medications[29].
Studies on youth population have suggested that children and adolescents have also been affected by the pandemic. During the first year of the pandemic, one in four young adults experienced a clinical increase in depressive symptoms, with older children being the most affected. In addition, one in five children and adolescents had clinically elevated anxiety levels. The prevalence rates of depression and anxiety in children and adolescents increased over time and doubled compared to estimates before the pandemic according to a recent meta-analysis[30]. Further, the global prevalence of depression and anxiety increased by 25% and 27.6% due to the COVID-19 pandemic in 2020, indicating the negative impact of COVID-19 on the mental health of people of all ages worldwide[31].
Several studies have shown that inflammation plays a key role in the pathophysiology of depressive disorders[12]. Preclinical studies have provided consistent evidence that exposure of rodents to chronic unpredictable and/or inescapable stress situations induces depressive-like behavior accompanied by peripheral and central activation of the immune, inflammatory, and oxidative and nitrosative stress pathways. Furthermore, chronic administration of antidepressants attenuates these effects[32]. Chronic stress can also induce neurotoxic effects on specific brain regions, either directly or indirectly, through the kynurenine pathway[33], causing a reduction in brain-derived neurotrophic factor with consequent impairment of adult hippocampal neurogenesis[32].
Individuals with depression present with high serum levels of pro-inflammatory cytokines and acute-phase proteins and an increased expression of adhesion molecules and chemokines[34-38]. These protein alterations suggest an association between depression and activation of pro-inflammatory responses. Depression has been associated with increased levels of peripheral and central tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and C-reactive protein[34,37,39]. Furthermore, studies have reported an increase in the levels of other acute-phase proteins (i.e., α-1-acid glycoprotein, α-1-antichymotrypsin, and haptoglobin) in the plasma of patients with depression[37,40-42]. Elevated levels of human macrophage chemoattractant protein-1, soluble intracellular adhesion molecule-1, and E-selectin have also been reported[43]. An apparent association between the severity of depressive symptoms and level of inflammatory mediators in the plasma of patients has also been shown[34,44]. In addition, functional variants of alleles of IL-1β and TNF-α genes influence different factors, either elevating the risk of depression or reducing the response to antidepressants[40,45,46].
Despite the frequent co-occurrence of anxiety and depression and their common association with cardiovascular[47] and metabolic diseases[48], the role of neuroinflammation in the pathophysiology of anxiety disorders has not been studied as extensively as that in depression[49]. Neuroinflammation may cause alterations in the structure or function of anxiety-related brain circuits (mainly the limbic and prefrontal regions), priming the brain to become vulnerable to anxiety disorders[33]. Studies have reported increased inflammation in patients of both sexes with late-onset anxiety disorder; however, they were unable to confirm it as an etiological factor[49]. Other studies have linked the immune system and CNS through key interactions that can influence behavioral changes; however, a causal relationship between anxiety and inflammation needs extensive investigation[49]. In preclinical studies, activation of the nucleotide-binding oligomerization domain-like receptor pyrin domain-containing-3 (NLRP3) inflammasome has been associated with anxiety-like behavior[50,51]. Clinical findings have suggested that increased cytokine levels affect neurotransmitters, such as monoamines and glutamate, in the amygdala, insula, and anterior cingulate cortex, which are brain regions related to anxiety[52]. Accordingly, inhibition of neuroinflammation has been accompanied by anxiolytic effects[51].
Increased levels of TNF-α, a cytokine important for cellular regulation and apoptosis, have been consistently associated with depression and anxiety in humans[53]. Similarly, central administration of TNF-α in mice resulted in depressive-like behavior, whereas TNF-α receptor 1 knockout mice exhibited antidepressant-like behavior in the forced swimming test and tail suspension test[54]. In addition, administration of TNF-α induced anxiety-related behavior in mice[55]. Administration of etanercept, a TNF-α blocker, reduced anxiety and depressive-like behavior in db/db mice exhibiting type-2 diabetes-related inflammation and mood alterations[56]. TNF-α blockade also caused an anxiolytic effect in mice with experimental autoimmune encephalomyelitis[55] and mice subjected to peripheral immune challenge with lipopolysaccharide[57]. Furthermore, administration of the TNF-α-neutralizing antibody infliximab in the basolateral amygdala reversed anxiety-like behaviors in mice with persistent inflammatory pain[58].
During the initial phases of inflammation, IL-6 is induced along with TNF-α and may represent a key inflammatory mediator in patients with COVID-19[59-61]. Similarly, IL-1β is the major cytokine (in association with IL-18) produced by the activation of the NLRP3 inflammasome and increases in depression. These cytokines modulate the neuroimmune pathways that regulate critical brain circuits involved in cognition, mood, and reward[62-64]. Notably, SARS-CoV-2 is postulated to directly activate the NLRP3 inflammasome, and patients with dysregulated NLRP3 inflammasome activity may develop COVID-19 with severe tissue damage and a cytokine storm[65].
Increased levels of pro-inflammatory cytokines such as IL-6 may repress brain-derived neurotrophic factor, contributing to the development of depressive behavior[66,67]. IL-6 is also associated with lymphocyte exhaustion, and its role in COVID-19 inflammation has propelled the use of IL-6 inhibitors, corticosteroids, antimalarial drugs, and intravenous immunoglobulin to oppose the effects of cytokine storms in individuals with COVID-19[68]. Therefore, a strong inflammatory response can be related to disease severity and death in patients with COVID-19[68]. In severely affected patients, increased levels of peripheral cytokines can cause lymphopenia and invasion of mononuclear cells in the heart, lungs, lymph nodes, spleen, and kidneys[69]. A study on COVID-19 survivors revealed elevated depression, anxiety, insomnia, post-traumatic stress disorder, and obsessive–compulsive symptoms one month after hospitalization[70]. These findings are consistent with those reported during the previous coronavirus outbreaks, in which 10%-35% patients in the post-disease recovery stage presented psychiatric comorbidities[10]. These psychiatric outcomes may be a consequence of neuroinflammation caused by COVID-19. Moreover, neuroimaging and CSF marker elevations in patients with COVID-19 have suggested that SARS-COV-2 causes CNS inflammation[71].
Individuals infected with SARS-CoV-2 can remain asymptomatic or develop COVID-19 symptoms. Hospitalized patients with COVID-19 commonly present with clinical sequelae that appear up to three months after discharge[72]. These sequelae are not limited to respiratory issues because patients can manifest cardiovascular, neurological, and psychosocial symptoms after discharge[11,72,73].
The neurological symptoms after COVID-19 may be associated with direct SARS-CoV-2 invasion of the CNS, where the virus has a high potential for replication, causing significant neuronal death[74]. Patient autopsies have revealed neuronal loss[75], often associated with an immune response against the virus in the CSF. Few reports showed that patients who tested positive for SARS-CoV-2 in their CSF but did not have any significant risk factors or a history of neurological diseases manifested neurological symptoms, such as seizures and loss of consciousness[76].
Most in vitro and in vivo experiments support the hypothesis that neuroinvasion by SARS-CoV-2 causes neurological symptoms in patients with COVID-19. The presence of the virus within neurons in multiple brain areas of infected animals resulted in a neuropathology similar to that observed in hospitalized patients[77]. Importantly, these alterations are not limited to adult patients; children also manifest the same critical developments after COVID-19, including thrombosis, inflammation, and secondary tissue ischemia[78,79]. Severe COVID-19 is rarely reported in children; however, there have been reports of children who developed acute fulminant cerebral edema, severe encephalopathy, and ischemic stroke despite being previously healthy[80,81].
Animal experiments have provided detailed information regarding the neuroinvasive potential of SARS-CoV-2. A study by Song et al[74] revealed that SARS-CoV-2 infects animal lungs at early time points, while it infects the brain much later. In the same study, electron microscopy to identify viral particles sprouting from the endoplasmic reticulum indicated that the virus could use cellular machinery for replication. Unlike other neurotropic viruses such as Zika, SARS-CoV-2 causes metabolic changes in the brain, as demonstrated using human brain organoids[74].
The literature further suggests SARS-CoV-2 neuroinvasion occurs through the trans-neuronal route, especially during the early stages of infection, in which SARS-CoV-2 invades the brain via the cranial nerve pathways such as the olfactory, gustatory, and trigeminal nerves[77]. This infiltration route is also associated with the severity of infection and neurological manifestations that lead to a higher risk of mortality in patients with COVID-19. Liu et al[77] reported that death occurred only in infected animals with neurological deficits, suggesting that disease progression is associated with the severity of neurological impairment.
Involvement of the trans-neuronal route suggests that SARS-CoV-2 enters the CNS through the olfactory nerves via angiotensin-converting enzyme 2 (ACE2; a part of the renin-angiotensin-aldosterone system) present on the cell membrane. The virus then migrates through the neuroepithelium and reaches the brain, consistent with the loss of smell observed in patients with COVID-19[82,83]. This route of SARS-CoV-2 neuroinvasion has been demonstrated by Song et al[74] in mice overexpressing human ACE2[74]. Accordingly, COVID-19 respiratory distress has been associated with increased nasopharyngeal expression of ACE2 and transmembrane serine protease 2[84]. In addition, clinical studies and post-mortem analyses have reported the presence of viral antigens in the olfactory tract[85-88]. Magnetic resonance imaging examination of patients with COVID-19 revealed structural changes throughout the olfactory pathway, including the nerve, bulb, and cerebral cortex, and supports the olfactory bulb route hypothesis[83,89,90]. Immunostaining for SARS-CoV-2 in animal models has revealed extensive staining in these regions[91,92].
Another plausible entry route for SARS-CoV-2 could be through the blood-brain barrier (BBB) by binding to ACE2 on endothelial cells[82]. This route, previously linked to infected individuals with high fever, may cause cytokine storms and increase the BBB permeability[93,94], thereby facilitating the access of SARS-CoV-2 to the brain[95]. As a consequence of BBB impairment, peripheral immune cells can enter the brain, increase the release of pro-inflammatory cytokines by microglial cells and sustain neuroinflammation[96].
Finally, post-mortem studies have reported the presence of ischemic damage and microinfarcts in brain samples of patients with COVID-19, supporting the assumption of SARS-CoV-2 neuroinvasion into the CNS[74].
As illustrated in Figure 1, the increased prevalence of depression and anxiety during the COVID-19 pandemic may be attributed to SARS-CoV-2 neuroinvasion and its harmful consequences on the CNS. Depression and anxiety may also occur because of peripheral inflammation caused by the virus and indirect negative effects on the brain function. Moreover, long-lasting social stressors linked to the pandemic may contribute to neuroinflammation and, consequently, to the development of these psychiatric symptoms. Therefore, anxiety and depression can affect the infected individuals and general population exposed to long-lasting pandemic stress. In the future, epidemiological studies should be conducted to elucidate the COVID-19 psychiatric burden, and public health control measures to help manage this burden must be provided.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garcia-Campayo J, Spain; Girardi P, Italy S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30097] [Article Influence: 6019.4] [Reference Citation Analysis (3)] |
| 2. | Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, Khaledi-Paveh B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2221] [Cited by in RCA: 1840] [Article Influence: 368.0] [Reference Citation Analysis (0)] |
| 3. | Zhou SJ, Zhang LG, Wang LL, Guo ZC, Wang JQ, Chen JC, Liu M, Chen X, Chen JX. Prevalence and socio-demographic correlates of psychological health problems in Chinese adolescents during the outbreak of COVID-19. Eur Child Adolesc Psychiatry. 2020;29:749-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 677] [Cited by in RCA: 817] [Article Influence: 163.4] [Reference Citation Analysis (0)] |
| 4. | Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1812] [Cited by in RCA: 2247] [Article Influence: 449.4] [Reference Citation Analysis (0)] |
| 5. | Kentikelenis A, Gabor D, Ortiz I, Stubbs T, McKee M, Stuckler D. Softening the blow of the pandemic: will the International Monetary Fund and World Bank make things worse? Lancet Glob Health. 2020;8:e758-e759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Khan K, Zhao H, Zhang H, Yang H, Shah MH, Jahanger A. The Impact of COVID-19 Pandemic on Stock Markets: An Empirical Analysis of World Major Stock Indices. J Asian Finance Econo Business. 2020;7:463-474. [DOI] [Full Text] |
| 7. | Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg. 2020;78:185-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2872] [Cited by in RCA: 2680] [Article Influence: 536.0] [Reference Citation Analysis (0)] |
| 8. | Moghanibashi-Mansourieh A. Assessing the anxiety level of Iranian general population during COVID-19 outbreak. Asian J Psychiatr. 2020;51:102076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 386] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 9. | Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, Ho RC. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4551] [Cited by in RCA: 5133] [Article Influence: 1026.6] [Reference Citation Analysis (1)] |
| 10. | Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1673] [Cited by in RCA: 1521] [Article Influence: 304.2] [Reference Citation Analysis (0)] |
| 11. | Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Brain Behav Immun. 2020;87:34-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 665] [Cited by in RCA: 620] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 12. | Felger JC. Role of Inflammation in Depression and Treatment Implications. Handb Exp Pharmacol. 2019;250:255-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Hirschfeld RM. The Comorbidity of Major Depression and Anxiety Disorders: Recognition and Management in Primary Care. Prim Care Companion J Clin Psychiatry. 2001;3:244-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 423] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 14. | Arora A, Jha AK, Alat P, Das SS. Understanding coronaphobia. Asian J Psychiatr. 2020;54:102384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 15. | Pirkis J, John A, Shin S, DelPozo-Banos M, Arya V, Analuisa-Aguilar P, Appleby L, Arensman E, Bantjes J, Baran A, Bertolote JM, Borges G, Brečić P, Caine E, Castelpietra G, Chang SS, Colchester D, Crompton D, Curkovic M, Deisenhammer EA, Du C, Dwyer J, Erlangsen A, Faust JS, Fortune S, Garrett A, George D, Gerstner R, Gilissen R, Gould M, Hawton K, Kanter J, Kapur N, Khan M, Kirtley OJ, Knipe D, Kolves K, Leske S, Marahatta K, Mittendorfer-Rutz E, Neznanov N, Niederkrotenthaler T, Nielsen E, Nordentoft M, Oberlerchner H, O'Connor RC, Pearson M, Phillips MR, Platt S, Plener PL, Psota G, Qin P, Radeloff D, Rados C, Reif A, Reif-Leonhard C, Rozanov V, Schlang C, Schneider B, Semenova N, Sinyor M, Townsend E, Ueda M, Vijayakumar L, Webb RT, Weerasinghe M, Zalsman G, Gunnell D, Spittal MJ. Suicide trends in the early months of the COVID-19 pandemic: an interrupted time-series analysis of preliminary data from 21 countries. Lancet Psychiatry. 2021;8:579-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 435] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 16. | Lopez AD, Murray CC. The global burden of disease, 1990-2020. Nat Med. 1998;4:1241-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1086] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 17. | World Health Organization. Mental health atlas 2020. [cited 10 January 2022]. Available from: https://apps.who.int/iris/handle/10665/345946. |
| 18. | Baxter AJ, Scott KM, Vos T, Whiteford HA. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med. 2013;43:897-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 859] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 19. | Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, Wittchen HU. Anxiety disorders. Nat Rev Dis Primers. 2017;3:1-19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 328] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 20. | Kessler RC, Angermeyer M, Anthony JC, DE Graaf R, Demyttenaere K, Gasquet I, DE Girolamo G, Gluzman S, Gureje O, Haro JM, Kawakami N, Karam A, Levinson D, Medina Mora ME, Oakley Browne MA, Posada-Villa J, Stein DJ, Adley Tsang CH, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell BE, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustün TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry. 2007;6:168-176. [PubMed] |
| 21. | Vinkers CH, Joëls M, Milaneschi Y, Kahn RS, Penninx BW, Boks MP. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety. 2014;31:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Polizzi C, Lynn SJ, Perry A. Stress and Coping in the Time of Covid-19: Pathways to Resilience and Recovery. Clin Neuropsychiatry. 2020;17:59-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 120] [Reference Citation Analysis (0)] |
| 23. | Lee SA, Jobe MC, Mathis AA, Gibbons JA. Incremental validity of coronaphobia: Coronavirus anxiety explains depression, generalized anxiety, and death anxiety. J Anxiety Disord. 2020;74:102268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 24. | Liu CH, Zhang E, Wong GTF, Hyun S, Hahm HC. Factors associated with depression, anxiety, and PTSD symptomatology during the COVID-19 pandemic: Clinical implications for U.S. young adult mental health. Psychiatry Res. 2020;290:113172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 807] [Cited by in RCA: 714] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 25. | Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2421] [Cited by in RCA: 3152] [Article Influence: 197.0] [Reference Citation Analysis (0)] |
| 26. | Li J, Yang Z, Qiu H, Wang Y, Jian L, Ji J, Li K. Anxiety and depression among general population in China at the peak of the COVID-19 epidemic. World Psychiatry. 2020;19:249-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 243] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 27. | Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, Yu Y, Kou C, Xu X, Lu J, Wang Z, He S, Xu Y, He Y, Li T, Guo W, Tian H, Xu G, Ma Y, Wang L, Yan Y, Wang B, Xiao S, Zhou L, Li L, Tan L, Zhang T, Ma C, Li Q, Ding H, Geng H, Jia F, Shi J, Wang S, Zhang N, Du X, Wu Y. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6:211-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1590] [Cited by in RCA: 1355] [Article Influence: 225.8] [Reference Citation Analysis (0)] |
| 28. | Islam MA, Barna SD, Raihan H, Khan MNA, Hossain MT. Depression and anxiety among university students during the COVID-19 pandemic in Bangladesh: A web-based cross-sectional survey. PLoS One. 2020;15:e0238162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 426] [Cited by in RCA: 291] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 29. | Digon S. Anti-Anxiety Prescription Meds Increase Amid COVID-19 Pandemic, Report Says. International Business Times. 2020. [cited 10 January 2022]. Available from: https://www.ibtimes.com/anti-anxiety-prescription-meds-increase-amid-covid-19-pandemic-report-says-2962093. |
| 30. | Racine N, McArthur BA, Cooke JE, Eirich R, Zhu J, Madigan S. Global Prevalence of Depressive and Anxiety Symptoms in Children and Adolescents During COVID-19: A Meta-analysis. JAMA Pediatr. 2021;175:1142-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1348] [Article Influence: 337.0] [Reference Citation Analysis (0)] |
| 31. | COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700-1712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2518] [Cited by in RCA: 2678] [Article Influence: 669.5] [Reference Citation Analysis (0)] |
| 32. | Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 338] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 33. | Won E, Kim YK. Neuroinflammation-Associated Alterations of the Brain as Potential Neural Biomarkers in Anxiety Disorders. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 34. | Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, Ayala AR, Licinio J, Gold HK, Kling MA, Chrousos GP, Gold PW. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 35. | Bouhuys AL, Flentge F, Oldehinkel AJ, van den Berg MD. Potential psychosocial mechanisms linking depression to immune function in elderly subjects. Psychiatry Res. 2004;127:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 393] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 37. | Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 397] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 38. | Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158:1252-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 308] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 39. | Mikova O, Yakimova R, Bosmans E, Kenis G, Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol. 2001;11:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 221] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 40. | Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1963] [Cited by in RCA: 2165] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 41. | Sluzewska A, Sobieska M, Rybakowski JK. Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology. 1997;35:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Tiemeier H, Hofman A, van Tuijl HR, Kiliaan AJ, Meijer J, Breteler MM. Inflammatory proteins and depression in the elderly. Epidemiology. 2003;14:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88:196-198, A7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 176] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90:1279-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 306] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 45. | Jun TY, Pae CU, Hoon-Han, Chae JH, Bahk WM, Kim KS, Serretti A. Possible association between -G308A tumour necrosis factor-alpha gene polymorphism and major depressive disorder in the Korean population. Psychiatr Genet. 2003;13:179-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Yu YW, Chen TJ, Hong CJ, Chen HM, Tsai SJ. Association study of the interleukin-1 beta (C-511T) genetic polymorphism with major depressive disorder, associated symptomatology, and antidepressant response. Neuropsychopharmacology. 2003;28:1182-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Vogelzangs N, Seldenrijk A, Beekman AT, van Hout HP, de Jonge P, Penninx BW. Cardiovascular disease in persons with depressive and anxiety disorders. J Affect Disord. 2010;125:241-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 48. | Carroll D, Phillips AC, Thomas GN, Gale CR, Deary I, Batty GD. Generalized anxiety disorder is associated with metabolic syndrome in the Vietnam experience study. Biol Psychiatry. 2009;66:91-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 50. | Lei Y, Chen CJ, Yan XX, Li Z, Deng XH. Early-life lipopolysaccharide exposure potentiates forebrain expression of NLRP3 inflammasome proteins and anxiety-like behavior in adolescent rats. Brain Res. 2017;1671:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Smith C, Trageser KJ, Wu H, Herman FJ, Iqbal UH, Sebastian-Valverde M, Frolinger T, Zeng E, Pasinetti GM. Anxiolytic effects of NLRP3 inflammasome inhibition in a model of chronic sleep deprivation. Transl Psychiatry. 2021;11:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 52. | Felger JC. Imaging the Role of Inflammation in Mood and Anxiety-related Disorders. Curr Neuropharmacol. 2018;16:533-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 53. | Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1713] [Cited by in RCA: 1762] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 54. | Kaster MP, Gadotti VM, Calixto JB, Santos AR, Rodrigues AL. Depressive-like behavior induced by tumor necrosis factor-α in mice. Neuropharmacology. 2012;62:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 55. | Haji N, Mandolesi G, Gentile A, Sacchetti L, Fresegna D, Rossi S, Musella A, Sepman H, Motta C, Studer V, De Chiara V, Bernardi G, Strata P, Centonze D. TNF-α-mediated anxiety in a mouse model of multiple sclerosis. Exp Neurol. 2012;237:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 56. | Alshammari MA, Khan MR, Majid Mahmood H, Alshehri AO, Alasmari FF, Alqahtani FM, Alasmari AF, Alsharari SD, Alhossan A, Ahmad SF, Nadeem A, Alshammari TK. Systemic TNF-α blockade attenuates anxiety and depressive-like behaviors in db/db mice through downregulation of inflammatory signaling in peripheral immune cells. Saudi Pharm J. 2020;28:621-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Camara ML, Corrigan F, Jaehne EJ, Jawahar MC, Anscomb H, Baune BT. Effects of centrally administered etanercept on behavior, microglia, and astrocytes in mice following a peripheral immune challenge. Neuropsychopharmacology. 2015;40:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Chen J, Song Y, Yang J, Zhang Y, Zhao P, Zhu XJ, Su HC. The contribution of TNF-α in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci Lett. 2013;541:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 59. | Yimin, Kohanawa M. A regulatory effect of the balance between TNF-alpha and IL-6 in the granulomatous and inflammatory response to Rhodococcus aurantiacus infection in mice. J Immunol. 2006;177:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Rubin EJ, Longo DL, Baden LR. Interleukin-6 Receptor Inhibition in Covid-19 - Cooling the Inflammatory Soup. N Engl J Med. 2021;384:1564-1565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 61. | Simpson RJ, Hammacher A, Smith DK, Matthews JM, Ward LD. Interleukin-6: structure-function relationships. Protein Sci. 1997;6:929-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 287] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 62. | Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30:30-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 301] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 63. | Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3039] [Cited by in RCA: 3402] [Article Influence: 226.8] [Reference Citation Analysis (0)] |
| 64. | Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY, Zhang T, Wang W, Wang YX, Jiang CL. NLRP3 Inflammasome Mediates Chronic Mild Stress-Induced Depression in Mice via Neuroinflammation. Int J Neuropsychopharmacol. 2015;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 65. | van den Berg DF, Te Velde AA. Severe COVID-19: NLRP3 Inflammasome Dysregulated. Front Immunol. 2020;11:1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 66. | Sharma RP, Tun N, Grayson DR. Depolarization induces downregulation of DNMT1 and DNMT3a in primary cortical cultures. Epigenetics. 2008;3:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9:1007-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 327] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 68. | Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front Immunol. 2020;11:1708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 768] [Cited by in RCA: 725] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 69. | Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1356] [Cited by in RCA: 1805] [Article Influence: 361.0] [Reference Citation Analysis (0)] |
| 70. | Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P; COVID-19 BioB Outpatient Clinic Study group, Benedetti F. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 967] [Cited by in RCA: 1013] [Article Influence: 202.6] [Reference Citation Analysis (0)] |
| 71. | Sriwastava S, Tandon M, Podury S, Prasad A, Wen S, Guthrie G, Kakara M, Jaiswal S, Subedi R, Elkhooly M, Lisak RP. COVID-19 and neuroinflammation: a literature review of relevant neuroimaging and CSF markers in central nervous system inflammatory disorders from SARS-COV2. J Neurol. 2021;268:4448-4478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 72. | Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, Dong W. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 444] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 73. | Adeloye D, Elneima O, Daines L, Poinasamy K, Quint JK, Walker S, Brightling CE, Siddiqui S, Hurst JR, Chalmers JD, Pfeffer PE, Novotny P, Drake TM, Heaney LG, Rudan I, Sheikh A, De Soyza A; International COVID-19 Airways Diseases Group. The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir Med. 2021;9:1467-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 74. | Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, Lu P, Weizman OE, Liu F, Dai Y, Szigeti-Buck K, Yasumoto Y, Wang G, Castaldi C, Heltke J, Ng E, Wheeler J, Alfajaro MM, Levavasseur E, Fontes B, Ravindra NG, Van Dijk D, Mane S, Gunel M, Ring A, Kazmi SAJ, Zhang K, Wilen CB, Horvath TL, Plu I, Haik S, Thomas JL, Louvi A, Farhadian SF, Huttner A, Seilhean D, Renier N, Bilguvar K, Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. bioRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 75. | Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF Jr, Sabeti P. Neuropathological Features of Covid-19. N Engl J Med. 2020;383:989-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 629] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 76. | Rezaeitalab F, Jamehdar SA, Sepehrinezhad A, Rashidnezhad A, Moradi F, Sadat Esmaeli Fard F, Hasanzadeh S, Etezad Razavi M, Gorji A, Sahab Negah S. Detection of SARS-coronavirus-2 in the central nervous system of patients with severe acute respiratory syndrome and seizures. J Neurovirol. 2021;27:348-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Liu JM, Tan BH, Wu S, Gui Y, Suo JL, Li YC. Evidence of central nervous system infection and neuroinvasive routes, as well as neurological involvement, in the lethality of SARS-CoV-2 infection. J Med Virol. 2021;93:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 78. | Lazzaroni MG, Piantoni S, Masneri S, Garrafa E, Martini G, Tincani A, Andreoli L, Franceschini F. Coagulation dysfunction in COVID-19: The interplay between inflammation, viral infection and the coagulation system. Blood Rev. 2021;46:100745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 79. | Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607-1608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1565] [Cited by in RCA: 1765] [Article Influence: 353.0] [Reference Citation Analysis (0)] |
| 80. | Gomes I, Karmirian K, Oliveira JT, Pedrosa CDSG, Mendes MA, Rosman FC, Chimelli L, Rehen S. SARS-CoV-2 infection of the central nervous system in a 14-month-old child: A case report of a complete autopsy. Lancet Reg Health Am. 2021;2:100046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92:699-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 689] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 82. | Siddiqui R, Mungroo MR, Khan NA. SARS-CoV-2 invasion of the central nervous: a brief review. Hosp Pract (1995). 2021;49:157-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Li CW, Syue LS, Tsai YS, Li MC, Lo CL, Tsai CS, Chen PL, Ko WC, Lee NY. Anosmia and olfactory tract neuropathy in a case of COVID-19. J Microbiol Immunol Infect. 2021;54:93-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 84. | Rossi ÁD, de Araújo JLF, de Almeida TB, Ribeiro-Alves M, de Almeida Velozo C, Almeida JM, de Carvalho Leitão I, Ferreira SN, da Silva Oliveira J, Alves HJ, Scheid HT, Faffe DS, Galliez RM, de Ávila RE, Resende GG, Teixeira MM; COVID-19 UFRJ Workgroup, da Costa Ferreira Júnior O, Castiñeiras TMPP, Souza RP, Tanuri A, Aguiar RS, Barroso SPC, Cardoso CC. Association between ACE2 and TMPRSS2 nasopharyngeal expression and COVID-19 respiratory distress. Sci Rep. 2021;11:9658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 85. | Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M, Smura T, Levanov L, Szirovicza L, Tobi A, Kallio-Kokko H, Österlund P, Joensuu M, Meunier FA, Butcher SJ, Winkler MS, Mollenhauer B, Helenius A, Gokce O, Teesalu T, Hepojoki J, Vapalahti O, Stadelmann C, Balistreri G, Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 1377] [Article Influence: 275.4] [Reference Citation Analysis (0)] |
| 86. | Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264-7275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 962] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 87. | Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, Chance R, Macaulay IC, Chou HJ, Fletcher RB, Das D, Street K, de Bezieux HR, Choi YG, Risso D, Dudoit S, Purdom E, Mill J, Hachem RA, Matsunami H, Logan DW, Goldstein BJ, Grubb MS, Ngai J, Datta SR. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 708] [Cited by in RCA: 727] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 88. | Bodnar B, Patel K, Ho W, Luo JJ, Hu W. Cellular mechanisms underlying neurological/neuropsychiatric manifestations of COVID-19. J Med Virol. 2021;93:1983-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 89. | Lin E, Lantos JE, Strauss SB, Phillips CD, Campion TR Jr, Navi BB, Parikh NS, Merkler AE, Mir S, Zhang C, Kamel H, Cusick M, Goyal P, Gupta A. Brain Imaging of Patients with COVID-19: Findings at an Academic Institution during the Height of the Outbreak in New York City. AJNR Am J Neuroradiol. 2020;41:2001-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 90. | Coolen T, Lolli V, Sadeghi N, Rovai A, Trotta N, Taccone FS, Creteur J, Henrard S, Goffard JC, Dewitte O, Naeije G, Goldman S, De Tiège X. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020;95:e2016-e2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 91. | Fagre A, Lewis J, Eckley M, Zhan S, Rocha SM, Sexton NR, Burke B, Geiss B, Peersen O, Kading R, Rovnak J, Ebel GD, Tjalkens RB, Aboellail T, Schountz T. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for reverse zoonosis to New World rodents. bioRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Zheng J, Wong LR, Li K, Verma AK, Ortiz ME, Wohlford-Lenane C, Leidinger MR, Knudson CM, Meyerholz DK, McCray PB Jr, Perlman S. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. 2021;589:603-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 378] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 93. | Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020;26:499-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 94. | Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 2020;296:E119-E120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 906] [Cited by in RCA: 1048] [Article Influence: 209.6] [Reference Citation Analysis (0)] |
| 95. | Perrin P, Collongues N, Baloglu S, Bedo D, Bassand X, Lavaux T, Gautier-Vargas G, Keller N, Kremer S, Fafi-Kremer S, Moulin B, Benotmane I, Caillard S. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur J Neurol. 2021;28:248-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 96. | Bossù P, Toppi E, Sterbini V, Spalletta G. Implication of Aging Related Chronic Neuroinflammation on COVID-19 Pandemic. J Pers Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |