Published online May 19, 2022. doi: 10.5498/wjp.v12.i5.708
Peer-review started: December 22, 2021
First decision: March 13, 2022
Revised: March 26, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 19, 2022
Processing time: 146 Days and 15.8 Hours
Major depressive disorder (MDD) tends to have a high incidence and high suicide risk. Electroconvulsive therapy (ECT) is currently a relatively effective treatment for MDD. However, the mechanism of efficacy of ECT is still unclear.
To investigate the changes in the amplitude of low-frequency fluctuations in specific frequency bands in patients with MDD after ECT.
Twenty-two MDD patients and fifteen healthy controls (HCs) were recruited to this study. MDD patients received 8 ECT sessions with bitemporal placement. Resting-state functional magnetic resonance imaging was adopted to examine regional cerebellar blood flow in both the MDD patients and HCs. The MDD patients were scanned twice (before the first ECT session and after the eighth ECT session) to acquire data. Then, the amplitude of low-frequency fluctuations (ALFF) was computed to characterize the intrinsic neural oscillations in different bands (typical frequency, slow-5, and slow-4 bands).
Compared to before ECT (pre-ECT), we found that MDD patients after the eighth ECT (post-ECT) session had a higher ALFF in the typical band in the right middle frontal gyrus, posterior cingulate, right supramarginal gyrus, left superior frontal gyrus, and left angular gyrus. There was a lower ALFF in the right superior temporal gyrus. Compared to pre-ECT values, the ALFF in the slow-5 band was significantly increased in the right limbic lobe, cerebellum posterior lobe, right middle orbitofrontal gyrus, and frontal lobe in post-ECT patients, whereas the ALFF in the slow-5 band in the left sublobar region, right angular gyrus, and right frontal lobe was lower. In contrast, significantly higher ALFF in the slow-4 band was observed in the frontal lobe, superior frontal gyrus, parietal lobe, right inferior parietal lobule, and left angular gyrus.
Our results suggest that the abnormal ALFF in pre- and post-ECT MDD patients may be associated with specific frequency bands.
Core Tip: In this study, we explored changes in the intrinsic neural activity in major depressive disorder (MDD) patients who underwent electroconvulsive therapy (ECT) procedures by calculating amplitude of low-frequency fluctuations (ALFF) values for different bands. Compared to pre-ECT values, the ALFF in the slow-5 band was significantly increased in the right limbic lobe, cerebellum posterior lobe, right middle orbitofrontal gyrus, and frontal lobe in post-ECT patients, whereas the ALFF in the slow-5 band in the left sublobar region, right angular gyrus, and right frontal lobe was lower. In contrast, significantly higher ALFF in the slow-4 band was observed in the frontal lobe, superior frontal gyrus, parietal lobe, right inferior parietal lobule, and left angular gyrus. Our findings demonstrated that the ALFF alterations in post-ECT patients are dependent on specific frequency bands. These results may help us to understand more fully the potential therapeutic mechanisms of ECT for MDD patients.
- Citation: Li XK, Qiu HT, Hu J, Luo QH. Changes in the amplitude of low-frequency fluctuations in specific frequency bands in major depressive disorder after electroconvulsive therapy. World J Psychiatry 2022; 12(5): 708-721
- URL: https://www.wjgnet.com/2220-3206/full/v12/i5/708.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i5.708
Depression is a common mental illness with a high recurrence rate and risk of suicide. The main clinical manifestations are persistent depression, lack of interest in and pleasure from normal activities, severe grief, and even stupor[1-3]. According to the latest report released by the World Health Organization in 2017[4], approximately 322 million people suffer from depression worldwide. The prevalence rate is 4.4%, and more than 1 million people commit suicide every year due to depression. The lifetime prevalence of major depression is 16.2%. Antidepressants and behavioral therapies are the most commonly used treatments, but as many as one in three patients remain unresponsive to initial treatment[5,6]. With rapid and high response rates, electroconvulsive therapy (ECT) is usually used when other treatments fail. It is particularly important in suicidal, psychotic, or catatonic depression[7]. Although clinical efficacy has suggested that ECT is the most effective treatment for major depressive disorder (MDD), the mechanism of action of ECT is unclear[8], and little is known about the relationship between symptom improvement and the neurobiological effects associated with ECT. Some neurobiological effects are not necessary for therapeutic effects during ECT[9], and the potential adverse reactions require its clinical application to be very cautious and limited.
Antidepressant treatment response studies have reported changes in gray matter volumes and cortical thickness associated with improvement in MDD patients[10-12]. For ECT treatment, some changes have been reported in the structure of the gray matter in MDD patients. Yrondi et al[13] reported that gray matter changes occurred after several ECT sessions. Some studies have confirmed that ECT can also induce changes in the hippocampal formation and other brain regions[14-18]. Abbott et al[14] found a significant increase in the volume of the right hippocampus. Bouckaert et al[15] found that the caudate nucleus increased in volume. ECT also had vital effects on the dentate gyrus[19].
In addition to measuring alterations in brain structure in patients with MDD after ECT treatment, functional magnetic resonance imaging (fMRI) also has been used to detect changes in brain activity. Beall et al[20] adopted task fMRI to find that remission after ECT for MDD is connected to decreased activation in emotional regulation but increased resting connectivity. Abbott et al[21] used resting-state fMRI to measure the variations in MDD patients after multiple ECT sessions. This research reported that functional connectivity increased in two networks: (1) Posterior default mode (p_DM) and the dorsomedial prefrontal cortex (DMPFC); and (2) The left dorsal lateral prefrontal cortex (l_DLPFC) and p_DM. The fronto-temporal connectivity and the functional connectivity strength of the left angular gyrus in MDD were also found to be responses to ECT[7,22]. Redlich et al[9] used fMRI to find an increase in amygdala activity in patients with ECT, whereas activity after ECT was significantly reduced. Sinha et al[23] applied graph theory to fMRI data and revealed significant differences in the brain regions of patients with depression before and after ECT. To assess the alterations in depressive patients with ECT, data-driven methods also have been adopted[24,25]. However, there were still no consistent antidepressant responses observed in previous studies[26].
Many studies have revealed different functional activities of the brain since rs-fMRI was adopted by Biswal et al[27] to study spontaneous brain activity. To date, most studies have examined spontaneous low-frequency oscillation (LFO) activities at the frequency band of 0.01-0.1 Hz. However, some studies observed that neuronal oscillations are distributed linearly on the natural logarithmic scale and that independent frequency bands are generated by distinct oscillators with specific properties and physiological functions[28-30]. Moreover, neighboring frequency bands within the same neuronal network may compete or interact with each other[31]. The rs-fMRI LFO can be decomposed into the following frequency bands: slow-6 (0-0.01 Hz), slow-5 (0.01-0.027 Hz), slow-4 (0.027-0.073 Hz), slow-3 (0.073-0.198 Hz), and slow-2 (0.198-0.25 Hz). Zuo et al[32] found that the low-frequency amplitudes in the slow-5 band are smaller than those in the slow-4 band in the basal ganglia, thalamus, precuneus, and so on. Meanwhile, many studies have presented different measures of the nature of rs-fMRI. Among them, the amplitude of low-frequency fluctuations (ALFF) is a reliable representation of whole-brain rs-fMRI signals[33-35]. ALFF has been widely adopted because it directly correlates with the intensity of spontaneous neural activity in the resting state with regard to energy metabolism[36,37]. Frequency-dependent changes in ALFF have already been used to investigate some brain network mechanisms and disease phenotypes, such as chronic schizophrenia, late-onset depression, chronic tinnitus, and social anxiety disorder[28,30-40]. These studies showed that intrinsic functional activities of brain networks are correlated with different frequency bands.
In the current study, we investigated the alterations of the ALFF at different frequency bands (slow-5 (0.01-0.027 Hz), slow-4 (0.027-0.08 Hz)) in MDD patients before and after ECT. Then, the differences before and after ECT were explored.
Twenty-two inpatients (14 females and 8 males, aged 34.4 ± 10.1, range 21-55 years old) who had been diagnosed with major depression at the Mental Health Center, the First Affiliated Hospital of Chongqing Medical University were recruited. Fifteen gender- and age-matched healthy controls (HCs) (10 females and 5 males, aged 36.1 ± 9.4, range 21-55 years old) were recruited to participate in the investigation. All patients underwent blood tests, electrocardiogram, electroencephalogram, X-ray, and physical examination before ECT[41]. The study was approved by the local ethics committee of Chongqing Medical University accordance with the ethical standards laid down in the Declaration of Helsinki. Each patient gave written informed consent.
The inclusion criteria for the MDD patients included the following: (1) Agreeing to receive ECT; (2) meeting the unipolar major depressive diagnostic criteria according to the Diagnostic Statistical Manual-IV[42] (two trained senior psychiatrists carried out the structured clinical interviews and made the diagnoses); (3) no contraindications to MRI scanning; (4) Hamilton Depression Scale (HAMD)[43] score greater than 21; and (5) age between sixteen and sixty years. The exclusion criteria for the patients were as follows: (1) Severe somatic disease; (2) substance abuse; (3) pregnancy or lactation; (4) depression with other mental illnesses[44]; and (5) exposure to ECT or mood stabilizers in the preceding one month. HCs had no history of their own or family mental illness.
The Thymatron DGx (Somatics LLC, Lake Bluff, IL, United States) was used to perform the ECT for all 22 MDD patients at the Mental Health Center of the First Affiliated Hospital of Chongqing Medical University. Each patient received eight ECT treatments within three weeks. Specifically, the procedures were administered 3 times per week (Monday, Wednesday, and Friday mornings) for the first two weeks and 2 times per week (Monday and Friday mornings) for the 3rd week. The time and frequency of ECT treatment were the same for all patients. Before ECT, water and food intake were restricted for the patients beginning at midnight. Before receiving the first ECT and after the eighth ECT, all patients were administered MRI scans, fMRI scans, and HAMD scores. Antidepressants and antipsychotics were not used during the ECT treatment period.
In every ECT process, the patients received anesthesia with sodium thiopental (3.0-5.0 mg/kg) and succinylcholine (0.5-1.0 mg/kg). In this study, the ECT electrodes were placed in the bitemporal position. According to the seizure response and adverse reactions (if any) during ECT, the electrical stimulation intensity was individually accommodated. In the first ECT, the seizure threshold was measured by the minimum electrical dose that elicited a seizure for at least 25 s[45]. Each time the initial dose failed to cause seizures, the output charge of the 5% ECT device was increased, and the patient was re-stimulated after 30 s. The patient underwent up to three electrical stimulations at one ECT. If the seizure threshold measurements failed in the first session, stimulation with 2 times the last dose was performed in the next session. To achieve a therapeutic effect and reduce side effects, the electrical dosage was set at 1.5-2 times the seizure threshold in subsequent ECT treatment sessions according to the extent of seizure. If the clinician determined that the clinical symptoms of depression had not been adequately improved after eight sessions, we continued the ECT course for the patients to up to 12 ECT sessions. For the sake of the comparison, each patient underwent MRI scanning after the eighth ECT treatment.
Depression symptoms of the patients were measured by the 24-item HAMD Rating Scale on the same day as brain scanning. The psychiatrists performed the clinical assessments of depression for all patients twice. The first time was within 24 h before the 1st ECT treatment (pre-ECT). The second time was within 24 h after the 8th ECT treatment (post-ECT).
Image data were collected with the MRI scanner system (3.0-T, GE Signa) at the Mental Health Center of Chongqing Medical University. Both the HCs and the MDD patients were instructed to relax, stay awake, keep their eyes closed, and avoid thinking during the scanning process. The resting-state functional images were collected with an echo planar imaging sequence. The image parameters were recorded as follows: repetition time/echo time, 2 s/30 milliseconds; field of view, 240 mm × 240 mm; data matrix, 64 × 64; flip angle, 90°; slices, 30; slice thickness, 5 mm; volumes, 200. The scan lasted 6 min and 50 s per scan.
Using the statistical parametric mapping software platform, functional image data preprocessing was carried out by DPABI (Data Processing Assistant for rs-fMRI, http://www.restfmri.net, by YAN Chao-Gan et al[46]). The preprocessing procedure on the rs-fMRI data included the following: (1) We abandoned the first 10 volumes because the signals of the participants’ adaptation to the scanning environment were unstable. Then, the remaining 190 volumes were retained; (2) Head motion correction was performed. Subjects with a head motion of more than 1.5 mm in any direction of the 3 coordinate axes (x, y, and z) or angular motion of more than 1.5° were excluded from this study; (3) Considering the delay of the acquisition, slice timing was conducted. There were 30 Layers in a scan. The odd-numbered layers started and were followed by the even-numbered layers; (4) Spatial normalization was carried out. The fMRI images were registered to the standard Montreal Neurological Institute space and were resampled to 3 mm × 3 mm; (5) We adopted the Gaussian kernel with full-width at half-maximum of eight mm to fulfill the spatial smoothing; and (6) The linear trend of the functional image data was removed. Finally, the normalized image data were subjected to bandpass filtering with frequency ranges of 0.01-0.08 Hz.
A fast Fourier transform can be used to obtain the frequency domain for the time series signal. Moreover, we adopted the average square root of the power spectrum to denote the ALFF value of a given voxel. Then, the intensity of spontaneous LFO can be measured by the ALFF. In the present study, the ALFF was performed by the REST software toolkit (Resting-State fMRI Data Analysis)[47] in two different frequency ranges (slow-5: 0.01-0.027 Hz, slow-4: 0.027-0.073 Hz) separately. The ALFF of the typical band (0.01-0.08 Hz) was also computed for comparative purposes.
To explore the changes in ALFF at different frequency bands before and after ECT, the effects of ECT treatment on MDD and frequency alterations were examined by REST[47]. Two-sample two-sided t-tests were adopted to assess the differences between the MDD group and the HC group. We applied paired t-tests to measure the ALFF alterations before and after ECT. The statistical maps were corrected by multiple comparisons with a significance level of P < 0.05 (bilateral) using AlphaSim as well as a height threshold of P < 0.01 and a minimum cluster size = 85. To find the difference between pre-ECT and post-ECT with the clinical measure, the significant alterations of ALFF values in the regions of interest (ROIs) in the brain were calculated. Moreover, the coordinates (x, y, and z) of the peak density of the ROIs were described in the ALFF map.
In the present study, twenty-two MDD patients (14 women, 8 men, right-handed, 34.4 ± 10.1 years old) were recruited from the Inpatient Department of Psychiatry at the First Affiliated Hospital of Chongqing Medical University. Among them, 21 patients took at least one antidepressant, and the remaining patients were receiving no medication. We adopted the 24-item HAMD to examine all MDD patients. The average score for the patients pre-ECT was 30.59 ± 4.35 (as seen in Table 1).
After 8 ECT sessions, the depression symptoms improved greatly for all patients (t21 = 12.61, P < 0.0001; paired t test). According to the clinical results, the HAMD scores of all 22 patients before and after ECT decreased by more than 50%. The HAMD scores of 10 patients were lower than 7. Therefore, they were considered to be remitted.
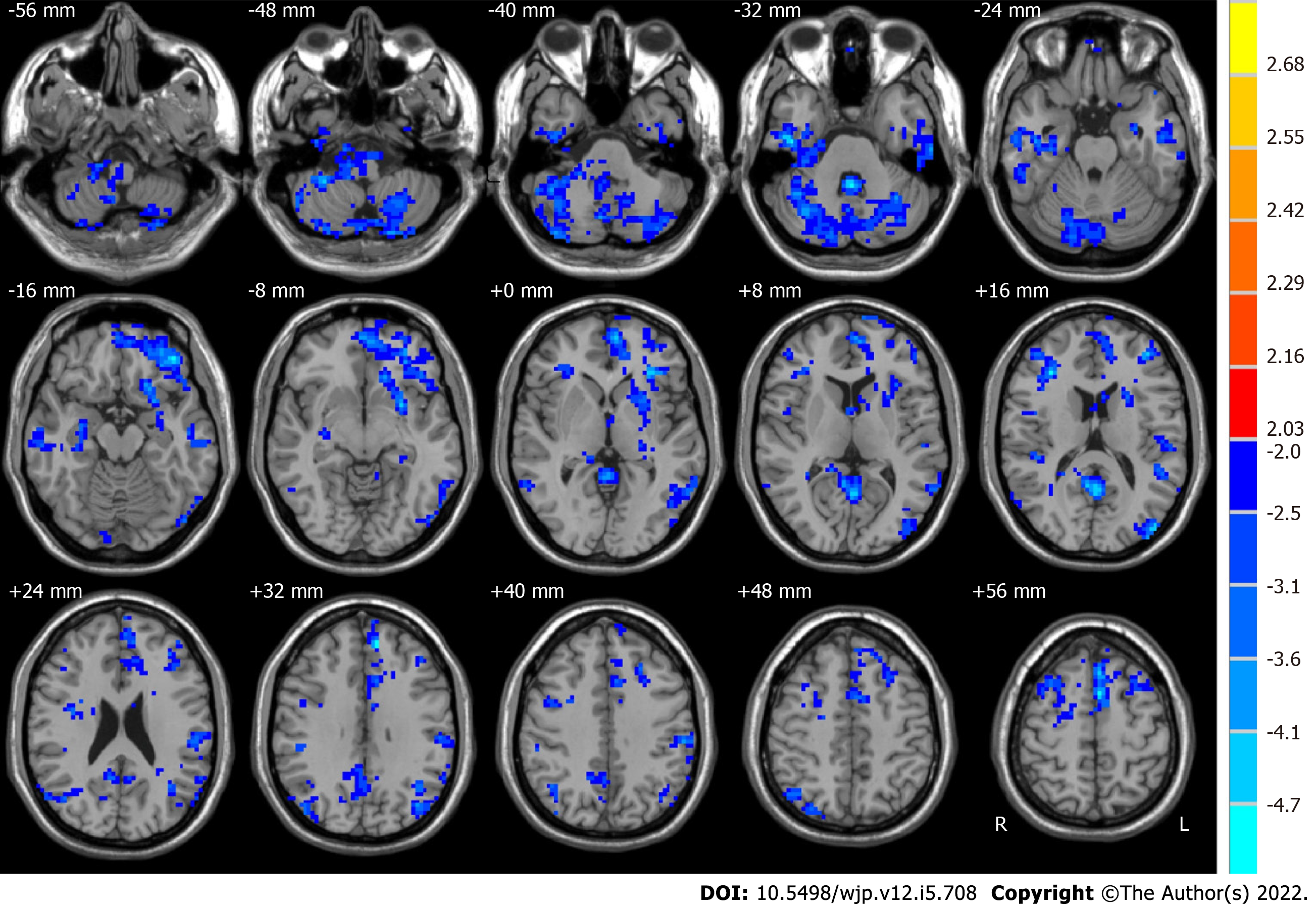
Compared with HCs, pre-ECT MDD patients had significant alterations in ALFF values in some brain regions (as shown in Figure 1). The typical frequency band (0.01-0.08 Hz) is reported as follows. The ALFF values in the brain areas in pre-ECT patients were lower than those in HCs, which included the posterior lobe of the cerebellum, the cerebellar tonsil, inferior semilunar lobule, temporal lobe, inferior temporal gyrus, frontal lobe, superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, limbic lobe, parietal lobe, occipital lobe, and inferior parietal lobule.
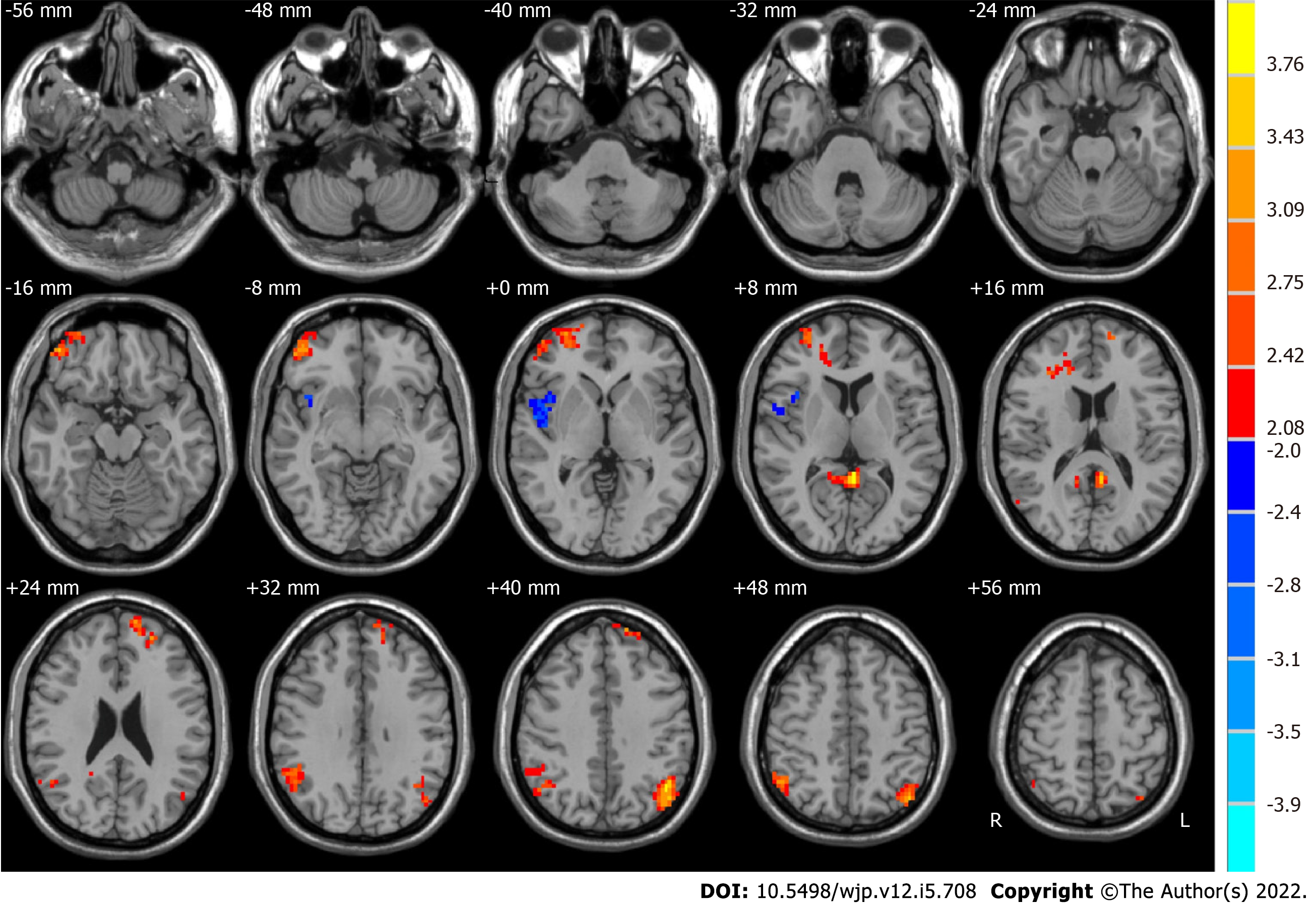
We used paired t-tests to identify differences, which are shown in Figure 2. Moreover, Table 2 shows the group differences in ALFF in the typical frequency band between before ECT and after ECT in patients with MDD. We found that the ALFF of the right middle frontal gyrus, posterior cingulate, right supramarginal gyrus, left superior frontal gyrus, and left angular gyrus increased significantly in MDD patients after ECT. However, that of the right superior temporal gyrus decreased significantly. In our study, Monte Carlo simulations were used to conduct the multiple comparison correction for all the statistical maps with a significance level of P < 0.05. The individual voxel P is lower than 0.05, and the cluster size is larger than 2079 mm3[48].
| Brain region | Side | BA | MNI coordinates | Voxels | t values | ||
| x | y | z | |||||
| Before ECT < after ECT | |||||||
| Middle frontal gyrus | R | 10, 11 | 30 | 54 | 3 | 262 | 3.5547 |
| Posterior cingulate | R and L | 29, 30 | -3 | -45 | 9 | 92 | 4.0993 |
| Supramarginal gyrus | R | 7, 39, 40 | 51 | -48 | 30 | 187 | 3.7424 |
| Superior frontal gyrus | L | 9, 10 | -12 | 63 | 24 | 96 | 3.4006 |
| Angular gyrus | L | 39, 40 | -42 | -72 | 42 | 193 | 4.0957 |
| Before ECT > after ECT | |||||||
| Superior temporal gyrus | R | 13, 22, 47 | 54 | -3 | 0 | 114 | -3.1055 |
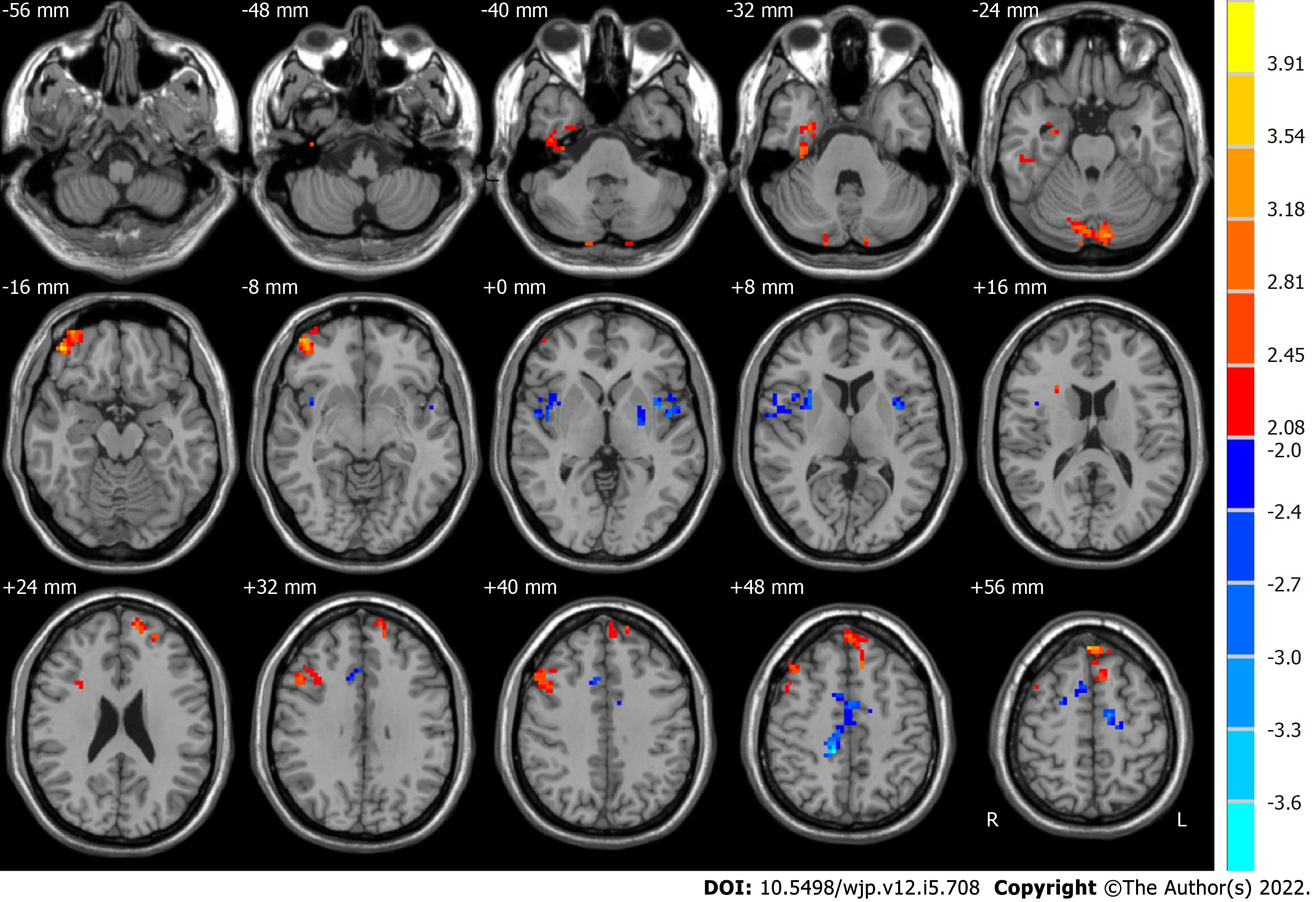
The post-ECT MDD patients, relative to the pre-ECT MDD patients, demonstrated significantly higher ALFF in the slow-5 band in the right limbic lobe, bilateral cerebellum posterior lobe, right middle orbitofrontal gyrus, and frontal lobe, whereas they had lower ALFF in the slow-5 band in the left sublobar region, right frontal lobe, and right angular gyrus, as shown in Figure 3 and Table 3.
| Brain region | Side | BA | MNI coordinates | Voxels | t values | ||
| x | y | z | |||||
| Before ECT < after ECT | |||||||
| Limbic lobe | R | 36 | 33 | -21 | -30 | 105 | 3.0807 |
| Cerebellum posterior lobe | R and L | 18 | -12 | -82 | -27 | 116 | 3.4515 |
| Frontal_Mid_Orb_R | R | 11 | 45 | 48 | -15 | 187 | 3.7424 |
| Frontal lobe | R | 22 | 48 | 18 | 36 | 147 | 3.3909 |
| Frontal lobe | R and L | 6, 8, 9, 10 | -6 | 48 | 54 | 233 | 4.2748 |
| Before ECT > after ECT | |||||||
| Sublobar | L | 22 | -42 | 3 | 3 | 111 | -4.015 |
| Angular gyrus | R | 13 | 39 | 12 | -3 | 124 | -3.1741 |
| Frontal lobe | R | 24 | 12 | -36 | 48 | 228 | -3.7067 |
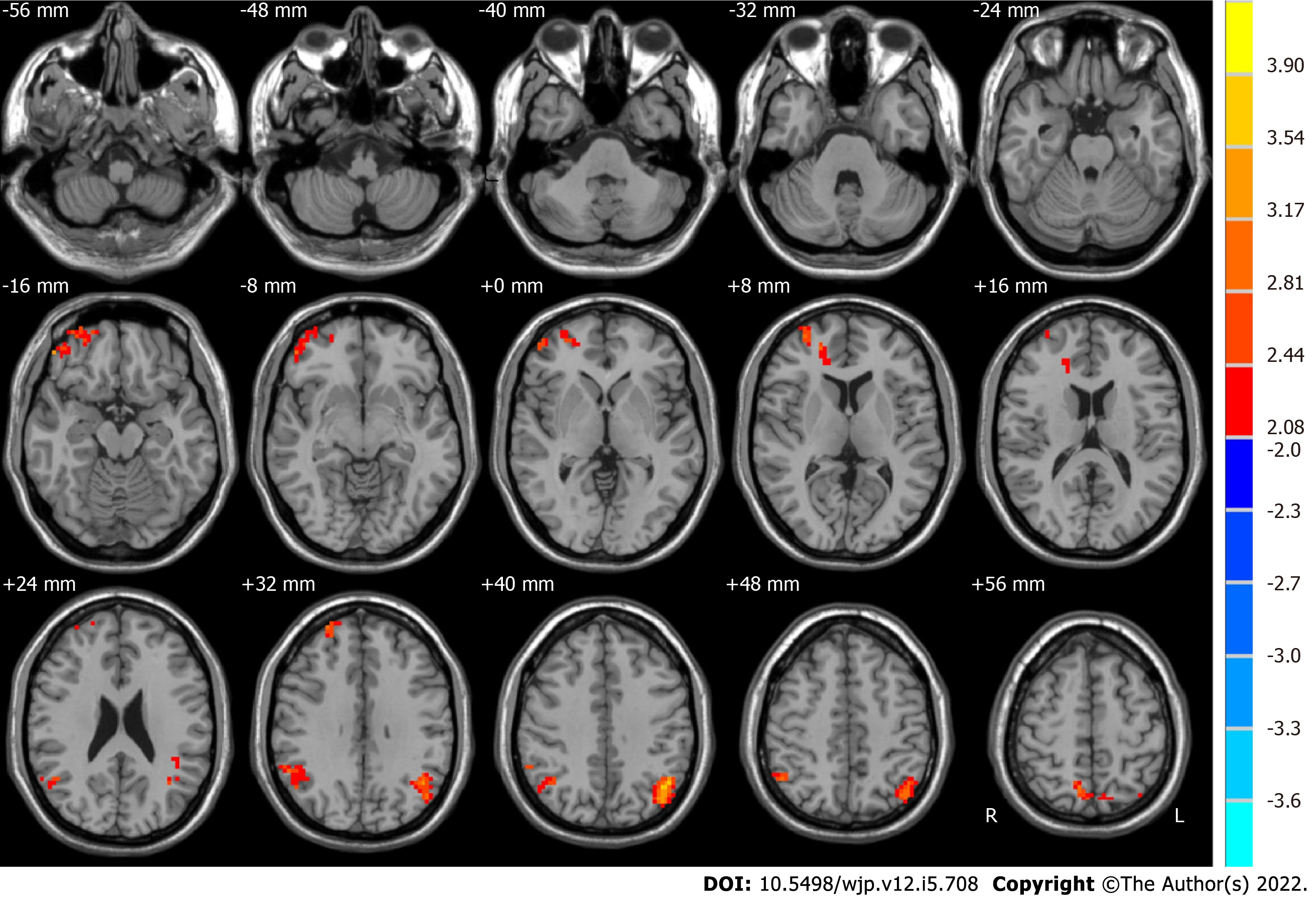
We measured the ALFF of two frequency bands (slow-4 and slow-5) in the groups after and before ECT. Significant difference maps using paired t-tests are shown in Figures 3 and 4.
Compared with the pre-ECT patients, the post-ECT patients showed significantly higher ALFF in the slow-4 band in the frontal lobe, superior frontal gyrus, bilateral parietal lobe, right inferior parietal lobule, and left angular gyrus (as shown in Figure 4 and Table 4).
| Brain region | Side | BA | MNI coordinates | Voxels | t values | ||
| x | y | z | |||||
| Before ECT < after ECT | |||||||
| Frontal lobe, superior frontal gyrus | R and L | 9, 10, 11, 47 | 51 | 45 | -15 | 243 | 3.3179 |
| Parietal lobe, inferior parietal lobule | R | 39, 40 | 57 | -60 | 21 | 131 | 2.8756 |
| Parietal lobe, angular gyrus | L | 39, 40, 19, 7 | -45 | -63 | 36 | 256 | 4.1322 |
| Parietal lobe | R | 7 | 15 | -69 | 63 | 129 | 3.9572 |
To improve MDD patients’ depressive symptoms, several treatments, including ECT and transcranial magnetic stimulation, can be applied[49]. There have also been some studies using resting-state fMRI to assess antidepressant treatment response[49]. Among these methods, ECT is an effective therapy for MDD patients. Abbott et al[21] investigated the differences between ECT remitters and nonremitters and suggested that thane increase in functional connectivity between p_DM network areas and the l_DLPFC is a potential biomarker of recovery from depressive disorder patients. Kong et al[34] used regional homogeneity and ALFF to measure changes in regional resting state function after ECT in elderly MDD patients. Their results demonstrated that ECT affected regional resting-state brain function in these patients. In this study, we investigated spontaneous neural activity changes in ALFF at different frequency bands (typical frequency, slow-5, and slow-4 bands) in patients with MDD before and after ECT. We found that post-ECT, compared to pre-ECT, patients showed significant alterations in ALFF within the frequency bands in some brain areas. Our findings further showed that the ALFF alterations in post-ECT patients were dependent on specific frequency bands.
Compared with HCs, MDD patients showed significant differences in ALFF with a frequency band of 0.01-0.08 Hz in numerous brain regions[50-53]. The present study also found that pre-ECT patients had lower ALFF values than HCs in widely distributed brain areas, including the cerebellum posterior lobe, cerebellar tonsil, inferior semilunar lobule, temporal lobe, inferior temporal gyrus, frontal lobe, superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, limbic lobe, parietal lobe, occipital lobe, and inferior parietal lobule. In the comparison of ALFF in the typical frequency band in MDD patients before and after ECT, we found that the right middle frontal gyrus, posterior cingulate, right supramarginal gyrus, left superior frontal gyrus, and left angular gyrus increased significantly after ECT. However, the right superior temporal gyrus decreased significantly. The results were similar to those of previous studies[34,41].
Baria et al[54] found that the lower frequency bands had higher power. Thus, subcortical structures with higher frequency bands usually have less power. In contrast, the brain cortexes, including the prefrontal and parietal cortexes, exhibit higher power[55]. In addition, Zuo et al[32] and Han et al[56] demonstrated that the regions of the default mode network are more active in the slow-5 band, whereas the basal ganglia are dominant in the slow-4 band. In this study, compared to pre-ECT patients, significantly higher ALFF was found in the slow-5 band in post-ECT patients in the right limbic lobe, cerebellum posterior lobe, right middle orbitofrontal gyrus, and frontal lobe. The sublobar region, angular gyrus, and frontal lobe were lower. Significantly higher ALFF in the slow-4 band was also observed in the frontal lobe, superior frontal gyrus, parietal lobe, right inferior parietal lobule, and left angular gyrus.
Many abnormal regions associated with the frontal lobe in MDD have been observed in previous studies[57,58]. Recently, a multisite rs-fMRI study reported MDD patients with hypoactivity in the medial orbitofrontal region[59]. We found a significantly higher ALFF both at the slow-5 band and slow-4 band in the frontal lobe post-ECT. As a result, abnormal activities in these brain areas might be normalized after ECT treatment[60], which might have improved the symptoms of depression. This may be considered evidence of the effectiveness of ECT for MDD.
Alterations of the limbic lobe have important effects in MDD patients[12,61]. For example, the amygdala and hippocampus are usually thought of as potential biomarkers for major depression. Compared to HCs, MDD patients illustrated decreased ALFF values in the limbic regions[50,51]. Jiao et al[62] and Liu et al[63] also demonstrated that MDD patients had abnormalities in prefrontal-limbic emotional processing. The results of the present study were consistent with these conclusions. Moreover, post-ECT patients, relative to pre-ECT patients, showed increased ALFF in the slow-5 band in the limbic lobe. This feature may also indicate an effective response to ECT treatment.
Recently, more interest has been drawn to the pathophysiology of the cerebellum in MDD[62,64,65]. Previous studies reported decreased ALFF values in the cerebellum in MDD patients[66,67]. Moreover, Zhou et al[66] concluded that reduced activity in the cerebellum in MDD might be a biomarker for patients. In the current study, compared with pre-ECT patients, post-ECT patients exhibited a significant increase in ALFF in the slow-5 band in the cerebellum posterior lobe. Thus, the hypothesis of Zhou et al[66] was supported by our results.
Post-ECT patients had lower ALFF values than pre-ECT patients for the angular gyrus in the slow-5 band. In contrast, the ALFF value was significantly increased in the slow-4 band. This was an interesting finding in the current study. The angular gyrus might play an important role in many functions, such as memory retrieval, spatial cognition, and semantic processing[68]. Previous studies reported significantly increased spontaneous brain activity in the angular gyrus in MDD patients[69,70]. The alterations of ALFF in the angular gyrus at the slow-5 band in our findings can be viewed as consistent with these studies. Our study also demonstrated that the effectiveness of ECT treatment for MDD may be partly proven by significantly decreased ALFF in the slow-5 band.
Compared with HCs, decreased ALFF was exhibited in the parietal lobe in MDD patients[50,71]. In this study, the results of the comparison between HCs and pre-ECT patients were similar to this point. In addition, we found that ALFF increased significantly in the parietal lobe at the slow-4 band in post-ECT patients compared to pre-ECT patients. However, there was no difference in the slow-5 band.
There are some limitations of our study. First, the MDD patients were scanned only twice (before the first ECT session and after the eighth ECT session). To observe more alterations in spontaneous neural activity, more scans should be carried out during ECT treatment. Second, different MDD patients had divergent responses in speed and effectiveness in the practical clinic. Some unresponsive patients may receive more than eight ECT sessions[16]. For comparison, all patients were scanned after the eighth ECT session in this study. To find a more reliable relationship between ALFF and clinical symptoms, a more detailed longitudinal study should be performed pre- and post-ECT. Finally, the number of MDD patients and control subjects in the present study was relatively small. The multiple comparison tests failed due to the insufficient number of subjects. A larger sample would help us to achieve more robust results.
In this study, we explored changes in the intrinsic neural activity in MDD patients who underwent ECT procedures by calculating ALFF values for different bands (typical frequency, slow-5, and slow-4 bands). For post-ECT patients, relative to pre-ECT patients, significantly higher ALFF in the slow-5 band was observed in the right limbic lobe, cerebellum posterior lobe, right middle orbitofrontal gyrus, and frontal lobe. The ALFF of the left sublobar region, right angular gyrus, and right frontal lobe were lower. Significantly higher ALFF in the slow-4 band was also observed in the frontal lobe, superior frontal gyrus, parietal lobe, right inferior parietal lobule, and left angular gyrus. Our findings demonstrated that the ALFF alterations in post-ECT patients are dependent on specific frequency bands. These results may help us to understand more fully the potential therapeutic mechanisms of ECT for MDD patients. In future work, we will recruit more patients and health controls to participate this investigation. More scans will be carried out for participants to obtain more robust results. The changes in cognitive function will also be monitored.
The mechanism of efficacy of electroconvulsive therapy (ECT) for major depressive disorder (MDD) is still unclear. Intrinsic functional activities of brain networks are correlated with different frequency bands.
The amplitude of low-frequency fluctuations (ALFF) at different frequency bands (slow-5 (0.01-0.027 Hz), slow-4 (0.027-0.08 Hz)) in MDD patients may be changed regularly before and after ECT.
To investigate the alterations of the amplitude of low-frequency fluctuations in slow-5 (0.01-0.027 Hz) and slow-4 (0.027-0.08 Hz) in patients with MDD after ECT.
Resting-state functional magnetic resonance imaging and the intrinsic neural oscillations in different bands were adopted to analyze the changes in MDD patients before and after ECT.
Compared to before ECT, we found that MDD patients after ECT had a higher ALFF in the typical band in some regions such as the right middle frontal gyrus and posterior cingulate. Moreover, there were other changes in slow-5 band and slow-4 band.
Our findings showed that the ALFF alterations in post-ECT patients were dependent on specific frequency bands.
These changes may reveal some mechanism of efficacy of electroconvulsive therapy for major depressive disorder.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chaudhury S, India; Kar SK, India S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 647] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 2. | Gong W, Liao W, Fang C, Liu Y, Xie H, Yi F, Huang R, Wang L, Zhou J. Analysis of Chronic Mild Stress-Induced Hypothalamic Proteome: Identification of Protein Dysregulations Associated With Vulnerability and Resiliency to Depression or Anxiety. Front Mol Neurosci. 2021;14:633398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | de la Peña FR, Cruz-Fuentes C, Palacios L, Girón-Pérez MI, Medina-Rivero E, Ponce-Regalado MD, Alvarez-Herrera S, Pérez-Sánchez G, Becerril-Villanueva E, Maldonado-García JL, Jiménez-Martínez MC, Pavón L. Serum levels of chemokines in adolescents with major depression treated with fluoxetine. World J Psychiatry. 2020;10:175-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | WHO. Depression and Other Common Mental Disorders: Global Health Estimates. In: World Health Organization 2017, Geneva, 2017. |
| 5. | Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 2693] [Article Influence: 141.7] [Reference Citation Analysis (0)] |
| 6. | Aryutova K, Paunova R, Kandilarova S, Todeva-Radneva A, Stoyanov D. Implications from translational cross-validation of clinical assessment tools for diagnosis and treatment in psychiatry. World J Psychiatry. 2021;11:169-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 7. | Wei Q, Bai T, Chen Y, Ji G, Hu X, Xie W, Xiong Z, Zhu D, Wei L, Hu P, Yu Y, Wang K, Tian Y. The Changes of Functional Connectivity Strength in Electroconvulsive Therapy for Depression: A Longitudinal Study. Front Neurosci. 2018;12:661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Argyelan M, Lencz T, Kaliora S, Sarpal DK, Weissman N, Kingsley PB, Malhotra AK, Petrides G. Subgenual cingulate cortical activity predicts the efficacy of electroconvulsive therapy. Transl Psychiatry. 2016;6:e789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Redlich R, Bürger C, Dohm K, Grotegerd D, Opel N, Zaremba D, Meinert S, Förster K, Repple J, Schnelle R, Wagenknecht C, Zavorotnyy M, Heindel W, Kugel H, Gerbaulet M, Alferink J, Arolt V, Zwanzger P, Dannlowski U. Effects of electroconvulsive therapy on amygdala function in major depression - a longitudinal functional magnetic resonance imaging study. Psychol Med. 2017;47:2166-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Sheline YI, Disabato BM, Hranilovich J, Morris C, D'Angelo G, Pieper C, Toffanin T, Taylor WD, MacFall JR, Wilkins C, Barch DM, Welsh-Bohmer KA, Steffens DC, Krishnan RR, Doraiswamy PM. Treatment course with antidepressant therapy in late-life depression. Am J Psychiatry. 2012;169:1185-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Li XK, Qiu HT. Current progress in neuroimaging research for the treatment of major depression with electroconvulsive therapy. World J Psychiatry. 2022;12:128-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 12. | Amidfar M, Quevedo J, Z Réus G, Kim YK. Grey matter volume abnormalities in the first depressive episode of medication-naïve adult individuals: a systematic review of voxel based morphometric studies. Int J Psychiatry Clin Pract. 2021;25:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Yrondi A, Nemmi F, Billoux S, Giron A, Sporer M, Taib S, Salles J, Pierre D, Thalamas C, Rigal E, Danet L, Pariente J, Schmitt L, Arbus C, Péran P. Grey Matter changes in treatment-resistant depression during electroconvulsive therapy. J Affect Disord. 2019;258:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Abbott CC, Jones T, Lemke NT, Gallegos P, McClintock SM, Mayer AR, Bustillo J, Calhoun VD. Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry. 2014;4:e483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | Bouckaert F, De Winter FL, Emsell L, Dols A, Rhebergen D, Wampers M, Sunaert S, Stek M, Sienaert P, Vandenbulcke M. Grey matter volume increase following electroconvulsive therapy in patients with late life depression: a longitudinal MRI study. J Psychiatry Neurosci. 2016;41:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Oltedal L, Narr KL, Abbott C, Anand A, Argyelan M, Bartsch H, Dannlowski U, Dols A, van Eijndhoven P, Emsell L, Erchinger VJ, Espinoza R, Hahn T, Hanson LG, Hellemann G, Jorgensen MB, Kessler U, Oudega ML, Paulson OB, Redlich R, Sienaert P, Stek ML, Tendolkar I, Vandenbulcke M, Oedegaard KJ, Dale AM. Volume of the Human Hippocampus and Clinical Response Following Electroconvulsive Therapy. Biol Psychiatry. 2018;84:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 17. | Sartorius A, Demirakca T, Böhringer A, Clemm von Hohenberg C, Aksay SS, Bumb JM, Kranaster L, Ende G. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016;26:506-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Gbyl K, Videbech P. Electroconvulsive therapy increases brain volume in major depression: a systematic review and meta-analysis. Acta Psychiatr Scand. 2018;138:180-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 19. | Takamiya A, Plitman E, Chung JK, Chakravarty M, Graff-Guerrero A, Mimura M, Kishimoto T. Acute and long-term effects of electroconvulsive therapy on human dentate gyrus. Neuropsychopharmacology. 2019;44:1805-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Beall EB, Malone DA, Dale RM, Muzina DJ, Koenig KA, Bhattacharrya PK, Jones SE, Phillips MD, Lowe MJ. Effects of electroconvulsive therapy on brain functional activation and connectivity in depression. J ECT. 2012;28:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Abbott CC, Lemke NT, Gopal S, Thoma RJ, Bustillo J, Calhoun VD, Turner JA. Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Front Psychiatry. 2013;4:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Leaver AM, Wade B, Vasavada M, Hellemann G, Joshi SH, Espinoza R, Narr KL. Fronto-Temporal Connectivity Predicts ECT Outcome in Major Depression. Front Psychiatry. 2018;9:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Sinha P, Reddy RV, Srivastava P, Mehta UM, Bharath RD. Network neurobiology of electroconvulsive therapy in patients with depression. Psychiatry Res Neuroimaging. 2019;287:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, Schwarzbauer C. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci U S A. 2012;109:5464-5468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 25. | Leaver AM, Espinoza R, Pirnia T, Joshi SH, Woods RP, Narr KL. Modulation of intrinsic brain activity by electroconvulsive therapy in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Leaver AM, Vasavada M, Joshi SH, Wade B, Woods RP, Espinoza R, Narr KL. Mechanisms of Antidepressant Response to Electroconvulsive Therapy Studied With Perfusion Magnetic Resonance Imaging. Biol Psychiatry. 2019;85:466-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6985] [Cited by in RCA: 7231] [Article Influence: 241.0] [Reference Citation Analysis (0)] |
| 28. | Yu R, Chien YL, Wang HL, Liu CM, Liu CC, Hwang TJ, Hsieh MH, Hwu HG, Tseng WY. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum Brain Mapp. 2014;35:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 188] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 29. | Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3926] [Cited by in RCA: 4203] [Article Influence: 200.1] [Reference Citation Analysis (0)] |
| 30. | Penttonen M, Buzsáki G. Natural logarithmic relationship between brain oscillators. Thalamus & Related Systems. 2 (2):145-152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2161] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 32. | Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP. The oscillating brain: complex and reliable. Neuroimage. 2010;49:1432-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 1172] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 33. | Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1781] [Cited by in RCA: 1623] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 34. | Kong XM, Xu SX, Sun Y, Wang KY, Wang C, Zhang J, Xia JX, Zhang L, Tan BJ, Xie XH. Electroconvulsive therapy changes the regional resting state function measured by regional homogeneity (ReHo) and amplitude of low frequency fluctuations (ALFF) in elderly major depressive disorder patients: An exploratory study. Psychiatry Res Neuroimaging. 2017;264:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Zhang B, Chang J, Park J, Tan Z, Tang L, Lyu T, Han Y, Fan R, Gao Y, Kong J. Uncinate fasciculus and its cortical terminals in aphasia after subcortical stroke: A multi-modal MRI study. Neuroimage Clin. 2021;30:102597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Qi R, Zhang L, Wu S, Zhong J, Zhang Z, Zhong Y, Ni L, Li K, Jiao Q, Wu X, Fan X, Liu Y, Lu G. Altered resting-state brain activity at functional MR imaging during the progression of hepatic encephalopathy. Radiology. 2012;264:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 2022] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 38. | Yue Y, Jia X, Hou Z, Zang Y, Yuan Y. Frequency-dependent amplitude alterations of resting-state spontaneous fluctuations in late-onset depression. Biomed Res Int. 2015;2015:505479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Chen YC, Xia W, Luo B, Muthaiah VP, Xiong Z, Zhang J, Wang J, Salvi R, Teng GJ. Frequency-specific alternations in the amplitude of low-frequency fluctuations in chronic tinnitus. Front Neural Circuits. 2015;9:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Ries A, Hollander M, Glim S, Meng C, Sorg C, Wohlschläger A. Frequency-Dependent Spatial Distribution of Functional Hubs in the Human Brain and Alterations in Major Depressive Disorder. Front Hum Neurosci. 2019;13:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Qiu H, Li X, Luo Q, Li Y, Zhou X, Cao H, Zhong Y, Sun M. Alterations in patients with major depressive disorder before and after electroconvulsive therapy measured by fractional amplitude of low-frequency fluctuations (fALFF). J Affect Disord. 2019;244:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York: Biometrics Research. 2002. |
| 43. | Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5785] [Cited by in RCA: 5844] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 44. | Cao B, Luo Q, Fu Y, Du L, Qiu T, Yang X, Chen X, Chen Q, Soares JC, Cho RY, Zhang XY, Qiu H. Predicting individual responses to the electroconvulsive therapy with hippocampal subfield volumes in major depression disorder. Sci Rep. 2018;8:5434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 45. | Abrams R. Electroconvulsive therapy (4th Edition). Oxford University Press. 2002. |
| 46. | Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1581] [Cited by in RCA: 2679] [Article Influence: 297.7] [Reference Citation Analysis (0)] |
| 47. | Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1442] [Cited by in RCA: 1614] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 48. | Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8:113-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 216] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2015;172:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 261] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 50. | Wang L, Dai W, Su Y, Wang G, Tan Y, Jin Z, Zeng Y, Yu X, Chen W, Wang X, Si T. Amplitude of low-frequency oscillations in first-episode, treatment-naive patients with major depressive disorder: a resting-state functional MRI study. PLoS One. 2012;7:e48658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 51. | Liu F, Guo W, Liu L, Long Z, Ma C, Xue Z, Wang Y, Li J, Hu M, Zhang J, Du H, Zeng L, Liu Z, Wooderson SC, Tan C, Zhao J, Chen H. Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. J Affect Disord. 2013;146:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 52. | Zhang X, Zhu X, Wang X, Zhong M, Yi J, Rao H, Yao S. First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PLoS One. 2014;9:e85241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Gong J, Wang J, Qiu S, Chen P, Luo Z, Huang L, Wang Y. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl Psychiatry. 2020;10:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 54. | Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011;31:7910-7919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 55. | Zhang J, Wei L, Hu X, Zhang Y, Zhou D, Li C, Wang X, Feng H, Yin X, Xie B, Wang J. Specific frequency band of amplitude low-frequency fluctuation predicts Parkinson's disease. Behav Brain Res. 2013;252:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Han Y, Wang J, Zhao Z, Min B, Lu J, Li K, He Y, Jia J. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. Neuroimage. 2011;55:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 374] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 57. | Brown EC, Clark DL, Hassel S, MacQueen G, Ramasubbu R. Intrinsic thalamocortical connectivity varies in the age of onset subtypes in major depressive disorder. Neuropsychiatr Dis Treat. 2019;15:75-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Husain SF, Yu R, Tang TB, Tam WW, Tran B, Quek TT, Hwang SH, Chang CW, Ho CS, Ho RC. Validating a functional near-infrared spectroscopy diagnostic paradigm for Major Depressive Disorder. Sci Rep. 2020;10:9740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 59. | Xia M, Si T, Sun X, Ma Q, Liu B, Wang L, Meng J, Chang M, Huang X, Chen Z, Tang Y, Xu K, Gong Q, Wang F, Qiu J, Xie P, Li L, He Y; DIDA-Major Depressive Disorder Working Group. Reproducibility of functional brain alterations in major depressive disorder: Evidence from a multisite resting-state functional MRI study with 1,434 individuals. Neuroimage. 2019;189:700-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 60. | Lanzenberger R, Baldinger P, Hahn A, Ungersboeck J, Mitterhauser M, Winkler D, Micskei Z, Stein P, Karanikas G, Wadsak W, Kasper S, Frey R. Global decrease of serotonin-1A receptor binding after electroconvulsive therapy in major depression measured by PET. Mol Psychiatry. 2013;18:93-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 61. | Xu H, Zhao T, Luo F, Zheng Y. Dissociative changes in gray matter volume following electroconvulsive therapy in major depressive disorder: a longitudinal structural magnetic resonance imaging study. Neuroradiology. 2019;61:1297-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Jiao Q, Ding J, Lu G, Su L, Zhang Z, Wang Z, Zhong Y, Li K, Ding M, Liu Y. Increased activity imbalance in fronto-subcortical circuits in adolescents with major depression. PLoS One. 2011;6:e25159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Liu Y, Du L, Li Y, Liu H, Zhao W, Liu D, Zeng J, Li X, Fu Y, Qiu H, Qiu T, Hu H, Meng H, Luo Q. Antidepressant Effects of Electroconvulsive Therapy Correlate With Subgenual Anterior Cingulate Activity and Connectivity in Depression. Medicine (Baltimore). 2015;94:e2033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 64. | Tepfer LJ, Alloy LB, Smith DV. Family history of depression is associated with alterations in task-dependent connectivity between the cerebellum and ventromedial prefrontal cortex. Depress Anxiety. 2021;38:508-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Zhu DM, Yang Y, Zhang Y, Wang C, Wang Y, Zhang C, Zhao W, Zhu J. Cerebellar-cerebral dynamic functional connectivity alterations in major depressive disorder. J Affect Disord. 2020;275:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 66. | Zhou M, Hu X, Lu L, Zhang L, Chen L, Gong Q, Huang X. Intrinsic cerebral activity at resting state in adults with major depressive disorder: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2017;75:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 67. | Liu J, Ren L, Womer FY, Wang J, Fan G, Jiang W, Blumberg HP, Tang Y, Xu K, Wang F. Alterations in amplitude of low frequency fluctuation in treatment-naïve major depressive disorder measured with resting-state fMRI. Hum Brain Mapp. 2014;35:4979-4988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 68. | Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 885] [Cited by in RCA: 1131] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 69. | Xue S, Wang X, Wang W, Liu J, Qiu J. Frequency-dependent alterations in regional homogeneity in major depression. Behav Brain Res. 2016;306:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | Zhu X, He Z, Luo C, Qiu X, He S, Peng A, Zhang L, Chen L. Altered spontaneous brain activity in MRI-negative refractory temporal lobe epilepsy patients with major depressive disorder: A resting-state fMRI study. J Neurol Sci. 2018;386:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Yang C, Zhang A, Jia A, Ma JX, Sun N, Wang Y, Li X, Liu Z, Liu S, Xu Y, Zhang K. Identify abnormalities in resting-state brain function between first-episode, drug-naive major depressive disorder and remitted individuals: a 3-year retrospective study. Neuroreport. 2018;29:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |