Published online May 19, 2022. doi: 10.5498/wjp.v12.i5.673
Peer-review started: April 15, 2021
First decision: June 17, 2021
Revised: July 16, 2021
Accepted: April 1, 2022
Article in press: April 1, 2022
Published online: May 19, 2022
Processing time: 397 Days and 18.8 Hours
Caused by the mutation of methyl-CpG binding protein 2 (MeCP2), Rett syn
Core Tip: In this mini-review, Zhang WJ summarized current findings for the synaptic plasticity in the cortex and related motor learning functions under the scenario of Rett syndrome. The discussion of neuropathological mechanisms can help us to better understand the disease progression and more importantly to develop more effective measures to counteract motor deficits.
- Citation: Zhang WJ, Shi LL, Zhang L. Dysregulated cortical synaptic plasticity under methyl-CpG binding protein 2 deficiency and its implication in motor impairments. World J Psychiatry 2022; 12(5): 673-682
- URL: https://www.wjgnet.com/2220-3206/full/v12/i5/673.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i5.673
Rett syndrome is one neurodevelopmental disorder that is caused by the genetic mutation of methyl-CpG binding protein 2 (MeCP2)[1]. Predominantly found in females with about a 0.01% incidence[2], Rett syndrome has been recognized as one of the major genetic conditions that affects neurodevelopment. As clinical features, about 61% of Rett syndrome patients developed autism spectrum disorder (ASD)-like symptoms[3], making it one major genetic contribution to autistic syndromes. Other behavioral features of Rett syndrome include cognitive and verbal disabilities[4] as well as the retardation of general development[5]. Among various clinical manifestations, deficits of motor function can be found in early stages of disease progression (around 12-18 mo in patients), as displayed by the gradual deterioration of normal motor functions and the occurrence of repetitive movements[6]. As a result, the gradual loss of acquired motor skill has been recognized as one prominent feature of Rett syndrome[7], further highlighting the relationship between motor functions and MeCP2. In this mini-review, we will summarize current major findings regarding motor dysfunctions in Rett syndrome and discuss their correlation with MeCP2-mediated synaptic plasticity of motor circuits, especially those in the motor cortex. In addition, we will also explore the possibility of non-drug intervention strategies including noninvasive neuromodulation and physical exercise in relieving these motor syndromes.
Recent studies have demonstrated the pleiotropic functions of MeCP2 in mediating early events of neurodevelopment including neurogenesis, migration and patterning[8-10]. Deficits of neural network formation frequently lead to abnormal functions. In the cortical region, MeCP2 mutation disrupts the normal excitatory-inhibitory (E/I) balance, resulting in altered synaptic computation[4,7,11-13]. In specific studies, MeCP2-null knockout mice presented elevated GABAA and N-methyl-D-aspartic acid (NMDA) receptors in the barrel cortex[13]. However, using MeCP2-mutant mice, both excitatory and inhibitory conductance were reduced in vivo while the E/I ratio was increased[11]. In another study using MeCP2-mutant mice, cortical pyramidal neurons (PNs) displayed decreased spontaneous activity probably due to the reduced miniature excitatory postsynaptic currents (mEPSCs) amplitude while the inhibitory input did not change[12]. Those seemingly contradictory results further suggested the complicated mechanism of MeCP2 in mediating cortical network. A possible approach for further investigation can be achieved via cell type-specific study of MeCP2 function. For example, parvalbumin (PV)-specific MeCP2 deletion recapitulated reduced cortical excitability by global MeCP2 deletion[11]. Multiple mechanisms including ion permeability, neurotransmitter receptor or synaptic structural proteins can be further interrogated, as MeCP2 works as a transcriptional regulatory factor to potentially affect their gene expression. Since the neural plasticity of the cortical network is closely correlated with motor learning[14,15], the dysregulated function of MeCP2 may confer motor deficits. Further interrogation of MeCP2-dependent synaptic regulation can help to reveal the pathological process of related motor impairments in order to provide diagnostic and treatment targets.
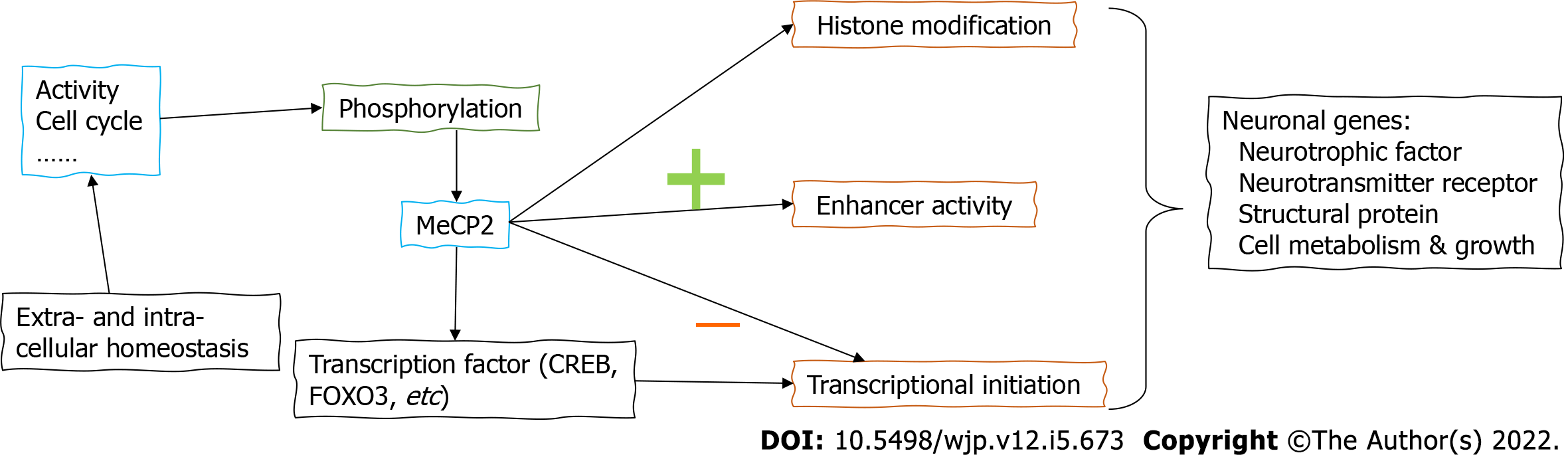
When examining the neural mechanism of Rett syndrome-associated behavioral symptoms, it is suggested that MeCP2 works as one methyl-DNA binding protein[16]. The loss-of-function mutation of MeCP2 in Rett syndrome thus can be generalized as the deprivation of transcription repression, although recent studies are suggesting its multifaceted roles including activation or suppression of specific genes[17]. Across different brain regions, MeCP2 mediates the gene expressional network in a similar pattern[18], suggesting the brain-wide effect. When examining the transcriptional regulatory mechanism, a recent study identified the prominent role of MeCP2 in suppressing the initiation of gene regions with high CG-methylation levels[19]. For those non-CG methylated gene regions, MeCP2 also exerts a suppressor role via repressing enhancer activity[20]. In the exploration of MeCP2-targeted molecules, key modulators of neural plasticity have been recovered. For example, MeCP2 affects the transcription of BDNF to affect myelination and remyelination[21]. An early study further showed that MeCP2 associated with the transcriptional activator CREB1 to mediate a wide range of brain genes[17]. Moreover, MeCP2 interacts with a lot of neuronal genes in positive or negative manners. The transcriptional factor forkhead box protein O3 (FOXO3) has been found to be positively regulated by MeCP2 via deacetylation[22]. Those effects on transcriptional factors highlight the role of MeCP in the top layer of the gene regulatory network. Besides those transcriptional factors and neurotrophic molecules, MeCP2 also affects the post-translational modification of neuronal genes. For example, the histone modification has been shown to be mediated by MeCP2 via recognizing H3K27me3[23]. Furthermore, the phosphorylation of MeCP2 itself adds further layers onto its regulatory network. The brain-specific phosphorylation of MeCP2 is known to regulate BDNF expression, contributing to neuronal growth and maturation[24]. In a broad sense, activity-dependent MeCP2 phosphorylation affects its interaction with transcriptional repressors[25], providing an epigenetic mechanism. During neurodevelopment, cell cycle-associated MeCP2 phosphorylation modulates adult neurogenesis[26] and nervous system functions[27]. Combining all these results, MeCP2 regulates the expression of neuronal genes via different pathways at transcriptional and post-transcriptional levels (Figure 1).
In neural tissues, gene transcription plays a critical role in various forms of synaptic plasticity such as the long-term potentiation (LTP) and long-term depression (LTD)[28]. People are thus beginning to dissect the neuropathological mechanism of Rett syndrome from the synaptic perspective[29]. Current knowledge has observed the disruption of normal synaptic plasticity under MeCP2 loss-of-function mutation across different brain regions including the hippocampus[30], the cerebellum[31], the visual pathway[32] and the amygdala nuclei[33]. As the critical region for high-order cognitive and mental regulation, the cortical region is also affected by MeCP2 mutations. For example, MeCP2 insufficiency in mouse auditory cortex affected the local network and disrupted maternal pup-retrieval behaviors[34]. In mouse primary visual cortex (V1), MeCP2 deficiency remarkably disrupted the early-stage development of neural plasticity during the so-called “critical period”[35,36]. The abnormal synaptic development resulted in the morphological deficits of synapse, including decreased spine density[37], altered spine morphology or dendritic complexity[38], shorter dendritic lengths[39] and alternation of synaptic protein expression in primary motor cortex (M1)[40,41]. Furthermore, the reduced neuronal size can be observed in layer V PNs of M1 in Rett syndrome model mice[42]. These findings provide the first-hand evidence for the disruption of structural and functional plasticity in the cortical region upon MeCP2 deprivation, highlighting the necessity and importance to elaborate the cortical neuropathology of Rett syndrome.
It is important to notice that both cell autonomous and non-autonomous mechanisms reside in MeCP2-mediated cell plasticity. For example, the loss of MeCP2 affects the autocrine brain derived neurotrophic factor (BDNF) signaling in excitatory neurons to affect neural plasticity, as wildtype neurons cannot rescue mutant cells in the area[43]. Such results provide further clues for clinical manifestations as mosaic patterns of mutations frequently occurs in Rett syndrome patients[44]. Although the primary cause of Rett syndrome is believed to be cell autonomous, non-autonomous mechanism has been revealed as the culture medium from MeCP2-mutated astrocytes disrupted dendritic morphology of wildtype hippocampal neurons[45]. Therefore, MeCP2 affects neural function via a complex network and further elaborations are required to study the cell-specific effect.
To attribute the factors for disrupted cortical synaptic plasticity under MeCP2 mutation, recent advances are highlighting the role of local inhibitory transmissions. In the mouse auditory cortex, independent lines of evidence are suggesting that the abolishment or insufficiency of MeCP2 suppresses normal activity of PV-interneurons, resulting in failures of maternal caring behaviors[34,46]. In primary somatosensory cortex (S1) and M1, the learning-associated modulation of plasticity of PV-interneurons was impaired in MeCP2 knockout mice as well as under heterogenous mutation of MeCP2[47]. In the barrel region, the loss of MeCP2 also enhanced glutamatergic transmission[13]. Such interruption of normal cortical network homeostasis might be explained by MeCP2 influence on synaptic plasticity during the critical period in early-stage development[36]. Such opinions were further supported by the conditional knockout of MeCP2 in PV-interneurons resulting in the absence of neural plasticity of V1 during the critical time[35]. To figure out the molecular mechanism, current studies are suggesting the role of neurotrophic factors. For example, BDNF was downregulated under MeCP2 deficiency[48]. As an intervention trial, insulin-like growth factor-1 (IGF-1) partially relieved such neurodevelopmental deficits under MeCP2 deficiency[49] and recovered cortical plasticity[50]. An alternative explanation exists in the cortical perineuronal nets (PNNs) whose formation is dependent on MeCP2[51]. Since PNNs are known to mainly surround PV-interneurons[52], the extracellular modulation may provide a model to explain how pan-neuronal mutation of MeCP2 leads to PV-interneuron specific defects.
The converging evidence of deficient GABAergic transmission upon MeCP2 mutation implies the hyper-excitation of the cortical network. In Rett syndrome patients, clinical recording supported such hypothesis by displaying significant increases of the excitation index of M1 in association with reduced short-interval inhibition[53] plus decreased inhibitory motor control[54]. Mouse model studies also suggested aberrantly high cortical excitability upon MeCP2 deficiency[49], probably due to diminished extracellular GABA transporter activity[55] or under-development of dendritic spines[40]. However, other studies supported the enhanced GABA transmission under MeCP2 knockout[13]. In a short summary, both presynaptic function such as GABA transporter and postsynaptic mechanism including spine formation and synaptic transmission are involved in MeCP2-mediated cortical plasticity. To better dissect the molecular pathway, cell-specific genetic manipulation and functional studies can be performed. For example, PV-specific MeCP2 deletion mimics the effect of global gene knockout[11]. In the future, MeCP2 can be studied in other neuronal and glial cell subpopulations in the cortex.
Based on these facts of disrupted cortical E/I balance, the application of neuromodulator drugs or neuromodulation stimulus may provide a promising future for region-specific intervention of motor symptoms under MeCP2 deficiency. In the last part of this article, we will summarize major findings and prospective regarding the neuromodulation approaches for alleviating motor symptoms of Rett syndrome.
Among the major clinical features of Rett syndrome, motor deficits occur early during the disease development and persist across the whole disease process: The motor delay becomes apparent among 1.5-years-old and 3-years-old, after a seemingly normal early postnatal period[4]. During the adolescent and adulthood period, the progressively declined motor function can be presented as Parkinsonism-like features[56]. Such progression of motor symptoms usually develops into severe ataxia and deprives the patients of the ability to walk or stand during the teenage period[7]. These clinical manifestations can be replicated in mouse models: In MeCP2-null knockout mice, early-onset motor abnormalities were found to induce higher lethal rates[57]. In addition, these model animals presented regression of acquired psychomotor skills under a social interaction scenario[58]. These behavioral deficits clearly suggested the involvement of the motor system in Rett syndrome pathology.
Distinct brain regions and neural ensembles regulate voluntary movement, including the forebrain sensorimotor region, the midbrain nuclei such as the thalamus and basal ganglia, as well as the hindbrain regions plus the cerebellum. The motor cortex is innervated by distinct neuromodulator systems including dopamine, noradrenaline and serotonin. The brain-wide deficiency of MeCP2 thus may affect motor cortical plasticity via disruption of subcortical inputs. For example, the ablation of MeCP2 in aminergic neurons produced cell autonomous effects resulting in behavioral abnormalities[59]. The pharmaceutical potentiation of the serotonergic pathway improved cortical microcircuits and recovered motor learning behaviors[60]. Another study further revealed that striatal MeCP2 was critical for maintaining dopaminergic transmission of psychomotor regulation[61]. These findings supported the indispensable role of MeCP2 in the neural network related with cortical activity.
Although the site-specific gene knockout study has suggested the role of MeCP2 in mediating motor behaviors across different neural networks such as the noradrenergic transmission, the motor cortex remains as the prominent brain region in which fine motor control is regulated. Within the motor cortex, both excitatory PNs and GABAergic interneurons form the local network to drive the voluntary movement. PNs were once believed to be the principal projecting neurons in the cortical region and their structural and functional plasticity largely affects motor functions[62,63]. MeCP2 was known to mediate synaptic structures in the motor cortex as it can regulate the dosage of gene expression via homeostatic control of DNA methylation. The over-expression of MeCP2, for instance, resulted in altered structural plasticity of cortical dendritic spines[64]. On the other hand, the deficiency of MeCP2 led to remarkably shorter dendrites of PNs in the motor cortex in human patients across different age groups[38]. Similar phenotypes were observed in mouse models, which presented reduced spine density, shorter dendrite lengths[37], irregular spine clustering or shapes[65] and reduced dendritic complexity[39]. Such evidence clearly demonstrates the relationship between MeCP2 and synaptic plasticity and implies the participation of MeCP2-mediated synaptic defects in Rett syndrome.
Besides the excitatory neurons, GABAergic inhibitory neurons in the motor cortex also tightly regulates motor coordination and motor learning functions, as they can provide both inhibitory synaptic inputs and subthreshold oscillation wave onto excitatory neurons. For example, the somatostatin (SST)-interneuron is found to actively participate in the acquisition and retrieval of complex motor skills as suggested by an in vivo recording study[66], and our recent work has revealed the abnormally suppressed activity of those SST-interneurons under a Parkinson’s disease (PD) mouse model, leading to pathologically over-excitation of pyramidal cells[67]. Such phenomena revealed cortical dysfunctions due to the loss of normal inhibitory inputs onto the pyramidal projecting neurons, leading to their hyperactivation and related neural symptoms. Besides the local regulation of cortical inhibition, GABAergic neurons received inputs from subcortical nuclei which consisted of multiple monoaminergic systems. For instance, the α2A -adrenoceptor was found to suppress the activity of cortical inhibitory neurons[68]. The dopamine receptor D1 and D2 have been known to affect the density of cortical inhibitory neurons, including PV- and SST-interneurons[69]. In the human motor cortex, serotonin was also reported to enhance GABAergic transmission[70]. No direct study, however, has investigated the modulation of cortical inhibitory neurons by the monoaminergic system under MeCP2 deficiency. Further work thus can be performed to dissect the circuitry pathway of MeCP2 in affecting motor learning functions.
When one broadens their scope of neurological diseases, it is interesting to find that the “cortical disinhibition” model can be found across different neurological disease models such as Alzheimer’s disease (AD)[71], amyotrophic lateral sclerosis (ALS)[72] and Huntington’s disease (HD)[73]. In a primate model of Rett syndrome, MeCP2 is expressed in both excitatory and inhibitory neurons in cortical regions[74], implying the possible role for mediating glutamatergic and GABAergic transmission. In specific, the conditional knockout of MeCP2 in cortical vasoactive intestinal peptide (VIP)-interneurons resulted in the deficits of social and mental functions[75]. It thus seems that the abovementioned correlation between MeCP2 and motor function may reside in the inhibitory neurons of the motor cortex. In fact, the cellular pathological studies have also attributed motor dysfunction to MeCP2 deficiency in PV-interneurons in the motor cortex as suggested by a conditional gene knockout model[76]. In a similar manner, the deletion of MeCP2 in SST-interneurons resulted in stereotypic and repetitive behaviors, highlighting the distinct functions of interneuron subtypes in fine motor control[76]. On the other hand, PNs may also be affected under MeCP2 deficits which can impair the structural or functional integrity of the excitatory synapse[11,38,42]. For example, MeCP2 deletion in glutamatergic neurons resulted in much more severe symptoms than those from inhibitory neuron-specific deletion[77]. As the restoration of MeCP2 in GABAergic neurons only partially rescued symptoms in null knockout mice[78], the integrity of local E/I homeostasis is of critical importance for relieving cortical neuropathology in Rett syndrome. Combining all data, it is promising that targeting the E/I balance in the motor cortex, especially by potentiating the inhibitory transmission, may aid in retarding or alleviating the motor syndrome in patients.
Based on motor deficits and dysregulated neural plasticity of motor circuits upon MeCP2 dysfunction as aforementioned, it is possible that certain neuromodulation approaches targeting circuitry function might help to ameliorate those motor symptoms. As supporting evidence, environmental enrichment helped to relieve the behavioral deficits including motor learning functions in MeCP2 null knockout mice, in addition to the rescue of cortical LTP function[31]. In a clinical trial of Rett syndrome patients under the age of 6 years, the 6-mo environmental enrichment training paradigm improved motor functions[79]. These examples clearly suggested the possibility of environmental intervention in relieving Rett syndrome symptoms.
Physical training, as one widely accepted life-style intervention to facilitate neurogenesis and cognitive functions[80], has been recently demonstrated by our group to improve motor learning abilities via stimulating structural and functional plasticity of synapses in mouse motor cortex[81]. Therefore, exercise training may work as one promising approach to relieve motor deficits of Rett syndrome patients. Such a proposal was supported by several clinical reports in which daily activities and rehabilitation helped to maintain motor abilities[82,83] or to prevent functional deterioration[84]. Specifically, a recently published case report found that periodic exercise rehabilitation at 2 years of age helped to maintain normal motor function[82]. Another study recruited 4 girls under the age of 11 years and found that 2-mo treadmill training helped to improve the general body fitness and behavioral scores[84]. Although these preliminary studies only included a small cohort of patients, the potency of physical exercise in early intervention of Rett syndrome-related motor dysfunction can be tested by large-scale clinical trials in the future.
To provide neurobiological evidence for physical exercise, Zoghbi et al[85] recently reported the effectiveness of pre-symptomatic training in the mitigation of specific motor impairments using a mouse Rett syndrome model. In particular, exercise training repeatedly activated a specific population of neurons that developed more dendritic arbors and higher excitability to enhance motor function[85]. These data suggested a possibly new intervention strategy by which endurance exercise works to retard the deterioration of motor dysfunctions. When examining the molecular mechanism underlying exercise intervention on Rett syndrome, BDNF upregulation has been reported upon exercise paradigm in both rodent models[86] and human cohorts[87]. At the downstream of BDNF activation, it is worth noting that physical training boosted the activity of the mechanistic target of rapamycin (mTOR) pathway for improving structural and functional plasticity of dendritic spines in the motor cortex[81]. Since previous knowledge has established the role of mTOR down-regulation upon Mecp2 mutation[88,89] to generate the phenotypes of Rett syndrome[90], it is highly likely that exercise may help to relieve neural dysfunctions via moderately stimulating mTOR pathways. As functional evidence, both in vitro and in vivo data have proved the down-sized neurons across multiple brain regions in mice carrying the A140V mutation of Mecp2, in association with mTOR activity inhibition[88]. On the other hand, human brain samples presented abnormally upregulated mTOR activity under Rett syndrome[91]. Such discrepancy between human patients and animal models may arise from the different mutational sites or distinct disease stages. Nevertheless, the critical role of the mTOR pathway in MeCP2-related dysfunction and the modulatory role of mTOR by exercise training cannot be neglected. This further highlights the promising future of using endurance training for alleviating cellular and behavioral deficits of Rett syndrome.
Currently, few available intervention strategies have been adopted to benefit Rett syndrome patients. Besides the potential usage of exercise training at early stages as aforementioned, non-invasive neuromodulation approaches provide alternative choices for alleviating behavioral deficits. Various methods including electric, magnetic and ultrasound stimulations have been approved as safe means to modulate neural functions, mainly focusing on the cortical region. The application of transcranial magnetic stimulation (TMS) has been accepted to evaluate the excitability and E/I balance of the M1 neural network[53,54], despite relatively small sample sizes. As an alternative neuromodulation approach, transcranial direct current stimulation (tDCS) has recently been tested on Rett syndrome patients. In one study recruiting 31 patients, tDCS effectively improved attention and verbal functions[92]. A second study also reported enhancement of language skills by tDCS[93]. These neuromodulation approaches thus may have potential values in improving neural functions. Due to the early-onset and persistency of motor deficits, the targeted intervention on the motor cortex may be worth further testing by employing large-scale and multi-centered clinical trials. When considering neuromodulation in large cohorts of patients, however, some concerns may arise as it may result in episodes of epilepsy[94], whose susceptibility rises in Rett syndrome patients[95]. These safety issues also remind that environmental intervention such as exercise training might be a more preferrable and safer way in treating Rett syndrome.
In summary, MeCP2 mediates the synaptic plasticity and neural circuitry in the motor cortex and its genetic mutation leads to the disruption of neural transmission, thereby causing the dysfunction of fine motor coordination and motor learning abilities in Rett syndrome. Targeting the motor cortex by either physical training or neuromodulation approaches thus have become accessible and promising strategies for alleviating motor symptoms in Rett syndrome and is worth of more investigations from both basic science and the clinical fields.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Smirnakis SM, United States S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 931] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 2. | Laurvick CL, de Klerk N, Bower C, Christodoulou J, Ravine D, Ellaway C, Williamson S, Leonard H. Rett syndrome in Australia: a review of the epidemiology. J Pediatr. 2006;148:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Richards C, Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 4. | Banerjee A, Miller MT, Li K, Sur M, Kaufmann WE. Towards a better diagnosis and treatment of Rett syndrome: a model synaptic disorder. Brain. 2019;142:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, Leonard H, Bailey ME, Schanen NC, Zappella M, Renieri A, Huppke P, Percy AK; RettSearch Consortium. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68:944-950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1076] [Cited by in RCA: 975] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 6. | Leonard H, Cobb S, Downs J. Clinical and biological progress over 50 years in Rett syndrome. Nat Rev Neurol. 2017;13:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Ip JPK, Mellios N, Sur M. Rett syndrome: insights into genetic, molecular and circuit mechanisms. Nat Rev Neurosci. 2018;19:368-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 8. | Feldman D, Banerjee A, Sur M. Developmental Dynamics of Rett Syndrome. Neural Plast. 2016;2016:6154080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Bedogni F, Cobolli Gigli C, Pozzi D, Rossi RL, Scaramuzza L, Rossetti G, Pagani M, Kilstrup-Nielsen C, Matteoli M, Landsberger N. Defects During Mecp2 Null Embryonic Cortex Development Precede the Onset of Overt Neurological Symptoms. Cereb Cortex. 2016;26:2517-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 345] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Banerjee A, Rikhye RV, Breton-Provencher V, Tang X, Li C, Li K, Runyan CA, Fu Z, Jaenisch R, Sur M. Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome. Proc Natl Acad Sci U S A. 2016;113:E7287-E7296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102:12560-12565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 496] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 13. | Lo FS, Blue ME, Erzurumlu RS. Enhancement of postsynaptic GABAA and extrasynaptic NMDA receptor-mediated responses in the barrel cortex of Mecp2-null mice. J Neurophysiol. 2016;115:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Sidarta A, Vahdat S, Bernardi NF, Ostry DJ. Somatic and Reinforcement-Based Plasticity in the Initial Stages of Human Motor Learning. J Neurosci. 2016;36:11682-11692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Guo ZV, Li N, Huber D, Ophir E, Gutnisky D, Ting JT, Feng G, Svoboda K. Flow of cortical activity underlying a tactile decision in mice. Neuron. 2014;81:179-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 476] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 16. | Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, Bird A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 989] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 17. | Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1494] [Cited by in RCA: 1382] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 18. | Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum Mol Genet. 2009;18:2431-2442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 19. | Boxer LD, Renthal W, Greben AW, Whitwam T, Silberfeld A, Stroud H, Li E, Yang MG, Kinde B, Griffith EC, Bonev B, Greenberg ME. MeCP2 Represses the Rate of Transcriptional Initiation of Highly Methylated Long Genes. Mol Cell. 2020;77:294-309.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Clemens AW, Wu DY, Moore JR, Christian DL, Zhao G, Gabel HW. MeCP2 Represses Enhancers through Chromosome Topology-Associated DNA Methylation. Mol Cell. 2020;77:279-293.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | KhorshidAhmad T, Acosta C, Cortes C, Lakowski TM, Gangadaran S, Namaka M. Transcriptional Regulation of Brain-Derived Neurotrophic Factor (BDNF) by Methyl CpG Binding Protein 2 (MeCP2): a Novel Mechanism for Re-Myelination and/or Myelin Repair Involved in the Treatment of Multiple Sclerosis (MS). Mol Neurobiol. 2016;53:1092-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Lyst MJ, Bird A. Rett syndrome: a complex disorder with simple roots. Nat Rev Genet. 2015;16:261-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 23. | Lee W, Kim J, Yun JM, Ohn T, Gong Q. MeCP2 regulates gene expression through recognition of H3K27me3. Nat Commun. 2020;11:3140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 678] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 25. | Ebert DH, Gabel HW, Robinson ND, Kastan NR, Hu LS, Cohen S, Navarro AJ, Lyst MJ, Ekiert R, Bird AP, Greenberg ME. Activity-dependent phosphorylation of MeCP2 threonine 308 regulates interaction with NCoR. Nature. 2013;499:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Li H, Zhong X, Chau KF, Santistevan NJ, Guo W, Kong G, Li X, Kadakia M, Masliah J, Chi J, Jin P, Zhang J, Zhao X, Chang Q. Cell cycle-linked MeCP2 phosphorylation modulates adult neurogenesis involving the Notch signalling pathway. Nat Commun. 2014;5:5601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, Harmin DA, Greenberg RS, Verdine VK, Zhou Z, Wetsel WC, West AE, Greenberg ME. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron. 2011;72:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 28. | Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 697] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 29. | Na ES, Nelson ED, Kavalali ET, Monteggia LM. The impact of MeCP2 Loss- or gain-of-function on synaptic plasticity. Neuropsychopharmacology. 2013;38:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Bertoldi ML, Zalosnik MI, Fabio MC, Aja S, Roth GA, Ronnett GV, Degano AL. MeCP2 Deficiency Disrupts Kainate-Induced Presynaptic Plasticity in the Mossy Fiber Projections in the Hippocampus. Front Cell Neurosci. 2019;13:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, Pizzorusso T. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol Psychiatry. 2010;67:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Noutel J, Hong YK, Leu B, Kang E, Chen C. Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice. Neuron. 2011;70:35-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Gambino F, Khelfaoui M, Poulain B, Bienvenu T, Chelly J, Humeau Y. Synaptic maturation at cortical projections to the lateral amygdala in a mouse model of Rett syndrome. PLoS One. 2010;5:e11399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Krishnan K, Lau BY, Ewall G, Huang ZJ, Shea SD. MECP2 regulates cortical plasticity underlying a learned behaviour in adult female mice. Nat Commun. 2017;8:14077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | He LJ, Liu N, Cheng TL, Chen XJ, Li YD, Shu YS, Qiu ZL, Zhang XH. Conditional deletion of Mecp2 in parvalbumin-expressing GABAergic cells results in the absence of critical period plasticity. Nat Commun. 2014;5:5036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | Krishnan K, Wang BS, Lu J, Wang L, Maffei A, Cang J, Huang ZJ. MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proc Natl Acad Sci U S A. 2015;112:E4782-E4791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 37. | Stuss DP, Boyd JD, Levin DB, Delaney KR. MeCP2 mutation results in compartment-specific reductions in dendritic branching and spine density in layer 5 motor cortical neurons of YFP-H mice. PLoS One. 2012;7:e31896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Armstrong D, Dunn JK, Antalffy B, Trivedi R. Selective dendritic alterations in the cortex of Rett syndrome. J Neuropathol Exp Neurol. 1995;54:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 271] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Robinson L, Guy J, McKay L, Brockett E, Spike RC, Selfridge J, De Sousa D, Merusi C, Riedel G, Bird A, Cobb SR. Morphological and functional reversal of phenotypes in a mouse model of Rett syndrome. Brain. 2012;135:2699-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci U S A. 2009;106:2029-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 436] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 41. | Varghese M, Keshav N, Jacot-Descombes S, Warda T, Wicinski B, Dickstein DL, Harony-Nicolas H, De Rubeis S, Drapeau E, Buxbaum JD, Hof PR. Autism spectrum disorder: neuropathology and animal models. Acta Neuropathol. 2017;134:537-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 349] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 42. | Rietveld L, Stuss DP, McPhee D, Delaney KR. Genotype-specific effects of Mecp2 Loss-of-function on morphology of Layer V pyramidal neurons in heterozygous female Rett syndrome model mice. Front Cell Neurosci. 2015;9:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Sampathkumar C, Wu YJ, Vadhvani M, Trimbuch T, Eickholt B, Rosenmund C. Loss of MeCP2 disrupts cell autonomous and autocrine BDNF signaling in mouse glutamatergic neurons. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Renthal W, Boxer LD, Hrvatin S, Li E, Silberfeld A, Nagy MA, Griffith EC, Vierbuchen T, Greenberg ME. Characterization of human mosaic Rett syndrome brain tissue by single-nucleus RNA sequencing. Nat Neurosci. 2018;21:1670-1679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 45. | Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 359] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 46. | Lau BYB, Krishnan K, Huang ZJ, Shea SD. Maternal Experience-Dependent Cortical Plasticity in Mice Is Circuit- and Stimulus-Specific and Requires MECP2. J Neurosci. 2020;40:1514-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Morello N, Schina R, Pilotto F, Phillips M, Melani R, Plicato O, Pizzorusso T, Pozzo-Miller L, Giustetto M. Loss of Mecp2 Causes Atypical Synaptic and Molecular Plasticity of Parvalbumin-Expressing Interneurons Reflecting Rett Syndrome-Like Sensorimotor Defects. eNeuro. 2018;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 48. | Kondo M, Gray LJ, Pelka GJ, Christodoulou J, Tam PP, Hannan AJ. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome--Mecp2 gene dosage effects and BDNF expression. Eur J Neurosci. 2008;27:3342-3350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Sun Y, Gao Y, Tidei JJ, Shen M, Hoang JT, Wagner DF, Zhao X. Loss of MeCP2 in immature neurons leads to impaired network integration. Hum Mol Genet. 2019;28:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Castro J, Garcia RI, Kwok S, Banerjee A, Petravicz J, Woodson J, Mellios N, Tropea D, Sur M. Functional recovery with recombinant human IGF1 treatment in a mouse model of Rett Syndrome. Proc Natl Acad Sci U S A. 2014;111:9941-9946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 51. | Lau BYB, Layo DE, Emery B, Everett M, Kumar A, Stevenson P, Reynolds KG, Cherosky A, Bowyer SH, Roth S, Fisher DG, McCord RP, Krishnan K. Lateralized Expression of Cortical Perineuronal Nets during Maternal Experience is Dependent on MECP2. eNeuro. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sánchez-Aguilera A, Mantoan L, Maeso P, Fernandes C, Ewers H, Rico B. Activity-Dependent Gating of Parvalbumin Interneuron Function by the Perineuronal Net Protein Brevican. Neuron. 2017;95:639-655.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 53. | Bernardo P, Cobb S, Coppola A, Tomasevic L, Di Lazzaro V, Bravaccio C, Manganelli F, Dubbioso R. Neurophysiological Signatures of Motor Impairment in Patients with Rett Syndrome. Ann Neurol. 2020;87:763-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Krajnc N, Zidar J. The role of transcranial magnetic stimulation in evaluation of motor cortex excitability in Rett syndrome. Eur J Paediatr Neurol. 2016;20:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Zhang L, Wither RG, Lang M, Wu C, Sidorova-Darmos E, Netchev H, Matolcsy CB, Snead OC, Eubanks JH. A Role for Diminished GABA Transporter Activity in the Cortical Discharge Phenotype of MeCP2-Deficient Mice. Neuropsychopharmacology. 2016;41:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Bahi-Buisson N, Nectoux J, Rosas-Vargas H, Milh M, Boddaert N, Girard B, Cances C, Ville D, Afenjar A, Rio M, Héron D, N'guyen Morel MA, Arzimanoglou A, Philippe C, Jonveaux P, Chelly J, Bienvenu T. Key clinical features to identify girls with CDKL5 mutations. Brain. 2008;131:2647-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 57. | Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1199] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 58. | Veeraragavan S, Wan YW, Connolly DR, Hamilton SM, Ward CS, Soriano S, Pitcher MR, McGraw CM, Huang SG, Green JR, Yuva LA, Liang AJ, Neul JL, Yasui DH, LaSalle JM, Liu Z, Paylor R, Samaco RC. Loss of MeCP2 in the rat models regression, impaired sociability and transcriptional deficits of Rett syndrome. Hum Mol Genet. 2016;25:3284-3302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 59. | Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, Hyland K, Thaller C, Maricich SM, Humphreys P, Greer JJ, Percy A, Glaze DG, Zoghbi HY, Neul JL. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci U S A. 2009;106:21966-21971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 60. | Villani C, Sacchetti G, Carli M, Invernizzi RW. Fluoxetine rescues rotarod motor deficits in Mecp2 heterozygous mouse model of Rett syndrome via brain serotonin. Neuropharmacology. 2020;176:108221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Su SH, Kao FC, Huang YB, Liao W. MeCP2 in the rostral striatum maintains local dopamine content critical for psychomotor control. J Neurosci. 2015;35:6209-6220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Li W, Ma L, Yang G, Gan WB. REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci. 2017;20:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 63. | Cichon J, Gan WB. Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature. 2015;520:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 335] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 64. | Jiang M, Ash RT, Baker SA, Suter B, Ferguson A, Park J, Rudy J, Torsky SP, Chao HT, Zoghbi HY, Smirnakis SM. Dendritic arborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. J Neurosci. 2013;33 19518-19533 [PMID:24336718] DOI: 10.1523/JNEUROSCI.1745-13.2013. |
| 65. | Belichenko NP, Belichenko PV, Mobley WC. Evidence for both neuronal cell autonomous and nonautonomous effects of methyl-CpG-binding protein 2 in the cerebral cortex of female mice with Mecp2 mutation. Neurobiol Dis. 2009;34:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Adler A, Zhao R, Shin ME, Yasuda R, Gan WB. Somatostatin-Expressing Interneurons Enable and Maintain Learning-Dependent Sequential Activation of Pyramidal Neurons. Neuron. 2019;102:202-216.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 67. | Chen K, Yang G, So KF, Zhang L. Activation of Cortical Somatostatin Interneurons Rescues Synapse Loss and Motor Deficits after Acute MPTP Infusion. iScience. 2019;17:230-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Ohshima M, Itami C, Kimura F. The α2A -adrenoceptor suppresses excitatory synaptic transmission to both excitatory and inhibitory neurons in layer 4 barrel cortex. J Physiol. 2017;595:6923-6937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 69. | Müller Smith K, Fagel DM, Stevens HE, Rabenstein RL, Maragnoli ME, Ohkubo Y, Picciotto MR, Schwartz ML, Vaccarino FM. Deficiency in inhibitory cortical interneurons associates with hyperactivity in fibroblast growth factor receptor 1 mutant mice. Biol Psychiatry. 2008;63:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Batsikadze G, Paulus W, Kuo MF, Nitsche MA. Effect of serotonin on paired associative stimulation-induced plasticity in the human motor cortex. Neuropsychopharmacology. 2013;38:2260-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Najm R, Jones EA, Huang Y. Apolipoprotein E4, inhibitory network dysfunction, and Alzheimer's disease. Mol Neurodegener. 2019;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 72. | Nardone R, Golaszewski S, Thomschewski A, Sebastianelli L, Versace V, Brigo F, Orioli A, Saltuari L, Höller Y, Trinka E. Disinhibition of sensory cortex in patients with amyotrophic lateral sclerosis. Neurosci Lett. 2020;722:134860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Kim EH, Thu DC, Tippett LJ, Oorschot DE, Hogg VM, Roxburgh R, Synek BJ, Waldvogel HJ, Faull RL. Cortical interneuron loss and symptom heterogeneity in Huntington disease. Ann Neurol. 2014;75:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Akbarian S, Chen RZ, Gribnau J, Rasmussen TP, Fong H, Jaenisch R, Jones EG. Expression pattern of the Rett syndrome gene MeCP2 in primate prefrontal cortex. Neurobiol Dis. 2001;8:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Mossner JM, Batista-Brito R, Pant R, Cardin JA. Developmental loss of MeCP2 from VIP interneurons impairs cortical function and behavior. Elife. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 76. | Ito-Ishida A, Ure K, Chen H, Swann JW, Zoghbi HY. Loss of MeCP2 in Parvalbumin-and Somatostatin-Expressing Neurons in Mice Leads to Distinct Rett Syndrome-like Phenotypes. Neuron. 2015;88:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 77. | Meng X, Wang W, Lu H, He LJ, Chen W, Chao ES, Fiorotto ML, Tang B, Herrera JA, Seymour ML, Neul JL, Pereira FA, Tang J, Xue M, Zoghbi HY. Manipulations of MeCP2 in glutamatergic neurons highlight their contributions to Rett and other neurological disorders. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 78. | Ure K, Lu H, Wang W, Ito-Ishida A, Wu Z, He LJ, Sztainberg Y, Chen W, Tang J, Zoghbi HY. Restoration of Mecp2 expression in GABAergic neurons is sufficient to rescue multiple disease features in a mouse model of Rett syndrome. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 79. | Downs J, Rodger J, Li C, Tan X, Hu N, Wong K, de Klerk N, Leonard H. Environmental enrichment intervention for Rett syndrome: an individually randomised stepped wedge trial. Orphanet J Rare Dis. 2018;13:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 80. | Horowitz AM, Fan X, Bieri G, Smith LK, Sanchez-Diaz CI, Schroer AB, Gontier G, Casaletto KB, Kramer JH, Williams KE, Villeda SA. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 303] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 81. | Chen K, Zheng Y, Wei JA, Ouyang H, Huang X, Zhang F, Lai CSW, Ren C, So KF, Zhang L. Exercise training improves motor skill learning via selective activation of mTOR. Sci Adv. 2019;5:eaaw1888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 82. | Imamura T, Nakayama T, Nakayama J, Iwasaki N. A Patient with Rett Syndrome Maintained Motor Function by Periodic Rehabilitation Therapy and Proactive Daily Activities. Prog Rehabil Med. 2020;5:20200014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Downs J, Lotan M, Elefant C, Leonard H, Wong K, Buckley N, Stahlhut M. Implementing telehealth support to increase physical activity in girls and women with Rett syndrome-ActivRett: protocol for a waitlist randomised controlled trial. BMJ Open. 2020;10:e042446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 84. | Lotan M, Isakov E, Merrick J. Improving functional skills and physical fitness in children with Rett syndrome. J Intellect Disabil Res. 2004;48:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Achilly NP, Wang W, Zoghbi HY. Presymptomatic training mitigates functional deficits in a mouse model of Rett syndrome. Nature. 2021;592:596-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 86. | Chen K, Zhang L, Tan M, Lai CS, Li A, Ren C, So KF. Treadmill exercise suppressed stress-induced dendritic spine elimination in mouse barrel cortex and improved working memory via BDNF/TrkB pathway. Transl Psychiatry. 2017;7:e1069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 87. | Skriver K, Roig M, Lundbye-Jensen J, Pingel J, Helge JW, Kiens B, Nielsen JB. Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol Learn Mem. 2014;116:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 88. | Rangasamy S, Olfers S, Gerald B, Hilbert A, Svejda S, Narayanan V. Reduced neuronal size and mTOR pathway activity in the Mecp2 A140V Rett syndrome mouse model. F1000Res. 2016;5:2269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 89. | Ricciardi S, Boggio EM, Grosso S, Lonetti G, Forlani G, Stefanelli G, Calcagno E, Morello N, Landsberger N, Biffo S, Pizzorusso T, Giustetto M, Broccoli V. Reduced AKT/mTOR signaling and protein synthesis dysregulation in a Rett syndrome animal model. Hum Mol Genet. 2011;20:1182-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 90. | Tsujimura K, Irie K, Nakashima H, Egashira Y, Fukao Y, Fujiwara M, Itoh M, Uesaka M, Imamura T, Nakahata Y, Yamashita Y, Abe T, Takamori S, Nakashima K. miR-199a Links MeCP2 with mTOR Signaling and Its Dysregulation Leads to Rett Syndrome Phenotypes. Cell Rep. 2015;12:1887-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 91. | Olson CO, Pejhan S, Kroft D, Sheikholeslami K, Fuss D, Buist M, Ali Sher A, Del Bigio MR, Sztainberg Y, Siu VM, Ang LC, Sabourin-Felix M, Moss T, Rastegar M. MECP2 Mutation Interrupts Nucleolin-mTOR-P70S6K Signaling in Rett Syndrome Patients. Front Genet. 2018;9:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 92. | Fabio RA, Gangemi A, Semino M, Vignoli A, Canevini MP, Priori A, Rosa GD, Caprì T. Effects of Combined Transcranial Direct Current Stimulation with Cognitive Training in Girls with Rett Syndrome. Brain Sci. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Fabio RA, Gangemi A, Capri T, Budden S, Falzone A. Neurophysiological and cognitive effects of Transcranial Direct Current Stimulation in three girls with Rett Syndrome with chronic language impairments. Res Dev Disabil. 2018;76:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 94. | Stultz DJ, Osburn S, Burns T, Pawlowska-Wajswol S, Walton R. Transcranial Magnetic Stimulation (TMS) Safety with Respect to Seizures: A Literature Review. Neuropsychiatr Dis Treat. 2020;16:2989-3000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 95. | Operto FF, Mazza R, Pastorino GMG, Verrotti A, Coppola G. Epilepsy and genetic in Rett syndrome: A review. Brain Behav. 2019;9:e01250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |