Published online Apr 19, 2022. doi: 10.5498/wjp.v12.i4.541
Peer-review started: February 26, 2021
First decision: July 15, 2021
Revised: July 28, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: April 19, 2022
Processing time: 411 Days and 5.2 Hours
Schizophrenia (SCZ) is a severe mental illness that affects several brain domains with relation to cognition and behaviour. SCZ symptoms are typically classified into three categories, namely, positive, negative, and cognitive. The etiology of SCZ is thought to be multifactorial and poorly understood. Accumulating evidence has indicated abnormal synaptic plasticity and cognitive impairments in SCZ. Synaptic plasticity is thought to be induced at appropriate synapses during memory formation and has a critical role in the cognitive symptoms of SCZ. Many factors, including synaptic structure changes, aberrant expression of plasticity-related genes, and abnormal synaptic transmission, may influence synaptic plasticity and play vital roles in SCZ. In this article, we briefly summarize the morphology of the synapse, the neurobiology of synaptic plasticity, and the role of synaptic plasticity, and review potential mechanisms underlying abnormal synaptic plasticity in SCZ. These abnormalities involve dendritic spines, postsynaptic density, and long-term potentiation-like plasticity. We also focus on cognitive dysfunction, which reflects impaired connectivity in SCZ. Additionally, the potential targets for the treatment of SCZ are discussed in this article. Therefore, understanding abnormal synaptic plasticity and impaired cognition in SCZ has an essential role in drug therapy.
Core Tip: Schizophrenia (SCZ) is a severe mental illness that affects several domains of cognition and behaviour. SCZ symptoms are typically classified into three categories, namely, positive, negative, and cognitive. The etiology of SCZ is thought to be multifactorial and poorly understood. Accumulating evidence has indicated abnormal synaptic plasticity and cognitive impairments in SCZ. This article will briefly review abnormalities in synaptic plasticity, including synaptic structure, synaptic plasticity-related genes, neuroplasticity, synaptic transmission, and cognitive dysfunction in SCZ.
- Citation: Wu XL, Yan QJ, Zhu F. Abnormal synaptic plasticity and impaired cognition in schizophrenia. World J Psychiatry 2022; 12(4): 541-557
- URL: https://www.wjgnet.com/2220-3206/full/v12/i4/541.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i4.541
Schizophrenia (SCZ) is a chronic, dangerous psychiatric disorder that affects about 1% of people worldwide. Typically, SCZ, occurring in late adolescence or early adulthood, often results in lifetime disability if not effectively controlled. The symptoms of SCZ are generally grouped into three categories, addressed as follows: Positive symptoms (auditory hallucinations and persecutory delusions), negative symptoms (social withdrawal, self-neglect, loss of motivation and initiative, emotional blunting, and paucity of speech), and cognitive symptoms (problems with attention, certain types of memory, and executive functions)[1]. There are numerous hypotheses postulated to elaborate the pathophysiology of SCZ, including the neurodevelopmental hypothesis and synaptic hypothesis. The synaptic hypothesis involves abnormal synaptic transmission and impaired synaptic plasticity.
Synaptic plasticity consists of structural plasticity and functional plasticity. Various evidence discloses abnormal structural and functional plasticity in the pathogenesis of SCZ. Postmortem studies in the brain of SCZ patients point out that there is a significant decrease in the density of dendritic spines (DSs) and the size of postsynaptic density (PSD) in SCZ compared to healthy controls[2,3]. Similarly, functional imaging has revealed that the expression levels of synaptic structure related genes have changed in SCZ[4,5]. Change in morphology or distribution of synaptic structure is related to synaptic plasticity and contributes to SCZ. Additionally, a mouse model of SCZ induced by MK801 also proves that abnormal structural and functional plasticity can constitute to the etiology of SCZ. MK-801-induced mice display the disruption of long-term potentiation (LTP) and change of excitatory postsynaptic potential[6,7]. Furthermore, LTP-like plasticity deficits may result in impairments of learning and memory[8,9].
Abnormal synaptic plasticity might lead to cognitive impairments, including deficits in learning and memory, attention, and social cognition, in SCZ[9,10]. Cognitive impairments refer to aberrant functional connectivity or transmission. Cognitive deficit is an early warning sign of SCZ and contributes to poor functional outcomes[11]. Conventional antipsychotic drugs targeted by dopamine receptors have beneficial effects on positive symptoms but offer minimal benefit for negative symptoms or cognitive symptoms[12]. Therefore, in-depth research on abnormal synaptic plasticity and impaired cognition in SCZ could help understand the underlying mechanism of SCZ and find new drugs to treat it.
This review will focus on recent advances in the understanding of impaired synaptic plasticity and cognitive dysfunction, including changes in synaptic structure, synaptic plasticity-related genes, dysregulation of synaptic transmission, and disconnection, in SCZ, as well as the potential targets for SCZ.
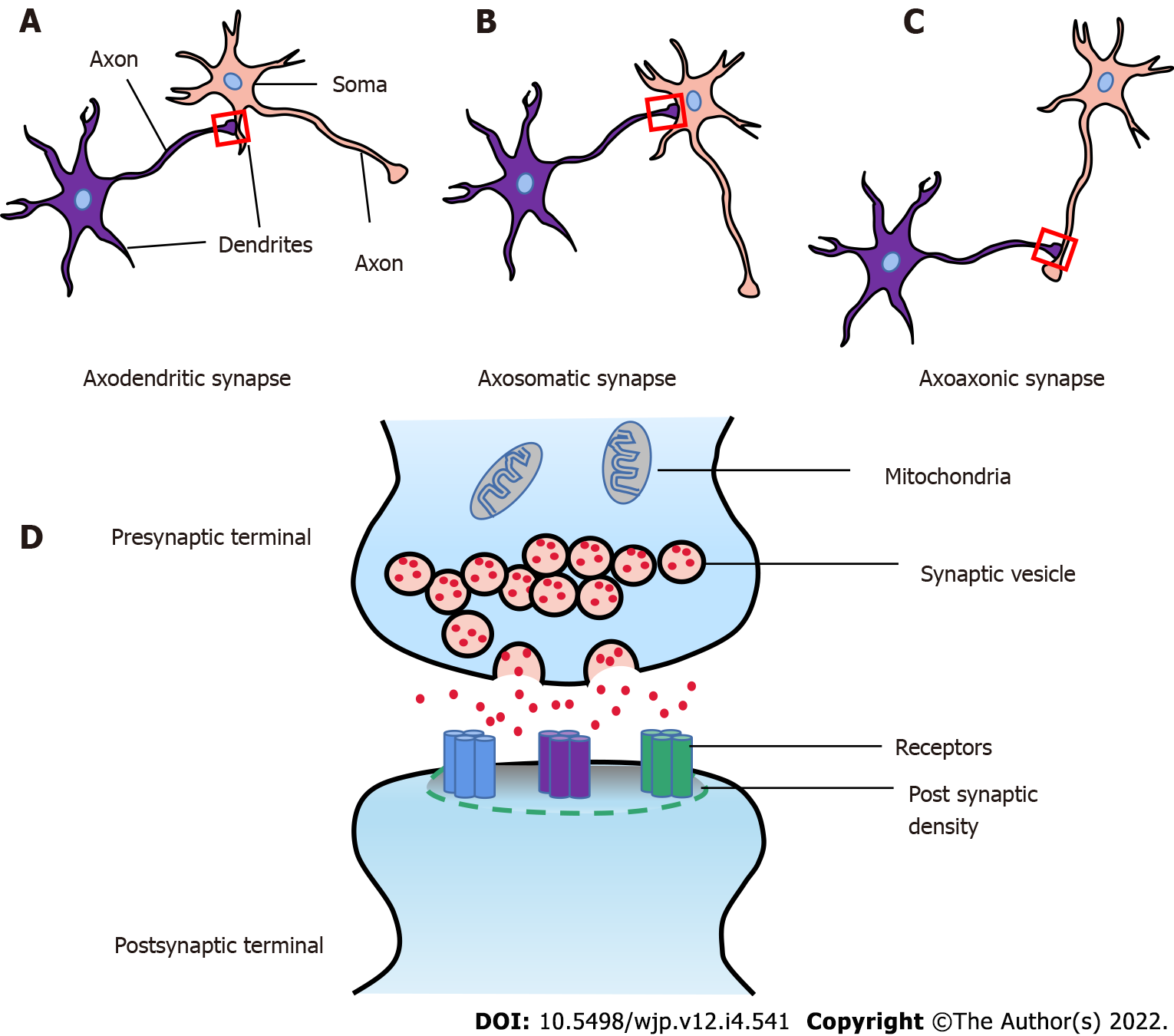
The synapse is a structure that allows a neuron (or nerve cell) to communicate electrical or chemical signals to another neuron or other target effector cell. There are three common types of synapses, respectively called axodendritic, axosomatic, and axoaxonic (Figure 1). In the mammalian brain, neuronal signals are transmitted by two fundamental types of synapses: The electrical synapse and the chemical synapse[13]. A classical chemical synapse is composed of three main parts: (1) The presynaptic components, enclosing neurotransmitter-filled synaptic vesicles (SVs) and proteins (SNARE complex, Munc13, and Munc18) which promote SV recruitment and neurotransmitters release[14]; (2) The postsynaptic components, containing specific receptors and proteins including scaffolding proteins, neurotransmitter receptors, enzymes, and cytoskeletal components, which receive and transmit signals and regulate the synaptic plasticity[15]; and (3) The synaptic cleft, physical space between the presynaptic and postsynaptic terminals which is 10-20 nm, also called synaptic gap (Figure 1D)[16].
Furthermore, the surface where the presynaptic component and the postsynaptic component are connected is usually called the synaptic interface. It is determined by the width of the synaptic cleft, length of the synaptic active zone, the thickness of PSDs, and curvature of the synaptic interface[17-19]. Changes of synaptic interface closely relate to synaptic function.
In vivo imaging studies have shown that the decreased density of DSs may be a loss of synapse[20]. Spines have a critical role in synaptic transmission. The reduced spines directly correlate with the loss of synaptic function[21,22]. Many factors, including specific gene expression, signal transduction, and new synapse formation, can change synapse level. The total number of synapses is controlled by forming new synapses and pruning old or inappropriate synapses, and finally contributes to synaptic plasticity and memory consolidation[23].
Synaptic plasticity (also called synaptic strengths) is the ability of neurons to modify synaptic strength in response to external stimuli. During this process, the structure and function of the synapse are highly dynamic.
Structurally, synaptic plasticity is characterized by the insertion or retention of neurotransmitter receptors, especially AMPAR, into the postsynaptic membrane. Many factors, including the size of DS, the pool of SVs, the areas of active zone, and the PSD, may influence synaptic plasticity[24-26]. Functionally, LTP and long-term depression (LTD) are two forms of synaptic plasticity. There are usually two LTP types, namely, NMDA receptor-dependent LTP and mossy fibre LTP (a cAMP-dependent presynaptic form of plasticity)[27]. The activation of NMDA receptors and increased calcium (Ca2+) concentration are essential for the induction of NMDA receptor-dependent LTP[28,29]. Noteworthy, the spine Ca2+ signal is required to trigger LTP[30,31]. Thus, calcium/calmodulin-dependent protein kinase II (CaMKII) has an important role in NMDA receptor-dependent LTP. Besides, various kinases, including protein kinase C, the mitogen-activated protein kinase, and the tyrosine kinase Src, have been implicated in LTP induction[32-34]. Interestingly, some forms of LTP can only maintain 30-60 min, but some can last a very long time, from several hours to days, even for many weeks. The possibilities for the longer-term maintenance of LTP is involved in synaptic structural remodeling, increased spines size, and enlargement of PSD[35,36].
In summary, synaptic structure, AMPAR trafficking, and DS dynamics are critical for the maintenance of synaptic plasticity.
The formation of memory involves four processes: Encoding, storing, consolidating, and retrieving information. Learning is viewed as the acquisition or encoding of the information to memory. The core hypothesis of synaptic plasticity and memory is as follows: Activity-dependent synaptic plasticity is induced at appropriate synapses during memory formation, and is both necessary and sufficient for the information storage underlying the type of memory mediated by the brain area in which plasticity is observed[37].
Changing the strength of synaptic connections is a prime process underlying learning and memory formation. Accumulative studies suggest that synaptic plasticity is necessary for learning and memory. The induction of synaptic plasticity requires NMDAR activation. NMDAR1 knockdown mice show deficit in spatial memory in the hippocampus[38]. Besides, synaptic plasticity may contribute to declarative and relational memory[39], sequence learning[40], motor learning[41,42], and perceptual learning at sensory cortex synapses[43]. The traditional view is that fast learning requires more robust synaptic changes[44]. However, some studies suggest that weak synaptic plasticity can support fast learning[45]. Synaptic plasticity has a requisite role in learning and memory across many regions of the brain.
Human brain maturation is a complex, dynamic, and lifelong process. Billions of cells proliferate, migrate, and maturate during early development, which leads to a brain with billions of neurons at birth, finally forming connections. As children become teenagers, the brain dynamically strengthens or weakens connections in response to environmental input[46]. Simultaneously, neural maturity is increased with age across various brain regions, including primary sensory, motor, associative learning, and cognition function[47]. The prefrontal cortex (PFC) is the last brain region to mature and can mediate executive function such as goal planning, working memory, and guided behavior[48].
Post-mortem studies suggest that the synaptic densities increase rapidly in the visual and auditory cortices, with a maximum of near 3 mo followed by pruning until the age of 12 years[49]. However, synaptic density in the PFC reaches the maximum during childhood, up to 150-200 percent of its adult level. Interestingly, synaptic elimination lasts to mid-adolescence in the PFC[50]. Furthermore, evidence shows that synaptic strength is reduced in the developing brain because it presents synaptic pruning[51]. The specialized and functionally-connected neural circuits accompany regional changes. Additionally, changes in brain volume occur in SCZ. Several reports suggest reducing cerebral cortical volume at premature birth compared to infants born at term[52]. Similarly, there are linearly decreased cortical gray matter and increased white matter across ages 4 years to 12 years[53,54]. In a word, the change of synaptic strength has an influential role in brain maturation and maintenance of a functional neuronal circuit.
Synaptic plasticity is mediated by structural changes (elongation, contraction, and shape changes) of DSs. DSs are tiny, actin-rich protrusions from the dendritic shaft of various types of neurons. Most of the excitatory synapses are on DSs. Postmortem studies suggest that the density of DSs is reduced in brain tissue of individuals with SCZ, including the neocortex (especially in layer deep 3) and hippocampus, while it may be increased in the dorsal striatum[3,55,56]. Moreover, reduced number of spines and decreased length of basilar dendrites have been observed in SCZ[3]. Deficits in DSs may contribute to the impairment of synaptic plasticity in SCZ.
DSs possess specialized subdomains, including PSD, scaffolding proteins, signal transduction molecules, ion channels, and cytoskeleton components. Under the electron microscope, PSD appears as a regular, dense band about 25 nm to 50 nm thick in the postsynaptic membrane. PSD has essentially different roles in the process of LTP formation[57]. Postmortem study demonstrates a drastic reduction of PSD in the nucleus accumbens in SCZ, especially in asymmetric synapse[2]. The alteration of the synaptic ultrastructure may result from overstimulation of the excitatory synapse. Thus, the alteration of PSD may contribute to SCZ.
LTP and LTD are two primary forms for studying synaptic plasticity. Many factors, including transmitter release and NMDAR function, can affect LTP[58,59]. The dopaminergic or serotonergic systems can also modulate LTP. Impaired LTP and LTD-like plasticity have been reported in SCZ[60,61].
Evidence has shown altered LTP-like plasticity in SCZ compared to healthy subjects[61,62]. Furthermore, NMDAR antagonists (phencyclidine, MK801, and ketamine) can induce SCZ-like symptoms in healthy individuals[63,64]. Studies reveal NMDAR hypofunction in SCZ[65]. Those changes are involved in excitation and inhibition imbalance, controlled by excitatory neurotransmission glutamate and inhibitory neurotransmission gamma-aminobutyric acid (GABA). Electrophysiological recordings reveal that MK801 treatment can significantly suppress the frequency of miniature excitatory postsynaptic current/miniature inhibitory postsynaptic current ratio of layer (L) 2/3 PNs[66]. Neurogranin, a calmodulin-binding protein, modulates LTP in the hippocampus. The lower level of neurogranin results in hypo-phosphorylation of NMDAR subunit NR2A and finally contributes to NMDAR current decay[67]. Maybe, NMDAR hypofunction accounts for the lack of associative LTP-like plasticity in patients with SCZ.
Ca2+ entry is another crucial factor for the induction of LTP-like plasticity. The voltage-gated calcium channel is critical for mediating intracellular Ca2+ entry, especially the Cav1.2 or Cav1.3 channel. Clinical findings reveal the alteration of intracellular calcium homeostasis in SCZ[68]. Calcium concentration level increases in the cerebrospinal fluid (CSF) of patients with SCZ when acute psychotic symptoms are in remission[69]. It means a positive correlation between SCZ and calcium dysregulation. Therefore, dysregulation of calcium concentration is responsible for changing neuronal excitability and LTP-like plasticity.
Gene expression studies, including microarray, have discovered the aberrant expression of synaptic plasticity-related genes in SCZ, such as GAP43 and PSD95. GAP43 is a phosphoprotein of the presynaptic membrane that regulates the growth state of axon terminals. Several postmortem studies show reduced GAP43 levels in the frontal cortex and the hippocampus of patients with SCZ[70,71]. What’s more, PSD95 is the most abundant protein in the postsynaptic membrane. Postmortem studies show decreased PSD95 protein and mRNA expression levels in SCZ[72,73]. Interestingly, PSD95 can directly interact with ARC or IL1RAPL1 to regulate spine density and function[74,75]. Besides, TAOK2 kinase could directly phosphorylate Septin7 to regulate PSD95 stability and DS maturation[76]. The PSD proteins can directly reflect the number of synapses.
Additionally, some genes regulate the development and function of neuronal synapses. KIF3B, a member of the kinesin superfamily proteins, supports the NR2A/APC complex transport. Its dysfunction relates to SCZ[77]. The dynamic regulation of NR2A and NR2B is critical to the function of NMDAR, which has a substantial role in regulating synaptic plasticity. Besides, CaMKII, ARP2/3, Arc, and PI4KA affect NMDAR function and mediate Ca2+ entry[78]. A recent study reports that an envelope protein encoded by human endogenous retrovirus type W (also called syncytin-1) regulates Ca2+ entry via activating the TRPC3 channel[79], indicating that syncytin-1 may also regulate the development and function of neuronal synapses. Intriguingly, our results show that syncytin-1 can increase the expression of BDNF and IL-6 in SCZ[80,81]. BDNF, an essential member of the nerve growth factor family, regulates synapse formation and contributes to impaired plasticity in SCZ[82]. These data predict that syncytin-1 may participate in the regulation of synaptic plasticity.
In summary, abnormality of synapse morphology, LTP-like plasticity, and synaptic plasticity-related genes may contribute to the pathogenesis of SCZ.
The hypothesis of dysconnectivity gives two inconsistent explanations: (1) Robust connectivity: The synapse has not been cleared in time in the process of neural system development; and (2) Weak connectivity: Synaptic connectivity decreases and is responsible for the processing information in the brain involving multi brain regions[83,84]. Impaired connectivity is a failure of proper functional integration within the brain, and the connection between different neuron systems influences the functional integration[85]. Effective and functional connectivity plays a prominent role in brain function. Functional magnetic resonance imaging (fMRI), positron emission tomography (PET), magnetic resonance imaging (MRI), computer-assisted tomography, and magnetic resonance spectroscopy have been used to study brain structure or function.
With the development of brain imaging technology, impaired connectivity has been observed in SCZ. Evidence suggests that prefrontal-limbic cortices are hyperconnected with the mediodorsal thalamus and ventral parts of the striatum and pallidum by fMRI[86]. Impaired connectivity correlates with cognitive impairments. Additionally, PET reveals that SCZ involves dysfunction of a widely distributed cortico-thalamic circuitry[87].
Moreover, an MRI study shows reduced synaptic connectivity in SCZ[88]. These reductions are widespread in the left fronto-parietal network, lateral and medial visual network, motor network, default mode network, and auditory network. Reduced synaptic connectivity is also present in the first episode of psychosis but appears to progress throughout the disorder[89]. The reduction of synaptic connectivity may disturb brain development, including myelogenesis and synaptic pruning or disruption of maturation of inhibitory neural networks such as GABAergic interneurons[90-93]. Maybe, reduced synaptic connectivity involves impaired γ synchronization and increased excitation/inhibition ratio[94]. In conclusion, impaired connectivity found in the brain of patients with SCZ is related to the cognitive dysfunction in SCZ.
Since the “dementia praecox” was proposed, cognitive dysfunction had received extensive attention and research in SCZ. It is until 1970s that Gallhofer proposed cognitive symptoms as the third symptoms of SCZ. Cognitive impairments are in the first episode of SCZ[95]. Those deficits include the speed of processing, attention vigilance, working memory, verbal learning, visual learning, reasoning problem solving, and social cognitive[96]. Kudo et al[97] report that increased MMP-9 levels are associated with cognitive impairments in SCZ. High concentrations of S100B correlates with memory impairments, and the variants of S100B may lead to poor performance in patients with SCZ[98,99].
Cognitive deficits may impair global functioning or contribute to poor functional outcomes in SCZ[11]. A four-year follow-up study shows that first-episode SCZ with severe cognitive impairments has no social functioning improvement, even after therapy[100]. Besides, the function and structure of frontal-limbic brain regions have a meaningful role in functional outcome in SCZ[101]. Conventional antipsychotic drug treatment has minimal benefits on cognitive symptoms in SCZ, and even some may impair certain aspects of cognition, such as attention, short-term memory, and learning. However, second-generation (atypical) antipsychotics, such as clozapine, improve several cognitive function domains, especially attention and verbal fluency in SCZ[102-104]. In summary, cognitive deficits are core symptoms of SCZ and result in severe disability.
SCZ is currently considered as a polygenic and multifactorial disorder, involving abnormality of synaptic function and neurotransmission, including dopaminergic pathway, serotoninergic pathway, glutamatergic pathway, GABAergic pathway, cholinergic pathway, and other neurotransmitter pathways, such as norepinephrine (NE) and neurosteroids.
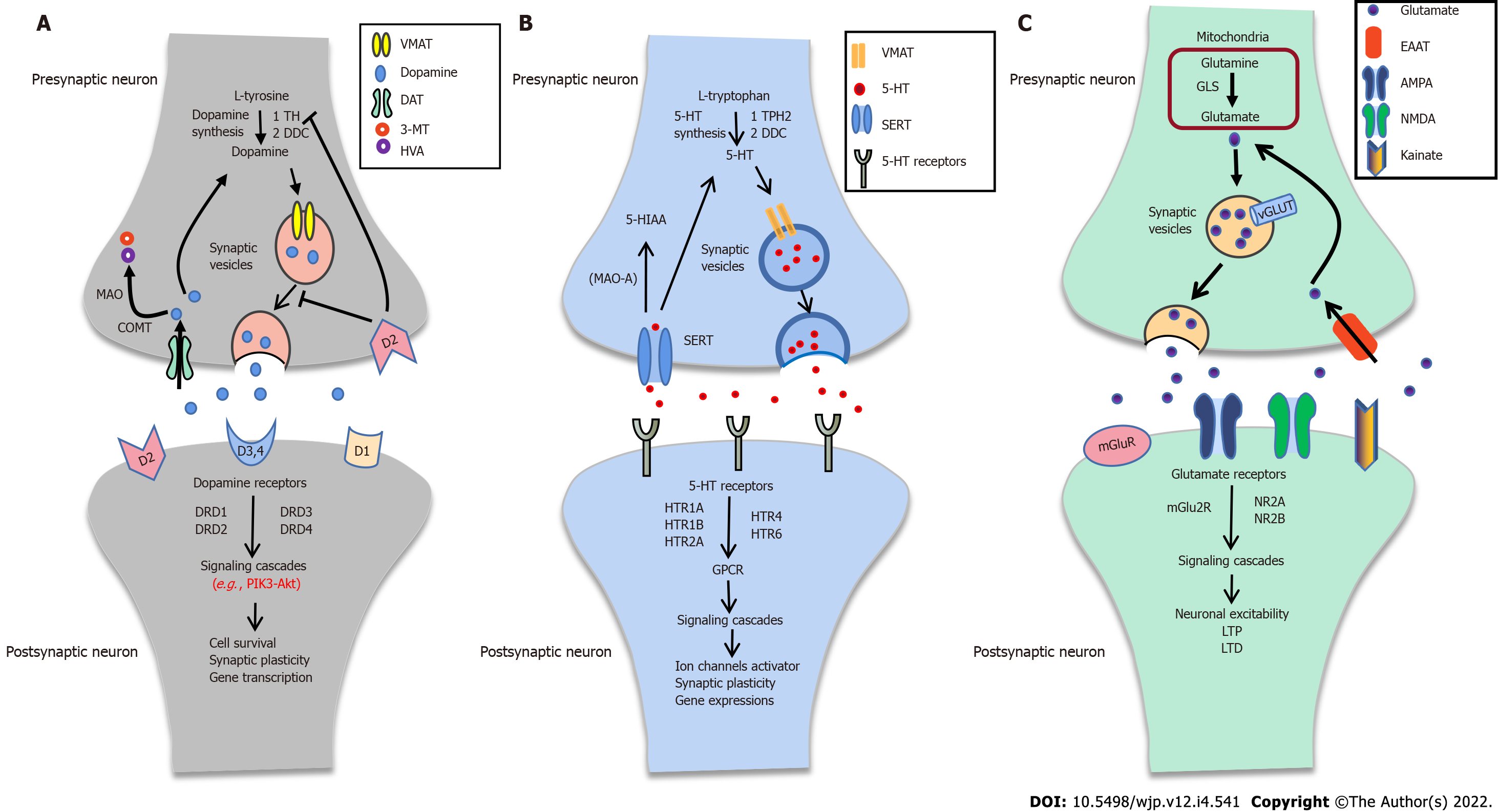
Typically, the dopaminergic pathway consists of dopamine synthesis, release, and reuptake. It can activate the downstream signal cascades, which play a critical role in synaptic plasticity (Figure 2A). Dopamine is synthesized from tyrosine through two steps: (1) Tyrosine hydroxylase catalyzes the tyrosine to L-DOPA by hydroxylation; and (2) L-DOPA is converted to dopamine by DOPA decarboxylase[105,106]. Dopamine can be stored into SVs, transported to the presynaptic membrane by the vesicular monoamine transporter 2, and finally released to the synaptic cleft[107]. There are five subtypes of dopamine receptors (DRD1, DRD2, DRD3, DRD4, and DRD5) known to mediate dopaminergic physiological functions. Dopamine receptors, especially DRD2, can couple to Gαi/o protein and modulate the PI3K-Akt signal pathway[108,109]. The PI3K-Akt signal pathway has a critical role in cell survival, proliferation, differentiation, glucose metabolism, and gene transcription[110].
Dopaminergic dysfunction has a prominent role in the development of symptoms of SCZ. High dopamine levels in SCZ support this hypothesis[111]. Postmortem studies have suggested a hyperactive dopaminergic system in SCZ, compared to healthy controls[112]. Nowadays, most antipsychotic drugs target dopamine receptors to block dopamine transmission. Notably, DRD2 is considered as the primary target for antipsychotics to alleviate positive symptoms. Moreover, dopamine transporter and vesicular monoamine transporter are decreased in SCZ. However, increased expression of monoamine oxidase A appears to occur in the substantia nigra of patients with SCZ[113].
Brain 5-HT plays a crucial role in affect and mood control, memory, reward, and modulation of developmental, physiological, and behavioral processes[114-116]. Typically, 5-HT synthesis needs two enzymes: Tryptophan hydroxylase and DOPA decarboxylase. After synthesizing, 5-HT can be transported into SVs and release to the synaptic cleft. Some 5-HT directly binds to its receptors (HTR1A, HTR1B, HTR2A, HTR4, and HTR6), activates downstream signaling pathways to trigger ion channels, and regulates synaptic plasticity (Figure 2B).
Alteration of serotonin transmission has been implicated in the processes of SCZ. Tryptophan hydroxylase 2 (TPH2), a rate-limiting enzyme for serotonin synthesis, is selectively expressed in the raphe serotonergic neurons[117]. Postmortem studies and single nucleotide polymorphism (SNP) studies show a significant association of TPH2 with SCZ in Han Chinese[118,119]. Additionally, the expression level of SERT (5-HT transporter, also named 5-HTT) is reduced in the frontal cortex of subjects with SCZ[120]. Recently, a SNP meta-analysis shows a strong association between SERT polymorphism and SCZ[121]. Indeed, the 5-HT receptor has an outstanding role in 5-HT transmission. 5-HT1A agonist can directly bind to atypical antipsychotic drugs (AAPDs) to treat cognitive impairments associated with SCZ[122-124]. Maybe as a compensatory mechanism, the expression of serotonin 1A is increased or maybe due to the beneficial effects of AAPDs in SCZ, the 5-HT1A receptor is activated.
Glutamate is the principal excitatory neurotransmitter in the central nervous system. Notedly, glutamate is converted from glutamine by phosphate-activated glutaminase in mitochondria and packaged into SVs by vesicular glutamate transporters (VGLUTs). Sequentially, the glutamate releases to the synaptic cleft. It then activates the downstream pathway or is re-uptaken into the presynaptic membrane by excitatory amino acid transporter after binding to the glutamate receptors (Figure 2C). Besides, the cystine/glutamate antiporter system xc-, which might exchange cystine for glutamate in a 1:1 ratio, has a vital role in releasing glutamate[125]. The “glutamate hypothesis” was first proposed by Kim et al[126]. They found that glutamate levels were decreased compared to healthy controls in CSF with SCZ[126]. The glutamatergic hypothesis of SCZ is based on the NMDAR hypofunction and the abnormality of glutamate transmission in SCZ.
Postmortem brain study shows a decreased expression level of VGLUT1 in the hippocampus of patients with SCZ[127]. However, VGLUT2 protein levels are increased in the inferior temporal gyrus (ITG) of SCZ[128]. The loss of VGLUT activity eliminates vesicular release and glutamatergic neurotransmission and regulates presynaptic quantal size or synaptic plasticity[129]. Postmortem studies have also revealed an increase in EAAT1 and EAAT2 transcripts in Brodmann's area (BA) 10 of subjects with SCZ, but not BA46[130]. Similar results have a relatively high agreement in the thalamus and cerebellar vermis[131,132]. These results indicate that EAAT is involved in glutamate reuptake in SCZ. Furthermore, evidence shows that mRNA expression levels of SLC3A2 and SLC7A11, two system xc- subunit genes, are decreased in peripheral white blood cells of SCZ patients compared to healthy controls. Abnormality of system xc- is involved in glutamatergic neurotransmission[125]. NMDAR-mediated glutamate transmission has been implicated in cognitive execution in the nucleus accumbens of SCZ[133]. Changes in the mRNA and protein levels of NMDAR subunits have been described in SCZ[134]. Suppressed NMDAR signaling through Src kinase may facilitate presynaptic glutamate release during synaptic activity[135]. In addition, the D-amino acid oxidase activator (DAOA, also called G72) protein, which has an important role in modulating NMDAR signaling, has a strong association with SCZ[136,137]. Those results indicate that alteration of glutamatergic transmission has a meaningful role in SCZ.
Reduced GABAergic neurotransmission is in support of the ‘GABA hypothesis’ for SCZ[138]. RNA-Seq analysis reveals the disruption of GABA metabolite levels in SCZ[139]. Moreover, postmortem studies suggest that subjects with SCZ have lower mRNA and protein levels of synthetic enzyme GAD67 compared to healthy controls[140]. Lower expression of GAD67 may be a consequence of a deficiency of the immediate early gene Zif268, suggesting a potential mechanistic basis for altered cortical GABA synthesis and impaired cognition in SCZ[141]. GAD67 promoter methylation levels are associated with the SCZ-risk SNP rs3749034 and with the expression of GAD25 in the dorsolateral prefrontal cortex (DLPFC). Alternative splicing of GAD67 may contribute to GABA dysfunction in SCZ[142]. Similarly, the immunoreactivity of GAT1, a protein responsible for the reuptake of GABA, is decreased in SCZ[143]. Furthermore, GAD1 knockout rats exhibit SCZ-related phenotypes, such as cognitive impairments in spatial reference and working memory in the hippocampus[144]. A PET study using [11C] Ro154513 has reported differential expression of GABA-A receptors in SCZ[145]. Therefore, the synthesis and reuptake of GABA are lower in SCZ. These abnormalities of GABAergic neurotransmission are related to cognitive impairments in SCZ.
Acetylcholine has a vital role in cognitive and behavioural/psychological function. Pharmacologic studies show that central cholinergic activity profoundly affects the storage and retrieval of information in memory. The choline acetyltransferase, a cholinergic function marker, is correlated with the severity of cognitive impairments in the parietal cortex of schizophrenic patients[146]. Furthermore, cholinesterase inhibitors (donepezil or rivastigmine) have positive effects on cognitive dysfunction in SCZ[147,148]. These inhibitions increase the synaptic concentration of acetylcholine and finally enhance and prolong acetylcholine action on muscarinic and nicotinic receptors in the postsynaptic membrane.
SCZ patients show decreased α4β2 nicotinic acetylcholine receptors (nAChR)[149]. However, the α7 nAChR level is increased in the DLPFC of SCZ patients[150]. Besides, functional polymorphisms of the α7 nAChR have shown genetic linkage in SCZ[151]. Muscarinic receptors, also called the metabotropic muscarinic acetylcholine receptors, have five subtypes (M1-M5 receptors), encoded by the CHRM1-5 genes. Postmortem studies suggest lower CHRM1 levels in the cortex of patients with SCZ[152]. The loss of cortical CHRM1 may be regulated by miR-107 in SCZ[153]. What’s more, CHRM1 is involved in memory processes, and blockade of hippocampal CHRM1 demonstrates a deficit in working memory[154]. Together, these results suggest that alterations in the cholinergic pathway may contribute to a breakdown in cholinergic homeostasis and have a key role in the pathophysiology of SCZ, particularly the cognitive impairments.
Other neurotransmitter pathways, such as NE and neurosteroids, have also been implicated in the cognitive dysfunction of SCZ.
NE is a significant neuromodulator of brain function and neural gain. NE exerts its effects through noradrenergic receptors (α1, α2, and β). The alteration of noradrenergic neurotransmission has been studied for years. It is a consensus that patients with SCZ have higher NE levels than the control group[155,156]. Furthermore, α2-adrenergic receptor antagonist idazoxan has antipsychotic efficacy in the treatment of SCZ, especially the anxiety or depression symptoms[157]. It may be associated with the increased output of DA.
Additionally, the abnormality of neurosteroid transmission also has a crucial role in the pathobiology and symptomatology of SCZ[158]. Both the levels of progesterone and allopregnanolone (ALLO) are decremented in SCZ in a postmortem study[159,160]. Studies suggest that ALLO enhances NMDA receptor neurotransmission by interaction with σ1 receptors in SCZ[161,162]. What’s more, decreased levels of ALLO may modulate GABAergic transmission in the brain and finally lead to impairments of GABAergic function in SCZ[163].
Most antipsychotic drugs target serotonin-dopamine receptors or serotonin-glutamate receptors, suggesting disarranged neurotransmitter interaction. Newer AAPDs, such as clozapine, olanzapine, and risperidone, have been developed because of their significant effects on dopaminergic receptor subtypes and serotonergic receptors[164]. Interestingly, co-immunoprecipitation studies verify that HTR2A and DRD2 physically interact in HEK293 cells. Furthermore, shreds of evidence reveal that HTR2A and mGlu2 receptors can assemble into a functional heteromeric complex to modulate each other’s function[165,166]. The expression of HTR2A is required for phosphorylation of mGlu2R at serine 843 and promotes mGlu2R-modulate G i/o signaling[167]. Therefore, there are potential antipsychotic drugs by targeting HTR2A, DRD2, and mGlu2R. DRD3 was found to be associated with SCZ in a case-control study[168]. Several pharmaceutical studies suggest that DRD1/5 agonists have potential therapeutic effects in SCZ by improving cognitive or negative symptoms[169,170]. What’s more, HTR4/6 agonists can improve cognitive symptoms in SCZ. HTR4/6 may be a promising target for treatment of cognitive dysfunction in SCZ[171]. Additionally, sarcosine (a competitive inhibitor of the type 1 glycine transporter) and D-amino acid oxidase (DAAO or DAO) inhibitor can improve the clinical symptoms in SCZ patients. Therefore, glycine transporter and DAO may offer potential therapeutic targets for SCZ[172,173].
There are many other potential targets for the treatment of SCZ. Accumulated pieces of evidence have revealed various susceptibility genes in SCZ, including STAB2, GRIN1, GRIN2A, ARC, BDNF, NRGN, syncytin-1, and others[67,81,174]. Interestingly, many of those genes appear to be related to the control of synaptic plasticity and cognitive impairments in SCZ. BDNF plays a principal role in regulating synaptic organization, neurotransmitter synthesis, and the maintenance of synaptic plasticity[175]. Data from our lab provide evidence that syncytin-1 can regulate the expression of BDNF and DISC1. Furthermore, GNbAC1, a monoclonal antibody targeting syncytin-1, has been implicated in the treatment of multiple sclerosis and type 1 diabetes[176,177]. Thus, syncytin-1 is a promising therapeutic target for SCZ in the future.
Accumulated shreds of evidence indicate that changes in the morphology of synapses have a vital role in the incidence of SCZ. The potential role of synapse in SCZ appears much more complicated. In conclusion, the synapse can be involved in three aspects as follows: (1) The change of synaptic plasticity (e.g., change in the dendrite spines, PSD, and alteration in LTP and LTD); (2) The abnormalities in neurotransmission (e.g., dopaminergic transmission, serotoninergic transmission, and glutamatergic transmission); and (3) The impairment of cognition (e.g., disconnection).
Impaired synaptic plasticity contributes to cognitive dysfunction in SCZ. These dysfunctions include abnormal brain connectivity and functional outcomes. With the development of brain imaging technology, research on cognitive impairments should do not focus on a single gene or brain regions but on neural circuits or brain networks to study the underlying mechanism in SCZ. SCZ is a complex disease, and there are still no available antipsychotic drugs to treat all symptoms of SCZ or accompany little side effects. Finding potential antipsychotic drug targets will help identify and develop novel therapeutic agents with fewer side effects.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lane HY, Taiwan S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Gao CC
| 1. | Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1067] [Cited by in RCA: 1283] [Article Influence: 142.6] [Reference Citation Analysis (1)] |
| 2. | McCollum LA, Walker CK, Roche JK, Roberts RC. Elevated Excitatory Input to the Nucleus Accumbens in Schizophrenia: A Postmortem Ultrastructural Study. Schizophr Bull. 2015;41:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71:1323-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 294] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 4. | Onwordi EC, Halff EF, Whitehurst T, Mansur A, Cotel MC, Wells L, Creeney H, Bonsall D, Rogdaki M, Shatalina E, Reis Marques T, Rabiner EA, Gunn RN, Natesan S, Vernon AC, Howes OD. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun. 2020;11:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 5. | Gulsuner S, Stein DJ, Susser ES, Sibeko G, Pretorius A, Walsh T, Majara L, Mndini MM, Mqulwana SG, Ntola OA, Casadei S, Ngqengelele LL, Korchina V, van der Merwe C, Malan M, Fader KM, Feng M, Willoughby E, Muzny D, Baldinger A, Andrews HF, Gur RC, Gibbs RA, Zingela Z, Nagdee M, Ramesar RS, King MC, McClellan JM. Genetics of schizophrenia in the South African Xhosa. Science. 2020;367:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Obi-Nagata K, Temma Y, Hayashi-Takagi A. Synaptic functions and their disruption in schizophrenia: From clinical evidence to synaptic optogenetics in an animal model. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95:179-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Frankiewicz T, Potier B, Bashir ZI, Collingridge GL, Parsons CG. Effects of memantine and MK-801 on NMDA-induced currents in cultured neurones and on synaptic transmission and LTP in area CA1 of rat hippocampal slices. Br J Pharmacol. 1996;117:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Pitkänen M, Sirviö J, MacDonald E, Niemi S, Ekonsalo T, Riekkinen P Sr. The effects of D-cycloserine and MK-801 on the performance of rats in two spatial learning and memory tasks. Eur Neuropsychopharmacol. 1995;5:457-463. [PubMed] |
| 9. | Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1529] [Cited by in RCA: 1528] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 11. | Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2300] [Cited by in RCA: 2254] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 12. | Monteleone P, Cascino G, Monteleone AM, Rocca P, Rossi A, Bertolino A, Aguglia E, Amore M, Collantoni E, Corrivetti G, Cuomo A, Bellomo A, D'Ambrosio E, Dell'Osso L, Frascarelli M, Giordano GM, Giuliani L, Marchesi C, Montemagni C, Oldani L, Pinna F, Pompili M, Roncone R, Rossi R, Siracusano A, Vita A, Zeppegno P, Galderisi S, Maj M; Italian Network for Research on Psychoses. Prevalence of antipsychotic-induced extrapyramidal symptoms and their association with neurocognition and social cognition in outpatients with schizophrenia in the "real-life". Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Pereda AE. Electrical synapses and their functional interactions with chemical synapses. Nat Rev Neurosci. 2014;15:250-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 361] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 14. | Siksou L, Triller A, Marty S. Ultrastructural organization of presynaptic terminals. Curr Opin Neurobiol. 2011;21:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Sheng M, Kim E. The postsynaptic organization of synapses. Cold Spring Harb Perspect Biol. 2011;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 16. | ROBERTSON JD. Ultrastructure of two invertebrate synapses. Proc Soc Exp Biol Med. 1953;82:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 57] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Marrone DF, Petit TL. The role of synaptic morphology in neural plasticity: structural interactions underlying synaptic power. Brain Res Brain Res Rev. 2002;38:291-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Jing Y, Wang Z, Song Y. Quantitative study of aluminum-induced changes in synaptic ultrastructure in rats. Synapse. 2004;52:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Desmond NL, Levy WB. Synaptic interface surface area increases with long-term potentiation in the hippocampal dentate gyrus. Brain Res. 1988;453:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | MacDonald ML, Alhassan J, Newman JT, Richard M, Gu H, Kelly RM, Sampson AR, Fish KN, Penzes P, Wills ZP, Lewis DA, Sweet RA. Selective Loss of Smaller Spines in Schizophrenia. Am J Psychiatry. 2017;174:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 21. | Herms J, Dorostkar MM. Dendritic Spine Pathology in Neurodegenerative Diseases. Annu Rev Pathol. 2016;11:221-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 22. | Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 23. | Bailey CH, Kandel ER, Harris KM. Structural Components of Synaptic Plasticity and Memory Consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 24. | Penn AC, Zhang CL, Georges F, Royer L, Breillat C, Hosy E, Petersen JD, Humeau Y, Choquet D. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature. 2017;549:384-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 25. | Meyer D, Bonhoeffer T, Scheuss V. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82:430-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 26. | Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci. 2007;1:131-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 400] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 27. | Lüscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol. 2012;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 719] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 28. | Stevens CF, Sullivan J. Synaptic plasticity. Curr Biol. 1998;8:R151-R153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 598] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 30. | Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 330] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 31. | Neveu D, Zucker RS. Postsynaptic levels of [Ca2+]i needed to trigger LTD and LTP. Neuron. 1996;16:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 157] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Lu YM, Roder JC, Davidow J, Salter MW. Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science. 1998;279:1363-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 252] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Wang JH, Feng DP. Postsynaptic protein kinase C essential to induction and maintenance of long-term potentiation in the hippocampal CA1 region. Proc Natl Acad Sci U S A. 1992;89:2576-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 126] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Izumi Y, Tokuda K, Zorumski CF. Long-term potentiation inhibition by low-level N-methyl-D-aspartate receptor activation involves calcineurin, nitric oxide, and p38 mitogen-activated protein kinase. Hippocampus. 2008;18:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Hill TC, Zito K. LTP-induced long-term stabilization of individual nascent dendritic spines. J Neurosci. 2013;33:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 36. | Desmond NL, Levy WB. Changes in the postsynaptic density with long-term potentiation in the dentate gyrus. J Comp Neurol. 1986;253:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1861] [Cited by in RCA: 1898] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 38. | Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1225] [Cited by in RCA: 1323] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 39. | Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3410] [Cited by in RCA: 3391] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 40. | Mehta MR. From synaptic plasticity to spatial maps and sequence learning. Hippocampus. 2015;25:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Hasan MT, Hernández-González S, Dogbevia G, Treviño M, Bertocchi I, Gruart A, Delgado-García JM. Role of motor cortex NMDA receptors in learning-dependent synaptic plasticity of behaving mice. Nat Commun. 2013;4:2258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | Hirano T. Regulation and Interaction of Multiple Types of Synaptic Plasticity in a Purkinje Neuron and Their Contribution to Motor Learning. Cerebellum. 2018;17:756-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O'Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 357] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 44. | Piette C, Touboul J, Venance L. Engrams of Fast Learning. Front Cell Neurosci. 2020;14:575915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Yger P, Stimberg M, Brette R. Fast Learning with Weak Synaptic Plasticity. J Neurosci. 2015;35:13351-13362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Galván A. Adolescence, brain maturation and mental health. Nat Neurosci. 2017;20:503-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Johnson MH. Functional brain development in humans. Nat Rev Neurosci. 2001;2:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 562] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 48. | Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 2013;3:e238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 279] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 49. | Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 50. | Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:13281-13286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 941] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 51. | Piochon C, Kano M, Hansel C. LTD-like molecular pathways in developmental synaptic pruning. Nat Neurosci. 2016;19:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 52. | Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, Gousias IS, Edwards AD, Counsell SJ. The effect of preterm birth on thalamic and cortical development. Cereb Cortex. 2012;22:1016-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 53. | Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1164] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 54. | Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174-8179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3626] [Cited by in RCA: 3719] [Article Influence: 177.1] [Reference Citation Analysis (0)] |
| 55. | Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 531] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 57. | Carlisle HJ, Fink AE, Grant SG, O'Dell TJ. Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. J Physiol. 2008;586:5885-5900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 58. | Guo F, Zhao J, Zhao D, Wang J, Wang X, Feng Z, Vreugdenhil M, Lu C. Dopamine D4 receptor activation restores CA1 LTP in hippocampal slices from aged mice. Aging Cell. 2017;16:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol. 2006;18:71-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 60. | Hasan A, Nitsche MA, Herrmann M, Schneider-Axmann T, Marshall L, Gruber O, Falkai P, Wobrock T. Impaired long-term depression in schizophrenia: a cathodal tDCS pilot study. Brain Stimul. 2012;5:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Hasan A, Nitsche MA, Rein B, Schneider-Axmann T, Guse B, Gruber O, Falkai P, Wobrock T. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behav Brain Res. 2011;224:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 62. | Hamilton HK, Roach BJ, Cavus I, Teyler TJ, Clapp WC, Ford JM, Tarakci E, Krystal JH, Mathalon DH. Impaired Potentiation of Theta Oscillations During a Visual Cortical Plasticity Paradigm in Individuals With Schizophrenia. Front Psychiatry. 2020;11:590567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Cadinu D, Grayson B, Podda G, Harte MK, Doostdar N, Neill JC. NMDA receptor antagonist rodent models for cognition in schizophrenia and identification of novel drug treatments, an update. Neuropharmacology. 2018;142:41-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 64. | Rung JP, Carlsson A, Rydén Markinhuhta K, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 65. | Nakazawa K, Sapkota K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther. 2020;205:107426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 66. | Huang Y, Jiang H, Zheng Q, Fok AHK, Li X, Lau CG, Lai CSW. Environmental enrichment or selective activation of parvalbumin-expressing interneurons ameliorates synaptic and behavioral deficits in animal models with schizophrenia-like behaviors during adolescence. Mol Psychiatry. 2021;26:2533-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 67. | Hwang H, Szucs MJ, Ding LJ, Allen A, Ren X, Haensgen H, Gao F, Rhim H, Andrade A, Pan JQ, Carr SA, Ahmad R, Xu W. Neurogranin, Encoded by the Schizophrenia Risk Gene NRGN, Bidirectionally Modulates Synaptic Plasticity via Calmodulin-Dependent Regulation of the Neuronal Phosphoproteome. Biol Psychiatry. 2021;89:256-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | Melkersson K. Introduction: clinical findings related to alterations of the intracellular calcium homeostasis in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1365-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 69. | Jimerson DC, Post RM, Carman JS, van Kammen DP, Wood JH, Goodwin FK, Bunney WE Jr. CSF calcium: clinical correlates in affective illness and schizophrenia. Biol Psychiatry. 1979;14:37-51. [PubMed] |
| 70. | Tian SY, Wang JF, Bezchlibnyk YB, Young LT. Immunoreactivity of 43 kDa growth-associated protein is decreased in post mortem hippocampus of bipolar disorder and schizophrenia. Neurosci Lett. 2007;411:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Weickert CS, Webster MJ, Hyde TM, Herman MM, Bachus SE, Bali G, Weinberger DR, Kleinman JE. Reduced GAP-43 mRNA in dorsolateral prefrontal cortex of patients with schizophrenia. Cereb Cortex. 2001;11:136-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Catts VS, Derminio DS, Hahn CG, Weickert CS. Postsynaptic density levels of the NMDA receptor NR1 subunit and PSD-95 protein in prefrontal cortex from people with schizophrenia. NPJ Schizophr. 2015;1:15037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Ohnuma T, Kato H, Arai H, Faull RL, McKenna PJ, Emson PC. Gene expression of PSD95 in prefrontal cortex and hippocampus in schizophrenia. Neuroreport. 2000;11:3133-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Fernández E, Collins MO, Frank RAW, Zhu F, Kopanitsa MV, Nithianantharajah J, Lemprière SA, Fricker D, Elsegood KA, McLaughlin CL, Croning MDR, Mclean C, Armstrong JD, Hill WD, Deary IJ, Cencelli G, Bagni C, Fromer M, Purcell SM, Pocklington AJ, Choudhary JS, Komiyama NH, Grant SGN. Arc Requires PSD95 for Assembly into Postsynaptic Complexes Involved with Neural Dysfunction and Intelligence. Cell Rep. 2017;21:679-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 75. | Pavlowsky A, Gianfelice A, Pallotto M, Zanchi A, Vara H, Khelfaoui M, Valnegri P, Rezai X, Bassani S, Brambilla D, Kumpost J, Blahos J, Roux MJ, Humeau Y, Chelly J, Passafaro M, Giustetto M, Billuart P, Sala C. A postsynaptic signaling pathway that may account for the cognitive defect due to IL1RAPL1 mutation. Curr Biol. 2010;20:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 76. | Yadav S, Oses-Prieto JA, Peters CJ, Zhou J, Pleasure SJ, Burlingame AL, Jan LY, Jan YN. TAOK2 Kinase Mediates PSD95 Stability and Dendritic Spine Maturation through Septin7 Phosphorylation. Neuron. 2017;93:379-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 77. | Alsabban AH, Morikawa M, Tanaka Y, Takei Y, Hirokawa N. Kinesin Kif3b mutation reduces NMDAR subunit NR2A trafficking and causes schizophrenia-like phenotypes in mice. EMBO J. 2020;39:e101090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 78. | Forsyth JK, Nachun D, Gandal MJ, Geschwind DH, Anderson AE, Coppola G, Bearden CE. Synaptic and Gene Regulatory Mechanisms in Schizophrenia, Autism, and 22q11.2 Copy Number Variant-Mediated Risk for Neuropsychiatric Disorders. Biol Psychiatry. 2020;87:150-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 79. | Chen Y, Yan Q, Zhou P, Li S, Zhu F. HERV-W env regulates calcium influx via activating TRPC3 channel together with depressing DISC1 in human neuroblastoma cells. J Neurovirol. 2019;25:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 80. | Wang X, Liu Z, Wang P, Li S, Zeng J, Tu X, Yan Q, Xiao Z, Pan M, Zhu F. Syncytin-1, an endogenous retroviral protein, triggers the activation of CRP via TLR3 signal cascade in glial cells. Brain Behav Immun. 2018;67:324-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 81. | Huang W, Li S, Hu Y, Yu H, Luo F, Zhang Q, Zhu F. Implication of the env gene of the human endogenous retrovirus W family in the expression of BDNF and DRD3 and development of recent-onset schizophrenia. Schizophr Bull. 2011;37:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 82. | Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J Cell Biol. 2006;174:289-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 83. | Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 626] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 84. | Rolls ET, Cheng W, Gilson M, Gong W, Deco G, Lo CZ, Yang AC, Tsai SJ, Liu ME, Lin CP, Feng J. Beyond the disconnectivity hypothesis of schizophrenia. Cereb Cortex. 2020;30:1213-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 85. | Moussa-Tooks AB, Kim DJ, Bartolomeo LA, Purcell JR, Bolbecker AR, Newman SD, O'Donnell BF, Hetrick WP. Impaired Effective Connectivity During a Cerebellar-Mediated Sensorimotor Synchronization Task in Schizophrenia. Schizophr Bull. 2019;45:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 86. | Avram M, Brandl F, Bäuml J, Sorg C. Cortico-thalamic hypo- and hyperconnectivity extend consistently to basal ganglia in schizophrenia. Neuropsychopharmacology. 2018;43:2239-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 87. | Avram M, Brandl F, Knolle F, Cabello J, Leucht C, Scherr M, Mustafa M, Koutsouleris N, Leucht S, Ziegler S, Sorg C. Aberrant striatal dopamine links topographically with cortico-thalamic dysconnectivity in schizophrenia. Brain. 2020;143:3495-3505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Sharma A, Kumar A, Singh S, Bhatia T, Beniwal RP, Khushu S, Prasad KM, Deshpande SN. Altered resting state functional connectivity in early course schizophrenia. Psychiatry Res Neuroimaging. 2018;271:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Das P, Alexander D, Boord P, Brown K, Flynn G, Galletly C, Gordon E, Harris A, Whitford T, Williams L, Wong W. Impaired connectivity in amygdala pathways may explain disorganization symptoms of patients with first-episode schizophrenia. Acta Neuropsychiatr. 2006;18:282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 90. | McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 438] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 91. | Rabe-Jabłońska J. [Significance of synaptic connectivity reduction for pathogenesis, clinical picture and course of schizophrenia]. Psychiatr Pol. 2003;37:951-964. [PubMed] |
| 92. | Warm D, Schroer J, Sinning A. Gabaergic Interneurons in Early Brain Development: Conducting and Orchestrated by Cortical Network Activity. Front Mol Neurosci. 2021;14:807969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 93. | Bitanihirwe BK, Woo TU. Perineuronal nets and schizophrenia: the importance of neuronal coatings. Neurosci Biobehav Rev. 2014;45:85-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 94. | Hirano Y, Oribe N, Onitsuka T, Kanba S, Nestor PG, Hosokawa T, Levin M, Shenton ME, McCarley RW, Spencer KM. Auditory Cortex Volume and Gamma Oscillation Abnormalities in Schizophrenia. Clin EEG Neurosci. 2020;51:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 95. | Olivier RM, Kilian S, Chiliza B, Asmal L, Oosthuizen PP, Emsley R, Kidd M. Cognitive-perceptual deficits and symptom correlates in first-episode schizophrenia. S Afr J Psychiatr. 2017;23:1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Solís-Vivanco R, Rangel-Hassey F, León-Ortiz P, Mondragón-Maya A, Reyes-Madrigal F, de la Fuente-Sandoval C. Cognitive Impairment in Never-Medicated Individuals on the Schizophrenia Spectrum. JAMA Psychiatry. 2020;77:543-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Kudo N, Yamamori H, Ishima T, Nemoto K, Yasuda Y, Fujimoto M, Azechi H, Niitsu T, Numata S, Ikeda M, Iyo M, Ohmori T, Fukunaga M, Watanabe Y, Hashimoto K, Hashimoto R. Plasma levels of matrix metalloproteinase-9 (MMP-9) are associated with cognitive performance in patients with schizophrenia. Neuropsychopharmacol Rep. 2020;40:150-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 98. | Chen S, Tian L, Chen N, Xiu M, Wang Z, Yang G, Wang C, Yang F, Tan Y. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 99. | Zhai J, Zhang Q, Cheng L, Chen M, Wang K, Liu Y, Deng X, Chen X, Shen Q, Xu Z, Ji F, Liu C, Dong Q, Chen C, Li J. Risk variants in the S100B gene, associated with elevated S100B levels, are also associated with visuospatial disability of schizophrenia. Behav Brain Res. 2011;217:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Fu S, Czajkowski N, Rund BR, Torgalsbøen AK. The relationship between level of cognitive impairments and functional outcome trajectories in first-episode schizophrenia. Schizophr Res. 2017;190:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 101. | Butler T, Weisholtz D, Isenberg N, Harding E, Epstein J, Stern E, Silbersweig D. Neuroimaging of frontal-limbic dysfunction in schizophrenia and epilepsy-related psychosis: toward a convergent neurobiology. Epilepsy Behav. 2012;23:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 102. | Bourque J, Lakis N, Champagne J, Stip E, Lalonde P, Lipp O, Mendrek A. Clozapine and visuospatial processing in treatment-resistant schizophrenia. Cogn Neuropsychiatry. 2013;18:615-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Lee MA, Thompson PA, Meltzer HY. Effects of clozapine on cognitive function in schizophrenia. J Clin Psychiatry. 1994;55 Suppl B:82-87. [PubMed] |
| 104. | Essali A, Al-Haj Haasan N, Li C, Rathbone J. Clozapine vs typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009;CD000059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 105. | Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. 2011;508:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 798] [Cited by in RCA: 713] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 106. | Elsworth JD, Roth RH. Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson's disease. Exp Neurol. 1997;144:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 175] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 107. | Eiden LE, Weihe E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann N Y Acad Sci. 2011;1216:86-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 108. | Beaulieu JM. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci. 2012;37:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 109. | Karam CS, Ballon JS, Bivens NM, Freyberg Z, Girgis RR, Lizardi-Ortiz JE, Markx S, Lieberman JA, Javitch JA. Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol Sci. 2010;31:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 110. | Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 872] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 111. | Mackay AV, Iversen LL, Rossor M, Spokes E, Bird E, Arregui A, Creese I, Synder SH. Increased brain dopamine and dopamine receptors in schizophrenia. Arch Gen Psychiatry. 1982;39:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 249] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 112. | Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1:133-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 556] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 113. | McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19:15-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 363] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 114. | Fernandez SP, Muzerelle A, Scotto-Lomassese S, Barik J, Gruart A, Delgado-García JM, Gaspar P. Constitutive and Acquired Serotonin Deficiency Alters Memory and Hippocampal Synaptic Plasticity. Neuropsychopharmacology. 2017;42:512-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 115. | Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Jia C, Hu F, Zeng J, Guo Q, Fu L, Luo M. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun. 2016;7:10503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 271] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 116. | Frick A, Åhs F, Engman J, Jonasson M, Alaie I, Björkstrand J, Frans Ö, Faria V, Linnman C, Appel L, Wahlstedt K, Lubberink M, Fredrikson M, Furmark T. Serotonin Synthesis and Reuptake in Social Anxiety Disorder: A Positron Emission Tomography Study. JAMA Psychiatry. 2015;72:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 117. | Pratelli M, Pasqualetti M. Serotonergic neurotransmission manipulation for the understanding of brain development and function: Learning from Tph2 genetic models. Biochimie. 2019;161:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 118. | Xu XM, Ding M, Pang H, Wang BJ. TPH2 gene polymorphisms in the regulatory region are associated with paranoid schizophrenia in Northern Han Chinese. Genet Mol Res. 2014;13:1497-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 119. | Zhang C, Li Z, Shao Y, Xie B, Du Y, Fang Y, Yu S. Association study of tryptophan hydroxylase-2 gene in schizophrenia and its clinical features in Chinese Han population. J Mol Neurosci. 2011;43:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE. Selective abnormalities of prefrontal serotonergic receptors in schizophrenia. A postmortem study. Arch Gen Psychiatry. 1993;50:810-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 162] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 121. | Vijayan NN, Iwayama Y, Koshy LV, Natarajan C, Nair C, Allencherry PM, Yoshikawa T, Banerjee M. Evidence of association of serotonin transporter gene polymorphisms with schizophrenia in a South Indian population. J Hum Genet. 2009;54:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 122. | Maeda K, Lerdrup L, Sugino H, Akazawa H, Amada N, McQuade RD, Stensbøl TB, Bundgaard C, Arnt J, Kikuchi T. Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350:605-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 123. | Sumiyoshi T, Higuchi Y, Uehara T. Neural basis for the ability of atypical antipsychotic drugs to improve cognition in schizophrenia. Front Behav Neurosci. 2013;7:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 124. | Ohno Y. New insight into the therapeutic role of 5-HT1A receptors in central nervous system disorders. Cent Nerv Syst Agents Med Chem. 2010;10:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 125. | Lin CH, Lin PP, Lin CY, Lin CH, Huang CH, Huang YJ, Lane HY. Decreased mRNA expression for the two subunits of system xc(-), SLC3A2 and SLC7A11, in WBC in patients with schizophrenia: Evidence in support of the hypo-glutamatergic hypothesis of schizophrenia. J Psychiatr Res. 2016;72:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 126. | Kim JS, Kornhuber HH, Schmid-Burgk W, Holzmüller B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci Lett. 1980;20:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 448] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 127. | Harrison PJ, Law AJ, Eastwood SL. Glutamate receptors and transporters in the hippocampus in schizophrenia. Ann N Y Acad Sci. 2003;1003:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 128. | Uezato A, Meador-Woodruff JH, McCullumsmith RE. Vesicular glutamate transporter mRNA expression in the medial temporal lobe in major depressive disorder, bipolar disorder, and schizophrenia. Bipolar Disord. 2009;11:711-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 129. | Pietrancosta N, Djibo M, Daumas S, El Mestikawy S, Erickson JD. Molecular, Structural, Functional, and Pharmacological Sites for Vesicular Glutamate Transporter Regulation. Mol Neurobiol. 2020;57:3118-3142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 130. | Parkin GM, Gibbons A, Udawela M, Dean B. Excitatory amino acid transporter (EAAT)1 and EAAT2 mRNA levels are altered in the prefrontal cortex of subjects with schizophrenia. J Psychiatr Res. 2020;123:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Wilmsdorff MV, Blaich C, Zink M, Treutlein J, Bauer M, Schulze T, Schneider-Axmann T, Gruber O, Rietschel M, Schmitt A, Falkai P. Gene expression of glutamate transporters SLC1A1, SLC1A3 and SLC1A6 in the cerebellar subregions of elderly schizophrenia patients and effects of antipsychotic treatment. World J Biol Psychiatry. 2013;14:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 132. | Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 133. | Ding X, Qiao Y, Piao C, Zheng X, Liu Z, Liang J. N-methyl-D-aspartate receptor-mediated glutamate transmission in nucleus accumbens plays a more important role than that in dorsal striatum in cognitive flexibility. Front Behav Neurosci. 2014;8:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Curr Opin Pharmacol. 2007;7:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 135. | Bialecki J, Werner A, Weilinger NL, Tucker CM, Vecchiarelli HA, Egaña J, Mendizabal-Zubiaga J, Grandes P, Hill MN, Thompson RJ. Suppression of Presynaptic Glutamate Release by Postsynaptic Metabotropic NMDA Receptor Signalling to Pannexin-1. J Neurosci. 2020;40:729-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 136. | Lin E, Lin CH, Hung CC, Lane HY. An Ensemble Approach to Predict Schizophrenia Using Protein Data in the N-methyl-D-Aspartate Receptor (NMDAR) and Tryptophan Catabolic Pathways. Front Bioeng Biotechnol. 2020;8:569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 137. | Jagannath V, Gerstenberg M, Correll CU, Walitza S, Grünblatt E. A systematic meta-analysis of the association of Neuregulin 1 (NRG1), D-amino acid oxidase (DAO), and DAO activator (DAOA)/G72 polymorphisms with schizophrenia. J Neural Transm (Vienna). 2018;125:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 138. | Orhan F, Fatouros-Bergman H, Goiny M, Malmqvist A, Piehl F; Karolinska Schizophrenia Project (KaSP) Consortium, Cervenka S, Collste K, Victorsson P, Sellgren CM, Flyckt L, Erhardt S, Engberg G. CSF GABA is reduced in first-episode psychosis and associates to symptom severity. Mol Psychiatry. 2018;23:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 139. | Ramaker RC, Bowling KM, Lasseigne BN, Hagenauer MH, Hardigan AA, Davis NS, Gertz J, Cartagena PM, Walsh DM, Vawter MP, Jones EG, Schatzberg AF, Barchas JD, Watson SJ, Bunney BG, Akil H, Bunney WE, Li JZ, Cooper SJ, Myers RM. Post-mortem molecular profiling of three psychiatric disorders. Genome Med. 2017;9:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 140. | Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 141. | Kimoto S, Bazmi HH, Lewis DA. Lower expression of glutamic acid decarboxylase 67 in the prefrontal cortex in schizophrenia: contribution of altered regulation by Zif268. Am J Psychiatry. 2014;171:969-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 142. | Tao R, Davis KN, Li C, Shin JH, Gao Y, Jaffe AE, Gondré-Lewis MC, Weinberger DR, Kleinman JE, Hyde TM. GAD1 alternative transcripts and DNA methylation in human prefrontal cortex and hippocampus in brain development, schizophrenia. Mol Psychiatry. 2018;23:1496-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 143. | Schleimer SB, Hinton T, Dixon G, Johnston GA. GABA transporters GAT-1 and GAT-3 in the human dorsolateral prefrontal cortex in schizophrenia. Neuropsychobiology. 2004;50:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 144. | Fujihara K, Yamada K, Ichitani Y, Kakizaki T, Jiang W, Miyata S, Suto T, Kato D, Saito S, Watanabe M, Kajita Y, Ohshiro T, Mushiake H, Miyasaka Y, Mashimo T, Yasuda H, Yanagawa Y. CRISPR/Cas9-engineered Gad1 elimination in rats leads to complex behavioral changes: implications for schizophrenia. Transl Psychiatry. 2020;10:426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 145. | Marques TR, Ashok AH, Angelescu I, Borgan F, Myers J, Lingford-Hughes A, Nutt DJ, Veronese M, Turkheimer FE, Howes OD. GABA-A receptor differences in schizophrenia: a positron emission tomography study using [11C]Ro154513. Mol Psychiatry. 2021;26:2616-2625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 146. | Karson CN, Mrak RE, Husain MM, Griffin WS. Decreased mesopontine choline acetyltransferase levels in schizophrenia. Correlations with cognitive functions. Mol Chem Neuropathol. 1996;29:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 147. | Shoja Shafti S, Azizi Khoei A. Effectiveness of rivastigmine on positive, negative, and cognitive symptoms of schizophrenia: a double-blind clinical trial. Ther Adv Psychopharmacol. 2016;6:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 148. | Thakurathi N, Vincenzi B, Henderson DC. Assessing the prospect of donepezil in improving cognitive impairment in patients with schizophrenia. Expert Opin Investig Drugs. 2013;22:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 149. | Durany N, Zöchling R, Boissl KW, Paulus W, Ransmayr G, Tatschner T, Danielczyk W, Jellinger K, Deckert J, Riederer P. Human post-mortem striatal alpha4beta2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson's syndrome. Neurosci Lett. 2000;287:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 150. | Dean B, Pavey G, Scarr E. Higher levels of α7 nicotinic receptors, but not choline acetyltransferase, in the dorsolateral prefrontal cortex from a sub-group of patients with schizophrenia. Schizophr Res. 2020;222:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 151. | De Luca V, Wang H, Squassina A, Wong GW, Yeomans J, Kennedy JL. Linkage of M5 muscarinic and alpha7-nicotinic receptor genes on 15q13 to schizophrenia. Neuropsychobiology. 2004;50:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 152. | Scarr E, Hopper S, Vos V, Seo MS, Everall IP, Aumann TD, Chana G, Dean B. Low levels of muscarinic M1 receptor-positive neurons in cortical layers III and V in Brodmann areas 9 and 17 from individuals with schizophrenia. J Psychiatry Neurosci. 2018;43:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 153. | Scarr E, Craig JM, Cairns MJ, Seo MS, Galati JC, Beveridge NJ, Gibbons A, Juzva S, Weinrich B, Parkinson-Bates M, Carroll AP, Saffery R, Dean B. Decreased cortical muscarinic M1 receptors in schizophrenia are associated with changes in gene promoter methylation, mRNA and gene targeting microRNA. Transl Psychiatry. 2013;3:e230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 154. | Ohno M, Yamamoto T, Watanabe S. Blockade of hippocampal M1 muscarinic receptors impairs working memory performance of rats. Brain Res. 1994;650:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 155. | Mäki-Marttunen V, Andreassen OA, Espeseth T. The role of norepinephrine in the pathophysiology of schizophrenia. Neurosci Biobehav Rev. 2020;118:298-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 156. | Fitzgerald PJ. Is elevated norepinephrine an etiological factor in some cases of schizophrenia? Psychiatry Res. 2014;215:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 157. | Hertel P, Nomikos GG, Svensson TH. Idazoxan preferentially increases dopamine output in the rat medial prefrontal cortex at the nerve terminal level. Eur J Pharmacol. 1999;371:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 158. | Cai H, Cao T, Zhou X, Yao JK. Neurosteroids in Schizophrenia: Pathogenic and Therapeutic Implications. Front Psychiatry. 2018;9:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 159. | Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31:1249-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 160. | Taherianfard M, Shariaty M. Evaluation of serum steroid hormones in schizophrenic patients. Indian J Med Sci. 2004;58:3-9. [PubMed] |
| 161. | Ratner MH, Kumaresan V, Farb DH. Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders. Front Endocrinol (Lausanne). 2019;10:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 162. | Jorratt P, Hoschl C, Ovsepian SV. Endogenous antagonists of N-methyl-d-aspartate receptor in schizophrenia. Alzheimers Dement. 2021;17:888-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 163. | Hantsoo L, Epperson CN. Allopregnanolone in premenstrual dysphoric disorder (PMDD): Evidence for dysregulated sensitivity to GABA-A receptor modulating neuroactive steroids across the menstrual cycle. Neurobiol Stress. 2020;12:100213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 164. | Kuroki T, Nagao N, Nakahara T. Neuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin-dopamine hypothesis. Prog Brain Res. 2008;172:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 165. | Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76-87. [PubMed] |
| 166. | González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 677] [Cited by in RCA: 622] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 167. | Murat S, Bigot M, Chapron J, König GM, Kostenis E, Battaglia G, Nicoletti F, Bourinet E, Bockaert J, Marin P, Vandermoere F. 5-HT2A receptor-dependent phosphorylation of mGlu2 receptor at Serine 843 promotes mGlu2 receptor-operated Gi/o signaling. Mol Psychiatry. 2019;24:1610-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 168. | Morozova A, Zorkina Y, Pavlov K, Pavlova O, Storozheva Z, Zubkov E, Zakharova N, Karpenko O, Reznik A, Chekhonin V, Kostyuk G. Association of rs4680 COMT, rs6280 DRD3, and rs7322347 5HT2A With Clinical Features of Youth-Onset Schizophrenia. Front Psychiatry. 2019;10:830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 169. | Homberg JR, Olivier JD, VandenBroeke M, Youn J, Ellenbroek AK, Karel P, Shan L, van Boxtel R, Ooms S, Balemans M, Langedijk J, Muller M, Vriend G, Cools AR, Cuppen E, Ellenbroek BA. The role of the dopamine D1 receptor in social cognition: studies using a novel genetic rat model. Dis Model Mech. 2016;9:1147-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 170. | De Bundel D, Femenía T, DuPont CM, Konradsson-Geuken Å, Feltmann K, Schilström B, Lindskog M. Hippocampal and prefrontal dopamine D1/5 receptor involvement in the memory-enhancing effect of reboxetine. Int J Neuropsychopharmacol. 2013;16:2041-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 171. | Kumar A, Yadav M, Parle M, Dhingra S, Dhull DK. Potential drug targets and treatment of schizophrenia. Inflammopharmacology. 2017;25:277-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 172. | Chang CH, Lin CH, Liu CY, Chen SJ, Lane HY. Efficacy and cognitive effect of sarcosine (N-methylglycine) in patients with schizophrenia: A systematic review and meta-analysis of double-blind randomised controlled trials. J Psychopharmacol. 2020;34:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 173. | Lin CH, Chen YM, Lane HY. Novel Treatment for the Most Resistant Schizophrenia: Dual Activation of NMDA Receptor and Antioxidant. Curr Drug Targets. 2020;21:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 174. | Wang Q, Chen R, Cheng F, Wei Q, Ji Y, Yang H, Zhong X, Tao R, Wen Z, Sutcliffe JS, Liu C, Cook EH, Cox NJ, Li B. A Bayesian framework that integrates multi-omics data and gene networks predicts risk genes from schizophrenia GWAS data. Nat Neurosci. 2019;22:691-699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 175. | Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol Neurobiol. 2018;38:579-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 703] [Cited by in RCA: 898] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 176. | Diebold M, Derfuss T. The monoclonal antibody GNbAC1: targeting human endogenous retroviruses in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419833574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 177. | Curtin F, Bernard C, Levet S, Perron H, Porchet H, Médina J, Malpass S, Lloyd D, Simpson R; RAINBOW-T1D investigators. A new therapeutic approach for type 1 diabetes: Rationale for GNbAC1, an anti-HERV-W-Env monoclonal antibody. Diabetes Obes Metab. 2018;20:2075-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |