Published online Feb 19, 2022. doi: 10.5498/wjp.v12.i2.264
Peer-review started: May 31, 2021
First decision: July 14, 2021
Revised: August 10, 2021
Accepted: January 17, 2022
Article in press: January 17, 2022
Published online: February 19, 2022
Processing time: 262 Days and 1.4 Hours
Schizophrenia and bipolar disorder are disabling psychiatric disorders with a worldwide prevalence of approximately 1%. Both disorders present chronic and deteriorating prognoses that impose a large burden, not only on patients but also on society and health systems. These mental illnesses share several clinical and neurobiological traits; of these traits, oligodendroglial dysfunction and alterations to white matter (WM) tracts could underlie the disconnection between brain regions related to their symptomatic domains. WM is mainly composed of heavily myelinated axons and glial cells. Myelin internodes are discrete axon-wrapping membrane sheaths formed by oligodendrocyte processes. Myelin ensheathment allows fast and efficient conduction of nerve impulses through the nodes of Ranvier, improving the overall function of neuronal circuits. Rapid and precisely synchronized nerve impulse conduction through fibers that connect distant brain structures is crucial for higher-level functions, such as cognition, memory, mood, and language. Several cellular and subcellular anomalies related to myelin and oligodendrocytes have been found in postmortem samples from patients with schizophrenia or bipolar disorder, and neuroimaging techniques have revealed consistent alterations at the macroscale connectomic level in both disorders. In this work, evidence regarding these multilevel alterations in oligodendrocytes and myelinated tracts is discussed, and the involvement of proteins in key functions of the oligodendroglial lineage, such as oligodendrogenesis and myelination, is highlighted. The molecular components of the axo-myelin unit could be important targets for novel therapeutic approaches to schizophrenia and bipolar disorder.
Core Tip: Schizophrenia and bipolar disorder are multifactorial neuropsychiatric entities that share clinical manifestations as well as alterations to brain structure and function, genetic characteristics, and neurobiological pathways. Among the main pathophysiological mechanisms shared by these conditions is oligodendroglial dysfunction. Scientific evidence that ranges from the microscale cellular and subcellular levels to the macroscale connectomic level strongly supports overall myelin dysfunction and brain disconnection as hallmarks of schizophrenia and bipolar disorder.
- Citation: Valdés-Tovar M, Rodríguez-Ramírez AM, Rodríguez-Cárdenas L, Sotelo-Ramírez CE, Camarena B, Sanabrais-Jiménez MA, Solís-Chagoyán H, Argueta J, López-Riquelme GO. Insights into myelin dysfunction in schizophrenia and bipolar disorder. World J Psychiatry 2022; 12(2): 264-285
- URL: https://www.wjgnet.com/2220-3206/full/v12/i2/264.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i2.264
Currently, it is widely understood that optimal functioning of the central nervous system (CNS) depends on synaptic connections and multidirectional interactions between neuronal and glial cells. One of the closest glial-neuronal interactions in the CNS occurs between oligodendrocytes and neurons through myelination. Myelin ensheathment induces axonal compartmentalization to form nodes of Ranvier, i.e., specialized domains that increase the conduction speed of action potentials. The saltatory propagation and speed of these electrical impulses depends on axon caliber, but primarily on myelin features such as the number and length of internodes as well as myelin width and compaction[1]. Therefore, myelination allows neuronal circuits to be finely tuned and synchronized and, as such, plays a key role in maintaining the proper connectivity between brain structures to support higher integrating processes, such as perception, memory, or cognition. Furthermore, through myelination, oligodendr
Myelination is a neurodevelopmental process that begins during the third trimester of pregnancy and increases steadily during childhood and early youth until it reaches a slow-increasing plateau in adult life[4,5]. This process is adaptive, with neural activity being one of the main factors driving myelin plasticity[6]. Moreover, because the myelin sheath is a specialized structure made up of multiple layers of plasma membrane, from which most cytoplasm is extruded, its composition is enriched with lipids (approximately 70% of its content) and proteins[7]. These proteins have important functional roles, such as providing anchorage between myelin lamellae, attaching axons and myelin at paranodal regions, signaling and interacting with cytoskeletal elements within oligodendrocytes[7,8]. As with any other cell type, the plasma membrane in oligodendrocytes is subject to homeostatic turnover; thus, to manage this large energy requirement, their metabolic rate is higher than that of other cell types[9]. This characteristic renders myelinating oligodendrocytes more vulnerable to cellular stress and oxidative damage generated by reactive oxygen/nitrogen species[10]. In the case of injury and myelin loss (demyeli
Due to the intrinsic complexity of oligodendrocyte morphology and functioning and the importance of myelination/remyelination processes for CNS homeostasis, impairments in oligodendroglial lineage may be associated with brain disorders. Within the last two decades, great effort has been made to determine and describe neuronal and glial alterations that contribute to the etiology of mental illnesses[15-18]. Of the studied mental disorders, we focused on schizophrenia and bipolar disorder because these neuropsychiatric illnesses present a chronic and deteriorating course that imposes a large burden, not only on patients but also on society and health systems. These disorders are long-lasting, severe mental health conditions that share genetic characteristics and alterations to brain structure and function, and neurobiological pathways[19-21]. Among the main pathophysiological mechanisms shared by these conditions is glial dysfunction[22,23], specifically related to myelination, which is the focus of this text.
Taking the above information into account, the aim of this work was to gather and discuss the evidence that myelin dysfunction at the cellular and subcellular levels may underlie the white matter (WM) macroscale connectome alterations evidenced by neuroimaging in schizophrenia and bipolar disorder, thereby supporting the disconnection hypothesis that explains the symptomatic domains of these clinical entities. For this purpose, we first provide a brief overview of the main structural features of myelin. Next, we present the evidence of myelin alterations at the microscale levels (cellular and subcellular) found in postmortem samples from schizophrenia and bipolar disorder patients. Then, we briefly compare the main findings at these levels. Finally, we review evidence at the macroscale level from neuroimaging techniques and find consistent support for dysconnectivity among key brain regions in these disorders. These neuroimaging techniques are the main methods that allow us to obtain information about brain structure and function from patients during the course of their illnesses.
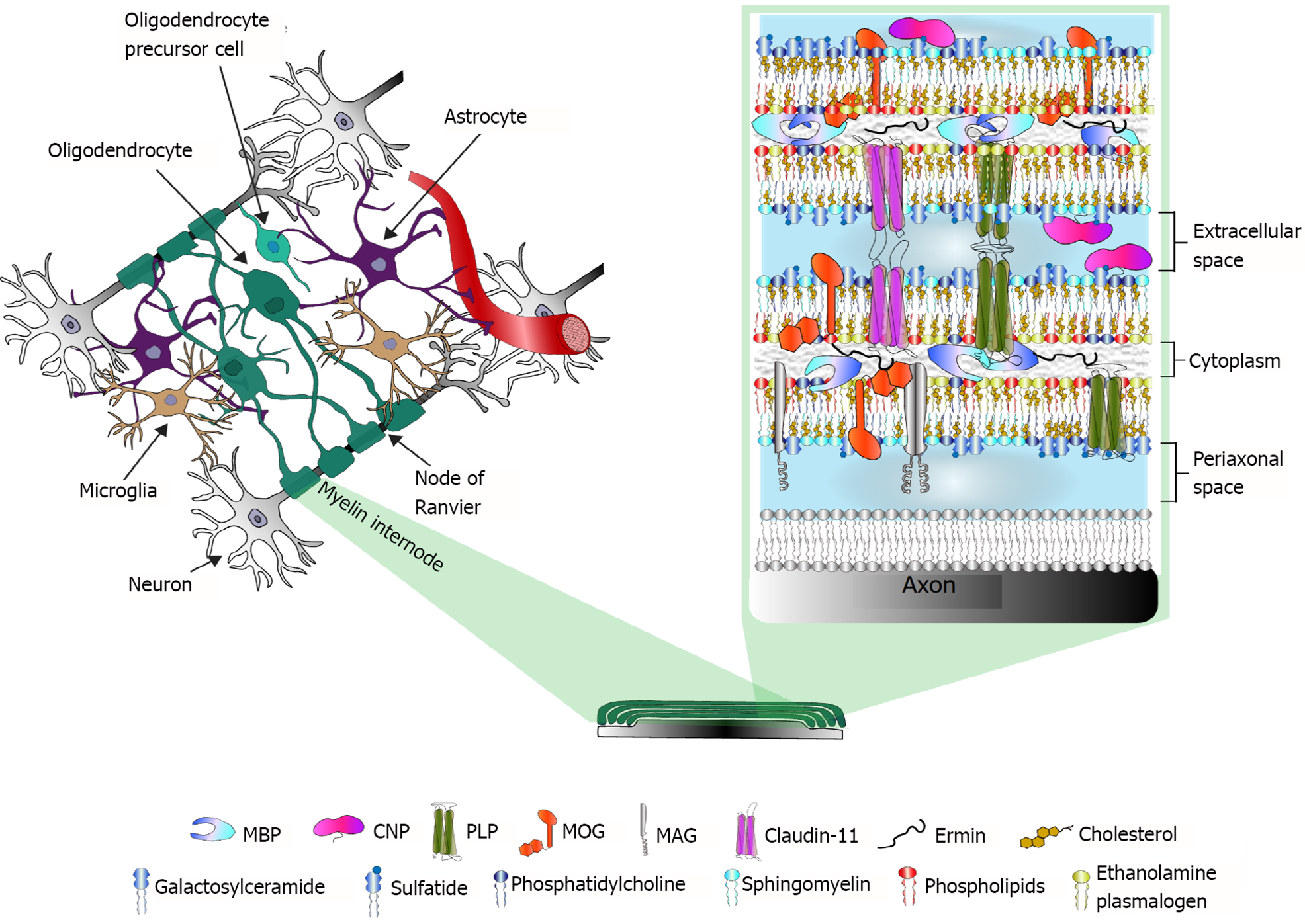
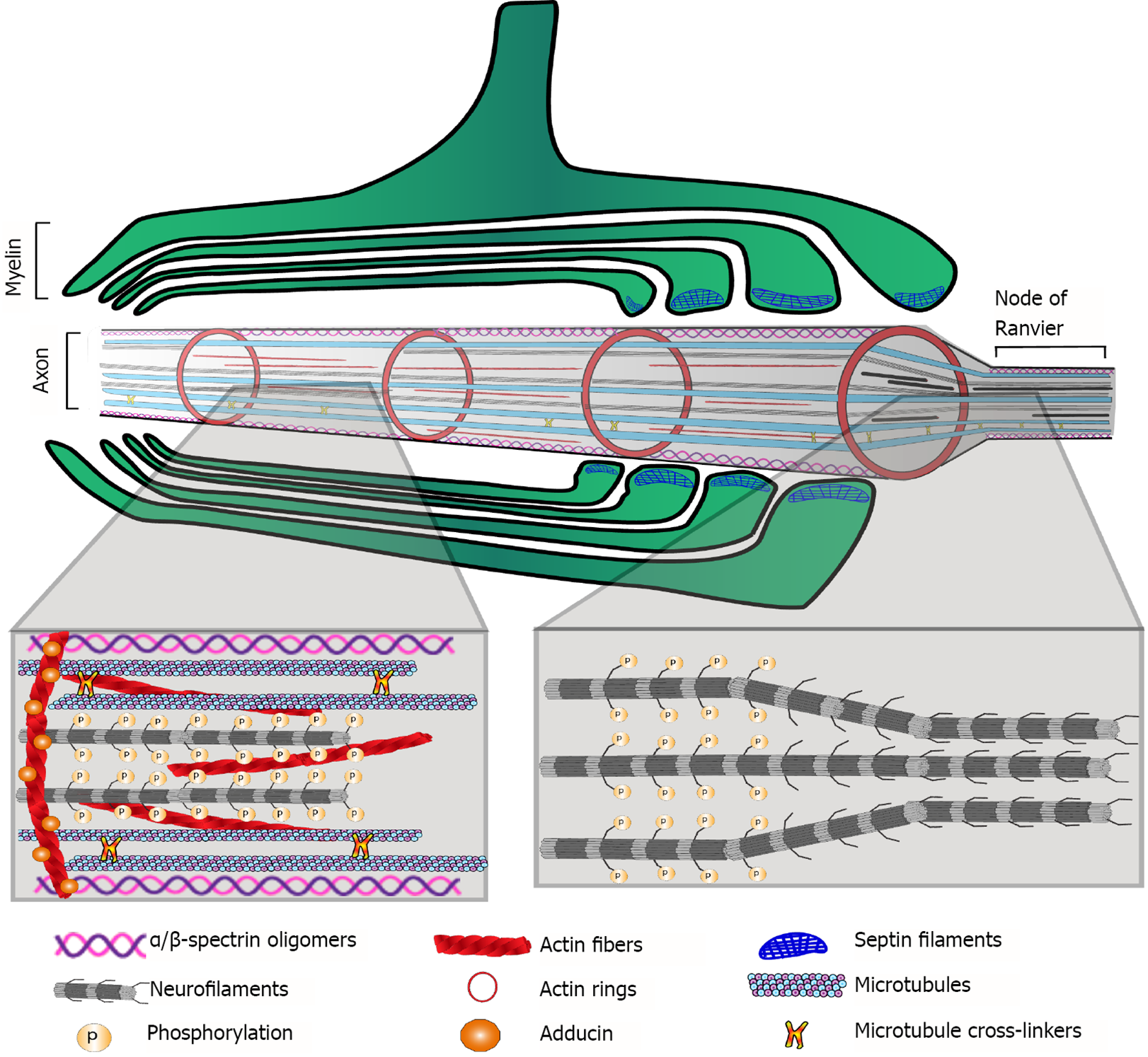
Each myelin internode is a specialized structure of multiple membrane lamellae. The first membrane layer-closest to the axon-is called the adaxonal membrane. Between the axon and the adaxonal membrane is the periaxonal space[7]. Flanking each internode, paranodal loops make contact with the axon through the cell adhesion proteins neurofascin 155 (NF155; on the oligodendrocyte) and contactin-associated protein 1 (Caspr1)/contactin 1 (on the axon). In juxtaparanodal regions, myelin-axon interactions are mediated by contactin 2 and Caspr2, and the voltage-gated K+ channels Kv1.1/1.2 are enriched at the axolemma. Contactin’s cytoplasmic domains provide anchors for scaffold molecules of the paranodal-nodal-paranodal cytoskeleton, specifically for the 4.1B protein, the αII/β2SP heterotetramers (both actin-interacting proteins) and ankyrin B (AnkB)[24].
In a mature myelin internode, the adaxonal layer is relatively loose compared with the tightly compacted myelin lamellae, and its cytoplasmic content is slightly higher, which allows the functional presence of signal transduction molecules and oligodendroglial cytoskeletal components such as septin filaments[25]. These components are also present at paranodal loops. In contrast, the structure of compact myelin is almost withdrawn from the cytoplasm; thus, intracellular membranes are in tight apposition, with myelin basic protein (MBP) playing a key role in regulating the hydrophobic forces between them[7].
As previously mentioned, the molecular composition of myelin is highly enriched in lipids, which account for approximately 70% of its wet weight. Myelin membranes have a higher cholesterol content than other membranes (at approximately 1.6-fold) and are characteristically enriched with galactosphingolipids and plasmalogens, which are asymmetrically distributed among the bilayer leaflets. The extracellular leaflet is enriched in galactosylceramide and its sulfated form, sulfatide, as well as phosphatidylcholine and sphingomyelin, whereas the intracellular leaflet is rich in ethanolamine plasmalogen and other phospholipids. The lipid components of the extracellular leaflet form discrete domains known as lipid rafts, which often contain membrane proteins and are frequently involved in signaling and/or myelin component turnover. For further review of myelin lipids, see[26,27].
Myelin-specific structural proteins also are distributed according to their functions. Interestingly, at least five out of eleven CNS myelin-specific proteins are categorized as intrinsically disordered proteins. This set of physicochemical attributes accounts for their flexibility and multifunctionality, which are important for a plastic structure such as myelin[28]. An exhaustive description of the structural and functional features of every myelin-specific protein is beyond the scope of this review and has been further addressed elsewhere[8,29]. Figure 1 illustrates the main CNS myelin-specific proteins at their common locations in the myelin sheath, as well as the lipidic composition of myelin membranes.
The analysis of postmortem samples provides valuable information about the structural and biochemical alterations present in the brains of patients with neuropsychiatric disorders. In the last 20 years, several reports by Uranova et al[30] have described the main ultrastructural alterations in oligodendrocytes and myelinated fibers found in patients with schizophrenia.
With electron microscopy and morphometry or with a stereological approach and Nissl-stained sections, they extensively analyzed the prefrontal cortex (PFC), specifically the gray matter layers of Brodmann’s area 9 (BA9) and BA10[30-33] and their adjacent WM[34-36], as well as the caudate nucleus[21,33,37,38], hippocampus[33,38,39] and anterior putamen[40].
Their analysis of myelinated fibers found concentric lamellar bodies and interlamellar abnormal inclusions, swelling of periaxonal oligodendrocyte processes and ultrastructural signs of axonal atrophy[21,33,38,39]. They characterized six types of abnormal myelinated fibers that were present in patients with schizophrenia and that could correlate with the predominant presence of positive or negative symptoms, age or illness duration[35].
Oligodendrocytes showed consistent signs of dystrophia, apoptosis and/or necrosis, and in most of the studies, their numerical density was significantly reduced in patient samples[31,33,37,40]. Oligodendrocyte clusters, which are thought to be involved in activity-dependent myelination, were also consistently reduced[37,40]. In the oligodendrocytes, mitochondria were the main altered organelle, with a significant reduction in numeric and volume density and even intramitochondrial accumulation of lipofuscin granules[30,34,36,38]. These findings suggest that not only is the numerical density of oligodendrocytes affected in schizophrenia, but that their energy and redox metabolism is also compromised.
Interestingly, both perineuronal and pericapillar oligodendrocytes showed signs of dystrophy in patient samples[33,34] suggesting that oligodendrocytes may be involved both in the disrupted transmission of neuronal information and in metabolic dysregulation. In addition, these studies found dystrophic ameboid microglia adjacent to dystrophic oligodendrocytes[30,34] and myelin concentric lamellar bodies engulfed by astrocytes[21], implying the involvement of other glial cells in myelin pathology in schizophrenia patients.
Hof et al[41,42] found a significant decrease in both the total number of oligodendrocytes and the number of oligodendrocytes expressing the 2',3'-Cyclic-nucleotide 3'-phosphodiesterase (CNP) marker in cortical layer III of BA9 and the WM of the superior frontal gyrus with a stereological analysis on samples from patients with schizophrenia. Additionally, the number of oligodendrocyte clusters in the WM was significantly reduced[42]. Other studies reported a decreased number of oligodendrocytes in the anterior principal thalamic nucleus[43,44], centromedian thalamic nucleus[44], thalamic internal capsule[45], hippocampus[46,47] and anterior cingulate WM[48] of schizophrenia patient samples. In the latter structure, oligodendrocytes expressing disintegrin and metalloproteinase domain-containing protein 12 (ADAM12) were examined[48]. ADAM12 is predominantly expressed in oligodendrocytes and has been suggested to play a role in myelination and neurodevelopmental processes, as well as in higher cognitive functions[49].
Not all neuropathological studies of postmortem schizophrenia brain samples showed significant differences in oligodendrocyte densities, e.g., in the cingulum bundle[50], BA9 adjacent WM[23,51], and BA10 adjacent WM[34], no changes in oligodendrocyte cell densities were found between schizophrenia and control samples. In contrast, an increased density of prohibitin(+)-oligodendrocytes was reported in the right dorsolateral prefrontal WM of schizophrenia patients[52]. The authors suggested that prohibitin may be upregulated in oligodendrocytes as a result of mitochondrial stress and/or dysfunction in schizophrenia.
mRNA expression of neural/glial antigen 2 (NG2) was augmented in the putamen of schizophrenia patients[53], suggesting that there could be an increased density of OPCs. Additionally, a study by Kerns et al[45] supported the hypothesis that in schizophrenia OPCs may fail to exit the cell cycle and differentiate into mature myelinating oligodendrocytes. In BA9 WM, there was no significant difference in NG2(+)-cells but a significant reduction in cells expressing oligodendrocyte transcription factor 2 (OLIG2), suggesting an overall reduction in the oligodendroglial lineage[54].
Proteomic approaches have been used to determine that the main myelin structural proteins are differentially expressed in schizophrenia postmortem brain samples; in most of the studies, these proteins were significantly downregulated. For most of the myelin structural proteins, altered transcriptomic levels have consistently been reported[53,55-57], and in some cases, single-nucleotide polymorphisms (SNPs) at their codifying genes have been associated with schizophrenia (Table 1). This is the case for MBP[58-61], CNP[57,60-63], proteolipid protein (PLP)[60,62,64], myelin-associated glycoprotein (MAG)[62,64,65], and transferrin[66-68]. The latter is not a structural myelin protein, but is essential for oligodendrocyte homeostasis and survival[69,70]. Downregulation of myelin oligodendrocyte glycoprotein (MOG)[58,60-62,71] and claudin-11[62,64] at the proteomic and transcriptomic levels has been reported, although no SNPs of the corresponding codifying genes have been associated with schizophrenia. Several brain regions have been analyzed by proteomic studies, such as the dorsolateral PFC BA46[59,62,71], PFC BA9 gray and WM[68], PFC BA10[60], anterior PFC[65], orbitofrontal cortex[64], anterior temporal lobe[58], corpus callosum[61], cerebellum, posterior cingulate cortex and caudate nucleus[63].
| Protein name | Gene | SNPs | Ref. |
| Myelin basic protein | MBP | rs12458282; rs2008323; rs721286 | Baruch et al[185], 2009 |
| 2’,3’-Cyclic nucleotide 3’-phosphodiesterase | CNP | rs2070106 | Peirce et al[186], 2006 |
| Voineskos et al[187], 2008 | |||
| Voineskos et al[175], 2013 | |||
| Proteolipid protein | PLP | rs475827 | Qin et al[188], 2005 |
| Myelin-associated glycoprotein | MAG | rs720308; rs720309; rs756796; rs2301600 | Wan et al[189], 2005 |
| Yang et al[190], 2005 | |||
| Voineskos et al[187], 2008 | |||
| Transferrin | TF | rs3811655; rs448115 | Qu et al[191], 2008 |
| Huo et al[192], 2019 | |||
| Oligodendrocyte lineage transcription factor 2 | OLIG2 | rs1059004; rs9653711 | Georgieva et al[73], 2006 |
| Voineskos et al[175], 2013 | |||
| Huo et al[192], 2019 | |||
| Komatsu et al[74], 2020 | |||
| Quaking | QKI | rs2784865 | Voineskos et al[175], 2013 |
Consistent with findings of overall downregulation of myelin-specific proteins, mRNA levels of OLIG1[22], OLIG2[22,55,72-74] and SOX10[22,55,72], corresponding to oligodendroglial lineage trans
In addition to the previously mentioned gene association studies, a functional glial-specific gene set analysis based on genome-wide association data reported three main oligodendroglial gene sets, i.e., lipid metabolism, gene transcription and oxidation–reduction, which were strongly associated with an increased risk for schizophrenia[78]. Furthermore, gene expression profile analysis of CNP(+)-cells revealed nine differentially regulated signaling pathways associated with oligodendrocyte differentiation[54], strongly suggesting oligodendrogenesis impairment in schizophrenia.
Proteomic studies of schizophrenia-derived postmortem brain samples have also consistently revealed that many cytoskeletal components are differentially expressed in this disorder. Dynamic cytoskeletal rearrangements are crucial for oligodendrogenesis since this process implies a dramatic increase in oligodendroglial morphologic complexity. Additionally, actin-cytoskeleton dynamic assembly and disassembly are critical for axon ensheathment during the myelination process[79-81]. Several actin-interacting proteins are involved in these rearrangements, including gelsolin and cofilin, actin filament-severing proteins that drive actin cytoskeleton disassembly, which is essential for proper myelin wrapping[79]. Gelsolin is specifically expressed in myelin-forming cells[82] and is present in the different stages of oligodendroglial lineage differentiation[83]. Transcriptomic and proteomic analyses of postmortem brain samples have shown that both gelsolin[61,68,72] and cofilin[59,63,64] are dysregulated in schizophrenia. Similarly, the oligodendrocyte-specific protein ermin, also known as juxtanodin, is downregulated in the anterior temporal lobe and upregulated in the dorsolateral PFC in patients with schizophrenia[58,59]. Ermin is an F-actin binding protein that is expressed at late stages of oligodendrocyte maturation. It may play a key role in the formation of multiple oligodendroglial processes and the dramatic changes in morphology as these cells acquire the capacity for myelination[84,85].
Septin heteromeric filaments (SEPT2/SEPT4/SEPT7/SEPT8) form at the adaxonal myelin layer and at paranodal loops. These filaments act as molecular scaffolds, mediating axo-glial signaling and compartmentalization of mature myelin. Their loss or deficit has been associated with the formation of myelin outfoldings that impair the rapid propagation of nerve impulses[25,86,87]. The four septins involved in these filaments are differentially expressed in proteomic analyses of postmortem schizophrenia brain samples[62,63].
α/β-Spectrin oligomers are important components of the membrane-bound cytoskeleton at the axolemma. At the paranodal and juxtaparanodal regions of the axon beneath a myelin internode, these oligomers interact with proteins such as 4.1B, adducin and AnkB to form a scaffold that mediates the interaction of the cytoplasmic tails of contactins and other axo-glial adhesion molecules with the actin filaments and the actin rings found along the axon. These proteins are also relevant because they are crucial for the paranodal-nodal-paranodal cytoskeleton, which is a specific arrangement of cytoskeletal protein oligomers and polymers underlying the proper assembly and plasticity of the nodes of Ranvier[24]. All of these proteins are differentially regulated in schizophrenia postmortem brain samples[58,59,62-64].
Neurofilaments are important axonal cytoskeletal components. They belong to the intermediate filament IV category, and their composition is heteromeric, with light (NEFL), medium (NEFM) and heavy (NEFH) polypeptides as their main constituents. Internexin (INA) is also a component of these axonal structural filaments. Repelling forces among negatively charged phosphorylated residues on the neurofilaments contribute to the enlargement of axon caliber, e.g., at internodes (Figure 2). Thus, phosphorylation/dephosphorylation of neurofilament polypeptides is a mechanism that regulates axon caliber, which influences molecular trafficking as well as the speed of nerve impulse conduction. Proteomic studies have found that the three neurofilament polypeptides NEFL, NEFM, and NEFH, as well as INA, are differentially regulated in postmortem brain samples from schizophrenia patients[57,61-64].
As expected, the actin and tubulin monomeric components of microfilaments and microtubules, respectively, as well as various microtubule-associated proteins, are altered in schizophrenia brain samples[58,61-63]. The cytoskeleton mediates the essential functions of every cell in the organism. In the axo-myelin functional unit[88], the cytoskeleton is crucial for the following: Oligodendrogenesis; myelin formation, turnover and plasticity; assembly and remodeling of axonal specialized domains, such as the axon initial segment and nodes of Ranvier; myelin and axonal compartmentalization; anchorage for cell adhesion molecules involved in axo-glial junctions; and scaffolds for molecules involved in signal transduction.
As most of the myelin structural proteins are affected by schizophrenia and most of the cytoskeletal components are dysregulated, it is plausible to infer that overall dysfunction of the axo-myelin unit may underlie the compromised integrity of gray and WM and thus the functional disconnection observed in schizophrenia.
Metabolic dysfunction in schizophrenia has been suggested by positron emission tomography (PET) and magnetic resonance imaging (MRI), and mitochondrial alterations have been documented as mentioned above. At the proteomic level, dysregulation in the expression of enzymes involved in energy metabolism and the antioxidant system has been observed. For example, Martins-de-Souza et al[59,89] found alterations in proteins involved in glycolysis (fructose-bisphosphate aldolase C and phosphoglycerate kinase 1), the Krebs cycle (citrate hydrolyase), the malate-aspartate shuttle (cytosolic malate dehydrogenase) and oxidative phosphorylation (mitochondrial ATP synthase F1 and F0 complexes) in postmortem dorsolateral PFC samples from schizophrenia patients[59,89]. In addition, four subunits of mitochondrial respiratory complex I (NADH dehydrogenase [ubiquinone] (NDU) flavoprotein 2 (NDUFV2), iron-sulfur protein 3 (NDUFS3) and 6 (NDUFS6), and 1 beta subcomplex subunit 5 (NDUFB5)) are downregulated in the anterior temporal lobe of schizophrenia patients[58,89].
Increased amounts of oxidative reactive species are produced under high energy demand or mitochondrial dysfunction, as is suggested to occur in schizophrenia. Therefore, antioxidant enzymatic systems in schizophrenia are expected to be upregulated to counteract oxidative damage. However, the expression levels of three members of the glutathione transferase (GST) family (GSTM3, GSTTLp28, and GSTP1), carbonyl reductase 1 (CBR1), carbonyl reductase 3 (CBR3) and quinoid dihydropteridine reductase (QDPR), are reduced in the thalamus and PFC of schizophrenia patients (reviewed in[89]). As these results were obtained from brain homogenates, an interesting follow-up would be to assess whether these metabolic and redox alterations are present in oligodendrocytes. For this purpose, enriched cultures of patient-derived oligodendrocytes differentiated from induced pluripotential stem cells (iPSCs) and/or cocultures of these induced oligodendrocytes with neurons and other glial cells could be useful in vitro tools for studying alterations in the oligodendroglial lineage in schizophrenia.
Uranova et al[51] also analyzed samples from patients with bipolar disorder, examining BA9 of the PFC, layers III and VI and the adjacent WM, BA10, the caudate nucleus and the anterior putamen. A stereological approach with Nissl-stained samples revealed a significant reduction in the numerical density of oligodendrocytes in the caudate nucleus and in the gray matter layers of BA9[32,37,51]. In the adjacent WM, they found no difference between bipolar disorder samples and samples from control subjects[51]. The number of oligodendrocyte clusters was also significantly reduced in the caudate nucleus[37] and in the anterior putamen, but the latter difference was observed only in male subjects[40]. Electron microscopy analysis of the samples showed ultrastructural signs of apoptosis and necrosis of oligodendrocytes[21].
Oligodendrocyte numbers were significantly reduced in the thalamic anterior principal and centromedian nuclei, in postmortem samples from bipolar disorder patients with a clinical history of psychotic episodes[44]. The age-related increase in oligodendrocyte number observed in control subjects was attenuated in this group of patients. The latter effect was also observed by Vostrikov and Uranova[90]. Vostrikov and Uranova[90] also found significantly reduced oligodendrocyte densities in samples from BA9 Layer VI from bipolar disorder patients younger than 50 years old compared with those from corresponding age-matched controls[90]. Hayashi et al[91] found a significant reduction in OLIG2(+)-cells using a flow cytometry approach in unfixed postmortem gray matter BA10 samples from bipolar disorder patients, which suggests an overall deficit in the oligodendroglial lineage. S100B(+)-oligodendrocyte density was decreased in the left alveus of the hippocampus from bipolar disorder patients[92]. In contrast, Hercher et al[23] found increased oligodendrocyte density and CNP protein levels in BA9-adjacent WM in bipolar disorder patients compared with control samples. A further study also showed an increase in oligodendrocyte density along with deficits in axonal markers in prefrontal WM in bipolar disorder patients[93]. In a systematic review of postmortem brain studies in bipolar disorder, Gigase et al[94] found no difference in either neurons or glial cells and suggested that findings from existing studies should be validated.
Significantly less intense myelin staining of the deep prefrontal WM was shown in bipolar disorder patients than in control subjects[95]. Additionally, MBP immunostaining revealed decreased myelination of the hippocampal formation in female bipolar disorder patients than a corresponding sex-matched control group[96]. In contrast, male patients showed increased MBP staining in the superior medullary lamina, which suggests sex differences in myelin alterations[96]. To the best of our knowledge, no ultrastructural analysis of myelinated fibers has been conducted on bipolar disorder postmortem samples.
Perineuronal oligodendrocytes are located in the cerebral gray matter in close proximity to neuronal perikarya and less frequently near dendrites and neurites. Although their morphology is indistinguishable from that of other oligodendrocytes, it remains unknown whether perineuronal oligo
Myelin structural proteins MBP, CNP, PLP and MOG were downregulated in postmortem brain samples from bipolar disorder patients[60]. Consistent downregulation at the transcriptomic level was reported for MBP and CNP, and at this level MAG, PLP, CLDN11, MOG, and MOBP were also downregulated[22]. Reduced mRNA levels were also reported for TF[22,53]. The gene expression of the oligodendroglial lineage transcription factors OLIG1, OLIG2 and SOX10 was downregulated. Additionally, transcript levels of NG2 and GALC, which correspond to markers of OPCs and immature oligodendrocytes, respectively, were significantly lower in bipolar disorder samples than in control samples[22].
Differential expression of cytoskeletal components of the axo-myelin unit has been reported in postmortem brain samples of bipolar disorder patients. In the WM adjacent to BA9, the β-tubulin protein level assessed by Western blot was significantly lower in patient samples than in controls[93]. A proteomic approach found that the NEFL level was downregulated in bipolar disorder samples of BA10[60]. Similarly, the neurofilament units NEFL and NEFM and INA, α-spectrin (SPTAN1), SEPT11 and tubulin polymerization-promoting protein (TPPP) were downregulated, whereas β-actin (ACBT) and the ARPC5 subunit of the actin-binding Arp2/3 complex were upregulated in hippocampal samples from bipolar disorder patients[99,100]. The actin-bundling protein fascin (FASC) was also dysregulated in these samples[99]. In samples of the dorsolateral PFC, NEFL, NEFM and INA were consistently downregulated, while α- and β-tubulins as well as SEPT5, SEPT6 and SEPT11 were upregulated[67].
As in schizophrenia, alterations in metabolic and redox pathways have been described for bipolar disorder. Studies using magnetic resonance spectroscopy have found a reduction in phosphocreatine and ATP in the frontal lobes and basal ganglia, while an increase in lactate levels was reported in postmortem gray matter samples from bipolar disorder patients. In addition, mitochondrial structure is altered, and mutations or polymorphisms in mitochondrial DNA associated with the respiratory chain have been reported[101]. Furthermore, high levels of lipid peroxidation, nitric oxide concentration, and DNA and RNA oxidative damage were found in patient samples[102]. There is evidence of dysfunctional attachment of the hexokinase 1 protein to the outer mitochondrial membrane in patient samples, which results in abnormal generation of mitochondrial reactive oxygen species and cellular oxidative stress[103]. Additionally, impairment of redox modulation pathways in the frontal cortex is found in bipolar disorder patients[104]. The antioxidant molecule glutathione has been reported at low concentrations in some brain regions and could contribute to oxidative stress[105,106]; however, some patients present a significant increase in this molecule in the anterior cingulate cortex[107]. These apparently contradictory results could reflect differential redox regulation or antioxidant capacity in diverse brain regions.
Due to their high metabolic rate and high lipid content in myelin-forming membranes, oligo
Several features are similarly altered by schizophrenia and bipolar disorder at the cellular level. Ultrastructural studies have revealed signs of oligodendrocyte apoptosis and necrosis[21], oligodendrocyte numerical density was significantly reduced in the caudate nucleus[37] and in BA9 gray matter layers of the PFC[32,51], and significantly fewer oligodendrocyte clusters were found in the caudate nucleus[37] and the anterior putamen[40]. In BA9-adjacent WM, a stereological analysis found no differences in oligodendrocyte numerical density in either schizophrenia or bipolar disorder patients compared to that of the control samples[51]. However, in schizophrenia, studies have reported decreased oligodendrocyte numerical density in the BA9- and BA10-adjacent WM[33,108], a significant reduction of OLIG2(+)-cells in the former[54], and a significant decrease in both total and CNP(+)-oligodendrocytes in the WM of the superior frontal gyrus[41,42]. Additionally, significantly fewer ADAM12(+)-oligodendrocytes were found in the anterior cingulate WM[48]. In contrast, two different studies reported increased oligodendrocyte density in the prefrontal WM in postmortem samples from bipolar disorder patients[23,93]. One of these studies reported a concomitant decrease in axonal markers[93], which may imply axonal degeneration due to demyelination, which is consistent with an increase in oligodendrocytes at early stages of differentiation. In schizophrenia-derived samples, significantly more oligodendrocytes expressing prohibitin were found in the right dorsolateral prefrontal WM[52]. Although prohibitin proteins can be found in other cell compartments, such as the nucleus or plasma membrane, their role in the inner mitochondrial membranes is key for modulating cell proliferation or apoptosis and for overall mitochondrial homeostasis[109-112]. Therefore, altered oligodendroglial prohibitin expression is consistent with a previous work suggesting dysregulation of the cell cycle in oligodendrocytes in schizophrenia[113]. Based on the findings of that work, Katsel et al[113] suggested that postmitotic oligodendrocytes may abnormally re-enter the cell cycle, while a significantly increased level of NG2 in the putamen of schizophrenia patients suggested that OPCs failed to exit the cell cycle. Dysregulation of p57Kip2 gene expression in schizophrenia patient samples[113] could also be related to impaired oligodendrocyte maturation, since this protein has been characterized as an oligodendroglial differentiation competence marker[114-116].
At the subcellular level, proteomic analyses have revealed that the four most abundant myelin structural proteins[117] (PLP, MBP, CNP, and MOG) are significantly reduced in schizophrenia and bipolar disorder. At the transcriptomic level, almost all myelin structural proteins, as well as the main oligodendroglial lineage markers and OPC markers, were significantly downregulated. This evidence strongly suggests that the oligodendroglial lineage is compromised at all differentiation stages in these disorders. Moreover, several axonal and oligodendroglial cytoskeletal components and cytoskeletal-interacting proteins are dysregulated in both schizophrenia and bipolar disorder. A deficit of myelin structural and cytoskeletal proteins in the axo-myelin functional unit may compromise myelin formation, compaction, remodeling and its overall integrity and functionality, which may imply a concomitant compromise in the assembly and functioning of the nodes of Ranvier and other axonal functional rearrangements. If nervous impulses are not properly conducted in terms of speed and precise timing, some connections would not be reinforced and could be lost, influencing the local connectome. At the macroscale connectomic level, which comprises long-range tracts, fine-tuning and synchronization of nervous impulse conduction is crucial, and even subtle alterations of myelin structural and functional features may have a detrimental impact on information processing and thus on cognitive functions and behavior.
These findings suggest that altered myelination, loss of oligodendrocytes and compromised energy and redox metabolism in oligodendrocytes of schizophrenia and bipolar disorder patients could correlate with the WM alterations observed by neuroimaging techniques. These mechanisms could explain, at least partially, the clinical manifestations observed in schizophrenia and bipolar disorder patients. The relationship between myelin and oligodendrocytes, WM and symptom domains can be systematically studied. In the following sections, we will address the evidence from imaging studies on dysfunctions in the nervous tracts and how the main symptoms correlate with these alterations, giving rise to the hypothesis of disconnection in mental disorders.
Structural and functional neuroimaging findings provide evidence of connectivity alterations that might be related to myelin dysfunction; the most extensive evidence comes from MRI studies[118]. In the field of structural magnetic resonance imaging (sMRI), WM volume and density have been measured using techniques such as voxel-based morphometry (VBM)[119]. VBM studies have found diminished WM volume and density in several brain regions of patients with schizophrenia, with main decreases in the frontal and temporal regions. A meta-analysis of VBM studies reported decreased WM in 150 foci. The affected tracts included the corpus callosum, internal capsule, fornix, anterior commissure, and an additional sixteen tracts[120].
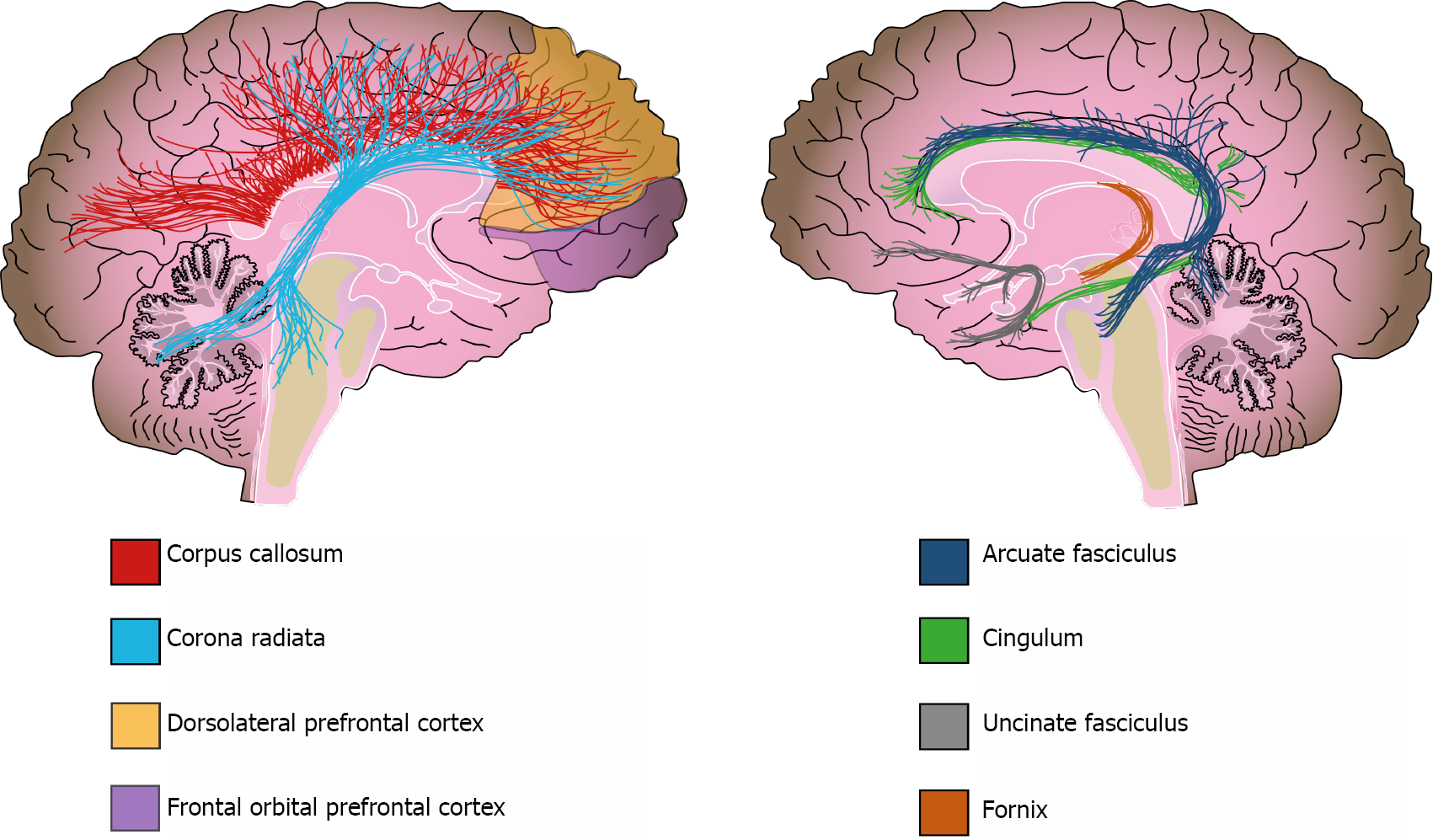
Additionally, WM alterations have been detected by diffusion tensor imaging (DTI); this method evaluates subtle changes in WM, determining fractional anisotropy (FA). FA expresses the diffusion of water molecules along neural fibers. Water movement is inhibited when myelin sheaths are thick and well preserved (FA = 1); in contrast, water moves easily along fibers in any direction when the myelin sheath is damaged (FA = 0)[118]. Although FA can indeed reflect changes in myelination, it could also reflect other tract properties, such as axonal ordering and axonal density[121]. Therefore, Jones et al[121] urge caution when interpretating DTI-based measurements and not assume that they are direct indicators of WM integrity[121]. Bearing this in mind, DTI studies have reported that patients with schizophrenia have a widespread decrease in FA[122]. For instance, the ENIGMA-Schizophrenia DTI group analyzed 4321 individuals and found widespread FA reductions in 20 of the 25 analyzed regions in schizophrenia patients when compared with those of the controls[123]. In addition, at least three meta-analyses have reported reduced FA in schizophrenia patients; they conclude that the tracts more frequently affected in these patients are the anterior corona radiata, the corpus callosum, the cingulate bundle, and the uncinate and arcuate fascicles[120,123,124] (Figure 3). Furthermore, functional MRI (fMRI) studies have also reported connectivity alterations in several circuits connecting frontal, limbic, temporal, and parietal regions in schizophrenia subjects, as well as alterations to the default network[125,126].
As the evidence is extensive and complex, we will discuss the neuroimaging evidence of WM alterations in schizophrenia based on each of its main clinical domains. This will integrate the findings and highlight the importance of WM. The structural and functional WM alterations associated with the psychotic domain of schizophrenia include tracts and circuits that connect the frontal, temporal, and parietal cortexes[127]. For instance, the arcuate fasciculus (AF) is frequently studied in regard to the psychotic domain; the AF connects temporal and parietal regions with the frontal lobe and is considered the main language processing tract of the brain because it connects Wernicke’s and Broca’s areas[128]. DTI studies on schizophrenia patients have reported diminished FA on the AF when compared with that of controls[123]. Additionally, some studies have related the decrease in AF integrity with thought disturbances, language alterations, and auditory hallucinations[129-131].
Furthermore, psychotic symptoms are related to functional connectivity alterations in the frontostriatal, frontotemporal, and frontoparietal circuits[132]. The frontostriatal circuit comprises the connections between the PFC and basal ganglia[128]. These regions have been extensively studied in relation to the dopaminergic hypothesis of schizophrenia[133]. Some studies of resting-state fMRI analysis have indicated functional dysconnectivity between the dorsolateral PFC and basal ganglia in patients with schizophrenia, which is related not only to psychotic symptoms but also to cognitive alterations[134]. In contrast, the frontotemporal circuit comprises connections from the PFC to temporal structures, including the auditory cortex and Wernicke’s area[128]. Functional connectivity alterations in this circuit have been related to auditory hallucinations and the perceived reality of those hallucinations[134].
Alterations to the cingulum bundle, fornix, and inferior fronto-occipital fascicle are related to cognitive symptoms of schizophrenia[127,135]. The cingulum bundle is a major connector between limbic, paralimbic, and neocortical structures, including the dorsolateral PFC, amygdala, paralimbic gyrus, and cingulate gyrus. This tract is implicated in self-monitoring, spatial orientation, and memory[128]. Subjects with schizophrenia have lower FA on the cingulum bundle than controls, which has also been linked with executive dysfunction and impaired working memory in these same patients[136,137]. The fornix is another WM structure implicated in cognitive function; this tract connects the hippocampus with other cortical structures and is implicated in memory and spatial learning[128]. Patients with schizophrenia have compromised fornix integrity and disrupted functional connectivity between the PFC and the hippocampus[138,139]. Further analysis of functional connectivity has provided evidence of alterations in the frontostriatal and frontoparietal circuits that are also related to cognitive dysfunctions in schizophrenia subjects[132].
Connectivity alterations have been associated with altered tract integrity of the uncinate fascicle; this tract connects the orbitofrontal and anterior dorsolateral cortex with the temporal lobe and is related to negative symptoms[128]. At least two studies have demonstrated an association between low FA of this tract and flattened affect and lack of social engagement[140,141]. As WM decline can be a consequence of demyelination, all of these neuroimaging results (that report WM reduction in important tracts underlying highly integrative brain functions) support the hypothesis that demyelination may be a key factor in explaining, at least in part, the symptoms of schizophrenia.
There are also extensive data on gray and WM changes that are associated with clinical characteristics, genetics, functional impairment, and treatment response for bipolar disorder[142,143]. Currently, one of the main hypotheses about the neurobiology of this disease centers on the disconnection of prefrontal-subcortical networks and limbic structures associated with mood regulation[144]. Diverse prefrontal-striatal-thalamic circuits that regulate the expression of sensorial, cognitive, and emotional data from cortical regions are altered in bipolar disorder patients. It is believed that the dysfunction of these networks explains the cognitive, behavioral, and affective manifestations of this disorder[145]. The current fronto-limbic circuit disconnection model highlights the importance of WM in bipolar disorder. Evidence of WM alterations can be provided through structural or functional findings from neuroimaging techniques, with the most extensive evidence coming from MRI studies.
White matter hyperintensities (WMHs) are evident bright areas on T2 MRI sequences. These alterations are one of the most replicated findings in bipolar disorder[144]. WMHs are lesions that are associated with vascular anomalies and neurodegenerative processes, such as demyelination, axon loss, or necrosis[146]. These lesions are frequently found around the lateral ventricles (periventricular), deep WM, and subcortical gray matter (basal ganglia, thalamus)[147,148]. At least three meta-analyses have linked the presence of WMHs with bipolar disorder, and it is estimated that approximately 39% of bipolar disorder patients have these lesions, compared with 18% of controls[149-151]. The presence of WMHs in patients has been associated with the worst outcomes of the disease, such as hospitalizations, psychotic symptoms, suicide attempts, cognitive impairment, and treatment resistance[151-155].
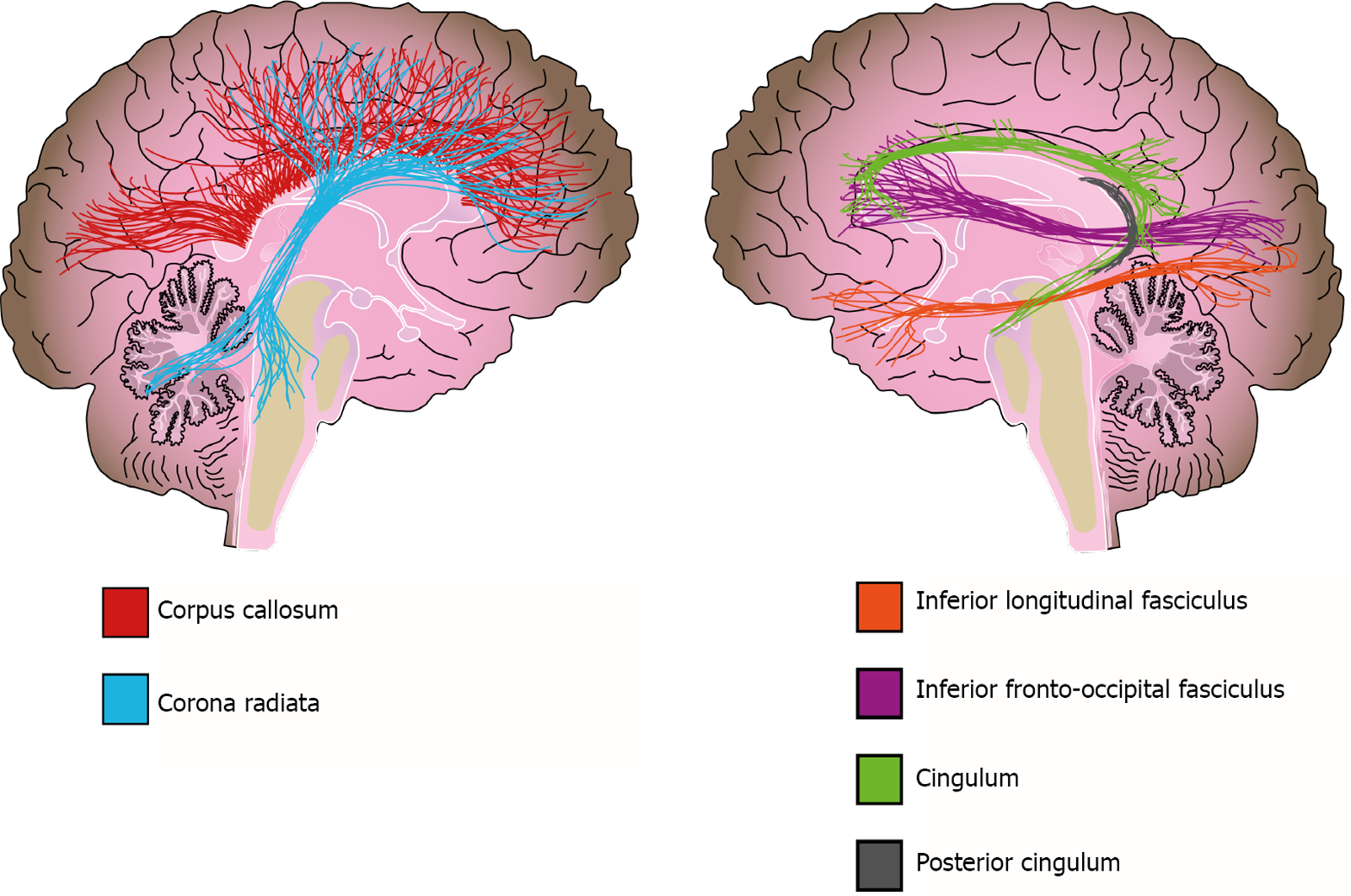
In addition to WMH, there is also extensive evidence about WM volume alterations from different methodologies[156]. Two meta-analyses that used a region-of-interest (ROI) approach reported a volume reduction in the corpus callosum of bipolar disorder patients, which is a structure of crucial importance for interhemispheric connectivity and is implicated in higher cognitive functions such as attention, memory, and language[124,156,157]. However, no clear association was found between altered corpus callosum volumes and psychotic symptoms or suicidal ideation in patients[157-159]. In contrast, Lavagnino et al[160] reported an association between volume reduction of the posterior corpus callosum and a higher number of affective episodes, hospitalizations, and incomplete remission of symptoms in female patients[160]. Other studies and meta-analyses used VBM to evaluate the whole brain and reported a reduction in WM volume of the corpus callosum, corona radiata, posterior cingulum, and inferior longitudinal fasciculus in bipolar disorder[142,161] (Figure 4).
DTI studies of bipolar disorder have reported diffuse WM microstructural alterations[124,162,163], which are evident when tract integrity and WM volume are measured. Recent meta-analyses have found FA reductions in all major classes of WM tracts (commissural, association and projection fibers) with frequent reports of alterations in temporoparietal WM, the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and left cingulum[124,164]. A mega- and meta-analysis of the ENIGMA group revealed decreased FA in 29 ROIs, with the greatest effect sizes in the corpus callosum and cingulum of patients compared with those of controls[162]. Voxel-based analysis of DTI (VBA-DTI) data has also found clusters of decreased FA and WM volume in prefrontal, temporal and parietal regions[164-166]. Emsell et al[165] conducted a study on euthymic bipolar disorder patients and found a cluster extending from the prefrontal WM to the splenium of the corpus callosum and posterior cingulum bundle[165], whereas a VBA-DTI meta-analysis reported another two clusters in areas involved in emotional processing[164]. Nortje’s meta-analysis identified a large cluster of decreased FA and mean diffusivity in the right temporoparietal WM, a region that is crossed by the inferior longitudinal fasciculus and inferior fronto-occipital fasciculus[164]. The evidence suggests that the posterior WM contributes to cognitive deficits, while the alterations of anterior fibers are associated with affective symptoms of bipolar disorder[164,167]. In conclusion, the previously discussed evidence suggests not only alterations to fronto-limbic connectivity but also dysfunction in parietal, fronto-occipital and interhemispheric connections, which may explain the cognitive and emotional manifestations of this disorder[167].
Taken together, the evidence discussed above highlights the importance of oligodendroglial cells for brain function; through myelin formation, they are involved in the precise synchronization of electrical impulses that propagate along nerve fibers connecting brain structures[168-170]. Most long-distance connecting tracts in the CNS are heavily myelinated and comprise the WM. Although structural and functional WM alterations have been described in other mental illnesses, such as major depression, obsessive-compulsive disorder, attention-deficit/hyperactivity disorder, autism spectrum disorders, Alzheimer’s disease, and drug addiction[16,171], in this review, we focused on schizophrenia and bipolar disorder because these two neuropsychiatric illnesses share several clinical and patho
As can be inferred from the previously mentioned findings, the focus of investigations on the pathophysiology of schizophrenia and bipolar disorder has changed from alterations in specific regions to dysfunction in the connectivity of brain structures. This shift occurred first for schizophrenia, when the disconnection hypothesis was postulated more than twenty years ago, in response to the fact that several manifestations of schizophrenia, such as negative symptoms, cannot be fully explained by structural alterations to a specific cortical area[172-174]. Researchers subsequently hypothesized that the clinical domains of schizophrenia might be due to widespread network dysfunction instead of only specific morphological alterations of specialized cortical regions[125]. This paradigm shift in schizophrenia research quickly translated to other psychiatric conditions, and many studies have since tested the disconnection hypothesis in bipolar disorder[153]. Functional MRI and DTI studies have reported an association between compromised WM integrity and clinical manifestations of these disorders[126,167].
In the following years, many neuroimaging studies have associated WM alterations found in psychiatric patients with executive function, functional impairment, affective symptoms, treatment response or resistance, suicidal thoughts and attempts, and the severity of symptoms, to name only a few traits[123,145,161,175]. This overwhelming evidence has helped researchers to frame schizophrenia and bipolar disorder as multidimensional conditions with strong brain correlates at the macroscale connectomic level[125]. Undoubtedly, further research from a neuroglial integrative perspective is necessary to unravel the anomalies at the cellular/subcellular level, i.e., the microscale connectomic level that may underlie the complex clinical manifestations of these patients.
The axo-myelin interaction is so close that, by itself, it constitutes a functional unit with a complex and deeply intermingled physiology. However, both astrocytes and microglia interact with axo-myelin units and influence their function[176-179]. Metabolic homeostasis and de novo formation or plasticity of myelin internodes and nodes of Ranvier are modulated by astrocytes and microglia. The main glial-mediated modulatory mechanisms of myelin homeostasis include physical intercellular interactions through gap junctions, secretion of soluble factors and clearance of myelin debris. Dysregulation of these modulatory mechanisms may also underlie the pathophysiology of mental illnesses such as schizophrenia and bipolar disorder; however, scientific research on this topic is still limited.
During the last two decades, great advances have been made in our understanding of human CNS physiology and pathophysiology, and glial cells have been recognized as key players in neuropsychiatric disorders[15,180-182]. Nevertheless, scientific psychiatry and patients with mental disorders would definitely benefit from a more integrative point of view at all research levels.
Schizophrenia and bipolar disorder are multifactorial neuropsychiatric illnesses that share clinical manifestations and alterations to brain structure and function, genetic characteristics, and neurobiological pathways. Both are chronic and severe conditions that cause disability, reduce lifespan and impose a high burden on patients and society. The disconnection hypothesis of the pathophysiology of these two disorders is supported by alterations in WM tracts revealed by neuroimaging techniques. Alterations at the macroscale connectome level strongly correlated with the multidimensional clinical manifestations of these disorders; however, to better understand the correlates at the cellular and subcellular levels, it is necessary to obtain deeper insight into the main components of WM, i.e., myelinated axons. Therefore, the pathophysiology of both the neuronal and oligodendroglial components of neural circuits and networks needs to be investigated. Twenty years since the first hypothesis implying oligodendrocyte/myelin failure as a hallmark of schizophrenia[183], a large amount of evidence at the connectomic, microscopic, proteomic, transcriptomic and genomic levels has accumulated for overall dysfunction of the axo-myelin functional unit in these patients. Although oligodendrocyte/myelin dysfunction has also been consistently reported in bipolar disorder, the same amount of scientific knowledge about axo-myelin pathophysiology in this psychiatric disorder is lacking, at least at the cellular and subcellular levels. Further research on schizophrenia and bipolar disorder is needed to better understand the axo-myelin molecular pathways that are dysregulated and to identify potential targets for the development of novel therapeutic alternatives. Several recent studies have focused on the effects of commonly prescribed antipsychotic drugs on oligodendrocytes/myelin[184]. However, testing the effects of novel compounds intended to induce oligodendrogenesis and (re)myelination[116] in preclinical models of schizophrenia and bipolar disorder could also hold great promise for future research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chakrabarti S, Manelis A S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Saab AS, Tzvetanova ID, Nave KA. The role of myelin and oligodendrocytes in axonal energy metabolism. Curr Opin Neurobiol. 2013;23:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Simons M, Nave KA. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb Perspect Biol. 2015;8:a020479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 515] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 4. | Abrahám H, Vincze A, Jewgenow I, Veszprémi B, Kravják A, Gömöri E, Seress L. Myelination in the human hippocampal formation from midgestation to adulthood. Int J Dev Neurosci. 2010;28:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Turner R. Myelin and Modeling: Bootstrapping Cortical Microcircuits. Front Neural Circuits. 2019;13:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci. 2015;16:756-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 498] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 7. | Aggarwal S, Yurlova L, Simons M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 2011;21:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Han H, Myllykoski M, Ruskamo S, Wang C, Kursula P. Myelin-specific proteins: a structurally diverse group of membrane-interacting molecules. Biofactors. 2013;39:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | White R, Krämer-Albers EM. Axon-glia interaction and membrane traffic in myelin formation. Front Cell Neurosci. 2014;7:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Roth AD, Núñez MT. Oligodendrocytes: Functioning in a Delicate Balance Between High Metabolic Requirements and Oxidative Damage. Adv Exp Med Biol. 2016;949:167-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Franklin RJM, Ffrench-Constant C. Regenerating CNS myelin - from mechanisms to experimental medicines. Nat Rev Neurosci. 2017;18:753-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 447] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 12. | Bechler ME, Swire M, Ffrench-Constant C. Intrinsic and adaptive myelination-A sequential mechanism for smart wiring in the brain. Dev Neurobiol. 2018;78:68-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Bauer NG, Richter-Landsberg C, Ffrench-Constant C. Role of the oligodendroglial cytoskeleton in differentiation and myelination. Glia. 2009;57:1691-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Thomason EJ, Escalante M, Osterhout DJ, Fuss B. The oligodendrocyte growth cone and its actin cytoskeleton: A fundamental element for progenitor cell migration and CNS myelination. Glia. 2020;68:1329-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res. 2015;161:4-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 16. | Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 941] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 17. | Pinto JV, Passos IC, Librenza-Garcia D, Marcon G, Schneider MA, Conte JH, da Silva JPA, Lima LP, Quincozes-Santos A, Kauer-Sant Anna M, Kapczinski F. Neuron-glia Interaction as a Possible Pathophysiological Mechanism of Bipolar Disorder. Curr Neuropharmacol. 2018;16:519-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Haroutunian V, Katsel P, Roussos P, Davis KL, Altshuler LL, Bartzokis G. Myelination, oligodendrocytes, and serious mental illness. Glia. 2014;62:1856-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Gavin DP, Akbarian S. Epigenetic and post-transcriptional dysregulation of gene expression in schizophrenia and related disease. Neurobiol Dis. 2012;46:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Shao L, Vawter MP. Shared gene expression alterations in schizophrenia and bipolar disorder. Biol Psychiatry. 2008;64:89-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 355] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 717] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 23. | Hercher C, Chopra V, Beasley CL. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci. 2014;39:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Arancibia-Carcamo IL, Attwell D. The node of Ranvier in CNS pathology. Acta Neuropathol. 2014;128:161-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Patzig J, Erwig MS, Tenzer S, Kusch K, Dibaj P, Möbius W, Goebbels S, Schaeren-Wiemers N, Nave KA, Werner HB. Septin/anillin filaments scaffold central nervous system myelin to accelerate nerve conduction. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Poitelon Y, Kopec AM, Belin S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 27. | Montani L. Lipids in regulating oligodendrocyte structure and function. Semin Cell Dev Biol. 2021;112:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Raasakka A, Kursula P. Flexible Players within the Sheaths: The Intrinsically Disordered Proteins of Myelin in Health and Disease. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Kursula P. Structural properties of proteins specific to the myelin sheath. Amino Acids. 2008;34:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Uranova NA, Vikhreva OV, Rakhmanova VI, Orlovskaya DD. Dystrophy of Oligodendrocytes and Adjacent Microglia in Prefrontal Gray Matter in Schizophrenia. Front Psychiatry. 2020;11:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Kolomeets NS, Uranova NA. Reduced oligodendrocyte density in layer 5 of the prefrontal cortex in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2019;269:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. 2007;94:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 33. | Uranova NA, Vostrikov VM, Vikhreva OV, Zimina IS, Kolomeets NS, Orlovskaya DD. The role of oligodendrocyte pathology in schizophrenia. Int J Neuropsychopharmacol. 2007;10:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Uranova NA, Vikhreva OV, Rakhmanova VI, Orlovskaya DD. Ultrastructural pathology of oligodendrocytes adjacent to microglia in prefrontal white matter in schizophrenia. NPJ Schizophr. 2018;4:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Uranova NA, Vikhreva OV, Rachmanova VI, Orlovskaya DD. Ultrastructural alterations of myelinated fibers and oligodendrocytes in the prefrontal cortex in schizophrenia: a postmortem morphometric study. Schizophr Res Treatment. 2011;2011:325789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Vikhreva OV, Rakhmanova VI, Orlovskaya DD, Uranova NA. Ultrastructural alterations of oligodendrocytes in prefrontal white matter in schizophrenia: A post-mortem morphometric study. Schizophr Res. 2016;177:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Vostrikov VM, Uranova NA. Reduced density of oligodendrocytes and oligodendrocyte clusters in the caudate nucleus in major psychiatric illnesses. Schizophr Res. 2020;215:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Uranova NA, Kolomeets NS, Vikhreva OV, Zimina IS, Rakhmanova VI, Orlovskaya DD. [Ultrastructural changes of myelinated fibers in the brain in continuous and attack-like paranoid schizophrenia]. Zh Nevrol Psikhiatr Im S S Korsakova. 2017;117:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Kolomeets NS, Uranova NA. [Pathology of oligodendroglia and myelinated fibers of the hippocampus in schizophrenia (an ultrastructural and morphometric study)]. Zh Nevrol Psikhiatr Im S S Korsakova. 2008;108:52-60. [PubMed] |
| 40. | Kolomeets NS, Uranova NA. Numerical density of oligodendrocytes and oligodendrocyte clusters in the anterior putamen in major psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. 2020;270:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res. 2002;27:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Hof PR, Haroutunian V, Friedrich VL Jr, Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 325] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 43. | Byne W, Kidkardnee S, Tatusov A, Yiannoulos G, Buchsbaum MS, Haroutunian V. Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res. 2006;85:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Byne W, Tatusov A, Yiannoulos G, Vong GS, Marcus S. Effects of mental illness and aging in two thalamic nuclei. Schizophr Res. 2008;106:172-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Kerns D, Vong GS, Barley K, Dracheva S, Katsel P, Casaccia P, Haroutunian V, Byne W. Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophr Res. 2010;120:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Schmitt A, Simons M, Cantuti-Castelvetri L, Falkai P. A new role for oligodendrocytes and myelination in schizophrenia and affective disorders? Eur Arch Psychiatry Clin Neurosci. 2019;269:371-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Falkai P, Malchow B, Wetzestein K, Nowastowski V, Bernstein HG, Steiner J, Schneider-Axmann T, Kraus T, Hasan A, Bogerts B, Schmitz C, Schmitt A. Decreased Oligodendrocyte and Neuron Number in Anterior Hippocampal Areas and the Entire Hippocampus in Schizophrenia: A Stereological Postmortem Study. Schizophr Bull. 2016;42 Suppl 1:S4-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Farkas N, Lendeckel U, Dobrowolny H, Funke S, Steiner J, Keilhoff G, Schmitt A, Bogerts B, Bernstein HG. Reduced density of ADAM 12-immunoreactive oligodendrocytes in the anterior cingulate white matter of patients with schizophrenia. World J Biol Psychiatry. 2010;11:556-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Bernstein HG, Keilhoff G, Dobrowolny H, Lendeckel U, Steiner J. From putative brain tumor marker to high cognitive abilities: Emerging roles of a disintegrin and metalloprotease (ADAM) 12 in the brain. J Chem Neuroanat. 2020;109:101846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Segal D, Schmitz C, Hof PR. Spatial distribution and density of oligodendrocytes in the cingulum bundle are unaltered in schizophrenia. Acta Neuropathol. 2009;117:385-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 471] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 52. | Bernstein HG, Smalla KH, Dürrschmidt D, Keilhoff G, Dobrowolny H, Steiner J, Schmitt A, Kreutz MR, Bogerts B. Increased density of prohibitin-immunoreactive oligodendrocytes in the dorsolateral prefrontal white matter of subjects with schizophrenia suggests extraneuronal roles for the protein in the disease. Neuromolecular Med. 2012;14:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res. 2009;112:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 54. | Mauney SA, Pietersen CY, Sonntag KC, Woo TW. Differentiation of oligodendrocyte precursors is impaired in the prefrontal cortex in schizophrenia. Schizophr Res. 2015;169:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Iwamoto K, Bundo M, Yamada K, Takao H, Iwayama-Shigeno Y, Yoshikawa T, Kato T. DNA methylation status of SOX10 correlates with its downregulation and oligodendrocyte dysfunction in schizophrenia. J Neurosci. 2005;25:5376-5381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 56. | Matthews PR, Eastwood SL, Harrison PJ. Reduced myelin basic protein and actin-related gene expression in visual cortex in schizophrenia. PLoS One. 2012;7:e38211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Martins-de-Souza D. Proteome and transcriptome analysis suggests oligodendrocyte dysfunction in schizophrenia. J Psychiatr Res. 2010;44:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Martins-de-Souza D, Gattaz WF, Schmitt A, Rewerts C, Marangoni S, Novello JC, Maccarrone G, Turck CW, Dias-Neto E. Alterations in oligodendrocyte proteins, calcium homeostasis and new potential markers in schizophrenia anterior temporal lobe are revealed by shotgun proteome analysis. J Neural Transm (Vienna). 2009;116:275-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | Martins-de-Souza D, Gattaz WF, Schmitt A, Maccarrone G, Hunyadi-Gulyás E, Eberlin MN, Souza GH, Marangoni S, Novello JC, Turck CW, Dias-Neto E. Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia. J Psychiatr Res. 2009;43:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 60. | Wesseling H, Gottschalk MG, Bahn S. Targeted multiplexed selected reaction monitoring analysis evaluates protein expression changes of molecular risk factors for major psychiatric disorders. Int J Neuropsychopharmacol. 2014;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Saia-Cereda VM, Cassoli JS, Schmitt A, Falkai P, Nascimento JM, Martins-de-Souza D. Proteomics of the corpus callosum unravel pivotal players in the dysfunction of cell signaling, structure, and myelination in schizophrenia brains. Eur Arch Psychiatry Clin Neurosci. 2015;265:601-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 62. | Martins-de-Souza D, Guest PC, Reis-de-Oliveira G, Schmitt A, Falkai P, Turck CW. An overview of the human brain myelin proteome and differences associated with schizophrenia. World J Biol Psychiatry. 2021;22:271-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Reis-de-Oliveira G, Zuccoli GS, Fioramonte M, Schimitt A, Falkai P, Almeida V, Martins-de-Souza D. Digging deeper in the proteome of different regions from schizophrenia brains. J Proteomics. 2020;223:103814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Velásquez E, Martins-de-Souza D, Velásquez I, Carneiro GRA, Schmitt A, Falkai P, Domont GB, Nogueira FCS. Quantitative Subcellular Proteomics of the Orbitofrontal Cortex of Schizophrenia Patients. J Proteome Res. 2019;18:4240-4253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, Smith GN, Arango V, Mann JJ, Dwork AJ, Falkai P, Honer WG. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 337] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 66. | Martins-de-Souza D, Maccarrone G, Wobrock T, Zerr I, Gormanns P, Reckow S, Falkai P, Schmitt A, Turck CW. Proteome analysis of the thalamus and cerebrospinal fluid reveals glycolysis dysfunction and potential biomarkers candidates for schizophrenia. J Psychiatr Res. 2010;44:1176-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 67. | Pennington K, Beasley CL, Dicker P, Fagan A, English J, Pariante CM, Wait R, Dunn MJ, Cotter DR. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry. 2008;13:1102-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 68. | Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684-697, 643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 594] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 69. | Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57:467-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 452] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 70. | Cheli VT, Correale J, Paez PM, Pasquini JM. Iron Metabolism in Oligodendrocytes and Astrocytes, Implications for Myelination and Remyelination. ASN Neuro. 2020;12:1759091420962681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 71. | Martins-de-Souza D, Gattaz WF, Schmitt A, Rewerts C, Maccarrone G, Dias-Neto E, Turck CW. Prefrontal cortex shotgun proteome analysis reveals altered calcium homeostasis and immune system imbalance in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2009;259:151-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 72. | Katsel P, Davis KL, Haroutunian V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophr Res. 2005;79:157-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 73. | Georgieva L, Moskvina V, Peirce T, Norton N, Bray NJ, Jones L, Holmans P, Macgregor S, Zammit S, Wilkinson J, Williams H, Nikolov I, Williams N, Ivanov D, Davis KL, Haroutunian V, Buxbaum JD, Craddock N, Kirov G, Owen MJ, O'Donovan MC. Convergent evidence that oligodendrocyte lineage transcription factor 2 (OLIG2) and interacting genes influence susceptibility to schizophrenia. Proc Natl Acad Sci U S A. 2006;103:12469-12474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Komatsu H, Takeuchi H, Kikuchi Y, Ono C, Yu Z, Iizuka K, Takano Y, Kakuto Y, Funakoshi S, Ono T, Ito J, Kunii Y, Hino M, Nagaoka A, Iwasaki Y, Yamamori H, Yasuda Y, Fujimoto M, Azechi H, Kudo N, Hashimoto R, Yabe H, Yoshida M, Saito Y, Kakita A, Fuse N, Kawashima R, Taki Y, Tomita H. Ethnicity-Dependent Effects of Schizophrenia Risk Variants of the OLIG2 Gene on OLIG2 Transcription and White Matter Integrity. Schizophr Bull. 2020;46:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Aberg K, Saetre P, Jareborg N, Jazin E. Human QKI, a potential regulator of mRNA expression of human oligodendrocyte-related genes involved in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:7482-7487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | McCullumsmith RE, Gupta D, Beneyto M, Kreger E, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophr Res. 2007;90:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 77. | Haroutunian V, Katsel P, Dracheva S, Davis KL. The human homolog of the QKI gene affected in the severe dysmyelination "quaking" mouse phenotype: downregulated in multiple brain regions in schizophrenia. Am J Psychiatry. 2006;163:1834-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Goudriaan A, de Leeuw C, Ripke S, Hultman CM, Sklar P, Sullivan PF, Smit AB, Posthuma D, Verheijen MH. Specific glial functions contribute to schizophrenia susceptibility. Schizophr Bull. 2014;40:925-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 79. | Zuchero JB, Fu MM, Sloan SA, Ibrahim A, Olson A, Zaremba A, Dugas JC, Wienbar S, Caprariello AV, Kantor C, Leonoudakis D, Lariosa-Willingham K, Kronenberg G, Gertz K, Soderling SH, Miller RH, Barres BA. CNS myelin wrapping is driven by actin disassembly. Dev Cell. 2015;34:152-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 80. | Nawaz S, Sánchez P, Schmitt S, Snaidero N, Mitkovski M, Velte C, Brückner BR, Alexopoulos I, Czopka T, Jung SY, Rhee JS, Janshoff A, Witke W, Schaap IAT, Lyons DA, Simons M. Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev Cell. 2015;34:139-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 81. | Brown TL, Macklin WB. The Actin Cytoskeleton in Myelinating Cells. Neurochem Res. 2020;45:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 82. | Tanaka J, Sobue K. Localization and characterization of gelsolin in nervous tissues: gelsolin is specifically enriched in myelin-forming cells. J Neurosci. 1994;14:1038-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Léna JY, Legrand C, Faivre-Sarrailh C, Sarliève LL, Ferraz C, Rabié A. High gelsolin content of developing oligodendrocytes. Int J Dev Neurosci. 1994;12:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Brockschnieder D, Sabanay H, Riethmacher D, Peles E. Ermin, a myelinating oligodendrocyte-specific protein that regulates cell morphology. J Neurosci. 2006;26:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 85. | Wang S, Wang T, Liu T, Xie RG, Zhao XH, Wang L, Yang Q, Jia LT, Han J. Ermin is a p116RIP -interacting protein promoting oligodendroglial differentiation and myelin maintenance. Glia. 2020;68:2264-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Erwig MS, Patzig J, Steyer AM, Dibaj P, Heilmann M, Heilmann I, Jung RB, Kusch K, Möbius W, Jahn O, Nave KA, Werner HB. Anillin facilitates septin assembly to prevent pathological outfoldings of central nervous system myelin. Elife. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 87. | Buser AM, Erne B, Werner HB, Nave KA, Schaeren-Wiemers N. The septin cytoskeleton in myelinating glia. Mol Cell Neurosci. 2009;40:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Stassart RM, Möbius W, Nave KA, Edgar JM. The Axon-Myelin Unit in Development and Degenerative Disease. Front Neurosci. 2018;12:467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 89. | Martins-de-Souza D, Harris LW, Guest PC, Bahn S. The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxid Redox Signal. 2011;15:2067-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 90. | Vostrikov V, Uranova N. Age-related increase in the number of oligodendrocytes is dysregulated in schizophrenia and mood disorders. Schizophr Res Treatment. 2011;2011:174689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Hayashi Y, Nihonmatsu-Kikuchi N, Hisanaga S, Yu XJ, Tatebayashi Y. Neuropathological similarities and differences between schizophrenia and bipolar disorder: a flow cytometric postmortem brain study. PLoS One. 2012;7:e33019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Gos T, Schroeter ML, Lessel W, Bernstein HG, Dobrowolny H, Schiltz K, Bogerts B, Steiner J. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: a postmortem study. J Psychiatr Res. 2013;47:1694-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Shao L, Golbaz K, Honer WG, Beasley CL. Deficits in axon-associated proteins in prefrontal white matter in bipolar disorder but not schizophrenia. Bipolar Disord. 2016;18:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Gigase FAJ, Snijders GJLJ, Boks MP, de Witte LD. Neurons and glial cells in bipolar disorder: A systematic review of postmortem brain studies of cell number and size. Neurosci Biobehav Rev. 2019;103:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Regenold WT, Phatak P, Marano CM, Gearhart L, Viens CH, Hisley KC. Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Res. 2007;151:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 96. | Chambers JS, Perrone-Bizzozero NI. Altered myelination of the hippocampal formation in subjects with schizophrenia and bipolar disorder. Neurochem Res. 2004;29:2293-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 97. | Bernstein HG, Keilhoff G, Dobrowolny H, Guest PC, Steiner J. Perineuronal oligodendrocytes in health and disease: the journey so far. Rev Neurosci. 2019;31:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Kim S, Webster MJ. Correlation analysis between genome-wide expression profiles and cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. Mol Psychiatry. 2010;15:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 99. | Schubert KO, Föcking M, Cotter DR. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: potential roles in GABAergic interneuron pathology. Schizophr Res. 2015;167:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 100. | Föcking M, Dicker P, English JA, Schubert KO, Dunn MJ, Cotter DR. Common proteomic changes in the hippocampus in schizophrenia and bipolar disorder and particular evidence for involvement of cornu ammonis regions 2 and 3. Arch Gen Psychiatry. 2011;68:477-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 101. | Wang JF. Defects of mitochondrial electron transport chain in bipolar disorder: implications for mood-stabilizing treatment. Can J Psychiatry. 2007;52:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | Kim Y, Vadodaria KC, Lenkei Z, Kato T, Gage FH, Marchetto MC, Santos R. Mitochondria, Metabolism, and Redox Mechanisms in Psychiatric Disorders. Antioxid Redox Signal. 2019;31:275-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 103. | Puthumana JS, Regenold WT. Glucose-6-phosphate dehydrogenase activity in bipolar disorder and schizophrenia: Relationship to mitochondrial impairment. J Psychiatr Res. 2019;112:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Kim HK, Tyryshkin K, Elmi N, Feilotter H, Andreazza AC. Examining redox modulation pathways in the post-mortem frontal cortex in patients with bipolar disorder through data mining of microRNA expression datasets. J Psychiatr Res. 2018;99:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 434] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 106. | Godlewska BR, Yip SW, Near J, Goodwin GM, Cowen PJ. Cortical glutathione levels in young people with bipolar disorder: a pilot study using magnetic resonance spectroscopy. Psychopharmacology (Berl). 2014;231:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 107. | Das TK, Javadzadeh A, Dey A, Sabesan P, Théberge J, Radua J, Palaniyappan L. Antioxidant defense in schizophrenia and bipolar disorder: A meta-analysis of MRS studies of anterior cingulate glutathione. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 108. | Vostrikov VM, Uranova NA, Rakhmanova VI, Orlovskaia DD. [Lowered oligodendroglial cell density in the prefrontal cortex in schizophrenia]. Zh Nevrol Psikhiatr Im S S Korsakova. 2004;104:47-51. [PubMed] |
| 109. | Merkwirth C, Langer T. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta. 2009;1793:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 303] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 110. | Merkwirth C, Martinelli P, Korwitz A, Morbin M, Brönneke HS, Jordan SD, Rugarli EI, Langer T. Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphosphorylation and neurodegeneration. PLoS Genet. 2012;8:e1003021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 111. | Chowdhury I, Thompson WE, Thomas K. Prohibitins role in cellular survival through Ras-Raf-MEK-ERK pathway. J Cell Physiol. 2014;229:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 112. | Signorile A, Sgaramella G, Bellomo F, De Rasmo D. Prohibitins: A Critical Role in Mitochondrial Functions and Implication in Diseases. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 113. | Katsel P, Davis KL, Li C, Tan W, Greenstein E, Kleiner Hoffman LB, Haroutunian V. Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology. 2008;33:2993-3009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 114. | Göttle P, Sabo JK, Heinen A, Venables G, Torres K, Tzekova N, Parras CM, Kremer D, Hartung HP, Cate HS, Küry P. Oligodendroglial maturation is dependent on intracellular protein shuttling. J Neurosci. 2015;35:906-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 115. | Göttle P, Küry P. Intracellular Protein Shuttling: A Mechanism Relevant for Myelin Repair in Multiple Sclerosis? Int J Mol Sci. 2015;16:15057-15085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Manousi A, Göttle P, Reiche L, Cui QL, Healy LM, Akkermann R, Gruchot J, Schira-Heinen J, Antel JP, Hartung HP, Küry P. Identification of novel myelin repair drugs by modulation of oligodendroglial differentiation competence. EBioMedicine. 2021;65:103276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 117. | Jahn O, Siems SB, Kusch K, Hesse D, Jung RB, Liepold T, Uecker M, Sun T, Werner HB. The CNS Myelin Proteome: Deep Profile and Persistence After Post-mortem Delay. Front Cell Neurosci. 2020;14:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 118. | Mulert C, Shenton ME. MRI in Psychiatry. Berlin, Heidelberg: Springer Berlin Heidelberg, 2014. |
| 119. | Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakowski SM. Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol Psychiatry. 2007;61:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 120. | Vitolo E, Tatu MK, Pignolo C, Cauda F, Costa T, Ando' A, Zennaro A. White matter and schizophrenia: A meta-analysis of voxel-based morphometry and diffusion tensor imaging studies. Psychiatry Res Neuroimaging. 2017;270:8-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 121. | Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1637] [Cited by in RCA: 1792] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 122. | Mighdoll MI, Tao R, Kleinman JE, Hyde TM. Myelin, myelin-related disorders, and psychosis. Schizophr Res. 2015;161:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 123. | Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, Andreassen OA, Arango C, Banaj N, Bouix S, Bousman CA, Brouwer RM, Bruggemann J, Bustillo J, Cahn W, Calhoun V, Cannon D, Carr V, Catts S, Chen J, Chen JX, Chen X, Chiapponi C, Cho KK, Ciullo V, Corvin AS, Crespo-Facorro B, Cropley V, De Rossi P, Diaz-Caneja CM, Dickie EW, Ehrlich S, Fan FM, Faskowitz J, Fatouros-Bergman H, Flyckt L, Ford JM, Fouche JP, Fukunaga M, Gill M, Glahn DC, Gollub R, Goudzwaard ED, Guo H, Gur RE, Gur RC, Gurholt TP, Hashimoto R, Hatton SN, Henskens FA, Hibar DP, Hickie IB, Hong LE, Horacek J, Howells FM, Hulshoff Pol HE, Hyde CL, Isaev D, Jablensky A, Jansen PR, Janssen J, Jönsson EG, Jung LA, Kahn RS, Kikinis Z, Liu K, Klauser P, Knöchel C, Kubicki M, Lagopoulos J, Langen C, Lawrie S, Lenroot RK, Lim KO, Lopez-Jaramillo C, Lyall A, Magnotta V, Mandl RCW, Mathalon DH, McCarley RW, McCarthy-Jones S, McDonald C, McEwen S, McIntosh A, Melicher T, Mesholam-Gately RI, Michie PT, Mowry B, Mueller BA, Newell DT, O'Donnell P, Oertel-Knöchel V, Oestreich L, Paciga SA, Pantelis C, Pasternak O, Pearlson G, Pellicano GR, Pereira A, Pineda Zapata J, Piras F, Potkin SG, Preda A, Rasser PE, Roalf DR, Roiz R, Roos A, Rotenberg D, Satterthwaite TD, Savadjiev P, Schall U, Scott RJ, Seal ML, Seidman LJ, Shannon Weickert C, Whelan CD, Shenton ME, Kwon JS, Spalletta G, Spaniel F, Sprooten E, Stäblein M, Stein DJ, Sundram S, Tan Y, Tan S, Tang S, Temmingh HS, Westlye LT, Tønnesen S, Tordesillas-Gutierrez D, Doan NT, Vaidya J, van Haren NEM, Vargas CD, Vecchio D, Velakoulis D, Voineskos A, Voyvodic JQ, Wang Z, Wan P, Wei D, Weickert TW, Whalley H, White T, Whitford TJ, Wojcik JD, Xiang H, Xie Z, Yamamori H, Yang F, Yao N, Zhang G, Zhao J, van Erp TGM, Turner J, Thompson PM, Donohoe G. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 503] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 124. | Koshiyama D, Fukunaga M, Okada N, Morita K, Nemoto K, Usui K, Yamamori H, Yasuda Y, Fujimoto M, Kudo N, Azechi H, Watanabe Y, Hashimoto N, Narita H, Kusumi I, Ohi K, Shimada T, Kataoka Y, Yamamoto M, Ozaki N, Okada G, Okamoto Y, Harada K, Matsuo K, Yamasue H, Abe O, Hashimoto R, Takahashi T, Hori T, Nakataki M, Onitsuka T, Holleran L, Jahanshad N, van Erp TGM, Turner J, Donohoe G, Thompson PM, Kasai K; COCORO. White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2937 individuals. Mol Psychiatry. 2020;25:883-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 125. | Ji E, Lejuste F, Sarrazin S, Houenou J. From the microscope to the magnet: Disconnection in schizophrenia and bipolar disorder. Neurosci Biobehav Rev. 2019;98:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 126. | Whitford TJ, Kubicki M, Shenton ME. Diffusion tensor imaging, structural connectivity, and schizophrenia. Schizophr Res Treatment. 2011;2011:709523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Yang X, Cao D, Liang X, Zhao J. Schizophrenia symptomatic associations with diffusion tensor imaging measured fractional anisotropy of brain: a meta-analysis. Neuroradiology. 2017;59:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 128. | Mesulam MM. Principles of Behavioral and Cognitive Neurology. Oxford University Press, 2000. |
| 129. | Chawla N, Deep R, Khandelwal SK, Garg A. Reduced integrity of superior longitudinal fasciculus and arcuate fasciculus as a marker for auditory hallucinations in schizophrenia: A DTI tractography study. Asian J Psychiatr. 2019;44:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 130. | Geoffroy PA, Houenou J, Duhamel A, Amad A, De Weijer AD, Curčić-Blake B, Linden DE, Thomas P, Jardri R. The Arcuate Fasciculus in auditory-verbal hallucinations: a meta-analysis of diffusion-tensor-imaging studies. Schizophr Res. 2014;159:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 131. | Psomiades M, Fonteneau C, Mondino M, Luck D, Haesebaert F, Suaud-Chagny MF, Brunelin J. Integrity of the arcuate fasciculus in patients with schizophrenia with auditory verbal hallucinations: A DTI-tractography study. Neuroimage Clin. 2016;12:970-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 132. | Vanes LD, Mouchlianitis E, Barry E, Patel K, Wong K, Shergill SS. Cognitive correlates of abnormal myelination in psychosis. Sci Rep. 2019;9:5162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 133. | McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019;42:205-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 507] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 134. | Rotarska-Jagiela A, van de Ven V, Oertel-Knöchel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 135. | Zhou Y, Fan L, Qiu C, Jiang T. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci Bull. 2015;31:207-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 136. | Fujiwara H, Namiki C, Hirao K, Miyata J, Shimizu M, Fukuyama H, Sawamoto N, Hayashi T, Murai T. Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2007;95:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 137. | Takei K, Yamasue H, Abe O, Yamada H, Inoue H, Suga M, Muroi M, Sasaki H, Aoki S, Kasai K. Structural disruption of the dorsal cingulum bundle is associated with impaired Stroop performance in patients with schizophrenia. Schizophr Res. 2009;114:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 138. | Fitzsimmons J, Hamoda HM, Swisher T, Terry D, Rosenberger G, Seidman LJ, Goldstein J, Mesholam-Gately R, Petryshen T, Wojcik J, Kikinis R, Kubicki M. Diffusion tensor imaging study of the fornix in first episode schizophrenia and in healthy controls. Schizophr Res. 2014;156:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 139. | Kantarci K. Fractional anisotropy of the fornix and hippocampal atrophy in Alzheimer's disease. Front Aging Neurosci. 2014;6:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 140. | Perlstein MD, Chohan MR, Coman IL, Antshel KM, Fremont WP, Gnirke MH, Kikinis Z, Middleton FA, Radoeva PD, Shenton ME, Kates WR. White matter abnormalities in 22q11.2 deletion syndrome: preliminary associations with the Nogo-66 receptor gene and symptoms of psychosis. Schizophr Res. 2014;152:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 141. | Singh S, Singh K, Trivedi R, Goyal S, Kaur P, Singh N, Bhatia T, Deshpande SN, Khushu S. Microstructural abnormalities of uncinate fasciculus as a function of impaired cognition in schizophrenia: A DTI study. J Biosci. 2016;41:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 142. | Ganzola R, Duchesne S. Voxel-based morphometry meta-analysis of gray and white matter finds significant areas of differences in bipolar patients from healthy controls. Bipolar Disord. 2017;19:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 143. | McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 144. | Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 584] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 145. | Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 373] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 146. | Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What are white matter hyperintensities made of? J Am Heart Assoc. 2015;4:001140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 645] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 147. | Coffey CE, Figiel GS, Djang WT, Saunders WB, Weiner RD. White matter hyperintensity on magnetic resonance imaging: clinical and neuroanatomic correlates in the depressed elderly. J Neuropsychiatry Clin Neurosci. 1989;1:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 121] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 148. | Ahn KH, Lyoo IK, Lee HK, Song IC, Oh JS, Hwang J, Kwon J, Kim MJ, Kim M, Renshaw PF. White matter hyperintensities in subjects with bipolar disorder. Psychiatry Clin Neurosci. 2004;58:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 149. | Altshuler LL, Curran JG, Hauser P, Mintz J, Denicoff K, Post R. T2 hyperintensities in bipolar disorder: magnetic resonance imaging comparison and literature meta-analysis. Am J Psychiatry. 1995;152:1139-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 150. | Beyer JL, Young R, Kuchibhatla M, Krishnan KR. Hyperintense MRI lesions in bipolar disorder: A meta-analysis and review. Int Rev Psychiatry. 2009;21:394-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 151. | Grangeon MC, Seixas C, Quarantini LC, Miranda-Scippa A, Pompili M, Steffens DC, Wenzel A, Lacerda AL, de Oliveira IR. White matter hyperintensities and their association with suicidality in major affective disorders: a meta-analysis of magnetic resonance imaging studies. CNS Spectr. 2010;15:375-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 152. | Aylward EH, Roberts-Twillie JV, Barta PE, Kumar AJ, Harris GJ, Geer M, Peyser CE, Pearlson GD. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry. 1994;151:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 166] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 153. | Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev. 2010;34:533-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 154. | Moore PB, Shepherd DJ, Eccleston D, Macmillan IC, Goswami U, McAllister VL, Ferrier IN. Cerebral white matter lesions in bipolar affective disorder: relationship to outcome. Br J Psychiatry. 2001;178:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 155. | Pompili M, Innamorati M, Mann JJ, Oquendo MA, Lester D, Del Casale A, Serafini G, Rigucci S, Romano A, Tamburello A, Manfredi G, De Pisa E, Ehrlich S, Giupponi G, Amore M, Tatarelli R, Girardi P. Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1501-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 156. | Pezzoli S, Emsell L, Yip SW, Dima D, Giannakopoulos P, Zarei M, Tognin S, Arnone D, James A, Haller S, Frangou S, Goodwin GM, McDonald C, Kempton MJ. Meta-analysis of regional white matter volume in bipolar disorder with replication in an independent sample using coordinates, T-maps, and individual MRI data. Neurosci Biobehav Rev. 2018;84:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 157. | Arnone D, McIntosh AM, Chandra P, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in bipolar disorder. Acta Psychiatr Scand. 2008;118:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 158. | Holleran L, Ahmed M, Anderson-Schmidt H, McFarland J, Emsell L, Leemans A, Scanlon C, Dockery P, McCarthy P, Barker GJ, McDonald C, Cannon DM. Altered interhemispheric and temporal lobe white matter microstructural organization in severe chronic schizophrenia. Neuropsychopharmacology. 2014;39:944-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 159. | Zhang R, Jiang X, Chang M, Wei S, Tang Y, Wang F. White matter abnormalities of corpus callosum in patients with bipolar disorder and suicidal ideation. Ann Gen Psychiatry. 2019;18:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 160. | Lavagnino L, Cao B, Mwangi B, Wu MJ, Sanches M, Zunta-Soares GB, Kapczinski F, Soares J. Changes in the corpus callosum in women with late-stage bipolar disorder. Acta Psychiatr Scand. 2015;131:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 161. | López-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 162. | Favre P, Pauling M, Stout J, Hozer F, Sarrazin S, Abé C, Alda M, Alloza C, Alonso-Lana S, Andreassen OA, Baune BT, Benedetti F, Busatto GF, Canales-Rodríguez EJ, Caseras X, Chaim-Avancini TM, Ching CRK, Dannlowski U, Deppe M, Eyler LT, Fatjo-Vilas M, Foley SF, Grotegerd D, Hajek T, Haukvik UK, Howells FM, Jahanshad N, Kugel H, Lagerberg TV, Lawrie SM, Linke JO, McIntosh A, Melloni EMT, Mitchell PB, Polosan M, Pomarol-Clotet E, Repple J, Roberts G, Roos A, Rosa PGP, Salvador R, Sarró S, Schofield PR, Serpa MH, Sim K, Stein DJ, Sussmann JE, Temmingh HS, Thompson PM, Verdolini N, Vieta E, Wessa M, Whalley HC, Zanetti MV, Leboyer M, Mangin JF, Henry C, Duchesnay E, Houenou J; ENIGMA Bipolar Disorder Working Group. Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacology. 2019;44:2285-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 163. | Lee DK, Lee H, Park K, Joh E, Kim CE, Ryu S. Common gray and white matter abnormalities in schizophrenia and bipolar disorder. PLoS One. 2020;15:e0232826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 164. | Nortje G, Stein DJ, Radua J, Mataix-Cols D, Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord. 2013;150:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 165. | Emsell L, Langan C, Van Hecke W, Barker GJ, Leemans A, Sunaert S, McCarthy P, Nolan R, Cannon DM, McDonald C. White matter differences in euthymic bipolar I disorder: a combined magnetic resonance imaging and diffusion tensor imaging voxel-based study. Bipolar Disord. 2013;15:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 166. | Marlinge E, Bellivier F, Houenou J. White matter alterations in bipolar disorder: potential for drug discovery and development. Bipolar Disord. 2014;16:97-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 167. | Bellani M, Boschello F, Delvecchio G, Dusi N, Altamura CA, Ruggeri M, Brambilla P. DTI and Myelin Plasticity in Bipolar Disorder: Integrating Neuroimaging and Neuropathological Findings. Front Psychiatry. 2016;7:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 168. | Fields RD, Woo DH, Basser PJ. Glial Regulation of the Neuronal Connectome through Local and Long-Distant Communication. Neuron. 2015;86:374-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 169. | Lago-Baldaia I, Fernandes VM, Ackerman SD. More Than Mortar: Glia as Architects of Nervous System Development and Disease. Front Cell Dev Biol. 2020;8:611269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 170. | Allen NJ, Lyons DA. Glia as architects of central nervous system formation and function. Science. 2018;362:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 595] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 171. | Feng Y. Convergence and divergence in the etiology of myelin impairment in psychiatric disorders and drug addiction. Neurochem Res. 2008;33:1940-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 172. | Connor CM, Crawford BC, Akbarian S. White matter neuron alterations in schizophrenia and related disorders. Int J Dev Neurosci. 2011;29:325-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 173. | Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 174. | Rubinov M, Bullmore E. Schizophrenia and abnormal brain network hubs. Dialogues Clin Neurosci. 2013;15:339-349. [PubMed] |
| 175. | Voineskos AN, Felsky D, Kovacevic N, Tiwari AK, Zai C, Chakravarty MM, Lobaugh NJ, Shenton ME, Rajji TK, Miranda D, Pollock BG, Mulsant BH, McIntosh AR, Kennedy JL. Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia. Cereb Cortex. 2013;23:2044-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 176. | Traiffort E, Kassoussi A, Zahaf A, Laouarem Y. Astrocytes and Microglia as Major Players of Myelin Production in Normal and Pathological Conditions. Front Cell Neurosci. 2020;14:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 177. | Tress O, Maglione M, May D, Pivneva T, Richter N, Seyfarth J, Binder S, Zlomuzica A, Seifert G, Theis M, Dere E, Kettenmann H, Willecke K. Panglial gap junctional communication is essential for maintenance of myelin in the CNS. J Neurosci. 2012;32:7499-7518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 178. | Ronzano R, Thetiot M, Lubetzki C, Desmazieres A. Myelin Plasticity and Repair: Neuro-Glial Choir Sets the Tuning. Front Cell Neurosci. 2020;14:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 179. | Hughes AN. Glial Cells Promote Myelin Formation and Elimination. Front Cell Dev Biol. 2021;9:661486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 180. | Keshavarz M. Glial cells as key elements in the pathophysiology and treatment of bipolar disorder. Acta Neuropsychiatr. 2017;29:140-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 181. | Raabe FJ, Slapakova L, Rossner MJ, Cantuti-Castelvetri L, Simons M, Falkai PG, Schmitt A. Oligodendrocytes as A New Therapeutic Target in Schizophrenia: From Histopathological Findings to Neuron-Oligodendrocyte Interaction. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 182. | Dong XH, Zhen XC. Glial pathology in bipolar disorder: potential therapeutic implications. CNS Neurosci Ther. 2015;21:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 183. | Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746-4751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 892] [Cited by in RCA: 917] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 184. | Gouvêa-Junqueira D, Falvella ACB, Antunes ASLM, Seabra G, Brandão-Teles C, Martins-de-Souza D, Crunfli F. Novel Treatment Strategies Targeting Myelin and Oligodendrocyte Dysfunction in Schizophrenia. Front Psychiatry. 2020;11:379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 185. | Baruch K, Silberberg G, Aviv A, Shamir E, Bening-Abu-Shach U, Baruch Y, Darvasi A, Navon R. Association between golli-MBP and schizophrenia in the Jewish Ashkenazi population: are regulatory regions involved? Int J Neuropsychopharmacol. 2009;12:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 186. | Peirce TR, Bray NJ, Williams NM, Norton N, Moskvina V, Preece A, Haroutunian V, Buxbaum JD, Owen MJ, O'Donovan MC. Convergent evidence for 2',3'-cyclic nucleotide 3'-phosphodiesterase as a possible susceptibility gene for schizophrenia. Arch Gen Psychiatry. 2006;63:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 187. | Voineskos AN, de Luca V, Bulgin NL, van Adrichem Q, Shaikh S, Lang DJ, Honer WG, Kennedy JL. A family-based association study of the myelin-associated glycoprotein and 2',3'-cyclic nucleotide 3'-phosphodiesterase genes with schizophrenia. Psychiatr Genet. 2008;18:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 188. | Qin W, Gao J, Xing Q, Yang J, Qian X, Li X, Guo Z, Chen H, Wang L, Huang X, Gu N, Feng G, He L. A family-based association study of PLP1 and schizophrenia. Neurosci Lett. 2005;375:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 189. | Wan C, Yang Y, Feng G, Gu N, Liu H, Zhu S, He L, Wang L. Polymorphisms of myelin-associated glycoprotein gene are associated with schizophrenia in the Chinese Han population. Neurosci Lett. 2005;388:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 190. | Yang YF, Qin W, Shugart YY, He G, Liu XM, Zhou J, Zhao XZ, Chen Q, La YJ, Xu YF, Li XW, Gu NF, Feng GY, Song H, Wang P, He L. Possible association of the MAG locus with schizophrenia in a Chinese Han cohort of family trios. Schizophr Res. 2005;75:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 191. | Qu M, Yue W, Tang F, Wang L, Han Y, Zhang D. Polymorphisms of Transferrin gene are associated with schizophrenia in Chinese Han population. J Psychiatr Res. 2008;42:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 192. | Huo Y, Li S, Liu J, Li X, Luo XJ. Functional genomics reveal gene regulatory mechanisms underlying schizophrenia risk. Nat Commun. 2019;10:670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |