Published online Feb 19, 2022. doi: 10.5498/wjp.v12.i2.212
Peer-review started: February 1, 2021
First decision: July 28, 2021
Revised: August 7, 2021
Accepted: January 13, 2022
Article in press: January 13, 2022
Published online: February 19, 2022
Processing time: 380 Days and 18.1 Hours
As we cycle between the states of wakefulness and sleep, a bilateral cholinergic nucleus in the pontine brain stem, the laterodorsal tegmentum (LDT), plays a critical role in controlling salience processing, attention, behavioral arousal, and electrophysiological signatures of the sub- and microstates of sleep. Disorders involving abnormal alterations in behavioral and motivated states, such as drug dependence, likely involve dysfunctions in LDT signaling. In addition, as the LDT exhibits connectivity with the thalamus and mesocortical circuits, as well as receives direct, excitatory input from the prefrontal cortex, a role for the LDT in cognitive symptoms characterizing attention-deficit/hyperactivity disorder (ADHD) including impulsivity, inflexibility, and dysfunctions of attention is suggested. Prenatal nicotine exposure (PNE) is associated with a higher risk for later life development of drug dependence and ADHD, suggesting alteration in development of brain regions involved in these behaviors. PNE has been shown to alter glutamate and cholinergic signaling within the LDT. As glutamate and acetylcholine are major excitatory mediators, these alterations would likely alter excitatory output to target regions in limbic motivational circuits and to thalamic and cortical networks mediating executive control. Further, PNE alters neuronal development and transmission within prefrontal cortex and limbic areas that send input to the LDT, which would compound effects of differential processing within the PNE LDT. When taken together, alterations in signaling in the LDT are likely to play a role in negative behavioral outcomes seen in PNE individuals, including a heightened risk of drug dependence and ADHD behaviors.
Core Tip: Offspring of women who used nicotine-containing products while pregnant exhibit risk factors for later-life development of cognitive deficits, including attention deficit/hyperactivity disorder and drug dependence. This suggests that circuits that play a role in cognition are being altered by nicotine. The laterodorsal tegmental nucleus of the pons plays a role in attention, motivation, and other cognitive-related processes, and nicotine during gestation has been shown in animal studies to alter functioning of this nucleus. In this review, we discuss the human and animal literature that indicate that alterations in functioning of the laterodorsal tegmental nucleus could arise following prenatal nicotine exposure, and that these alterations could play a role in the negative risks associated with early-life nicotine exposure.
- Citation: Polli FS, Kohlmeier KA. Prenatal nicotine alters development of the laterodorsal tegmentum: Possible role for attention-deficit/hyperactivity disorder and drug dependence . World J Psychiatry 2022; 12(2): 212-235
- URL: https://www.wjgnet.com/2220-3206/full/v12/i2/212.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i2.212
Smoking during pregnancy exposes the fetus to a variety of chemicals known to have effects on development. Arguably, the most influential of these is nicotine, which crosses the placenta and sequesters within the fetal compartment, which is unfortunate as nicotine is a known teratogen likely involved in differences seen in the development of neural structures characterized in functional imaging studies (for review, see[1]). Many of the brain regions that have been found to be altered are known to play a role in cognitive processing and behavioral control, and differences in their development associated with prenatal nicotine exposure (PNE) could underlie negative cognitive and other behavioral outcomes. Clinically, among other maladaptive, neurally-based behavioral outcomes, PNE individuals exhibit a higher degree of drug dependence[2-6], impulsivity[7,8], and dysfunctions in attention[9-12]. Given the occurrence of these later two behaviors in PNE, an association between attention-deficit/hyperactivity disorder (ADHD) and PNE has been explicitly examined, with studies suggesting that there is a higher incidence of ADHD in offspring of women who smoked while pregnant[10,12-14]. Although few studies have addressed the topic, some reports detail sex-based differences in behavioral outcomes following PNE, which could be due to hormonally-based differential sensitivities to nicotine’s ability to alter structural development[15]. In studies examining the damaging neural effects of nicotine that could underlie negative behavioral outcomes, the focus has been directed to changes within neural structures well known to play a role in cognition- and motivation control, such as the prefrontal cortex (PFC), amygdala, hippocampus, and mesoaccumbal circuits comprising the nucleus accumbens (NAc) and the ventral tegmental area (VTA). Unsurprisingly, the role of changes imposed by PNE in the brain stem in PNE-associated behavioral risks has been much less well studied.
While the literature supporting a role of the brain stem in cognitive functioning is scant, since the 1930s, it has been known that damage to the brain stem causes dysfunctions in executive control, suggesting that the brain stem transmits signals that are incorporated into high-order, cognitive processing[16,17]. While sparse, anatomical lesion, pharmacological, and stimulation data began to emerge supporting the interpretation that the brain stem plays a role that extends beyond simply receiving information, and its role may include participation as an inherent functional player in shaping cognitive function. Thanks in large part to the advent of optogenetics, which allows selective dissection of cellular contributions to behavior, the amount of data showing a role of the laterodorsal tegmentum (LDT) in cognitive-based behaviors has exploded, and when taken together, suggest that the brain stem is an integral functional component of the circuits that are involved with executive functions[18-20]. Specifically, determination of the role played by neurons within the LDT of the pons in motivation, attention, and other facets of goal-directed behaviors[21-25] suggests that a re-evaluation is warranted of the perception that the brain stem receives input from top-down circuits and passively transmits it onwards. At this time, it appears clear that the LDT is not a region that passively complies with and executes commands from higher order centers, but rather that the LDT plays a significant role in the coding of information in associative circuits in a bottom-up direction. Further, data suggest that the LDT could be involved in behavioral and mental behaviors known to be altered in PNE individuals. In this review, we discuss data from recent studies that should lead to redefinitions of the extent of control of behavior played by what is considered the most ancestral region of the brain, the brain stem. In addition, results from these studies should raise alarm that early life exposures to nicotine could alter the way by which the LDT responds to input, which would subsequently impact LDT output. This alteration could participate in the generation of PNE-associated behavioral abnormalities in motivation and executive control.
Cognitive functioning, including that involved in attention, relies on acetylcholine (ACh) acting at neuronal nicotinic ACh receptors (nAChRs) and muscarinic ACh receptors. Cholinergic dysfunction has been correlated with impairment of long-term memory[26-28], and manipulations of cholinergic systems have been shown to play a role in attentional states[29]. In humans, augmentation of cholinergic signaling at nAChRs in individuals not sensitized by nicotine has been shown to improve cognitive functions, such as memory and attention[30,31]. Transdermal nicotine delivery in non-smoking subjects increases attention by reducing omission errors and response time variability in the human continuous performance task[32]. Further, nicotine has been shown to improve attentional performance in a variety of cognitive disorders in non-sensitized adults, including ADHD[33], Alzheimer’s disease[34], and schizophrenia[35]. These and other studies have focused on the development of cognitive enhancing drugs based upon agonism or potentiation of nAChRs.
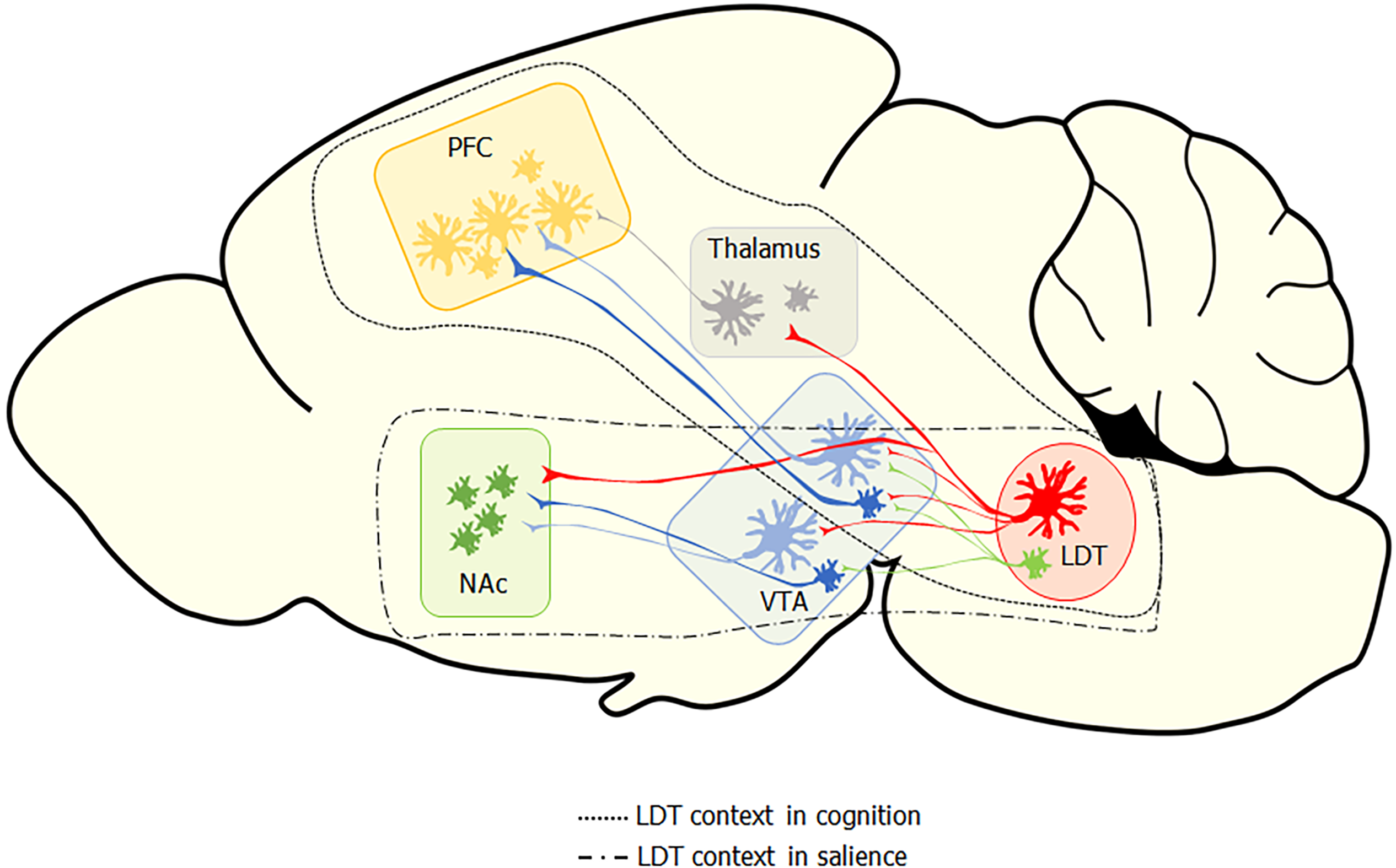
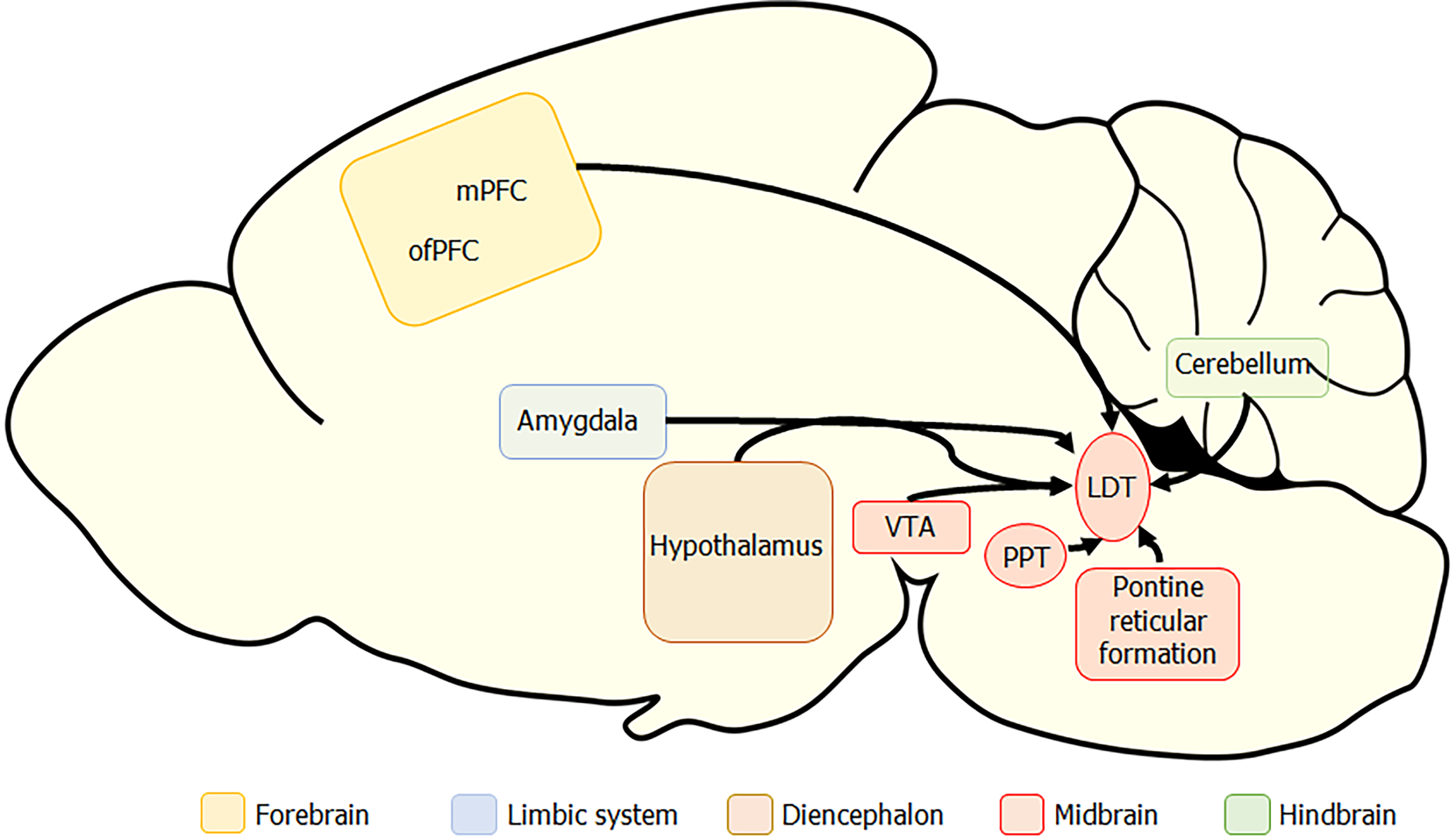
While nicotine is an excellent agonist for the nAChR, endogenous signaling at nAChRs is mediated by ACh. The majority of neuronal ACh is sourced from two main clusters in the brain, one within the forebrain and another within the pontine brain stem, with both clusters sending diffuse projections to a variety of targets. The LDT and the pedunculopontine tegmental nucleus (PPT) comprise the cholinergic cluster in the pontine brain stem and send ACh projections widely to both caudal and rostral targets. Both the PPT and the LDT participate in the reticular activating system and, as part of it, exert cholinergic control over the thalamus, which has been implicated in behavioral state control and electroencephalographic states of arousal and attention. Both cholinergic brain stem nuclei also play a role in sensorimotor integration, reinforcement, and learning; however, their contribution to the control of these processes differs, which is supported by the distinct segregation in the projection patterns of the two nuclei and by divergent functional outcomes upon stimulation[18,36]. The PPT appears particularly involved in control of gait and posture, which is supported by a heavy innervation of structures involved in motor functions, and findings that, when stimulated, the PPT modulates activity in the basal ganglia as well as in the formation and updating of action-outcome associations and rapid decision making[37,38]. The LDT does send projections to the substantia nigra, suggesting it could participate in control of movement; however, optogenetic stimulation of this projection did not result in locomotion, which provides functional evidence in line with the interpretation that the projection from the LDT to the portion of the striatum involved in motor control is not as involved as the PPT projections are in movement control[18,25]. The LDT appears to be more involved than the PPT in the control of cognition and behavior, as suggested from a plethora of anatomical, behavioral, and stimulation studies. This control appears to be exerted directly via connectivity of the LDT to limbic structures as well as indirectly via synapses within specific thalamic nuclei (Figure 1).
Projections from the LDT synapsing within the principle relay nuclei of the thalamus suggest control of the LDT over thalamic cellular activity that would impact output to cortical regions via thalamo-cortical tracts. Thalamo-cortical radiations are involved in relaying information critical in mediation of consciousness, arousal, and alertness. While thought to be a passive relay station, recent evidence suggests the possibility that the thalamus may govern amplification of cortical signaling and therefore be involved more centrally in cognitive behaviors, including behavioral flexibility, than previously appreciated. The more active role emerging of the thalamus in cognitive processes highlights that afferent input to the thalamus, such as that sourcing from the LDT, likely plays a modulatory role in cognitive control[39]. Retrograde studies revealed that the major cholinergic input into the thalamus, particularly in the cognitive-relevant anterior, reticular, ventroposterior, mediodorsal, and central medial nuclei, sources from LDT neurons through both ipsi- and counter-lateral projections[40-43]. Double retrograde labeling approaches showed that many of the LDT neurons that send projections to the thalamus also send collaterals to extra thalamic targets. One of these extra thalamic targets is the VTA, which is also involved in cognitive and limbic functioning through dopamine (DA)-mediated transmission in the mesoaccumbal and mesocortical pathways[18]. Since both the thalamus and VTA project to the NAc, a central nucleus in limbic processing that sends input both to the thalamus and to cortical targets via the mesocortical pathway, this collateralized projection pattern provides the LDT with direct and indirect control of striatal regions that project to and release DA within the cortex. Therefore, regulation of cognitive functions via variations in DA levels in the PFC, which exerts executive functioning, could occur via LDT actions within the thalamus or, more indirectly, via LDT collaterals in the VTA, suggesting a complex potential for LDT control over DA transmission. However, this dual projection pattern is not consistently seen in all LDT thalamic targets.
The anterior thalamus, which is known to be involved in alertness, learning, and memory, receives cholinergic projections from the LDT that arise from a different population of cholinergic LDT cells than those sending input to the VTA, suggesting presence of distinct sub-populations of LDT cells[44].
Functional activation of the LDT-thalamic pathway has been shown. LDT cholinergic neurons fire action potentials most vigorously during rapid eye movement sleep and aroused wakefulness[45,46], which has been shown with in vivo micro dialysis to result in increases in the levels of ACh within the thalamus during these states[47]. Functional connections between the LDT and different thalamic centers have also been shown by in vivo electrophysiology, combined with pharmacological approaches. Electrical stimulation of the LDT, as well as pharmacological stimulation of the thalamus via application of the muscarinic ACh receptor agonist carbachol, enhanced firing rates of ventroposterior medial thalamic cells, indirectly modulating sensory-related cortical areas involved in selective attention[19,48]. Actions of cholinergic agonists in the ventroposterior medial thalamus were associated with modulation of tonic firing patterns and activation of thalamic-cortical projecting centers, such as the somatosensory cortex responsible for processing sensory perception[49,50]. Lesions of the mediodorsal thalamic nucleus, one of the thalamic regions that receives the heaviest cholinergic inputs from the LDT and exhibits reciprocal innervation with the PFC, resulted in working memory deficits in rats, as assayed by impaired radial maze performance[51]. Injection of cholinergic agents enhanced mediodorsal thalamus-PFC synaptic plasticity and inhibited mechanisms underlying depotentiation, which is a mechanism behind the weakening of strength of synapses[52]. Weak in vivo stimulation of the LDT nucleus was shown to eliminate spontaneous and evoked burst-firing in the reticular nucleus of the thalamus in anesthetized rats, whereas strong LDT activation induced discharge within this region[53]. In addition, pulse trains injected within the LDT enhanced the responsiveness of anterior thalamic neurons to cortical stimuli[54]. Further, lesions of the anterior or central thalamic nuclei reduced performance in memory testing and diminished attention, which were effects also seen upon local infusion of cholinergic antagonists at these sites, thereby linking the deficits in ACh in the thalamus to working memory and attentional impairments[55-57]. Functional imaging studies in humans have shown that the improvement of attention induced by nicotine is associated with increased activation of the thalamus[58,59], and, furthermore, functional magnetic resonance imaging has provided evidence that more general cognitive improvements observed upon nicotine exposure could be due to activation of nAChRs in the thalamus[60]. When taken together, it is clear that cholinergic actions in the thalamus are involved in attention and cognition, and cholinergic input is provided by a functional connection between the LDT and the thalamus. Therefore, LDT-thalamic cholinergic projections confer upon the LDT an indirect control of cortical excitability through thalamic relay centers and could be involved in amplification of cognitive processing controlled by the thalamus.
Besides participating in cholinergic modulation of cortico-thalamic circuits, ultrastructural, immunolabeling, and optogenetic studies indicate that the LDT exerts a cholinergic modulatory role within structures and circuits associated with the limbic system that underlie motivation and salience, including the VTA and the NAc[61,62]. The LDT has been shown to be the main source of cholinergic inputs into the NAc core and the VTA, and studies have suggested that LDT cholinergic inputs onto VTA cells modulate activity of DA-containing VTA neurons that participate in both the mesocortical and mesoaccumbal pathways[61-65]. The LDT has been shown to form mainly asymmetric, putatively excitatory, synapses within the striatal complex, particularly onto DA-containing VTA cells and within the NAc core[64]. While the majority of VTA neurons are DA-containing, 35% of the cells in the VTA are non-dopaminergic, with 25 % of these being inhibitory gamma-aminobutyric acid (GABA)ergic neurons[66]. GABAergic VTA neurons in both mesoaccumbal and mesocortical pathways were found to receive symmetric synapses, putatively inhibitory inputs, from LDT projections, which led to the suggestion that the LDT could participate in disinhibitory mechanisms by inhibiting striatal GABAergic interneurons[22,63]. This point was reinforced by findings that following optogenetic activation of LDT cholinergic cells projecting to DA VTA neurons, a late activation could be observed, consistent with a rebound excitation after the stimulation of GABAergic interneurons[18]. Inhibitory input from the LDT directed to GABAergic cells of the mesocortical pathways could also participate in disinhibitory processes occurring indirectly between the LDT and PFC[63], which could occur in combination with thalamic inhibitory input. Interestingly, symmetric synapses from the LDT were selectively found on DA neurons in the mesocortical pathway, as there was no evidence for their presence in mesoaccumbal DA neurons, indicating that directly mediated, inhibitory influences of the LDT on limbic DA output are mainly targeted to mesocortical DA pathways[63].
Activation of the mesoaccumbal DA system resulting in DA output to the NAc is involved in reward reinforcement to natural stimuli including sex[67], social interaction[68], and food[69]. Large rises in DA encode a positive valence to these triggering stimuli, which is reinforcing and leads to incentive for repeat of behaviors leading to acquisition of the triggering stimulus. However, in addition to activation of this system by stimuli promotive of health and continuation of our species, drugs of abuse also activate this system and do so to a greater extent than natural stimuli, leading to rises in DA of several fold greater than those evoked by non-drug stimuli[70]. All drugs of abuse share the common ability to activate the mesoaccumbal system, whereas this property is not shared by the majority of drugs that do not exhibit dependence-inducing effects[70]. While drugs of abuse lead to rises in DA, diverse pharmacologic properties across drug classes confer differences in the way by which rises in DA are elicited. The pharmacologic actions leading to rises in DA can be directly-mediated excitatory cellular effects on DA cells, or actions can be indirectly-mediated via afferent input to DA cells, which can include cells within the VTA that are not DA-containing neurons, including GABAergic cells and glutamate cells[71] or non VTA sourced projections. Following a large body of studies showing the critical role of VTA DA in incentive salience, the central paradigm regarding the neural processes underlying development of dependence to drugs of abuse involves a high degree of drug-induced DA activation of the mesoaccumbal pathway via actions on the heterogenous VTA cell population but also activation of extra-VTA input terminating in the VTA or NAc.
Extensive evidence shows that the connectivity of the LDT to the VTA and NAc plays a role in drug addiction behaviors suggestive of drug actions on the LDT-VTA-NAc circuit. Early microdialysis studies showed that electrical stimulation of the LDT resulted in large rises in DA in the NAc, which was reduced by intra-VTA application of nAChR, muscarinic ACh receptor, and ionotropic glutamate receptor antagonists, suggesting ACh and glutamate output from the LDT play a role in DA rises[21]. Further, rises in DA induced by morphine were reduced in LDT lesioned rats[72]. Behaviorally relevant, large rises in DA were found to result from high frequency, burst firing of VTA DA neurons, which was a firing pattern impossible to elicit in VTA brain slices[73-76]. This finding suggested that afferent input severed in the slice preparation was crucial for firing activity of VTA DA-neurons. Consistent with this, in vivo studies revealed that DA VTA burst firing was reliant on an intact LDT, since pharmacologic inactivation of the LDT eliminated this firing pattern[77]. The influence of the LDT was thought to be mediated via cholinergic inputs[77], and further work showed that cholinergic output from the LDT shapes the firing of VTA neurons and biases VTA activity towards a burst pattern from a more disorganized discharge that likely results in higher release of DA to levels sufficient to underlie the encoding of value of stimulus value, as the rises were associated with evidence of changes in motivated behaviors[18].
Initial optogenetic studies of the role of the LDT in motivated behaviors showed that stimulation of the LDT engendered conditioned place preference (CPP), a model of both associative learning and drug-dependent behavior, which was an effect attributed to the demonstrated presence of glutamatergic output in the LDT-VTA circuit, albeit direct in vivo evidence of the role of this circuit in behavioral outcome was not provided[22]. Further optogenetic work confirmed the ability of stimulation of the LDT to induce CPP, and a role of the cholinergic LDT population was shown[64,78]. The role of the cholinergic LDT cells in motivated behaviors mediated by the VTA was additionally supported by loss of CPP conditioning to cocaine when associated with pharmacologic inactivation of the cholinergic LDT cells as well as failure to induce CPP when muscarinic and nicotinic receptors were blocked in the VTA[79]. In addition, photo excitation of LDT cholinergic terminals in the VTA was shown to cause positive reinforcement as subjects spent more time in the compartment in which they received photo stimulation, which was an effect similar to that induced when cholinergic LDT-NAc input was activated[25]. In a study designed to tease apart the relative contribution of excitatory LDT neurons to motivated behavior, the role in CPP of both glutamate and cholinergic LDT cell populations was examined under identical laboratory conditions[80]. Selective activation of either the glutamate or cholinergic LDT projections to the VTA by light pulses resulted in induction of CPP in mice, leading to the conclusion that both glutamate and cholinergic LDT inputs to the VTA play a role in the net rewarding effects of drugs of abuse[80]. However, the role played by the two excitatory transmitters was found to differ, suggesting that glutamatergic LDT projections may be important for initial reinforcement of place preference, whereas cholinergic mechanisms underlie continued reinforcement, as longer stays in the light drug-paired chamber were seen upon stimulation of cholinergic LDT projections[80]. Glutamatergic neurons, which exhibit very different connectivity to limbic structures and different firing patterns due to differences in intrinsic membrane properties to that exhibited by cholinergic neurons, likely do play a role in the control of VTA neurons, but that role is probably complementary to that served by ACh-containing cells[18,80]. Whether or not the ACh or the glutamatergic LDT afferents to the VTA play a more relevant role in drug dependence behaviors remains an open and very interesting question to address, especially vis a vis treatment targets; however, what is clear from the data is that the LDT can control DA efflux from the VTA in a behaviorally relevant fashion via both major excitatory transmitter systems that project to the mesoaccumbal pathway.
The role of the GABAergic LDT neurons, which can be local or projecting, and their impact on eventual VTA DA efflux have been less well examined. A role of LDT-mediated disinhibition of VTA GABAergic cells, especially those within mesocortical circuits, has been proposed[63]. Stimulation of GABAergic VTA cells was found to inhibit firing of DA cells, whereas their optogenetic activation led to conditioned place aversion, a behavioral model of aversive stimulation, suggesting that their inhibition would be promotive of DA release and the encoding of stimuli with a positive valence[81,82]. However, excitation of GABAergic LDT neurons was found to mediate innate fear responses following exposure to predator odorant in rodents[81]. This action was found to be mediated by the lateral habenula, which sends input to the VTA and the GABAergic rostromedial tegmental nucleus, also identified as the tail of the VTA, known to mediate aversive responses. While direct evidence is needed, this raises the interpretation that GABAergic LDT neurons projecting to the VTA do not play a functionally relevant role in inhibiting GABAergic VTA cells, leading to rises in DA sufficient for reinforcement. It is also possible that they inhibit a subset of the remaining 75% of the non-GABAergic VTA population, and/or LDT input directed to the lateral habenula and rostromedial tegmental nucleus supersedes any effect of local VTA disinhibition. A non-mutually exclusive possibility is that different populations of LDT GABAergic projection neurons exist. The role of the local GABAergic interneurons in the LDT, which abut the cholinergic neurons, and their impact on excitatory output are unexplored. When taken together, while it remains to be determined how the three main neuronal phenotypes of the LDT work in concert as a whole, output from the LDT results in significant changes in DA-VTA neuronal activity. As rises in DA efflux from the VTA are involved in reward prediction of salient stimuli and the LDT has been shown to control DA VTA output, the LDT is believed to be critically involved in DA-mediated striatal-control of behavior[83].
In addition to the heavy projections in the mesoaccumbal pathway, the LDT also provides the major cholinergic input to VTA neurons participating in the mesocortical pathway, which suggests the LDT has control over DA output to cortical regions as the direct projection from the striatum to the PFC of the mesocortical pathway provides the major DA innervation of the PFC. Connections from PFC back to limbic regions are present, creating a striatum cortical loop that is importantly involved in controlling behavioral flexibility and decision making[22,63,65]. Two such loops, comprised of dorsolateral PFC-striatum and anterior cingulate cortex-striatum connections, are suggested to control sustained and selective attention, respectively[84-86]. Dysfunctions within the mesocortical loop have been associated in humans with the expression of ADHD cardinal phenotypes. ADHD is a predominantly childhood mental disorder characterized by a combination of persistent maladaptive behaviors, including hyperactivity as well as cognitive impairments leading to failure to sustain directed attention and impulsivity, which involves decision making before full cognitive processing has occurred[87,88]. The classic triad of symptoms can manifest in several different negative ways, however, effects on emotionality and cognition tend to become exacerbated with age, likely due to increased stresses, whereas motor overactivity tends to abate in adulthood, which are clinical features relevant to note in animal experimentation of ADHD mechanisms[88].
Given the role of striatal cortical loops in control of behavior and the role of DA within behavioral controlling networks, DA dysfunctions within the PFC have been hypothesized to be involved in ADHD[87,89,90]. One of the most compelling findings supporting the hypothesis of a role of DA in ADHD is that stimulants of catecholaminergic systems have been among the most successful treatment of ADHD patients[90]. In addition, reductions in striatal DA transmission have been reported in ADHD patients[91]. Several animal studies have implicated DA function in mesocortical circuits encompassing the PFC as involved in control of executive functions shown to be altered in ADHD as well as in the control of one of the hallmarks of ADHD, hyperactivity. Lesions of DA-containing mesocortical inputs to the PFC were associated with a hyperactive phenotype in rats[92]. Extracellular DA levels were increased in the PFC during the training phase of a radial maze task in rats, which assays working memory performance[93]. In addition, DA depletion in the PFC was associated with working memory deficits in a T-maze paradigm[94], and similar cognitive deficits were seen after intra-PFC administration of type I DR receptor (DR1) antagonists[95,96]. Depletion of DA release into the PFC was shown to induce cognitive deficits in rhesus monkeys[97], and subsequent studies found that application of D1R antagonists into the PFC promoted deficits in oculomotor delay responses and working memory tasks[98,99]. Behavioral flexibility and decision making were reduced following antagonism of D1R and type 2 DA receptors in the PFC[65]. Interestingly, while D1R agonists injected at low doses within the PFC increased visual attentional performance in rats[100], increased activation by higher concentrations of the D1R agonists impaired performance in both rodents and primates, suggesting optimal D1R activation in the PFC is necessary for proper working memory performance[101-104]. These data support the hypothesis that DA levels within the PFC exert cognitive effects; however, this control is likely exerted in an “inverted U shape” manner, as originally suggested more than 100 years ago[105]. According to this suggestion, optimal dopamine levels within the PFC are believed to be associated with maximum behavioral performance, and either hyper- or hypo-DA function in this brain region compromises executive behaviors[106].
Since PFC-projecting DA VTA neurons contribute to the DA tone of cortical circuits brain regions, which modulate mesocortical VTA activity, the LDT could indirectly contribute to cortical DA functioning. Therefore, activity in the LDT could be indirectly involved in ADHD behaviors via the control exerted on mesocortical pathways via excitatory synapses on DA mesocortical VTA cells directed to the PFC. A role of the LDT in control of PFC DA levels is indirectly supported by findings that local infusions of nAChR antagonists in the rat VTA resulted in deficits in PFC-controlled behaviors that are DA dependent[107]. Further, control of PFC DA levels could be exerted by the LDT via disinhibitory mechanisms mediated by inhibitory LDT inputs directed to GABAergic mesocortical neurons controlling PFC function[63]. In addition, non-DA control of the LDT in ADHD behaviors could be mediated by LDT-thalamic connections, since thalamic-cortical circuits associated with ADHD-related hyperactivity receive input from the LDT[84-86,88]. In addition, the LDT could be involved in other ADHD features, including impulsive behavior. This conclusion is supported by findings that reductions in activation in thalamic relay nuclei are seen in gamblers exhibiting poor impulse control[108]. When taken together, altered neurotransmitter signaling from the LDT could be involved in increasing susceptibility for dysfunctions of attention and cognition. Thus, although no direct evidence has linked LDT function or dysfunction with ADHD-related phenotypes, studies investigating dysfunction of DA mesocortical, mesostriatal, and thalamo-cortical pathways in working memory and other cognitive-related behaviors support the assumption that alterations in the LDT-VTA and LDT-thalamic circuitry could contribute to ADHD-related behavioral deficits. In support of this hypothesis, the brain stem reticular activating system has been suggested to contribute to attention and filtration of interfering stimuli and, accordingly, was thought to play a potential role in disorders involving disorganization in cognitive processes[109]. However, detection of structural abnormities in the brain stem of human patients suffering from ADHD-associated cognitive dysfunctions awaits as these structures have proven to be more challenging than forebrain structures to image[87].
When taken together, anatomical and functional studies suggest a complex and regulatory role of LDT neurons on VTA functioning and provide further support of the influence of the LDT on mesoaccumbal DA transmission as a relevant step in encoding the valence of environmental stimuli[21]. In addition, these data suggest that the LDT plays a regulatory role in other cognitive functions via actions in PFC through direct striatal influence on DA transmission or through indirect actions mediated by thalamic relay centers. However, the circuits in which the LDT participates are not one way, and the LDT receives dense afferent input from many extra-LDT regions throughout the brain (Figure 2). A high number of LDT inputs were shown to source from pontine and midbrain nuclei, including the PPT, central gray, and deep mesencephalic nucleus[61]. In addition, afferents sourcing from cerebellum, spinal cord, basal ganglia, medulla, the bed nucleus of the stria terminalis, and the hypothalamus, particularly from the lateral hypothalamus that houses neurons importantly involved in state-control, were noted[61,110]. Relevant to circuits involved in motivated behaviors and cognition, substantial projections sourced from the VTA as well as the cerebral cortex, including the medial and orbitofrontal PFC[61,111]. These studies demonstrate that LDT afferents source from cortical, limbic, and somatosensory systems, which do, in some cases, themselves receive projections from the LDT. If the LDT passively transmitted information from higher order brain regions, alterations in LDT functioning would not be expected to have a significant impact on downstream signals. However, as the LDT processes signals before they are transmitted, alterations in LDT functioning would be expected to have an exponential effect on signal transmission if processing occurs on signals that themselves are altered. Therefore, in dysfunctional conditions, the effect of alterations in LDT functioning would be expected to have a high degree of impact via alteration of input and output transmission within networks important in behavioral outputs.
PNE has been associated with a higher risk of several adverse behaviors that are controlled by signaling in the striatum, thalamus, and PFC. Functional and anatomical studies have shown that molecular, cellular, and structural changes present in these regions are found following PNE. Control over these regions is exerted by the LDT either via direct or indirect pathways. When taken together with the fact that there is currently no human data available regarding structural development within the LDT associated with PNE, experimentally examining the issue of PNE-associated changes in the LDT is warranted if we wish to understand fully the mechanisms underlying the higher risk of these maladaptive, cognitive-based behaviors in PNE individuals. While three-dimensional human-derived brain organoid models have recently been used to examine effects on neural development of environmental factors, including nicotine, they do not allow for examination of behavioral associations[112] (for review, see[113]). Accordingly, for studies examining synaptic changes that could underlie behavioral outcomes, we require animal models of PNE in which both cellular and behavioral studies can be conducted. However, PNE animal models vary in several very important factors, making it difficult to choose the model best suited for translational significance.
One major difference in PNE models to date in the choice and breed of animal that have been used, which is a not insignificant confound as different species, and strains within the same species can respond with diverse behavioral outcomes suggestive of different cellular changes[114]. PNE studies also have varied in the experimental design regarding the method by which nicotine was applied, which has included subcutaneous application either via injection or implantation of osmotic mini pumps, intravenous application, intraperitoneal injection, or inhalation of cigarette smoke. In a less invasive approach, nicotine can be applied via the drinking water of the pregnant dams. Each of these methods would be expected to result in blood nicotine levels that are different and perhaps not similar in kinetics to those seen in humans, as nicotine concentrations in the blood of regular smokers are usually constant during periods of wakefulness in order to abate symptoms of withdrawal.
Other variations in the model utilized have sourced from differences in the nicotine dose utilized, the age at which the animal behaviors were assessed, the behavioral tests which were employed, and the time during pregnancy at which nicotine exposure occurred. This later point is relevant to physiological, peripheral effects engendered by first time exposures to nicotine, and concern of induction of stress, which is known to induce neural changes in offspring and maternal behaviors, as nicotine can be aversive in drug naïve individuals. If the first-time exposure occurs to the pregnant dam during the gestational period, which has been necessary in experimental designs when pumps with limited lifetimes have been utilized, the confound of stress’ role in physiological responses complicate attribution of effects to nicotine. As it is known that sex plays a role in PNE behavioral outcomes, interpretation of data where sexes have been pooled, or extrapolation to the opposite sex when single sex selective studies have been conducted, limits applicability of the data. These and other variables inherent to any laboratory study with rodents make it difficult to compare results across studies and further complicate determination as to which is the superior model in order to make conclusions relevant to the human situation [see[115] for a full discussion of the issue].
Despite these complications, examination of results from many PNE studies has led to the conclusion that the most robust rodent model of PNE is the oral nicotine intake method during pregnancy[115]. Arguments for this model include that it reflects pharmacodynamics/kinetics observed in human smokers, ADHD- and addiction-related behaviors have been seen in the rodent offspring with features similar to those seen in humans exposed to nicotine in utero, the nicotine exposure pattern is very similar to that seen in humans as it occurs during wakefulness, and stress levels are minimized, as no manipulations or surgical procedures are required. Finally, it avoids the issue of first-time exposure to nicotine to the dam occurring during gestational periods, which could introduce confounding factors. Accordingly, this model has been utilized by many laboratories to examine alterations in excitatory signaling within several brain regions associated with PNE. Further, in work conducted in the LDT, an outbred strain of mouse, the Naval Medical Research Institute (NMRI) mouse, was used in our investigations in order to attempt to reflect better the genetic diversity of the human population.
Validation of the PNE NMRI model via maternal drinking water model was provided by evaluation and detection of high cotinine levels in newborn PNE pups, confirming the gestational nicotine exposure of the fetus following maternal ingestion of nicotine via the drinking water[116]. Behavioral tasks were also employed in order to characterize the behavioral phenotype associated with early-life exposure to nicotine via the drinking water[116]. Although an extensive review of the behavioral deficits associated with PNE treatment in rodents is beyond the scope of this article, it is of interest when comparing dysfunctions of behaviors in which the LDT plays a role to compare sex-based findings of PNE-associated effects on affective state, cognition, and locomotion in the NMRI mouse exposed to nicotine via maternal drinking water with data from other laboratories using different PNE models, and with human clinical data, in order to evaluate the face validity of the oral NMRI PNE model.
Anxiety and PNE: Within young adulthood [postnatal day (PND)42-48], PNE treatment in NMRI mice was associated with anxiety-like behaviors that were effects only seen in male offspring[116]. In inbred C57BL/6J mice exposed to nicotine via maternal drinking water, anxiety levels of males have been reported to be increased[114,117]. However, it appears that the nicotine concentration is relevant for the anxiolytic action, since in another study using lower concentrations PNE did not alter anxiety levels in the offspring[118]. The method of nicotine administration is also likely important, as failure to detect anxiolytic-like behavior is common in studies in which nicotine was administered subcutaneously[119-121]. Early life exposures to nicotine have been suggested to heighten the risk of anxiety disorders in humans[122]. However, very few studies have been conducted examining the influence of smoking during pregnancy on anxiety in offspring, and in those conducted, mixed results have been reported with no gender segregation[123-125]. In perhaps the largest and well-characterized cohort examined, the Norwegian Mother and Child Cohort (1999-2009), maternal smoking was associated with an increase in externalizing behaviors, including anxiety; however, unfortunately, sex-based effects were not taken into account[122]. Interestingly, a larger impact was noted when the amount of cigarettes was considered as well as the time during gestation when smoking was present, with a more negative effect on anxiety the earlier nicotine was present in the pregnancy. This later finding was supported by a study of a much smaller population of Australian mother and child pairs[124-126]. In conclusion, while an enhanced risk of anxiety-like behavior remains a point to be examined in both human and animal studies, available data suggest that the PNE mouse model, in which nicotine is provided in the drinking water, represents a reasonable, translational model that can be used to study the mechanistic neural link between anxiety and PNE.
Hyperactivity and PNE: The oral administration method of PNE in NMRI mice was associated with hyperactivity in the offspring of both sexes in the open arena test (PND42-48)[116]. When nicotine was delivered via drinking water to pregnant rodent dams in other studies, PNE treatment was associated with hyperactivity in males, albeit some data showed that this effect could be present in both sexes or it could be linked to the genetic background of the mouse employed[114,127-130]. Differences can also be due to strain, as in a study using outbred mice, hyperactivity was seen in male PNE Swiss mice during late adolescence[119]. Further, it is relevant to consider the nicotine concentrations employed to draw associations between PNE and locomotor behavior, as no locomotor effects were seen in both sexes in a study employing a lower nicotine level before and during pregnancy[118], contrasting with previous findings showing PNE hyperactivity in similar models and ages investigated when higher doses of nicotine were utilized[116,127,130,131]. Although one study reported greater hyperactivity in 3-year-old boys following exposure to tobacco during gestation[132], another study suggested that prenatal tobacco exposure could have a causal relationship with hyperactivity seen in both adolescent and adult women[8]. Thus, the sex-dependency of hyperactive effects on offspring following PNE in experimental studies and prenatal tobacco exposure in clinical investigations is still unclear. Further, sex-dependent effects on motor activity of nicotine exposure via e-cigarette usage during pregnancy need to be examined as neurobehavioral evaluation of a small population of neonates exposed to e-cigarettes reported abnormal motor reflexes linked to later life motor development that were similar to those seen in prenatally cigarette exposed infants[133]. The small sample size precluded sex-based comparisons.
Cognitive deficits, ADHD, and PNE: In the oral PNE NMRI model, poorer outcomes have been detected in the spontaneous alternate behavior test, which is a Y-maze based test quantifying performance of a cognitive-dependent behavior. Scores indicative of cognitive impairments and working memory deficits were found in both sexes in young adult NMRI PNE offspring. In the outbred NMRI PNE model, both male and female offspring displayed deficits in the percentage of correct alternate behavior in the Y-maze, suggesting deficits in hippocampal-dependent working memory[116]. Moreover, this same model was associated with performance impairments in the rodent continuous performance task, particularly in scores related with learning, impulsivity, and attention, but only male offspring were investigated[134]. In inbred mice in which nicotine was delivered via drinking water of pregnant dams, deficits in the spontaneous alternate behavior performance assessed in adult offspring were seen only in males[118,135]. However, another study using twice the concentration of nicotine in the same inbred strain found that PNE cognitive deficits in this test were present in both PNE males and females[131], suggesting that the concentration of nicotine given could play a role in the sex-dependent outcomes. Further, young adult rats exposed prenatally to nicotine through the drinking water displayed impaired performance in another test of working spatial memory, the radial maze test. This effect was seen in both sexes[136], but little or no effect was found in PNE models using minipumps or subcutaneous injections[137-139]. A higher risk of cognitive deficits has been found in children born from pregnant smokers[9,12,140]. This association was also found in a study with a cohort of 574 children born from mothers who used NRTs during pregnancy[141]. Additionally, children prenatally exposed to smoke exhibited alterations in cognitive control circuitry and exhibited attention dysfunctions[142]. When taken together, the data strongly support the conclusion that nicotine during the prenatal period is associated with cognitive deficits. PNE individuals show up to a three-fold higher risk of ADHD, and a strong association has been made between nicotine levels in the mother during the first and second trimesters and diagnosis of ADHD[9]. Interestingly, ADHD has shown a sex bias, with reports of the male/female ratio being 4:1. However, carefully controlled, large population studies indicate the ratio is more likely 2:1 in adolescence, which was a proportion maintained into adulthood, leading the authors to suggest the possibility that males exhibit a greater level of hyperactivity/ impulsive symptoms that are disruptive than manifestations of these behaviors in females, and that female ADHD behaviors tend to be more cognitive-based and require more probing to detect[143]. Although clinical studies have employed both sexes to draw associations between prenatal tobacco exposure and ADHD, sex-dependent effects in the offspring were not taken into consideration to date in these studies, as genders were pooled together[144]. Our findings and others suggest that perhaps more clinical investigative attempts to identify and better recognize ADHD symptoms, especially in females, are warranted.
Conclusions on the animal models of PNE: In conclusion, we found a greater level of anxiety, locomotion, and cognitive deficiencies, with sex-specificity regarding emotional behaviors, in young adult NMRI mice prenatally exposed to nicotine via the drinking water[116,134]. The behavioral associations seen reproduced some of the relevant features observed in ADHD patients, which are associated with exposures prenatally to nicotine. When taken together, behavioral outcomes associated with PNE models in which gestational nicotine exposure occurs via maternal drinking water suggest that this model provides reasonable face validity relative to others by recapitulation of risk outcomes of individuals exposed to prenatal tobacco which have been seen in epidemiological investigations. This conclusion leads us to suggest that this model displays high translational potential for research focused on the connection of developmental exposure to nicotine to later-life appearance of ADHD-associated symptoms,= as well as in the search of relevant brain circuit alterations that could contribute to this phenotype. Studies using other rodent models of PNE have provided data that these models do exhibit characteristics of drug dependence and in some cases, recapitulate sex-differences seen in humans[3,145-147]. However, whether the NMRI PNE drinking water model exhibits features seen related to drug dependence and whether sex-based differences exist remains an open question which must be experimentally addressed.
The oral administration PNE NMRI model has demonstrated many of the behavioral risks associated with gestational nicotine exposure in humans that could involve the LDT, and other models of PNE have shown the heightened risk of drug dependency, suggesting a role of nicotine in this outcome. This model has been utilized to explore the molecular changes occurring in the LDT during development when nicotine is present in order to gain insight into alterations that could contribute to the behavioral risks found in PNE individuals in which this brain stem nucleus is implicated.
We have reported that gestational exposure to nicotine induces cellular changes in cholinergic signaling within the LDT that are findings in line with other studies, which have shown alterations in players in cholinergic transmission in diverse regions of the brain using alternative PNE models[145,148]. Indeed, reductions in the expression of nAChRs in different regions of the PNE brain, including the brain stem= as well as lower striatal and cortical DA levels[149-151], led to the suggestion that alterations in nAChRs induced by PNE are involved in dysfunctions in DA functioning in these regions underlying the higher drug dependence and ADHD risks in PNE individuals[152], which could involve changes in function of nAChRs in the LDT. Consistent with this, we have provided evidence that PNE is associated with alterations in functioning of nAChRs in the LDT. Nicotine application ex vivo in LDT-containing brain slices resulted in significantly smaller rises in calcium in LDT cells from PNE individuals when compared to rises elicited in control LDT cells. Further, in the PNE LDT, a reduced proportion of cells responded with rises in calcium upon nicotine application[153]. Although the mechanism of altered nAChR-stimulated calcium was not examined, changes in calcium responses seen could be due to reductions in numbers of nAChRs and/or could be due to alterations in nAChR subunit composition, as the subunit composition determines calcium permeability.
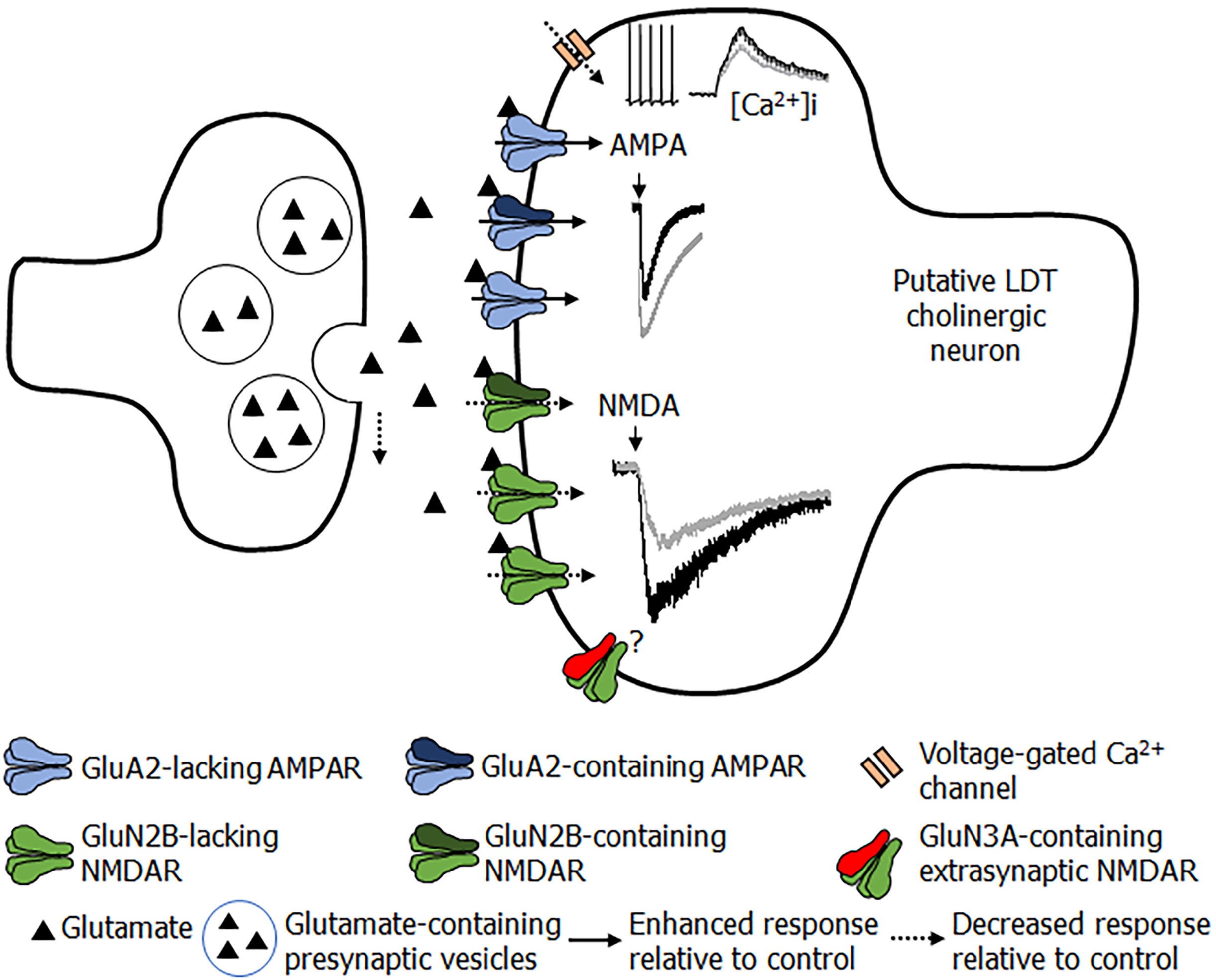
Glutamate transmission was also altered in the LDT of PNE mice, which has been seen in other regions of the rodent brain examined across several different PNE models. Glutamate mediates fast excitatory transmission via actions at three ionotropic receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA), and kainate. AMPA receptors (AMPARs) are tetramers composed of different assemblies of subunits (GluA1-4)[154], exhibit widespread expression in the brain, and are the major mediators of fast glutamate synaptic transmission[155]. Further, expression of AMPAR subunits follows a distinct ontogenetic pattern, which suggests specific functional roles at different periods during development. In the rat hippocampus, GluA1 expression remains constant until young adulthood, whereas GluA3 increases, and both GluA2 and GluA4 expressions are reduced over time[156], with GluA2 expression mostly limited to interneurons[157]. Within the VTA, electrophysiological evidence has suggested that GluA2-lacking AMPARs are abundant during the first postnatal days with a reduction in functional presence across age, with similar findings in cortical pyramidal cells and other brain areas[158-160]. NMDA receptors (NMDARs) are composed of heteromeric assemblies of GluN1-3 subunits, with obligatory presence of GluN1 with four GluN2 (GluN2A, GluN2B, GluN2C and GluN2D) and two GluN3 (GluN3A and GluN3B) possible isoforms. NMDAR subunit expression levels also shift during ontogeny, particularly among GluN2 and GluN3 subunits. GluN2A expression starts after birth, with a steady rise during development so that levels are at their highest in the adult brain. GluN2B/D subunits are expressed during the intra-uterine period, with GluN2B expression maintained at high levels up to the first postnatal week, and progressively decreasing in expression across age, culminating gradually in a limited restriction of presence within the forebrain, whereas GluN2D is markedly reduced in expression immediately after birth. GluN2C subunits appear late during development, at PND10, and exhibit a restricted expression and are primarily found within cerebellum and olfactory bulb. Finally, GluN3A subunits increase expression following birth, but thereafter, decline progressively to low levels; whereas, conversely, GluN3B expression shows a slow and steady increase throughout development[161-164].
Investigations from other laboratories have reported alterations in glutamate receptor subunit expression from expected patterns in the PNE brain. Differences in levels of expression of the GluA2, GluN1, and GluN2C subunits were seen in the PNE hippocampus at PND63 following osmotic pump-mediated PNE for 14 gestational days, whereas changes in glutamate signaling-related molecules were observed at younger ages[165]. Using a similar PNE model in which nicotine exposure was also provided by osmotic minipumps, a reduced expression of GluA1 subunits, smaller amplitudes in glutamate-mediated, miniature postsynaptic excitatory currents, reduced long-term potentiation, and increased long-term depression in hippocampal CA1 neurons associated with PNE treatment were reported[166-168]. As changes in synaptic strength are mediated by alterations in AMPA and NMDA receptor functioning, PNE-associated differences in long-term potentiation and long-term depression suggest changes in the functionality of glutamate receptors. Further, PNE from gestational day 5 was associated with a reduced frequency of excitatory postsynaptic currents and altered AMPA-mediated synaptic transmission in hypoglossal motoneurons in brain slices from rat neonates[169,170]. In addition, reduced glutamatergic input was found in the PNE auditory brainstem[171]. Finally, gestational nicotine exposure was associated with suppression of progenitor cell differentiation in the glutamatergic-projecting granule cells within the hippocampal dentate gyrus at PND21 in rats[172] as well as with the impairment of progenitor cell proliferation during gestation, resulting in reductions in the availability of pyramidal glutamate neurons within the postnatal medial PFC in mice[173]. Overall, these studies indicate that the teratogenic effects of nicotine can affect glutamate signaling in different brain regions, which could affect both pre- and postsynaptic mechanisms in neuronal circuits.
Our studies in the LDT extend the observations of PNE effects on glutamate functionality. When the effects of PNE treatment in the NMRI model in male offspring were examined, early life exposure to nicotine was associated with larger, AMPA receptor-mediated intracellular calcium rises and inward currents in LDT cells (Figure 3)[174]. Pharmacological examination suggested a delayed switching of GluA2-lacking AMPA receptors in PNE LDT neurons, suggesting a time lag in appropriate development of AMPA receptors associated with early exposure to nicotine. Presynaptic release of glutamate was lower in PNE LDT cells, which would contribute to reductions in postsynaptic excitability of these neurons[174]. Notably, an unpublished observation in our group was that, despite the finding that PNE LDT neurons exhibited significantly higher AMPAR-stimulated current amplitudes, enhanced membrane responsiveness was not sufficient to activate these neurons to fire action potentials to the same extent as observed in control cells, further suggesting reduced excitability. NMDA receptors in the LDT were also shown to be associated with alterations in functionality following PNE. Our data indicated that PNE was associated with changes in both synaptic and extrasynaptic NMDAR function, which was cell-type specific. In putatively GABAergic inhibitory LDT cells, PNE treatment was associated with higher functional presence of GluN2B-containing synaptic NMDARs and higher levels of silent synapses, without major functional effects detected in extrasynaptic NMDARs. Further, putatively cholinergic cells displayed reduced functional presence of GluN2B subunits in synaptic NMDARs, and changes in extrasynaptic NMDARs[175]. Our electrophysiological findings were in line with a previous calcium imaging study conducted in our group that did not include electrophysiology, suggesting lower intracellular calcium increases upon a second bath application of NMDA, which was interpreted to reflect a shift in properties of NMDARs in LDT cells following PNE treatment[176].
Passive and active properties of cholinergic neurons of the LDT were also examined in the PNE as membrane properties underlie cellular excitability. Lower neuronal excitability among LDT cells in PNE mice was exhibited in several different paradigms. PNE LDT neurons exhibited a higher rheobase, which is defined as the minimum amount of current necessary to elicit an action potential[177], and smaller activity-induced rises in calcium putatively due to PNE-associated alterations in voltage-operated calcium channels, although this point was not directly examined[174]. Examination of the action potential revealed a broader spike in the PNE, due to a slower decay slope that was likely reflective of differences in ionic conductance underlying the kinetics of the rise and decay times. Further studies revealed data consistent with a reduction in function of K+-channels activated by Ca+2[174]. In addition, the amplitude of the afterhyperpolarization was significantly larger in the PNE, which also suggested alterations in ionic conductance[153]. When taken together, the effects on the kinetics of the action potential and the amplitude of the afterhyperpolarization would likely result in a limitation in the firing frequency.
In summary, our studies of PNE LDT neurons found reductions in membrane excitability, effects on the action potential kinetics and the amplitude of the afterhyperpolarization that likely resulted in limitations in firing frequency, reductions in nAChR-induced calcium rises suggestive of a reduction in excitability mediated by nAChRs, and changes in glutamate signaling that would lead to decreases in excitability in cholinergic neurons, with concurrent increases in activity of GABAergic cells, which could be local or projection neurons. Interestingly, some of these changes were present in young animals but did not persist into adulthood, suggesting that nicotine-associated alterations in development of LDT transmission would result in changes in output that would participate differentially across ontogeny and thereby, affect neuronal excitability differentially across age. When taken together, our studies have led us to the working hypothesis that PNE is associated with a hypofunctioning LDT, which would lead to reductions in output of excitatory neurotransmitters onto projection targets, including those within the VTA, NAc, and thalamus.
Reductions in cholinergic transmission from the LDT to target areas would be expected to have a significant effect on behaviors controlled by those target regions. Data from the oral NMRI PNE model have led to our development of the ‘hypocholinergic hypothesis’, and predictions from this hypothesis could mechanistically play a role in adverse behavioral outcomes associated with PNE.
PNE has been associated with a higher risk of later life development of drug dependence, especially to nicotine as well as a higher risk of drug experimentation and abuse, which is an association seen in studies correcting for confounds such as maternal cigarette consumption after birth[2-5]. Importantly, an increased risk for nicotine dependence was also seen in investigations including sibling-pairs discordant for prenatal tobacco exposure that were controlled for such confounds as postnatal maternal smoking, which linked prenatal cigarette exposure with increased liability for nicotine addiction[178-180]. Gestational exposure to nicotine has been associated with a higher likelihood for abusing drugs, particularly during the adolescent time, including marijuana and cocaine, which is only explained in part by increased experimentation during the adolescent period and incomplete development of cortical regions of the brain[6,181]. An association has also been found during adulthood, in which gestationally-exposed young adults displayed significantly higher rates of cigarette smoking and nicotine dependence, which has been shown in independent studies[3,182].
As burst firing in mesoaccumbal circuits leads to behaviorally relevant levels and temporal patterns of DA in the NAc, which signal salience and engender continued usage of drugs, if PNE was associated with alterations in DA VTA burst firing engendered by exposure to drugs of abuse, or endogenous rewarding stimuli, this could alter coding of salience to the triggering stimuli. Interestingly, PNE was associated with alterations in the burst firing pattern of DA-VTA neurons of adolescent rats who were exposed to nicotine prenatally via mini-pump implantation[183], and several different models have identified an association between PNE treatment with lower DA release within the striatum[149,151,184]. When findings from these and other PNE cellular studies conducted in the VTA are taken together with our PNE LDT data, it is tempting to speculate that since the LDT is a critical modulator of burst firing in the VTA[77], PNE-associated alterations in LDT excitability are likely involved in differences seen in VTA neuronal firing in PNE. As a working hypothesis, our LDT cellular data have led us to propose that the alterations seen in the PNE LDT would result in a reduced cholinergic tone into target brain areas upon activation of the LDT following exposure to drugs of abuse. As ACh is excitatory to DA VTA neurons, among other consequences, a hypocholinergic tone from the LDT in PNE individuals would be expected to reduce, or at least drastically alter, behaviorally relevant, excitatory drive mediated, phasic DA VTA firing in response to stimuli. This hypothesis is also in line with very recent findings that inhibition of cholinergic transmission from the LDT influences neuronal firing of striatal neurons, associated with blockade of goal-directed behaviors, resulting in a more habitually-directed brain reflective of reduced flexibility in development of action strategies[18]. This hypothesis is somewhat in line with the ‘hypoexcitability hypothesis’ of drug dependence, which postulates that individuals with a higher liability for drug dependence possess a hypodopaminergic function within the mesolimbic circuit, a condition that could be due to genetic and epigenetic factors as well as in utero insults, including drug exposure[178,185-188]. Arguably, hypodopaminergic functioning within the mesoaccumbal circuit could lead to a bias towards coding a relatively higher reward value upon drug intake when compared to natural rewards, or when compared to coding conferred by normal functioning of the mesolimbic circuit[189], and this higher reinforcement could underlie continuous usage and engender escalation in drug consumption[190,191]. Further, a progressive development towards a switch to habitual and non-flexible responses to stimuli, rather than development of novel adaptive strategies integrated within experienced behaviors, has been noted as a feature of drug dependence, although drug dependency can be seen perhaps more correctly as an imbalance between habit and goal-directed behaviors[192]. Neuroimaging studies are in line with our hypothesis, since a weaker response in striatum to reward anticipation was noted in adolescents born to smoking mothers, which was suggested to contribute to an increased risk factor for substance dependence[193]. Although our hypothesis requires experimental validation, it places the PNE-associated changes in the LDT as critically involved in the negative behavioral outcomes related to a higher risk of drug dependence in this population.
PNE has also been associated with a higher risk of later life development of ADHD-like behaviors. Modulation of catecholamine levels within the PFC has provided compelling experimental evidence of the role of DA pathways in impulsivity and attention deficits in behavioral performances[87,89,90]. Studies employing the PNE model in which nicotine was delivered via drinking water reported a reduced DA content in the PFC of adolescent male PNE mice[151], which corroborates findings of lower levels of DA in the cortex associated with PNE induced by minipump nicotine delivery model, an effect more pronounced at juvenile and adolescent stages[184], but which does not support findings in a later study with the minipump method in which DA levels were greater in the PFC of males and female offspring; however, the turnover ratio from DA to the DA metabolite homovanillic acid (HVA) was reduced only in the PNE males, suggestive of a sex-based PFC DA alteration[194]. Gestational tobacco smoke exposure was associated with a reduction in the DA and tyrosine hydroxylase levels within the striatum of PTE adult mice[149]. In adult PNE mice born to mothers exposed to nicotine via the drinking water, microdialysis of medial PFC showed reduced basal extracellular levels of DA[151]. Reductions in tyrosine hydroxylase, which catalyzes the conversion of L-tyrosine to L-DOPA, a precursor of DA, were detected using immunohistochemistry in DA-positive cells in the medial PFC and in the NAc core and shell in PNE animals[151].
As further evidence that alterations in DA signaling might be a common outcome following PNE, diminished levels of HVA were noted in the PFC in the mouse and rat PNE[151,195], which interestingly, while seen in the males of another study, was not noted in the female mice in that same work[194]. As lower HVA levels in spinal fluid and urine has been seen in clinical studies with both children and adult ADHD patients[196,197], alterations in DA turnover in the PFC could represent a common signaling dysfunction in both PNE and ADHD individuals. Accordingly, alterations in DA levels within the PFC seen in PNE rodents could underlie the higher risk of ADHD-type behaviors following early life exposure to nicotine. While it remains to be explored, alterations in LDT output to the DA cells of the mesocortical pathway could be involved in alterations of DA release in the PFC and NAc, which could represent a circuit-based alteration with great relevance for the heightened risks seen in PNE individuals to the development of ADHD. In line with this possibility, imaging studies on ADHD individuals have reported reduced activation of the ventral striatum in response to rewards, which is a similar response detected following PTE[193,198].
The ascending cholinergic projections which encompass LDT output to thalamic regions suggest that the LDT could play a role in cognitive functions by modulating cortico-projecting thalamic neurons. Therefore, changes induced by gestational nicotine associated with synaptic alterations in the LDT could lead to alterations in cholinergic output terminating in the thalamus, which could also play a role in ADHD-like phenotypes associated with PNE. Interestingly, it has been hypothesized that alterations in cholinergic signaling in corticothalamic circuits induced by PNE could underlie deficits in sensory processing, contributing to the behavioral alterations seen in these individuals in response to environmental stimuli, including ADHD related behaviors[199]. The majority of studies of effects of PNE on cholinergic transmission in cognition-associated regions have focused on alterations in nAChRs; however, deficits suggestive of reduced cholinergic transmission were noted in cerebral regions[145,199]. When taken together, PNE-induced alterations in neuronal excitability and cholinergic and glutamate signaling within the LDT nucleus presumably affect LDT cholinergic input to thalamic relay nuclei. Our working hypothesis is that cholinergic output from the LDT to targets including those within the thalamo-cortical circuit is reduced in PNE, altering cortical activation in this network, leading to higher risks in this population of negative, cognitive behaviors controlled by the cortex. This conclusion is paralleled by findings of a reduced activation of the thalamus seen in conditions exhibiting poor impulse control characteristic of both ADHD and drug dependence[108]. Interestingly, PNE-associated alterations in cortical transmission were found to be sex-dependent, with a striking effect in males[145]. Although females were not as affected, PNE appeared to sensitize females to a greater extent as they exhibited poorer cognitive outcomes upon later life exposure to nicotine when compared to those in males[145]. In summary, PNE-induced alterations in excitability, cholinergic, and glutamate signaling within the LDT nucleus would presumably affect LDT cholinergic tone present in thalamic centers leading to a dysfunction in thalamo-cortical brain circuits. This dysfunction in input could lead to altered processing of sensory stimuli and to cognitive deficits seen in ADHD present in those gestationally exposed to nicotine. Further, while this effect might be more prominent in males, early life exposure appears to leave behind a liability in females, in that later life exposure to nicotine could result in reductions in cholinergic transmission, which could have deleterious behavioral consequences on processes controlled by cortico-thalamic loops.
While the brain stem might not be the obvious neural target in studies interested in cognitive processing, or in studies focused on cognitively-based disorders, over time, irrefutable evidence of the role the LDT plays in cognitive processes has been provided, and accordingly, alterations in LDT neuronal output could play a significant role in dysfunctions of cognitively-based behaviors. As regions of the brain known to modulate psychomotor, reward, memory, and attentional behaviors[93,200-202] are altered in PNE, and since the LDT exerts direct or indirect control over these regions, it would be expected that changes in glutamate and cholinergic receptor signaling, as well as in excitatory membrane processes in this nucleus seen in experimental models of gestational exposure to nicotine, would lead to reductions in excitatory cholinergic and glutamatergic output from the LDT to target regions. This scenario would lead in the PNE to a hypodopaminergic midbrain function, lower cholinergic tone in the NAc, and reduced cholinergic strength within ascending reticular activating system participating pathways to thalamic relay centers. Many of the regions targeted by LDT afferent input are DA releasing, which strongly suggests that DA release would be altered, as has been seen in the PNE brain. In addition, ACh stimulatory input to thalamic nuclei that control the cortex, including the PFC, would be altered, implying that changes in DA are also likely to be accompanied by non-DA changes due to PNE-associated differences in cholinergic tone within cortico-thalamic circuits. Finally, resulting postsynaptic processing of afferent input to the LDT would be altered, as cellular changes impacting on synaptic integration would likely be affected in this nucleus. Given the neural regions under control by activity of the LDT, PNE-associated alterations in LDT function would likely contribute to the enhanced risk of drug dependence and ADHD-like behaviors seen in PNE individuals, placing the brain stem as notably involved in these cognitively-based risks following PNE.
Increases in magnetic strength is allowing functional magnetic resonance imaging to reveal unprecedented details of the human brain, and as improvements are made in spatial resolution, it may become possible to conduct studies in humans to evaluate potential structural changes in LDT in PNE. Moreover, powerful in vivo electrophysiological techniques such as utilization of Neuropixels probes have emerged, allowing unprecedented recordings of deep brain structures in rodent models. Future studies employing in vivo electrophysiology, pharmacology, and optogenetic approaches in animal models should be used to determine the extent of LDT involvement in demonstrated PNE-induced alterations of midbrain DA functioning. Such studies could also dissect the effects of the LDT-thalamo-cortical pathway in cognitive and behavioral control. If our working hypothesis of PNE-associated reductions in ACh transmission sourcing from the LDT is confirmed, data obtained from future studies could identify a target brain substrate for therapeutic interventions involving cholinergic function within the LDT to VTA, NAc, and thalamic circuits in order to ameliorate drug dependence and ADHD-like associated behaviors, such as those seen in PNE individuals.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li A S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry. 2003;160:1978-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Cornelius MD, Goldschmidt L, Day NL. Prenatal cigarette smoking: Long-term effects on young adult behavior problems and smoking behavior. Neurotoxicol Teratol. 2012;34:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | De Genna NM, Goldschmidt L, Day NL, Cornelius MD. Prenatal tobacco exposure, maternal postnatal nicotine dependence and adolescent risk for nicotine dependence: Birth cohort study. Neurotoxicol Teratol. 2017;61:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Lotfipour S, Ferguson E, Leonard G, Miettunen J, Perron M, Pike GB, Richer L, Séguin JR, Veillette S, Jarvelin MR, Moilanen I, Mäki P, Nordström T, Pausova Z, Veijola J, Paus T. Maternal cigarette smoking during pregnancy predicts drug use via externalizing behavior in two community-based samples of adolescents. Addiction. 2014;109:1718-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Hayatbakhsh MR, Alati R, Hutchinson DM, Jamrozik K, Najman JM, Mamun AA, O'callaghan M, Bor W. Association of maternal smoking and alcohol consumption with young adults' cannabis use: a prospective study. Am J Epidemiol. 2007;166:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Fitzpatrick C, Barnett TA, Pagani LS. Parental bad habits breed bad behaviors in youth: exposure to gestational smoke and child impulsivity. Int J Psychophysiol. 2014;93:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Gard AM, Owens EB, Hinshaw SP. Prenatal Smoke Exposure Predicts Hyperactive/Impulsive but Not Inattentive ADHD Symptoms in Adolescent and Young Adult Girls. Infant Child Dev. 2016;25:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Sourander A, Sucksdorff M, Chudal R, Surcel HM, Hinkka-Yli-Salomäki S, Gyllenberg D, Cheslack-Postava K, Brown AS. Prenatal Cotinine Levels and ADHD Among Offspring. Pediatrics. 2019;143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Biederman J, Martelon M, Woodworth KY, Spencer TJ, Faraone SV. Is Maternal Smoking During Pregnancy a Risk Factor for Cigarette Smoking in Offspring? J Atten Disord. 2017;21:975-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Schwenke E, Fasching PA, Faschingbauer F, Pretscher J, Kehl S, Peretz R, Keller A, Häberle L, Eichler A, Irlbauer-Müller V, Dammer U, Beckmann MW, Schneider M. Predicting attention deficit hyperactivity disorder using pregnancy and birth characteristics. Arch Gynecol Obstet. 2018;298:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Thakur GA, Sengupta SM, Grizenko N, Schmitz N, Pagé V, Joober R. Maternal smoking during pregnancy and ADHD: a comprehensive clinical and neurocognitive characterization. Nicotine Tob Res. 2013;15:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Daseking M, Petermann F, Tischler T, Waldmann HC. Smoking during Pregnancy Is a Risk Factor for Executive Function Deficits in Preschool-aged Children. Geburtshilfe Frauenheilkd. 2015;75:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Melchior M, Hersi R, van der Waerden J, Larroque B, Saurel-Cubizolles MJ, Chollet A, Galéra C; EDEN Mother-Child Cohort Study Group. Maternal tobacco smoking in pregnancy and children's socio-emotional development at age 5: The EDEN mother-child birth cohort study. Eur Psychiatry. 2015;30:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P 3rd. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 405] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 16. | Keschner M, Bender MB, Strauss I. Mental symptoms in cases of subtentorial tumor. Arch Neurol Psychiatr. 1937;37:1-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Cairns H. Mental disorders with tumours of the pons. Folia Psychiatr Neurol Neurochir Neerl. 1950;53:193-203. [PubMed] |
| 18. | Dautan D, Souza AS, Huerta-Ocampo I, Valencia M, Assous M, Witten IB, Deisseroth K, Tepper JM, Bolam JP, Gerdjikov TV, Mena-Segovia J. Segregated cholinergic transmission modulates dopamine neurons integrated in distinct functional circuits. Nat Neurosci. 2016;19:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol. 2002;541:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Cissé Y, Toossi H, Ishibashi M, Mainville L, Leonard CS, Adamantidis A, Jones BE. Discharge and Role of Acetylcholine Pontomesencephalic Neurons in Cortical Activity and Sleep-Wake States Examined by Optogenetics and Juxtacellular Recording in Mice. eNeuro. 2018;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596-3604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 199] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 834] [Cited by in RCA: 999] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 23. | Laviolette SR, Priebe RP, Yeomans JS. Role of the laterodorsal tegmental nucleus in scopolamine- and amphetamine-induced locomotion and stereotypy. Pharmacol Biochem Behav. 2000;65:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 2009;30:1358-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Xiao C, Cho JR, Zhou C, Treweek JB, Chan K, McKinney SL, Yang B, Gradinaru V. Cholinergic Mesopontine Signals Govern Locomotion and Reward through Dissociable Midbrain Pathways. Neuron. 2016;90:333-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 26. | Kopelman MD. The cholinergic neurotransmitter system in human memory and dementia: a review. Q J Exp Psychol A. 1986;38:535-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 112] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Shannon HE, Bemis KG, Hart JC. Assessment of working memory in rats using spatial alternation behavior with variable retention intervals: effects of fixed-ratio size and scopolamine. Psychopharmacology (Berl). 1990;100:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 477] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 30. | Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 393] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 31. | Schredl M, Weber B, Leins ML, Heuser I. Donepezil-induced REM sleep augmentation enhances memory performance in elderly, healthy persons. Exp Gerontol. 2001;36:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology (Berl). 1998;140:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 243] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl). 1996;123:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 270] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Sahakian B, Jones G, Levy R, Gray J, Warburton D. The effects of nicotine on attention, information processing, and short-term memory in patients with dementia of the Alzheimer type. Br J Psychiatry. 1989;154:797-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 340] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 35. | Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 222] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | Mena-Segovia J. Structural and functional considerations of the cholinergic brainstem. J Neural Transm (Vienna). 2016;123:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Gut NK, Winn P. The pedunculopontine tegmental nucleus-A functional hypothesis from the comparative literature. Mov Disord. 2016;31:615-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 38. | Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 231] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM. Thalamic amplification of cortical connectivity sustains attentional control. Nature. 2017;545:219-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 426] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 40. | Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983;10:1185-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1850] [Cited by in RCA: 1821] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 41. | Shiromani PJ, Floyd C, Velázquez-Moctezuma J. Pontine cholinergic neurons simultaneously innervate two thalamic targets. Brain Res. 1990;532:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Smith Y, Paré D, Deschênes M, Parent A, Steriade M. Cholinergic and non-cholinergic projections from the upper brainstem core to the visual thalamus in the cat. Exp Brain Res. 1988;70:166-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 522] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 44. | Holmstrand EC, Sesack SR. Projections from the rat pedunculopontine and laterodorsal tegmental nuclei to the anterior thalamus and ventral tegmental area arise from largely separate populations of neurons. Brain Struct Funct. 2011;216:331-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | el Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 259] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | Steriade M. Acetylcholine systems and rhythmic activities during the waking--sleep cycle. Prog Brain Res. 2004;145:179-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Williams JA, Comisarow J, Day J, Fibiger HC, Reiner PB. State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J Neurosci. 1994;14:5236-5242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 112] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. J Neurophysiol. 2010;103:1147-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 536] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 50. | Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex. 1997;7:583-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 285] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 51. | Stokes KA, Best PJ. Mediodorsal thalamic lesions impair radial maze performance in the rat. Behav Neurosci. 1988;102:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Bueno-Junior LS, Lopes-Aguiar C, Ruggiero RN, Romcy-Pereira RN, Leite JP. Muscarinic and nicotinic modulation of thalamo-prefrontal cortex synaptic plasticity [corrected] in vivo. PLoS One. 2012;7:e47484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Kayama Y, Sumitomo I, Ogawa T. Does the ascending cholinergic projection inhibit or excite neurons in the rat thalamic reticular nucleus? J Neurophysiol. 1986;56:1310-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Paré D, Steriade M, Deschênes M, Bouhassira D. Prolonged enhancement of anterior thalamic synaptic responsiveness by stimulation of a brain-stem cholinergic group. J Neurosci. 1990;10:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Aggleton JP, Keith AB, Sahgal A. Both fornix and anterior thalamic, but not mammillary, lesions disrupt delayed non-matching-to-position memory in rats. Behav Brain Res. 1991;44:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Mitchell AS, Dalrymple-Alford JC, Christie MA. Spatial working memory and the brainstem cholinergic innervation to the anterior thalamus. J Neurosci. 2002;22:1922-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Newman LA, Mair RG. Cholinergic modulation of visuospatial responding in central thalamus. Eur J Neurosci. 2007;26:3543-3552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 244] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 59. | Kumari V, Gray JA, ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, Vythelingum GN, Williams SC, Simmons A, Sharma T. Cognitive effects of nicotine in humans: an fMRI study. Neuroimage. 2003;19:1002-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 60. | Freo U, Pizzolato G, Dam M, Ori C, Battistin L. A short review of cognitive and functional neuroimaging studies of cholinergic drugs: implications for therapeutic potentials. J Neural Transm (Vienna). 2002;109:857-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Satoh K, Fibiger HC. Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol. 1986;253:277-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 337] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 62. | Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 232] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 64. | Dautan D, Huerta-Ocampo I, Gut NK, Valencia M, Kondabolu K, Kim Y, Gerdjikov TV, Mena-Segovia J. Cholinergic midbrain afferents modulate striatal circuits and shape encoding of action strategies. Nat Commun. 2020;11:1739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 65. | Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl). 2006;188:567-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 295] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 66. | Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 448] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 67. | Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 160] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 69. | Kiyatkin EA, Gratton A. Electrochemical monitoring of extracellular dopamine in nucleus accumbens of rats lever-pressing for food. Brain Res. 1994;652:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274-5278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3125] [Cited by in RCA: 3247] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 71. | Root DH, Barker DJ, Estrin DJ, Miranda-Barrientos JA, Liu B, Zhang S, Wang HL, Vautier F, Ramakrishnan C, Kim YS, Fenno L, Deisseroth K, Morales M. Distinct Signaling by Ventral Tegmental Area Glutamate, GABA, and Combinatorial Glutamate-GABA Neurons in Motivated Behavior. Cell Rep. 2020;32:108094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 72. | Forster GL, Falcon AJ, Miller AD, Heruc GA, Blaha CD. Effects of laterodorsal tegmentum excitotoxic lesions on behavioral and dopamine responses evoked by morphine and d-amphetamine. Neuroscience. 2002;114:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Ungless MA, Grace AA. Are you or aren't you? Trends Neurosci. 2012;35:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 340] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 74. | Mercuri NB, Stratta F, Calabresi P, Bernardi G. A voltage-clamp analysis of NMDA-induced responses on dopaminergic neurons of the rat substantia nigra zona compacta and ventral tegmental area. Brain Res. 1992;593:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Seutin V, Verbanck P, Massotte L, Dresse A. Evidence for the presence of N-methyl-D-aspartate receptors in the ventral tegmental area of the rat: an electrophysiological in vitro study. Brain Res. 1990;514:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Wang T, French ED. L-glutamate excitation of A10 dopamine neurons is preferentially mediated by activation of NMDA receptors: extra- and intracellular electrophysiological studies in brain slices. Brain Res. 1993;627:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167-5172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 78. | Steidl S, Veverka K. Optogenetic excitation of LDTg axons in the VTA reinforces operant responding in rats. Brain Res. 2015;1614:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Shinohara F, Kihara Y, Ide S, Minami M, Kaneda K. Critical role of cholinergic transmission from the laterodorsal tegmental nucleus to the ventral tegmental area in cocaine-induced place preference. Neuropharmacology. 2014;79:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 80. | Steidl S, Wang H, Ordonez M, Zhang S, Morales M. Optogenetic excitation in the ventral tegmental area of glutamatergic or cholinergic inputs from the laterodorsal tegmental area drives reward. Eur J Neurosci. 2017;45:559-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 81. | Yang H, Yang J, Xi W, Hao S, Luo B, He X, Zhu L, Lou H, Yu YQ, Xu F, Duan S, Wang H. Laterodorsal tegmentum interneuron subtypes oppositely regulate olfactory cue-induced innate fear. Nat Neurosci. 2016;19:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 82. | Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 492] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 83. | Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153 Suppl 1:S438-S445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99:211-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 85. | Dalley JW, Robbins TW. Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci. 2017;18:158-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 421] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 86. | Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2008;47:1233-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 87. | Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35:278-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 88. | Stahl SM. Stahl’s essential psychopharmacology: Neuroscientific basis and practical applications. 4th ed. New York: Cambridge University; 2013: 471-502. |
| 89. | Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 768] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 90. | Pliszka SR. The neuropsychopharmacology of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 91. | Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 985] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 92. | Jones GH, Robbins TW. Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor activity. Pharmacol Biochem Behav. 1992;43:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci. 2004;24:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 94. | Bubser M, Schmidt WJ. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav Brain Res. 1990;37:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 190] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 95. | Broersen LM, Heinsbroek RP, de Bruin JP, Joosten RN, van Hest A, Olivier B. Effects of local application of dopaminergic drugs into the dorsal part of the medial prefrontal cortex of rats in a delayed matching to position task: comparison with local cholinergic blockade. Brain Res. 1994;645:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 319] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 97. | Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1061] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 98. | Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 938] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 99. | Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 443] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 100. | Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004;29:1628-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 101. | Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528-8535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 550] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 102. | Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 319] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 103. | Danilova LIa. [The effect of desoxyconticosterous acetate (DOCA) on carbohydrate metabolism during artificial and natural hypothermia (hibernation)]. Patol Fiziol Eksp Ter. 1966;10:15-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 104. | Lidow MS, Koh PO, Arnsten AF. D1 dopamine receptors in the mouse prefrontal cortex: Immunocytochemical and cognitive neuropharmacological analyses. Synapse. 2003;47:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 105. | Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459-482. [DOI] [Full Text] |
| 106. | Chamberlain SR, Robbins TW. Noradrenergic modulation of cognition: therapeutic implications. J Psychopharmacol. 2013;27:694-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 107. | Levin ED, Briggs SJ, Christopher NC, Auman JT. Working memory performance and cholinergic effects in the ventral tegmental area and substantia nigra. Brain Res. 1994;657:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Potenza MN, Steinberg MA, Skudlarski P, Fulbright RK, Lacadie CM, Wilber MK, Rounsaville BJ, Gore JC, Wexler BE. Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:828-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 260] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 109. | Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 382] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 110. | Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 307] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 111. | Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 393] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 112. | Notaras M, Lodhi A, Barrio-Alonso E, Foord C, Rodrick T, Jones D, Fang H, Greening D, Colak D. Neurodevelopmental signatures of narcotic and neuropsychiatric risk factors in 3D human-derived forebrain organoids. Mol Psychiatry. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 113. | Sarieva K, Mayer S. The Effects of Environmental Adversities on Human Neocortical Neurogenesis Modeled in Brain Organoids. Front Mol Biosci. 2021;8:686410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 114. | Balsevich G, Poon A, Goldowitz D, Wilking JA. The effects of pre- and post-natal nicotine exposure and genetic background on the striatum and behavioral phenotypes in the mouse. Behav Brain Res. 2014;266:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 115. | Polli FS, Kohlmeier KA. Prenatal Nicotine Exposure in Rodents: Why Are There So Many Variations in Behavioral Outcomes? Nicotine Tob Res. 2020;22:1694-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 116. | Polli FS, Scharff MB, Ipsen TH, Aznar S, Kohlmeier KA, Andreasen JT. Prenatal nicotine exposure in mice induces sex-dependent anxiety-like behavior, cognitive deficits, hyperactivity, and changes in the expression of glutamate receptor associated-genes in the prefrontal cortex. Pharmacol Biochem Behav. 2020;195:172951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 117. | Alkam T, Kim HC, Hiramatsu M, Mamiya T, Aoyama Y, Nitta A, Yamada K, Nabeshima T. Evaluation of emotional behaviors in young offspring of C57BL/6J mice after gestational and/or perinatal exposure to nicotine in six different time-windows. Behav Brain Res. 2013;239:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 118. | Zhang L, Spencer TJ, Biederman J, Bhide PG. Attention and working memory deficits in a perinatal nicotine exposure mouse model. PLoS One. 2018;13:e0198064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 119. | Ajarem JS, Ahmad M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav. 1998;59:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 120. | Sobrian SK, Marr L, Ressman K. Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:501-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 121. | Santiago SE, Huffman KJ. Prenatal nicotine exposure increases anxiety and modifies sensorimotor integration behaviors in adult female mice. Neurosci Res. 2014;79:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 122. | Moylan S, Gustavson K, Øverland S, Karevold EB, Jacka FN, Pasco JA, Berk M. The impact of maternal smoking during pregnancy on depressive and anxiety behaviors in children: the Norwegian Mother and Child Cohort Study. BMC Med. 2015;13:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 123. | Brion MJ, Victora C, Matijasevich A, Horta B, Anselmi L, Steer C, Menezes AM, Lawlor DA, Davey Smith G. Maternal smoking and child psychological problems: disentangling causal and noncausal effects. Pediatrics. 2010;126:e57-e65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 124. | Williams GM, O'Callaghan M, Najman JM, Bor W, Andersen MJ, Richards D, U C. Maternal cigarette smoking and child psychiatric morbidity: a longitudinal study. Pediatrics. 1998;102:e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 125. | Robinson M, McLean NJ, Oddy WH, Mattes E, Bulsara M, Li J, Zubrick SR, Stanley FJ, Newnham JP. Smoking cessation in pregnancy and the risk of child behavioural problems: a longitudinal prospective cohort study. J Epidemiol Community Health. 2010;64:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 126. | Carter S, Paterson J, Gao W, Iusitini L. Maternal smoking during pregnancy and behaviour problems in a birth cohort of 2-year-old Pacific children in New Zealand. Early Hum Dev. 2008;84:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 127. | Paz R, Barsness B, Martenson T, Tanner D, Allan AM. Behavioral teratogenicity induced by nonforced maternal nicotine consumption. Neuropsychopharmacology. 2007;32:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 128. | Schneider T, Bizarro L, Asherson PJ, Stolerman IP. Hyperactivity, increased nicotine consumption and impaired performance in the five-choice serial reaction time task in adolescent rats prenatally exposed to nicotine. Psychopharmacology (Berl). 2012;223:401-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 129. | Zhu J, Zhang X, Xu Y, Spencer TJ, Biederman J, Bhide PG. Prenatal nicotine exposure mouse model showing hyperactivity, reduced cingulate cortex volume, reduced dopamine turnover, and responsiveness to oral methylphenidate treatment. J Neurosci. 2012;32:9410-9418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 130. | Pauly JR, Sparks JA, Hauser KF, Pauly TH. In utero nicotine exposure causes persistent, gender-dependant changes in locomotor activity and sensitivity to nicotine in C57Bl/6 mice. Int J Dev Neurosci. 2004;22:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 131. | Alkam T, Kim HC, Mamiya T, Yamada K, Hiramatsu M, Nabeshima T. Evaluation of cognitive behaviors in young offspring of C57BL/6J mice after gestational nicotine exposure during different time-windows. Psychopharmacology (Berl). 2013;230:451-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 132. | Hutchinson J, Pickett KE, Green J, Wakschlag LS. Smoking in pregnancy and disruptive behaviour in 3-year-old boys and girls: an analysis of the UK Millennium Cohort Study. J Epidemiol Community Health. 2010;64:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 133. | Froggatt S, Reissland N, Covey J. The effects of prenatal cigarette and e-cigarette exposure on infant neurobehaviour: A comparison to a control group. EClinicalMedicine. 2020;28:100602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 134. | Polli FS, Ipsen TH, Caballero-Puntiverio M, Østerbøg TB, Aznar S, Andreasen JT, Kohlmeier KA. Cellular and Molecular Changes in Hippocampal Glutamate Signaling and Alterations in Learning, Attention, and Impulsivity Following Prenatal Nicotine Exposure. Mol Neurobiol. 2020;57:2002-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 135. | Zhu J, Fan F, McCarthy DM, Zhang L, Cannon EN, Spencer TJ, Biederman J, Bhide PG. A prenatal nicotine exposure mouse model of methylphenidate responsive ADHD-associated cognitive phenotypes. Int J Dev Neurosci. 2017;58:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 136. | Sorenson CA, Raskin LA, Suh Y. The effects of prenatal nicotine on radial-arm maze performance in rats. Pharmacol Biochem Behav. 1991;40:991-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 137. | Cutler AR, Wilkerson AE, Gingras JL, Levin ED. Prenatal cocaine and/or nicotine exposure in rats: preliminary findings on long-term cognitive outcome and genital development at birth. Neurotoxicol Teratol. 1996;18:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 138. | Levin ED, Wilkerson A, Jones JP, Christopher NC, Briggs SJ. Prenatal nicotine effects on memory in rats: pharmacological and behavioral challenges. Brain Res Dev Brain Res. 1996;97:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 139. | Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol. 1993;15:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 140. | Lindblad F, Hjern A. ADHD after fetal exposure to maternal smoking. Nicotine Tob Res. 2010;12:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 141. | Zhu JL, Olsen J, Liew Z, Li J, Niclasen J, Obel C. Parental smoking during pregnancy and ADHD in children: the Danish national birth cohort. Pediatrics. 2014;134:e382-e388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 142. | Margolis AE, Pagliaccio D, Ramphal B, Banker S, Thomas L, Robinson M, Honda M, Sussman T, Posner J, Kannan K, Herbstman J, Rauh V, Marsh R. Prenatal environmental tobacco smoke exposure alters children's cognitive control circuitry: A preliminary study. Environ Int. 2021;155:106516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 143. | Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. 2010;49:217-28.e1. [PubMed] |
| 144. | Wiebe SA, Clark CA, De Jong DM, Chevalier N, Espy KA, Wakschlag L. Prenatal tobacco exposure and self-regulation in early childhood: Implications for developmental psychopathology. Dev Psychopathol. 2015;27:397-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 145. | Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology. 2007;32:1082-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 146. | Lacy RT, Hord LL, Morgan AJ, Harrod SB. Intravenous gestational nicotine exposure results in increased motivation for sucrose reward in adult rat offspring. Drug Alcohol Depend. 2012;124:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 147. | Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol Biochem Behav. 2006;85:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 148. | Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: Serotonin receptors and cell signaling. Neuropsychopharmacology. 2006;31:2462-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 149. | Yochum C, Doherty-Lyon S, Hoffman C, Hossain MM, Zelikoff JT, Richardson JR. Prenatal cigarette smoke exposure causes hyperactivity and aggressive behavior: role of altered catecholamines and BDNF. Exp Neurol. 2014;254:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 150. | Vivekanandarajah A, Chan YL, Chen H, Machaalani R. Prenatal cigarette smoke exposure effects on apoptotic and nicotinic acetylcholine receptor expression in the infant mouse brainstem. Neurotoxicology. 2016;53:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 151. | Alkam T, Mamiya T, Kimura N, Yoshida A, Kihara D, Tsunoda Y, Aoyama Y, Hiramatsu M, Kim HC, Nabeshima T. Prenatal nicotine exposure decreases the release of dopamine in the medial frontal cortex and induces atomoxetine-responsive neurobehavioral deficits in mice. Psychopharmacology (Berl). 2017;234:1853-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 152. | Miao H, Liu C, Bishop K, Gong ZH, Nordberg A, Zhang X. Nicotine exposure during a critical period of development leads to persistent changes in nicotinic acetylcholine receptors of adult rat brain. J Neurochem. 1998;70:752-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 153. | Christensen MH, Ishibashi M, Nielsen ML, Leonard CS, Kohlmeier KA. Age-related changes in nicotine response of cholinergic and non-cholinergic laterodorsal tegmental neurons: implications for the heightened adolescent susceptibility to nicotine addiction. Neuropharmacology. 2014;85:263-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 154. | Borges K, Dingledine R. AMPA receptors: molecular and functional diversity. Prog Brain Res. 1998;116:153-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 155. | Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2956] [Cited by in RCA: 2739] [Article Influence: 182.6] [Reference Citation Analysis (0)] |
| 156. | Ritter LM, Vazquez DM, Meador-Woodruff JH. Ontogeny of ionotropic glutamate receptor subunit expression in the rat hippocampus. Brain Res Dev Brain Res. 2002;139:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 157. | Akgül G, Abebe D, Yuan XQ, Auville K, McBain CJ. The Role of AMPARs in the Maturation and Integration of Caudal Ganglionic Eminence-Derived Interneurons into Developing Hippocampal Microcircuits. Sci Rep. 2019;9:5435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 158. | Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW, McBain CJ. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci. 2013;16:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 159. | Simeone TA, Sanchez RM, Rho JM. Molecular biology and ontogeny of glutamate receptors in the mammalian central nervous system. J Child Neurol. 2004;19:343-60; discussion 361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 160. | Bellone C, Mameli M, Lüscher C. In utero exposure to cocaine delays postnatal synaptic maturation of glutamatergic transmission in the VTA. Nat Neurosci. 2011;14:1439-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 161. | Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2476] [Cited by in RCA: 2680] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 162. | Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1040] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 163. | Pachernegg S, Strutz-Seebohm N, Hollmann M. GluN3 subunit-containing NMDA receptors: not just one-trick ponies. Trends Neurosci. 2012;35:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 164. | Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1879] [Article Influence: 156.6] [Reference Citation Analysis (0)] |
| 165. | Wang H, Dávila-García MI, Yarl W, Gondré-Lewis MC. Gestational nicotine exposure regulates expression of AMPA and NMDA receptors and their signaling apparatus in developing and adult rat hippocampus. Neuroscience. 2011;188:168-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 166. | Vaglenova J, Parameshwaran K, Suppiramaniam V, Breese CR, Pandiella N, Birru S. Long-lasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiol Learn Mem. 2008;90:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 167. | Parameshwaran K, Buabeid MA, Karuppagounder SS, Uthayathas S, Thiruchelvam K, Shonesy B, Dityatev A, Escobar MC, Dhanasekaran M, Suppiramaniam V. Developmental nicotine exposure induced alterations in behavior and glutamate receptor function in hippocampus. Cell Mol Life Sci. 2012;69:829-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 168. | Parameshwaran K, Buabeid MA, Bhattacharya S, Uthayathas S, Kariharan T, Dhanasekaran M, Suppiramaniam V. Long term alterations in synaptic physiology, expression of β2 nicotinic receptors and ERK1/2 signaling in the hippocampus of rats with prenatal nicotine exposure. Neurobiol Learn Mem. 2013;106:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 169. | Pilarski JQ, Wakefield HE, Fuglevand AJ, Levine RB, Fregosi RF. Developmental nicotine exposure alters neurotransmission and excitability in hypoglossal motoneurons. J Neurophysiol. 2011;105:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 170. | Buls Wollman L, Fregosi RF. Chronic, Episodic Nicotine Alters Hypoglossal Motor Neuron Function at a Critical Developmental Time Point in Neonatal Rats. eNeuro. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 171. | Baumann VJ, Koch U. Perinatal nicotine exposure impairs the maturation of glutamatergic inputs in the auditory brainstem. J Physiol. 2017;595:3573-3590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 172. | Ohishi T, Wang L, Akane H, Shiraki A, Itahashi M, Mitsumori K, Shibutani M. Transient suppression of late-stage neuronal progenitor cell differentiation in the hippocampal dentate gyrus of rat offspring after maternal exposure to nicotine. Arch Toxicol. 2014;88:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 173. | Aoyama Y, Toriumi K, Mouri A, Hattori T, Ueda E, Shimato A, Sakakibara N, Soh Y, Mamiya T, Nagai T, Kim HC, Hiramatsu M, Nabeshima T, Yamada K. Prenatal Nicotine Exposure Impairs the Proliferation of Neuronal Progenitors, Leading to Fewer Glutamatergic Neurons in the Medial Prefrontal Cortex. Neuropsychopharmacology. 2016;41:578-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 174. | Polli FS, Kohlmeier KA. Prenatal nicotine exposure alters postsynaptic AMPA receptors and glutamate neurotransmission within the laterodorsal tegmentum (LDT) of juvenile mice. Neuropharmacology. 2018;137:71-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 175. | Polli FS, Kohlmeier KA. Alterations in NMDAR-mediated signaling within the laterodorsal tegmental nucleus are associated with prenatal nicotine exposure. Neuropharmacology. 2019;158:107744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 176. | McNair LF, Kohlmeier KA. Prenatal nicotine is associated with reduced AMPA and NMDA receptor-mediated rises in calcium within the laterodorsal tegmentum: a pontine nucleus involved in addiction processes. J Dev Orig Health Dis. 2015;6:225-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 177. | Christensen MH, Nielsen ML, Kohlmeier KA. Electrophysiological changes in laterodorsal tegmental neurons associated with prenatal nicotine exposure: implications for heightened susceptibility to addict to drugs of abuse. J Dev Orig Health Dis. 2015;6:182-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 178. | Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84:1407-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 170] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 179. | Shenassa ED, Papandonatos GD, Rogers ML, Buka SL. Elevated risk of nicotine dependence among sib-pairs discordant for maternal smoking during pregnancy: evidence from a 40-year longitudinal study. Epidemiology. 2015;26:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 180. | Weber TL, Selya A, Wakschlag LS, Dierker L, Rose JS, Hedeker D, Mermelstein RJ. The Effect of Maternal Smoking on Offspring Smoking Is Unrelated to Heritable Personality Traits or Initial Subjective Experiences. Nicotine Tob Res. 2021;23:1754-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 181. | Lotfipour S, Ferguson E, Leonard G, Perron M, Pike B, Richer L, Séguin JR, Toro R, Veillette S, Pausova Z, Paus T. Orbitofrontal cortex and drug use during adolescence: role of prenatal exposure to maternal smoking and BDNF genotype. Arch Gen Psychiatry. 2009;66:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 182. | O'Callaghan FV, Al Mamun A, O'Callaghan M, Alati R, Najman JM, Williams GM, Bor W. Maternal smoking during pregnancy predicts nicotine disorder (dependence or withdrawal) in young adults - a birth cohort study. Aust N Z J Public Health. 2009;33:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 183. | Dragomir A, Akay YM, Zhang D, Akay M. Ventral Tegmental Area Dopamine Neurons Firing Model Reveals Prenatal Nicotine Induced Alterations. IEEE Trans Neural Syst Rehabil Eng. 2017;25:1387-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 184. | Navarro HA, Seidler FJ, Whitmore WL, Slotkin TA. Prenatal exposure to nicotine via maternal infusions: effects on development of catecholamine systems. J Pharmacol Exp Ther. 1988;244:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 185. | Kane VB, Fu Y, Matta SG, Sharp BM. Gestational nicotine exposure attenuates nicotine-stimulated dopamine release in the nucleus accumbens shell of adolescent Lewis rats. J Pharmacol Exp Ther. 2004;308:521-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 186. | Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 416] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 187. | Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol. 2005;63:101-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 188. | Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267-1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 953] [Cited by in RCA: 884] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 189. | Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1607] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 190. | Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 891] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 191. | Franke RM, Park M, Belluzzi JD, Leslie FM. Prenatal nicotine exposure changes natural and drug-induced reinforcement in adolescent male rats. Eur J Neurosci. 2008;27:2952-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 192. | Vandaele Y, Janak PH. Defining the place of habit in substance use disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 193. | Müller KU, Mennigen E, Ripke S, Banaschewski T, Barker GJ, Büchel C, Conrod P, Fauth-Bühler M, Flor H, Garavan H, Heinz A, Lawrence C, Loth E, Mann K, Martinot JL, Pausova Z, Rietschel M, Ströhle A, Struve M, Walaszek B, Schumann G, Paus T, Smolka MN; IMAGEN Consortium. Altered reward processing in adolescents with prenatal exposure to maternal cigarette smoking. JAMA Psychiatry. 2013;70:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 194. | Dwyer JB, Cardenas A, Franke RM, Chen Y, Bai Y, Belluzzi JD, Lotfipour S, Leslie FM. Prenatal nicotine sex-dependently alters adolescent dopamine system development. Transl Psychiatry. 2019;9:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 195. | Muneoka K, Ogawa T, Kamei K, Muraoka S, Tomiyoshi R, Mimura Y, Kato H, Suzuki MR, Takigawa M. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Brain Res Dev Brain Res. 1997;102:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 196. | Shaywitz BA, Cohen DJ, Bowers MB Jr. CSF monoamine metabolites in children with minimal brain dysfunction: evidence for alteration of brain dopamine. A preliminary report. J Pediatr. 1977;90:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 103] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 197. | Shekim WO, Dekirmenjian H, Javaid J, Bylund DB, Davis JM. Dopamine-norepinephrine interaction in hyperactive boys treated with d-amphetamine. J Pediatr. 1982;100:830-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 198. | Ströhle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M, Hein J, Nedderhut A, Neumann B, Gregor A, Juckel G, Knutson B, Lehmkuhl U, Bauer M, Heinz A. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 199. | Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56 Suppl 1:254-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 200. | Lacroix L, Broersen LM, Feldon J, Weiner I. Effects of local infusions of dopaminergic drugs into the medial prefrontal cortex of rats on latent inhibition, prepulse inhibition and amphetamine induced activity. Behav Brain Res. 2000;107:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 201. | Miyazaki M, Noda Y, Mouri A, Kobayashi K, Mishina M, Nabeshima T, Yamada K. Role of convergent activation of glutamatergic and dopaminergic systems in the nucleus accumbens in the development of methamphetamine psychosis and dependence. Int J Neuropsychopharmacol. 2013;16:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 202. | Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 1034] [Article Influence: 86.2] [Reference Citation Analysis (0)] |