Published online Dec 19, 2021. doi: 10.5498/wjp.v11.i12.1191
Peer-review started: March 1, 2021
First decision: July 15, 2021
Revised: July 29, 2021
Accepted: November 2, 2021
Article in press: November 2, 2021
Published online: December 19, 2021
Processing time: 289 Days and 5.3 Hours
Major depressive disorder (MDD) is highly prevalent and is a significant cause of mortality and morbidity worldwide. Currently, conventional pharmacological treatments for MDD produce temporary remission in < 50% of patients; therefore, there is an urgent need for a wider spectrum of novel antidepressants to target newly discovered underlying disease mechanisms. Accumulated evidence has shown that immune inflammation, particularly inflammasome activity, plays an important role in the pathophysiology of MDD. In this review, we summarize the evidence on nuclear receptors (NRs), such as glucocorticoid receptor, mineralocorticoid receptor, estrogen receptor, aryl hydrocarbon receptor, and peroxisome proliferator-activated receptor, in modulating the inflammasome activity and depression-associated behaviors. This review provides evidence from an endocrine perspective to understand the role of activated NRs in the pathophysiology of MDD, and to provide insight for the discovery of antidepressants with novel mechanisms for this devastating disorder.
Core Tip: We summarize the evidence on nuclear receptors (NRs), such as glucocorticoid receptor, mineralocorticoid receptor, estrogen receptor, aryl hydrocarbon receptor, and peroxisome proliferator-activated receptor, in modulating inflammasome activity and depression-associated behaviors. This review provides evidence from an endocrine perspective to understand the role of activated NRs in the pathophysiology and treatment of major depressive disorder. Hopefully, the modulation of NRs with hormones and metabolites may become one of the key endocrinologic mechanisms for the development of novel therapeutics to increase the likelihood of therapeutic efficacy.
- Citation: Wang H, Kan WJ, Feng Y, Feng L, Yang Y, Chen P, Xu JJ, Si TM, Zhang L, Wang G, Du J. Nuclear receptors modulate inflammasomes in the pathophysiology and treatment of major depressive disorder. World J Psychiatr 2021; 11(12): 1191-1205
- URL: https://www.wjgnet.com/2220-3206/full/v11/i12/1191.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i12.1191
Major depressive disorder (MDD) is common, has a high recurrence rate and disability rate, and affects approximately 300 million people worldwide[1]. However, the underlying pathophysiological mechanisms of MDD have yet to be completely understood. Although effective treatments are available, market-approved antidepressants have many problems, such as a single mechanism of action, delayed effect[2], and numerous side effects[3], and approximately one third of all patients fail to respond to conventional antidepressants[4]. Accordingly, there is an urgent need for new conceptual frameworks and perspectives to understand the occurrence and development of depression to develop better treatments. As another important hypothesis of depression, several lines of evidence have established an association between MDD and the neuroimmune pathway, although some psychiatrists have argued about the causal relationship between inflammation and depression[5-7]. In this review, we outline emerging data that point to nuclear receptors (NRs) as potentially important contributors to the pathophysiology of depression. We first review the current research on the inflammatory hypothesis of depression, and investigate the role of inflammasomes in the neuroimmune pathway of depression. The regulatory roles of NRs [including glucocorticoid receptor (GR), mineralocorticoid receptor (MR), estrogen receptor (ER), aryl hydrocarbon receptor (AHR), and peroxisome proliferator-activated receptor (PPAR)] in inflammasome activation and pathophysiology of depression are also investigated. Finally, these interactions are discussed as a foundation for new therapeutics that target the NRs to treat depression.
Inflammatory response is a survival mechanism in human self-protection, which is the defensive response of the body to various traumatic stimuli. Endogenous or exogenous pathogens and tissue damage are initially detected by pattern recognition receptors (PRRs), such as Toll-like receptors and nucleotide-binding oligomerization domain (NOD)-like receptors, mainly expressed by cells that participate in the innate immune response[8]. Following the activation of such receptors, signals are then transmitted to activate transcription factors. These factors regulate hundreds of genes that increase the initial inflammatory response. The brain has its own highly complex immune regulation system and is closely connected with the peripheral immune system[9]. Crosstalk between the immune system and the central nervous system (CNS) is very important for the establishment of appropriate immunity against infection and injury, the maintenance of mental health, and the influence of behavioral response[10].
The role of inflammation in the causation and exacerbation of MDD is supported by the findings from clinical studies that patients with chronic inflammation (e.g., asthma[11,12] and meningitis[13,14]), tumors, and autoimmune diseases (e.g., multiple sclerosis[15,16], Guillain-Barre syndrome[17], and systemic lupus erythematosus[18,19]) are more likely to suffer from depression, the secretion of inflammation-activated cytokines [interleukin (IL)-1β, IL-6, tumor necrosis factor α (TNFα), and C-reactive protein] in the peripheral blood and cerebrospinal fluid of patients with depression is increased[20,21], microglial activation and neuro-inflammation were found in the brain of patients with depression examined post mortem[22], and both nonsteroidal anti-inflammatory drugs and cytokine inhibitors have an active therapeutic effect on depression[23-25]. Preclinical studies have demonstrated that repeated stress events cause neurobiological changes including synaptic plasticity deficits[26] and neurotransmitter system dysregulation[27,28], leading to depressive-like behavior. Apart from these neurobiological responses, exposure to stress also has physiological and immunological consequences such as increased expression of inflammatory cytokines (such as IL-1β, TNFα, and IL-6) in the blood and brain[29]. Although cumulative evidence supports that immune inflammation plays a very important role in the pathogenesis of depression, the exact mechanism remains unclear.
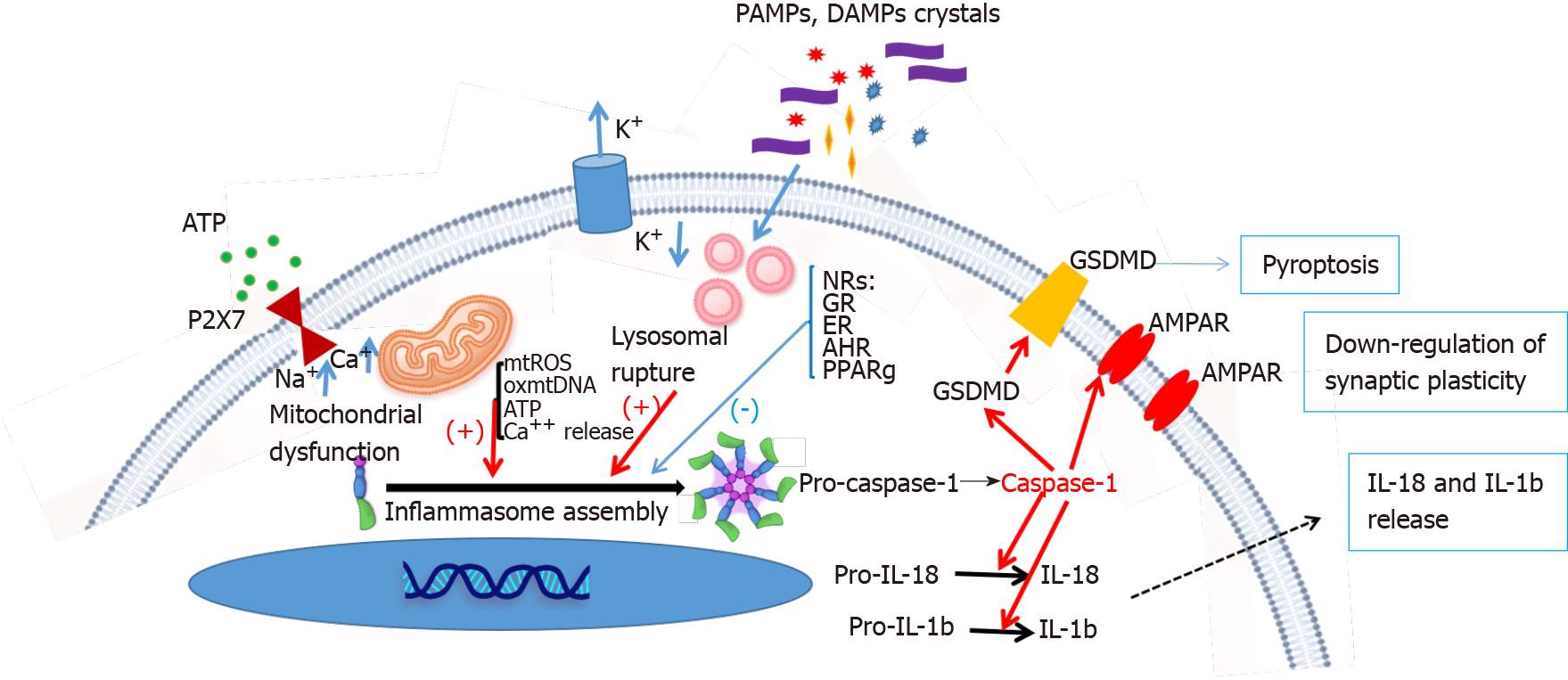
The term ‘inflammasome’ was first proposed by the Tschopp research group in 2002[30]. Inflammasomes are multiprotein complexes (-700 KD) composed of intracellular PRRs, and are an important part of the innate immune system. They can recognize pathogen-associated molecular patterns (PAMPs, such as lipopolysaccharide and bacteria) or host-derived danger signaling molecular patterns [DAMPs, including adenosine triphosphate (ATP), heat shock proteins (Hsp), glucose, uric acid, high mobility group box 1, and molecules associated with oxidative stress], and can recruit and activate pro-caspase-1. Activated caspase-1 cleaves the precursors of IL-1β and IL-18 to produce corresponding mature cytokines[31]. Activated inflammasomes can also induce apoptosis. Over the past 18 years, extensive research in this area has illustrated the key components of inflammasome activation and its role in disease processes. To date, five receptor proteins have been found to assemble inflammasomes, consisting of the NOD, leucine rich repeat (LRR)-containing protein (NLR) family members NLRP1, NLRP3, and NLRC4, as well as the proteins absent in melanoma 2 and pyrin[32]. The existing evidence suggests that NLRP1 and NLRP3 inflammasomes, especially NLRP3, play an important role in the neuroimmune pathway of MDD[33].
NLRP1 is the first identified inflammasome sensor protein[31]. Humans only have one NLRP1 protein, containing PYD, NOD, and LRRs domains, a function-to-find domain, and a carboxy-terminal caspase-associated recruitment domain[31]. The NLRP1 inflammasome, mainly expressed in neurons, is predominantly implicated in pathologies of neuronal injury and cognitive impairment, which are core features of MDD[34,35]. Although no clinical studies have reported the NLRP1 inflammasome changes in the pathogenesis of MDD patients, animal studies suggest that the NLRP1 inflammasome may play an important regulatory role in depressive-like behavior. Li et al[36] found that inhibiting the product of NLRP1 inflammasome could eliminate the depression-like behaviors caused by a chronic constriction injury. Recent studies showed that chronic unpredictable mild stress (CUMS) increased the expression of NLRP1 inflammasome complexes and pro-inflammatory cytokines. Hippocampal Nlrp1a knockdown prevented the NLRP1 inflammasome-driven inflammatory response and improved CUMS-induced depressive-like behaviors[37]. The above results suggest that NLRP1 inflammasome may be a potential antidepressant target, and further mechanisms need to be clarified.
Unlike NLRP1, NLRP3, mostly expressed in microglia cells, is activated by the most diverse array of danger signals[33,34]. NLRP3 has been reported to participate in the pathophysiology of depression in animal models and MDD patients. Supporting the hidden role of the NLRP3 inflammasome in MDD patients are data demonstrating that NLRP3 activation is increased in peripheral blood mononuclear cells[38,39]. Preclinical evidence linking the NLRP3 inflammasome to depressive-like behaviors has been found in numerous animal models, including an acute model of systemic lipopolysaccharide administration[40], chronic stress models[33], and ovariectomy and estrogen-deficient mice. These models can lead to depressive-like behavior and up-regulation of NLRP3 expression in rodents. Down-regulation of the expression of NLRP3 by some biological methods can reverse depression-like behavior[41]. NLRP3 inflammasome-driven pathways in depression have been widely reviewed[42]. In brief, psychological stress and danger substances can activate the NLRP3 inflammasome, which may lead to the release of pro-inflammatory cytokines and induction of depression. Next, we will focus on the role of NRs in the activation of inflammatory bodies in the following chapters.
The NR superfamily is a family of ligand-regulated transcription factors that are widely expressed throughout the body[43]. NRs are activated by steroid hormones, such as androgen, estrogen, and progesterone, and other lipid-soluble signals, inclu
GR is a member of the steroid receptors, and is activated by the endogenous steroid hormone cortisol[46]. Unliganded GR is predominantly localized within the cytoplasm[47]. Glucocorticoid (GC) binding causes conformational changes of the GR and activates multiple functional domains, including the hinge and LBD regions. After rapidly and efficiently being transported to the nucleus, the GR binds to the specific GC response elements of the genome to form a nuclear complex containing the GR and co-regulatory factors, which jointly activate or inhibit the transcription of GC res
The participation of GR down-regulation in the pathophysiology of MDD has been demonstrated in clinical and preclinical studies. Drug-free MDD patients have reduced GR mRNA expression together with increased expression of the FK506 binding protein 5[49,50], which reduces GR function and promotes inflammation by coordinating with Hsp90. Kang et al[51] found an association between the methylation of GRs and depression later in life. A meta-analysis demonstrated that the NR3C1 (GR) rs41423247 homozygous mutation may be a risk factor for MDD [odds ratio (OR): 0.77, 95% cumulative incidence (CI): 0.64-0.94, P = 0.01][52]. Studies on transgenic mice and a mouse stress model found that the down-regulation of GR expression is significantly related to depressive-like behavior[53]. Exogenous GC exacerbates depressive-like behavior, and down-regulates GR expression. In addition, accumulating evidence has illustrated that GR antagonists, such as mifepristone, ameliorate psychotic symptoms and cognitive deficits in MDD and bipolar disorder[54,55]. However, this seems to contradict the hypothesis of enhanced immune inflammatory response in MDD, as GC is one of the most effective anti-inflammatory hormones in the body.
It is also understandable that the effect of GR on the immune system and synapse is highly dependent on the time and dose. Mounting data indicate that innate immune cytokines cause insufficient GC signals by decreasing GR expression, blocking translocation of the GR from the cytoplasm to the nucleus, and disrupting GR-DNA binding through nuclear protein-protein interactions, which may be a reasonable explanation for this problem. Escoter-Torres et al[56] have reviewed the mechanisms of inflammatory gene regulation by the GR. Here, we will mainly explore the relationship between GR and inflammasomes in the pathophysiological mechanism of MDD.
Hypothalamic-pituitary-adrenal (HPA) axis dysfunction was assumed to be due to aberrant adrenal GC secretion and disorderly hormone feedback loops in MDD patients[57]. GC-induced activation of the NLRP3 inflammasome may mediate the potentiated neuroinflammation[58]. However, whether the effects of inflammasome activation and the HPA axis are regulated through GR-related pathways is still un
Negative feedback regulation of the HPA axis requires the participation of the dual-receptor system of MR and GR[63]. Similar to GR, MR is another member of steroid receptor and ligand-inducible transcription factors. In the brain, MR has approximately 10-fold higher affinity for CORT than the GR[64]. Due to the differences in affinity, CORT at the basal level largely occupies the MR, whereas higher hormone levels progressively occupy the GR after stress and circadian/ultradian peaks[65]. Early research results showed that brain MRs did not play an important role in the regulation of the stress response; however, subsequent studies demonstrated that MRs were essential for nongenomic regulation of glutamate transmission in the hippocampus by CORT. Based on this, considering that MRs are expressed abundantly in the limbic circuitry, a number of studies have focused on their regulatory role in depression and cognitive dysfunction[66].
The expression of MRs was decreased in the hippocampus, inferior frontal gyrus, and cingulate gyrus in depressed patients[67,68]. In addition, neuroendocrine studies also indicated abnormal MR function in MDD[63]. Otte C et al[69] found that the administration of an MR agonist (fludrocortisone) in drug-free patients with de
Research on the role of MR in the pathogenesis of depression is still in its infancy, and its possible mechanism has not been fully explained. Chen et al[80] reviewed the possible mechanism of MR in regulating depression, learning, and memory from different perspectives, such as HPA-axis activity, 5-HT transmitter system, adult-neurogenesis, and inflammation. Considering that MR can participate in the regulation of other and immune-related diseases by activating NLRP3 inflammasome[81,82], whether the role of MR in the pathogenesis of depression is involved in inflammasomes and modulation of inflammasomes will be important research directions in the future.
Given that the prevalence of depression in women is 2-3 times higher than that in men and changes in mood are simultaneously associated with estrogen levels[83], a potential role for estrogen in the pathophysiology of depression has generated substantial interest. It is well documented that estrogen can regulate neurotransmission, enhance the levels of serotonin and noradrenaline, and plays a vital role in emotion processing, cognition regulation, and motivation triggers[84,85]. The data from clinical and preclinical research show that estrogen is involved in modulation of depression and anxiety. For example, cumulative clinical studies found that men
Estrogen plays its biological role mainly through activating ERs. The ERs including ERα (ESR1) and ERβ (ESR2) are members of a superfamily of hormone-regulated transcription factors, and regulate the gene transcription of estrogen by binding to specific DNA sequences[91]. Genetic variation in ERs may therefore modify estrogen signaling, such as altering binding efficiency and disrupting normal gene regulation, thus increasing susceptibility to developing depression in women. Ryan J et al[87] carried out a detailed review and pointed out that there was a significant correlation between ESR1 gene polymorphism and severe depression in women. Preclinical research has demonstrated that ERα and ERβ agonists can reverse stress-induced depressive behavior and cognitive deficits[92]. However, the specific mechanism of ER in stress-induced depression remains unclear. Some studies have found that NLRP3 inflammasome activation mediates estrogen deficiency-induced depressive-like behavior and neuroinflammation in the hippocampus of mice[93]. In other inflammation-related diseases, such as endometriosis and breast cancer, the ER regulates the activation of NLRP3, which leads to inflammation[94,95].
AHR is a ligand-activated transcription factor which was first identified as a contaminant of the chemical herbicide Agent Orange[96]. However, AHR has been proved to be a crucial modulator of host-environment interactions in recent years, espe
Increased kynurenine (KYN) production from Trp metabolism, mediated by indole-amine 2,3-dioxygenase (IDO), is a biomarker of immune dysregulation in depression[99]. Clinical and preclinical data have consistently shown an elevated KYN level with depressive behavior after immune disturbance. The activation of AHR signaling may play an important role in immune regulation. Preclinical evidence has shown that blocking the AHR can reverse KYN-induced monocyte trafficking, neuroimmune disorder, and depression-like behavior in mice[99]. Recent clinical studies have also confirmed that the AHR is related to the individual difference in plasma KYN concentration in MDD patients[100]. The AHR regulates the expression of Trp-2,3-dioxygenase 2 (TDO2) and IDO1/2, and downstream enzymes kynurenase and kynurene 3-monooxygenase (KMO). The results of in vitro cell culture showed that AHR knockdown resulted in a decrease of KYN concentration in the cell culture medium, which may be due to the increase in quinolinic acid, a downstream metabolite of KYN[97]. Quinolinic acid is a neurotoxic NMDA receptor agonist and contributes to MDD symptoms[100]. Although cumulative data have confirmed the regulatory role of AHR in depression-like behavior induced by an abnormal KYN metabolic pathway, the specific mechanism has not been clearly elucidated. A significant result showed that AHR can regulate the activity of NLRP3 inflammasome by inhibiting the transcription of NLRP3[101]. The proposed model is as follows: Following engagement by AHR cognate ligands, it forms a heterodimer with ARNT in the nucleus, binds to the xenobiotic response element (XRE) regions located at the NF-κB site in the promoter of NLRP3 and then inhibits NF-κB transcription activity, finally decreasing NLRP3 transcription and subsequent inflammasome activation[101]. In view of the role of NLRP3 in the neuroimmune mechanism of depression, this may be the potential mechanism of AHR in regulating depressive episodes. In addition, the AHR acts as a potential crosstalk mediator between the adaptive immune system in the gut and gut microbiota-derived metabolites. Whether AHR has a certain role in the brain-gut axis dysfunction of MDD should be investigated in subsequent research.
PPARs are ligand-activated transcription factors and members of the NR receptor superfamily. Three isotypes of PPARs have been identified, namely, PPARα, PPARβ/δ, and PPARγ[102]. Despite the three PPAR isoforms having a high degree of structural homology, they have distinct tissue distribution, ligand-binding properties, and functional roles. Endogenous and natural ligands of PPARs mainly include fatty acids and fatty-acid derivatives. PPARs translocate into the nucleus upon ligand binding, where they form heterodimers with the RXR and then bind to peroxisome proliferator response elements to regulate transcriptional target genes. The physiological characteristics of PPARα, β/δ, and γ and their role in other diseases have been extensively reviewed[103,104], and will not be elaborated here. Next, we will discuss the role of PPARs in depression.
PPARα is distributed in many peripheral tissues which catabolize high amounts of fatty acids. In the CNS, PPARα is highly expressed in the basal ganglia, prefrontal cortex, thalamic nuclei, hippocampus, and ventral and tegmental areas[105]. In these regions, the distribution of PPARα in neurons is higher than that in glial cells. Recent research found that PPARα modulates the stress response, neurotransmission, neuroinflammation, and neurogenesis and plays an important regulatory role in some neuropsychiatric diseases, such as depression, post-traumatic stress disorder, and neurodegenerative diseases[106]. Preclinical studies found that knockout or overexpression of PPARα in rodent brain could imitate or reverse the depressive-like behavior induced by chronic stress. In addition, PPARα selective agonists (WY14643 and fenofibrate) have been associated with antidepressant effects in stress-induced depression models[107,108]. Some antidepressants, such as venlafaxine and fluoxetine, need PPARα to play an antidepressant role[109]. The antidepressant effect may be mediated by acting on the cAMP response element-binding (CREB)-mediated biosynthesis of brain-derived neurotrophic factor (BDNF)[109-111]. Some studies have also indicated that PPARα can modulate mesolimbic dopamine transmission and improve depression-related behavior[112]. Furthermore, N-palmitoylethanolamine, which stimulates PPARα, induced a dose-dependent antidepressant effect by engaging neurosteroid biosynthesis[113]. In summary, PPARα may play an important role in the pathogenesis of MDD and the effects of antidepressant medications, and it may be a new target for developing novel antidepressants.
PPARβ/δ is the most widely expressed isoform in the brain, with particularly high levels in the hippocampus, entorhinal cortex, and hypothalamus[105]. Compared with the other two subtypes, PPARβ/δ showed a higher expression level in neurons, and had neuroprotective effects in some CNS disease models[114]. Recent studies have found that overexpression of PPARβ/δ in the hippocampus can inhibit depressive-like behavior induced by chronic stress in rats, which corresponds to a significant down-regulation of PPARβ/δ expression in the hippocampus when rats experience chronic unpredictable stress[115]. Subsequent studies have found that when PPARβ/δ is knocked down, rats show depressive-like behavior[116]. Similar to the antidepressant effect of PPARα, the CREB-BDNF pathway may also be involved in the antidepressant effect of PPARβ/δ. Furthermore, chronic stress can increase the expression of TWIST1, which will lead to mitochondrial damage and ATP deficiency by down-regulating PPARβ/δ expression, and eventually leads to depression-like behavior in mice[116]. How overexpression of PPARβ/δ and its agonists play an antidepressant role is still unclear.
PPARγ is highly expressed in the amygdala, dental gyrus, prefrontal cortex, ventral tegmental area, and basal ganglia[105]. Under normal physiological conditions, PPARγ can co-localize with neurons and astrocytes in human and mouse brain, but not with microglia. However, PPARγ can also be expressed in microglia when the functional status of microglia changes. PPARγ agonists have been synthesized for the treatment of metabolic diseases, especially dyslipidemia and type 2 diabetes mellitus, as well as non-metabolic diseases including neurodegenerative diseases, cancer, and inflammatory diseases due to their important metabolic regulation and excellent druggability[117,118]. Compared with the above two subtypes, the relationship between PPARγ and depression has been more widely recognized, and clinical trials on the antidepressant effects of PPARγ agonists are in full swing. Some gratifying results have been found and were well reviewed[117].
In conclusion, all isotypes of PPAR may participate in the pathophysiology of depression, and even antidepressants based on PPAR agonists have been developed. However, how PPARs play an antidepressant role seems unclear, although some studies have shown that this occurs by regulating the biosynthesis of BDNF and regulating the 5-HT neurotransmitter system. Activation of PPARs inhibits the activation of inflammasomes (in particular NLRP3) and the release of inflammatory cytokines, which is similar to the changes in patients with depression and in de
Given the relatively low overall response rates and the wide range of ‘adverse’ events associated with current antidepressants, there is an urgent need for novel therapeutics to treat specific underlying disease mechanisms that are not addressed by the antidepressants targeting the serotonergic and/or noradrenergic system. Hopefully, the modulation of NRs with hormones and metabolites may become one of the key endocrinologic mechanisms for the development of novel therapeutics to increase the likelihood of therapeutic efficacy. Here, we reviewed the regulatory role of NRs (including the GC, MR, ER, AHR, and PPAR) in inflammasome activation and the pathophysiology of depression (Figure 1). Indeed, a major breakthrough in the pathophysiology of depression was the discovery that DAMPs and PAMPs activate inflammasomes, which enhance caspase-1 activity, and subsequently inhibit excitatory AMPA receptor synaptic plasticity in the brain circuitry to change mood-associated behaviors[124,125]. Cumulative studies have shown that activation of the NRs may directly change the activity of inflammasomes to modulate the levels of mature forms of caspase-1 and IL-1β. Caspase-1-mediated programmed cell death and surface stability of the AMPA receptor in the hippocampus, are essential for depression-like behavior[125]. Current data suggest that direct modulation of NRs may offer new opportunities to mitigate depressive disorders. However, several directions are warranted for future studies: (1) To identify more NR activators for the treatment of MDD; (2) To address the detailed mechanism of how NRs modulate inflammasomes; and (3) To perform clinical trials to prove the role of NR modulators in the treatment of MDD. These NR modulators can be safely used in combination with currently available antidepressants to simultaneously target multiple disease mechanisms and increase the likelihood of therapeutic success.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The National Clinical Research Center for Mental Disorders & Beijing Key Laboratory of Mental Disorders.
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotzalidis GD S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1254] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 2. | Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2618] [Cited by in RCA: 3368] [Article Influence: 177.3] [Reference Citation Analysis (0)] |
| 3. | Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, Demyttenaere K, de Girolamo G, Haro JM, Jin R, Karam EG, Kovess-Masfety V, Levinson D, Medina Mora ME, Ono Y, Ormel J, Pennell BE, Posada-Villa J, Sampson NA, Williams D, Kessler RC. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66:785-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1032] [Cited by in RCA: 877] [Article Influence: 54.8] [Reference Citation Analysis (9)] |
| 4. | Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, Fava M, Nierenberg AA, McGrath PJ, Warden D, Niederehe G, Hollon SD, Rush AJ. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Leonard BE. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 271] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 6. | Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 659] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 7. | Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1671] [Cited by in RCA: 2421] [Article Influence: 269.0] [Reference Citation Analysis (0)] |
| 8. | Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5434] [Cited by in RCA: 6541] [Article Influence: 436.1] [Reference Citation Analysis (0)] |
| 9. | Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 437] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Jeltsch-David H, Muller S. Autoimmunity, neuroinflammation, pathogen load: A decisive crosstalk in neuropsychiatric SLE. J Autoimmun. 2016;74:13-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Choi HG, Kim JH, Park JY, Hwang YI, Jang SH, Jung KS. Association Between Asthma and Depression: A National Cohort Study. J Allergy Clin Immunol Pract. 2019;7:1239-1245.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Choi S, Kim SH, Lee JS. Association between depression and asthma in Korean adults. Allergy Asthma Proc. 2017;38:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Irby IT, Leja P, Manning D, Limaye K, Lahoti S. Aseptic Meningitis and Depression: The Neuropsychiatric Manifestations of a Patient with Systemic Lupus Erythematosus. Cureus. 2019;11:e5424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Barichello T, Simões LR, Generoso JS, Sharin VS, Souza LB, Jornada LK, Dominguini D, Valvassori SS, Teixeira AL, Quevedo J. Depression-Like Adult Behaviors may be a Long-Term Result of Experimental Pneumococcal Meningitis in Wistar Rats Infants. Neurochem Res. 2016;41:2771-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Feinstein A. Multiple sclerosis, depression, and suicide. BMJ. 1997;315:691-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Caine ED, Schwid SR. Multiple sclerosis, depression, and the risk of suicide. Neurology. 2002;59:662-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Merkies IS, Kieseier BC. Fatigue, Pain, Anxiety and Depression in Guillain-Barré Syndrome and Chronic Inflammatory Demyelinating Polyradiculoneuropathy. Eur Neurol. 2016;75:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Figueiredo-Braga M, Cornaby C, Cortez A, Bernardes M, Terroso G, Figueiredo M, Mesquita CDS, Costa L, Poole BD. Depression and anxiety in systemic lupus erythematosus: The crosstalk between immunological, clinical, and psychosocial factors. Medicine (Baltimore). 2018;97:e11376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Zhang L, Fu T, Yin R, Zhang Q, Shen B. Prevalence of depression and anxiety in systemic lupus erythematosus: a systematic review and meta-analysis. BMC Psychiatry. 2017;17:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 20. | Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 397] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3039] [Cited by in RCA: 3402] [Article Influence: 226.8] [Reference Citation Analysis (0)] |
| 22. | Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014;42:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 396] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 23. | Shaikh NF, Sambamoorthi U. Prescription Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Depression among Adults with Inflammatory Chronic Conditions in the United States. Psychiatr Q. 2020;91:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Lehrer S, Rheinstein PH. Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce suicidal ideation and depression. Discov Med. 2019;28:205-212. [PubMed] |
| 25. | Kohler O, Krogh J, Mors O, Benros ME. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr Neuropharmacol. 2016;14:732-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 373] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 26. | Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22:535-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 27. | Seo JS, Wei J, Qin L, Kim Y, Yan Z, Greengard P. Cellular and molecular basis for stress-induced depression. Mol Psychiatry. 2017;22:1440-1447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 28. | Mahar I, Bambico FR, Mechawar N, Nobrega JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 459] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 29. | Gómez-Lázaro E, Arregi A, Beitia G, Vegas O, Azpiroz A, Garmendia L. Individual differences in chronically defeated male mice: behavioral, endocrine, immune, and neurotrophic changes as markers of vulnerability to the effects of stress. Stress. 2011;14:537-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4047] [Cited by in RCA: 4681] [Article Influence: 203.5] [Reference Citation Analysis (0)] |
| 31. | Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3880] [Cited by in RCA: 4543] [Article Influence: 302.9] [Reference Citation Analysis (0)] |
| 32. | Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 274] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY, Zhang T, Wang W, Wang YX, Jiang CL. NLRP3 Inflammasome Mediates Chronic Mild Stress-Induced Depression in Mice via Neuroinflammation. Int J Neuropsychopharmacol. 2015;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 34. | Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 409] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 35. | Freeman LC, Ting JP. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016;136 Suppl 1:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 36. | Li Q, Liu S, Zhu X, Mi W, Maoying Q, Wang J, Yu J, Wang Y. Hippocampal PKR/NLRP1 Inflammasome Pathway Is Required for the Depression-Like Behaviors in Rats with Neuropathic Pain. Neuroscience. 2019;412:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Song AQ, Gao B, Fan JJ, Zhu YJ, Zhou J, Wang YL, Xu LZ, Wu WN. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J Neuroinflammation. 2020;17:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 38. | Tian H, Li G, Xu G, Liu J, Wan X, Zhang J, Xie S, Cheng J, Gao S. Inflammatory cytokines derived from peripheral blood contribute to the modified electroconvulsive therapy-induced cognitive deficits in major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2021;271:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Taene A, Khalili-Tanha G, Esmaeili A, Mobasheri L, Kooshkaki O, Jafari S, Shokouhifar A, Sarab GA. The Association of Major Depressive Disorder with Activation of NLRP3 Inflammasome, Lipid Peroxidation, and Total Antioxidant Capacity. J Mol Neurosci. 2020;70:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 40. | Arioz BI, Tastan B, Tarakcioglu E, Tufekci KU, Olcum M, Ersoy N, Bagriyanik A, Genc K, Genc S. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front Immunol. 2019;10:1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 383] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 41. | Alcocer-Gómez E, Casas-Barquero N, Williams MR, Romero-Guillena SL, Cañadas-Lozano D, Bullón P, Sánchez-Alcazar JA, Navarro-Pando JM, Cordero MD. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol Res. 2017;121:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 42. | Kaufmann FN, Costa AP, Ghisleni G, Diaz AP, Rodrigues ALS, Peluffo H, Kaster MP. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav Immun. 2017;64:367-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 282] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 43. | Sever R, Glass CK. Signaling by nuclear receptors. Cold Spring Harb Perspect Biol. 2013;5:a016709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 44. | Porter BA, Ortiz MA, Bratslavsky G, Kotula L. Structure and Function of the Nuclear Receptor Superfamily and Current Targeted Therapies of Prostate Cancer. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 45. | Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5272] [Cited by in RCA: 5178] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 46. | Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1236] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 47. | Heitzer MD, Wolf IM, Sanchez ER, Witchel SF, DeFranco DB. Glucocorticoid receptor physiology. Rev Endocr Metab Disord. 2007;8:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 48. | Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17:245-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 575] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 49. | Cattaneo A, Ferrari C, Turner L, Mariani N, Enache D, Hastings C, Kose M, Lombardo G, McLaughlin AP, Nettis MA, Nikkheslat N, Sforzini L, Worrell C, Zajkowska Z, Cattane N, Lopizzo N, Mazzelli M, Pointon L, Cowen PJ, Cavanagh J, Harrison NA, de Boer P, Jones D, Drevets WC, Mondelli V, Bullmore ET; Neuroimmunology of Mood Disorders and Alzheimer’s Disease (NIMA) Consortium, Pariante CM. Whole-blood expression of inflammasome- and glucocorticoid-related mRNAs correctly separates treatment-resistant depressed patients from drug-free and responsive patients in the BIODEP study. Transl Psychiatry. 2020;10:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 50. | Lukic I, Mitic M, Soldatovic I, Jovicic M, Maric N, Radulovic J, Adzic M. Accumulation of cytoplasmic glucocorticoid receptor is related to elevation of FKBP5 in lymphocytes of depressed patients. J Mol Neurosci. 2015;55:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Kang HJ, Bae KY, Kim SW, Shin IS, Kim HR, Shin MG, Yoon JS, Kim JM. Longitudinal associations between glucocorticoid receptor methylation and late-life depression. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Peng Q, Yan H, Wen Y, Lai C, Shi L. Association between NR3C1 rs41423247 polymorphism and depression: A PRISMA-compliant meta-analysis. Medicine (Baltimore). 2018;97:e12541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Farrell C, O'Keane V. Epigenetics and the glucocorticoid receptor: A review of the implications in depression. Psychiatry Res. 2016;242:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Block T, Petrides G, Kushner H, Kalin N, Belanoff J, Schatzberg A. Mifepristone Plasma Level and Glucocorticoid Receptor Antagonism Associated With Response in Patients With Psychotic Depression. J Clin Psychopharmacol. 2017;37:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, Ferrier IN. Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology. 2004;29:1538-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 225] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 56. | Escoter-Torres L, Caratti G, Mechtidou A, Tuckermann J, Uhlenhaut NH, Vettorazzi S. Fighting the Fire: Mechanisms of Inflammatory Gene Regulation by the Glucocorticoid Receptor. Front Immunol. 2019;10:1859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 57. | Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 500] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 58. | Frank MG, Watkins LR, Maier SF. The permissive role of glucocorticoids in neuroinflammatory priming: mechanisms and insights. Curr Opin Endocrinol Diabetes Obes. 2015;22:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Zhao Q, Wu CS, Fang Y, Qian Y, Wang H, Fan YC, Wang K. Glucocorticoid Regulates NLRP3 in Acute-On-Chronic Hepatitis B Liver Failure. Int J Med Sci. 2019;16:461-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Feng X, Zhao Y, Yang T, Song M, Wang C, Yao Y, Fan H. Glucocorticoid-Driven NLRP3 Inflammasome Activation in Hippocampal Microglia Mediates Chronic Stress-Induced Depressive-Like Behaviors. Front Mol Neurosci. 2019;12:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 61. | Bharti V, Tan H, Zhou H, Wang JF. Txnip mediates glucocorticoid-activated NLRP3 inflammatory signaling in mouse microglia. Neurochem Int. 2019;131:104564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Hu W, Zhang Y, Wu W, Yin Y, Huang D, Wang Y, Li W. Chronic glucocorticoids exposure enhances neurodegeneration in the frontal cortex and hippocampus via NLRP-1 inflammasome activation in male mice. Brain Behav Immun. 2016;52:58-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Mineralocorticoid receptor function in major depression. Arch Gen Psychiatry. 2003;60:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Grossmann C, Scholz T, Rochel M, Bumke-Vogt C, Oelkers W, Pfeiffer AF, Diederich S, Bahr V. Transactivation via the human glucocorticoid and mineralocorticoid receptor by therapeutically used steroids in CV-1 cells: a comparison of their glucocorticoid and mineralocorticoid properties. Eur J Endocrinol. 2004;151:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 65. | Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102:19204-19207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 579] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 66. | Otte C, Wingenfeld K, Kuehl LK, Kaczmarczyk M, Richter S, Quante A, Regen F, Bajbouj M, Zimmermann-Viehoff F, Wiedemann K, Hinkelmann K. Mineralocorticoid receptor stimulation improves cognitive function and decreases cortisol secretion in depressed patients and healthy individuals. Neuropsychopharmacology. 2015;40:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Medina A, Seasholtz AF, Sharma V, Burke S, Bunney W Jr, Myers RM, Schatzberg A, Akil H, Watson SJ. Glucocorticoid and mineralocorticoid receptor expression in the human hippocampus in major depressive disorder. J Psychiatr Res. 2013;47:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Klok MD, Alt SR, Irurzun Lafitte AJ, Turner JD, Lakke EA, Huitinga I, Muller CP, Zitman FG, de Kloet ER, Derijk RH. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J Psychiatr Res. 2011;45:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 69. | Otte C, Hinkelmann K, Moritz S, Yassouridis A, Jahn H, Wiedemann K, Kellner M. Modulation of the mineralocorticoid receptor as add-on treatment in depression: a randomized, double-blind, placebo-controlled proof-of-concept study. J Psychiatr Res. 2010;44:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 70. | Kuningas M, de Rijk RH, Westendorp RG, Jolles J, Slagboom PE, van Heemst D. Mental performance in old age dependent on cortisol and genetic variance in the mineralocorticoid and glucocorticoid receptors. Neuropsychopharmacology. 2007;32:1295-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 71. | Bogdan R, Perlis RH, Fagerness J, Pizzagalli DA. The impact of mineralocorticoid receptor ISO/VAL genotype (rs5522) and stress on reward learning. Genes Brain Behav. 2010;9:658-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, Arolt V, Baune BT, Blackwood D, Cichon S, Coventry WL, Domschke K, Farmer A, Fava M, Gordon SD, He Q, Heath AC, Heutink P, Holsboer F, Hoogendijk WJ, Hottenga JJ, Hu Y, Kohli M, Lin D, Lucae S, Macintyre DJ, Maier W, McGhee KA, McGuffin P, Montgomery GW, Muir WJ, Nolen WA, Nöthen MM, Perlis RH, Pirlo K, Posthuma D, Rietschel M, Rizzu P, Schosser A, Smit AB, Smoller JW, Tzeng JY, van Dyck R, Verhage M, Zitman FG, Martin NG, Wray NR, Boomsma DI, Penninx BW. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2009;14:359-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 73. | Vinkers CH, Joëls M, Milaneschi Y, Gerritsen L, Kahn RS, Penninx BW, Boks MP. Mineralocorticoid receptor haplotypes sex-dependently moderate depression susceptibility following childhood maltreatment. Psychoneuroendocrinology. 2015;54:90-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 74. | Stonawski V, Frey S, Golub Y, Rohleder N, Kriebel J, Goecke TW, Fasching PA, Beckmann MW, Kornhuber J, Kratz O, Moll GH, Heinrich H, Eichler A. Associations of prenatal depressive symptoms with DNA methylation of HPA axis-related genes and diurnal cortisol profiles in primary school-aged children. Dev Psychopathol. 2019;31:419-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 75. | Galbally M, Watson SJ, van IJzendoorn M, Saffery R, Ryan J, de Kloet ER, Oberlander TF, Lappas M, Lewis AJ. The role of glucocorticoid and mineralocorticoid receptor DNA methylation in antenatal depression and infant stress regulation. Psychoneuroendocrinology. 2020;115:104611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 76. | Avital A, Segal M, Richter-Levin G. Contrasting roles of corticosteroid receptors in hippocampal plasticity. J Neurosci. 2006;26:9130-9134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 77. | Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, Chepkova AN, Welzl H, Haas HL, Lipp HP, Schütz G. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci U S A. 2006;103:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 78. | Arp JM, ter Horst JP, Kanatsou S, Fernández G, Joëls M, Krugers HJ, Oitzl MS. Mineralocorticoid receptors guide spatial and stimulus-response learning in mice. PLoS One. 2014;9:e86236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | ter Horst JP, van der Mark MH, Arp M, Berger S, de Kloet ER, Oitzl MS. Stress or no stress: mineralocorticoid receptors in the forebrain regulate behavioral adaptation. Neurobiol Learn Mem. 2012;98:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Chen J, Wang ZZ, Zhang S, Zuo W, Chen NH. Does mineralocorticoid receptor play a vital role in the development of depressive disorder? Life Sci. 2016;152:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 81. | Brocca ME, Pietranera L, Meyer M, Lima A, Roig P, de Kloet ER, De Nicola AF. Mineralocorticoid receptor associates with pro-inflammatory bias in the hippocampus of spontaneously hypertensive rats. J Neuroendocrinol. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Ding W, Guo H, Xu C, Wang B, Zhang M, Ding F. Mitochondrial reactive oxygen species-mediated NLRP3 inflammasome activation contributes to aldosterone-induced renal tubular cells injury. Oncotarget. 2016;7:17479-17491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 83. | Brody DJ, Pratt LA, Hughes JP. Prevalence of Depression Among Adults Aged 20 and Over: United States, 2013-2016. NCHS Data Brief. 2018;1-8. [PubMed] |
| 84. | Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 514] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 85. | Parker MG, Arbuckle N, Dauvois S, Danielian P, White R. Structure and function of the estrogen receptor. Ann N Y Acad Sci. 1993;684:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Fernandez JW, Grizzell JA, Wecker L. The role of estrogen receptor β and nicotinic cholinergic receptors in postpartum depression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Ryan J, Ancelin ML. Polymorphisms of estrogen receptors and risk of depression: therapeutic implications. Drugs. 2012;72:1725-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | de Kruif M, Molendijk ML, Haffmans PM, Spijker AT. [Depression during the perimenopause]. Tijdschr Psychiatr. 2015;57:795-804. [PubMed] |
| 89. | Fang YY, Zeng P, Qu N, Ning LN, Chu J, Zhang T, Zhou XW, Tian Q. Evidence of altered depression and dementia-related proteins in the brains of young rats after ovariectomy. J Neurochem. 2018;146:703-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 90. | Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 91. | Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1305] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 92. | Eid RS, Lieblich SE, Duarte-Guterman P, Chaiton JA, Mah AG, Wong SJ, Wen Y, Galea LAM. Selective activation of estrogen receptors α and β: Implications for depressive-like phenotypes in female mice exposed to chronic unpredictable stress. Horm Behav. 2020;119:104651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Liu T, Ma Y, Zhang R, Zhong H, Wang L, Zhao J, Yang L, Fan X. Resveratrol ameliorates estrogen deficiency-induced depression- and anxiety-like behaviors and hippocampal inflammation in mice. Psychopharmacology (Berl). 2019;236:1385-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 94. | García-Gómez E, Vázquez-Martínez ER, Reyes-Mayoral C, Cruz-Orozco OP, Camacho-Arroyo I, Cerbón M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front Endocrinol (Lausanne). 2019;10:935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 95. | Raut PK, Kim SH, Choi DY, Jeong GS, Park PH. Growth of breast cancer cells by leptin is mediated via activation of the inflammasome: Critical roles of estrogen receptor signaling and reactive oxygen species production. Biochem Pharmacol. 2019;161:73-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 96. | Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 697] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 97. | Neavin DR, Liu D, Ray B, Weinshilboum RM. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 98. | Busbee PB, Rouse M, Nagarkatti M, Nagarkatti PS. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr Rev. 2013;71:353-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 99. | Zang X, Zheng X, Hou Y, Hu M, Wang H, Bao X, Zhou F, Wang G, Hao H. Regulation of proinflammatory monocyte activation by the kynurenine-AhR axis underlies immunometabolic control of depressive behavior in mice. FASEB J. 2018;32:1944-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 100. | Ogyu K, Kubo K, Noda Y, Iwata Y, Tsugawa S, Omura Y, Wada M, Tarumi R, Plitman E, Moriguchi S, Miyazaki T, Uchida H, Graff-Guerrero A, Mimura M, Nakajima S. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 101. | Huai W, Zhao R, Song H, Zhao J, Zhang L, Gao C, Han L, Zhao W. Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription. Nat Commun. 2014;5:4738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 102. | Corrales P, Vidal-Puig A, Medina-Gómez G. PPARs and Metabolic Disorders Associated with Challenged Adipose Tissue Plasticity. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 103. | Mirza AZ, Althagafi II, Shamshad H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur J Med Chem. 2019;166:502-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 362] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 104. | Tan NS, Vázquez-Carrera M, Montagner A, Sng MK, Guillou H, Wahli W. Transcriptional control of physiological and pathological processes by the nuclear receptor PPARβ/δ. Prog Lipid Res. 2016;64:98-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 105. | Warden A, Truitt J, Merriman M, Ponomareva O, Jameson K, Ferguson LB, Mayfield RD, Harris RA. Localization of PPAR isotypes in the adult mouse and human brain. Sci Rep. 2016;6:27618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 106. | Tufano M, Pinna G. Is There a Future for PPARs in the Treatment of Neuropsychiatric Disorders? Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 107. | Jiang B, Huang C, Zhu Q, Tong LJ, Zhang W. WY14643 produces anti-depressant-like effects in mice via the BDNF signaling pathway. Psychopharmacology (Berl). 2015;232:1629-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 108. | Jiang B, Wang YJ, Wang H, Song L, Huang C, Zhu Q, Wu F, Zhang W. Antidepressant-like effects of fenofibrate in mice via the hippocampal brain-derived neurotrophic factor signalling pathway. Br J Pharmacol. 2017;174:177-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 109. | Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 643] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 110. | Caviedes A, Lafourcade C, Soto C, Wyneken U. BDNF/NF-κB Signaling in the Neurobiology of Depression. Curr Pharm Des. 2017;23:3154-3163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 111. | Roy A, Jana M, Corbett GT, Ramaswamy S, Kordower JH, Gonzalez FJ, Pahan K. Regulation of cyclic AMP response element binding and hippocampal plasticity-related genes by peroxisome proliferator-activated receptor α. Cell Rep. 2013;4:724-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 112. | Scheggi S, Melis M, De Felice M, Aroni S, Muntoni AL, Pelliccia T, Gambarana C, De Montis MG, Pistis M. PPARα modulation of mesolimbic dopamine transmission rescues depression-related behaviors. Neuropharmacology. 2016;110:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 113. | Locci A, Pinna G. Stimulation of Peroxisome Proliferator-Activated Receptor-α by N-Palmitoylethanolamine Engages Allopregnanolone Biosynthesis to Modulate Emotional Behavior. Biol Psychiatry. 2019;85:1036-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 114. | Das NR, Gangwal RP, Damre MV, Sangamwar AT, Sharma SS. A PPAR-β/δ agonist is neuroprotective and decreases cognitive impairment in a rodent model of Parkinson's disease. Curr Neurovasc Res. 2014;11:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 115. | Chen F, Yu X, Meng G, Mei Z, Du Y, Sun H, Reed MN, Kong L, Suppiramaniam V, Hong H, Tang S. Hippocampal Genetic Knockdown of PPARδ Causes Depression-Like Behaviors and Neurogenesis Suppression. Int J Neuropsychopharmacol. 2019;22:372-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 116. | He JG, Zhou HY, Xue SG, Lu JJ, Xu JF, Zhou B, Hu ZL, Wu PF, Long LH, Ni L, Jin Y, Wang F, Chen JG. Transcription Factor TWIST1 Integrates Dendritic Remodeling and Chronic Stress to Promote Depressive-like Behaviors. Biol Psychiatry. 2021;89:615-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 117. | Colle R, de Larminat D, Rotenberg S, Hozer F, Hardy P, Verstuyft C, Fève B, Corruble E. PPAR-γ Agonists for the Treatment of Major Depression: A Review. Pharmacopsychiatry. 2017;50:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 118. | Cheng HS, Tan WR, Low ZS, Marvalim C, Lee JYH, Tan NS. Exploration and Development of PPAR Modulators in Health and Disease: An Update of Clinical Evidence. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 119. | Liu Y, Bi X, Zhang Y, Wang Y, Ding W. Mitochondrial dysfunction/NLRP3 inflammasome axis contributes to angiotensin II-induced skeletal muscle wasting via PPAR-γ. Lab Invest. 2020;100:712-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 120. | Chen L, Xue L, Zheng J, Tian X, Zhang Y, Tong Q. PPARß/δ agonist alleviates NLRP3 inflammasome-mediated neuroinflammation in the MPTP mouse model of Parkinson's disease. Behav Brain Res. 2019;356:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 121. | Liu P, Du J. Oridonin is an antidepressant molecule working through the PPAR-γ/AMPA receptor signaling pathway. Biochem Pharmacol. 2020;180:114136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 122. | Song MT, Ruan J, Zhang RY, Deng J, Ma ZQ, Ma SP. Astragaloside IV ameliorates neuroinflammation-induced depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3 inflammasome axis. Acta Pharmacol Sin. 2018;39:1559-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 123. | Li R, Wang X, Qin T, Qu R, Ma S. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav Brain Res. 2016;296:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 124. | Lu C, Wang Y, Furukawa K, Fu W, Ouyang X, Mattson MP. Evidence that caspase-1 is a negative regulator of AMPA receptor-mediated long-term potentiation at hippocampal synapses. J Neurochem. 2006;97:1104-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 125. | Li MX, Zheng HL, Luo Y, He JG, Wang W, Han J, Zhang L, Wang X, Ni L, Zhou HY, Hu ZL, Wu PF, Jin Y, Long LH, Zhang H, Hu G, Chen JG, Wang F. Gene deficiency and pharmacological inhibition of caspase-1 confers resilience to chronic social defeat stress via regulating the stability of surface AMPARs. Mol Psychiatry. 2018;23:556-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |