Published online Jul 19, 2020. doi: 10.5498/wjp.v10.i7.150
Peer-review started: December 31, 2019
First decision: April 3, 2020
Revised: April 25, 2020
Accepted: May 26, 2020
Article in press: May 26, 2020
Published online: July 19, 2020
Processing time: 198 Days and 14 Hours
Epilepsy is a complex neurological disorder characterized by recurrent, unprovoked seizures resulting from the sudden abnormal discharge of brain neurons. It leads to transient brain dysfunction, manifested by abnormal physical movements and consciousness. It can occur at any age, affecting approximately 65 million worldwide, one third of which are still estimated to suffer from refractory seizures. There is an urgent need for further establishment of seizure models in animals, which provides an approach to model epilepsy and could be used to identify novel anti-epileptic therapeutics in the future.
To compare three administration modes for establishing a seizure model caused by N-Methyl-D-aspartic acid (NMDA) in zebrafish.
Three administration routes of NMDA, including immersion, intravitreal injection and intraperitoneal injection, were compared with regard to their effects on inducing seizure-like behaviors in adult zebrafish. We evaluated neurotoxicity by observing behavioral changes in zebrafish and graded those behaviors with a seizure score. In addition, the protective effects of MK-801 (Dizocilpine) and natural active constituent resveratrol against NMDA-induced alterations were studied.
The three NMDA-administration methods triggered different patterns of the epileptic process in adult zebrafish. Seizure scores were increased after increasing NMDA concentration regardless of the mode of administration. However, the curve of immersion continuously rose to a high plateau (after 50 min), while the curves of intravitreal injection and intraperitoneal injection showed a spike in the early stage (10-20 min) followed by a steady decrease in seizure scores. Furthermore, pretreatment with resveratrol and MK-801 significantly delayed seizure onset time and lowered seizure scores.
By comparing the three methods of administration, intravitreal injection of NMDA was the most suitable for establishing an acute epileptic model in zebrafish. Thus, intraperitoneal injection in zebrafish can be applied to simulate diseases such as epilepsy. In addition, NMDA immersion may be an appropriate method to induce persistent seizures. Moreover, MK-801 and resveratrol showed strong anti-epileptic effects; thus, both of them may be clinically valuable treatments for epilepsy.
Core tip: This is the first study to systematically compare the three main administration modes to establish a seizure model in zebrafish. A newly developed zebrafish model with acute and sustained experimental epileptic behavior enables us to study and identify potential mechanisms and screen anti-epileptic drugs. Direct administration of N-Methyl-D-aspartic acid stimulates abnormal excitations of brain nerve cells to simulate epileptic seizures. This study demonstrated that intravitreal injection can be used to establish an acute epilepsy model and immersion can be used as a persistent epilepsy model. The protective effects of resveratrol and MK-801 on the epileptic process were also confirmed, which may have clinical application value.
- Citation: Long XY, Wang S, Luo ZW, Zhang X, Xu H. Comparison of three administration modes for establishing a zebrafish seizure model induced by N-Methyl-D-aspartic acid. World J Psychiatr 2020; 10(7): 150-161
- URL: https://www.wjgnet.com/2220-3206/full/v10/i7/150.htm
- DOI: https://dx.doi.org/10.5498/wjp.v10.i7.150
Epilepsy is a chronic brain disorder caused by abnormal, excessive and synchronous neuronal activities in the brain. The clinical manifestations are characterized by paroxysmal, transient, repetitive and stereotyped. The location of abnormal discharge neurons and the range of abnormal discharge spread are different, leading to different forms of seizure, manifested as sensory, motor, conscious, mental, behavioral, autonomic dysfunction or a combination of multiple dysfunctions[1]. According to the World Health Organization report, there are many causes of epilepsy, such as stroke, brain trauma, and central nervous system infection[2]. Although it is generally believed that about two-thirds of epilepsy is idiopathic, most of which are now considered to be hereditary[1]. Epilepsy also has various psychiatric complications such as depression, anxiety and cognitive defects[3,4]. It has been reported that peroxisome proliferator-activated receptor γ and mutations in genes involved in GABA-mediated inhibitory neurotransmission are highly associated with the pathogenesis of epilepsy[1,5]. Moreover, it is widely accepted that glutamate overstimulation of the N-Methyl-D-aspartic acid (NMDA) receptor is an important pathogenesis of epilepsy, which leads to continuous internal flow of calcium ions and excessive excitement of the hippocampal networks[6,7]. The molecular mechanism of epilepsy is still not fully understood, and thus there is a lack of effective clinical treatment[8,9]. Therefore, building relevant preclinical models is imperative for therapeutics screening in this disease.
NMDA is an amino acid derivative that exists naturally in the animal body. It is an analog of L-glutamate, an important excitatory neurotransmitter in the mammalian central nervous system. It has been used to model a series of neurodegenerative diseases such as epilepsy, glaucoma, Alzheimer's disease, Parkinson's disease and Huntington's disease[10-15]. NMDA-induced cellular excitotoxicity may be the consequence of overstimulating NMDA receptors at high concentrations of NMDA, causing a massive calcium influx. The overproduction of nucleases, proteinases, lipase, free radicals as well as the activation of nitric oxide pathway ultimately then give rise to cell death[16,17].
As a vertebrate model, zebrafish have received a great deal of attention in the field of developmental biology and genetics over the last decade as a cost-efficient and relevant alternative for human disease modeling and large-scale drug screening[18]. There are many reasons for its popularity, for example, zebrafish share high genetic, cellular and organ homologies to humans over the evolutionary process[19]. Besides their homology, zebrafish are much easier to breed than other types of experimental animals due to ease of handling and fast reproduction rate. These advantages indicate that zebrafish has outstanding value in preclinical drug screening[20,21]. Furthermore, its central nervous system is structurally similar to that of mammals. Some signaling systems such as serotonin energy and GABAergic neurotransmission are also highly similar[18,22]. When it comes to studying brain disease, these aspects have always been the advantages of zebrafish. Establishing a reliable zebrafish epilepsy model not only contributes to a better understanding of the molecular pathology of zebrafish seizures, but may also be conducive to screen drugs that protect the brain from seizure damage. Both of these directions will contribute to better clinical treatment of epilepsy.
The principal methods of administration used in the present study were immersion, intravitreal injection and intraperitoneal injection. Some studies have demonstrated that drugs can be absorbed directly from the water environment through the skin of zebrafish[23,24], while intraperitoneal injection of drugs can induce epileptic behaviors in zebrafish[25,26]. Ouabain was injected into the eyeballs of zebrafish through the vitreous cavity, resulting in nerve cell damage[27,28]. Although there are other alternative modeling methods, such as intraperitoneal perfusion, they were not adopted in this study.
MK-801 is a non-competitive antagonist of the NMDA receptor and can directly prevent NMDA-induced excitatory toxicity. Therefore, it was used as a positive control. Resveratrol is a biologically active constituent extracted from many plants. As previously reported, it has a multitude of health benefits including the ability to prolong life and prevent certain diseases such as heart disease, autoimmune diseases, metabolic disorders[29], inflammation[30], neurodegeneration[31,32], and epilepsy[33,34]. In addition, it also plays an active role in retinal degeneration models[35]. However, it is not clear whether the three modes of NMDA administration cause changes in epileptic behavior, and whether resveratrol or MK-801 can protect against this brain disorder. This study aims to investigate these two key aspects to further understand the establishment of seizure models in zebrafish and lay the foundation for drug screening and treatment development.
The study was approved by the Ethical Review Committee of Nanchang University (Nanchang, Jiangxi Province, China). The China Zebrafish Resource Center (Wuhan, Hubei Province, China) provided adult male and female wild-type zebrafish (Danio rerio, AB strain). All adult zebrafish were raised in a temperature-controlled (28°C) zebrafish breeding system (Thmorgan Biotechnology Corp., Ltd. Beijing, China) and all zebrafish were propagated in a cycle of 14 h light/10 h dark in the experiment, and they were fed with brine shrimp twice daily (Wudi, Shandong Province, China).
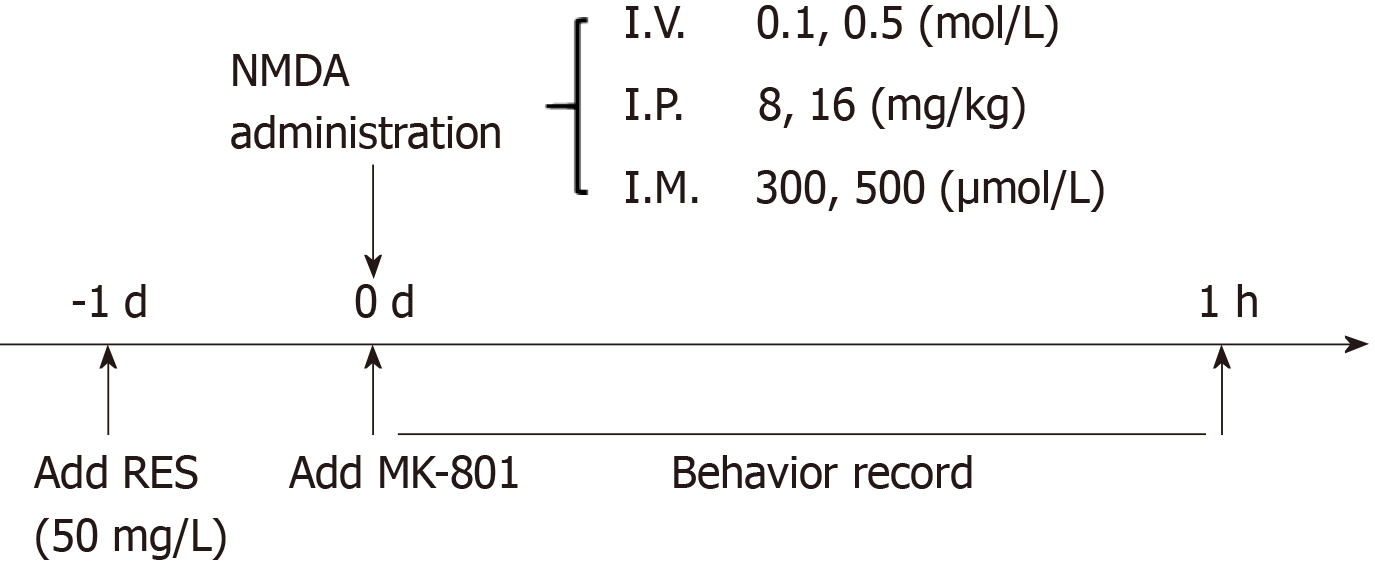
NMDA (M3262, Sigma, United States) was dissolved in phosphate buffered saline (PBS) to prepare solutions for different modeling methods (immersion: 300 and 500 μmol/L; intraperitoneal injection: 8 and 16 mg/kg; intravitreal injection: 0.1 and 0.5 mol/L). MK-801 (M107, Sigma, United States) was soluble in 500 mL/L ethanol (intraperitoneal injection: 3 mg/kg, intravitreal injection: 0.05 mol/L). Resveratrol (R5010, Sigma, United States) was dissolved in 1000 mL/L ethanol and kept in the dark during storage and during the whole experiment (40 mg/L). Distilled water was used to dissolve MS-222 (A5040, Sigma, United States) (0.2 g/L). The timeline of drug delivery is shown in Figure 1.
Immersion: Before NMDA treatment, zebrafish were soaked in resveratrol solution for 1 d in advance. Resveratrol was dissolved in 10 μL of 1000 mL/L ethanol, and then mixed with 100 mL of distilled water for 1 h until completely dissolved. During resveratrol treatment, the reaction tank was completely covered to avoid photodegradation of the compound. Then adult zebrafish (n = 6 in each group) were immersed in 500 μmol/L NMDA solution for 1 h (the solution was prepared at 28°C) and seizure-like behaviors were observed for 1 h. Another two groups of zebrafish, 12 in total without resveratrol immersion were separately placed into the other two 2 L tanks, respectively, filled with 300 μmol/L and 500 μmol/L concentration of NMDA for 1 h to record behavioral changes. Zebrafish in the MK-801 group were intraperitoneally injected with 10 μL MK-801 1 h before 500 μmol/L NMDA immersion. The remaining zebrafish were set as the control group, and except for drug treatment, the rest of the process was the same as the experimental groups.
Intravitreal injection: Zebrafish were anesthetized with MS-222 before intravitreal injection. According to previous research methods, the volume of the vitreous cavity measured by digital caliper is approximately 200-500 nL[36]. In the preliminary experiment to determine the appropriate amount of injection, we found that 100 nL PBS did not cause any retinal damage or behavior changes in zebrafish. The freshly prepared 100 nL of 0.1 and 0.5 mol/L NMDA solution was then aspirated with an acupuncture needle (0.20 mm) and inserted through a small incision between the vitreous body and the retina and delivered into the right eyes of zebrafish. The syringe pumps (HARVARD, C-14171) helped to deliver the appropriate amount of NMDA. The drug treatment time was 1 h. For the resveratrol + NMDA group, these zebrafish were treated with resveratrol for 1 d before 0.5 mol/L NMDA injection. In the 0.5 mol/L NMDA + MK-801 group, 100 nL MK-801 was injected intravitreally. 1× PBS injection was performed in the same manner to the control group. After the injection, all the fish were kept out for at least 1 min to allow for drug absorption and then returned to their normal living environment to record their behaviors.
Intraperitoneal injection: Each zebrafish was weighed before the injection and then adult zebrafish were anesthetized with MS-222 solution. NMDA 8 mg/kg and 16 mg/kg (the doses used here were chosen by comparing those used in rodents) was carefully injected into the abdomen in the operating area when the zebrafish temporarily lost body control. For the resveratrol + NMDA group, these zebrafish were treated with resveratrol for 1 d before the NMDA injection. Next, 10 μL MK-801 was injected intraperitoneally in the NMDA+MK-801 group. The remaining fish were injected with 1× PBS and set as the control group. Their 1 h behaviors were recorded via camera.
Following intravitreal and intraperitoneal injection, the zebrafish were individually placed into 2 L tanks after NMDA treatment; for immersion, the fish were directly placed in 2 L NMDA solution. We manipulated a specific camera to monitor the behavior of each fish to determine its seizure score. The behaviors of all zebrafish were photographed for 60 min to assess the degree of epilepsy. The seizure score was quantified by the following criteria: 1 point, immobility and hyperventilation; 2 points, whirlpool swimming; 3 points, rapid movement from right to left; 4 points, abnormal and spastic muscle contraction; 5 points, rapid clonic convulsion of the whole body; 6 points, submergence and spasm for several minutes; 7 points, death[25].
Statistical analysis was performed based on records to calculate time points and duration of abnormal behavior in zebrafish. The experimental data were expressed as mean ± SE. All data were analyzed by t-test using GraphPad PRISM 7.00. ANOVA was then performed to assess the differences in seizure and latency between the experimental groups, with P < 0.05 considered statistically significant.
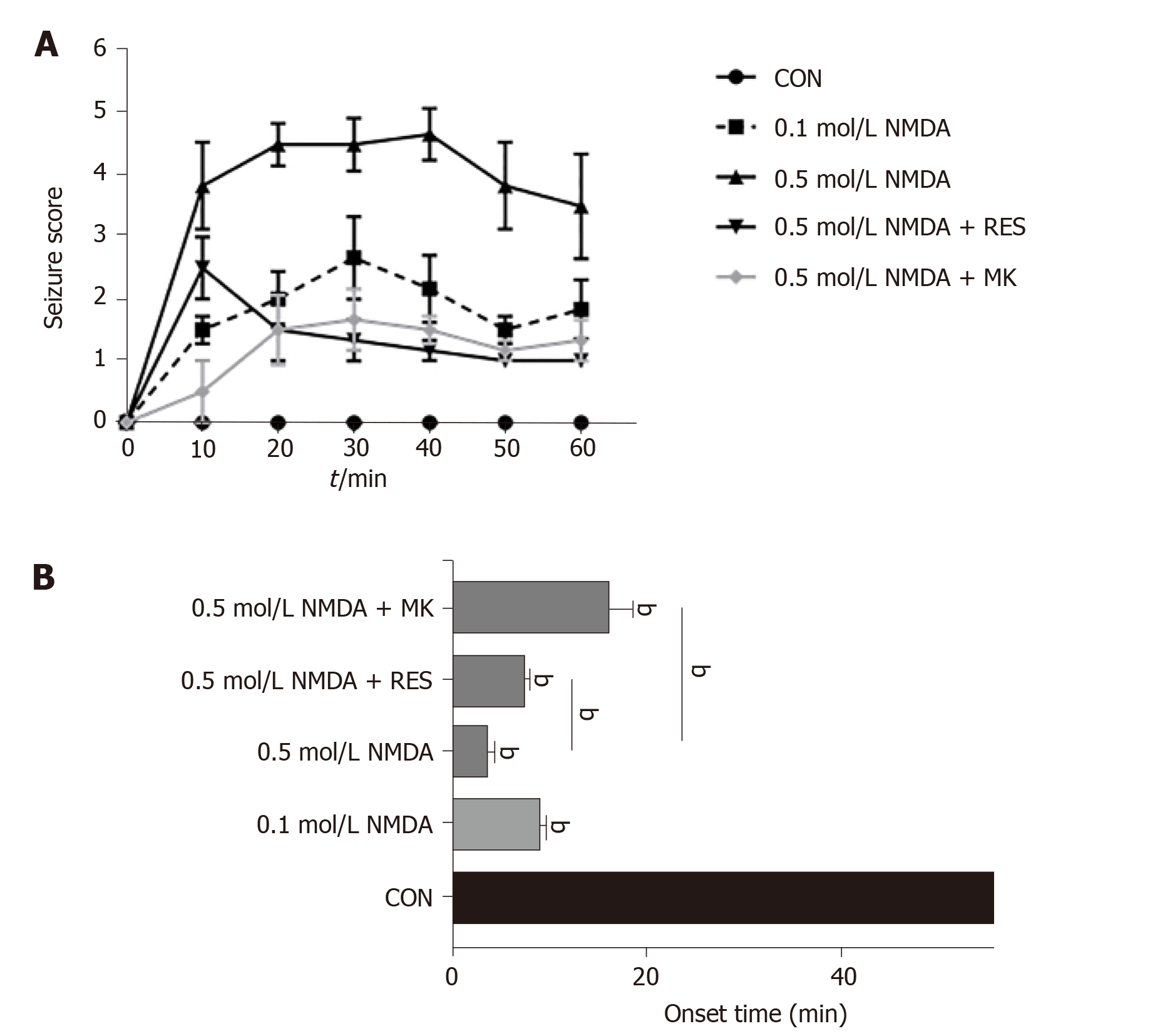
Prior to NMDA treatment, zebrafish were immersed in 40 mg/L resveratrol for 1 d. Zebrafish behavior was then observed and recorded for 60 min after intravitreal injection of NMDA and graded by the seizure score. NMDA treatment caused a seizure-like syndrome characterized by rapid movement, jumping, swimming in circles, and an intense response to stimulation. As shown in Figure 2A, the overall curve presented a trend of an intial rise and then a decrease, and the highest seizure score was approximately up to 5. The mean seizure score in the high-dose (0.5 mol/L) NMDA treatment group was significantly higher than that in the low-dose (0.1 mol/L) NMDA treatment group (4 vs 2, P < 0.0001), while co-injection of 50 mmol/L MK-801 with 100 nL 0.5 mol/L NMDA decreased the score to lower than 2. The shortest seizure onset time in zebrafish given intravitreal injection was about 3 min in the high-dose NMDA group. Additionally, the seizure onset time following high-dose NMDA treatment was significantly prolonged by MK-801 (> 10 min), to a level even lower than that of low-dose (0.1 mol/L) NMDA treatment (Figure 2B). Pretreatment with resveratrol also lowered the seizure score from approximately 4 to 1 and significantly delayed seizure onset from 3 to around 7 min (P = 0.0024) (Figure 2A and B). These behavioral changes indicate that intravitreal injection of NMDA leads to seizure-like behavior in zebrafish within a short time that can be significantly prevented by resveratrol pretreatment, consistent with prior studies showing the anti-epileptic effects of resveratrol.
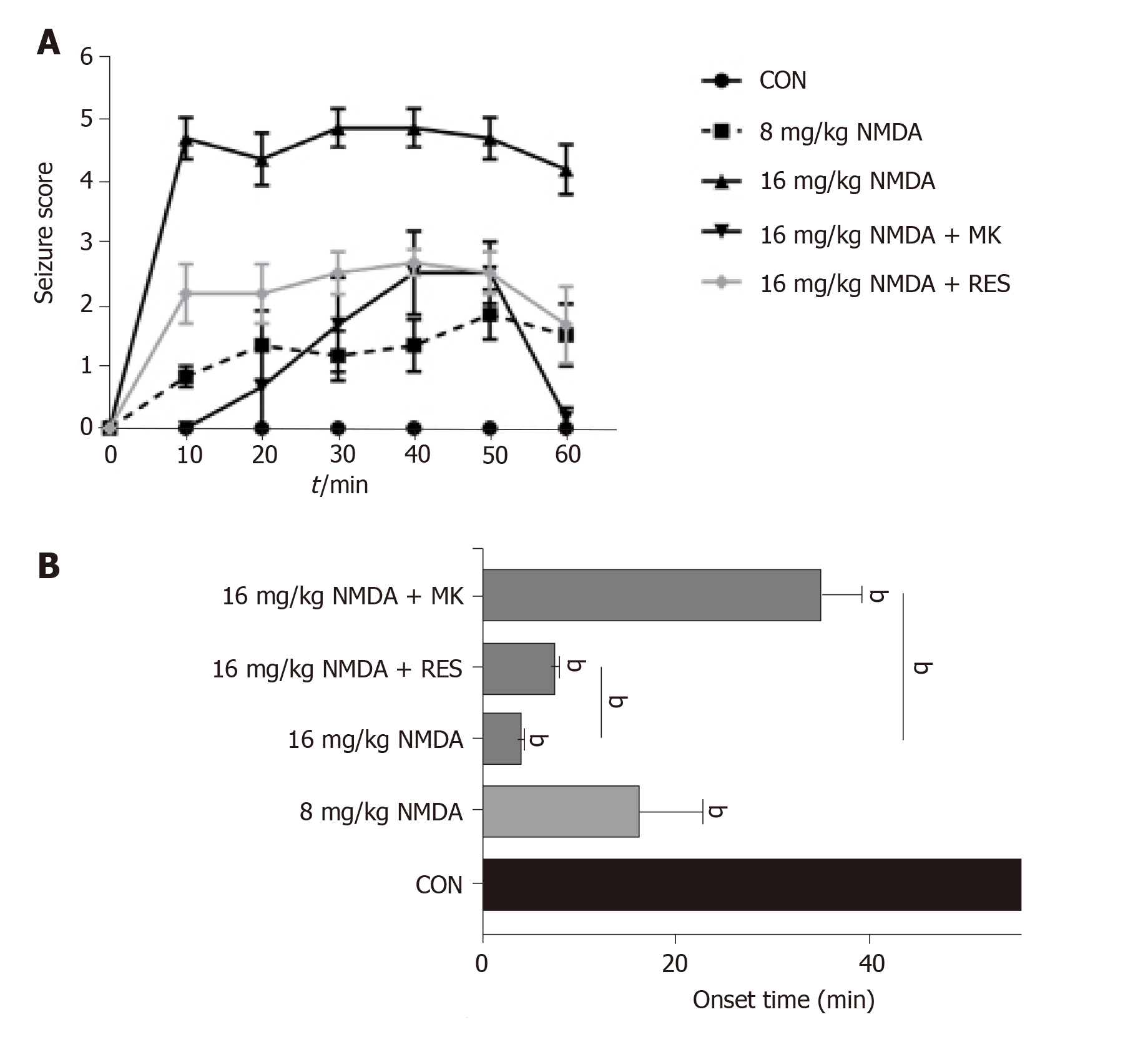
For resveratrol pretreatment, we immersed zebrafish in 50 mg/kg resveratrol for 1 d and then performed intraperitoneal injection of NMDA. Zebrafish behaviors were recorded for 60 min, and were similar to those following intravitreal injection of NMDA. According to the analysis of seizure score, the overall curve first increased within 30 min and then decreased following intraperitoneal injection. The degree of epilepsy in zebrafish injected with high dose NMDA was significantly higher than that in zebrafish injected with low dose NMDA. The seizure score in the high-dose (16 mg/kg) NMDA group was 4-5, while the low-dose (8 mg/kg) group had a score of around 2 (Figure 3A). The same trend in seizure onset time was identified: 16 mg/kg NMDA-treated zebrafish had a seizure onset time of less than 10 min while the 8 mg/kg NMDA-treated group had a seizure onset time closer to 20 min (Figure 3B). On the other hand, zebrafish treated intraperitoneally with 10 μL 3 mg/kg MK-801 + high-dose NMDA, had a significantly reduced seizure score at all time points analyzed (P < 0.001) (Figure 3A) as well as a very significantly delayed seizure onset time (from approximately 6 min to over 30 min) (P < 0.001) (Figure 3B). Resveratrol pretreatment also had a highly significant effect in lowering the seizure score from approximately 5 to 2 min (Figure 3A) and delayed seizure onset time from around 4 min to about 8 min (P = 0.0024) (Figure 3B). These data indicate that seizures can be more intensely induced in zebrafish by intraperitoneal injection of NMDA as well as intravitreal injection and that MK-801 and resveratrol have anti-epileptic effects in this model system.
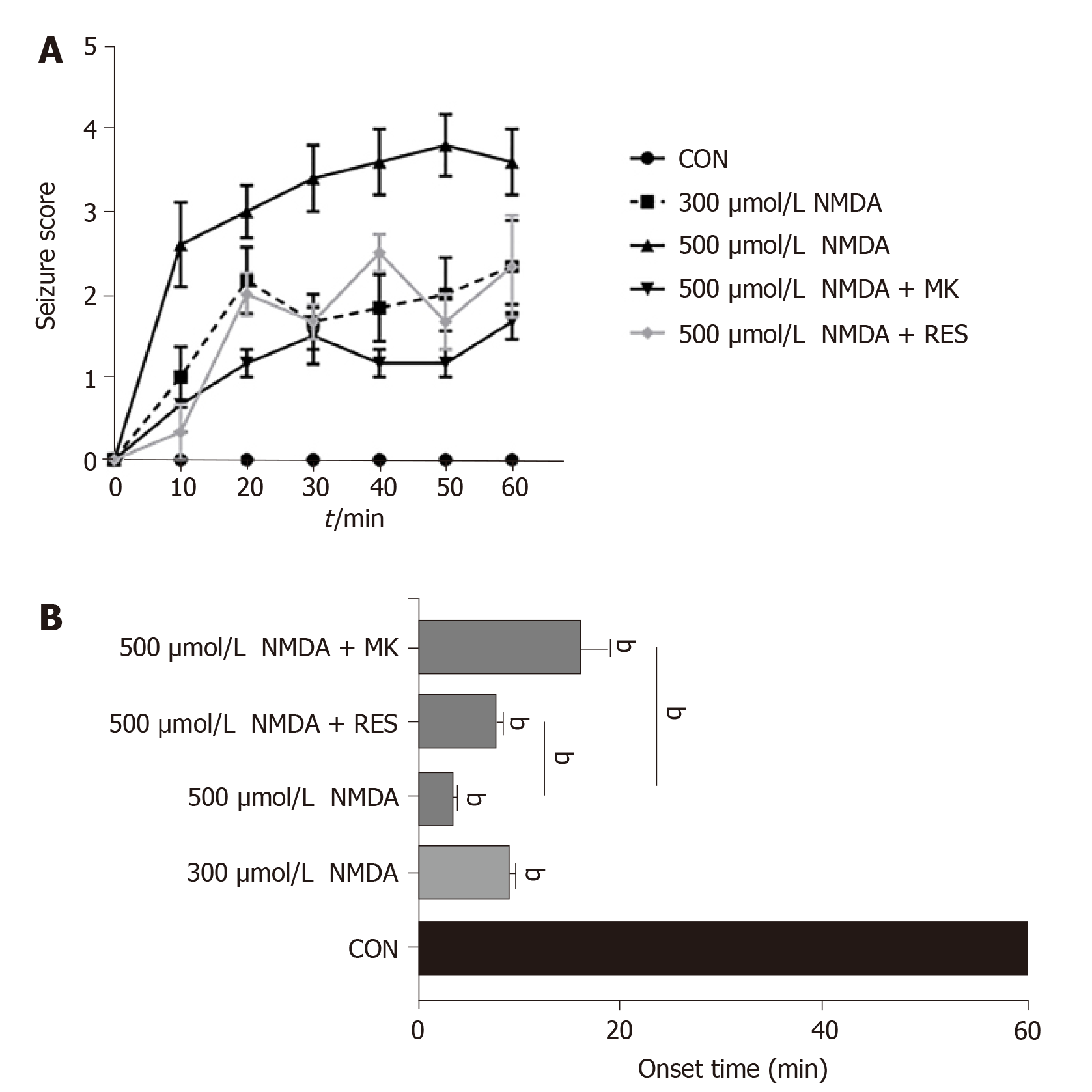
Zebrafish in one experimental group were placed into 2 L tanks with 40 mg/L resveratrol for 1 d before NMDA immersion. By recording zebrafish behaviors over 60 min, we found that different groups almost showed an increasing trend. Seizure scores continued to rise steadily within 0-20 min and 30-50 min. In addition, seizure scores following high-dose NMDA immersion were statistically higher than those following low-dose NMDA immersion. High-dose (500 μmol/L) NMDA treatment resulted in a seizure score of 2-4 while low-dose (300 μmol/L) treatment resulted in a score of around 1 to 2 (Figure 4A). The same trend was identified for seizure onset time: The 500 μmol/L NMDA-treated group had a seizure onset time of fewer than 5 min, while the 300 μmol/L NMDA-treated group had a seizure onset time of nearly 10 min (Figure 4B). On the other hand, zebrafish immersed in high-dose NMDA after 10 μL 3 mg/kg MK-801, had a significantly reduced seizure score at all time points analyzed (P < 0.001) (Figure 4A) as well as a delayed seizure onset time (from approximately 5 min to 9 min) (P < 0.001) (Figure 4B). Resveratrol pretreatment also had a highly significant effect in lowering the seizure score from approximately 4 to 1 (Figure 4A) and delayed seizure onset time from around 5 min to about 10 min (P = 0.0024) (Figure 4B). These data indicate that seizures can be induced in zebrafish by immersion in NMDA in a slow and unremitting way and that MK-801 and resveratrol have anti-epileptic effects in this model system.
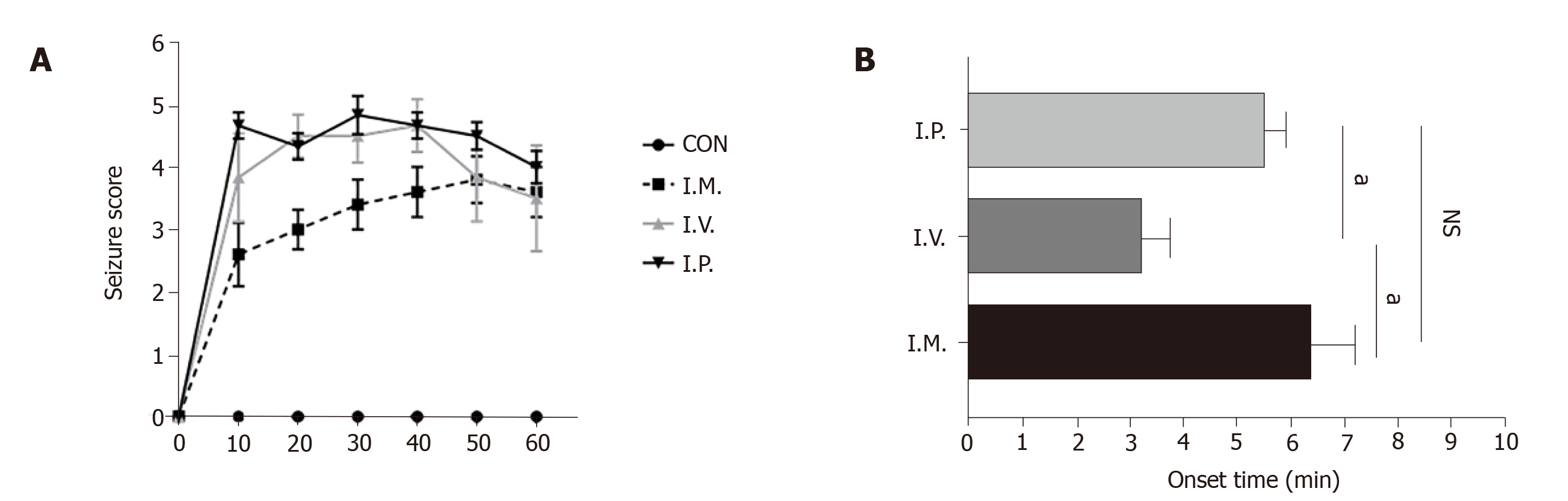
By comparing 1 h seizure scores and onset time of the three administration modes, we found high-dose NMDA immersion maintained the seizure score at about 2.5 to 3.5, the score caused by intraperitoneal injection was 4 to 5, and that following intravitreal injection was maintained at approximately 3.2 to 4.8 (Figure 5A). The trend in epilepsy caused by the three modes of administration generally increased initially and then decreased. In addition, the most severe stage of epilepsy caused by intraperitoneal injection of high-dose NMDA took about 30 min, while intravitreal injection took 40 min and immersion took 50 min. With regard to seizure onset time, immersion had the longest onset time of the three methods (approximately 6.3 min), which was followed by intraperitoneal injection (5.5 min). Intravitreal injection induced seizure-like behaviors within 3 min (P < 0.001) (Figure 5B). These data show that NMDA induces epilepsy-like behavior, while administration patterns alter seizure progression of epilepsy diversely.
Numerous animal models have been used in epilepsy research. However, choosing the best experimental model mainly depends on the problem to be solved, the type of epilepsy simulated, whether it is consistent with the clinical characteristics, and whether it is simple and reliable. Therefore, selecting an appropriate and valuable animal model of epilepsy is undoubtedly an effective shortcut to better study the mechanism and treatment of epilepsy. Our preliminary experiment showed that intravitreal injection, intraperitoneal injection, and immersion all caused seizure-like behaviors in zebrafish. By using different NMDA concentrations, we utilized these three methods of drug delivery to establish seizure models associated with brain damage, which are also less harmful to adult zebrafish.
It has been reported that intravitreal injection of NMDA induces seizure-like behaviors in zebrafish. We found that intravitreal injection of NMDA was the most suitable for the establishment of an acute seizure model in zebrafish, as it caused seizure-like manifestations in a short time with a high concentration of NMDA in the central nervous system, which greatly reduces drug waste and shortens the time to disease. Intraperitoneal injection of NMDA did cause seizure-like behavioral changes in adult zebrafish, which was similar to analogous seizure models in rodents[37,38]. The effect caused by this method depends on the amount of NMDA injected due to strong intestinal absorptive capacity. When comparing the three modeling methods, immersion was less harmful to zebrafish in a short period of time and hence contributes to observing long-term and chronic epileptic behavior. As the drug was directly dissolved in water, and zebrafish can continuously take in the drug from the surrounding environment, the efficacy or toxicity of drugs is not observed in mammals and can be observed using this methd in a short time.
However, these three methods still have some limitations. Although intravitreal injection of NMDA exerts the same effect on zebrafish as other animal models, the difficulty of microinjection may hinder its application in drug screening. Therefore, to improve the potential of this model system, it is necessary to perfect each detailed procedure. In addition, the ocular pharmacokinetics of different drugs are sensitive to different clearance mechanisms[39], which is mainly affected by the permeability of retinal pigment epithelium. Understanding the clearance mechanism of different drugs in zebrafish will ensure the effectiveness of drug delivery, which provides a basis for ideal animal modeling and further drug screening. Intraperitoneal administration is relatively easy, but it is worth noting that the procedure demands care in order to avoid injury to zebrafish organs such as the heart. With respect to the operational skills, immersion requires only control of the drug concentration.
According to the results of induced behavior changes, all three delivery modes were effective in delivering NMDA to the brain. Given that immersion lasts longer and takes effect later, the effects induced by intraperitoneal and intravitreal injection of NMDA on zebrafish appeared earlier. We hypothesized that intraperitoneal and vitreous injection would cause a sharp increase in NMDA concentration in the blood and target cells, resulting in acute pathophysiological changes, but NMDA concentration would then drop rapidly to baseline levels, and thus the dramatic response would quickly disappear. Of these three administration modes, the seizures induced by intravitreal injection appeared faster than the other two modes, which suggests that different administration routes into the zebrafish capillary network may have distinct effects of drug delivery. Intraperitoneal injection of NMDA has poor target specificity, rendering it hard to accumulate in the brain, and this drawback may cause uncontrollable damage to other non-targeted organs, such as the heart, which can interfere with the experiment results. In contrast, immersion can result in a relatively steady increase in NMDA concentration in the blood, which is enough to cause a long-term epileptic response; therefore, is more suitable for the model of sustained epilepsy. Unfortunately, due to the late onset time of seizures induced by drug immersion, it is not suitable for large-scale drug screening. At the operational level, immersion is the simplest and most convenient way to administer drugs, while intravitreal injection is relatively complicated but also the most effective way to establish the zebrafish model of brain disorder.
Antisense morpholine oligonucleotides (MOs) and hyperthermia have been used to construct zebrafish epilepsy models in previous studies. Although MOs can effectively interfere with protein synthesis of target genes, it can induce p53-dependent apoptosis and non-targeted cell-specific effects in gene expression, which in turn affect behavioral phenotype analysis[40]. The hyperthermia-induced zebrafish seizure model is more suitable for studying the mechanism of epileptic seizures in vivo and for acute seizure of chronic processes, but it does not show any persistence[41]. Both methods are appropriate for studying the mechanism of zebrafish seizures during innate or embryonic development. However, the methods we use can be applied to study the process of seizures in adulthood. Not only can they induce characteristic seizures which are similar to the reactions observed in mammalian seizures, but also emphasize the role of the zebrafish model in glutamate excitatory neurotransmission. For example, clomizole (a histamine receptor antagonist) is effective for gene-induced epilepsy of SCN1lab zebrafish (a model of Dravet syndrome caused by SCN1a mutation), a persistent drug-resistant epilepsy[42]. In addition, the methods we proposed can screen out the effect of psychotropic drugs and toxicity in the animal at a glance and reduce twists and turns in the drug development process.
It is noteworthy that one main defect in this NMDA-induced neurotoxicity model is that it only focuses on a single pathological mechanism (glutamate excitotoxicity) of epileptic seizures. Considering that the pathogenesis of human epilepsy is more complex, these models may not fully represent the pathogenesis of epilepsy; thus, may not be used to carry out clinical research on effective treatment methods for epilepsy[43]. In the current study, we only focused on seizure-like behavioral changes but did not carry out a specific analysis of pathological brain alterations or distortion of electrical signal transduction caused by excessive glutamate signaling. Therefore, further research is needed to fully establish the intravitreal administration route as a relevant model of epilepsy and other brain diseases. Although zebrafish seizure models are valuable for discovering anticonvulsants and studying ictogenesis, they are inadequate for the entire disease process. When studying epilepsy and screening anti-epileptic drugs, there is still a lack of epilepsy models that truly reflect the pathogenesis and characteristics of different forms of human epilepsy.
In conclusion, these results show that intravitreal injection of NMDA is an effective model for inducing acute epilepsy in zebrafish, and NMDA immersion can be used as a suitable model for persistent epilepsy. Additionally, intravitreal and intraperitoneal injection of NMDA may both be useful for modeling epilepsy. By comparing the three different drug administration patterns comprehensively, these models are valuable for identifying the potential mechanisms of epilepsy and drug screening. Last but not the least, our study provides convincing evidence for the potential application of MK-801 and resveratrol, a safe plant extract which is available for the treatment of epilepsy.
Epilepsy is a complex neurological disorder characterized by recurrent, unprovoked seizures resulting from the sudden abnormal discharge of brain neurons. It leads to transient brain dysfunction, manifested by abnormal physical movements and consciousness. It can occur at any age, affecting approximately 65 million worldwide, one third of which are still estimated to suffer from refractory seizures. The molecular mechanism of epilepsy is still not fully understood; thus, there is a lack of effective clinical treatment. Therefore, building relevant preclinical models is imperative for screening therapeutics for this disease.
There is an urgent need for further establishment of seizure models in animals, including acute epilepsy models and persistent epilepsy models. These models could be used to study the mechanism of epilepsy and identify novel anti-epileptic therapeutics in the future.
The main objective was to compare three administration modes for establishing a seizure model caused by N-Methyl-D-aspartic acid (NMDA) in zebrafish.
Three administration modes of NMDA, including immersion, intravitreal injection and intraperitoneal injection, were compared with regard to their effects on inducing seizure-like behaviors in adult zebrafish. We evaluated neurotoxicity by observing behavioral changes in zebrafish and graded those behaviors with a seizure score. Statistical analysis was performed based on records to calculate time points and duration of abnormal behavior in zebrafish. All data were analyzed by t-test using GraphPad PRISM 7.00. Analysis of variance was then performed to assess the differences in seizure and latency between experimental groups.
The three NMDA-administration methods triggered different patterns of the epileptic process in adult zebrafish. Seizure scores were increased after increasing NMDA concentration regardless of the mode of administration. However, the curve of immersion continuously rose to a high plateau (after 50 min), while the curves of intravitreal injection and intraperitoneal injection showed a spike in the early stage (10-20 min) followed by a steady decrease in seizure scores. Furthermore, pretreatment with resveratrol and MK-801 significantly delayed seizure onset time and lowered seizure scores.
Intravitreal injection of NMDA was the most suitable route for establishing an acute epileptic model in zebrafish, while immersion with NMDA may be an appropriate method for inducing persistent seizures. Additionally, MK-801 and resveratrol showed strong anti-epileptic effects; thus, both of them may be clinically valuable treatments for epilepsy.
Further study using our models to perform antiepileptic drug screening is necessary, and further work is needed to explore the mechanism of resveratrol against epilepsy.
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ng QX S-Editor: Dou Y L-Editor: Webster JR E-Editor: Wu YXJ
| 1. | Liao M, Kundap U, Rosch RE, Burrows DRW, Meyer MP, Ouled Amar Bencheikh B, Cossette P, Samarut É. Targeted knockout of GABA-A receptor gamma 2 subunit provokes transient light-induced reflex seizures in zebrafish larvae. Dis Model Mech. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. WHO’s first Global Epilepsy Report. Available from: https://www.who.int/en/news-room/fact-sheets/detail/epilepsy. |
| 3. | Canzian J, Müller TE, Franscescon F, Michelotti P, Fontana BD, Costa FV, Rosemberg DB. Modeling psychiatric comorbid symptoms of epileptic seizures in zebrafish. J Psychiatr Res. 2019;119:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Wang Z, Luo Z, Li S, Luo Z, Wang Z. Anxiety screening tools in people with epilepsy: A systematic review of validated tools. Epilepsy Behav. 2019;99:106392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Jin M, Zhang B, Sun Y, Zhang S, Li X, Sik A, Bai Y, Zheng X, Liu K. Involvement of peroxisome proliferator-activated receptor γ in anticonvulsant activity of α-asaronol against pentylenetetrazole-induced seizures in zebrafish. Neuropharmacology. 2020;162:107760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 700] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | Rosenberg EC, Patra PH, Whalley BJ. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 2017;70:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 8. | Alsharafi WA, Xiao B, Li J. MicroRNA-139-5p negatively regulates NR2A-containing NMDA receptor in the rat pilocarpine model and patients with temporal lobe epilepsy. Epilepsia. 2016;57:1931-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Hui Yin Y, Ahmad N, Makmor-Bakry M. Pathogenesis of epilepsy: challenges in animal models. Iran J Basic Med Sci. 2013;16:1119-1132. [PubMed] |
| 10. | Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 328] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 11. | Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5 Suppl:1039-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 363] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 659] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 13. | Davies SJ, Burhan AM, Kim D, Gerretsen P, Graff-Guerrero A, Woo VL, Kumar S, Colman S, Pollock BG, Mulsant BH, Rajji TK. Sequential drug treatment algorithm for agitation and aggression in Alzheimer's and mixed dementia. J Psychopharmacol. 2018;32:509-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Toscano CD, Kingsley PJ, Marnett LJ, Bosetti F. NMDA-induced seizure intensity is enhanced in COX-2 deficient mice. Neurotoxicology. 2008;29:1114-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Luo ZW, Wang HT, Wang N, Sheng WW, Jin M, Lu Y, Bai YJ, Zou SQ, Pang YL, Xu H, Zhang X. Establishment of an adult zebrafish model of retinal neurodegeneration induced by NMDA. Int J Ophthalmol. 2019;12:1250-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Soriano FX, Papadia S, Hofmann F, Hardingham NR, Bading H, Hardingham GE. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J Neurosci. 2006;26:4509-4518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, Lichnerova K, Cerny J, Krusek J, Dittert I, Horak M, Vyklicky L. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63 Suppl 1:S191-S203. [PubMed] |
| 18. | Bibliowicz J, Tittle RK, Gross JM. Toward a better understanding of human eye disease insights from the zebrafish, Danio rerio. Prog Mol Biol Transl Sci. 2011;100:287-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Barbazuk WB, Korf I, Kadavi C, Heyen J, Tate S, Wun E, Bedell JA, McPherson JD, Johnson SL. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000;10:1351-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 462] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 20. | Chakraborty C, Hsu CH, Wen ZH, Lin CS, Agoramoorthy G. Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab. 2009;10:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther. 2007;82:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 312] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 22. | Bouhenni RA, Dunmire J, Sewell A, Edward DP. Animal models of glaucoma. J Biomed Biotechnol. 2012;2012:692609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Schirmer H, Pereira TC, Rico EP, Rosemberg DB, Bonan CD, Bogo MR, Souto AA. Modulatory effect of resveratrol on SIRT1, SIRT3, SIRT4, PGC1α and NAMPT gene expression profiles in wild-type adult zebrafish liver. Mol Biol Rep. 2012;39:3281-3289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Torres-Hernández BA, Del Valle-Mojica LM, Ortíz JG. Valerenic acid and Valeriana officinalis extracts delay onset of Pentylenetetrazole (PTZ)-Induced seizures in adult Danio rerio (Zebrafish). BMC Complement Altern Med. 2015;15:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Alfaro JM, Ripoll-Gómez J, Burgos JS. Kainate administered to adult zebrafish causes seizures similar to those in rodent models. Eur J Neurosci. 2011;33:1252-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Menezes FP, Rico EP, Da Silva RS. Tolerance to seizure induced by kainic acid is produced in a specific period of zebrafish development. Prog Neuropsychopharmacol Biol Psychiatry. 2014;55:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 28. | McGinn TE, Mitchell DM, Meighan PC, Partington N, Leoni DC, Jenkins CE, Varnum MD, Stenkamp DL. Restoration of Dendritic Complexity, Functional Connectivity, and Diversity of Regenerated Retinal Bipolar Neurons in Adult Zebrafish. J Neurosci. 2018;38:120-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Szkudelski T, Szkudelska K. Resveratrol and diabetes: from animal to human studies. Biochim Biophys Acta. 2015;1852:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 30. | Yar AS, Menevse S, Alp E, Helvacioglu F, Take G. The effects of resveratrol on cyclooxygenase-1 and cyclooxygenase-2 mRNA and protein levels in diabetic rat kidneys. Mol Biol Rep. 2010;37:2323-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Marques FZ, Markus MA, Morris BJ. Resveratrol: cellular actions of a potent natural chemical that confers a diversity of health benefits. Int J Biochem Cell Biol. 2009;41:2125-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Luo H, Zhou M, Ji K, Zhuang J, Dang W, Fu S, Sun T, Zhang X. Expression of Sirtuins in the Retinal Neurons of Mice, Rats, and Humans. Front Aging Neurosci. 2017;9:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Ethemoglu MS, Seker FB, Akkaya H, Kilic E, Aslan I, Erdogan CS, Yilmaz B. Anticonvulsant activity of resveratrol-loaded liposomes in vivo. Neuroscience. 2017;357:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Shetty AK. Promise of resveratrol for easing status epilepticus and epilepsy. Pharmacol Ther. 2011;131:269-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Wang N, Luo Z, Jin M, Sheng W, Wang HT, Long X, Wu Y, Hu P, Xu H, Zhang X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging (Albany NY). 2019;11:3117-3137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 36. | Reymond L. Spatial visual acuity of the falcon, Falco berigora: a behavioural, optical and anatomical investigation. Vision Res. 1987;27:1859-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Sierra S, Alfaro JM, Sánchez S, Burgos JS. Administration of docosahexaenoic acid before birth and until aging decreases kainate-induced seizures in adult zebrafish. Brain Res Bull. 2012;88:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Yum MS, Lee M, Woo DC, Kim DW, Ko TS, Velíšek L. β-Hydroxybutyrate attenuates NMDA-induced spasms in rats with evidence of neuronal stabilization on MR spectroscopy. Epilepsy Res. 2015;117:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Zhang Y, Bazzazi H, Lima E Silva R, Pandey NB, Green JJ, Campochiaro PA, Popel AS. Three-Dimensional Transport Model for Intravitreal and Suprachoroidal Drug Injection. Invest Ophthalmol Vis Sci. 2018;59:5266-5276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Horie M, Kotani T. Formation of mos RNA granules in the zebrafish oocyte that differ from cyclin B1 RNA granules in distribution, density and regulation. Eur J Cell Biol. 2016;95:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Hunt RF, Hortopan GA, Gillespie A, Baraban SC. A novel zebrafish model of hyperthermia-induced seizures reveals a role for TRPV4 channels and NMDA-type glutamate receptors. Exp Neurol. 2012;237:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Baraban SC, Dinday MT, Hortopan GA. Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun. 2013;4:2410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 302] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 43. | Staley K. Molecular mechanisms of epilepsy. Nat Neurosci. 2015;18:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |