Copyright
©The Author(s) 2025.
World J Psychiatry. Sep 19, 2025; 15(9): 107498
Published online Sep 19, 2025. doi: 10.5498/wjp.v15.i9.107498
Published online Sep 19, 2025. doi: 10.5498/wjp.v15.i9.107498
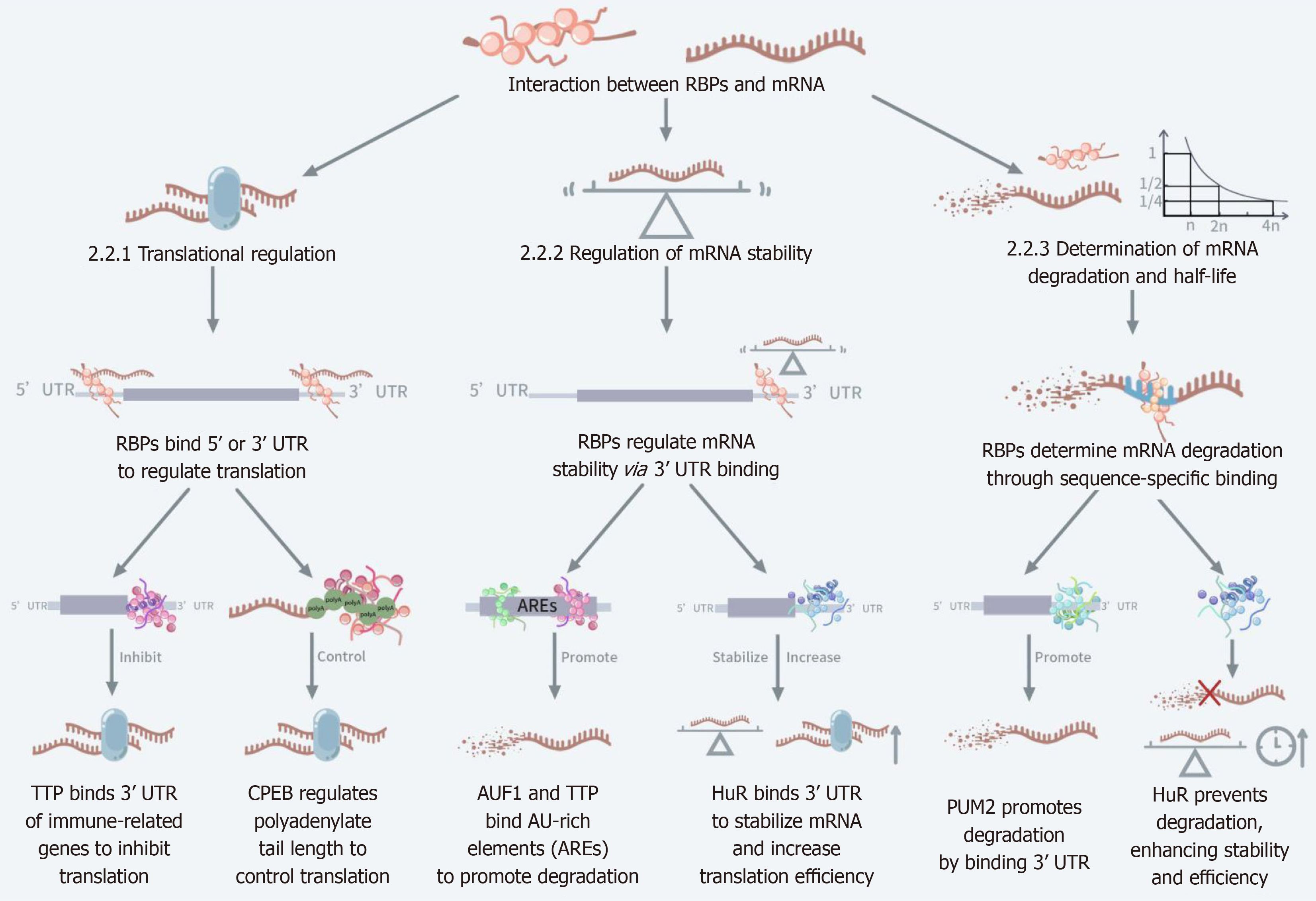
Figure 1 Mechanisms of interaction between RNA binding proteins and mRNA.
This figure illustrates how RNA binding proteins (RBPs) regulate their fate by attaching to different regions of the mRNA. This figure is divided into three main modules: Translational regulation, stability regulation, and regulation of degradation and half-life. In translational regulation, RBPs initiate or inhibit translation by attaching to the 5’ untranslated region (UTR) or 3’ UTR regions of mRNAs, such as tristetraprolin proteins and cytoplasmic polyadenylation element-binding protein proteins. In stability regulation, RBPs regulate mRNA stability by attaching to the 3’ UTR, AU-rich element RNA-binding protein 1 and tristetraprolin bind AU-rich regions to enhance degradation, while human antigen R increases mRNA stability and improves translation efficiency. In the regulation of degradation and half-life, RBPs enhance or prevent mRNA degradation through specific sequence recognition, e.g., Pumilio RNA-binding protein 2 enhances degradation and human antigen R prevents degradation to enhance stability. RBPs: RNA binding proteins; AREs: AU-rich regions; TTP: Tristetraprolin; HuR: Human antigen R; PUM2: Pumilio RNA-binding protein 2.
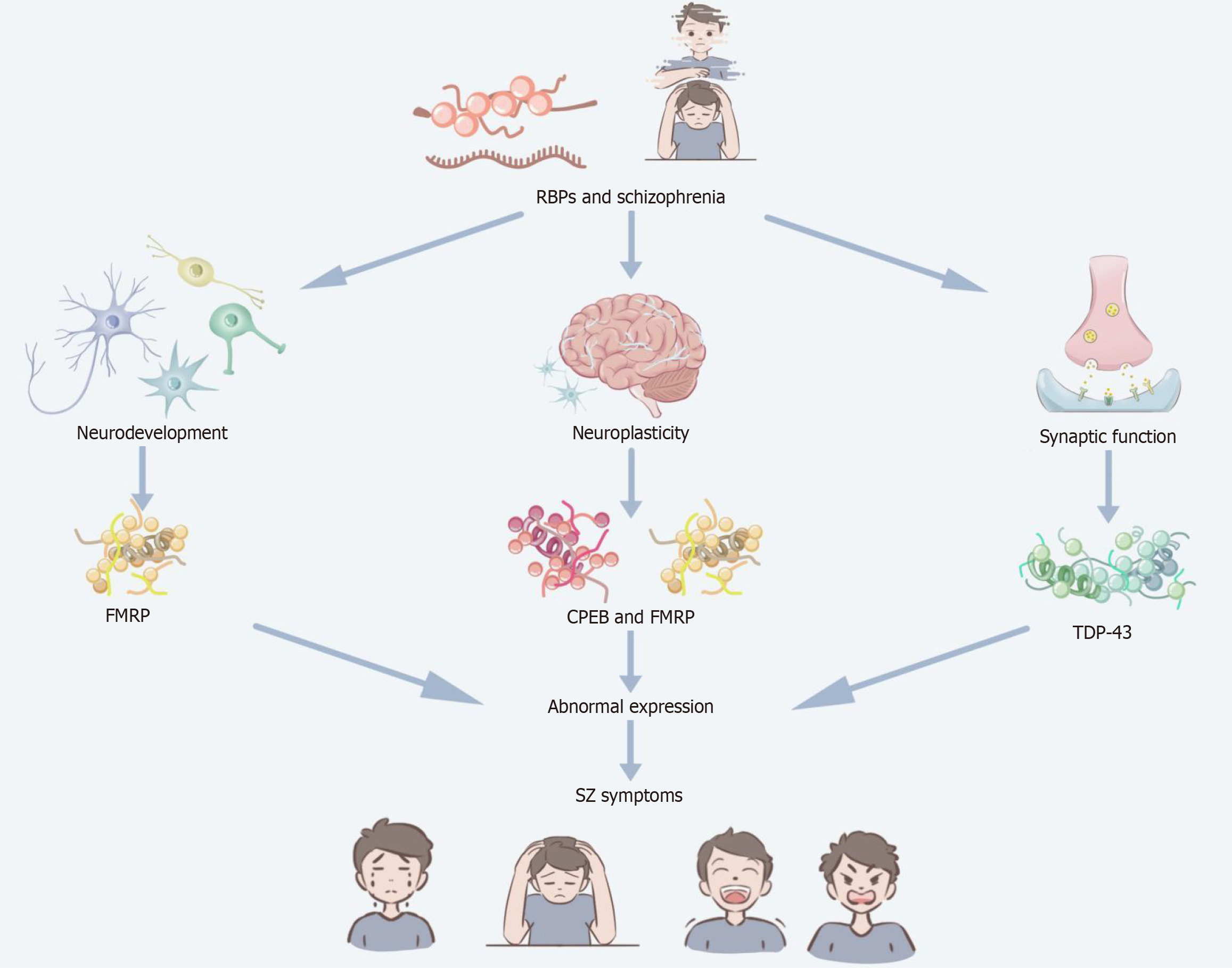
Figure 2 Relationship between RNA binding proteins and schizophrenia.
This figure depicts the relationship between the roles of RNA binding proteins (RBPs) in schizophrenia (SZ), showing how RBPs are associated with SZ symptoms by shaping neurodevelopment, neuroplasticity, and synaptic function. RBPs have been associated with neurodevelopmental abnormalities through their impact on the role of fragile X mental retardation protein (FMRP) in neural differentiation and synapse formation. RBPs (e.g., cytoplasmic polyadenylation element-binding protein and FMRP) regulate synaptic plasticity and have been associated with impaired cognitive and affective functioning in SZ patients. TAR DNA-binding protein 43 in RBPs plays a crucial role in synaptic function and its abnormalities have been associated with SZ. The relationship between abnormal expression of RBPs (especially FMRP, cytoplasmic polyadenylation element-binding protein, and TAR DNA-binding protein 43) and SZ symptoms, such as cognitive impairment and affective dysregulation, is demonstrated below. RBPs: RNA binding proteins; FMRP: Fragile X mental retardation protein; SZ: Schizophrenia; CPEB: Cytoplasmic polyadenylation element-binding protein; TDP-43: TAR DNA-binding protein 43.
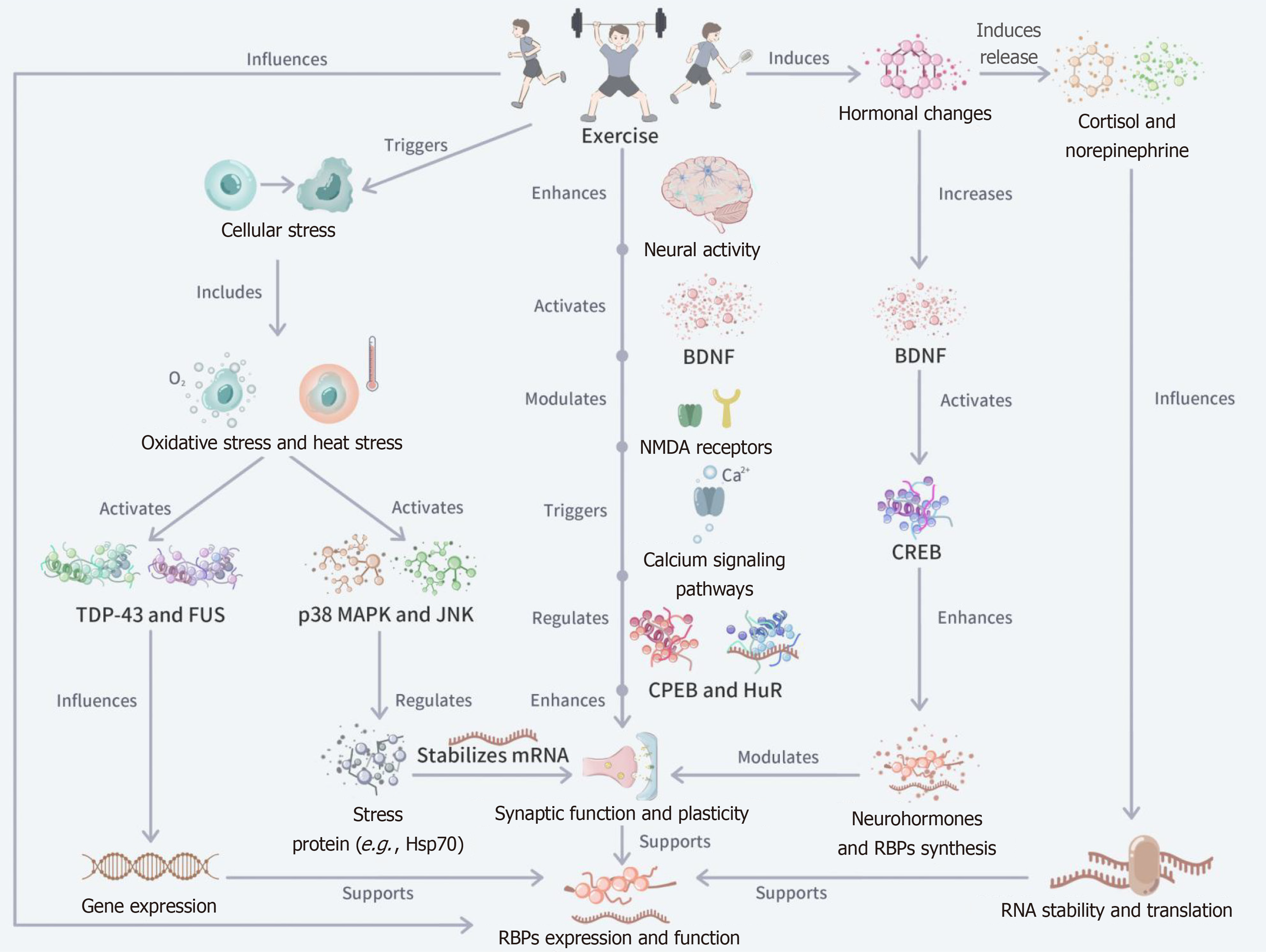
Figure 3 Mechanism of the effect of exercise on the expression and function of RNA binding proteins.
The figure starts with exercise and describes its regulation of RNA binding proteins (RBPs) through three major pathways - neural activity, hormonal changes, and cellular stress. Role of RBPs. In the neural activity pathway, exercise activated brain-derived neurotrophic factor, N-methyl-D-aspartate receptors, and calcium signaling pathways to regulate RBPs such as cytoplasmic polyadenylation element-binding protein and human antigen R to enhance synaptic function and neuroplasticity. The hormonal change pathway reveals that exercise raises the levels of brain-derived neurotrophic factor and stress hormones (e.g., cortisol), which alters RNA stability and translation efficiency. The cellular stress pathway, on the other hand, reveals that exercise-induced oxidative stress and heat stress ultimately support gene expression and adaptation in the nervous system by activating RBPs such as stress proteins (e.g., Hsp70), TAR DNA-binding protein 43, and fused in sarcoma. BDNF: Brain-derived neurotrophic factor; NMDA: N-methyl-D-aspartate; CPEB: Cytoplasmic polyadenylation element-binding protein; HuR: Human antigen R.
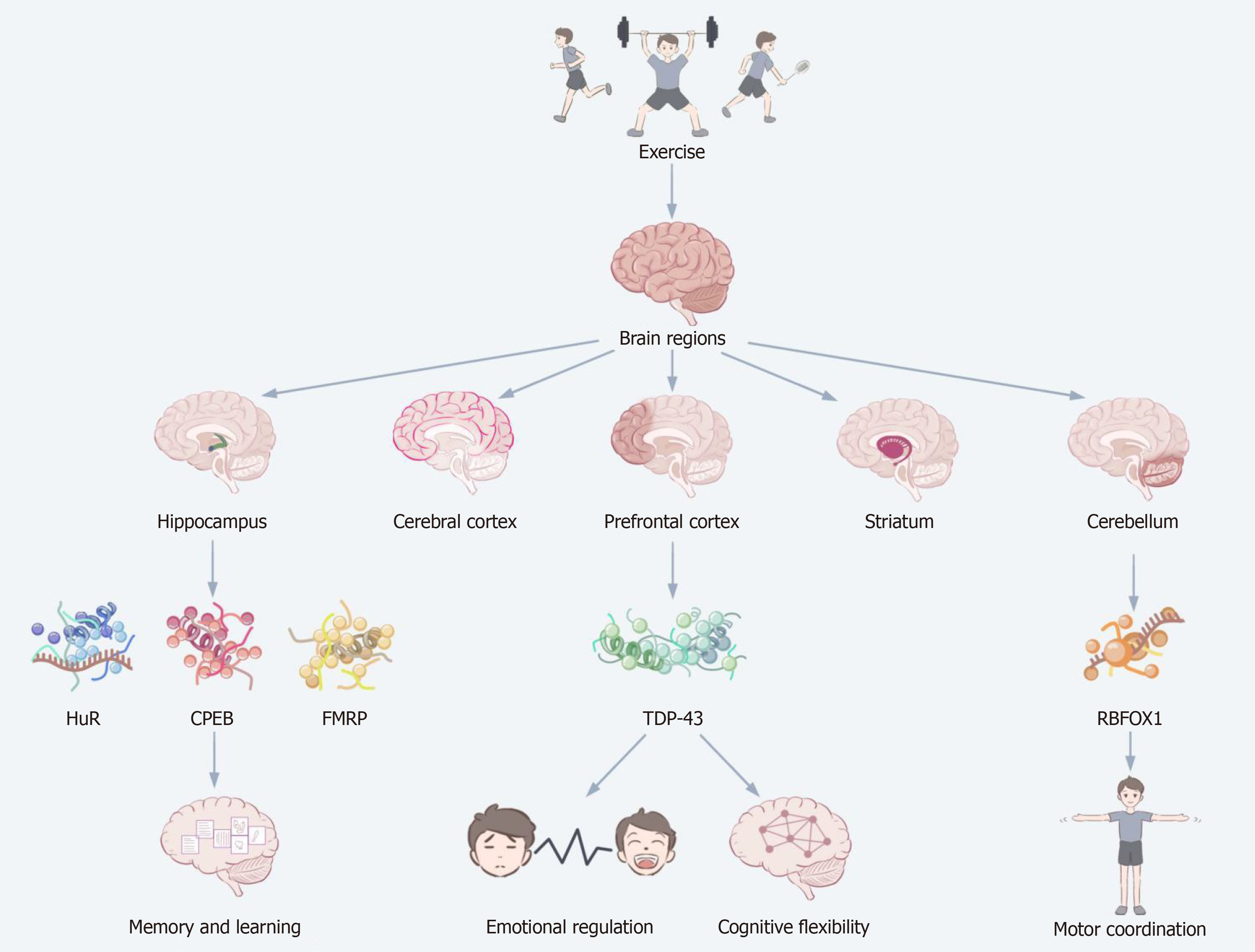
Figure 4 Mechanisms of exercise-induced changes in brain RNA binding proteins.
This figure demonstrates how exercise exerts a multilevel modulatory effect on brain function by shaping RNA binding protein (RBP) expression in different brain regions. Exercise activates multiple brain regions, namely the hippocampus, prefrontal cortex, cerebral cortex, striatum, and cerebellum, with each region responding uniquely to the expression of RBPs. In the hippocampus, enhanced expression of RBPs such as human antigen R, CPEB, and cytoplasmic polyadenylation element-binding protein enhances memory and learning ability; in the prefrontal cortex, the role of TAR DNA-binding protein 43 contributes to emotion regulation and cognitive flexibility; and in the cerebellum, the regulation of RNA binding fox-1 homolog contributes to motor coordination. This figure reveals the region-specific regulation of RBPs in the brain by exercise, which in turn has a profound effect on neural function. HuR: Human antigen R; CPEB: Cytoplasmic polyadenylation element-binding protein; FMRP: Fragile X mental retardation protein; TDP-43: TAR DNA-binding protein 43; RBFOX1: RNA binding fox-1 homolog.
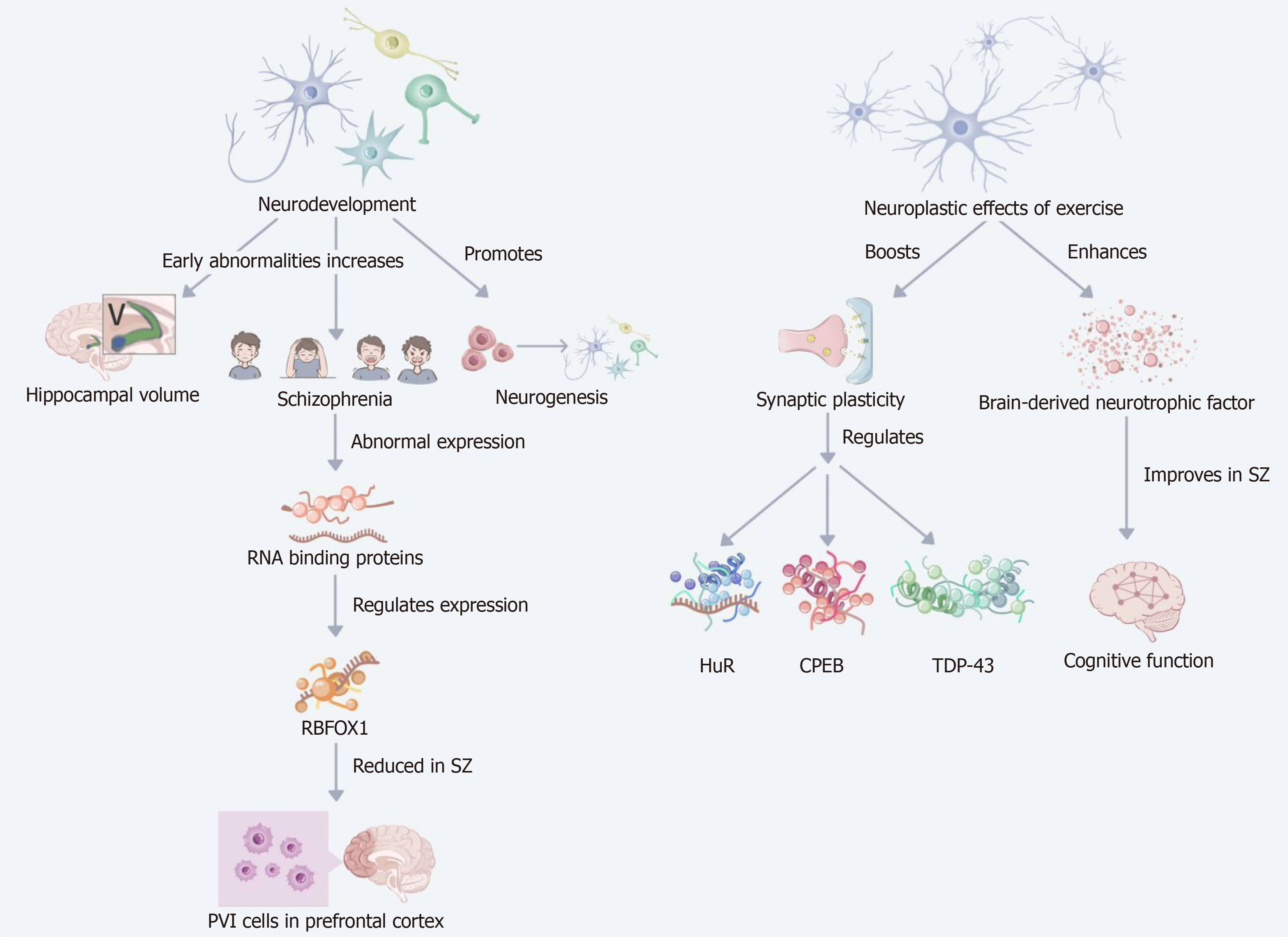
Figure 5 Regulatory mechanisms of RNA binding proteins associated with schizophrenia through exercise.
This figure demonstrates the mechanism of regulation of the expression of RNA binding proteins (RBPs) associated with schizophrenia through exercise. The figure first appears the association between schizophrenia and aberrant expression of RBPs, specifically RNA binding fox-1 homolog expression is decreased in prefrontal cortex PVI cells from schizophrenia patients. Exercise regulates the expression of several RBPs (e.g., human antigen R, cytoplasmic polyadenylation element-binding protein, and TAR DNA-binding protein 43) by enhancing synaptic plasticity and neuroplasticity, thereby restoring normal synaptic transmission function to some extent. In addition, exercise positively alters neurodevelopment by increasing the health of brain structures (e.g., promoting neurogenesis and hippocampal volume increase) and increasing brain-derived neurotrophic factor levels, thereby improving cognitive function and emotional stability in SZ patients. SZ: Schizophrenia; HuR: Human antigen R; CPEB: Cytoplasmic polyadenylation element-binding protein; TDP-43: TAR DNa-binding protein 43; RBFOX1: RNA binding fox-1 homolog.
- Citation: Lu Y, Kong JD, Zhao LN. Role of RNA-binding proteins in exercise-induced mRNA regulation: Unveiling biomarkers and therapeutic targets for schizophrenia. World J Psychiatry 2025; 15(9): 107498
- URL: https://www.wjgnet.com/2220-3206/full/v15/i9/107498.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i9.107498