Published online Jun 9, 2015. doi: 10.5497/wjp.v4.i2.227
Peer-review started: October 13, 2014
First decision: December 26, 2014
Revised: January 9, 2015
Accepted: April 8, 2015
Article in press: April 9, 2015
Published online: June 9, 2015
Processing time: 250 Days and 4.6 Hours
AIM: To investigate the efficacy of fu-qi granule (FQG) on carbon tetrachloride (CCl4) induced liver fibrosis in rats and the underlying mechanisms.
METHODS: Sixty rats were randomly divided into six groups: normal control group, CCl4 induced liver fibrosis group, AnluoHuaxianWan group and three treatment groups of FQG. Treatment of rats with intraperitoneal injection of carbon tetrachloride solution at 0.3 mL per 100 g body weigh twice a week for 8 wk. The normal control group the rats were given the media (olive oil) at the same time. In the first 2 wk, rats were raised with feedstuff (80% corn meal, 20% lard, 0.5% cholesterol). Serum samples were collected for alanine transaminase, aspartate aminotransferase, alkaline phosphatase, albumin, total protein assay and typical histopathological changes was observed in Hematoxylin-eosin staining sections. Smooth muscle alpha actin (α-SMA) was analyzed with immunohistochemistry. Mammalian target of rapamycin (mTOR) and hypoxia-inducible factor-1 (HIF-1α) expressions were detected by Western blotting. Tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) and matrix metalloproteinases-9 (MMP-9) were measured with semi-quantitative reverse transcriptase-polymerase chain reaction.
RESULTS: FQG significantly reduced the serum levels of alanine transaminase, aspartate aminotransferase, alkaline phosphatase and increased the serum contents of albumin, total protein in rats with liver fibrosis. Moreover, FQG promoted extracellular matrix degradation by increasing MMP-9 and inhibiting TIMP-1 and α-SMA. mTOR and HIF-1α expression in liver significantly decreased in the rats treated with FQG.
CONCLUSION: The results indicated that FQG significantly reverse fibrosis induced by CCl4, which should be developed as a new and promising preparation for the prevention of liver fibrosis.
Core tip: Fu-qi granule (FQG) is traditional Chinese medicine preparation to prove the antifibrotic properties of traditional Chinese drug composed of six medicinal herbs in rats treated with carbon tetrachloride. we checked activities of liver enzymes, histopathological changes within the liver as well as the expression of mammalian target of rapamycin (mTOR) and hypoxia-inducible factor-1 and tissue inhibitor of matrix metalloproteinases-1 and matrix metalloproteinases-9. FQG can attenuate liver fibrosis induced by carbon tetrachloride via mTOR/p70S6K signal transduction pathway.
- Citation: Zhong L, Sun YL, Shi WL, Ma X, Chen Z, Wang JB, Li RS, Song XA, Liu HH, Zhao YL, Xiao XH. Protective effect of fu-qi granule on carbon tetrachloride-induced liver fibrosis in rats. World J Pharmacol 2015; 4(2): 227-235
- URL: https://www.wjgnet.com/2220-3192/full/v4/i2/227.htm
- DOI: https://dx.doi.org/10.5497/wjp.v4.i2.227
As one of the common response to different kinds of liver injuries, such as autoimmune diseases, metabolic diseases, alcohol abuse, cholestatic liver disease[1], together with hepatitis. Liver fibrosis usually results in hepatic microstructure distortion and liver dysfunction with characterization of extracellular matrix (ECM) overproduction and irregular deposition in liver tissues[2], which seriously threatens human health. Therefore, it could be given a hint that the prevention of the progression from liver injury to liver cirrhosis may due to interruption and reversion of hepatic fibrosis.
Although there are many pathways and targets involving in liver fibrogenesis, however, by now, the mammalian targets of rapamycin (mTOR)/p70S6 kinase (p70S6K) signal pathways are receiving more and more attentions. It is reported that mTOR which were an regarded as an internal evolutionarily hidebound kinase, are taking advantage of controling serine threonine synthesis via phosphorylation of its downstream targets[3]. Being a mitogen-activated protein kinase, the p70S6K is playing an extremely major role in cell life cycle survival, proliferation or regulation[4]. Additionally, mTOR/p70S6K signaling pathways inhibited hepatic stellate cell (HSC) proliferation, which could be as an effective anti-fibrotic strategy[5].
Currently, several agents have showed promising anti-fibrogenesis effect in liver[6]. However, there still seems a long way to apply the agents in the clinical application[7]. Having been used for thousands of years in China, Traditional Chinese medicines (TCM) has been playing a special role in liver diseases treatment from a unique perspective[8]. With good therapeutic effects on liver fibrosis, traditional Chinese medicine has attracted more and more attentions and people are trying to explore new preparation of TCM and investigating the potential mechanisms[9]. Since TCM has specific characteristics with multi-constituents, multi-ways and less side effects, studies on traditional Chinese medicine with anti-fibrosis effects have been shown more important in today[10].
Fu-qi granule (FQG) is a new type with traditional Chinese medicine preparation. It was prepared by the astragalus membranaceus (Fisch.), broussonetia papyrifera (L.), poria cocos (Schw.) and angelica sinensis (Oliv.) via water extraction. Then the extraction was filtrated and the filtration liquid was enriched and dried to powder. The powder was mixed with Equus asinus L and Fermentative cordycepic fungal powder and the mixture was added dextrin to the preparation of FQG. These plants are chosen for the anti-fibrosis agent is because of their pharmacological properties and clinical curative effect is better against liver fibrosis in 302 Military Hospital of China.
Based on the theory of TCM, liver fibrosis is characterized by humidity, fever, blood-stasis, poison, and both gas and yin asthenia[11,12]. Therefore, FQG is used to treat liver fibrosis by clearing heart and damp, removing stasis and toxin in the liver. In modern pharmacy, Astragalus membranaceus and Poria cocos were also widely investigated in liver desease. Astragalosides was a potent chemical ingredient and it can protect acute liver injury and fibrogensis[13]. In addition, in order to compare the anti-fibrotic effecacy of FQG, AnluoHuaxianWan group (ALHXW) was also used as a positive-control drug in the experiment. According to the basic theories of TCM and results of modern pharmacology, the aim of this research is looking into the function of FQG regarding anti-liver fibrosis. Meanwhile, its underlying mechanisms on FQG for liver fibrosis were also investigated.
The composition of FQG included Astragalus membranaceus (Fisch.), Angelica sinensis (Oliv.), Poria cocos (Schw.), Broussonetia papyrifera (L.), Equus asinus L. and Fermentative cordycepic fungal powder. It was prepared by the Astragalus membranaceus (Fisch.), Angelica sinensis (Oliv.), Poria cocos (Schw.) and Broussonetia papyrifera (L.) via water extraction. Then the extraction was filtrated and the filtration liquid was enriched and dried to powder. The powder were mixed with Equus asinus L and Fermentative cordycepic fungal powder and the mixture was added dextrin to the preparation of FQG.
Sixty Sprague-Dawley male rats (180-200 g) were obtained from Experimental Animal Center of Medical Sciences of Chinese People’s Liberation Army (Beijing, China). Animal certificate was SCXK-(Army) 2012-0004.
Rats were housed 5 per cage with food and water ad libitum. All procedures involving animals and their care were following the regulations of the Committee on use of Human and Animal Subjects in Teaching and Research of the 302 Military Hospital of China. A total of 60 rats were randomly divided into six groups: normal control group, CCl4 induced liver fibrosis group, ALHXW and three treatment groups of FQG (n = 10, respectively). Except for the normal control group, all the rats were administered with carbon tetrachloride solution (CCl4 dissolved in olive oil, 5:5, v/v) at 0.3 mL/100 g body weight for the first time by intraperitoneal injection, and then with carbon tetrachloride solution (CCl4 dissolved in olive oil, 3:7, v/v) at 0.3 mL per 100 g body weigh twice a week for 8 wk[14,15]. The normal control group the rats were given the media (olive oil) at the same time. In the first 2 wk, rats were raised with feedstuff (80% corn meal, 20% lard, 0.5% cholesterol)[16].
At the end of the 8th week, the ALHXW was oral administered with ALHXW (2.16 g/kg per day), which used for a positive-control drug. The treatment group was treated with high, medium and low doses of FQG (5.4, 2.7 and 1.35 g/kg per day, respectively) by oral administration. The control group and CCl4 induced liver fibrosis group were given equivalent saline every day for 6 wk. All animals were anesthetized after the last administration. Blood was taken from the inferior vena cava, centrifuged at 3000 r/min, 4 °C, for 10 min, and serum was kept at -20 °C for assay. Liver samples were taken and washed immediately with ice cold saline. Subsequently, the liver was divided into two parts. One was immediately stored at -80 °C for future experiment, and the other one was fixed in 4% formalin solution for histopathologic examination.
Serum levels of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin (ALB) and total protein (TP) were measured by commercially available kits (Mindray bio-medical electronics co, LTD. Shenzhen, China) according to the manufacturer’s instructions.
Left lobe liver tissues were fixed in 4% buffered paraformaldehyde and dehydrated with different graded alcohol series. After paraffin embedded, and cut into 5 μm sections, they were stained with hematoxylin and eosin (HE) for histopathological examination.
The same part of liver in each group was fixed with 4% paraformaldehyde, dehydrated by ethanol gradients, paraffin embedded, sectioned into thickness of 5 microns, and underwent regular dewaxing. Endogenous peroxidase activity was blocked with 3% H2O2. After microwave treatment with 0.1 mol/L citrate buffer and blockage of non-specific antigen with horse serum, rabbit polyclonal antibody against rat Smooth muscle alpha actin (α-SMA) (Calbiochem Biotechnology, San Diego, CA, United States) (1:150 diluted in blocking buffer) was added, followed by overnight incubation at 4 °C. The membrane was washed three times with 0.1% Tween-PBS. Antibody-antigen complexes were detected with DAB as the substrate. An interstitial brown stellate structure was regarded as positive for α-SMA.
The liver tissues were washed with PBS and total cell lysates were prepared by adding cell lysis buffer (50 mmol/L Tris·HCl, pH = 8.0, 150 mmol/L solid acid sodium, 1% TritonX-100). The proteins were separated by electrophoresis on 10% SDS-PAGE gel with Bio-Rad electrophoresis system[17], (Bio-Rad Laboratories, Hercules, CA, United States). The membrane was blocked and incubated with primary antibodies overnight at 4 °C. The primary antibodies, phospho-mTOR (serine 2448) and hypoxia-inducible factor-1α (HIF-1α) (Cell signaling TECHNOLOGY), were used for detection of mTOR and HIF-1α, respectively. GAPDH protein was used as the internal control.
Total RNA was extracted from liver tissues of each group with Trizol reagent (Takara Biotechnology Dalian CO., LTD.) according to the manufacturer’s protocol. The isolated RNA was dissolved in RNase-free water stored at -80 °C immediately. RNA was quantified by optical density measurement at 260 nm on a spectrophotometer.
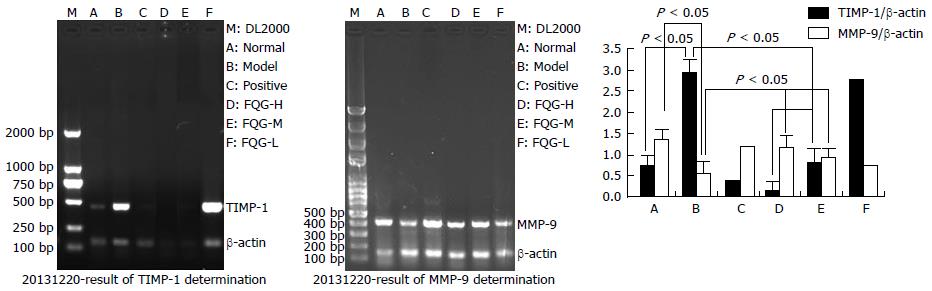
Reverse transcription reaction was performed with 2 μg of total RNA, which was used for polymerase chain reaction (PCR) amplification of cDNA products. The PCR of β-actin cDNA, which was used as an internal control, was carried out in the same tubes as for the genes. The products of PCR amplification were analyzed by electrophoresis on 1.5% agarose gel. The PCR product signal intensities were measured by scanning the gels. Tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) and matrix metalloproteinases-9 (MMP-9) densitometric measurement was normalized with the internal control β-actin. The primers used were as follows: TIMP-1, Forward GACCACCTTATACCAGCGTT and Reverse TCGAGACCCCAAGGTATTG; MMP-9, Forward CTGTATGGCTTCTGTCCTA and Reverse GGCTTCCTCCGTGATT.
Results were expressed as mean ± SD. Test data were analyzed with one-way variance (SPSS 20.0). Deviations with P < 0.05 were considered the presence of statistically significant.
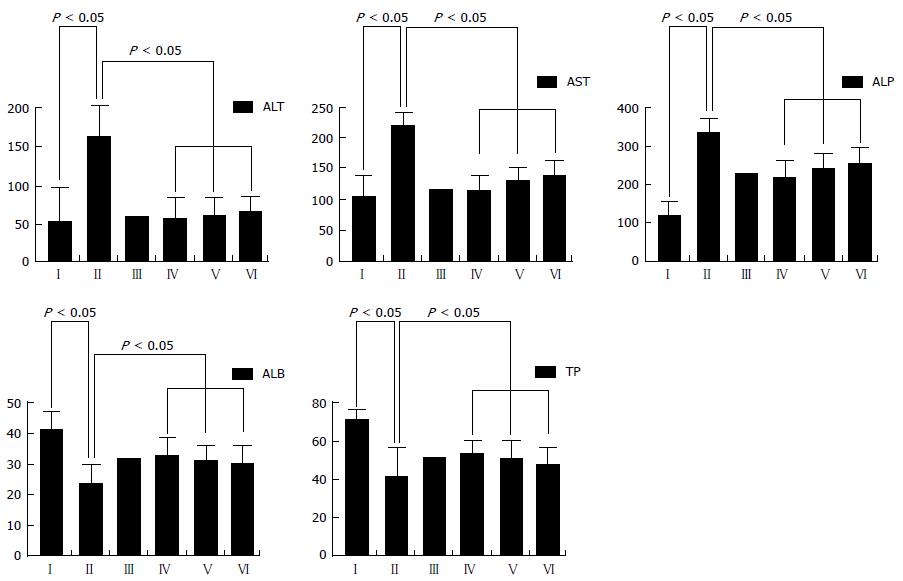
The blood serum AST, ALT, ALB, ALP and TP activity in various experiment groups is seen in Figure 1. Activity of ALT, AST, ALP have increased markedly, both serum ALB and TP activity were created a dramatic decreased in CCl4-duplicate liver fibrosis group (P < 0.05). Whereas three treatment groups of FQG showed the opposite response, which serum ALT, AST and ALP activities in rats were significantly improvement and the levels of ALB, TP were increased respectively higher than model group.
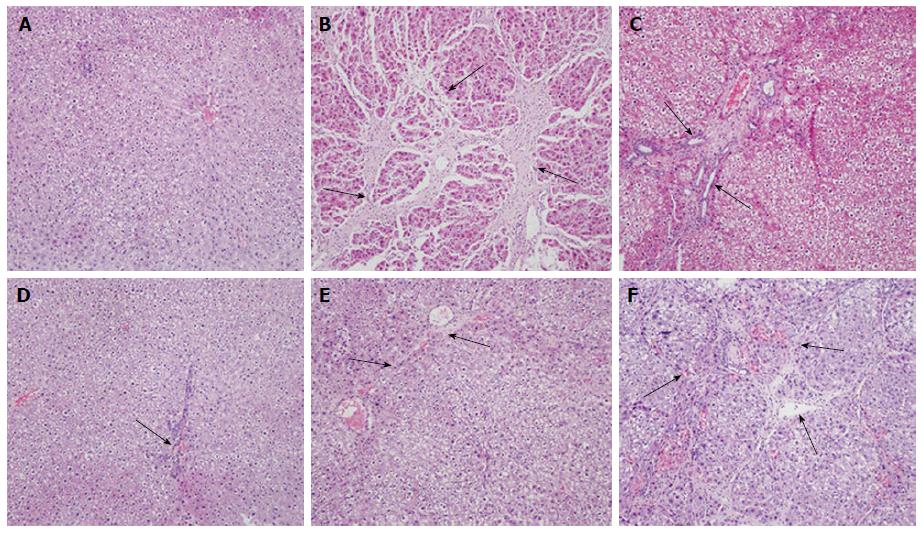
In order to evaluate the pathological changes in liver tissue, HE stain methods were employed in examination of liver tissue. Liver tissues of the group with normal saline have inerratic lobular composition with central veios and hepatic cords (Figure 2A). In the CCl4 group, serious injuries such as fibrous tissues hyperplasia, formed complete septa and pseudo lobule was observed in liver morphology (P < 0.05) (Figure 2B). In the groups treated with ALHXW, high dosages and middle dosages with FQG groups correspondingly appear alleviate tissue destroy compared with model group (Figure 2C-E). However, low-dose of FQG groups had no obvious effect (P < 0.05, Figure 2F).
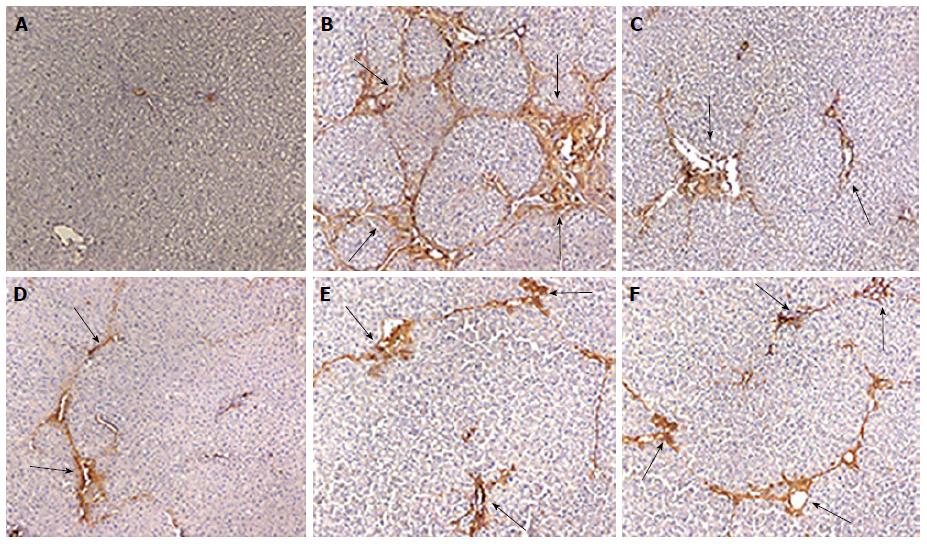
Compared with control group, α-SMA expression was increased significantly in the model group by method of immunohistochemical assay (Figure 3A and B). In the FQG treated rats groups with high-dose and meddle-dose, α-SMA expression of the liver tissues was noticeable reduction compared with CCl4 induced model groups (Figure 3D and E). Besides, expression of α-SMA was drastically diminished in rats does by high-dose of FQG compare with ALHXW and low-dose of FQG (Figure 3C and F).
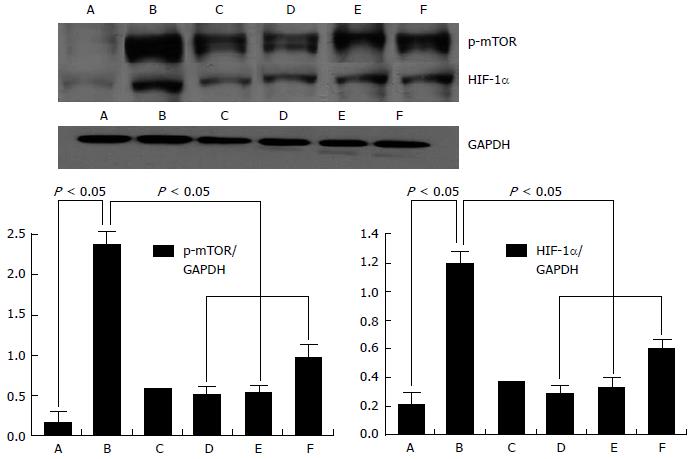
The mTOR and HIF-1α expression was detected undergo Western blotting. Studies have discovered that mTOR and HIF-1α expressions with hepatic tissue in models group were markedly increased, however, it was to observe the expression of mTOR and HIF-1α was significantly lowered in experimental rats with FQG intragastric administration group (Figure 4).
The results of semiquantitative reverse transcription-PCR (RT-PCR) expressions of MMP-9 and TIMP-1 in the hepatic tissues of different groups are exhibited in Figure 5. Experimental results showed that TIMP-1 expression dramatically stronger in treated with carbon tetrachloride than normal group, and its expression declined sharply in rats treated with high dosage of FQG. In contrast, the expression of MMP-9 was inferior in rats injected with CCl4 relatively physiological saline, which down-regulation could obviously inhibit by treated with FQG.
Liver fibrosis induced by CCl4 works as a kind of classic model in anti-fibrosis agents exploring and evaluation[18]. In the course of CCl4 being transformed to free radicals, the cytochrome P450 plays a most important role. Furtherly, lipid oxidation reactions were initiated by free radicals and leaded to liver cell damnification, retrograde, and even death. This kind of stimulation again and again can definitely form liver fibrosis[19]. It will further worsening or permanence of cirrhosis. Thus, the prevention and reversal of fibrosis is an important means to prevent the formation of liver cirrhosis[20]. Some studies evidence suggests that liver is likely to recover from fibrosis[21]. Recently research on treatment of liver fibrosis by TCM preparation has made some progress, such as ALHXW was typically used to cure this disease in this area, and marketed in china (National Drug permit Registry Z20010098). Chinese medicinal preparations showed the influences on liver fibrosis mainly embodied itself in composition with abundant and complex[22,23].
The research adopt FQG to examine the therapeutic effects on hepatic fibrosis. ALT and AST is an enzyme, Increasing of which were considered due to damage of liver cell. ALP is an ectoenzyme of plasma membrane, its ascension is partly a reflection of hepatocyte plasma membrane was damaged. The other, ALB, TP were depressed. In this study, compared with model rats via CCl4 treatment, effect of FQG makes serum ALT, AST and ALP levels significantly lowered, in the same time, it also elevated activity of serum ALB and TP in rats with treated FQG. These results exhibited an obvious therapeutic effect for liver fibrosis.
From a cellular perspective, in general, HSCs activation is the most important characteristic of liver fibrosis. During the process of HSC activation, α-SMA has started to overexpression, which is mostly found in smooth muscle cell. Increasing of α-SMA activated HSCs resulting in collagen fibers protein were secretion, ultimately leads to fibrosis. In this research, immunohistochenical suggested that α-SMA expression was obviously increased in rats with CCl4 stimulate. However, its expression was markedly improvement in rats with treated FQG. This study showed that FQG could inhibit the activity of HSC, thus emerging anti-fibrosis effect.
TIMPs was served as a vital factor in the process of liver fibrosis. It was secreted through activated HSC and can produce a variety of cytokines that are significantly increased in liver fibrosis[24]. MMPs are able to degrade ECM, and play a critical role in preventing inflammation and tumor progression[25]. Under physiological conditions, the expressions of TIMPs and MMP-9 are in dynamic equilibrium to maintain the stability of ECM in liver[26]. RT-PCR analysis of TIMP-1 and MMP-9 showed that FQGs could effectively inhibit TIMP-1 protein expressions, meanwhile, MMP-9 was enhanced during hepatic fibrosis in rats. This result also indicated that the regulation of TIMP-1 and MMP-9 levels can promote degradation of ECM.
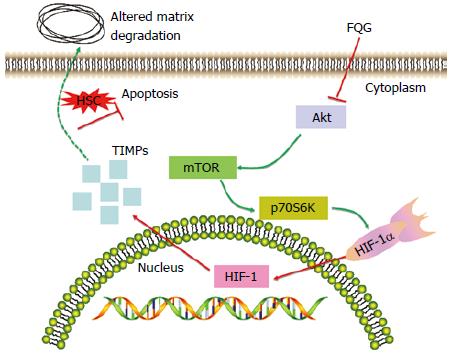
Activation of HSC is the core of liver fibrosis, clinical treatment of hepatic fibrosis is sticks chiefly to intervene activation process[27]. mTOR is one of the phosphoinositide 3-kinase related kinases family members and plays a vital role in cell proliferation regulation[28]. Previous research have showed that liver fibrosis could alleviated by mTOR inhibitor[29]. p70S6K is directly concerned to the matrix with mTOR, while the mTOR/p70S6K pathways is related to regulate of cell proliferation[30]. HIF-1 was used to grasp the expression of hypoxia inducible genes and further to decrease oxidation ability in cells[31]. Some studies have proved that increasing expression of HIF-1α though mTOR signaling can significantly result in pulmonary fibrosis, renal fibrosis or peritoneal angiogenesis, whereas mTOR inhibitor such as FQG is able to effectively alleviate liver fibrosis[32,33]. In fact, the expression intensity of mTOR and HIF-1α in experiment rats with CCl4 injected was up-regulated by western blot analysis. Moreover, anoxia is likely what lead HIF-1α to up-regulation in hepatic tissues. Nevertheless, it was down-regulated for FQG treat group, which perhaps raising matrix to degrade and promoting HSC to apoptosis, consequently inhibiting deterioration of hepatic fibrosis. From what has been discussed above, we speculate that the FQG effect might be due to inhibition of CCl4-induced p70S6K activation (Figure 6).
The incidence rate was high in patients with liver fibrosis in world, accordingly, if the issue was appropriately to regard liver fibrosis during this stage. It will prevent the development of cirrhosis and relieve the pain of the patients. However, there still lack of satisfactory treat medical for liver fibrosis at present. Based on the theories of Traditional Chinese medicines, humid, blocked lifeblood circulation, imbalance of yin and yang will give rise to liver fibrosis. Fu-qi granule (FQG) can activate blood and remove stasis, therefore, the current situation is to explore the effect and its underlying mechanisms of FQG on liver fibrosis duplicated by carbon tetrachloride (CCl4) in rats.
Recent research showed liver fibrosis can be relieved by regulating collagen metabolism, inhibiting hepatic stellate cell (HSC) activation. Moreover, amelioration of hepatic fibrosis was regulated by mammalian target of rapamycin (mTOR) inhibitors. mTOR/p70S6K pathway is blocked will lead to decrease of HSCs proliferation.
This study has confirmed that FQG can improve liver function, alleviate liver fibrosis, which is probably associated with its regulating mTOR/p70S6K signal transduction pathway.
The FQG can prevent liver fibrosis, which implies that it will be a good medicine and promising preparation for patients with liver fibrosis, this study can provide some scientific data for its application and development.
Liver fibrosis is a chronically ill, which was caused by excessive cumulation of extracellar stroma proteins. HSCs become a crucial role in liver fibrosis and cirrhosis with portal hypertension incidence of pathological basis.
This paper reinforced my conviction that there is protective effect of FQG on liver fibrosis rats with CCl4-stimulated. The study is interesting and the analysed parameters are well matched to the mechanism of hepatic fibrosis. Data are clear and convincing.
P- Reviewer: Bourgoin SG, Haidara M, Hohenegger M S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Lee TY, Chang HH, Chen JH, Hsueh ML, Kuo JJ. Herb medicine Yin-Chen-Hao-Tang ameliorates hepatic fibrosis in bile duct ligation rats. J Ethnopharmacol. 2007;109:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1292] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 3. | Osaka E, Suzuki T, Osaka S, Yoshida Y, Sugita H, Asami S, Tabata K, Hemmi A, Sugitani M, Nemoto N. Survivin as a prognostic factor for osteosarcoma patients. Acta Histochem Cytochem. 2006;39:95-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Zhou Q, Deng Z, Zhu Y, Long H, Zhang S, Zhao J. mTOR/p70S6K signal transduction pathway contributes to osteosarcoma progression and patients’ prognosis. Med Oncol. 2010;27:1239-1245. [PubMed] |
| 5. | Gäbele E, Reif S, Tsukada S, Bataller R, Yata Y, Morris T, Schrum LW, Brenner DA, Rippe RA. The role of p70S6K in hepatic stellate cell collagen gene expression and cell proliferation. J Biol Chem. 2005;280:13374-13382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Friedman SL. Reversibility of hepatic fibrosis and cirrhosis--is it all hype. Nat Clin Pract Gastroenterol Hepatol. 2007;4:236-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 397] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Li WL, Zheng HC, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 552] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 9. | Zou YH, Yang Y, Li J, Wu Q, Li WP, Lu JT, Roberts MS. Potential therapeutic effects of a traditional Chinese formulation, BJ-JN, on liver fibrosis induced by carbon tetrachloride in rats. J Ethnopharmacol. 2008;120:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Lin X, Zhang S, Huang Q, Wei L, Zheng L, Chen Z, Jiao Y, Huang J, Fu S, Huang R. Protective effect of Fufang-Liu-Yue-Qing, a traditional Chinese herbal formula, on CCl4 induced liver fibrosis in rats. J Ethnopharmacol. 2012;142:548-556. [PubMed] [DOI] [Full Text] |
| 11. | Liu P, Hu YY, Ni LQ. [On establishing comparative reference system for syndrome classification study from the thinking characteristics of syndrome differentiation dependent therapy]. Zhongguo Zhongxiyi Jiehe Zazhi. 2006;26:451-454. [PubMed] |
| 12. | Arias M, Lahme B, Van de Leur E, Gressner AM, Weiskirchen R. Adenoviral delivery of an antisense RNA complementary to the 3’ coding sequence of transforming growth factor-beta1 inhibits fibrogenic activities of hepatic stellate cells. Cell Growth Differ. 2002;13:265-273. [PubMed] |
| 13. | Zhang YD, Shen JP, Zhu SH, Huang DK, Ding Y, Zhang XL. [Effects of astragalus (ASI, SK) on experimental liver injury]. Yaoxue Xuebao. 1992;27:401-406. [PubMed] |
| 14. | Nakamura T, Akiyoshi H, Saito I, Sato K. Adenovirus-mediated gene expression in the septal cells of cirrhotic rat livers. J Hepatol. 1999;30:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Yang FR, Fang BW, Lou JS. Effects of Haobie Yangyin Ruanjian decoction on hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol. 2010;16:1458-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (5)] |
| 16. | Mullen KD, McCullough AJ. Problems with animal models of chronic liver disease: suggestions for improvement in standardization. Hepatology. 1989;9:500-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Yen CC, Lai YW, Chen HL, Lai CW, Lin CY, Chen W, Kuan YP, Hsu WH, Chen CM. Aerosolized human extracellular superoxide dismutase prevents hyperoxia-induced lung injury. PLoS One. 2011;6:e26870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Brautbar N, Williams J. Industrial solvents and liver toxicity: risk assessment, risk factors and mechanisms. Int J Hyg Environ Health. 2002;205:479-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Berger ML, Bhatt H, Combes B, Estabrook RW. CCl4-induced toxicity in isolated hepatocytes: the importance of direct solvent injury. Hepatology. 1986;6:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Ueberham E, Löw R, Ueberham U, Schönig K, Bujard H, Gebhardt R. Conditional tetracycline-regulated expression of TGF-beta1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology. 2003;37:1067-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Lou JL, Jiang MN, Li C, Zhou Q, He X, Lei HY, Li J, Jia YJ. Herb medicine Gan-fu-kang attenuates liver injury in a rat fibrotic model. J Ethnopharmacol. 2010;128:131-138. [PubMed] [DOI] [Full Text] |
| 22. | Zimmermann GR, Lehár J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 786] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 23. | Gu P, Chen H, Yu T. Ontology-oriented diagnostic system for traditional Chinese medicine based on relation refinement. Comput Math Methods Med. 2013;2013:317803. [PubMed] [DOI] [Full Text] |
| 24. | Cheung KF, Ye DW, Yang ZF, Lu L, Liu CH, Wang XL, Poon RT, Tong Y, Liu P, Chen YC. Therapeutic efficacy of Traditional Chinese Medicine 319 recipe on hepatic fibrosis induced by carbon tetrachloride in rats. J Ethnopharmacol. 2009;124:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Jackson BC, Nebert DW, Vasiliou V. Update of human and mouse matrix metalloproteinase families. Hum Genomics. 2010;4:194-201. [PubMed] |
| 26. | Zhou X, Hovell CJ, Pawley S, Hutchings MI, Arthur MJ, Iredale JP, Benyon RC. Expression of matrix metalloproteinase-2 and -14 persists during early resolution of experimental liver fibrosis and might contribute to fibrolysis. Liver Int. 2004;24:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Ueberham E, Löw R, Ueberham U, Schönig K, Bujard H, Gebhardt R. Conditional tetracycline-regulated expression of TGF-beta1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology. 2003;37:1067-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151-3171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 967] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 29. | Patsenker E, Schneider V, Ledermann M, Saegesser H, Dorn C, Hellerbrand C, Stickel F. Potent antifibrotic activity of mTOR inhibitors sirolimus and everolimus but not of cyclosporine A and tacrolimus in experimental liver fibrosis. J Hepatol. 2011;55:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Shamji AF, Nghiem P, Schreiber SL. Integration of growth factor and nutrient signaling: implications for cancer biology. Mol Cell. 2003;12:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 31. | Sun S, Gao YQ, Gao WX, Fan M. Hypoxia-inducible factor-1 and PI3K/Akt/mTOR signaling pathway. Chinese Bulletin of Life Sciences. 2005;17:311-314. |
| 32. | Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708-1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Rozen-Zvi B, Hayashida T, Hubchak SC, Hanna C, Platanias LC, Schnaper HW. TGF-β/Smad3 activates mammalian target of rapamycin complex-1 to promote collagen production by increasing HIF-1α expression. Am J Physiol Renal Physiol. 2013;305:F485-F494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |