Revised: October 1, 2014
Accepted: October 28, 2014
Published online: December 9, 2014
Processing time: 136 Days and 13.2 Hours
Telomeres are non-coding tandem repeats of 1000-2000 TTAGGG nucleotide DNA sequences on the 3’ termini of human chromosomes where they serve as protective “caps” from degradation and loss of genes. The “cap” at the end of chromosome required to protect its integrity is a 150-200 nucleotide-long single stranded G-rich 3’ overhang that forms two higher order structures, a T-loop with Sheltering complex, or a G-quadruplex complex. Telomerase is a human ribonucleoprotein reverse transcriptase that continually added single stranded TTAGGG DNA sequences onto the single strand 3’ of telomere in the 5’ to 3’ direction. Telomerase activity is detected in male germ line cells, proliferative cells of renewal tissues, some adult pluripotent stem cells, embryonic cells, but in most somatic cells is not detected. Re-expression or up-regulation of telomerase in tumours cells is considered as a critical step in cell tumorigenesis and telomerase is widely considered as a tumour marker and a target for anticancer drugs. Different approaches have been used in anticancer therapeutics targeting telomerase. Telomerase inhibitors can block directly Human TElomerase Reverse Transcriptase (hTERT) or Human TElomerase RNA telomerase subunits activity, or G-quadruplex and Sheltering complex components, shortening telomeres and inhibiting cell proliferation. Telomerase can become an immune target and GV1001, Vx-001, I540 are the most widespread vaccines used with encouraging results. Another method is to use hTERT promoter to drive suicide gene expression or to control a lytic virus replication. Recently telomerase activity was used to activate pro-drugs such as Acycloguanosyl 5’-thymidyltriphosphate, a synthetic ACV-derived molecule when it is activated by telomerase it does not require any virus or host active immune response to induce suicide gene therapy. Advantage of all these therapies is that target only neoplastic cells without any effects in normal cells, avoiding toxicity and adverse effects of the current chemotherapy. However, as not all the approaches are equally efficient, further studies will be necessary.
Core tip: One of the hallmark of cancer is the replicative immortality of tumor cells guaranteed by telomerase activity that counteracts progressive telomere shortening during cellular replication: this makes telomerase a tumor marker and a target for anticancer drugs. In this review we summarize and update the most recent innovative studies and results on the different strategies that consider telomerase as a target for cancer therapy. In particular, we try to point out the advantages and the potentialities of some innovative approaches, compared to other, equally promising, but that need further investigations.
- Citation: Picariello L, Grappone C, Polvani S, Galli A. Telomerase activity: An attractive target for cancer therapeutics. World J Pharmacol 2014; 3(4): 86-96
- URL: https://www.wjgnet.com/2220-3192/full/v3/i4/86.htm
- DOI: https://dx.doi.org/10.5497/wjp.v3.i4.86
Telomeres are non-coding tandem repeats of 1000-2000 TTAGGG nucleotide DNA sequences on the 3’ termini of human chromosomes[1-3] where they serve as protective “caps” from degradation and loss of genes. In this way cells can discriminate between double strand breaks and natural chromosome ends[4,5]. In human somatic cells, telomeres become critically short after successive cell divisions (number of divisions depending on the length of their telomeres), cells stop division and replicative senescence occurs[6]. As a consequence, telomeres can reach a critical length that is no longer suitable to assemble into T-loop: this triggers a localized DNA damage response and p53-mediated cell cycle arrest[7-9]. However, cells that have inactivated the p53-pathway cell cycle checkpoint, are able to continue dividing, bypassing senescence, loosing telomeric sequence with each division[9,10] and reach a “crisis” stage[11,12]. In this way telomeres become so short that cannot protect chromosome ends, so that they fuse together to produce a dicentric chromosome, inducing an increase aneuploidy and genomic instability that finally will lead to p53-independent apoptosis[13,14]. Bypassing crisis rarely occurs in human cells (1 in 10-6 in epithelial cells and 1 in 10-7 in human fibroblasts) and this leads to cell immortality and cancer cell progression, characterized by capability to continue to proliferate without limits.
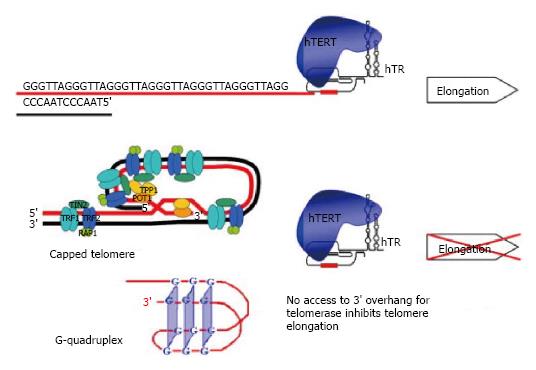
The “cap” at the end of chromosome required to protect its integrity is a 150-200 nucleotide-long single stranded G-rich 3’ overhang that forms two higher order structures, a T-loop with Sheltering complex, or a G-quadruplex complex. Sheltering complex is represented by six proteins (TRF1 and TRF2, POT1, TPP1, TIN2, RAP1) responsible for maintaining the T-loop structure. G-quadruplex is stabilized with BRACO19, RHS4 and telomestatin proteins. Sheltering complex with T-loop, G-quadruplex and its stabilizers can lock the telomeric 3’ overhang and block telomerase from accessing telomeres[15] (Figure 1).
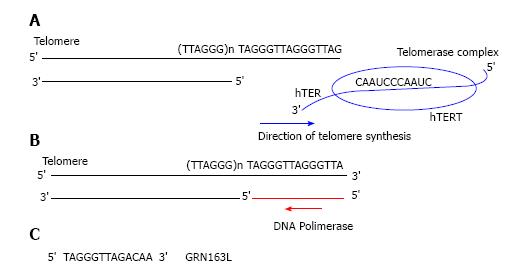
Telomerase is a human ribonucleoprotein reverse transcriptase that continually adds single stranded TTAGGG DNA sequences onto the single strand 3’ of telomere in the 5’ to 3’ direction and translocates to the new terminus[16,17]. This cycle goes on as far as telomerase dissociates from telomere[18,19]. Telomerase is composed of two main subunits: the catalytic protein Human TElomerase Reverse Transcriptase (hTERT) and the ribonucleoprotein template Human TElomerase RNA (hTER)[15-17]. In particular hTER consists of 451 nucleotides of which only nucleotides 46 through 56 (5’-CUAACCCUAAC-3’) represent a template for new telomeric added DNA sequences (Figure 2).
Many proteins associated to the core components hTERT and hTER are required and are necessary for stability regulation, recruitment and activity of the holoenzime[20]. hTER is expressed in all human cells, as well as normal and tumour cells, so telomerase activity is limited by of hTERT expression, whereas is present[21,22].
Telomerase activity is detected in proliferative cells of renewal tissues, in some adult pluripotent stem cells, male germ line cells, embryonic cells, but not in most somatic cells[23]. However, telomerase activity is found in almost all human cancer cell lines and in about 85%-90% of primary tumours[24]. In fact, one of the hallmark of cancer is the replicative immortality and so the ability to endlessly growth is synonymous of telomerase reverse transcriptase reactivation. Up-regulation or re-expression of telomerase in tumour cells is considered as a critical step in cell tumorigenesis and telomerase is widely considered as a tumour marker and a target for anticancer drugs. Progressive telomere shortening during cellular replication is counteracted by telomerase activity[1,25].
One of the advantages of anticancer therapies targeting telomerase is that the telomeres of highly proliferating cancer cells are shorter (5 kb) compared to that in normal somatic cells and stem cells (10-20 kb) that have not yet reached critical lengths as a result of aging[26,27]. The difference in telomerase activity and telomere lengths in normal and cancer cells leads to a more selective therapeutics cytotoxicity on cancer cells and a minimal impact on normal cells with a limitation of collateral effects that can be evaluated[28].
Telomerase inhibitors can be employed as a selective anti-cancer therapy, disrupting telomerase-positive cancer cells replicative capacity[29].
To target telomerase in cancer treatment we can find two types of approaches: the first one is blocking directly telomerase hTERT or hTER subunits activity, with consequent shortening of telomeres leading to the arrest of cell replication. The second approach is to block telomerase by an indirect method, targeting G-quadruplex stabilizers or Sheltering complex components with the consequence of preventing telomerase interaction with telomeres or binding of proteins associated with telomerase; this leads to telomere uncapping and cell apoptosis[30].
One of the most recent strategy for a direct telomerase enzymatic inhibition, is the use of antisense oligonucleotides inhibitors. These molecules are complementary to the 11-base template region of telomerase (hTER) and can be used to block the translation of sense RNA. In order to hybridize the hTER-template the antisense oligonucleotides must get to the hTER region without being degraded by nucleases. For this reason the challenge for this kind of drugs is both access and stability. To better get its target, antisense oligonucleotides have been modified and significantly improved in the past years.
Currently GRN163L (Imetelstat®) is one of the first generation most promising telomerase inhibitor targeting hTER used in cancer treatment; it is a lipid modified version of GRN163, a 13-mer oligonucleotide N3’-P5’-thio-phosphoramidate, that required a lipid carrier molecule and a lipid-base transfection agent to adequately enter tissue and cellular membranes[31,32]. On the contrary, GRN163L with a covalently bound lipophylic palmitoyl (C16) group linked to its 5’-thio-phosphate[33] is lipid soluble, and shows an higher drug availability and bio-distribution, without any lipid carrier supply[32]. GRN163L in part overlaps the hTER template region by binding with high affinity and specificity at its active site, acting as competitive telomerase inhibitor and causing a total enzyme inhibition[32,33] (Figure 2).
The GRN163L inhibitory effect on telomerase activity has been evaluated in different cancer cell lines[34] and its effects were evident as well as “in vitro” and “in vivo” models; in fact, long term treatment with GRN163L reduced cell viability in cancer cells derived from bladder[33] glioblastoma[35], multiple myeloma[36], Barrett’s adenocarcinoma[37], as well as breast[38,39], lung[40], liver cancer[41] and prostate[42].
Recently, the effects of GRN163L have been tested on a panel of ten pancreatic cancer cell lines, and the results indicated that the inhibitory effect of the drug was maintained also after its removal[43]: in fact, only three weeks after the GRN163L removal, a telomerase recovery was observed, but the enzyme was less processive. This suggests that to maintain continuous telomerase inhibition and to reduce side effects risk after a pharmacological treatment of a patient with GRN163L, a maintenance dose given once every other week might be sufficient. However, the reversible effects of Imetelstat have been also previously demonstrated on rat mesenchymal stem cells[44].
A combined treatment where homologous recombination and telomerase inhibition are associated, causes a significant increase in telomeres attrition, relative to each treatment alone, leading to senescence and apoptosis in Barrett’s adenocarcinoma[45].
Tamakawa et al[46] showed that the DNA damage induced in S/G2 phase of the cell cycle, by genotoxic stimulus was potentiated by the telomerase inhibition induced by GRN163L in breast and colorectal cancer cells[46].
In previous studies, synergies between GRN163L and various anticancer treatments such as microtubule inhibition, inhibition of oncogenic signals and ionizing radiation, were considered to be dependent on longer-term changes associated with chromatin status[47] and telomere length[48].
Telomere shortening induced by telomerase inhibitors would affect the self-renewal properties of cancer stem cells (CSCs), normally not responding to standard chemotherapy, but capable of inducing initiation and currency in different hematologic and solid tumours[49,50].
Many studies showed that CSCs can represent the Imetelstat target in different cancers[35,42,51], and that a telomere shortening-independent as well as dependent Imetelstat mechanism of action on CSCs subpopulation, can be suggested[52,53]. The effect of Imetelstat was evaluated on both the bulk cancer cells and putative CSCs of breast and pancreatic cancer cell lines. The in vitro treatment inhibited telomerase activity, cell growth, self renewal in bulk cancer cells and putative CSCs, with a consequent reduced cancer engraftment in nude mice[52]; in particular an increased sensitivity of CSCs to Imetelstat did not correlate with differences between telomerase activity expression levels or telomere length of CSCs and bulk tumour cells suggesting a telomere shortening- independent mechanism of action for the Imetelstat effects on CSCs subpopulation.
All these studies support the hypothesis that conventional therapies often fail to target CSCs while the use of telomerase inhibitor could have the potential role for more durable clinical response in many tumors, reducing relapse recurrence.
Imetelstat is currently in phase II clinical development for breast cancer, non-small cell lung carcinoma, multiple myeloma, and other tumor types[30].
BIBR1532 [2-(E-3-naphtalen-2-yl-but-2-enylylamino]-benzoic acid] is actually a promising hTERT inhibitor among the few TERT inhibitors developed. BIBR1532 is a small synthetic non-nucleic compound that linking hTERT in its active site, inhibits telomerase in a non-competitive manner: BIBR1532 does not cause chain termination events but rather leads to an overall reduction in the number of added TTAGGG repeats[54]; in particular the drug could act translocating the enzyme-DNA-substrate complex, or favouring the DNA substrate disjunction from the enzyme during the copy of the template[55].
In the last few years, different studies showed that BIBR1532 treatment induced telomerase activity reduction with consequent cell growth arrest in different human cancer cell lines[54,56-60], without affecting normal stem cells[61]. In addition telomeres targeting might represent a valid strategy for the re-sensitization of chemoresistant chondrosarcomas[56], and a rapid induction of a high level telomere dysfunction appears to be a crucial parameter for the development of future telomerase-based therapeutic[62]. However, although some human squamous cell carcinoma cell lines are resistant to telomerase inhibition[63] some works suggest that a valid strategy for the treatment of both drug-resistant and drug-sensitive cancers may be pharmacological telomerase inhibition in combination therapy[64-66].
As previously described, nearly all cancer cells over-express functional active telomerase, and hTERT-specific epitopes are expressed on tumour cells, but not on normal cells. In this way, telomerase become an immune target, and can be eradicated by the stimulation of the immune system with specific vaccines. Telomerase-target immunotherapy sensitizes immune cells against tumor cells expressing hTERT peptides as surface antigens[67]. The consequent expansion of telomerase-specific CD8+ cytotoxic T lymphocytes is directed to target and kill telomerase positive cancer cells[68,69].
Recently, multiple peptides are known to induce hTERT-specific immune responses[68] and several vaccine strategies are being developed and used: among these GV1001, Vx-001, I540, are the most widespread therapeutic approaches. As almost all human tumor-associated antigens are self-proteins, their specific T cells are often tolerated: this is the major problem of cancer immunotherapy. For this reason, overcoming tumor-specific self-tolerance is a principal goal in cancer immunotherapy.
Self-tolerance is commonly directed against ‘‘dominant’’ (high affinity for HLA) but not against ‘‘cryptic’’ (low affinity for HLA) peptides[70,71], so the simplest way to circumvent tolerance is to use these cryptic peptides[72] as for example Vx-001 (9-mer cryptic TERT 572 peptide) that was developed as tumour-associated antigen of hTERT to induce cytotoxic T lymphocyte responses[73,74].
Immunological response associated with extended survival were evident in patients with advanced non-small-cell lung cancer treated with Vx-001 vaccine (TERT572Y peptide)[74]; in patients with various types of chemo-resistant advanced solid tumours (stages III and IV) the vaccination with Vx-001 stimulates TERT572-specific reactive T cells in a great number of patients independently of the disease stage or clinical status before vaccination and a late immune response correlated with longer survival was induced[73,75].
State of the art of clinical trials using anti-telomerase cancer immunotherapy is encouraging. In fact, vaccines are tested in breast, lung, melanoma, prostate, and pancreatic cancer[76-82] and these trials have widely induced a specific immune response against hTERT positive cancer cells. Encouraging results have been also obtained in patients with advanced melanoma, where immunity to hTERT has been safely generated[83]. The combination of cancer vaccination with chemotherapy showed that temozolomide and GV1001 induced immune and clinical response in 78% of stage IV melanoma patients, that developed long-term T-cell memory and survived more than those rapidly losing their responses[84]. Vaccination with GV1001 was well tolerated and immunized the great part of non-small cell lung cancer patients establishing durable T-cell memory[85]. However, GV1001 vaccination was not effective in cutaneous T cell lymphoma patients, raising concerns about also its safety[86]. The survival data indicated that patients with non-resectable pancreatic cancer treated with GV1001 showed that immune response correlated with an extended survival, suggesting that the vaccine could be the new goal for pancreatic cancer patients treatment and encouraging further clinical studies[82]. On the contrary, in patients with advanced and metastatic pancreatic cancer the use of GV1001 telomerase vaccination in combination with chemotherapy, induced a weak and transient immune response and did not improve overall survival[80,81]. Likewise, a low dose cyclophosphamide treatment in combination with GV1001 vaccination in patients with advanced hepatocellular carcinoma did not show antitumor efficacy[87]. Further studies and new strategies are needed to analyze and to enhance the immune response effect of telomerase vaccination during chemotherapy, in patients with both pancreatic and hepatocellular cancers.
Vaccination with autologous dendritic cells transfected with hTERT mRNA (GRNVAC1) represents another anticancer approach that induced immunological response in human. Immunotherapy targeting the hTERT subunit of telomerase has been demonstrated to induce an important immune responses in cancer patients after vaccination with single hTERT peptides, while vaccination with dendritic cells transfected with hTERT mRNA has a key role in inducing efficient immune responses to multiple hTERT epitopes. In this way this kind of therapy can be an attractive approach to more efficient immunotherapy[88-90].
Recently has been shown that the use of hTERT promoter to drive the expression of a suicide gene and/or control the replication of a lytic virus, can be a successful approach to target cancer cells.
To drive the expression of a suicide gene, the expression of a pro-apoptotic protein, like TRAIL (tumour necrosis factor-related apoptosis-inducing ligand) or prodrug-activating enzyme[91-96] is controlled by the hTERT promoter, generally active in cancer cells expressing telomerase. These cells are injected with viruses carrying the suicide gene and then killed by a toxin derived from the administration of a pro-drug activated by the pro-drug-activating enzyme.
A second clinical approach, is to use the hTERT promoter to control the replication of a lytic virus. Oncolytic effects on tumors can be mediated by oncolytic viruses, tumor selective viruses genetically modified and engineered to replicate in and kill only cancer cells. For this purpose, the E1 gene expresses viral proteins E1A and E1B necessary for adenovirus replication, but the modified virus can replicate only in cells which express telomerase if gene itself is redesigned to be controlled by the hTERT promoter[97-100]. One such virus is telomelysin (OBP-301) that in pre-clinical studies targets selectively only telomerase-expressing cells.
The modified viruses induce cytolysis in several kinds of human cancer cell lines in which can replicate; when human lung, prostate or liver cancer cells were used in xenotransplantation models, intratumoral injection of the virus reduced tumor growth and improved mice survival[97-100].
The potential role of oncolytic virotherapy has recently been demonstrated to be a promising strategy in the management of human gastrointestinal cancer[101]. Studies about OBP-301 have been shown that it mediates the effective in vivo purging of metastatic tumor cells from regional lymph nodes and moreover it co-operates to optimize treatment of human gastrointestinal malignancies[102]. Moreover, telomerase-specific oncolytic viruses is a potential treatment of human squamous cell carcinoma of head and neck[103], while in pancreatic cancer the combination therapy with gemcitabine has been tried, exhibiting enhanced cytotoxic effects both “in vitro” and “in vivo”[104]. In addition, preclinical study showed that OBP-301 can be used for treatment of human hepatocellular carcinoma and that its tumor-killing activity persists after multiple injections[105].
Data regarding combination therapy with OBP-301 and chemotherapeutic agents are preliminary but encouraging[106]. In particular Boozari et al[107] showed that the combination of intratumoural virotherapy with an antitumoural vaccine, could represent a promising immunotherapeutic strategy against hepatocellular carcinoma and metastasis.
Recent studies revealed that telomerase canonical activity can be exploited for therapeutic purpose.
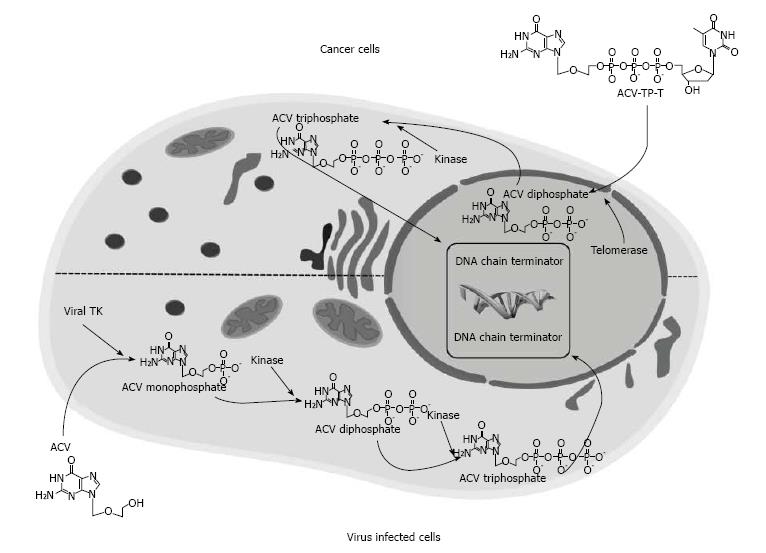
The evidence that telomerase is expressed in almost all tumor cells, preventing telomeres shortening by continually adding single stranded TTAGGG DNA sequences, prompted us to develop a thymidine analogue pro-drug, acycloguanosyl 5’-thymidyltriphosphate (ACV-TP-T) (Figure 3). This molecule is a synthetic ACV modification that is metabolized by telomerase, and this reaction releases the active form of acyclovir able to reduce pancreatic and hepatocellular carcinoma cells growth as well as “in vitro” and “in vivo”[108,109].
ACV is a nucleoside analogue acting as a DNA chain terminator that could be used in the suicide gene therapy[110]. ACV or the ACV analogue ganciclovir[110,111] when used as antiviral agent needs a first phosphorylation to ACV monophosphate by herpes virus thymidine kinase (TK) carried by wild-type herpes virus or, in the suicide gene therapy, by engineered adenovirus (Figure 3), then cellular kinases perform the two remaining phosphorylation to obtain the ACV triphosphate. This active metabolite is incorporated into DNA during its replication causing DNA chain termination.
On the contrary, ACV-TP-T, may be metabolized by telomerase that incorporates thymidine in replicating telomeres and releases ACV diphosphate. This process skips the viral TK phosphorylation, allowing the cellular kinases to go on with further phosphorylation to obtain the active drug[108,109]. The results showed that after activation of ACV-TP-T by telomerase, cell proliferation is significantly reduced and apoptosis is increased in different human pancreatic adenocarcinoma cell lines. High and low telomerase activity is related with low and high IC50 of the drug, respectively. On the other hand, the cytosine-containing pro-drug ACV-TP-dC, which is not a telomerase substrate, is not able to reduce pancreatic cancer cell proliferation. Moreover, ACV-TP-T administration increases apoptosis, reduces growth, proliferation and vascularization of pancreatic xenograft tumors in mice[108].
Analogue results were obtained in human and murine hepatocellular carcinoma cell lines and in transgenic and orthotopic murine models of hepatic cancers[109]. Furthermore, in orthotopic syngenic mice, ACV-TP-T has been used alone or in combination with the approved standard of care, Sorafenib, a multikinase inhibitor. Combination therapy showed a synergistic effect between Sorafenib and ACV-TP-T.
Advantages of this strategy are evident. Despite recent improvements in suicide gene therapy, the application of adenovirus-mediated therapy is limited by many factors: the low and transient expression levels of the transgene[110,112,113], the induction of immune response in the host[108], and a late carcinogenesis[112]. In addition ethical concerns regarding the use of virus in patients[112,113] could be a limitation.
The use of telomerase promoter[114] and the introduction of conditionally replication-competent adenovirus[115] only partially overcome the above mentioned disadvantages. Moreover, the immunotherapy based on vaccination for telomerase[84] relies on the induction of an active immune response that often is deregulated in the oncology treated patients[116].
In this contest, the use of ACV-TP-T represents a new therapeutic strategy that exploits the enzymatic activity of telomerase. This approach is efficient only in neoplastic cells without any effects in normal cells, it avoids the toxicity and the adverse effects of the current chemotherapy, and finally, it does not require the use of any viruses or an active immune response of the host.
As a paradox in this contest telomerase switches from being a target of anticancer therapy, to an integral part of the therapy. Preliminary evidences suggest the possible use of ACV-TP-T molecule for the treatment of other tumors characterized by high telomerase expression and activity such as ovarian and adrenocortical cancers.
Telomerase activation may have both telomere-dependent and telomere-independent implications for cancer progression: in particular, telomerase reverse transcriptase may exert some biological functions independently of its telomere maintenance enzymatic activity.
Different studies support a role of telomerase in some telomere-independent activities in cancer progression; nevertheless, apart from its role in telomere maintenance, the molecular mechanism by which telomerase promotes cancer is still not fully understood. Zhou et al[117] showed that hTER regulated vascular endothelial growth factor (VEGF) expression at the transcriptional level, independently of telomerase activity[117]; previous studies reported that VEGF induced hTERT expression and activity in normal[118] and cancer cells[119]. All these results suggested a positive feedback regulation that could contribute to a mutual and collaborative function of VEGF and telomerase in cancer progression.
Wu et al[120] in a recent review focused on various signaling pathways and genes involved in the feedback regulation of TERT. The expression of numerous genes involved in different cellular processes, as well as cell cycle and cellular signaling, could be regulated by TERT, indicating that telomerase is both an effector and a regulator in carcinoma. However, the mechanisms underlying the interaction between TERT and its target genes are still not completely understood.
Ghosh et al[121] suggested a functional interplay between TERT and nuclear factor (NF-κB) signaling, further reinforced by the observation that telomerase over expression resulted in enhanced expression of NF-κB target genes, whereas telomerase null mice were refractory to NF-κB activation; in addition, it seems that also hTER could regulate the expression of some NF-κB target genes. The function of hTER in gene expression regulation is not clear, in fact, hTERT can form complexes with or without hTER[122].
hTERT could be involved also in a negative feedback loop system with pRb/E2F pathway in cancer, as well as in a positive feedback loop with Wnt/β-catenin signalling, or in multiple interactions with phosphoinositide 3 kinase/Akt pathway[120]. In addition, Liu et al[123] demonstrated a potential role of hTERT in epithelial mesenchymal transition.
Although the mechanisms underlying the interaction between TERT and its target genes are still not completely understood, all the above observations, strengthen the idea that telomerase non-telomeric functions could be used as a new therapeutic target for cancer.
Although recent and ongoing results support an important role for telomerase targeting therapeutics in cancer treatment, additional preclinical and clinical trials are necessary to improve some of these strategies.
In fact, if difficulties with dendritic cells derivation will be easily overcome[124], vaccination with dendritic cells transfected with hTERT mRNA could potentially become an attractive approach to a more potent immunotherapy. In addition, further studies are necessary to enhance the effects of telomerase vaccination in combination with intratumoral virotherapy and with standard chemotherapeutic agents.
On the contrary, beside more promising approach offered by GRN163L that seems to target also CSC, BIBR1532 could be preferred therapy if used also in combination with standard chemotherapy for the treatment of drug-resistant cancers.
Finally, ACV-TP-T use is very promising and deserves further studies. In fact, preclinical evidences showed that this new pro-drug may be considered for treatment of hepatocellular and pancreatic carcinoma, as well as of other tumors characterized by high telomerase expression and activity.
P- Reviewer: Majumdar APN, Paraskevas KI S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 570] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 2. | Meyerson M. Role of telomerase in normal and cancer cells. J Clin Oncol. 2000;18:2626-2634. [PubMed] |
| 3. | Batista LF, Artandi SE. Telomere uncapping, chromosomes, and carcinomas. Cancer Cell. 2009;15:455-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622-6626. [PubMed] |
| 6. | Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4007] [Cited by in RCA: 4039] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 7. | Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1041] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 8. | d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194-198. [PubMed] |
| 9. | Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 781] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 10. | Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 514] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 11. | Wright WE, Pereira-Smith OM, Shay JW. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989;9:3088-3092. [PubMed] |
| 12. | Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontol. 1992;27:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 263] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Macera-Bloch L, Houghton J, Lenahan M, Jha KK, Ozer HL. Termination of lifespan of SV40-transformed human fibroblasts in crisis is due to apoptosis. J Cell Physiol. 2002;190:332-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Mar V, Zhou W, Harrington L, Robinson MO. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 1999;13:2388-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 477] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Ruden M, Puri N. Novel anticancer therapeutics targeting telomerase. Cancer Treat Rev. 2013;39:444-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 16. | Blackburn EH. Telomerase and Cancer: Kirk A. Landon--AACR prize for basic cancer research lecture. Mol Cancer Res. 2005;3:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Tian X, Chen B, Liu X. Telomere and telomerase as targets for cancer therapy. Appl Biochem Biotechnol. 2010;160:1460-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407-425, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 648] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 19. | Kelleher C, Teixeira MT, Förstemann K, Lingner J. Telomerase: biochemical considerations for enzyme and substrate. Trends Biochem Sci. 2002;27:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850-1853. [PubMed] |
| 21. | Ducrest AL, Szutorisz H, Lingner J, Nabholz M. Regulation of the human telomerase reverse transcriptase gene. Oncogene. 2002;21:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 1998;58:1558-1561. [PubMed] |
| 23. | Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173-179. [PubMed] |
| 24. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5156] [Cited by in RCA: 5233] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 25. | Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 440] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 26. | Kelland LR. Overcoming the immortality of tumour cells by telomere and telomerase based cancer therapeutics--current status and future prospects. Eur J Cancer. 2005;41:971-979. [PubMed] |
| 27. | Phatak P, Burger AM. Telomerase and its potential for therapeutic intervention. Br J Pharmacol. 2007;152:1003-1011. [PubMed] |
| 28. | Corey DR. Telomerase: an anusual target for cytotoxic agents. Chem res Toxicol. 2000;13:957-960. [PubMed] |
| 29. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19503] [Article Influence: 780.1] [Reference Citation Analysis (0)] |
| 30. | Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 537] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 31. | Rankin AM, Faller DV, Spanjaard RA. Telomerase inhibitors and ‚T-oligo‘ as cancer therapeutics: contrasting molecular mechanisms of cytotoxicity. Anticancer Drugs. 2008;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Röth A, Harley CB, Baerlocher GM. Imetelstat (GRN163L)--telomerase-based cancer therapy. Recent Results Cancer Res. 2010;184:221-234. [PubMed] |
| 33. | Dikmen ZG, Wright WE, Shay JW, Gryaznov SM. Telomerase targeted oligonucleotide thio-phosphoramidates in T24-luc bladder cancer cells. J Cell Biochem. 2008;104:444-452. [PubMed] |
| 34. | Herbert BS, Gellert GC, Hochreiter A, Pongracz K, Wright WE, Zielinska D, Chin AC, Harley CB, Shay JW, Gryaznov SM. Lipid modification of GRN163, an N3’--& gt; P5’ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene. 2005;24:5262-5268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 35. | Marian CO, Cho SK, McEllin BM, Maher EA, Hatanpaa KJ, Madden CJ, Mickey BE, Wright WE, Shay JW, Bachoo RM. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and tumor growth. Clin Cancer Res. 2010;16:154-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 36. | Shammas MA, Koley H, Bertheau RC, Neri P, Fulciniti M, Tassone P, Blotta S, Protopopov A, Mitsiades C, Batchu RB. Telomerase inhibitor GRN163L inhibits myeloma cell growth in vitro and in vivo. Leukemia. 2008;22:1410-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Shammas MA, Qazi A, Batchu RB, Bertheau RC, Wong JY, Rao MY, Prasad M, Chanda D, Ponnazhagan S, Anderson KC. Telomere maintenance in laser capture microdissection-purified Barrett’s adenocarcinoma cells and effect of telomerase inhibition in vivo. Clin Cancer Res. 2008;14:4971-4980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Gellert GC, Dikmen ZG, Wright WE, Gryaznov S, Shay JW. Effects of a novel telomerase inhibitor, GRN163L, in human breast cancer. Breast Cancer Res Treat. 2006;96:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Hochreiter AE, Xiao H, Goldblatt EM, Gryaznov SM, Miller KD, Badve S, Sledge GW, Herbert BS. Telomerase template antagonist GRN163L disrupts telomere maintenance, tumor growth, and metastasis of breast cancer. Clin Cancer Res. 2006;12:3184-3192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Dikmen ZG, Gellert GC, Jackson S, Gryaznov S, Tressler R, Dogan P, Wright WE, Shay JW. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005;65:7866-7873. [PubMed] |
| 41. | Djojosubroto MW, Chin AC, Go N, Schaetzlein S, Manns MP, Gryaznov S, Harley CB, Rudolph KL. Telomerase antagonists GRN163 and GRN163L inhibit tumor growth and increase chemosensitivity of human hepatoma. Hepatology. 2005;42:1127-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Marian CO, Wright WE, Shay JW. The effects of telomerase inhibition on prostate tumor-initiating cells. Int J Cancer. 2010;127:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Burchett KM, Yan Y, Ouellette MM. Telomerase inhibitor Imetelstat (GRN163L) limits the lifespan of human pancreatic cancer cells. PLoS One. 2014;9:e85155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Tokcaer-Keskin Z, Dikmen ZG, Ayaloglu-Butun F, Gultekin S, Gryaznov SM, Akcali KC. The effect of telomerase template antagonist GRN163L on bone-marrow-derived rat mesenchymal stem cells is reversible and associated with altered expression of cyclin d1, cdk4 and cdk6. Stem Cell Rev. 2010;6:224-233. [PubMed] |
| 45. | Lu R, Pal J, Buon L, Nanjappa P, Shi J, Fulciniti M, Tai YT, Guo L, Yu M, Gryaznov S. Targeting homologous recombination and telomerase in Barrett’s adenocarcinoma: impact on telomere maintenance, genomic instability and tumor growth. Oncogene. 2014;33:1495-1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Tamakawa RA, Fleisig HB, Wong JM. Telomerase inhibition potentiates the effects of genotoxic agents in breast and colorectal cancer cells in a cell cycle-specific manner. Cancer Res. 2010;70:8684-8694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Goldblatt EM, Gentry ER, Fox MJ, Gryaznov SM, Shen C, Herbert BS. The telomerase template antagonist GRN163L alters MDA-MB-231 breast cancer cell morphology, inhibits growth, and augments the effects of paclitaxel. Mol Cancer Ther. 2009;8:2027-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Gomez-Millan J, Goldblatt EM, Gryaznov SM, Mendonca MS, Herbert BS. Specific telomere dysfunction induced by GRN163L increases radiation sensitivity in breast cancer cells. Int J Radiat Oncol Biol Phys. 2007;67:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Allan AL, Vantyghem SA, Tuck AB, Chambers AF. Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis. 2006;26:87-98. [PubMed] |
| 50. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2140] [Article Influence: 118.9] [Reference Citation Analysis (0)] |
| 51. | Castelo-Branco P, Zhang C, Lipman T, Fujitani M, Hansford L, Clarke I, Harley CB, Tressler R, Malkin D, Walker E. Neural tumor-initiating cells have distinct telomere maintenance and can be safely targeted for telomerase inhibition. Clin Cancer Res. 2011;17:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Joseph I, Tressler R, Bassett E, Harley C, Buseman CM, Pattamatta P, Wright WE, Shay JW, Go NF. The telomerase inhibitor imetelstat depletes cancer stem cells in breast and pancreatic cancer cell lines. Cancer Res. 2010;70:9494-9504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Brennan SK, Wang Q, Tressler R, Harley C, Go N, Bassett E, Huff CA, Jones RJ, Matsui W. Telomerase inhibition targets clonogenic multiple myeloma cells through telomere length-dependent and independent mechanisms. PLoS One. 2010;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | El-Daly H, Kull M, Zimmermann S, Pantic M, Waller CF, Martens UM. Selective cytotoxicity and telomere damage in leukemia cells using the telomerase inhibitor BIBR1532. Blood. 2005;105:1742-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Pascolo E, Wenz C, Lingner J, Hauel N, Priepke H, Kauffmann I, Garin-Chesa P, Rettig WJ, Damm K, Schnapp A. Mechanism of human telomerase inhibition by BIBR1532, a synthetic, non-nucleosidic drug candidate. J Biol Chem. 2002;277:15566-15572. [PubMed] |
| 56. | Parsch D, Brassat U, Brümmendorf TH, Fellenberg J. Consequences of telomerase inhibition by BIBR1532 on proliferation and chemosensitivity of chondrosarcoma cell lines. Cancer Invest. 2008;26:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Röth A, Dürig J, Himmelreich H, Bug S, Siebert R, Dührsen U, Lansdorp PM, Baerlocher GM. Short telomeres and high telomerase activity in T-cell prolymphocytic leukemia. Leukemia. 2007;21:2456-2462. [PubMed] |
| 58. | Brassat U, Balabanov S, Bali D, Dierlamm J, Braig M, Hartmann U, Sirma H, Günes C, Wege H, Fehse B. Functional p53 is required for effective execution of telomerase inhibition in BCR-ABL-positive CML cells. Exp Hematol. 2011;39:66-76.e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Bashash D, Ghaffari SH, Zaker F, Hezave K, Kazerani M, Ghavamzadeh A, Alimoghaddam K, Mosavi SA, Gharehbaghian A, Vossough P. Direct short-term cytotoxic effects of BIBR 1532 on acute promyelocytic leukemia cells through induction of p21 coupled with downregulation of c-Myc and hTERT transcription. Cancer Invest. 2012;30:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Nakashima M, Nandakumar J, Sullivan KD, Espinosa JM, Cech TR. Inhibition of telomerase recruitment and cancer cell death. J Biol Chem. 2013;288:33171-33180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 61. | El Daly H, Martens UM. Telomerase inhibition and telomere targeting in hematopoietic cancer cell lines with small non-nucleosidic synthetic compounds (BIBR1532). Methods Mol Biol. 2007;405:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Pantic M, Zimmermann S, Waller CF, Martens UM. The level of telomere dysfunction determines the efficacy of telomerase-based therapeutics in a lung cancer cell line. Int J Oncol. 2005;26:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 63. | Bojovic B, Crowe DL. Resistance to telomerase inhibition by human squamous cell carcinoma cell lines. Int J Oncol. 2011;38:1175-1181. [PubMed] |
| 64. | Ward RJ, Autexier C. Pharmacological telomerase inhibition can sensitize drug-resistant and drug-sensitive cells to chemotherapeutic treatment. Mol Pharmacol. 2005;68:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Park YP, Kim KD, Kang SH, Yoon do Y, Park JW, Kim JW, Lee HG. Human telomerase reverse transcriptase (hTERT): a target molecule for the treatment of cisplatin-resistant tumors. Korean J Lab Med. 2008;28:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Meng E, Taylor B, Ray A, Shevde LA, Rocconi RP. Targeted inhibition of telomerase activity combined with chemotherapy demonstrates synergy in eliminating ovarian cancer spheroid-forming cells. Gynecol Oncol. 2012;124:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 415] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 68. | Liu JP, Chen W, Schwarer AP, Li H. Telomerase in cancer immunotherapy. Biochim Biophys Acta. 2010;1805:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Vonderheide RH. Prospects and challenges of building a cancer vaccine targeting telomerase. Biochimie. 2008;90:173-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Robbins PF, Kawakami Y. Human tumor antigens recognized by T cells. Curr Opin Immunol. 1996;8:628-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 142] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Van Pel A, van der Bruggen P, Coulie PG, Brichard VG, Lethé B, van den Eynde B, Uyttenhove C, Renauld JC, Boon T. Genes coding for tumor antigens recognized by cytolytic T lymphocytes. Immunol Rev. 1995;145:229-250. [PubMed] |
| 72. | Tourdot S, Scardino A, Saloustrou E, Gross DA, Pascolo S, Cordopatis P, Lemonnier FA, Kosmatopoulos K. A general strategy to enhance immunogenicity of low-affinity HLA-A2. 1-associated peptides: implication in the identification of cryptic tumor epitopes. Eur J Immunol. 2000;30:3411-3421. [PubMed] |
| 73. | Vetsika EK, Konsolakis G, Aggouraki D, Kotsakis A, Papadimitraki E, Christou S, Menez-Jamet J, Kosmatopoulos K, Georgoulias V, Mavroudis D. Immunological responses in cancer patients after vaccination with the therapeutic telomerase-specific vaccine Vx-001. Cancer Immunol Immunother. 2012;61:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Bolonaki I, Kotsakis A, Papadimitraki E, Aggouraki D, Konsolakis G, Vagia A, Christophylakis C, Nikoloudi I, Magganas E, Galanis A. Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J Clin Oncol. 2007;25:2727-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Kotsakis A, Vetsika EK, Christou S, Hatzidaki D, Vardakis N, Aggouraki D, Konsolakis G, Georgoulias V, Christophyllakis Ch, Cordopatis P. Clinical outcome of patients with various advanced cancer types vaccinated with an optimized cryptic human telomerase reverse transcriptase (TERT) peptide: results of an expanded phase II study. Ann Oncol. 2012;23:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Vonderheide RH, Domchek SM, Schultze JL, George DJ, Hoar KM, Chen DY, Stephans KF, Masutomi K, Loda M, Xia Z. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828-839. [PubMed] |
| 77. | Brunsvig PF, Aamdal S, Gjertsen MK, Kvalheim G, Markowski-Grimsrud CJ, Sve I, Dyrhaug M, Trachsel S, Møller M, Eriksen JA. Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55:1553-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 78. | Inderberg-Suso EM, Trachsel S, Lislerud K, Rasmussen AM, Gaudernack G. Widespread CD4+ T-cell reactivity to novel hTERT epitopes following vaccination of cancer patients with a single hTERT peptide GV1001. Oncoimmunology. 2012;1:670-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 79. | Chen Z, Koeneman KS, Corey DR. Consequences of telomerase inhibition and combination treatments for the proliferation of cancer cells. Cancer Res. 2003;63:5917-5925. [PubMed] |
| 80. | Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014;15:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 81. | Staff C, Mozaffari F, Frödin JE, Mellstedt H, Liljefors M. Telomerase (GV1001) vaccination together with gemcitabine in advanced pancreatic cancer patients. Int J Oncol. 2014;45:1293-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 82. | Bernhardt SL, Gjertsen MK, Trachsel S, Møller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer. 2006;95:1474-1482. [PubMed] |
| 83. | Hunger RE, Kernland Lang K, Markowski CJ, Trachsel S, Møller M, Eriksen JA, Rasmussen AM, Braathen LR, Gaudernack G. Vaccination of patients with cutaneous melanoma with telomerase-specific peptides. Cancer Immunol Immunother. 2011;60:1553-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Kyte JA. Cancer vaccination with telomerase peptide GV1001. Expert Opin Investig Drugs. 2009;18:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Brunsvig PF, Kyte JA, Kersten C, Sundstrøm S, Møller M, Nyakas M, Hansen GL, Gaudernack G, Aamdal S. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res. 2011;17:6847-6857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 86. | Schlapbach C, Yerly D, Daubner B, Yawalkar N, Hunger RE. Telomerase-specific GV1001 peptide vaccination fails to induce objective tumor response in patients with cutaneous T cell lymphoma. J Dermatol Sci. 2011;62:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Greten TF, Forner A, Korangy F, N’Kontchou G, Barget N, Ayuso C, Ormandy LA, Manns MP, Beaugrand M, Bruix J. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10:209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 88. | Su Z, Vieweg J, Weizer AZ, Dahm P, Yancey D, Turaga V, Higgins J, Boczkowski D, Gilboa E, Dannull J. Enhanced induction of telomerase-specific CD4(+) T cells using dendritic cells transfected with RNA encoding a chimeric gene product. Cancer Res. 2002;62:5041-5048. [PubMed] |
| 89. | Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, Sichi S, Niedzwiecki D, Boczkowski D, Gilboa E. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798-3807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 90. | Suso EM, Dueland S, Rasmussen AM, Vetrhus T, Aamdal S, Kvalheim G, Gaudernack G. hTERT mRNA dendritic cell vaccination: complete response in a pancreatic cancer patient associated with response against several hTERT epitopes. Cancer Immunol Immunother. 2011;60:809-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 91. | Katz MH, Spivack DE, Takimoto S, Fang B, Burton DW, Moossa AR, Hoffman RM, Bouvet M. Gene therapy of pancreatic cancer with green fluorescent protein and tumor necrosis factor-related apoptosis-inducing ligand fusion gene expression driven by a human telomerase reverse transcriptase promoter. Ann Surg Oncol. 2003;10:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Liu J, Zou WG, Lang MF, Luo J, Sun LY, Wang XN, Qian QJ, Liu XY. Cancer-specific killing by the CD suicide gene using the human telomerase reverse transcriptase promoter. Int J Oncol. 2002;21:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 93. | Majumdar AS, Hughes DE, Lichtsteiner SP, Wang Z, Lebkowski JS, Vasserot AP. The telomerase reverse transcriptase promoter drives efficacious tumor suicide gene therapy while preventing hepatotoxicity encountered with constitutive promoters. Gene Ther. 2001;8:568-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 94. | Plumb JA, Bilsland A, Kakani R, Zhao J, Glasspool RM, Knox RJ, Evans TR, Keith WN. Telomerase-specific suicide gene therapy vectors expressing bacterial nitroreductase sensitize human cancer cells to the pro-drug CB1954. Oncogene. 2001;20:7797-7803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Schepelmann S, Ogilvie LM, Hedley D, Friedlos F, Martin J, Scanlon I, Chen P, Marais R, Springer CJ. Suicide gene therapy of human colon carcinoma xenografts using an armed oncolytic adenovirus expressing carboxypeptidase G2. Cancer Res. 2007;67:4949-4955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Zhou JH, Tang B, Liu XL, He DW, Yang DT. hTERT-targeted E. coli purine nucleoside phosphorylase gene/6-methylpurine deoxyribose therapy for pancreatic cancer. Chin Med J. 2007;120:1348-1352. |
| 97. | Irving J, Wang Z, Powell S, O’Sullivan C, Mok M, Murphy B, Cardoza L, Lebkowski JS, Majumdar AS. Conditionally replicative adenovirus driven by the human telomerase promoter provides broad-spectrum antitumor activity without liver toxicity. Cancer Gene Ther. 2004;11:174-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Kawashima T, Kagawa S, Kobayashi N, Shirakiya Y, Umeoka T, Teraishi F, Taki M, Kyo S, Tanaka N, Fujiwara T. Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res. 2004;10:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 99. | Lanson NA, Friedlander PL, Schwarzenberger P, Kolls JK, Wang G. Replication of an adenoviral vector controlled by the human telomerase reverse transcriptase promoter causes tumor-selective tumor lysis. Cancer Res. 2003;63:7936-7941. [PubMed] |
| 100. | Wirth T, Zender L, Schulte B, Mundt B, Plentz R, Rudolph KL, Manns M, Kubicka S, Kühnel F. A telomerase-dependent conditionally replicating adenovirus for selective treatment of cancer. Cancer Res. 2003;63:3181-3188. [PubMed] |
| 101. | Fujiwara T. A novel molecular therapy using bioengineered adenovirus for human gastrointestinal cancer. Acta Med Okayama. 2011;65:151-162. |
| 102. | Fujiwara T, Shirakawa Y, Kagawa S. Telomerase-specific oncolytic virotherapy for human gastrointestinal cancer. Expert Rev Anticancer Ther. 2011;11:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Fujiwara T. Telomerase-specific virotherapy for human squamous cell carcinoma. Expert Opin Biol Ther. 2009;9:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 104. | Onimaru M, Ohuchida K, Nagai E, Mizumoto K, Egami T, Cui L, Sato N, Uchino J, Takayama K, Hashizume M. Combination with low-dose gemcitabine and hTERT-promoter-dependent conditionally replicative adenovirus enhances cytotoxicity through their crosstalk mechanisms in pancreatic cancer. Cancer Lett. 2010;294:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Lin WH, Yeh SH, Yang WJ, Yeh KH, Fujiwara T, Nii A, Chang SS, Chen PJ. Telomerase-specific oncolytic adenoviral therapy for orthotopic hepatocellular carcinoma in HBx transgenic mice. Int J Cancer. 2013;132:1451-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Fujiwara T, Kagawa S, Tazawa H. Synergistic interaction of telomerase-specific oncolytic virotherapy and chemotherapeutic agents for human cancer. Curr Pharm Biotechnol. 2012;13:1809-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 107. | Boozari B, Mundt B, Woller N, Strüver N, Gürlevik E, Schache P, Kloos A, Knocke S, Manns MP, Wirth TC. Antitumoural immunity by virus-mediated immunogenic apoptosis inhibits metastatic growth of hepatocellular carcinoma. Gut. 2010;59:1416-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 108. | Polvani S, Calamante M, Foresta V, Ceni E, Mordini A, Quattrone A, D’Amico M, Luchinat C, Bertini I, Galli A. Acycloguanosyl 5’-thymidyltriphosphate, a thymidine analogue prodrug activated by telomerase, reduces pancreatic tumor growth in mice. Gastroenterology. 2011;140:709-720.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 109. | Tarocchi M, Polvani S, Peired AJ, Marroncini G, Calamante M, Ceni E, Rhodes D, Mello T, Pieraccini G, Quattrone A. Telomerase activated thymidine analogue pro-drug is a new molecule targeting hepatocellular carcinoma. J Hepatol. 2014;61:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Ghosh SS, Gopinath P, Ramesh A. Adenoviral vectors: a promising tool for gene therapy. Appl Biochem Biotechnol. 2006;133:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 111. | Kaplan JM. Adenovirus-based cancer gene therapy. Curr Gene Ther. 2005;5:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 112. | Painter RG, Lanson NA, Jin Z, Park F, Wang G. Conditional expression of a suicide gene by the telomere reverse transcriptase promoter for potential post-therapeutic deletion of tumorigenesis. Cancer Sci. 2005;96:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 113. | Bonini C, Bondanza A, Perna SK, Kaneko S, Traversari C, Ciceri F, Bordignon C. The suicide gene therapy challenge: how to improve a successful gene therapy approach. Mol Ther. 2007;15:1248-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 114. | Wirth T, Kühnel F, Kubicka S. Telomerase-dependent gene therapy. Curr Mol Med. 2005;5:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 115. | Kirby TO, Rivera A, Rein D, Wang M, Ulasov I, Breidenbach M, Kataram M, Contreras JL, Krumdieck C, Yamamoto M. A novel ex vivo model system for evaluation of conditionally replicative adenoviruses therapeutic efficacy and toxicity. Clin Cancer Res. 2004;10:8697-8703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 116. | Steer HJ, Lake RA, Nowak AK, Robinson BW. Harnessing the immune response to treat cancer. Oncogene. 2010;29:6301-6313. [PubMed] |
| 117. | Zhou L, Zheng D, Wang M, Cong YS. Telomerase reverse transcriptase activates the expression of vascular endothelial growth factor independent of telomerase activity. Biochem Biophys Res Commun. 2009;386:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 118. | Zaccagnini G, Gaetano C, Della Pietra L, Nanni S, Grasselli A, Mangoni A, Benvenuto R, Fabrizi M, Truffa S, Germani A. Telomerase mediates vascular endothelial growth factor-dependent responsiveness in a rat model of hind limb ischemia. J Biol Chem. 2005;280:14790-14798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 119. | Bermudez Y, Yang H, Saunders BO, Cheng JQ, Nicosia SV, Kruk PA. VEGF- and LPA-induced telomerase in human ovarian cancer cells is Sp1-dependent. Gynecol Oncol. 2007;106:526-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 120. | Wu XQ, Huang C, He X, Tian YY, Zhou DX, He Y, Liu XH, Li J. Feedback regulation of telomerase reverse transcriptase: new insight into the evolving field of telomerase in cancer. Cell Signal. 2013;25:2462-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 121. | Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK. Telomerase directly regulates NF-κB-dependent transcription. Nat Cell Biol. 2012;14:1270-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 122. | Zhou J, Ding D, Wang M, Cong YS. Telomerase reverse transcriptase in the regulation of gene expression. BMB Rep. 2014;47:8-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 123. | Brower V. Telomerase-based therapies emerging slowly. J Natl Cancer Inst. 2010;102:520-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |