Copyright
©2014 Baishideng Publishing Group Inc.
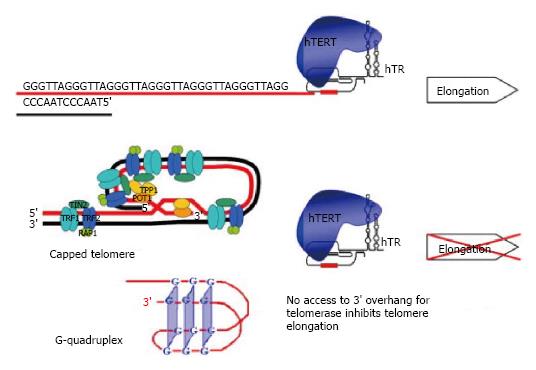
Figure 1 Impact of open or capped telomere structure on the telomerase activity.
(Reproduced with permission from Philippi C, Loretz B, Schaefer UF, Lehr CM. J Control Release 2010; 146: 228-240. Copyright Clearance Center, Inc.). hTERT: Human TElomerase Reverse Transcriptase.
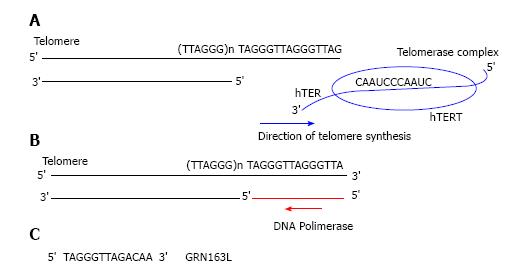
Figure 2 Telomeres an telomerase complex.
(TTAGG)n sequences form a 3’-overhang on the 3’ end of chromosome. Telomerase is composed by hTERT and hTER subunits; hTER is the RNA template for DNA synthesis to add new telomeric TTAGG sequences on 3’-overhang; B: DNA polymerase completes the lagging strand; C: GRN163L sequence complementary. hTERT: Human TElomerase Reverse Transcriptase; hTER: Human TElomerase RNA.
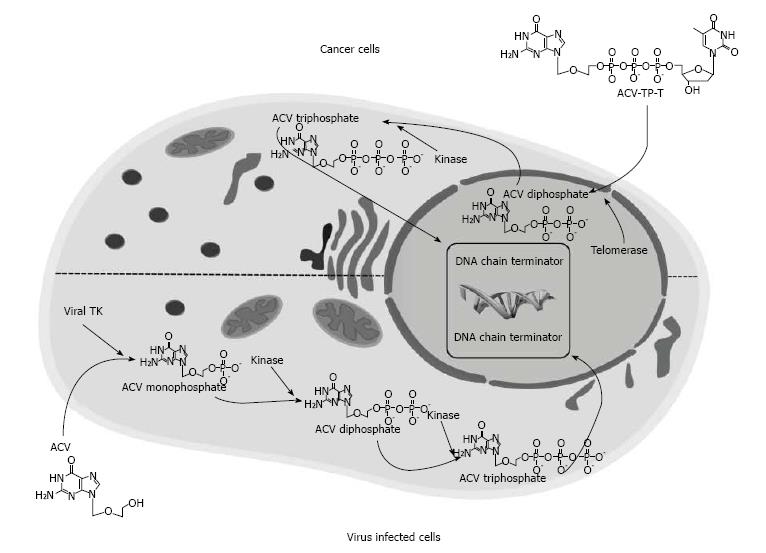
Figure 3 Structure and schematic mode of action of Acycloguanosyl 5’-thymidyltriphosphate in comparison with ACV.
For activation, ACV requires to be phosphorylated to ACV monophosphate by viral TK carried either by wildtype herpes virus or, in the suicide gene therapy, engineered adenovirus. ACV monophosphate is then further phosphorylated by cellular kinases to the triphosphated active form. Conversely, ACV-TP-T is substrate of telomerase that incorporates the thymidine in the replicating telomeres and directly release ACV diphosphate skipping the viral TK phosphorylation step. (Reproduced with permission from Ref [108]. Copyright 2011 AGA Institute). ACV-TP-T: Acycloguanosyl 5’-thymidyltriphosphate; TK: Thymidine kinase.
- Citation: Picariello L, Grappone C, Polvani S, Galli A. Telomerase activity: An attractive target for cancer therapeutics. World J Pharmacol 2014; 3(4): 86-96
- URL: https://www.wjgnet.com/2220-3192/full/v3/i4/86.htm
- DOI: https://dx.doi.org/10.5497/wjp.v3.i4.86