INTRODUCTION
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice, with high prevalence in the geriatric population[1]. AF is a condition in which heart-rhythm control is displaced from the normal sinus node pacemaker by rapid activity in different areas of the atria, resulting in rapid and irregular atrial activity. This pathology is often underestimated because it is usually asymptomatic and rarely life-threatening. However, it can induce palpitations, fainting, congestive heart failure, and stroke due to electrical disturbance, as well as thromboembolism, all of which are associated with high morbidity and mortality[2]. Therefore, a better understanding of the mechanisms of and molecular basis for AF may uncover safer, more effective therapeutic targets and new treatment methods[3].
AF progresses as three different clinical forms, which have been previously well described. Initially, AF occurs in a paroxysmal form characterized by episodes of the arrhythmia that terminate spontaneously. Persistent AF is defined by episodes that are sustained longer than 7 d and require termination by pharmacological or direct-current electric cardioversion. In permanent AF the normal heart rhythm cannot be converted to sinus rhythm. It is believed that AF development from paroxysmal to persistent and permanent forms occurs through the influence of atrial remodelling caused by the arrhythmia itself and/or the progression of underlying heart disease[3]. Therefore, the molecular and electrical remodelling of ion channels determining the potential duration has been proposed as a major mechanism in chronic atrial fibrillation. Prolonged episodes of AF alter atrial properties (“atrial remodelling”), promoting AF persistence. Electrical remodelling alters ion-channel expression and/or function, increasing the likelihood of ectopic firing or re-entry, thereby promoting AF initiation and/or recurrence. Ion-channel remodelling shortens the atrial effective refractory period (ERP), favouring re-entrant arrhythmias, mainly due to the shortening of atrial action potential duration (APD)[4-6]. ERP reduction promotes the conduction block and wave break underlying fibrillatory conduction. APD shortening may lead to AF-related atrial contractile dysfunction[7-11]. The balance between inward currents that depolarize and outward currents that repolarize cardiac myocytes determines APD, and thus ERP. Thus, deregulation of ion expression as a molecular mechanism for AF has been considered quite interesting by many researchesIn addition to electrical remodelling it has been shown that structural alterations and fibrosis are implicated in AF generation and perpetuation[12,13].
Recently microRNA (miRNA) research has evolved rapidly, therefore it is important to comment on the roles of these non-coding short RNA molecules in the cardiovascular system and their involvement in AF. Primarily, miRNAs suppress protein synthesis by inhibiting the translation of protein from mRNA or by promoting the degradation of mRNA, thereby silencing gene expression on the post-transcriptional level. Notably, many recent reports have demonstrated that miRNAs regulate several properties of cardiac physiology and excitability, including automaticity, Ca2+ handling, conduction, and repolarization[14]. Several miRNAs have been shown to have a role in the triggering or prolonging AF, while other miRNAs target several genes considered to have the potential to regulate AF. Avaliable results show the potential role of miRNAs involved in AF.
In this review, we summarize the mechanism of miRNAs as regulators of ion-channels genes and their role in electrical remodelling caused by AF.
ROLE OF MIRNAS IN CARDIAC DISEASES, REMODELLING, AND HOMEOSTASIS
MiRNAs are endogenous, conserved, single-stranded, small (c. 22 nucleotides in length), noncoding RNAs that repress gene expression at the post-transcriptional level by targeting mRNAs[15,16]. A single miRNA can regulate multiple targets; therefore, modulating the expression of one miRNA often results in the modulation of an entire gene network. Genes coding for miRNAs, such as the protein-coding genes, are located in chromosomes as an integral part of the genome; however, these genes may appear more genomically diverse and dynamic. Based on the genomic organization of miRNA genes, miRNAs can be grouped into two classes: (1) intergenic miRNAs, when the miRNA-coding genes are located in genomic regions distinct from known transcription units and are found between protein-coding genes; and/or (2) intragenic miRNAs, if the miRNA-coding genes are located within annotated genes either of protein-coding genes or of long noncoding RNAs as their host genes and they are transcribed by using the host regulatory elements. Curiously, some miRNAs are transcribed from multiple copies of their genes whereas many other miRNAs appear in clusters on a single polycistronic transcript.
Two converging pathways have been demonstrated for the biogenesis of miRNAs: the canonical pathway and the noncanonical pathway[17]. The canonical pathway is Drosha-dependent and is usually used to generate intergenic miRNAs and intronic miRNAs. The first step of this pathway, transcription of miRNAs genes yields transcripts, termed primary miRNA transcripts (pri-miRNAs) with a characteristic hairpin morphology, comprising a loop and an imperfectly paired stem incorporating the mature miRNA sequence on on of the strands, operated mainly by RNA polymerase II. Subsequently, these pri-miRNAs are processed by the Class 2 Rnase enzyme Drosha together with Pasha protein to become precursor miRNAs (pre-miRNAs), which are then transported to the cytoplasm through the Ran-GTP-dependent nuclear pore exportin-5, where they are processed by Dicer (another RNase III enzyme) to generate the final approximately 22 nucleotide product of mature miRNAs. The mature miRNAs are then loaded into the miRNA-associated multi-protein RNA-induced silencing complex (RISC) that includes the Argonaute Proteins (mature miRNAs). A perfect base-pairing of the miRNA (within miRISC complex) motif(s), mainly in the 3’-untranslated region termed the “seed sequence”, a critical site for miRNA actions[18,19], is required for effective repression of translation of the protein. Also, some miRNAs are involved in gene silencing through mRNA degradation. Alternatively, Drosha-independent and either Dicer-dependent or Dicer-independent noncanonical pathways are used to generate Mirtrons, small nucleolar miRNAs and short hairpin miRNAs transfer miRNAs. It is important to highlight that some properties of the miRNAs, such as their ability to influence whole cellular processes instead of single genes, the easy manipulation of miRNA activity in vivo using miRNA inhibitors, and the high intracellular stability of miRNAs, make them a promising therapeutic target[20].
To date, over a thousand miRNAs have been identified and they have been reported to participate in many fundamental biological processes, including cell proliferation, growth, differentiation, apoptosis, and tissue remodelling. The essential role of miRNA in early heart development was first revealed by a Dicer knockout. The early lethality of Dicer mutants, along with the complete depletion of mature miRNAs, indicated the crucial role of miRNAs in early heart development[21]. Additionally, Dicer activity is also required for normal functioning of the mature heart, as adult mice lacking Dicer in the myocardium suffer from high incidence of sudden death, cardiac hypertrophy, and reactivation of the foetal cardiac-gene programme[22]. These data demonstrate that miRNAs play an important role during normal heart development, cardiac remodelling, and homeostasis. Dysregulated miRNAs have been shown to be related to the pathology of many cardiovascular diseases, including arrhythmia, hypertrophy, and heart failure, gaining a critical position as potential therapeutical targets for cardiac diseases. For instance, multiple miRNAs, such as miR-1, miR-133, miR-208, miR-195, miR-21, and miR-18b, have been identified to participate in cardiac hypertrophy and can independently determine this pathological process. Importantly, miR-1, miR-133, miR-21, miR-29, miR-199a, and miR-320 have also been reported to be involved in myocardial infarction[23]. Among them, miR-1 and miR-133 are considered muscle-specific miRNAs and they represent the most abundant miRNAs expressed in the heart[14] .
Interestingly, recent studies have highlighted circulating micRNAs as biomarkers for the AF[24] Moreover, several miRNAs such as miR-24, miR-29b and miR133 have been shown to be involved in myocardial fibrosis[25,26] and open new perspectives to analyse the role of miRNAs in the structural alterations and fibrosis involved in AF generation and perpetuation.
Finally, a few miRNAs have been reported to regulate cardiac excitability through targeting genes encoding ion channels[27-30]. Here we focus on miRNAs which target different ion channels that play key roles in the electrical remodelling of AF.
ION-CHANNEL DYSFUNCTION IN ATRIAL FIBRILLATION
It has been previously shown that the factors responsible for onset of AF include triggers that induce arrhythmia. Triggers include not only sympathetic or parasympathetic stimulation, bradycardia, atrial premature beats or tachy-cardia, accessory AV pathways, and acute atrial stretch, but also ectopic foci occurring in “sleeves” of atrial tissue within the pulmonary veins or vena caval junctions as well[31].
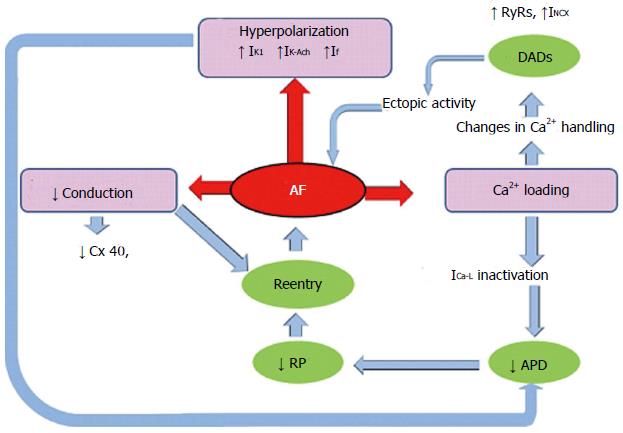
After the onset of AF, prolonged episodes alter atrial properties (“atrial remodelling”) promoting AF persistence. AF-associated remodelling events within the atria include electrical remodelling and, secondarily, structural remodelling and autonomic-nervous-system changes. AF-related electrical remodelling can result from altered expression and/or function of cardiac ion channels, leading to the development of functional re-entry substrates and finally contributing to persistent AF. Therefore, electrical remodelling shortens the atrial ERP and APD, and reduces its physiological rate of adaptation as a result of ion-channel remodelling and abnormal Ca2+ handling (Figure 1).
Figure 1 Mechanisms of atrial fibrillation.
Cytotoxic overloading of Ca2+ occurs by a rapid atrial rate. As a result, this acts as a mechanism of cell protection via the inactivation of L-type Ca2+ current (ICa-L), which in turn reduces the action potential duration (APD) and this consequently shortens the refractory period (RP). This overloading of Ca2+ also contributes to an abnormal function of ryanodine receptor channels (RyRs) and intensifies the inward Na+-Ca2+-exchange current (INCX), leading to delayed afterpolarizations (DADs) and ectopic activity. In addition, AF produces hyperpolarization due to increased ICa-L, which increases the inward rectifier current (IK1) and acetylcholine-regulated K+ current (IK-Ach). This also reduces APD and promotes conduction by decreasing of connexin-40 (Cx40).
The inward sodium current (INa), which is encoded by the voltage-gated cardiac sodium channel SCN5A, initiates the cardiac action potential and this INa is involved in repolarization and refractoriness. It bears highlight that rapid cardiac rates can significantly reduce (INa) density and conduction velocity, triggering AF (Figure 1)[32]. In addition, mutations in SCN5A are related to diseases caused by abnormalities in cardiac conduction, including AF. In this context, Darbar et al[11] tested the hypothesis that vulnerability to AF is associated with variation in SCN5A, the gene encoding the cardiac sodium channel. They found novel as well as rare variants in nearly 6% of the population, including alleles that segregate with AF in other family members, supporting the hypothesis that SCN5A is an important AF-susceptibility gene.
It has been well documented that increased K+ currents or decreased Ca2+ currents shorten APD, promoting re-entrant AF. Because Ca2+ enters atrial cells during AP depolarization, the high atrial rate in AF increase Ca2+ loading, which leads to activation of auto-protective mechanisms to reduce chronic Ca2+ overload. Therefore, in patients with chronic AF, mRNA transcription of the auxiliary L-type Ca2+-channel subunits are significantly diminished reducing the inward Ca2+ currents, maintaining the AP plateau, shortening the AP duration, and finally contributing to re-entry[33]. Moreover, alterations in Ca2+ handling promote diastolic Ca2+ release by increasing open probability of the sarcoplasmic reticulum Ca2+ release channels (RyR2), this in turn promoting delayed after-depolarizations (DADs) and ectopic activity (Figure 1)[5,6,34].
Additionally, AF-related shortening of APD can also result from an increase in K+ channel gene products and/or activity. The inward rectified K+ current (IK1) and IK-ACh are also altered in AF and both AF-related IK1 up-regulation and enhancement of the constitutive form of IK-ACh promote the persistence of AF by reducing APD (Figure 1)[35]. KCNN3, which encodes the small conductance calcium-activated potassium channel 3 (SK3), is a new locus strongly associated with AF[36]. The role of SK channels remains unclear, but it is believed that they contribute to the repolarization of cardiac action[37,38]. A recent study showed SK3 to be strongly down-regulated in permanent AF, which contributed to AF persistence and susceptibility by abbreviating APD[39].
Similarly, it has been well established that chronic AF in humans reduces the transient outward potassium currents (Ito) by transcriptional down-regulation of the Kv4.3 potassium channel[7,8]. The ultrarapid delayed rectifier K+ channel (Ikur) also plays a major part since it has been shown that the amplitude of Ikur is significantly reduced and the current density is lower in patients with AF[10]. More recently it has been demonstrated in human that chronic AF modifications in transcriptional and posttranscriptional mechanisms of HCN channels occur and are associated with a slight yet significant gain-of-function of the hyperpolarization activated “funny” current (If), which may contribute to enhanced atrial ectopy[40].
The slow delayed rectifier potassium channel (IKs), made up of a pore-forming subunit encoded by KCNQ1, has also been implicated in AF. Thus, several mutations in KCNQ1 have been described in AF patients[41,42], some of which are associated with IKs block and AP[43]. The potassium channel voltage-gated subfamily member 2-(KCNH2) is an ion-channel gene that encodes an inwardly rectifying potassium channel (IKr), and a genetic variation in the KCNH2 gene reportedly predisposes Chinese Han individuals to the risk of acquired AF[44]. In addition, lower expression levels of the ATP-sensitive potassium channel (Kir6.2); the acetylcholine-dependent potassium channel (Kir3.4) and the ultra-rapid delayed rectifier potassium channel (Kv1.5) in patients with AF have also been described[10,45]. However, the underlying mechanisms leading to these changes remain unclear.
Finally, although relatively little is known about gap-junction remodelling in the atria, changes in ion channels involved in cardiac conduction have also been reported in relation to AF. Atrial gap junctions are comprosed of two different subunit proteins, i.e., Connexin40 (Cx40) and Connexin43 (Cx43). In the goat, Cx40 expression decreases and the protein becomes more heterogeneously distributed after long periods of fast pacing, contributing to the onset and self-perpetuation of re-entry pathways and AF[46]. Moreover, pacing can induce atrial tachyarrhythmias or fibrillation in Cx40-null mice[47]. These results are consistent with the extensive clinical evidence pointing to disturbances in connexin-40 as a basis for genetic AF predisposition. Importantly, a very recent report has shown a clear reduction in Cx40 but not Cx43 expression (both mRNA and protein) in atrial appendage tissues from patients with lone AF with no Cx40 coding-region mutations identified[47].
In summary, despite that many studies have pointed out the relevance of the AF-related electrical remodelling, our understanding of the underlying molecular mechanisms leading to and perpetuating ion-channel remodelling during AF is very limited. Better knowledge and deeper insights into the molecular mechanisms underlying AF may help to identify new and selective drug targets for improved AF treatment.
MICRORNAS AND ELECTRICAL REMODELLING IN AF
As mentioned above, electrical remodelling is a maladaptive process that can trigger and prolong AF. Recently, multiple research groups have uncovered an important role of several miRNAs in regulating cardiac excitability and arrhythmogenesis by targeting specific ion channels, which might have an important impact on AF onset and/or persistence[48,49].
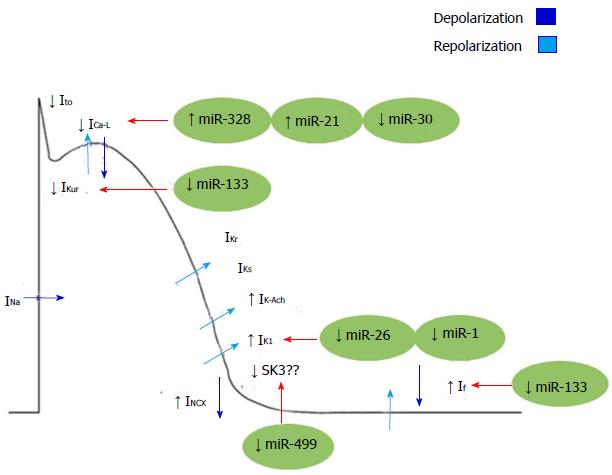
Microarray analyses in a canine model of AF showed that miR-223, miR-328, and miR-664 were significantly up-regulated, whereas miR-101, miR-320, and miR-499 were down-regulated[14,50]. Among these, miR-328 was the most significantly up-regulated and forced expression of miR-328 through adenovirus infection in canine atrium as well as a transgenic approach in mice recapitulated the phenotypes of AF. Enhanced AF vulnerability by diminished L-type Ca2+ current, led to a shortened atrial action potential. Normalization of miR-328 levels with antagomiR reversed the conditions, and genetic knockdown of endogenous miR-328 dampened AF vulnerability, highlighting the importance of miR-328 as a potential therapeutic target for AF[49].
Other miRNAs such as miR-21 can also act as regulators of L-type Ca2+ channel. Barana et al[51] showed that miR-21 was expressed in human myocytes with chronic AF. They demonstrated that this disease increases the expression of miR-21, which decreases ICa-L. Down-regulation of ICa-L by miR-21, suggests that this miR could have an important function in AF through electrical remodelling (Figure 2).
Figure 2 Mechanisms of electrical remodelling in atrial fibrillation regulated by microRNAs.
INa: Inward sodium current; IK1: Inward rectified K+ current; Ito: Transient outward potassium currents; ICa-L: Inactivation of L-type Ca2+ current; IKur: Ultrarapid delayed rectifier K+ channel; IKs: Slow delayed rectifier potassium channel; IK-Ach: Acetylcholine-activated inward-rectifing potassium channel; INCX: Inward Na+-Ca2+-exchange current; SK3: Small conductance calcium-activated potassium channel 3; If: Funny current.
On the other hand, miR-26 has been demonstrated to target KCNJ2, the gene encoding the main component of the inward rectifier potassium-channel current IK1 (KIR 2.1). As stated above, the IK1 is an important regulator of reentrant-spiral dynamics and a major component of AF-related electrical remodelling. Recently, Luo et al[52] has found that miR-26 was down-regulated in AF patients accompanied by IK1/KIR2.1 protein up-regulation. Moreover, by gain and loss-of-function experiments in mice, these authors demonstrated that miR-26 controls the expression of KCNJ2 and thereby promotes AF.
In addition, it has also been shown that expression levels of miR-1 (a muscle specific miRNA) are greatly reduced in the atrial tissue of AF patients. Yang et al[53] showed that the elimination of miR-1 by an antisense inhibitor in infarcted rat hearts relieved arrhythmogenesis, whereas miR-1 overexpression slowed conduction and depolarized the cytoplasmic membrane by post-transcriptionally repressing KCNJ2 and GJA1 (which encodes connexin 43). More recently it has been documented that increased IK1 currents in left atrial cardiomyocytes of AF patients are associated with greater Kir2.1 expression and lower levels of miR-1, reinforcing the notion of a role for miR-1 in regulating atrial remodelling in AF[48]. However, no significant changes in Cx43 mRNA and protein expression nor in Cx43 localization were found in those cardiomyocytes, suggesting that miR-1 may differentially regulate subunits such as Kir2.1 and Cx43 in different cell subtypes[48]. Moreover, a very recent miR array analysis in humans has revealed that miR-1 is greater in the left atrium than in the right one, underscoring the notion that AF pathophysiology may affect the two atria differently[54] (Figure 2).
The possibility that another muscle-specific miRNA such as miR-133 plays a role in AF has also been proposed, since down-regulation of miR-133 favours atrial remodelling by derepression of KCNH2/IKr and KCNH1/IKs. However, there is no direct evidence on how miR-133 affects IKs or IKr current[55,56]. In this way, Luo et al[52] found that miR-1 and miR-133 act to restrict overexpression of HCN2 and HCN4 (encoding for important components of If currents), and down-regulation of miR-1/miR-133 acts partially causing the anomalistic re-expression of HCN2 and HCN4 in cardiac hypertrophy. Yet it remains unclear whether miR-1 and miR-133 affect HCN channels in the context of AF[57]. Notwithstanding, mirR-133 together with miR-30 were found to be down-regulated in a canine model of AF, reinforcing the potential role of miR-133 in AF[58] (Figure 2).
Together with miR-1 and miR-133, miR-208a is also a muscle-specific miRNA, which is encoded within an intron of the α-cardiac muscle myosin heavy-chain gene (Myh6). Although no direct evidence for the involvement of miR-208a in atrial electrical remodelling have been reported, miR-208a transgenic gain of function in mice lead to arrhythmias. In addition, Cx40 expression was increased in those transgenic mice, indicating that miR-208a could modulate electrical conduction in the heart[59] (Figure 2).
Finally, in a recently reported finding, a potential role of miR-499 in AF-related electrical remodelling has been proposed. Atrial miRNA-499 is significantly upregulated in AF, (Figure 2), leading to down-regulation of the small-conductance SK3, possibly contributing to the electrical remodelling in AF[39].
MiRNA research has highlighted the roles of miRNAs in the cardiovascular system and their involvement in AF. Further studies on miRNA and AF will provide new insights into the mechanisms underlying that disorder and may offer new strategies for the treatment of AF.
CONCLUDING REMARKS AND CLINICAL PERSPECTIVES
Electrical remodelling secondary to the onset of atrial fibrillation dramatically contributes to the recurrence of AF, eventually leading to persistent AF. Many recent studies demonstrates that miRNAs are becoming one of the most fascinating regulatory molecules, due to their critical roles in fine-tuning of physiological processes and their deregulation in several disorders, including AF. The functional significance of miRs as direct or indirect post-transcriptional regulators of ion channels involved in electrical remodelling strongly suggests that these miRNAs may serve as potential biomarkers or promising drug targets in the treatment, and management of AF, though considerable work remains to be done. Further studies making use of existing AF models, miRNA expression signatures and miRNA target gene prediction should provide information finally applicable to clinical practice.