Revised: May 9, 2013
Accepted: May 20, 2013
Published online: May 23, 2013
Processing time: 86 Days and 16 Hours
AIM: To evaluate the role of chymase in blood pressure regulation and its actions on tissue renin-angiotensin system.
METHODS: A two-kidney, one-clip (2K1C) hypertension model was developed in Syrian hamsters, which have a human-type chymase. Either an angiotensin (Ang) converting enzyme (ACE) inhibitor (ACE-I; temocapril, 30 mg/kg per day), Ang II type 1 receptor antagonist (ARB; CS866, 10 mg/kg per day), or vehicle was administered, beginning 2 wk after renal artery clipping and continued for 16 wk. At the end of this protocol, hearts, aortas, and lungs were removed, and total Ang II-forming activities and ACE- and chymase-dependent Ang II-forming activities were determined.
RESULTS: After renal artery clipping, systolic blood pressure in the vehicle group was significantly higher compared with that in a sham-operated group throughout the experimental period. Both ACE-I and ARB treatments revealed similar antihypertensive effects. Moreover, in the vehicle group, cardiac total and chymase-dependent Ang II-forming activities significantly increased at 18 wk after clipping. Further, cardiac total and chymase-dependent Ang II-forming activities decreased significantly after ACE-I or ARB treatment for 16 wk. In addition, chymase-dependent Ang II-forming activity significantly increased in the aorta, although these changes were inhibited only by ARB. ARB treatment was more effective compared with ACE-I treatment in reversing the changes in tissue Ang II formation, particularly in the aorta, despite their similar antihypertensive effects.
CONCLUSION: Chymase does not play a major role in maintaining blood pressure and tissue ACE and chymase are regulated in a tissue-dependent manner in 2K1C hamster.
Core tip: There are several pathways that can produce angiotensin (Ang) II in human tissues, which are involved in remodeling of the cardiovascular system. Among these, chymase has been exhibited the greatest Ang II-forming enzyme in human heart. In hypertensive hamster, blood pressure was significantly elevated, and both Ang converting enzyme (ACE) inhibitor (ACE-I) and Ang II type 1 receptor antagonist (ARB) revealed similar antihypertensive effects. Interestingly, cardiac chymase-dependent Ang II-formation decreased after ACE-I or ARB treatment. In addition, chymase-dependent Ang II-formation increased in the aorta, although these changes were inhibited only by ARB. ACE and chymase were regulated in a tissue-dependent manner in hypertensive hamsters, and the both enzymes were independently regulated.
- Citation: Uehara Y, Fujimi K, Yahiro E, Abe S, Devarajan S, Saku K, Urata H. Induction of tissue angiotensin II-forming activity in two-kidney, one-clip hypertensive hamster model. World J Hypertens 2013; 3(2): 9-17
- URL: https://www.wjgnet.com/2220-3168/full/v3/i2/9.htm
- DOI: https://dx.doi.org/10.5494/wjh.v3.i2.9
Angiotensin (Ang) II is generated as a final active product of the renin-angiotensin system (RAS). Skeggs et al[1] reported that Ang II was generated from the inactive precursor Ang I by angiotensin-converting enzyme (ACE). Ang II plays an important role not only in regulating blood pressure by inducing aldosterone synthesis and secretion and vasopressin secretion but also in promoting cardiovascular tissue remodeling[2]. Previous clinical studies have reported that ACE inhibitors revealed beneficial effects in patients with hypertension, congestive heart failure, and myocardial infarction[3-5].
Several Ang II-generating systems that are independent of ACE have been reported[6]. In a previous study, we reported that serine proteases such as trypsin, tissue kallikrein, and tonin directly formed Ang II from angiotensinogen in a low pH condition in addition to forming bradykinin from kininogen at physiological pH[7,8]. Moreover, we demonstrated that tissue kallikrein purified from the rat submandibular gland or human urine can convert Ang I to Ang II[7]. Several serine proteinases, including chymase, kallikrein, and cathepsin G, are probably responsible for ACE-independent Ang II formation in human tissues; in particular, chymase has been reported as a major Ang II-generating enzyme in human heart[9].
Chymase is a chymotrypsin-like serine protease that is stored in a complex with heparin proteoglycan in the secretary granules of mast cells. Chymase is found in human blood vessels, heart, and several other tissues[10], but not in plasma[11]. Chymase remains in this complex, which binds to the extracellular matrix, and remains active for several weeks after it is released from these granules. In the cardiovascular system, chymase is primarily produced by mast cells in the heart[9] and in the blood vessels[12].
Human chymase represents more than 80% of the Ang II-forming activity in tissue extracts from human myocardium, which suggests that ACE is not the major Ang II-forming enzyme in the human left ventricle in vitro[9,13]. Furthermore, our studies have demonstrated that most Ang II-forming activity was dependent on chymase but not on ACE in other human tissues and that there were marked species differences among rat, hamster, rabbit, dog, and human species with regard to the Ang II-forming capacity of ACE versus that of chymase[14]; however, the physiological roles of chymase have not yet been determined. Interestingly, human chymase primarily forms Ang II from Ang I, whereas rat chymase I generates Ang I (5-10) rather than Ang II[13]; thus, while human chymase is an Ang II-forming enzyme, rat chymase I is an Ang II-degrading enzyme.
Because of these species differences, care must be taken when studying the physiological roles of human chymase in animals with human-type chymase. Hamsters are suitable for studies of this type because their chymase primarily generates Ang II from Ang I[15,16]; therefore, we compared the effects of an ACE inhibitor and an Ang II type 1 receptor antagonist (ARB) in two-kidney, one-clip (2K1C) hypertensive hamsters to examine a possible role for chymase in regulating blood pressure and tissue renin-angiotensin systems.
Eight-week-old male Syrian hamsters (100-130 g) were obtained from Biotec Co. (Saga, Japan). They were housed in separate cages and had free access to tap water and hamster chow. The study protocol was approved by the Animal Care and Use Committee of Fukuoka University.
The hamsters were anesthetized using pentobarbital. After making an incision in the back, a silver clip (internal diameter, 0.15 mm) was attached on the left renal artery. The right kidney and renal artery were not touched. The control hamsters underwent a sham operation under anesthesia. All the hamsters were again given free access to tap water and standard hamster chow.
CS-866, a nonpeptide angiotensin II type I receptor antagonist: olmesartan, temocapril, and inactin were kindly provided by Daiichi-Sankyo, Co. (Tokyo, Japan). Ang I, Ang II, captopril, and other chemicals were purchased from Sigma (St. Louis, MO, United States), unless otherwise noted.
Systolic blood pressure (SBP) was measured in the right hind leg with a pulse transducer and inflation cuff (MK-1100, Muromachi K.K. Co., Tokyo, Japan) under inactin (115 mg/kg) anesthesia on days 0 and 2, and at 18 and 34 wk after surgery. The accuracy of this method was confirmed by comparing the SBP values obtained using the following two methods in the same hamster. Carotid artery SBP was measured directly using a cannula and a pressure transducer. SBP was measured indirectly in the right hind leg under thiobutabarbital (inactin) anesthesia. A highly significant correlation (r = 0.923) was observed between the indirect and direct SBP values.
The hamsters were divided into four groups for measurements on day 14 after surgery. (1) Sham Group; Sham operation; (2) Vehicle Group; 2K1C + saline; (3) ACE-I Group; 2K1C + ACE-I; and (4) ARB Group; 2K1C + ARB.
Either ACE-I (temocapril, 30 mg/kg per day), ARB (CS866, 10 mg/kg per day), or vehicle was administered, beginning 14 d after clipping and continued for 16 wk. At the end of this experimental period, hearts, aortas, and lungs were removed to determine total and ACE- and chymase-dependent Ang II-forming activities.
Heart, aorta, and lung samples were homogenized in sodium phosphate buffer (50 mmol/L; pH 7.4) and centrifuged at 40000 g for 20 min. Ang II forming activity from Ang I was determined as described elsewhere with some modification[16,17]. The samples prepared as above were incubated with synthetic Ang I (0.2 mmol/L) at 37 °C for 30 min. The Ang II formed was analyzed by high-performance liquid chromatography (HPLC) using a C18 reverse-phase column (2.2 cm × 25 cm; Vydac) with a 15-min liner acetonitrile gradient (5% to 16%) in 25 mmol/L triethylamine-phosphate buffer, pH 3, at a flow rate of 2 mL/min. Ang II forming activity were expressed as nanomoles or picomoles of Ang II formed per minute per milligram of protein. Captopril (1 mmol/L)- or chymostatin (0.1 mmol/L)-inhibitable (both from Sigma Chemical Co) and aprotinin (0.24 mmol/L) (Bayer)-insensitive Ang II formations were expressed as ACE- and chymase-dependent Ang II forming activity, and the aprotinin-inhibitable Ang II forming activity was presented as others activity which is mainly dependent on cathepsin G. Ang II forming activity analyses for each sample were performed in duplicate, and the reproducibility and quality of all data were confirmed before statistical analyses.
At 18 and 34 wk after clipping, RNA was extracted from hamster tissues in the sham-operated and vehicle groups. Total RNA was isolated from the heart, aorta, and lung. Single-strand cDNA was synthesized from total RNA (5 μg) that was hybridized with 20 pmol of a random primer by heating at 72 °C for 10 min. A reverse transcriptase reaction was run in 20-μL reaction volume. ACE, β-actin, and chymase mRNA levels were assessed by reverse transcription polymerase chain reaction.
The polymerase chain reaction (PCR) primers for hamster chymase, ACE, and β-actin mRNA were selected on the basis of the hamster cDNA sequences: chymase: sense primer, 5′-AAT CGC TCA CCC AAA CTA CAG C-3′, antisense, 5′-GCA GAC CTG AAA ATA ACT G-3′; ACE: sense primer, 5′-GCT TGC CCA ACA AGA CTG CCA -3′, antisense, 5′-CCA CAT GTC TCC CAG CAG ATG -3′; β-actin: sense primer, 5′-TCC TGA CCG AGC GTG GCT ACA GC-3′, antisense, 5′-CTC CTG GAA GGT GGA CAG TGA GG -3′. The PCR products were electrophoretically separated on 1%-2% agarose gel, which was stained with ethidium bromide, and photographed.
Results are presented as means ± SEMs. Statistical analysis was performed using Statview J 5.0 software. Results were compared by two-way ANOVA for treatment and time effects. A P value of < 0.05 was considered statistically significant.
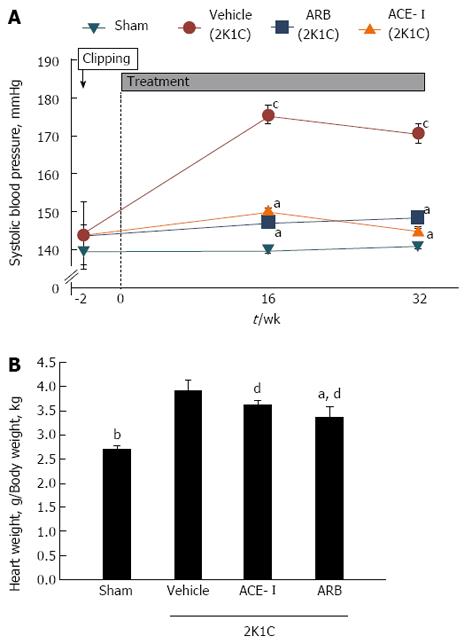
Systolic blood pressure in the vehicle group was significantly higher compared with that in the sham-operated group throughout the experimental period. Both ACE-I and ARB treatment revealed similar antihypertensive effects (Figure 1A). At the end of the experimental protocol, hamster hearts were removed and weighed. Heart weight as a proportion of total body weight increased in the vehicle group compared with the sham-operated group (P < 0.01). Heart weights significantly decreased after 16 wk of ARB treatment (Figure 1B). Furthermore, right and left kidneys were removed and weighed. The weight of the left kidney (clipped side) decreased in the vehicle group compared with the sham-operated group. In contrast, right kidney weight increased in the vehicle group compared with the sham-operated group.
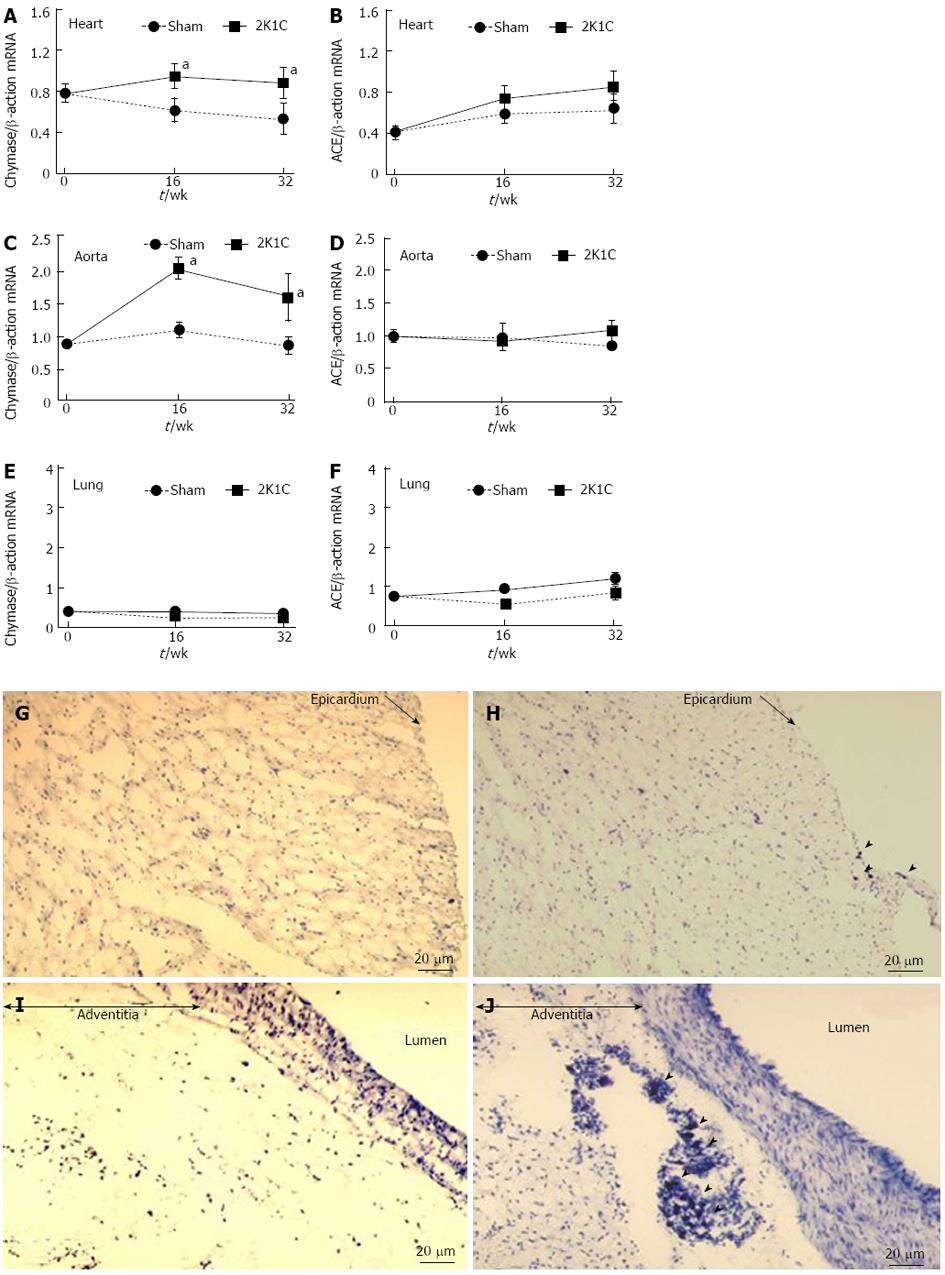
Cardiac chymase mRNA expression significantly increased in the 2K1C hypertensive model, whereas this expression slightly decreased at 16 and 32 wk in the sham-operated group (Figure 2A). However, cardiac ACE mRNA levels did not change (Figure 2B). Aortic chymase mRNA expression dramatically increased in the 2K1C group compared with the sham-operated group (Figure 2C, D). On further comparison, pulmonary chymase mRNA expressions were at lower levels in the 2K1C hamsters, and both chymase and ACE mRNA expressions did not reveal any significant changes in the lungs of 2K1C hamsters (Figure 2E, F).
The numbers of cardiac mast cells (MCs) significantly increased at 18 wk after clipping. Cardiac mast cells were localized in the subepicardial tissue in the hamster heart (Figure 2G, H). On aorta sections, the numbers of MCs were significantly increased and were only localized in the aortic adventitia (Figure 2I, J). These changes were also inhibited by both ARB and ACE-I treatment (data not shown).
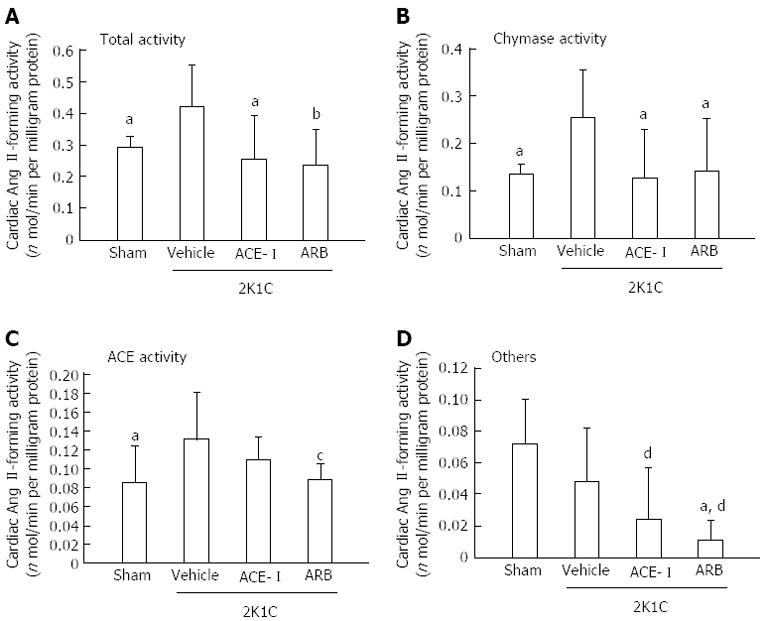
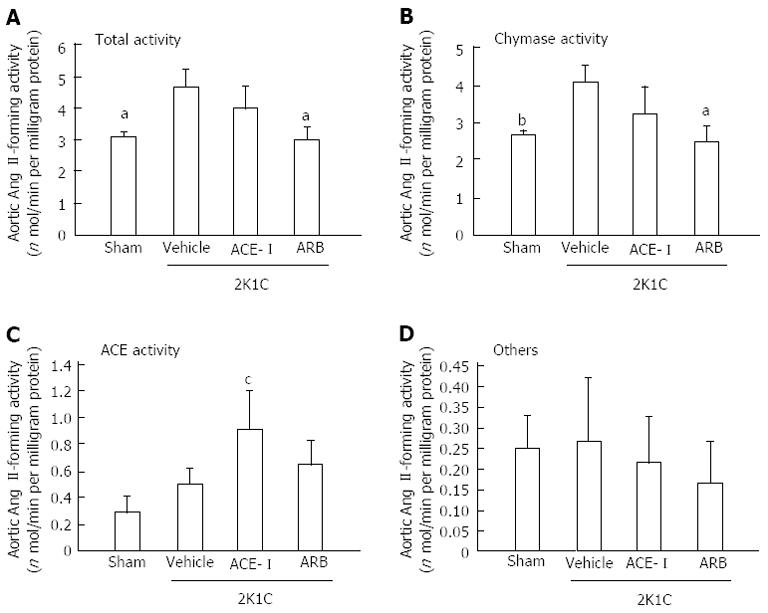
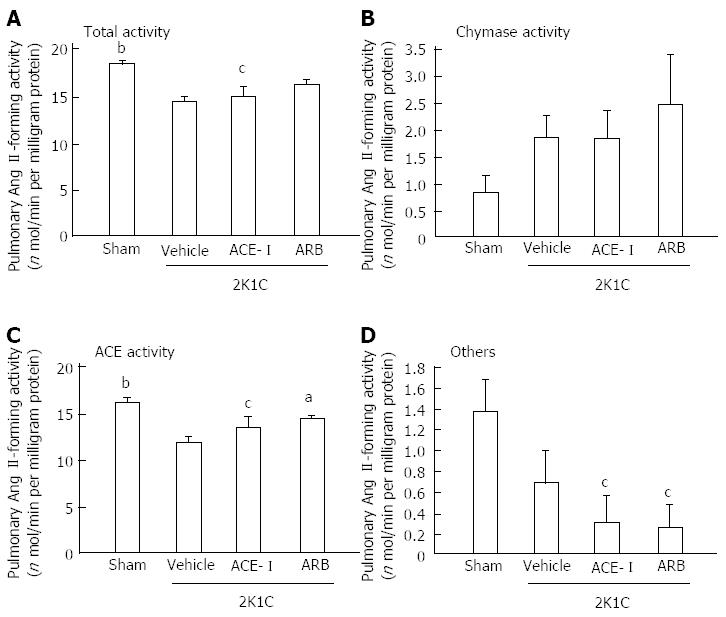
Because the increased chymase mRNA levels in the heart and aorta peaked at 16 wk after clipping, tissue Ang II formation was assessed at 18 wk after clipping (16 wk of treatment). In the vehicle group, cardiac total and chymase- and ACE-dependent Ang II-forming activities significantly increased at 16 wk. Both ACE-I and ARB treatment significantly suppressed these increases in cardiac total and chymase-dependent Ang II-forming activities (Figure 3). In addition to hamster heart, total and chymase-dependent Ang II-forming activities significantly increased in the aorta at 18 wk after clipping. However, aortic tissue ACE-dependent Ang II-forming activity did not change. These changes in aortic Ang II formation were inhibited by ARB but not by ACE-I treatment (Figure 4).
In contrast to the hamster heart and aorta, ACE rather than chymase was the major Ang II-forming enzyme in the lung (Figure 5). Pulmonary tissue ACE activity significantly decreased at 18 wk after clipping, and pulmonary tissue ACE-dependent Ang II-forming activity significantly increased after 16 wk of ARB treatment; however, ACE-I treatment resulted in no change. These results suggested that tissue-specific Ang II forming systems were independently regulated.
The aim of this study was to evaluate the role of chymase in blood pressure regulation and its actions on tissue RAS in 2K1C hypertensive hamsters. With this model, we compared the depressor effects of ACE-I and ARB. Each drug was used at its sufficient dosage[18-20]. We expected that blood pressure would decrease to a greater extent in ARB- than in ACE-I-treated hamsters if an ACE-independent Ang II-forming pathway (i.e., chymase-dependent Ang II formation) played a role in maintaining high blood pressure; however, we observed that both ARB and ACE-I treatments provided similar antihypertensive effects. This suggested that elevating and maintaining blood pressure in the 2K1C hypertensive hamster model was not dependent on tissue ACE-independent Ang II formation.
Chymase-dependent Ang II formation has been previously reported in hamsters, for which ACE inhibitors could not prevent the pressor response to [Pro11, D-Ala12] Ang I; Ang II could be produced by incubating Ang I with purified chymase[21]. Human chymase appears to be involved in clinical disorders such as atherosclerotic lesions[22], acute coronary syndrome[23], disorders in the metabolism of apolipoprotein[24], and extracellular matrix degradation[25-28].
Some remarkable differences in Ang II-forming pathways have been reported among various species[13]. In situ hybridization and electron microscopic and immunocytochemical studies have demonstrated that mast cells, endothelial cells, and other cell types within the interstitial, extracellular matrix were sites for synthesis and storage of chymase. In this study, we counted the numbers of mast cells in the hamster heart and aorta. Cardiac mast cell number changes paralleled cardiac tissue chymase activity. Furthermore, aortic mast cell numbers changed in parallel with aortic tissue chymase activity. Moreover, cardiac and aortic mast cells were localized in the subepicardial and adventitia layers in the hamster heart and aorta, respectively.
In contrast, ACE is bound to the cell membranes of endothelial cells and fibroblasts and has its catalytic site exposed to the extracellular space; therefore, compared with chymase, ACE may be more readily accessible to plasma Ang I. Moreover, these discrepancies may be because of the different models of hypertension used. We investigated the changes in blood pressure during the early phase of renin-angiotensin-dependent hypertension. However, the etiology of essential hypertension in humans is more heterogeneous, and hypertension develops more slowly than in the 2K1C model. Further studies are needed to determine the role of chymase in human hypertension.
With regard to chymase-like activity and ACE activity on the heart in hypertensive hamsters, we observed decreased total Ang II-forming activity. ACE-dependent Ang II-forming activity increased, whereas chymase-dependent activity decreased in the hearts of untreated hypertensive hamsters. In the lungs of untreated hamsters, both total Ang II-forming activity and ACE-dependent Ang II-forming activity significantly increased, whereas chymase-dependent Ang II-forming activity did not change. These changes in Ang II-forming activity in untreated hamsters reversed on treatment with ACE-I and ARB.
Hoit et al[29] reported that chymase activity decreased in all the cardiovascular tissues in 2K1C hypertensive baboons, although significant changes were observed only in aortic tissues. In contrast, increased ACE mRNA levels and ACE activity were observed in the cardiovascular tissues of 2K1C hypertensive rats. Our findings regarding the changes in chymase-like activity vs ACE activity of hypertensive hamsters were essentially consistent with these earlier findings. These changes suggest that elevated blood pressure and/or increased plasma Ang II levels might be regulated differently by the activities of chymase and ACE in the 2K1C hypertensive model.
The importance of ACE-independent Ang II formation in human pathophysiology has not been clarified yet. Nevertheless, the results of our previous studies suggested the significance of ACE-independent Ang II formation. First, we demonstrated that nafamostat, a serine protease inhibitor, was effective for improving blood flow in patients with peripheral vascular disease, probably by inhibiting the local formation of Ang II by a serine protease[30]. In addition, we observed that a serine protease-dependent Ang II-forming pathway was activated during exercise in normal volunteers[31]. Although the clinical significance of chymase is not well understood, we demonstrated that vascular chymase-like activity but not ACE activity increased markedly in atherosclerotic and/or aortic aneurysm lesions in humans and a hamster model[14,16,22].
Although previous studies suggested that an increase in tissue RAS activity may be associated with atherosclerotic development, it remains unclear whether vascular RAS activation is a cause or a consequence of this phenomenon. Few reports strongly suggested that increased arterial Ang II production was the primary event rather than a secondary event during the development of atherosclerosis. Arterial chymase- and ACE-dependent (but not cathepsin G-dependent) Ang II formation is upregulated in the histologically normal internal thoracic artery. Increased tissue Ang II formation initiates a chain of events that can result in atherosclerosis in arteries that are exposed to hypercholesterolemia[14] .
Moreover, we have evaluated the association between cholesterol and arterial chymase activity in a hamster model: when Syrian hamsters were fed a high fat diet, we observed marked lipid deposition in the aortic cusp, and the plasma cholesterol levels positively correlated with aortic chymase activity[16]. When an orally active nonpeptide chymase inhibitor (SUN-C8257) was combined with a high-cholesterol diet in this model, aortic lipid deposition nearly disappeared. In the present study, aortic tissue Ang II formation was dramatically increased in our 2K1C hypertensive model. Surprisingly, increased vascular Ang II formation can be suppressed only by treatment with ARB but not by ACE-I. These results suggest that arterial chymase-dependent Ang II production because of high blood pressure may promote atherosclerosis; furthermore, ARB may suppress the development of atherosclerosis.
Ang-(1-7) is also important biological active peptide of RAS, and it exerts vasoactive actions and can be considered as a physiological Ang II counter-regulatory peptide[32]. Resent study has demonstrated that the intrarenal generation of Ang-(1-7) is reduced in 2K1C rat[33]. This result suggests that the induction of tissue Ang II level might be attributed to not only activation of Ang II-generating enzyme, but also deficiency of Ang II-degradative system such as ACE2-Ang-(1-7) pathway in 2K1C hypertensive model.
In summary, the results of the present study indicated that chymase did not play a major role in elevating and maintaining blood pressure in this 2K1C hypertensive hamster model. Tissue Ang II-forming enzymes, ACE and chymase, in 2K1C hypertensive hamsters were regulated in a tissue-dependent manner, and both of these enzymes were regulated independently of each other. In addition, ARB treatment was more effective than ACE-I treatment for reversing the changes in tissue Ang II formation, particularly in the aorta, despite having similar antihypertensive effects.
Angiotensin (Ang) II plays an important role not only in regulating blood pressure but also in promoting tissue remodeling. There are several pathways that can produce Ang II in human tissues, which are involved in structural remodeling of the cardiovascular system. Among these, chymase has been demonstrated to exhibit the greatest Ang II-forming activity in human heart.
Inhibitors of the angiotensin converting enzyme (ACE) have been widely used to protect against organ damage in the treatment of hypertension, cardiac hypertrophy, and congestive heart failure. ACE contributes to the tissue remodeling through the generation of Ang II from Ang I; nevertheless, the results of some studies using ACE inhibitors have suggested the existence of alternative Ang II-generating pathways, since Ang II levels do not diminish in the plasma and tissues after long-term ACE inhibition. Local renin-angiotensin system (RAS) exists in human as well as animal models, and it has an important role in tissue remodeling such as cardiac hypertrophy and atherosclerosis, independent of systemic RAS.
Various large-scale clinical trials have not shown a predominance of Ang II type 1 receptor antagonist (ARB) in the treatment of heart failure as compared with ACE-I. In this hypertensive model, both ACE-I and ARB treatment suppress cardiac total and chymase-dependent Ang II-forming activities equally. Nevertheless, aortic Ang II formation are inhibited by ARB but not by ACE-I treatment and ARB treatment was more effective than ACE-I treatment for reversing the changes in tissue Ang II formation in the aorta. These findings strongly suggest that a predominance of ARB for tissue remodeling works in the aortic tissues.
A suppression of local Ang II-formation can lead to reducing of cardiovascular disease; in particular the treatment with ARB might contribute to anti-atherosclerotic effects.
Ang II, a final active product of RAS was generated from the inactive precursor Ang I by ACE, whereas there are several Ang II-generating systems that are independent of ACE have been existed in tissue local RAS. Above all, chymase is a major Ang II-generating enzyme in human heart and vessels. There are species differences for Ang II generating system, and hamster has human-type chymase which formed Ang II from Ang I.
The authors examined the study evaluated the role of chymase in blood pressure regulation and its actions on tissue RAS in 2K1C hypertensive hamsters. The methods of the study were novel to analyze the same problem.
P- Reviewers Chen Y, Mukaddam-Daher S, Tan XR, Wang G S- Editor Wen LL L- Editor A E- Editor Liu XM
| 1. | Skeggs LT, Lentz KE, Kahn JR, Dorer FE, Levine M. Pseudorenin. A new angiotensin-forming enzyme. Circ Res. 1969;25:451-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Uehara Y, Miura S, Yahiro E, Saku K. Non-ACE pathway-induced angiotensin II production. Curr Pharm Des. 2013;19:3054-3059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Parratt JR. Cardioprotection by angiotensin converting enzyme inhibitors--the experimental evidence. Cardiovasc Res. 1994;28:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Low-dose captopril for the treatment of mild to moderate hypertension. I. Results of a 14-wk trial. Veterans Administration Cooperative Study Group on Antihypertensive Agents. Arch Intern Med. 1984;144:1947-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5265] [Cited by in RCA: 4966] [Article Influence: 146.1] [Reference Citation Analysis (0)] |
| 6. | Campbell DJ. Extrarenal renin and blood pressure regulation. An alternative viewpoint. Am J Hypertens. 1989;2:266-275. [PubMed] |
| 7. | Arakawa K, Maruta H. Ability of kallikrein to generate angiotensin II-like pressor substance and a proposed ‘kinin-tensin enzyme system’. Nature. 1980;288:705-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ideishi M, Ikeda M, Arakawa K. Direct angiotensin II formation by rat submandibular gland kallikrein. J Biochem. 1987;102:859-868. [PubMed] |
| 9. | Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348-22357. [PubMed] |
| 10. | Urata H, Strobel F, Ganten D. Widespread tissue distribution of human chymase. J Hypertens Suppl. 1994;12:S17-S22. [PubMed] |
| 11. | Urata H, Healy B, Stewart RW, Bumpus FM, Husain A. Angiotensin II-forming pathways in normal and failing human hearts. Circ Res. 1990;66:883-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 348] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Okunishi H, Oka Y, Shiota N, Kawamoto T, Song K, Miyazaki M. Marked species-difference in the vascular angiotensin II-forming pathways: humans versus rodents. Jpn J Pharmacol. 1993;62:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Akasu M, Urata H, Kinoshita A, Sasaguri M, Ideishi M, Arakawa K. Differences in tissue angiotensin II-forming pathways by species and organs in vitro. Hypertension. 1998;32:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Uehara Y, Urata H, Sasaguri M, Ideishi M, Sakata N, Tashiro T, Kimura M, Arakawa K. Increased chymase activity in internal thoracic artery of patients with hypercholesterolemia. Hypertension. 2000;35:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Takai S, Song K, Tanaka T, Okunishi H, Miyazaki M. Antinociceptive effects of angiotensin-converting enzyme inhibitors and an angiotensin II receptor antagonist in mice. Life Sci. 1996;59:PL331-PL336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Uehara Y, Urata H, Ideishi M, Arakawa K, Saku K. Chymase inhibition suppresses high-cholesterol diet-induced lipid accumulation in the hamster aorta. Cardiovasc Res. 2002;55:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Murakami K, Uehara Y, Abe S, Inoue Y, Ideishi M, Saku K, Urata H. Positive correlation between chymase-like angiotensin II-forming activity in mononuclear cells and serum cholesterol level. J Cardiol. 2007;50:291-298. [PubMed] |
| 18. | Nakamura Y, Yoshiyama M, Omura T, Yoshida K, Izumi Y, Takeuchi K, Kim S, Iwao H, Yoshikawa J. Beneficial effects of combination of ACE inhibitor and angiotensin II type 1 receptor blocker on cardiac remodeling in rat myocardial infarction. Cardiovasc Res. 2003;57:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Taniyama Y, Morishita R, Nakagami H, Moriguchi A, Sakonjo H, Shokei-Kim K, Nakamura T, Higaki J, Ogihara T. Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation. 2000;102:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Shimizu H, Nakagami H, Osako MK, Hanayama R, Kunugiza Y, Kizawa T, Tomita T, Yoshikawa H, Ogihara T, Morishita R. Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB J. 2008;22:2465-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Nishimura H, Buikema H, Baltatu O, Ganten D, Urata H. Functional evidence for alternative ANG II-forming pathways in hamster cardiovascular system. Am J Physiol. 1998;275:H1307-H1312. [PubMed] |
| 22. | Ihara M, Urata H, Kinoshita A, Suzumiya J, Sasaguri M, Kikuchi M, Ideishi M, Arakawa K. Increased chymase-dependent angiotensin II formation in human atherosclerotic aorta. Hypertension. 1999;33:1399-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Kaartinen M, Penttilä A, Kovanen PT. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation. 1994;90:1669-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 269] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Paananen K, Kovanen PT. Proteolysis and fusion of low density lipoprotein particles independently strengthen their binding to exocytosed mast cell granules. J Biol Chem. 1994;269:2023-2031. [PubMed] |
| 25. | Banovac K, De Forteza R. The effect of mast cell chymase on extracellular matrix: studies in autoimmune thyroiditis and in cultured thyroid cells. Int Arch Allergy Immunol. 1992;99:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Saarinen J, Kalkkinen N, Welgus HG, Kovanen PT. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem. 1994;269:18134-18140. [PubMed] |
| 27. | Vartio T, Seppä H, Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 1981;256:471-477. [PubMed] |
| 28. | Kofford MW, Schwartz LB, Schechter NM, Yager DR, Diegelmann RF, Graham MF. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem. 1997;272:7127-7131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 141] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Hoit BD, Shao Y, Kinoshita A, Gabel M, Husain A, Walsh RA. Effects of angiotensin II generated by an angiotensin converting enzyme-independent pathway on left ventricular performance in the conscious baboon. J Clin Invest. 1995;95:1519-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Urabe Y, Ideishi M, Sasaguri M, Ikeda M, Arakawa K. Beneficial effects of a serine protease inhibitor in peripheral vascular disease. Am J Cardiol. 1993;72:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Miura S, Ideishi M, Sakai T, Motoyama M, Kinoshita A, Sasaguri M, Tanaka H, Shindo M, Arakawa K. Angiotensin II formation by an alternative pathway during exercise in humans. J Hypertens. 1994;12:1177-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Varagic J, Trask AJ, Jessup JA, Chappell MC, Ferrario CM. New angiotensins. J Mol Med (Berl). 2008;86:663-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Prieto MC, González-Villalobos RA, Botros FT, Martin VL, Pagán J, Satou R, Lara LS, Feng Y, Fernandes FB, Kobori H. Reciprocal changes in renal ACE/ANG II and ACE2/ANG 1-7 are associated with enhanced collecting duct renin in Goldblatt hypertensive rats. Am J Physiol Renal Physiol. 2011;300:F749-F755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |