Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.103948
Revised: March 12, 2025
Accepted: April 27, 2025
Published online: September 20, 2025
Processing time: 250 Days and 3.4 Hours
We aimed to identify the key proteins of miR-142-3p that regulate ferroptosis and ultimately control the downstream effectors of cardiomyocyte growth.
To investigate the role of miR-142-3p in regulating ferroptosis and its impact on diabetes-induced myocardial infarction via the PI3K/AKT/GSK3β pathway.
We constructed bones mesenchymal stem cells (BMCs) with low miR-142-3p expression and investigated its role using cell flow cytometry and western blotting (WB). A diabetes myocardial infarction model was established using streptozotocin and coronary artery ligation. The rats were divided into six groups (n = 15 per group): Control, sham surgery model, liraglutide intervention, BMCs intervention, and low miR-142-3p BMCs intervention. Interventions lasted for 7 days and BMCs injected for once. Blood glucose levels were monitored, and myocardial infarction improvements were assessed via electrocardiogra, general heart observation, staining techniques, and WB analysis.
We observed that miR-142-3p increased BMC apoptosis and affected AKT and GSK3β. The myocardial infarction drug, liraglutide, BMCs, and miR-142-3p low expression BMCs intervention showed improvement in differing degrees. The liraglutide and BMCs showed significant blood glucose reduction (0.05). BMCs increased the expression of PI3K, AKT, and GSK3, leading to an increase in the myocardial infarction intervention group, liraglutide, and BMCs intervention groups. The low miR-142-3p expression intervention with BMCs group had the lowest PI3K and AKT protein expression. Liraglutide improved ferroptosis markers (increased COX-2, decreased GPX4 and CHCHD6). Low miR-142-3p BMCs increased COX-2, GPX4, and CHCHD6. CCM3 and VEGFR2 expression increased in BMCs and low miR-142-3p groups, promoting myocardial repair, but decreased in the low miR-142-3p groups.
The preliminary results showed that the therapeutic mechanism of BMCs in diabetes myocardial infarction may involve miR-142-3p via the PI3K/AKT/GSK-3β axis, which jointly inhibits ferroptosis and programmed death.
Core Tip: This study demonstrated that using bones mesenchymal stem cells (BMCs) as an intervention can simultaneously reduce blood sugar levels and promote hypoxic myocardial recovery. However, when miR-142-3p expression in BMCs was low, there was a reduced therapeutic efficacy of BMCs and activation of PI3K/AKT/GSK-3β. The regulatory axis regulates changes in the protein expression levels of the ferroptosis-related genes GPX4/CHCHD6. These findings suggest that miR-142-3p via the PI3K/AKT/GSK-3β regulation axis that jointly inhibits ferroptosis, may be one of the therapeutic mechanisms of BMCs in diabetic myocardial infarction.
- Citation: Gao N, Wu PF, Wu MW, Li YM, Liang X, Wang FF, Li XJ, Shen QQ, Zheng TP, Liu XL, Sun Y, Yang LX. PI3K/AKT/GSK3β regulatory axis in bone mesenchymal stem cells initiates diabetic myocardial infarction via miR-142-3p. World J Exp Med 2025; 15(3): 103948
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/103948.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.103948
In 2021, the International Diabetes Federation released a report indicating that the prevalence of diabetes will be one in every 10 adults aged 20–79 years, for a total of 537 million people worldwide. By 2045, the number of diagnosed patients with diabetes is projected to reach 73.6 million globally, with one in four diabetes patients being from China[1]. Diabetes is an independent risk factor for cardiovascular disease, and research has shown that women aged < 55 with type 2 diabetes have a tenfold increased risk of coronary heart disease[2]. The risk of myocardial infarction in patients with diabetes without a history of coronary heart disease in the next 8–10 years is as high as 20%[3-5], and the risk of recurrent myocardial infarction in patients with diabetes with a history of myocardial infarction in the future is over 40%. Diabetes complicated by myocardial infarction has a high incidence and fatality rate and typically affects younger individuals. However, due to its complex etiology, potential long course, and lack of accurate and effective prevention and treatment measures, there is an urgent need for treatments that can rapidly restore myocardial function. Therefore, it is important to explore treatment methods for myocardial cell regeneration following myocardial infarction caused by diabetes.
Mesenchymal stem cells (MSCs) are adult pluripotent stem cells isolated from bone marrow. They possess self-renewal and multi-directional differentiation abilities and can differentiate into cardiomyocytes through transformation, paracrine signaling, and other methods[6-9]. MSCs have been widely used as part of an important method to promote myocardial cell repair and regeneration after myocardial infarction. However, the key genes that play a therapeutic role in MSCs cannot be fully expressed, and their therapeutic effects need improvement. MicroRNAs (miRNAs) are a class of small non-coding RNAs that regulate gene expression. MiRNAs combine with intracellular cofactors to form protein RNA complexes, which play an important role in the onset and development of various diseases, particularly cardiovascular diseases[10,11], and have complex mechanisms involved in the pathological processes of myocardial hypertrophy, myocardial fibrosis, myocardial apoptosis, arrhythmia, and cardiac failure after myocardial infarction. Studies have shown that miRNAs are involved in the regulation of physiological left ventricular hypertrophy caused by exercise, suggesting that miRNAs may be involved in the transcriptional regulation of cardiac myocyte hypertrophy[12,13]. Bioinformatics revealed that miR-142 can bind to the bases 389-395 of the 3′ untranslated region of the chemokine receptor 7 mRNA on the cell surface. Simultaneously, gene chip technology has been used to screen the differential expression profile of miRNAs in the myocardial tissue of diabetic rats. It was found that miR-142-3p expression was downregulated, suggesting that miR-142-3p might be associated with the development of diabetic cardiomyopathy[10,12].
The characteristics of ferroptosis, such as iron metabolism disorder, lipid peroxide accumulation, and ROS overload, are present at different stages of cardiovascular disease progression. Regulatory factors related to ferroptosis, such as toll-like receptor 4, are up-regulated[14-16], suggesting that the regulation of ferroptosis may be a key event in the improvement of myocardial cells in myocardial infarction. The expression of miR-142-3p significantly increased in the exosomes extracted from the peripheral blood of patients with hepatitis B virus-positive liver cancer. Genes related to intracellular iron metabolism and homeostasis, such as ferritin heavy chain 1, transferrin receptor 1, recombinant GPX4, and activating transcription factor 4, had abnormal expression levels in M1 macrophages. However, the relationship between miR-142-3p in BMCs and ferroptosis during the repair of damaged cardiomyocytes remains unclear.
PI3Ks is a family of lipid kinases composed of phosphorylated phosphatidylinositol eukaryotic cell membranes that can recruit signaling proteins including AKT. AKT, also known as protein kinase B, can control multiple biological processes, including cell survival, proliferation, growth, and glycogen metabolism. The PI3K/AKT signaling pathway is closely related to metabolism, and the regulatory factors and downstream molecules of PI3K/AKT are important targets for the treatment of type 2 diabetes mellitus. Cellular AKT regulates two processes by glycolysis of glucose 6-phosphate and GSK3 to produce cellular energy, which promotes glycogen production inhibition through glycolysis. AKT promotes phosphorylation of GSK3, resulting in inhibitory effects. Therefore, the PI3K/AKT/GSK3β signaling pathway is a key regulator of cell survival, proliferation, and metabolism[17-19].
Therefore, this study aimed to utilize miR-142-3p as an entry point to further investigate PI3K/AKT/GSK3β after the low expression of miRNA-142-3p in BMSCs in an in vivo experimental model of diabetic myocardial infarction. The study further aimed to elucidate the regulatory mechanisms involved in the proliferation and directional activation of damaged cardiomyocytes and explore whether MSCs, using miRNA-142-3p interference, can repair myocardial cell damage caused by high glucose. The study also explored the modulation of gene expression related to ferroptosis and programmed death to achieve efficient regulation of MSCs' directional differentiation. The aim was to establish MSC cell therapy as an effective treatment for challenging diseases.
The T25 cell culture bottle was placed in a 37 °C, 5% CO2 cell culture incubator for 3–4 hours for cell state stabilization. Pancreatic digestion was performed, and the bones mesenchymal stem cells (BMCs) were passaged. A cell counter was used for measuring cell numbers.
Well-growing BMSCs of 4 × 104/mL density were transferred into a 24-well plate and transfected when the convergence rate was approximately 50%–70%. A culture medium containing a final concentration of 5 µg/mL polybrene, and 10.8 µL of virus solution was added. After 12 hours of infection, a half-change of the solution was performed. Cell fluorescence was observed under an inverted fluorescence microscope at 72 hours to determine transfection efficiency. Upon successful cell transfection, a culture medium containing 3 µg/mL of puromycin was added for screening. The selected BMSCs were then transferred from a 24-well plate to a 6-well plate and subsequently transferred to a culture bottle for cultivation. Cells were frozen and resuspended when required.
The calculation method is as follows:
miR-142-3P (MOI = 100):
= Virus titer (TU/mL) × virus volume (mL)/number of cells
= 2.8 × 108 (TU/mL) × 7.2 µl/4 × 104 × 0.5
miR-142-NC (MOI = 100):
= Virus titer (TU/mL) × virus volume (mL)/number of cells
= 1 × 109 (TU/mL) × 2 µl/4 × 104 × 0.5
When the cell fusion degree reached 80%–90%, the cells were collected in a 15 mL centrifuge tube and centrifuged at 1000 rpm at room temperature for 5 minutes. After centrifugation, the concentration was adjusted to 2 × 107 cells/mL, and the cells were resuspended in 1 mL of binding buffer. Subsequently, 100 µL of the prepared cell suspension (approximately 1 × 106 cells) was added to a 5 mL flow cytometry tube, and 5 µL of Annexin V-APC/PI reagent was added. After thorough blowing and mixing, the cells were incubated at room temperature in the dark for 15 minutes, and then 200 µL of binding buffer was added to each tube. The prepared cell suspension was filtered using a cell filter and analyzed using flow cytometry.
The experiment used 90specific pathogen-free grade 8-week-old Sprague Dawley male rats purchased from Hunan Slake Jingda Company (Certificate No.430727220100671527). The experiment was approved by the Ethics Committee of Guilin Medical University (GLMC20200209)[1].
The rats were anesthetized using 1% pentobarbital sodium. Tracheal intubation was performed, and the rats were connected to a ventilator (tidal volume 3 mL/kg, respiratory ratio 1:1, respiratory rate 80–100 times/min). The intercostal space was exposed, and the chest cavity was opened at the 4th and 5th intercostal spaces. The centrifugal capsule was carefully peeled off to fully expose the left atrial appendage and left ventricle. A No. 7 suture needle was used to penetrate the surface of the myocardium approximately 2 mm from the lower edge of the left atrial appendage. This step involved ligating the left anterior descending branch of the coronary artery. If the color of the left ventricular anterior wall myocardium became lighter or whiter, surgery was considered successful. In the sham-surgery group, the chest was opened and sutured without ligation. One week later, after the wounds of the rats had healed, the diabetes model was induced by intraperitoneal injection of streptozotocin (STZ) in rats. The administered dose of STZ was 60 mg/kg. After 72 hours, glucose and fasting blood sugar levels were measured. The model was deemed successful when the fasting blood sugar level measured during the glucose test was > 16.7 mmol/L.
One week after the successful modeling of diabetes, 90 rats were selected from 105 diabetes rats models according to the random number table method to establish the model of diabetes combined with cardiac infarction: After fasting for 10 hours before operation, the rata were anesthetized by intraperitoneal injection of 0.2 mL/10 g ready to use mouse anesthetic, and then the left front chest of the rats was prepared for skin preparation. After that, the rats were fixed in a self-made foam plate with adhesive tape: The two upper incisors of the rats were hung with the surgical suture of the shell, the right upper limb was attached to the foam plate perpendicular to the longitudinal axis of the body, the left upper limb was attached to the foam plate at a 45° angle with the longitudinal axis of the body, and the upper and lower limbs were fixed on the right side of the body side together. After fixing the body position, erect the foam plate, aim the halogen cold light source at the mouse's trachea, gently clamp out the tongue with sterilized tweezers to fully expose the open and closed valves, intubate the trachea and connect the small animal ventilator (tidal volume 1.5, respiratory ratio 1:2, respiratory rate 130 times/minutes). After connecting the small animal ventilator, fix the cannula and place the foam plate flat. After fully disinfected by iodophor, passively separate the skin and muscle layer by layer. Open the chest cavity at the fourth and fifth rib spaces. After being fixed by the chest opener, strip the centrifugal capsule to fully expose the left atrial appendage and left ventricle. A needle is inserted horizontally through the surface of the myocardium at a depth of approximately 1.5 mm, approximately 2.0 mm below the left atrial appendage. The needle is then withdrawn near the pulmonary artery cone and the left anterior descending branch of the coronary artery is ligated. When the color of the left ventricular anterior wall myocardial tissue becomes lighter or whiter, successful modeling of myocardial infarction is achieved. To prevent infection of the wound in rats during surgery, penicillin powder is applied to the wound site during suturing, and to restore negative pressure in the mouse chest cavity, excess gas in the mouse chest cavity is squeezed out during suturing. After suturing, a small animal electric blanket is used to maintain the body temperature of the rats. After the rats are anesthetized and conscious, they are placed in a mouse cage disinfected with alcohol beforehand.
The rats were randomly divided into a control group, sham surgery group, model group, myocardial infarction drug intervention group (pre-intervention group), hypoglycemic drug liraglutide intervention group (pre-intervention group), BMCs intervention group, and miR-142-3p low expression BMCs intervention group [Knockdown (KD) group], with 15 rats per group. The control group consisted of rats without any treatment. The rats with thoracotomy and threading but without ligation were used as the sham surgery group. The model group consisted of rats with successful modeling without any intervention, and the rats with successful modeling were administered clopidogrel bisulfate tablets (60 mg/kg/day) + Atorvastatin calcium tablets (8 mg/kg/day) by gavage as the myocardial infarction intervention group. The rats successfully modeled by intraperitoneal injection of liraglutide (200 µg/kg/12 hours) were used as the hypoglycemic drug intervention group. The BMSCs group and miR-143-3p low-expression BMSCs intervention group were created from the successful model rats. BMSCs (1 mL, 1 × 107/mL) were injected once into the BMSCs group, while the miR-143-3p low expression BMSCs intervention group received the same intervention. On day seven of the intervention, all rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium (3.5 mL/kg), and fixed on the operating table. The rats were examined using a Cardiofax electrocardiograph, and their hearts were immediately collected after the electrocardiograms.
The rats were weighed every seven days. On the third day after the STZ injection, fasting was implemented during the night, and the fasting blood sugar was measured the following day. Blood glucose modeling was deemed successful when the fasting blood sugar was > 16.7 mmol/L. After the measurements were taken, a normal diet was restored.
Routine dehydration embedding of mouse heart tissue fixed in 10% formalin fixative. Slice thickness of 6 μ m, dewaxed with xylene, followed by rinsing, alcohol gradient dehydration, transparency, wax immersion, embedding, and slicing. After drying the slices, dewaxing treatment was performed, and the slices were dewaxed to water. Specific operations: Xylene I 5-10 minutes, xylene II 5-10 minutes, anhydrous ethanol I 5 minutes, anhydrous ethanol II 5 min, 95% alcohol 5 min, 85% alcohol 5 min, 75% alcohol 5 minutes, soaking in UP water 5 minutes; Hematoxylin staining for 10-20 minutes; Rinse with tap water for 1-3 minutes; Hydrochloric acid alcohol differentiation takes 5-10 seconds; Rinse with tap water for 1-3 minutes; Put it in warm water at 50 °C or weakly alkaline aqueous solution to turn blue until blue appears; Rinse with tap water for 1-3 minutes; Add 85% alcohol for 3-5 minutes; Eosin staining for 3-5 minutes; Wash with water for 3-5 seconds; Gradient alcohol dehydration; Xylene transparent; Seal with neutral gum. Leica DM4B upright fluorescence microscope is used for observation and photography. A panoramic 250 digital slice scanner produced by 3DHISTECH (Hungary) was used to obtain images of the slices. Each slice was first examined at low magnification to observe the overall pathological changes in the entire tissue. Subsequently, 40 × and 200 × images were collected from the area, and specific lesions were observed.
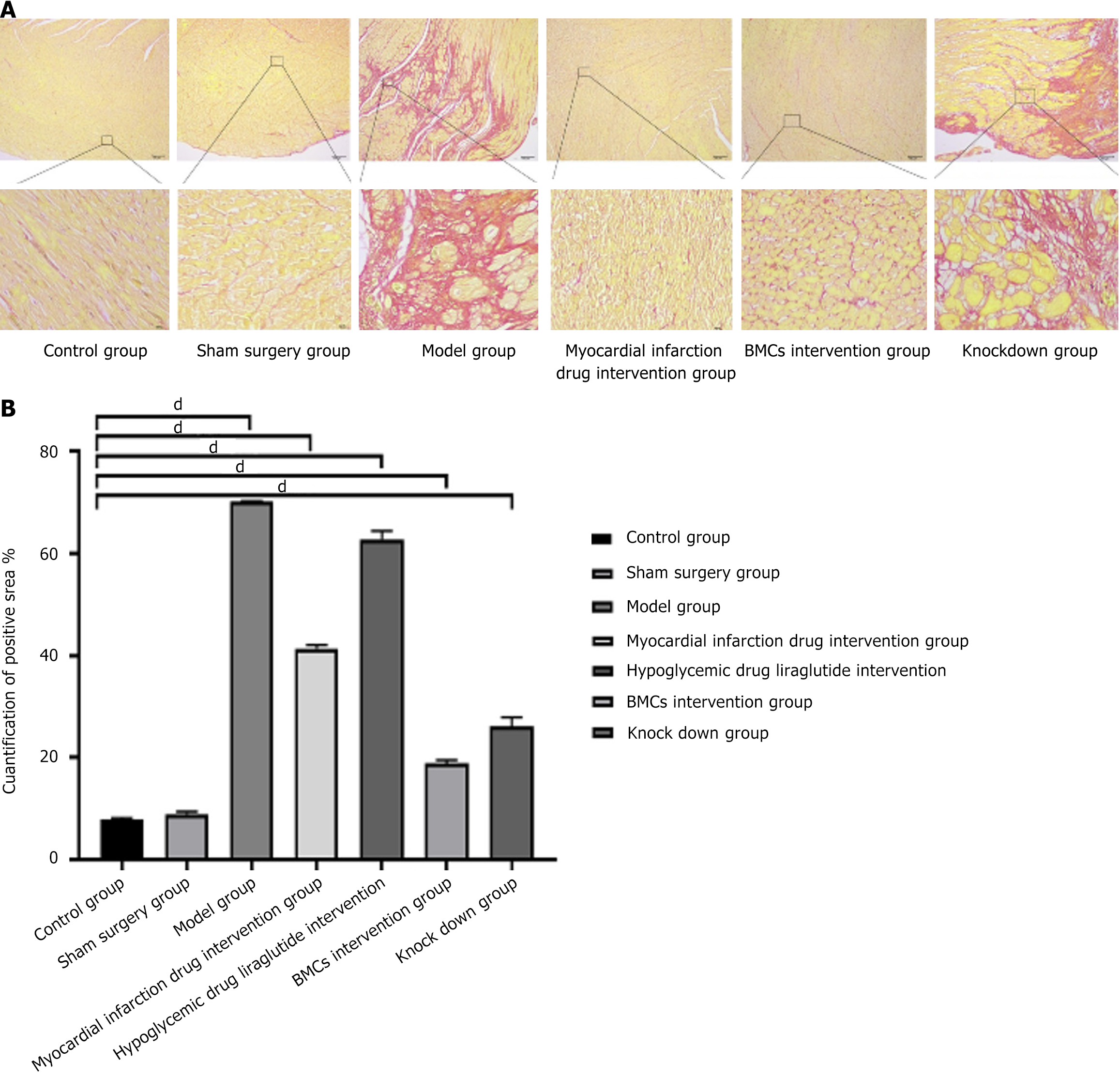
Sirius red staining was used to detect myocardial infarction, and the heart tissue was fixed and embedded in 10% formalin for cardiac perfusion and fluid replacement. After routine dehydration treatment, the tissue was embedded. Take a slice with a thickness of about 6 μm, dewaxing it with water and then bleaching it until it is clear and anhydrous. Lightly drop the Sirius Red staining solution into the nuclear solution for 1 hour, rinse it with running water to remove the surface staining solution of the slice, and then spray dye the surface of the cell nucleus with Mayer's hematoxylin staining solution for about 10 minutes according to the conventional method. Rinse it with running water for about 10 minutes. Dehydrate and float it to a clear and transparent state using the conventional method, and seal it with neutral gum. Ordinary light mirrors can capture and observe; Collagen fibers appear red, cell nuclei appear green, and other components appear yellow. It can be photographed and observed using a standing fluorescence ultraviolet microscope and Leica DM4B mirror. Each slice was first observed at low magnification for all tissues, and then three representative regions at 400 × magnification were selected based on the tissue size and expression to capture the images. The percentage of type I and III collagen expression in the collected images was measured using the Image-Pro Plus 6.0 image analysis system (Media Cybernetics, United States).
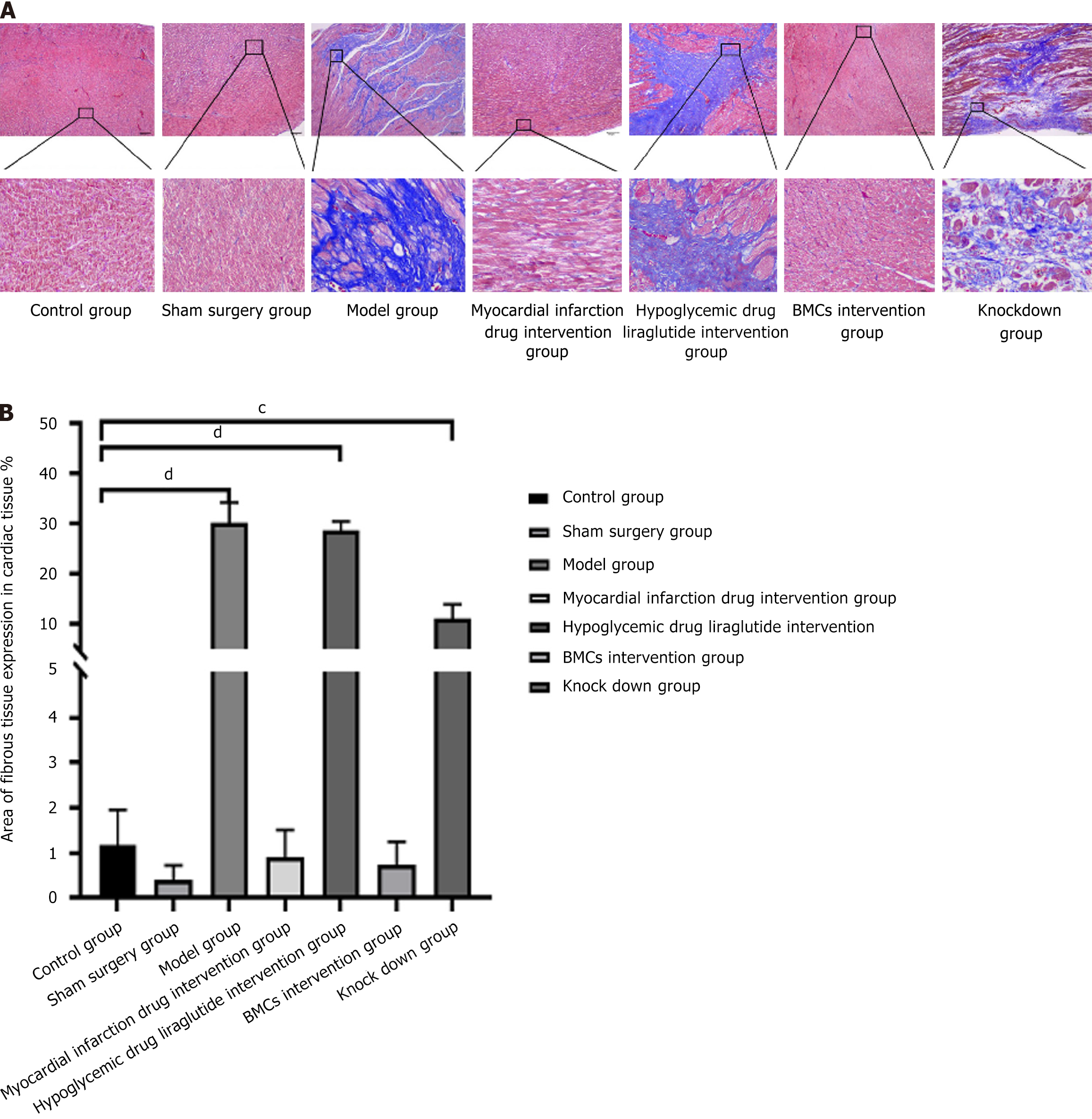
Heart tissue is fixed in 10% formalin fixative and embedded after routine dehydration. The thickness of the heart slice is about 6 μm. The heart slice tissue is conventionally dewaxed to dehydration, fixed with formaldehyde, and then soaked with an appropriate amount of tap water and a small amount of distilled water for rinsing. The nuclei are stained with Regaud hematoxylin staining solution or directly stained with Weigert hematoxylin semen for 10 minutes. Thoroughly wash with water. In case of excessive staining, use concentrated hydrochloric acid alcohol solution for staining and differentiation. Rinse repeatedly with distilled water and stain with Masson's solution or acidic counterstain solution for 10 minutes. Then immerse and stain with approximately 2% glacial acetic acid solution for a moment. Stain and differentiate with 1% ammonium phosphomolybdate aqueous solution for 5 minutes, with aniline blue solution or light green solution for 5 minutes, and then immerse in water again with approximately 0.2% acetic acid aqueous solution for differentiation and staining for a moment. Seal 95% or more with alcohol, anhydrous alcohol, xylene solution, or other transparent, neutral gum agents. Observe using a Leica DM4B upright fluorescence microscope. Each slice was first observed at low magnification for all tissues, and then three regions at 400 × magnification were selected based on tissue size and expression to capture the images. Image-Pro Plus 6.0 image analysis system (Media Cybernetics, United States) was used to measure the area of fibrous tissue in the collected images and calculate the percentage of fibrous tissue expression area: Fibrous tissue area/field of view area (pixel area).
BMCs in a good logarithmic growth phase were added to each group of cells. Protein was extracted using lysate, and the protein was denatured at 100 °C. Precordial myocardial tissue (100 mg/mL) was added to the RIPA lysis buffer, the protein was extracted, and the BCA protein was quantified. Conventional gel preparation, sample loading, electrophoresis, and film transfer were performed. A horizontal shaking table was used for TBST cleaning for 5 minutes per time. Subsequently, 5% skimmed milk powder was added at room temperature, and mixing on the horizontal shaking table continued for 1 h. TBST cleaning was performed on the horizontal shaking table for 5 min per time. The primary antibodies PI3K (1:1000), AKT (1:10000), GSK3 (1:500), and β- Actin (1:1000) were incubated overnight at 4 °C. The corresponding secondary antibodies, PI3K secondary antibody (1:10000), AKT secondary antibody (1:1000), and GSK3 and β-actin antibodies (1:5000), were incubated at room temperature for 2 hours, and the FluorChem M multicolor fluorescence and chemiluminescence imaging systems were used for detection. ImageJ software was used for protein grayscale quantitative analyses.
Statistical analyses were performed using SPSS version 21.0 software (SPSS Inc., United States). The independent sample t-test and χ2 test were used to compare the two groups of data. A single-factor analysis of variance was used to compare multiple data groups. The Bonferroni test was used to compare two groups of data. A rank-sum test was used if the data did not follow a normal distribution. Normally distributed data were expressed as mean ± SD. Otherwise, it was expressed as the median and interquartile range. A P value < 0.05 was considered statistically significant.
All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Ethics Committee of Guilin Medical University (IACUC protocol number: GLMC-IACUC-2023009).
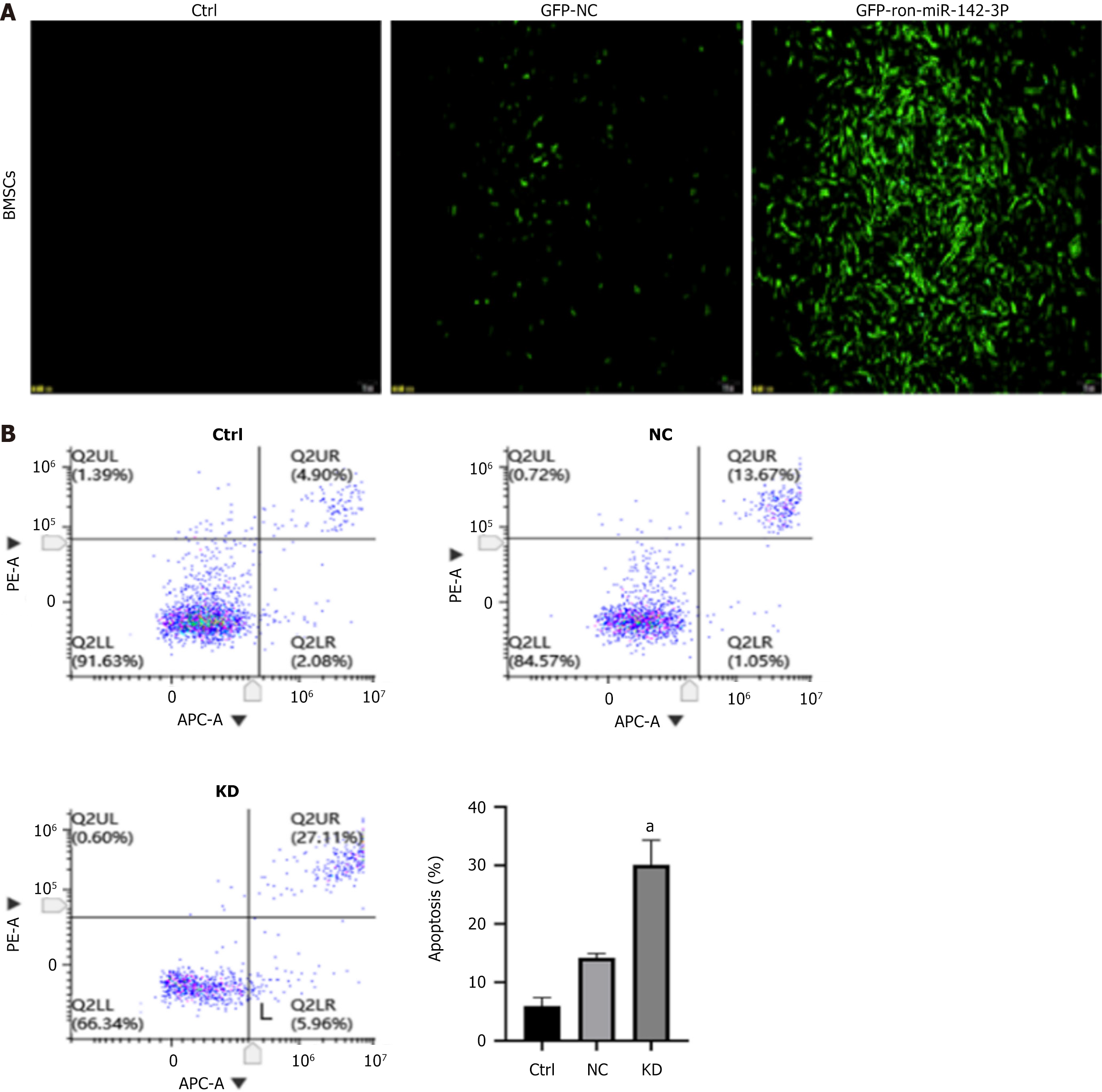
The expression of green fluorescent protein was observed 72 h after lentivirus infection of BMSCs with different multiplicity of infection (MOI) values (MOI = 50, 100, and 150). When MOI was 100 and transfection time was 72 hours, the expression of green fluorescence in each group was significantly enhanced, meeting the experimental requirements, and there was no significant difference in morphology between BMSCs that were not transfected with the lentivirus. Therefore, an MOI of 100 and a transfection time of 72 hours were initially selected as the optimal MOI value and time for lentiviral transfection into BMSCs for subsequent formal experiments. The results are shown in Figure 1A.
After transfecting BMSCs with the miR-142-3p low-expression lentivirus, cells in each group were collected and analyzed for early and late apoptosis using an APC/PI assay kit. The flow cytometry results indicated that among the results of each group, the overall apoptosis rate (early withering + late withering) of untreated BMSCs was the lowest. In contrast, the overall apoptosis rate of NC group cells increased; however, there was no significant difference. Additionally, the overall apoptosis rate of the KD group was significantly increased (P < 0.05) after one-way analysis of variance (ANOVA) analysis, as shown in Figure 1B.
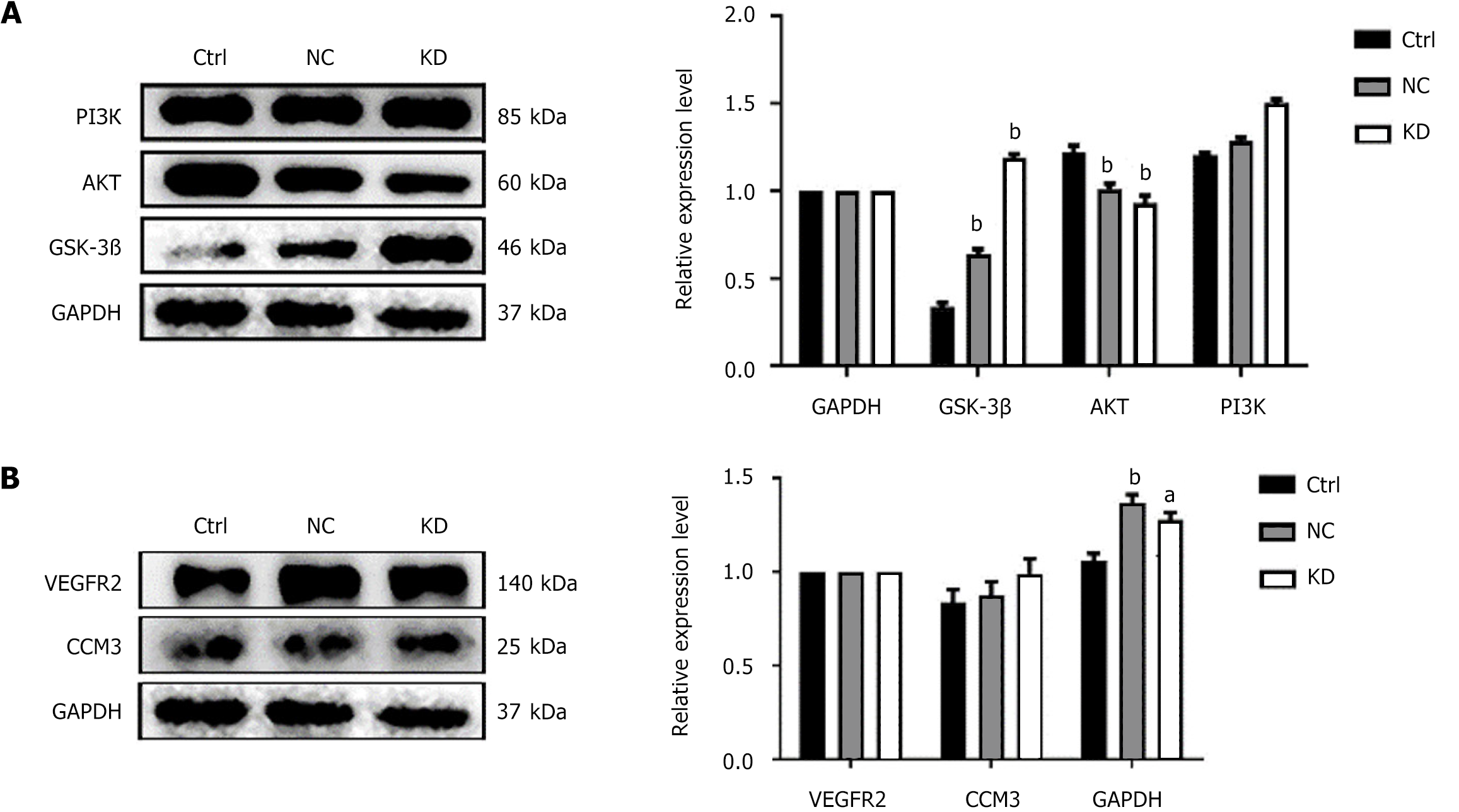
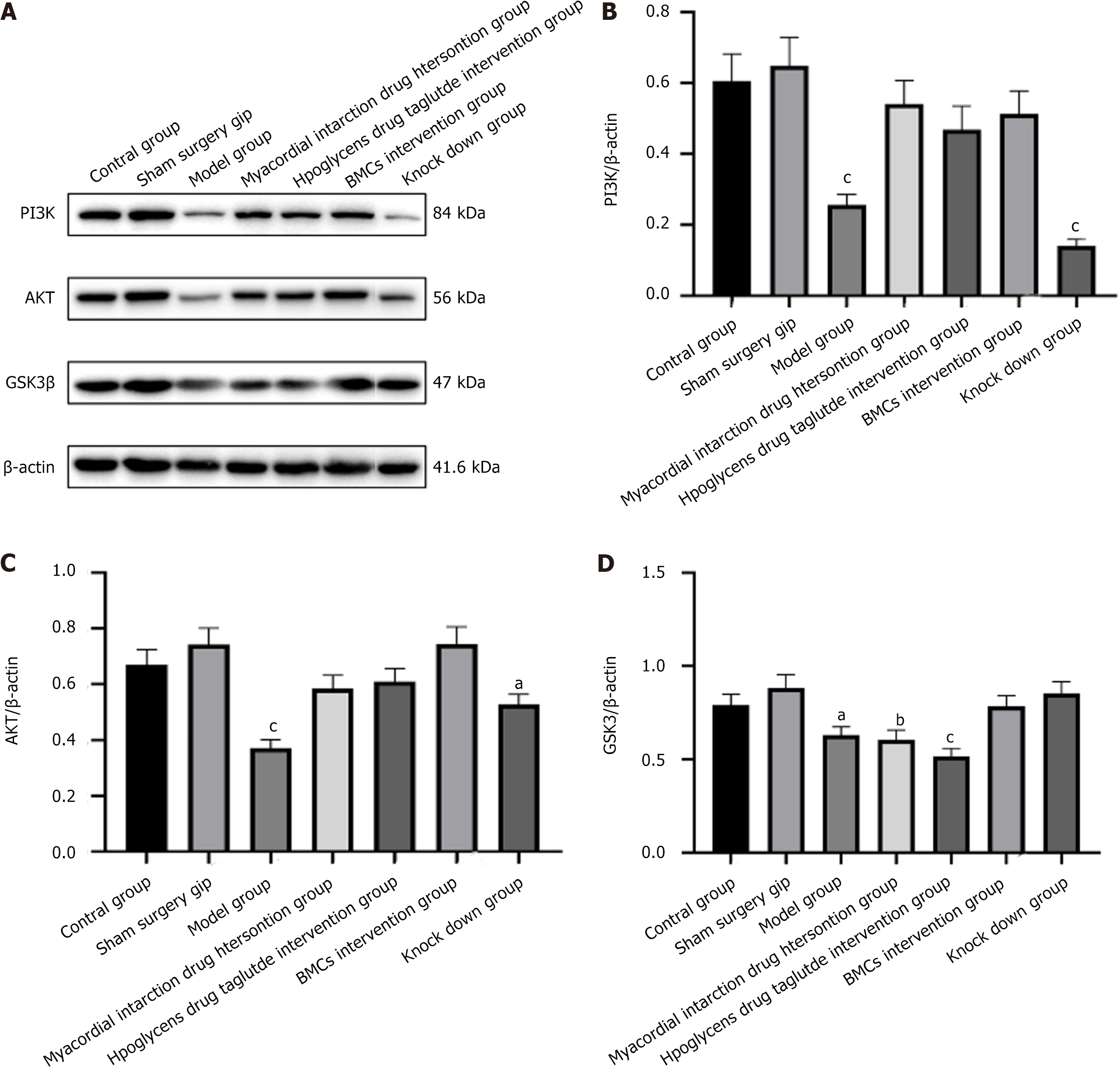
To assess changes in the PI3K/AKT/GSK3β regulatory axis after lentiviral transfection of miR-142-3p with low expression, western blotting (WB) analysis was used to detect the expression of related proteins in BMCs and miR-142 depleted BMSCs cells in each group. As shown in Figure 2A, miR-142-3 low expression BMCs cells GSK3β was higher than in control group. The expression level of AKT was higher than that of the control group, and the expression level of AKT was lower than that of the control group, with a statistically significant difference (P < 0.01). Moreover, the expression level of PI3K was increased.
To determine the changes in ferroptosis marker proteins after lentivirus transfection of miR-142-3 with low expression, WB was used to detect the expression of GPX4in BMCs and miR-142-depleted BMSCs. As shown in Figure 2B, the expression level of GPX4 in miR-142-3 Low-expression BMCs was higher than that in the control group. Additionally, the expression levels of CCM3 and its related factor VEGFR2 associated with programmed cell death were significantly higher than those in the control group (P < 0.01) after ANOVA analysis, however, the change in CCM3 expression level was not statistically significant.
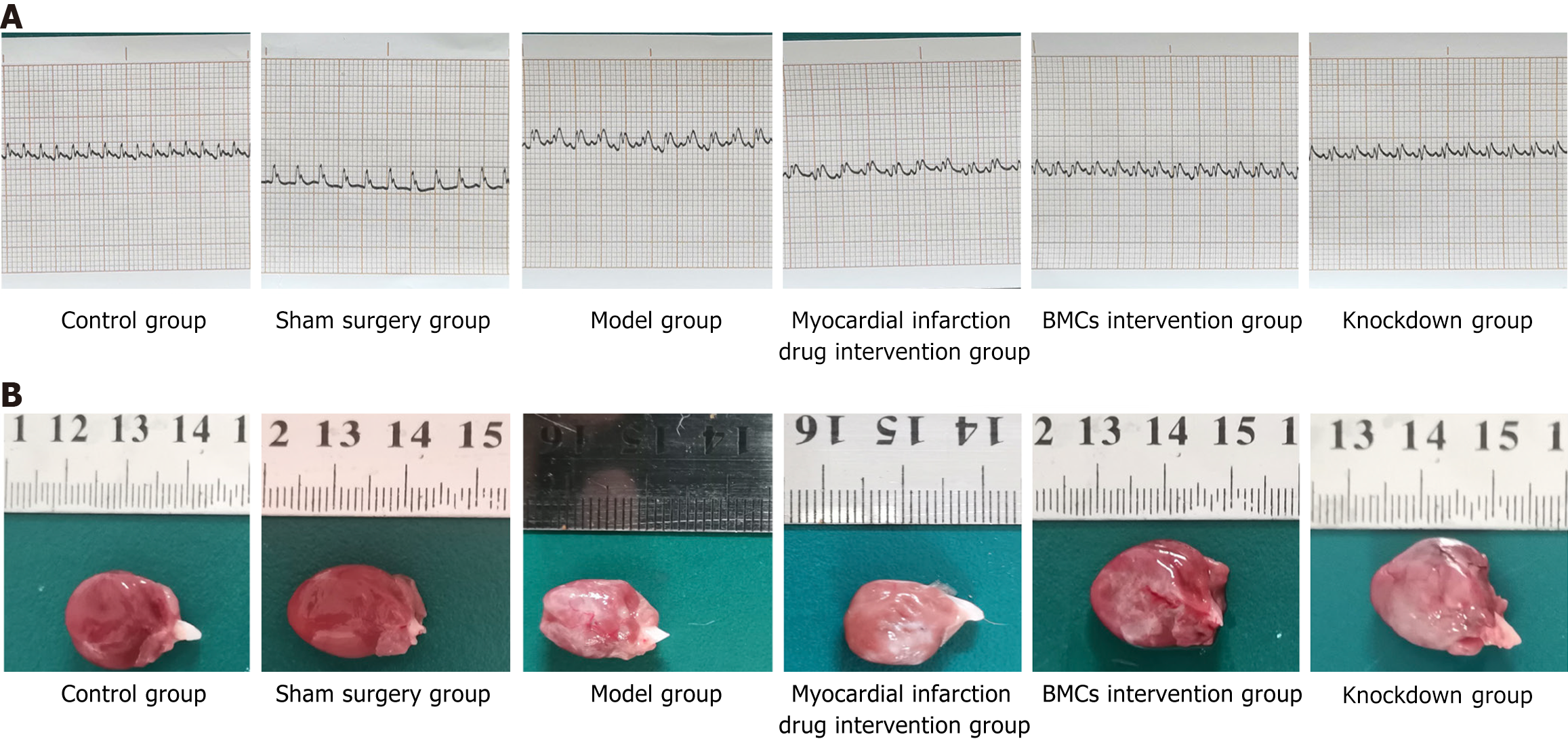
It was revealed that there was no abnormalities in the electrocardiogram examinations of rats before modeling. Among the rats with successful modeling, there was no abnormality in the electrocardiogram of the control and sham surgery groups after surgery following the administration of the intervention drugs for myocardial infarction and diabetes. In contrast, the rats in the model group showed significant ST segment elevation and variant T waves. The electrocardiograms of rats with myocardial infarction and diabetes drug intervention showed lower ST-segment elevation than the rats in the model group. Additionally, myocardial infarction and diabetes drugs inhibited the elevation of the T-segment of the electrocardiogra (ECG) and improved cardiac function. The drug effect of the myocardial infarction intervention group was better than that of the diabetes drug intervention group. Moreover, the ECG of the BMCs and KD groups was significantly lower than that of the myocardial infarction and diabetes drug intervention groups, and the effect in the BMCs group was significantly higher than that in the KD group. There was no significant abnormalities between the ECG of the BMC-treated group and the normal group. The results are shown in Figure 3A.
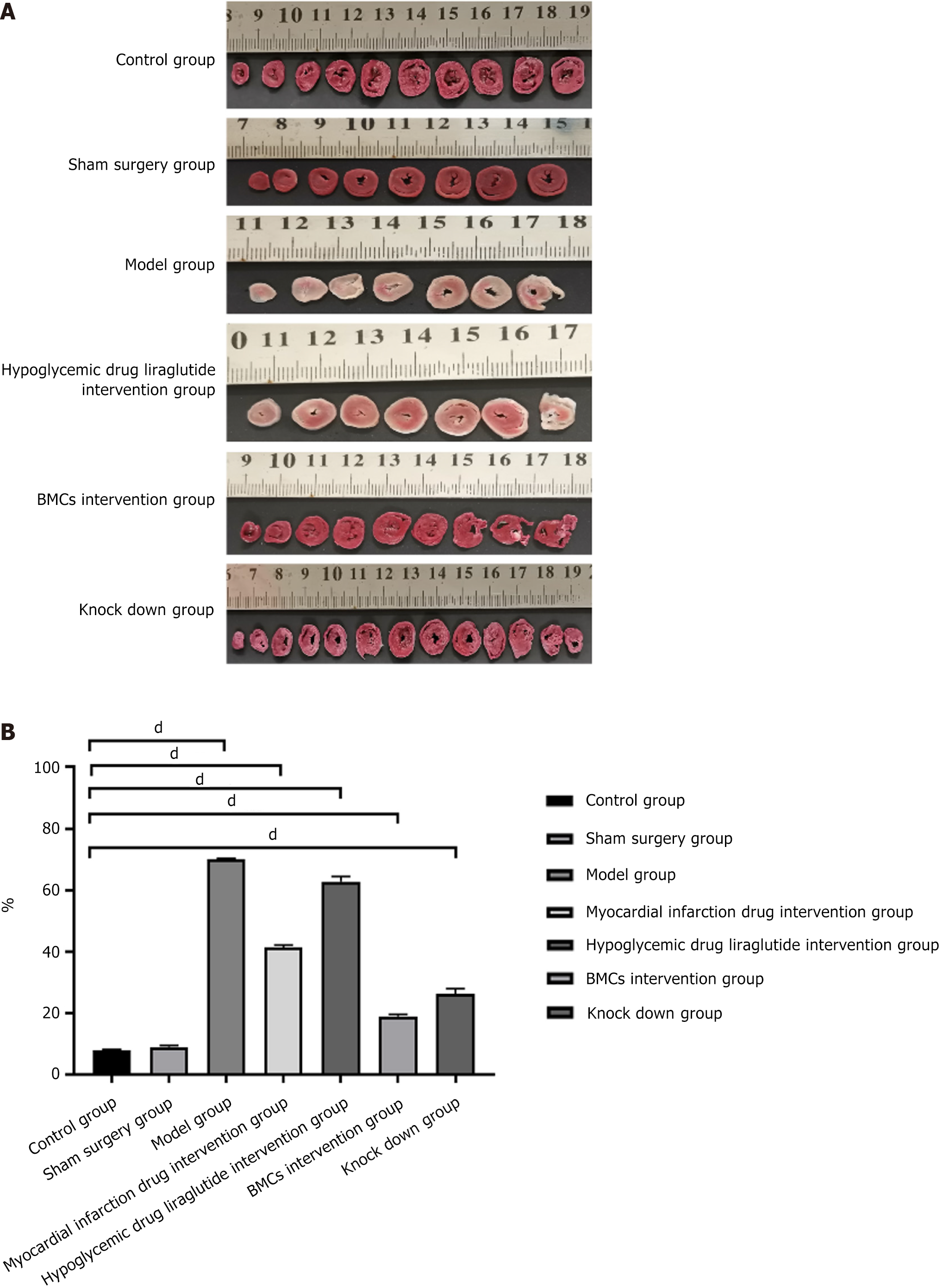
General observations showed that there were no abnormalities in the heart tissues of the control and sham surgery groups. The heart tissue in the model group began to turn white after ligation of the left anterior descending branch and spread further, involving almost the entire anterior wall of the heart. The apex was round and blunt, indicating ventricular enlargement. In the myocardial infarction drug intervention group and the diabetes drug intervention group, the white area of the anterior cardiac wall of the heart tissue was small. The white area in the myocardial infarction drug intervention group was limited to the ligation point and spread downward without affecting the apex. The white area in the diabetes drug intervention group spread from the ligation point to the apex, and the diffusion range was small compared with model group. there also some white myocardial infarction in The BMCs and miR-142-3p low-expression BMCs groups rats, but only below the ligation site without diffusion. The area of the BMCs group was significantly smaller than that of the low-expression group formiR-142-3p, and the effect of the BMCs group was greater than that of the low-expression group for miR-142-3p. The results are shown in Figure 3B.
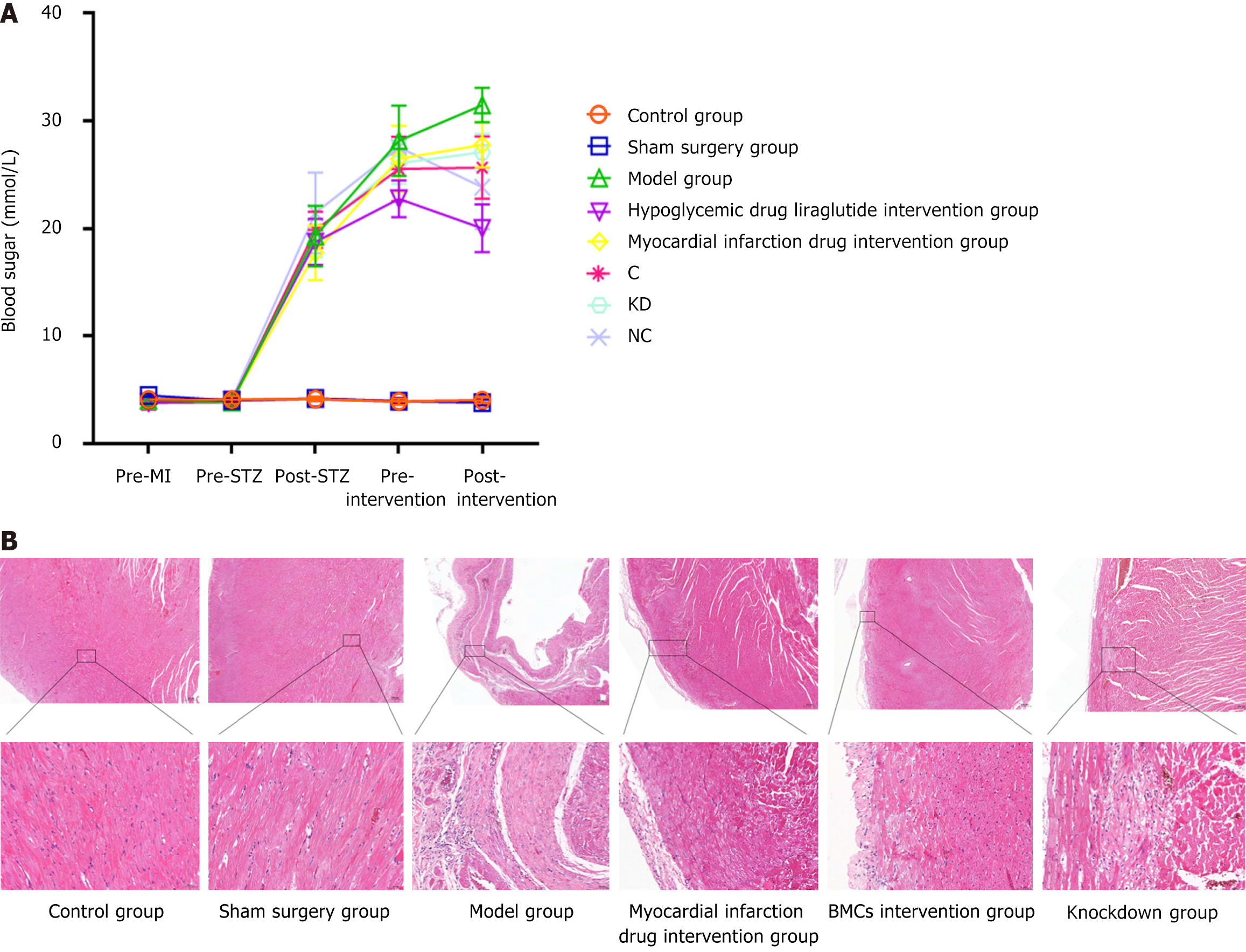
To explore the effects of myocardial infarction and diabetes drugs on blood glucose levels after myocardial infarction, blood glucose change ratios were compared at three time points (before modeling, before intervention, and before material extraction). The results demonstrated that, compared with the above initial values, except for the rats without diabetes (control group and sham surgery group), the steady-state blood glucose index of each group of rats (model group, glucose pre-group, stem pre-group, BMCs intervention group, and KD group) increased significantly 1 week before intervention (P < 0.05). After intervention in each group, the blood glucose in the liraglutide and BMCs intervention groups decreased most significantly (P< 0.05) compared with the model group with ANOVA analysis. The blood glucose in the model group decreased to the same level as the initial value, and the blood glucose in the pre-infarction group remained higher than the initial value, suggesting that both intervention drugs can help improve the hyperglycemic status, as shown in Figure 4A.
The HE pathological analysis results showed that compared with the control and sham surgery groups, the heart tissue structure of the model group rats was significantly damaged; the left ventricular wall was significantly thinner; the myocardial fibers were degenerated and necrotic; the broken cytoplasm was vacuolated; a large number of myocardial fibers were replaced by proliferative fibrous tissue; and a small number of lymphocytes and pigment deposition were observed in the proliferative fibrous tissue. Myocardial fiber degeneration and cytoplasmic vacuolization were observed in some areas of the heart tissue of animals in the myocardial infarction drug intervention group. In the diabetes drug intervention group, the myocardial fibers around the epicardium of the heart became degenerated and necrotic, with light staining, pyknosis, and lysis of the nucleus and cytoplasm. The epicardium of the heart tissue of the animals in the myocardial infarction drug intervention, diabetes drug intervention, and BMC cell control groups was slightly thickened, and the ventricular wall was wrapped in hypertrophic fibrous tissue. However, in the miR-142knockdown group, the epicardial structure of the cardiac tissue was intact, no significant proliferation or inflammatory infiltration was observed, and the myocardial fibers in the myocardial membrane were arranged in a spiral shape with a small amount of local myocardial fiber degeneration and necrosisac companied by fibrous tissue proliferation. The necrotic areas of the myocardial fibers exhibited breaking or dissolution into vacuoles. The results are presented in Figure 4B.
The contents of myocardial interstitial and perivascular collagen fibers in the model group was found to be significantly higher than that in the control group, while myocardial infarction drugs reduced the deposition of myocardial interstitial and perivascular collagen in diabetic rats. The stem cell intervention group showed the most significant reduction in fibrosis (P < 0.01), with ANOVA analysis, however, BMCs with low miR-142-3p expression did not show a significant improvement in myocardial fibrosis. Additionally, the above results were further confirmed by the collagen content percentage analysis based on Sirius red staining. The results are presented in Figure 5.
Collagen deposition in the myocardial interstitium was detected using Masson’s trichrome staining. Compared to the myocardium of rats in the control and sham surgery groups, the infarcted area in the heart of the model group showed a continuously large area of loose and disordered cardiomyocytes, the gap between cells was enlarged, and the blue collagen fibers distributed in the stroma were significantly increased. The fibrous rings around the blood vessels were significantly thickened, and some blood vessels showed luminal stenosis and closure. Compared to the model group, both the myocardial infarction drug and BMCs intervention groups were mainly composed of purplish-red myocardial tissue, with a significant decrease in blue collagen fibers. However, the BMCs miR-142-3p intervention group still had more blue collagen fiber tissue, and the tissue distribution was relatively disordered. The results are shown in Figure 6.
The results of TTC staining showed that, compared with the control and sham surgery groups, the infarcted area in the model group was white, while the non-infarcted area was red. In this experiment, the maximum size of the infarct area in the myocardial infarction model group reached 70.30%, involving almost the entire heart, and the minimum was 70.10% and mainly concentrated in the pical region. The average size of the infarcted area was 70.17% ± 0.09%. There was no significant difference in the infarct area of myocardial infarction between the control and sham surgery groups. However, the infarct area of the myocardial infarction intervention group (41.40% ± 0.57%) and diabetes intervention group (62.80% ± 1.34%) decreased, suggesting that drug intervention can improve the heart function and infarct area of rats. The infarct area of myocardial infarction in the BMCs control group (18.97% ± 0.49%) and BMCs miR 142 interference group (26.17% ± 1.43%) decreased significantly, indicating that BMCs intervention can significantly improve heart function and myocardial infarction area in rats. Further, the intervention effect of BMCs was better than that of the myocardial infarction intervention group and the diabetes intervention group, as shown in Figure 7.
WB results showed that, compared with the control and sham surgery groups, the expression of PI3K and AKT in the heart tissue of the model group decreased. Additionally, the protein expression of GSK3β was lower than that in the other groups. The BMCs increased the expression of PI3K, AKT, and GSK3β, and moreover the expressions of PI3K and AKT proteins were most pronounced. In contrast, the BMCs miR-142-3p group had the lowest expression of PI3K and AKT proteins; however, there was no significant difference in the expression of the GSK3β protein, as shown in Figure 8.
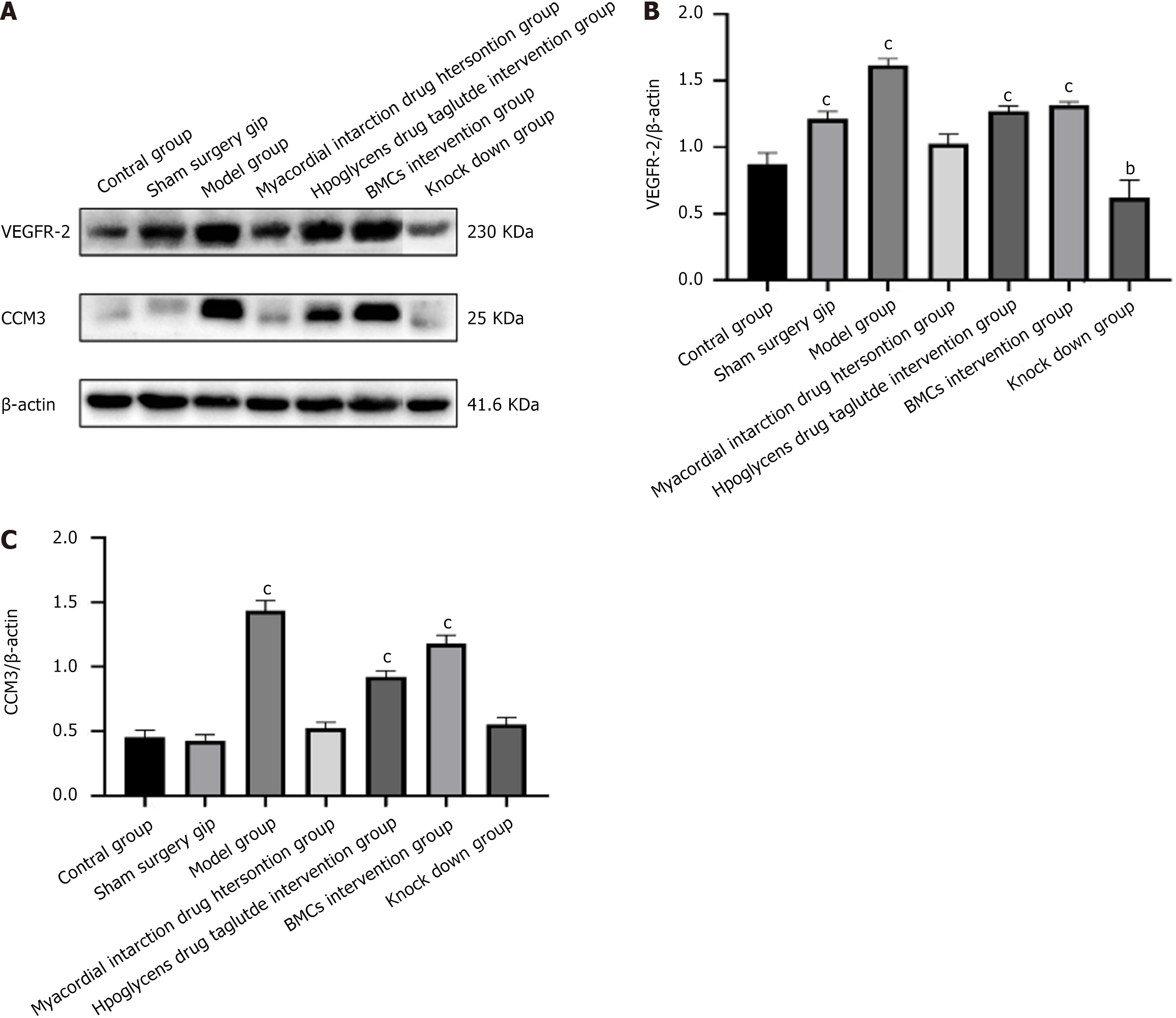
WB results revealed that, compared with the control and sham surgery groups, the expression of CCM3 and VEGFR2 in the heart tissue of the model group was significantly increased. In the myocardial infarction and liraglutide intervention groups, CCM3 and VEGFR2 showed opposite expression trends. The expression of CCM3 in the myocardial infarction drug intervention group decreased to the same level as in the control group, whereas the expression of CCM3 and VEGR2 in the liraglutide intervention group was similar to that in the model group. In the BMCs intervention group with low expression of BMCs and miR-142, the expression trends of CCM3 and VEGR2 were inconsistent. In the BMCs group, the expression of CCM3 and VEGFR2 increased, whereas in the KD group, their expression decreased, as shown in Figure 9.
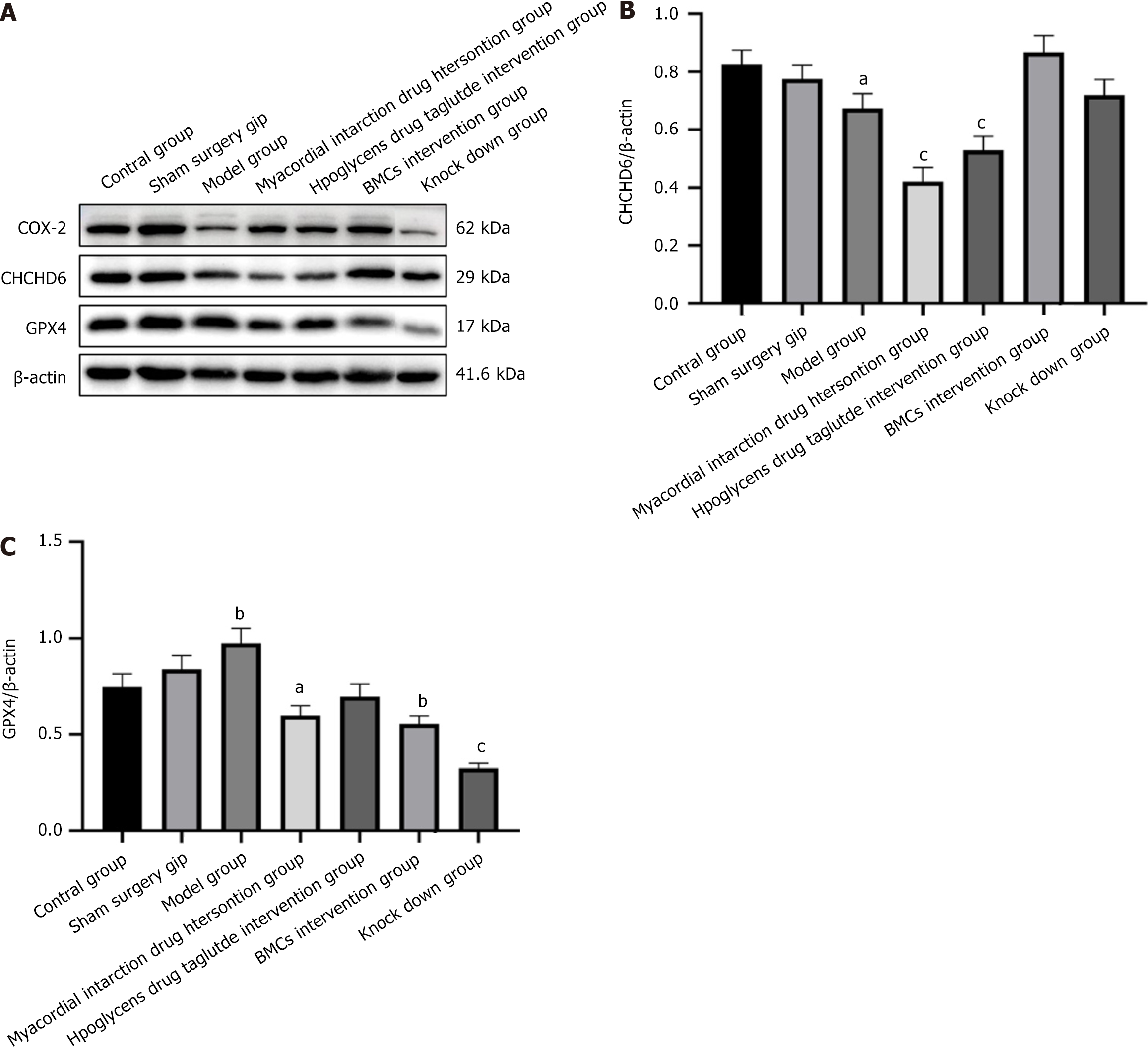
WB results showed that compared with the control and sham surgery groups, the model group showed increased expression of the ferroptosis protein GPX4, decreased expression of COX2, and decreased expression of the CHCHD6 protein in the heart tissue. In the myocardial infarction and liraglutide intervention groups, the expression of ferroptosis related to cardiac tissue in the myocardial infarction drug intervention group was significantly improved, COX-2 was increased, and GPX4 and CHCHD6 were decreased. In the intervention groups of BMCs with low miR-142-3p expression, the expression of COX-2, GPX4, andCHCHD6 was significantly increased. In contrast, the BMC miR-142-3p group was comparable to the model group and showed no significant changes. The results are shown in Figure 10.
To investigate the effect of miR-142-3p on the function of BMCs cells, we constructed stable, low-expression miR-142-3p rat BMCs cells transfected with lentivirus. Apoptosis flow cytometry detection was performed, and the expression of the PI3K/AKT/GSK3β regulatory axis, ferroptosis-related protein GPX4, and programmed apoptosis-related protein CCM3 were examined.
The results showed that the low expression of miR-142-3p in BMCs promotes the protein expression of GPX4, a marker of ferroptosis, and is accompanied by changes in the programmed death gene CCM3 and the related protein VEGFR2.The results provide preliminarily evidence that low expression of miR-142-3pcan be mediated by PI3K/AKT/GSK3β. The related proteins that regulate the axis to induce ferroptosis and programmed death will ultimately alter the activity of BMCs.
In this study, the STZ induction and coronary artery ligation methods were used to successfully establish a rat model of diabetic myocardial infarction, and the therapeutic effects of BMCs and miR-142-3p low-expression BMCs on diabetes complicated with myocardial infarction were examined. After inducing myocardial infarction in diabetes, the effects of myocardial infarction drugs, liraglutide, BMCs, and BMCs with low miR-142-3p expression were compared. The results showed that compared with the control and the sham-operated groups, the model group had elevated blood sugar, and the ECG and hematoxylin and eosin staining results showed apparent lesions. These include dabnormal elevation of the ST segment on the ECG and the variation of the T wave, irregular arrangement of myocardial cells in the infarct area and surrounding areas, accompanied by a large amount of inflammatory cell infiltration and fibrous tissue proliferation. Additionally, Sirius red and Masson's staining revealed a large amount of collagen fiber deposition and myocardial interstitial fibrosis. The myocardial infarction drug intervention, liraglutide intervention, BMCs intervention, and miR-142-3p low expression BMC intervention groups showed varying degrees of improvement, and the blood glucose in the liraglutide and BMCs groups showed the most significant decrease (P < 0.05). The drug effect of the myocardial infarction intervention group was greater than that of the diabetes drug intervention group. The effect of the BMCs intervention group was comparable to that of the myocardial infarction drugs; however, there was no significant improvement in cardiac function in the group with low expression of miR-142-3p BMCs. However, the BMCs group was significantly more effective than the group with lowmiR-142-3p expression. In the evaluation results of myocardial infarction fibrosis degree, including TTC staining, Sirius red staining, and Masson staining, the results of the myocardial infarction drug intervention group and BMCs intervention group were equivalent, while the liraglutide intervention group and miR-142-3p low-expression BMCs group showed no significant reduction in fibrosis area. Myocardial infarction drug intervention reduced myocardial interstitial and perivascular collagen deposition in diabetic rats, suggesting that myocardial fibrosis improved. It also reduced myocardial cell loss and collagen fiber deposition, improved myocardial interstitial fibrosis, and promoted myocardial remodeling after myocardial infarction. The BMCs intervention group showed the most significant reduction in the degree of fibrosis; however, there was no significant improvement in myocardial fibrosis after BMCs intervention with low expression of miR-142-3p. These results indicate that for the treatment of diabetic myocardial infarction, the hypoglycemic drug liraglutide can significantly reduce blood sugar levels in diabetic myocardial infarction model rats; nonetheless, the short-term effect of liraglutide is not better than that of myocardial infarction intervention drugs. The effect of the BMC intervention group on lowering blood sugar and alleviating myocardial infarction was excellent, equivalent to that of the liraglutide and myocardial infarction intervention groups. Simultaneously, it was observed that interference with miR-142-3p expression in BMCs significantly reduced the effects of the intervention in both aspects, indicating that miR-142-3p plays a crucial role in the therapeutic effects of BMCs. But we also found that Liraglutide has a hypoglycemic effect but does not significantly improve myocardial infarction, the limitations of this animal model and a longer treatment time or different doses may result in better outcomes.
PI3K can phosphorylate the 3-hydroxy group on the inositol ring, generate PIP3, and transmitextra cellular signals to the downstream target protein AKT[17–19]. AKT is a serine/threonine-specific protein kinase that can protect against ischemia and delay the progression of coronary heart disease and other diseases by inhibiting apoptosis[20]. The PI3K/AKT signaling pathway is a keyset of signaling pathways in the body, regulated by multiple upstream factors, and can directly affect various downstream effector factors, there by participating in the regulation of angiogenesis, oxidative stress, the inflammatory response, and apoptosis after myocardial infarction[21–23]. Activated AKT negatively regulates GSK3β, which has important regulatory functions in glucose metabolism, insulin activity, and energy homeostasis and participates in regulating various cell life activities, such as protein synthesis, glycogen metabolism, and cell mobility. During glycogen metabolism, GSK3 is phosphorylated and inactivated in response to insulin, which activates the GS and promotes glucose utilization to synthesize glycogen[24]. The WB results of the central muscle tissue in this study showed that compared with the control and sham surgery groups, the expression of PI3K, AKT, and GSK3β in the heart tissue of the model group rats was significantly reduced in all groups, with an increase in three key proteins in the myocardial infarction intervention group, liraglutide, and BMCs intervention groups. BMCs showed the most significant increase in PI3K, AKT, and GSK3 expression. This indicates that all three intervention groups can activate PI3K/AKT/GSK3β, the regulatory axis, and repair damaged myocardium, and BMCs intervention has the best effect on key regulatory proteins. Compared to the model group, the intervention group with low expression of miR-142-3p and BMCs showed the lowest expression of PI3K and AKT proteins; however, there was no significant difference in GSK3 expression. This indicates that the repair effect of BMCs is closely related to the regulation of miR-142-3p in myocardial cells by PI3K/AKT. Low expression of miR-142-3p can reduce the protective effect of BMCs but may not directly affect GSK3 as a downstream factor via AKT. PI3K is a common regulator of blood glucose levels and myocardial infarction.
Ferroptosis is a unique form of iron-dependent cell death driven by lipid peroxidation. It is also involved in the pathogenesis of diabetes, cardiomyopathy, cardiac ischemia, reperfusion/myocardial infarction, atherosclerosis, and other cardiovascular diseases. GPX4 is a glutathione-dependent selenium-containing enzyme. GPX4 is primarily located in the cytoplasm, mitochondria, endoplasmic reticulum, and nucleus[25-27]. It is involved in the regulation of cell death, inflammation, and other signaling pathways and is a crucial factor in the regulation of ferroptosis. The WB and QPCR results showed that the model group had increased expression of the ferroptosis protein GPX4 and decreased expression of COX2 in the heart tissue compared to the control and sham surgery groups. Mitochondrial pyknosis is also a classic change in ferroptosis, and CHCHD6 is a protein closely related to the mitochondria. In mammals, CHCHD6 forms a core component of the approximately 700 kD mitochondrial contact site and cristae organizing system with mitofilin and CHCHD3 complexes, controlling the integrity of the mitochondrial membrane structure and biogenesis. The expression of the CHCHD6 protein in the heart tissue of the model group also decreased[28,29]. In the myocardial infarction and the liraglutide intervention groups, the expression of ferroptosis related to cardiac tissue in the myocardial infarction drug intervention group was significantly improved, COX-2 was increased, and GPX4and CHCHD6 were decreased. In the intervention groups of BMCs with low miR-142-3p expression, the expression of COX-2, GPX4, and CHCHD6 was significantly increased, while the BMCs miR-142-3p group showed no significant changes and was comparable to the model group[30].
Many previous studies have suggested that CCM3, which was initially cloned from tumor cell lines that induce apoptosis, is mainly related to apoptosis. Prior to 2005, research suggested that CCM3 was an anti-apoptotic gene[31]. However, recent studies have found that under oxidative stress conditions, CCM3 andSTK25 work together to promote cell apoptosis. whereas CCM3 and MST4 work together to promote cell proliferation. CCM3interacts with various proteins, enabling it to exhibit functional pleiotropy. VEGF regulates vascular development by promoting the binding of VEGFR2 to the C-terminal domain of PDCD10 during PI3K upstream signaling[32]. Computational modeling by Manning et al[33] demonstrated that PDCD10 participates in the PI3K signaling pathway. The WB results in this study showed that compared with the control and sham surgery groups, the expression of CCM3 and VEGFR2 in the heart tissue of the model group rats was increased, indicating that CCM3/VEGFR2 may play an important role in inducing cardiomyocyte apoptosis in diabetes with myocardial infarction. Myocardial infarction intervention drugs significantly reduced its expression, whereas the liraglutide group, which had no significant effect on myocardial repair, showed no significant change. However, in the intervention group with BMCs and their low expression of miR-142-3p, CCM3/VEGFR2 showed opposite expression changes in myocardial tissue. Other indicators revealed an increase in CCM3/VEGFR2 expression in the BMCS group with good myocardial tissue repair, whereas low expression of miR-142-3p decreased. This indicates that in the treatment mechanism of BMCs, CCM3 may play a role in promoting cell proliferation, which also suggests that there are certain differences in the mechanism of action between clinical myocardial infarction treatment drugs and BMCs. Since there are no previous studies on the mechanism of the interaction between BMCs via miR-142-3p and CCM3 to promote the recovery of hypoxic myocardium in a model of diabetic myocardial infarction, further investigation is required to clarify the role of CCM3.
This study has certain limitations. First, liraglutide is an effective drug used clinically to improve cardiovascular disease in diabetes. However, in this experiment, only a hypoglycemic effect was observed, and no repair effect was observed in the myocardial infarction area. A possible reason for this discrepancy is the short duration of administration, as the clinical use of this application is generally 1 year. Meanwhile, the rat model used may not fully replicate human diabetes and myocardial infarction, and the short duration lacks long-term data. The small sample size limits statistical power, and the interventions tested were limited. Blood glucose measurements were infrequent, and the focus on the PI3K/AKT/GSK3β pathway neglects other relevant pathways. Clinicalvalidation in humans is needed, and the mechanisms of miR-142-3p require further investigation. Further investigation is necessary to explore the downstream pathways related to ferroptosis and CCM3. Additionally, Detection technology needs improvement. In clinical practice, diabetes myocardial infarction can be detected and screened using endoscopic imaging to avoid missed diagnosis of traditional imaging. It also has a good intervention and guidance role in the treatment of diabetes myocardial infarction, and can carry out prognosis evaluation, so as to visualize microcirculation disorders, dynamically monitor the degree of myocardial fibrosis, and evaluate the heart protection effect of anti diabetes drugs. Although it has been well used clinically, it is still rarely used in animal models. If it can be used in animal models in the future, it will better detect and test the intervention effect of the test substance.
The potential off target effects of knockout mainly include the following risks: Immune regulation imbalance, abnormal Treg cell function; uncontrolled release of pro-inflammatory cytokines; tumor related risks, accelerated proliferation of tumor cells; tumor stemness enhancement; abnormal metabolism and repair function, reprogramming of cellular metabolism; vascular regeneration disorder; Technical specificity risk, shRNA non-specific binding; organizational heterogeneity effects: The target gene profile of miR-142-3pvaries significantly in macrophages, neurons, and tumor microenvironments, and systemic knockout may lead to multi system side effects due to tissue-specific regulatory differences. At the same time, the off target effect of miR-142-3pknockout can be mitigated through certain strategies, such as targeted delivery systems, multi omics validation, and functional compensation mechanisms to jointly inhibit key downstream targets such as IRAK-1 or CDK4, partially offsetting the negative effects of knockout.
The clinical applications of miR-142-3pinclude regulating cardiac protection and repair mechanisms, inhibiting myocardial hypertrophy and fibrosis, and promoting vascular regeneration; Regulating immune homeostasis and inflammatory response, improving Treg cell function; Inhibit the release of inflammatory factors. The potential ofmiR-142-3p as a diagnostic and therapeutic target, biomarker value, and gene therapy application. In the future, this study still faces many challenges, including clarifying the differential roles of miR-142-3p in the heart and other organs such as the pancreas and immune system, avoiding the side effects of systemic intervention, and determining tissue specificity and safety; At the same time, in the clinical translation verification of Fang Xu, further clinical trials are needed to verify its efficacy and safety based on existing animal models and cell experiments. To sum up, miR-142-3p shows the potential of regulating immunity, repairing myocardium and promoting vascular regeneration in the treatment of diabetes myocardial infarction through multi-channel synergy, which is an important research direction of precision medicine in the future.
This study demonstrated that using BMCs as an intervention could simultaneously reduce blood sugar levels and promote hypoxic myocardial recovery. However, when miR-142-3p expression in BMCs was low, there was a reduced therapeutic efficacy of BMCs and activation of PI3K/AKT/GSK-3β. The regulation axis regulates changes in protein expression levels of apoptosis-related genes CCM3 and VEGFR2 and ferroptosis-related genes GPX4/COX-2/CHCHD6. These findings suggest that miR-142-3p via the PI3K/AKT/GSK-3β regulation axis, which jointly inhibits ferroptosis and programmed death, may be one of the therapeutic mechanisms of BMCs in diabetic myocardial infarction.
| 1. | International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Available from: https://diabetesatlas.org/. |
| 2. | Alonaizan R, Carr C. Cardiac regeneration following myocardial infarction: the need for regeneration and a review of cardiac stromal cell populations used for transplantation. Biochem Soc Trans. 2022;50:269-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Gyldenkerne C, Mortensen MB, Kahlert J, Thrane PG, Warnakula Olesen KK, Sørensen HT, Thomsen RW, Maeng M. 10-Year Cardiovascular Risk in Patients With Newly Diagnosed Type 2 Diabetes Mellitus. J Am Coll Cardiol. 2023;82:1583-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Goodarzi MO, Rotter JI. Genetics Insights in the Relationship Between Type 2 Diabetes and Coronary Heart Disease. Circ Res. 2020;126:1526-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 5. | Katakami N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J Atheroscler Thromb. 2018;25:27-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 6. | Huang Y, Yang L. Mesenchymal stem cells and extracellular vesicles in therapy against kidney diseases. Stem Cell Res Ther. 2021;12:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Shao L, Shen Y, Ren C, Kobayashi S, Asahara T, Yang J. Inflammation in myocardial infarction: roles of mesenchymal stem cells and their secretome. Cell Death Discov. 2022;8:452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 8. | Yan W, Xia Y, Zhao H, Xu X, Ma X, Tao L. Stem cell-based therapy in cardiac repair after myocardial infarction: Promise, challenges, and future directions. J Mol Cell Cardiol. 2024;188:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 9. | Guo R, Morimatsu M, Feng T, Lan F, Chang D, Wan F, Ling Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res Ther. 2020;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Xiao Y, Zhao J, Tuazon JP, Borlongan CV, Yu G. MicroRNA-133a and Myocardial Infarction. Cell Transplant. 2019;28:831-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 11. | Mirzavi F, Ebrahimi S, Ghazvini K, Hasanian SM, Hashemy SI. Diagnostic, Prognostic, and Therapeutic Potencies of Circulating miRNAs in Acute Myocardial Infarction. Crit Rev Eukaryot Gene Expr. 2019;29:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Mir R, Elfaki I, Khullar N, Waza AA, Jha C, Mir MM, Nisa S, Mohammad B, Mir TA, Maqbool M, Barnawi J, Albalawi SO, Abu-Duhier FM. Role of Selected miRNAs as Diagnostic and Prognostic Biomarkers in Cardiovascular Diseases, Including Coronary Artery Disease, Myocardial Infarction and Atherosclerosis. J Cardiovasc Dev Dis. 2021;8:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Zhou SS, Jin JP, Wang JQ, Zhang ZG, Freedman JH, Zheng Y, Cai L. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018;39:1073-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 470] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 14. | Zhao WK, Zhou Y, Xu TT, Wu Q. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxid Med Cell Longev. 2021;2021:9929687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 15. | Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11:3052-3059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 408] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 16. | Han X, Zhang J, Liu J, Wang H, Du F, Zeng X, Guo C. Targeting ferroptosis: a novel insight against myocardial infarction and ischemia-reperfusion injuries. Apoptosis. 2023;28:108-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 17. | Ghafouri-Fard S, Khanbabapour Sasi A, Hussen BM, Shoorei H, Siddiq A, Taheri M, Ayatollahi SA. Interplay between PI3K/AKT pathway and heart disorders. Mol Biol Rep. 2022;49:9767-9781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 18. | Walkowski B, Kleibert M, Majka M, Wojciechowska M. Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells. 2022;11:1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 19. | Gulluni F, De Santis MC, Margaria JP, Martini M, Hirsch E. Class II PI3K Functions in Cell Biology and Disease. Trends Cell Biol. 2019;29:339-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1103] [Article Influence: 157.6] [Reference Citation Analysis (0)] |
| 21. | Ghafouri-Fard S, Abak A, Shoorei H, Mohaqiq M, Majidpoor J, Sayad A, Taheri M. Regulatory role of microRNAs on PTEN signaling. Biomed Pharmacother. 2021;133:110986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 22. | Smith CC, Yellon DM. Adipocytokines, cardiovascular pathophysiology and myocardial protection. Pharmacol Ther. 2011;129:206-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Liang T, Gao F, Chen J. Role of PTEN-less in cardiac injury, hypertrophy and regeneration. Cell Regen. 2021;10:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Chen ZQ, Chen F, Li L. Role of glycogen synthase kinase following myocardial infarction and ischemia-reperfusion. Apoptosis. 2019;24:539-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Komai K, Kawasaki NK, Higa JK, Matsui T. The Role of Ferroptosis in Adverse Left Ventricular Remodeling Following Acute Myocardial Infarction. Cells. 2022;11:1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 26. | Shaghaghi Z, Motieian S, Alvandi M, Yazdi A, Asadzadeh B, Farzipour S, Abbasi S. Ferroptosis Inhibitors as Potential New Therapeutic Targets for Cardiovascular Disease. Mini Rev Med Chem. 2022;22:2271-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 27. | Wang XD, Kang S. Ferroptosis in myocardial infarction: not a marker but a maker. Open Biol. 2021;11:200367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Mistry P, Reitz CJ, Khatua TN, Rasouli M, Oliphant K, Young ME, Allen-Vercoe E, Martino TA. Circadian influence on the microbiome improves heart failure outcomes. J Mol Cell Cardiol. 2020;149:54-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Shang Y, Sun X, Chen X, Wang Q, Wang EJ, Miller E, Xu R, Pieper AA, Qi X. A CHCHD6-APP axis connects amyloid and mitochondrial pathology in Alzheimer's disease. Acta Neuropathol. 2022;144:911-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Chen C, Chen J, Wang Y, Liu Z, Wu Y. Ferroptosis drives photoreceptor degeneration in mice with defects in all-trans-retinal clearance. J Biol Chem. 2021;296:100187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 31. | Sun Y, Zhao Z, Zhang H, Li J, Chen J, Luan X, Min W, He Y. The interaction of lead exposure and CCM3 defect plays an important role in regulating angiogenesis through eNOS/NO pathway. Environ Toxicol Pharmacol. 2020;79:103407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | He Y, Zhang H, Yu L, Gunel M, Boggon TJ, Chen H, Min W. Stabilization of VEGFR2 signaling by cerebral cavernous malformation 3 is critical for vascular development. Sci Signal. 2010;3:ra26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Manning BD, Dibble CC. Growth Signaling Networks Orchestrate Cancer Metabolic Networks. Cold Spring Harb Perspect Med. 2024;14:a041543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |