Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.101277
Revised: February 26, 2025
Accepted: April 14, 2025
Published online: September 20, 2025
Processing time: 336 Days and 17.2 Hours
The review examines the intricate relationship between the microbiota and its host, highlighting how these microbial communities influence various phy
Core Tip: This review article explores the crucial role of gut microbiota and their metabolites in immune regulation, gene expression, and disease progression. It introduces the gut-organ axis hypothesis, linking systemic genetic factors with inflammatory bowel disease progression and treatment response. The study emphasizes microbiota-targeted therapies, such as probiotics, fecal microbiota transplantation, and dietary interventions, as promising strategies in precision medicine. Future research should focus on personalized microbiome-based treatments, leveraging machine learning and advanced analytics to optimize therapies. These insights pave the way for microbiome-driven interventions, revolutionizing treatments for inflammatory bowel disease, autoimmune diseases, and cancer.
- Citation: Khan AS, Kamthan M, Ali A. Understanding the intricate interactions between microbiota and host. World J Exp Med 2025; 15(3): 101277
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/101277.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.101277
The human microbiome comprises various microorganisms residing in and on the human body, including bacteria, protozoa, viruses, and archaea[1]. These microbes also interact with the human metabolic system, influencing the physiological processes. The gastrointestinal (GI) tract alone harbours the largest microbial community, comprising of trillions of microbes, including bacteria, protozoa, viruses, and fungi[2]. A growing body of evidence in the recent years has demonstrated the role of the GI tract microbiota in influencing pathogenesis of various disorders, both local and systemic[3]. Thereby, improved understanding of the microbiota, their metabolites, their influence on human physiology and disease development, etc. is warranted, to expand the advancements in the clinical field[4]. Although the intestines of a new-born are devoid of any microorganisms, the intestines gradually become home to a wide variety of complex populations of bacteria, archaea, viruses and eukaryotes, which are collectively referred to as the gut microbiota[5]. The gut microbiota alone consists of some 1014 bacteria[6] belonging to at least 1000 different species[7]. The intestines are known to harbour the major portion of the microbial population, and these organisms play a major role in maintaining and sustaining human health. The Bacteroides and Firmicutes are the two most prominent phyla found in the gut of healthy individuals[8]. Other than the intestines, every external surface of the mammalian body houses a large and complex composition of microorganisms, especially bacteria. These organisms play a key role in several essential bodily functions such as digestion of the plants complex carbohydrates, increasing digestive efficiency, and providing essential micronutrients, along with the regulation of the immune system[9]. There has been a co-evolution of the human system and the commensal microbiota over hundreds of millions of years[10]. The gut microbiota essentially shapes various characteristics of the host, including its overall physiological well-being and its immune system functioning[5]. Various studies based on culture-dependent and independent approaches have confirmed the large interpersonal variations that occur in the microbiota, i.e., each individual carries within them a unique set of genes contributed by their unique microbiota composition, which is subject to variation based on various factors, including the host’s geographical location, the host’s diet, and the intake of antibiotics[11].
Gut-microbiota interactions play a significant role in determining the optimal well-being of the human system[12] such that small alterations in the gut microbiome (dysbiosis) can lead to various fatal illnesses[13]. The symbiosis between the gut microbiota and the human body allows for a co-evolution between the host factors and the microorganisms[14]. Any sort of dysbiosis may lead to problems life-threatening conditions including allergies, bacterial infections, cancer, and cardiovascular diseases, which may not be directly linked to microbial activity, but are indirectly associated with imbalances in the gut-host interactions[15-17].
The microbial metabolites produced by the gut microbiome provide a vast range of bioactive substances in the host body, many of which serve as precursors for various important biomolecules in the host system[9]. The microbial metabolites involved in such stimulations include fatty acids, neurotransmitters, bile acids, etc.[18]. Among the various metabolites produced by the gut microbes, the short chain fatty acids (SCFAs) are probably the best studied[19]. Metabolites such as butyrate and propionate have been implicated in anticancer research, with butyrate-producing bacteria such as Clostridium, Ruminococcus, etc. exhibiting reduced tumor growth in tumor-bearing mouse models, contrary to other murine models that do not bear butyrate-producing microbes[20]. According to a study conducted by Das et al[21], Lactobacillus species in the gut influence the iron absorption ability of the host, by attenuating intestinal iron absorption via ferritin regulation. This further implicates the application of gut microbiota in iron-related disorders.
The gut microbiome contains an estimated 3 million genes, and being part of the human system, these microbial genes further expand the human genetic catalogue[22]. The gut microbiome has a significant influence on the host gene expression and vice versa[23]. The genetic, proteomic, and metabolomic abundance of the microbiota implies that the microbiome comprises a more extensive set of genes than the host itself. This abundance naturally leads to a wide variety of metabolic and immunological interactions between the microbiota and the host, including influence on host gene expression and the immune processes[24]. Various studies in this regard have shown that the gut microbiome can regulate the host pathways related to immune development and energy metabolism, as well as directly affect host signaling cascades and chromatin remodeling, which in turn has significant impacts on the host’s immunity and cancer resilience[23,25,26]. The gut microbiota has a widespread and modifiable influence in the expression of host genes, Exposure to gut microbiota induces changes in the transcriptional regulation and chromatin accessibility in host cells[27]. The GI tract is a host to trillions of microbial species that are actively involved in maintaining the homeostasis, any disturbance may lead to the development of autoimmune diseases (AIDs) and other pathogenic diseases[28]. The microbially-produced metabolites are known to play an important role in regulating the expression of the microRNAs (miRNAs), and together, the miRNAs and the microbiota influence host gene expression[29]. Such crosstalk between the miRNAs and the gut microbes paves the way for the idea that changing the diet via intake of certain dietary supplements may influence such RNA molecules, by regulating abnormal gene expression in the host, and ultimately be a simple and low-cost mode of treating certain diseases[30]. It was also shown that over 5000 host genes are influenced to change their expression, in response to the gut microbiota. Furthermore, 588 taxa were identified that modulate such alterations in the host gene expression[27]. More often than not, the taxa with the strongest influence on host gene expression were found to be associated with genes that encode complex traits[27]. Such studies point towards the plausible role of gut microbiota in regulating the expression of individual host genes. This in turn influences complex human traits and also lays evidence for the idea that manipulation of the resident microbial composition of the host could be employed in novel therapies in the future[31]. However, it must also be noted that the crosstalk between the microbiome and the host can regulate gene expressions in a beneficial as well as detrimental manner[32]. Recent studies have pointed towards the role of microbiota in carcinogenesis, its promotion, enhancement, or inhibition being influenced by the interactions between the microbiota and the host[32]. The microbes are known to interact with various components of the host’s immune system, and by modulating the immune cells and their products, and producing various genotoxins and virulence factors, play their part in cancer progression or regression[33]. For example, the enterotoxigenic strain of Bacteroidetes fragilis is known to induce colitis by producing metalloproteases, thereby promoting cell proliferation via the nuclear factor-kappa B and the mitogen-activated protein kinases signaling pathways. Studies on murine models further confirmed the role of enterotoxigenic strain of Bacteroidetes fragilis in oncogenesis by stimulating colorectal cancer. Products such as colibactin, produced by bacteria such as Klebsiella and Escherichia coli (E. coli), a normal resident of the intestines, have been implicated in DNA damage, ultimately leading to mutagenesis and carcinogenesis, posing the risk of colon cancer[34,35].
The gut microbiota remains the most extensively studied group of microbes that inhabit the human system[14]. The microbiota-host crosstalk is not just limited to one particular organ but extends to link various parts of the body together[36]. The gut microbiota is perhaps the most studied group of the human microbiome, with implications suggested in the regulation of homeostasis, affecting various organs, including the lungs, the heart, the gut, and even parts of the brain, systematically[3]. The importance of homeostasis of the microbiome is implicated in the fact that disruption of such balance, i.e., dysbiosis in the microbiome-host relationship is the cause of disease progression in various cases, including pulmonary diseases, cardiovascular diseases, neurological disorders, metabolic dysfunctions, and dermatological issues as well[37]. Such dysbiosis, i.e., the imbalance between the microbiota and the host’s immune system functioning can lead to opportunistic infections[38]. A decreased diversity of the gut microbiota serves as an indicator of an impending pathophysiological condition, such as inflammatory and metabolic disorders in Figure 1. An extensive amount of work done in the past years has established their role in disease progression under circumstances of dysbiosis, which may be associated with pathologies including cardiovascular diseases, carcinogenesis, dental caries, chronic disorders of the kidney and the lungs, and chronic inflammations of the bowel[39-41].
A growing body of evidence in literature suggests the role of gut microbiota and its influence which extends beyond just the GI tract, suggesting bidirectional crosstalk between the gut microbiome and inflammation and disorders in various other parts of the body as well. The gut microbiome has a well-established role in influencing various parts of the human system, including the immune system, the nervous system, the endocrine system, the musculoskeletal system, the pulmonary system and the cardiovascular system[38,42].
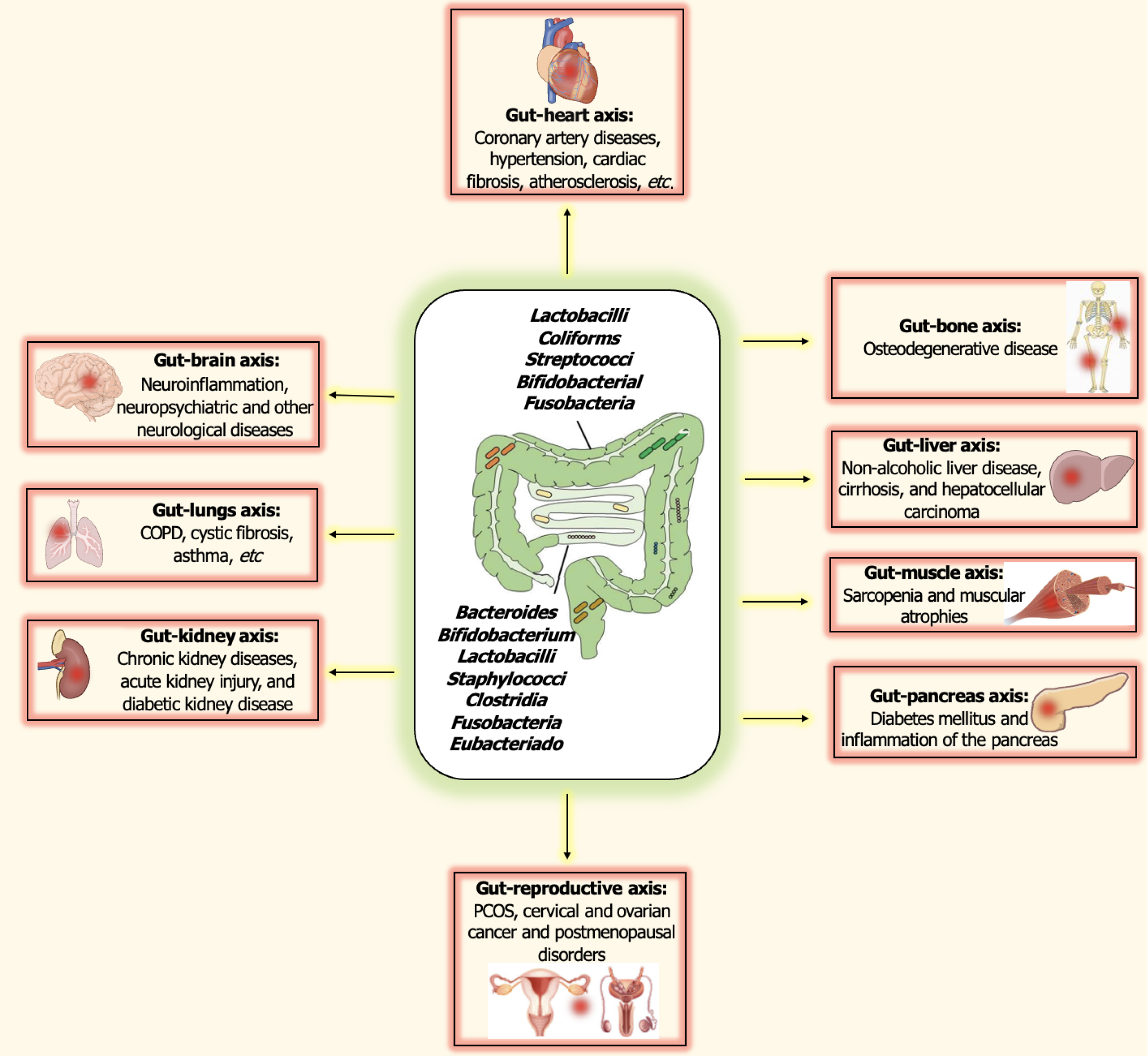
Various evidence in this regard has suggested that the influence of host-microbiota interactions extends far beyond just local impacts, influencing other organ systems as well. In this sense, many authors regard the gut microbiome as an organ system in itself, exerting a significant impact on extraintestinal sites as well. These interactions have been summarized in Table 1[43-53]. Some of these crosslinks have been discussed in the following sections.
| Gut-organ axis | Interactions | Important bacteria associated | Ref. |
| Gut-brain axis | Communication between CNS, ANS, and ENS. Significant role in neuroinflammation, and neuropsychiatric disorders and neurological diseases, interaction with neurotransmitters like serotonin, noradrenaline, norepinephrine dopamine, and GABA | Candida, Enterococcus, Escherichia, Lactobacillus, Bifidobacterium, etc. | [43,44] |
| Gut-heart axis | Significant role in development of coronary artery diseases, hypertension, cardiac fibrosis, atherosclerosis, etc. | Escherichia spp., Shigella, Streptococcus, Ruminococcus, Veillonella, etc., and a decline in the abundance of Prevotella, Faecalibacterium, Megamonas | [45,46] |
| Gut-lung axis | Onset and progression of tuberculosis, chronic obstructive pulmonary disease, cystic fibrosis, asthma, etc. | Decreased abundance of Eubacterium, Bacteroides, Bifidobacterium and Feacalibacterium, and abundance of propionate and butyrate producers like Facelibacterium, Eubacterium and Phascolarctobacterium | [47] |
| Gut-liver axis | Development of non-alcoholic liver disease, cirrhosis, and hepatocellular carcinoma | Bifidobacterium, Lactobacillus, Enterobacteriaceae, Clostridiales, Bacteroides, Prevotella | [48] |
| Gut-pancreas axis | Significant effects on insulin signaling and glucose and lipid metabolism, along with onset and progression of diabetes mellitus and inflammation of the pancreas | ||
| Gut-bone axis | Dysbiois may result in osteoporosis, and other osteodegenerative diseases | [49] | |
| Gut-muscle axis | Development of sarcopenia and muscular atrophy | [50] | |
| Gut-skin axis | Skin-related disorders such as psoriasis, alopecia, rosacea, acne and skin cancer may result due to dysbiosis | Bacteroides and Faecalibacterium, Clostridium, Escherichia | [51] |
| Gut-reproductive axis | Suggested role in spermatogenesis and development of PCOS, cervical and ovarian cancer and postmenopausal disorders | Escherichia, Clostridium and Citrobacter, an increased abundance of microbiota such as Phocaeicola vulgatus, Bacillota, Streptococcus and Escherichia/Shigella complex, and a decreased population of Akkermansiaa and Oscillospiraceae | [52] |
| Gut-kidney axis | Onset and progression of chronic kidney diseases, acute kidney injury, and diabeteic kidney disease | Increased occurrence of bacteria including Clostridium spp., Enterobacteria, Eggerthella spp., and a decreased occurrence of Prevotella, Bacteroides, and Roseburia | |
| Gut-bladder axis | Recurring urinary tract infections and overactive bladder | [53] |
The bioactive metabolites produced by gut microbiota are further involved in communication between the GI tract and the brain, i.e., with the central nervous system (CNS)[22]. Hence, the gut microbes have the potential to modulate the physiological and pathological processes influenced by the CNS of the host[54]. Various mechanisms exist that may help in modulating such communications between the gut microbiota and the brain, including binding of the microbial metabolites to the host receptors of the CNS, stimulation of the vagus nerve, and regulation of neuroinflammation[43]. The gut-brain axis serves as a bidirectional route for communication between the GI tract and the brain or the CNS. This bidirectional communication involves components of the CNS, the autonomic nervous system (comprising the sympathetic and the parasympathetic nervous system), and the enteric nervous system (ENS)[55]. Various microbial metabolites are known to interact with different components of the CNS, influencing its function and behaviour. The gut-brain axis involves communication between the CNS and the ENS, thereby linking the cognitive centres of the brain with the intestinal functions[43]. In recent years, growing evidence has pointed towards the role of the gut microbiota in influencing such interactions. Microbiota of the gut such as Candida, Enterococcus, Escherichia, Lactobacillus, Bifidobacterium, etc. are known to modulate or influence neurochemicals such as serotonin, noradrenaline, norepinephrine, dopamine, and gamma-aminobutyric acid, and even produce such neurotransmitters as well[44]. In fact, the gut microbiota composition has recently been suggested to play an important role in various neuropsychiatric and neurological issues, including anxiety, depression, autism, schizophrenia, epilepsy, and neurodegenerative diseases such as Parkinson’s and Alzheimer’s[55]. The gut microbiota is also implicated to play an important role in maintaining the selectivity of the blood-brain barrier, as germ-free mouse models have demonstrated greater blood-brain barrier permeability[56]. This further suggests a protective role of the gut microbiota against pathogenesis in components of the CNS.
The gut microbiome can affect the well-being of the cardiovascular system, as evidenced by various recent studies[57]. Dysbiosis in the gut microbiome may lead to the development of diseases such as coronary artery diseases, hypertension, atherosclerosis, strokes, etc. Significant changes in the gut microbiome makeup, including alterations in the ratio of Firmicutes and Bacteroides, or a relative abundance of gut microbes including Escherichia spp., Shigella, Streptoccus, Ruminococcus, Veillonella, etc., and a decline in the abundance of Prevotella, Faecalibacterium, Megamonas, etc. may lead to cardiovascular issues including heart failure[57,58]. Such dysbiosis may be affected by fluctuations in microbial metabolites such as an increase in trimethylamine N-oxide and a decrease in substances like short-chain fatty acids, which may lead to alterations in the gut microbiota composition, resulting in problems including cardiac fibrosis, atherosclerosis, hypertension, and heart failure[59]. Gut metabolites produced by the microbiome including trimethylamine N-oxide and trimethylamine have been positively correlated with cardiovascular dysfunction and diseases[60,61].
Despite the distance between the two organs, the gut and the pulmonary system remain interconnected via an intricate crosstalk along the gut-lung axis (GLA), mediated by the gut microbiota which is both, inter-organ, and inter-kingdom[62]. Various studies have pointed towards a correlation between development of pulmonary disorders and imbalance in the microbiota along the GLA. For example, chronic obstructive pulmonary disease (COPD) is a disorder of the lungs, linked with decreased diversity among the lung microbiota and a particular increase in the population of Proteobacteria[63]. Additionally, chronic lung diseases such as asthma, cystic fibrosis and other viral infections have also been associated with imbalances or dysbiosis of the intestinal microflora[64]. Changes in the intestinal microbiota have evidently been linked to the development of various lung disorders, suggesting an intricate crosstalk between these two sites of the body. In a study conducted in 2014, cases of cystic fibrosis in children were linked to a decreased abundance of Eubacterium, Bacteroides, Bifidobacterium and Feacalibacterium, leading to some conclusive evidence that lung disorders such as cystic fibrosis can be qualitatively and quantitatively linked to disrupted composition of the intestinal microbiota. Interestingly, administration of probiotics can potentially restore the original microbiome, thereby reducing the exacerbations that lead to pulmonary diseases. Although initially believed to be a sterile organ, the lung harbours at least 10-100 bacterial cells per 1000 human cells, as revealed by 16S rRNA based characterizations[65]. An increasing number of studies have linked changes in the gut microbial composition to the development of pulmonary disorders, and vice versa through the bloodstream, i.e., disorders such as acute lung injury, which disrupts the lung microbiota, can effectively cause an increase in the microbial load of the cecum[66].
Analysis of the gut microbiota in patients suffering from bacterial infections of the lung such as tuberculosis have revealed a decreased/altered bacterial diversity of the gut of infected patients, which may further be linked to the progression of the disease[67]. Furthermore, whole-genome sequencing and 16S rRNA sequencing-based studies revealed an abundance of propionate and butyrate producers like Facelibacterium, Eubacterium and Phascolarctobacterium in infected tuberculosis patients, suggesting a strong correlation between microbial dysbiosis in the gut and pathophysiology of tuberculosis[68].
A study conducted by Wang et al[69] suggests that the interactions between the gut and the lungs occur through inflammatory cells and chemical mediators. Altered gut microbiota composition can lead to disturbed mucosa, which further affects both the lungs and the gut, and has extended effects on the immune system of COPD individuals. Systemic hypoxia and oxidative stress, which are often found to occur in patients suffering from COPD may lead to intestinal dysbiosis and dysfunction, with a negative impact extended to the GLA as well. Hence, bidirectional crosstalk seems to exist between the gut-lung microbiota[69].
The gut-liver axis refers to the relationship between the microbiota of the gut and the well-being of the liver[70]. Various signals and metabolites generated through microbial, genetic, dietary, and environmental factors are integrated to ensure the optimal performance of the liver[71]. Homeostasis of the liver is influenced by the composition and functioning of the gut microbes, and, the liver itself is implicated in shaping the composition of the gut microbes. The communication between the gut and liver is mediated via the portal vein which transfers products of the gut to the liver and carries the liver products such as bile and antibodies to the intestine[71].
Naturally, disruption of the homeostasis between the gut and the liver can result in disruption of the microbiota, i.e., dysbiosis, leading to inflammation of the liver and development of chronic liver diseases such as alcoholic liver disease non-alcoholic liver disease, cirrhosis and hepatocellular carcinoma (HCC). Gut microbiotas have also been suggested to play a role in the onset and progression of HCC. The gut-liver axis has been identified as one of the key pathophysiological players involved in HCC[72]. Presence of increased levels of endotoxemia in the blood, which is associated with bacterial translocation, has been associated with the onset of HCC[73]. Considering the role of gut microbes in such diseases, probiotics-based therapeutic interventions have shown potential in regulating and improving conditions of endotoxemia[74]. Furthermore, gut microbiotas have been implicated in HCC immunotherapy as well[75]. Understanding the intricacies of the gut-liver axis and the interaction between the microbiota and the liver is imperative in the prevention and management of ailments of the liver.
The gut-pancreas axis, similar to the GLA is implicated in the development of various pathophysiological conditions. Alterations in the microbiota of the intestines can lead to disturbances in the gut-liver-pancreas axis, and result in the development of autoimmune diabetes, and the pathogenesis of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis[76]. The pancreas is known to play an important role in the regulation of homeostasis of the intestines, and also in the mucosal defense against pathogens and pathobionts. Furthermore, the pancreatic proteins and lipids influence the regulation of the gut microbiota and the retroaction of the gut on the liver is suggested to have a role in the development of inflammatory bowel disease (IBD) and pancreatic diseases as well[77]. The composition and functioning of the gut microbiota can influence the inflammation of the pancreas, insulin signaling, and glucose and lipid metabolism, along with blood glucose levels. More importantly, the gut microbiota such as Bifidobacterium spp. and Lactobacillus spp. play a significant role in hyperinsulinemia and the development of diabetes, including gestational diabetes mellitus and type 1 diabetes mellitus[78,79]. The gut microbiota of diabetic patients shows a prevalence of opportunistic pathogens, especially those belonging to the Enterobacteriaceae family, along with members of Clostridiales, Bacteroides, and Prevotella spp. as well[80].
The gut microbes and their products are also suggested to play an important role in the regulating the health of the skeletal system, and modulate bone formation and resorption processes. Close coordination of the gut-bone axis has also been found to influence osteoarthritis, a chronic degenerative condition affecting the articular cartilage, via modulation of the intestinal mucosal barrier, intestinal metabolites, and modulation of the immune system as well[81]. Furthermore, gut microbiota is also implicated in bone remodeling and the development of osteodegenerative diseases such as osteoporosis. Studies conducted in this regard, involving germ-free-mice and ovariectomized mice have confirmed a connection between the microbiota of the intestines and bone mineral density and bone mass[82,83]. This role of the gut microbiota and their metabolites in the pathogenesis of bone-related disorders further presents a potential therapeutic target as well. The influence of the gut microbiota on bine remodeling and disease development is associated with the production of SCFAs, hydrogen sulphide and polyamines, extracellular vesicles, and via interaction with the components of the immune system such as the regulatory and helper T cells[84].
The gut-muscle crosstalk involves significant impact on muscle mass, proteins, and function. Specifically, metabolic products such as the SCFAs are involved in the fermentation of dietary substrates, which further has significant impacts on the regulatory functions of the muscular system. Furthermore, the gut microbiota is suggested to have a role in the development of sarcopenia, which is the loss of muscular mass and function. In a study conducted by Lahiri et al[85], germ-free mice lacking the gut microbiota were employed to study the effects of the gut microbiota on muscular mass, when compared to the pathogen-free, gut microbiota-equipped mice models. Significant results included muscular atrophy, decreased levels of insulin-like growth factors, and reduced mitochondrial functioning and expression of genes involved in muscle growth. Interestingly, reduced levels of choline, suggest the role of gut microbiota in influencing the production of acetylcholine, which is imperative in the signaling at the neuromuscular junctions[85]. Recently, the role of gut microbes as a probable target for the treatment of Duchenne muscular dystrophy, a disorder involving the gradual wasting of muscles, has also been suggested[86]. Various studies in this regard have also confirmed that probiotic supplementation or transplantation can exert significant effects on muscle mass and functioning, suggesting the role of gut microbes in treating muscle-related disorders[87].
The gut microbiota is suggested to play a significant role in skin health as well. Research has shown that alterations in the composition and function of the gut microbiota can result in the development of skin-related issues including aging skin, pigmentation and dryness[88]. Studies have shown that gut microbiota can influence skin health by decreasing oxidative stress, suppressing inflammatory responses, regulating immune functions, and exerting a pharmacological action on local skin pathways[89].
Dysbiosis of the gut microbiota results in compromise of the epithelial barrier function, and decreased permeability of the intestinal barrier. This dysbiosis further affects the production of the SCFAs which could interact with the receptors in the skin, and this could directly affect the commensal microbial composition on the skin. The gut microbiota has been associated with some common skin disorders, including acne vulgaris, dermatitis, psoriasis, rosacea, and suspected interference leading to skin cancer as well, with higher levels of bacteria such as Bacteroides and Faecalibacterium, Clostridium, Escherichia, etc.
The gut-reproductive axis has been found to influence both male and female reproductive health, with dysbiosis of the gut microbiome leading to diminished fertility and impaired spermatogenesis in the case of the gut-testis axis[90]. In females, the microbial composition of the gut has been implicated in the genesis of various gynaecological disorders, including polycystic ovarian syndrome, cancers such as breast cancer, ovarian cancer and cervical cancer, and post-menopausal issues. According to a study conducted by Yang et al[91], opportunistic pathogens of the gut such as Escherichia, Clostridium, and Citrobacter are suggested to play a role in the development and progression of breast cancer. In cases of polycystic ovarian syndrome, research has revealed an increased abundance of microbiota such as Phocaeicola vulgatus, Bacillota, Streptococcus and Escherichia/Shigella complex, and a decreased population of Akkermansiaa and Oscillospiraceae[92]. Additionally, gut microbes are also related to the development of post-menopausal issues like postmenopausal osteoporosis, which results from the deficiency of estrogen, causing a reduction in bone mass and increased incidence of fragility fractures[93].
The gut microbes have been associated with the development of disorders related to the kidney as well, including the onset of chronic kidney diseases (CKD)[94]. CKD models have shown an overall increment in the occurrence of bacteria including Clostridium spp., Enterobacteria, Eggerthella spp., and a decreased occurrence of Prevotella, Bacteroides, and Roseburia. Bacterial metabolites including butyrate, indole, and those involved in mucin degradation have been linked to the development of CKD as well[95]. In CKD, dysbiosis in the gut microbiota may result in the production of uremic solutes that lead to renal damage, further complicating the situation[96]. Additionally, intestinal microbiota is also associated with acuter kidney injury, diabetic kidney disease and nephrolithiasis as well. Patients suffering from acuter kidney injury and CKD have shown an altered gut microbiome, suggesting a potential role in disease outcomes[2]. Identification of gut microbiota in CKD can potentially help in understanding the pathogenic mechanisms and development of novel therapeutic strategies in this regard[97]. An imbalance between the symbionts and the pathobionts can lead to loss of intestinal barrier integrity, activation of the immune cells, and cytokine secretion, all of which have detrimental effects on kidney function and symbiosis[96].
The dysbiosis in the gut has been associated with increased susceptibility to recurring urinary tract infections[98]. Studies have shown that the gut microbiome of individuals affected by urinary tract infection is depleted in microbial diversity and specifically, the butyrate producers, in comparison to the controls[99]. Additionally, the gut-bladder axis has also been linked to the disorder of overactive bladder, and benign conditions such as lower urinary tract symptoms[100]. Interestingly, in a study conducted by Li et al[100], increased diversity and species richness of the gut microbiome were associated with increased severity of conditions such as overactive bladder, further indicating the role if the microbiome in bladder dysfunctions and disorders.
The interactions between the commensal microbiota and the mammalian system development and functions include multifold interactions in homeostasis as well as disease development[101,102]. Although many of the microbes that reside in the mammalian system are known to carry out important physiological functions, they may also occasionally become pathogenic, and breach the safe zone[24]. Opportunistic pathogens residing in the gut, for example, may under certain circumstances invade the host epithelial tissues, and lead to severe and fatal issues including sepsis[103]. Thereby, the immune system is critical in maintaining a healthy balance between the resident microbial communities and the well-being of the human body, ensuring a safe interaction between the two[24], and further extending the host-microbiota relationship beyond just metabolic functions. Co-evolution between the immune system and the microbiota has indeed ensured the maintenance of a mutual relationship between the host and the microbiota. The resident microbial communities are known to play a significant role in influencing the functions of the immune system as well[104,105]. The dynamic interactions between the gut microbiota and the host’s immune systems play a key role in maintaining homeostasis and inhibiting inflammation[106]. Such homeostasis between the microbiota and the host involves two elements, one, maintaining optimal host-microbe interactions, and secondly, conserving the composition of the microbial consortium in the host body[36]. Deviations from the proper microbiota composition and establishment can alter the immune system development and lead to inflammatory diseases as well[107]. The microbiota has a profound impact on the development of the immune system, and understanding this interplay has the potential to change the very nature of immunological research[106]. Several chemical modulators of the immune system are essentially involved in the sustenance of microbiota-host interactions[28]. The interactions between the immune system and the microbiota are bidirectional, i.e., the immune system controls the microbiota, and the microbiota influences the immunity of the host too. An impairment in the interaction between the gut microbiota and the mucosal immune system can disrupt the epithelial barrier and increase the susceptibility to infections. Antibacterial lectins such as RegIIIγ, secreted by the epithelial cells, under the regulation of Toll-like receptors, are known to restrict bacterial cells from breaching the epithelium, thereby allowing minimal epithelial contact[108]. Furthermore, immunoglobulins such as the immunoglobulin A, produced in the intestine by the dendritic cells of the lamina propria mediate the stratification of the bacteria of the gut[109]. The microbiota further shape the immunity of the host, by exercising impact on the development and functioning of the lymphoid tissues and epithelial functioning of the GI tract[105].
There is growing evidence of studies that point towards the profound impact of the gut microbiota on the functions of the immune system of the host. Various gnotobiotic studies involving faecal transplantation from affected individuals to healthy, germ-free gnotobiotic mice. The results are often the development of features related to such diseases, including autoimmune issues, malnutrition, obesity, etc.[5]. Such studies point towards the possible role of the microbiota in the development and exacerbation of diseases and in the optimal functioning of the immune system, and the impact of the immune system on the microbiota composition as well. Germ-free gnotobiotic mice present numerous defects such as weakened production of T-cells, decreased production of immunoglobulins like immunoglobulin A, and increased production of immunoglobulins like immunoglobulin E, when transplanted with faecal microbiota from healthy hosts (murine or human donors), showed appreciable reversal of the above mentioned defects, pointing towards the role of the gut microbiota in the optimal functioning of the immune system[5]. Gut dysbiosis or the negative alterations in the gut microbial composition can dysregulate the immune responses and lead to oxidative stress, insulin resistance, inflammation, and sepsis[107]. The gut microbiota and their metabolites such as SCFAs play a pivotal role in mucosal immunity[19]. The microbiota is actively involved in influencing the development and functioning of the components of the host’s innate and adaptive system, and in turn, the immune system regulates the maintenance of the host-microbe symbiosis[101]. The potential role of gut microbiome in the development of disease is actively being studied in the recent years. In a study conducted by Montgomery et al[110], the AID multiple sclerosis was linked with imbalances in the gut microbiome. A murine model was employed to study the interactions between the host and the microbiome, followed by transplantation-based manipulation of the git microbiome, which can modulate the experimental autoimmune encephalomyelitis (a model for multiple sclerosis) susceptibility and the metabolic profiles of the host. The gut microbe Lactobacillus reuteri was identified to be associated with the exacerbation of experimental autoimmune encephalomyelitis. Such findings highlight the complex interactions between the host genetics and the gut microbiota in modulating the CNS-mediated autoimmunity. The gut microbiota and the immune system interact with each other very closely, via the Toll-like receptors and the microbial-associated molecular patterns and the SCFAs[62]. The microbiome may react with the immune system and modulate its functioning via various mechanisms including adenomatous polyposis coli activation and T-cell mediated reactions resulting in tumor surveillance, inhibition of regulatory T cell induction, modulation of inflammatory signals, etc.[33]. Continuous changes in lifestyle, overuse and misuse of antibiotics and other drugs, etc. in different parts of the world, particularly the high-income countries are proposed to have led to a significant alteration in the microbiota composition of individuals living in such countries. These may have further selected for a group of microbes that have negatively impacted the immune system, leading to a dramatic rise in cases of inflammatory issues and increased cases of AIDs[36]. The interaction of the microbiome and the immune system is implicated in several non-communicable diseases, including celiac disorders[111], IBD, and also various extra-intestinal problems including rheumatoid arthritis, neurodegenerative disorders, and even malignant cancers[112-114]. Such dynamic interactions are very complex and dependent on several factors and contexts.
The proof of concept on the influence of gut microbes on the overall physiological well-being of the host has been demonstrated through various gnotobiotic studies. The function of the mammalian system is to not only exclude potential pathogens but also maintain amicable relationships with the commensal microbiota, which regulates mammalian immune functions to ensure it responds appropriately to friends and foes. In effect, the interaction between the immune system and the microbiota ensures a balance between the proinflammatory defensive responses and the regulatory responses against inflammation[115]. As a result of the co-evolution between the microbiota and the host immune system, the immune system is largely involved in maintaining the symbiotic relationship between the host and its resident microbiota, while the microbiota continues to evolve and diversify. Understanding the interplay between the microbiota and the immune system is crucial to develop novel strategies to modulate immune responses improve cancer therapies, and maintain overall health.
Massive improvements in genomic, proteomic, and metabolomics studies in recent years have led to unprecedented advancements in understanding the role of the microbiome as a potential mode of treatment and prophylaxis of many health issues and the development of various problematic and often life-threatening disorders in humans[4]. The microbiota makeup, especially that of the gut microbiome, is easily influenced by various factors, including dietary habits, intake of antibiotics and other drugs, health status of the individual, and even environmental factors[116].
Although we are still understanding the relationship between microbiota and the host, clinical advancements are rapidly focusing on developing newer strategies that are aimed at altering/manipulating the gut microbial composition, and the implication of such strategies in dealing with the onset and progression of systemic and local disorders and diseases. Gut microbiota dysbiosis has also been linked to metabolic issues including hyperglycemia, hyperlipidemia, and obesity[117]. Changes in diet, especially a heavy intake of fat, has led to alterations in the gut microbiome, which may have a role in induction of metabolic disorders such as diabetes and obesity[118,119]. Cancer development has also been linked to dysbiosis of the gut microbiota. Periodontal bacteria such as Porphyromonas, Prevotella, Fusobacterium, etc. have been implicated in the onset of colorectal cancer and malignant tumors of the pancreas[120,121]. Another bacterium of the gut that has been closely associated with the development of cancer is Helicobacter pylori, which is the only bacterium to be listed as a class 1 carcinogen, for its role in the development of gastric cancer[122]. Additionally, E. coli strains producing the metabolite colibactin have been associated with the development of colorectal cancers, as confirmed by in vivo studies held in this regard. Oftentimes, the bacterial association with carcinogenesis is linked to the influence of bacteria on the production of interleukins such as interleukin-1β, and interleukin-6, tumor necrosis factors such as tumor necrosis factor-alpha, etc., and their influence on cell signaling molecules involved in cell proliferation, including cyclin/CDK, signal transducer and activator of transcription 3 signaling, protein kinase B signaling, etc., and the production of reactive oxygen species, RNS, volatile sulfur compounds, etc., which are carcinogenic[121]. Additionally, other bacterial toxins such as cytolethal distending toxin, and cytotoxic necrotizing factors may contribute to DNA damage and onset of cancers of various kinds, including gastric and colorectal cancer[117,123]. Given their role in the development of cancer, microbes also present themselves as interesting and novel therapeutic targets for the treatment of such ailments as well. However, such research is still in its infancy and requires extensive and robust technology and standardized methods.
Administration of beneficial bacteria via probiotics or fecal transplantation, or administration of bacterial components/metabolites have recently emerged as interesting and novel strategies to impart protection to the host, positively modulate the performance of the immune system, and also potentially treat certain diseases that have been linked to microbial dysbiosis in the host[124].
Ingestion of desirable microbes (probiotics) or their metabolites is also expected to restore the original gut microbiota composition and also provide an energy source for the bacteria to produce SCFAs and other desirable metabolites[125]. Additionally, probiotics have also shown therapeutic potential in various areas of health, including their use in treating psychiatric disorders in children and adolescents, and potential in attention deficit hyperactivity disorder and autism[126]. Interestingly, probiotics have also been studied for their role in cancer prevention, as various studies in this regard have already implicated the role of the gut microbiome in cancer onset and progression. Their relationship was discovered as early as 1980, when a study conducted by Goldin and Gorbach[127] indicated a strong correlation between ingestion of Lactobacillus-based probiotics and a reduction in rate of colorectal cancer by as much as 37%. Probiotic strains including those of Lactobacilli (Lactobacilli plantarum, Lactobacilli rhamnosus, Lactobacilli acidophilus, Lactobacilli pentosus, Lactobacilli paracasei, etc.) Bacillus spp., Bifidobacterium spp. and Propionibacterium spp. have shown significant potential in decreasing cell proliferation and modulation of apoptosis, and prevention of cancers including colorectal cancer, liver cancer, cervical cancer, etc.[128]. Attempts at clinical studies to validate the use of probiotics have yielded positive results. In a study conducted by Kotzampassi et al[129], strains of Lactobacillus acidophilus, Lactobacilli plantarum, Bifidobacterium lactis, and Saccharomyces boulardii were tested for their potential in treatment of cancer patients undergoing colorectal surgery. The ingestion of these probiotics decreased the occurrence of postoperative complications, and a correlation between the suppressor of cytokine signaling 3 gene and the tumor necrosis factor gene was observed. Probiotics have also been found to have a significant role in the prevention or treatment of acute respiratory tract infections, colic, acute infectious diarrhea, and enterocolitis. Their mechanism of action includes the inhibition of adhesion of pathogenic bacteria, enhancement of mucosal barrier functioning, secretion of various bioactive metabolites, modulation of immune responses of the innate and the adaptive systems, and regulation of the ENS and the CNS[130].
Faecal microbiome transplantation (FMT) is an unconventional technique that involves transplanting faecal solution from a donor with a healthy gut microbiome, into the gut of a patient. Such transplantation is expected to restore desirable gut bacteria and other microbes, thereby restoring the health of the affected individual[58]. One of the more successful applications of FMT to treat infection caused by Clostridium difficile[131]. In a randomized control trial to measure the success of FMT in the treatment of Clostridium difficile infection, a cure rate of 81% was obtained upon duodenal infusion of donor fences, in comparison to the control group, which was treated with vancomycin, and showed 31% cure rate[132]. Although various promising studies have been conducted in this regard, FMT therapies also pose the risk of infectious diseases being transferred from the donor to the patient, and also the stimulation of chronic diseases, such as obesity and atherosclerosis, due to an altered microbiota[133]. In this context, establishing a standardized protocol for faecal solution preparation, optimising the route for transplantation/administration, and maintenance databases carrying information on the type of disease, and the best-suited faecal microbiota composition, along with a record of the donors and the recipients is expected to enhance the clinical implications of this therapeutic strategy[134]. Some successful research involving the therapeutic potential of gut microbiota on human health has been summarised in Table 2[135-140].
| Microbiota-based intervention | Disease treated | Observations | Ref. |
| Bifidobacterium lactis and Lactobacillus rhamnosus | Colorectal cancer | Significant reduction in cancer proliferation and improved epithelial barrier function in patients under placebo-controlled trials | [135] |
| Lactobacillus reuteri | Gastric cancer | Human adenocarcinoma epithelial cells of the gastric tissue (AGS) treated with test strain showed reduced proliferation in dose-dependent manner | [136] |
| Lactobacillus casei and Lactobacillus paracasei | Cervical cancer | Upregulation of apoptotic genes such as BAX, caspase 3, caspase 8 and caspase 9, downregulation of BCl-2 gene observed in HeLa cells | [137] |
| Fecal microbiota transplantation | Clostridium difficile infection | Full primary or secondary response was achieved in 7 patients, with no serious after-effects contrary to metronidazole-treated control group | [138] |
| Faecal microbiota transplantation | Ulcerative colitis | [139] | |
| Fecal microbiota transplant | Recurrent hepatic encephalopathy | Improved cognition and dysbiosis | [140] |
Gut microbiota and their metabolites have been recognised as important biomarkers for disease phenotypes, prognosis, and treatment response as well[16]. As discussed in the previous sections, dysbiosis of the microbiome-host interaction can lead to the development of various complications and diseases including IBD, cancers, cardiovascular disorders, etc.[141]. These findings further implicate the role of the microbiome in the development of novel therapeutic approaches against these diseases[142]. Exploring areas of microbiomics can help in understanding the intimacies linked to disease development and progression in relation to gut microbes. The variability of the gut microbiome among individuals paves the way for the idea that a precision-based personalized approach in the treatment of gut-microbiome-related diseases and disorders may provide a sustainable and efficient way of treating diseases[143]. Furthermore, machine learning, improved data transparency and facilitation in effective clinical trials are expected to overcome the challenges in analysing and applying gut microbiota in therapy.
While numerous studies have explored the correlation between the microbiota and host well-being, our understanding of its functional implications in health and disease is still in its beginnings. Gnotobiotic studies using germ-free animals have been crucial in deciphering the complex interactions between the gut microbiota and host physiology. Insights into these molecular interactions may lead to novel microbially-derived therapies that optimize host health, though identifying effective microbial strains remains challenging due to the microbiota’s vast genomic complexity. Moreover, germ-free models, though useful, exhibit significant anatomical and immunological differences. The microbiome significantly affects the immune, pulmonary, and cardiovascular systems, influencing disease development and immunological signaling, including cancer. Dysbiosis has been linked to various pathologies, highlighting the need for in-depth research to leverage the microbiome in preventing, diagnosing, and treating diseases. Interdisciplinary approaches, molecular studies, standardized analyses, and effective clinical trials are essential to fully understand the potential of microbiome-based therapies.
| 1. | Dekaboruah E, Suryavanshi MV, Chettri D, Verma AK. Human microbiome: an academic update on human body site specific surveillance and its possible role. Arch Microbiol. 2020;202:2147-2167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 2. | Kim MG, Yang J, Jo SK. Intestinal microbiota and kidney diseases. Kidney Res Clin Pract. 2021;40:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787-8803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1421] [Cited by in RCA: 1834] [Article Influence: 183.4] [Reference Citation Analysis (57)] |
| 4. | Gebrayel P, Nicco C, Al Khodor S, Bilinski J, Caselli E, Comelli EM, Egert M, Giaroni C, Karpinski TM, Loniewski I, Mulak A, Reygner J, Samczuk P, Serino M, Sikora M, Terranegra A, Ufnal M, Villeger R, Pichon C, Konturek P, Edeas M. Microbiota medicine: towards clinical revolution. J Transl Med. 2022;20:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 5. | Ahern PP, Maloy KJ. Understanding immune-microbiota interactions in the intestine. Immunology. 2020;159:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 1468] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 7. | Feng T, Elson CO. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol. 2011;4:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 969] [Cited by in RCA: 2069] [Article Influence: 344.8] [Reference Citation Analysis (0)] |
| 9. | Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 993] [Cited by in RCA: 1604] [Article Influence: 200.5] [Reference Citation Analysis (0)] |
| 10. | Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70 Suppl 1:S38-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 714] [Article Influence: 54.9] [Reference Citation Analysis (1)] |
| 11. | Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (3)] |
| 12. | Quigley EM. Gut bacteria in health and disease. Gastroenterol Hepatol (N Y). 2013;9:560-569. [PubMed] |
| 13. | Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract--a role beyond infection. Nat Rev Urol. 2015;12:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 410] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 14. | Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823-1836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1710] [Cited by in RCA: 2052] [Article Influence: 256.5] [Reference Citation Analysis (7)] |
| 15. | Pascal M, Perez-Gordo M, Caballero T, Escribese MM, Lopez Longo MN, Luengo O, Manso L, Matheu V, Seoane E, Zamorano M, Labrador M, Mayorga C. Microbiome and Allergic Diseases. Front Immunol. 2018;9:1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 16. | Morgan XC, Huttenhower C. Chapter 12: Human microbiome analysis. PLoS Comput Biol. 2012;8:e1002808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 336] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 17. | Ali A, AlHussaini KI. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms. 2024;12:222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 48] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 18. | Caspani G, Kennedy S, Foster JA, Swann J. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microb Cell. 2019;6:454-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 19. | Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020;11:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 1598] [Article Influence: 319.6] [Reference Citation Analysis (0)] |
| 20. | Donohoe DR, Holley D, Collins LB, Montgomery SA, Whitmore AC, Hillhouse A, Curry KP, Renner SW, Greenwalt A, Ryan EP, Godfrey V, Heise MT, Threadgill DS, Han A, Swenberg JA, Threadgill DW, Bultman SJ. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 345] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 21. | Das NK, Schwartz AJ, Barthel G, Inohara N, Liu Q, Sankar A, Hill DR, Ma X, Lamberg O, Schnizlein MK, Arqués JL, Spence JR, Nunez G, Patterson AD, Sun D, Young VB, Shah YM. Microbial Metabolite Signaling Is Required for Systemic Iron Homeostasis. Cell Metab. 2020;31:115-130.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 22. | Caspani G, Swann J. Small talk: microbial metabolites involved in the signaling from microbiota to brain. Curr Opin Pharmacol. 2019;48:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Nichols RG, Davenport ER. The relationship between the gut microbiome and host gene expression: a review. Hum Genet. 2021;140:747-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 24. | Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3003] [Cited by in RCA: 2986] [Article Influence: 229.7] [Reference Citation Analysis (0)] |
| 25. | Mody D, Verma V, Rani V. Modulating host gene expression via gut microbiome-microRNA interplay to treat human diseases. Crit Rev Microbiol. 2021;47:596-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Fellows R, Varga-Weisz P. Chromatin dynamics and histone modifications in intestinal microbiota-host crosstalk. Mol Metab. 2020;38:100925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Richards AL, Muehlbauer AL, Alazizi A, Burns MB, Findley A, Messina F, Gould TJ, Cascardo C, Pique-Regi R, Blekhman R, Luca F. Gut Microbiota Has a Widespread and Modifiable Effect on Host Gene Regulation. mSystems. 2019;4:e00323-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 896] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 29. | Li M, Chen WD, Wang YD. The roles of the gut microbiota-miRNA interaction in the host pathophysiology. Mol Med. 2020;26:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 30. | Guz M, Jeleniewicz W, Malm A, Korona-Glowniak I. A Crosstalk between Diet, Microbiome and microRNA in Epigenetic Regulation of Colorectal Cancer. Nutrients. 2021;13:2428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Young VB. Therapeutic manipulation of the microbiota: past, present, and considerations for the future. Clin Microbiol Infect. 2016;22:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Lozenov S, Krastev B, Nikolaev G, Peshevska-Sekulovska M, Peruhova M, Velikova T. Gut Microbiome Composition and Its Metabolites Are a Key Regulating Factor for Malignant Transformation, Metastasis and Antitumor Immunity. Int J Mol Sci. 2023;24:5978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Garg S, Sharma N, Bharmjeet, Das A. Unraveling the intricate relationship: Influence of microbiome on the host immune system in carcinogenesis. Cancer Rep (Hoboken). 2023;6:e1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Strakova N, Korena K, Karpiskova R. Klebsiella pneumoniae producing bacterial toxin colibactin as a risk of colorectal cancer development - A systematic review. Toxicon. 2021;197:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Iftekhar A, Berger H, Bouznad N, Heuberger J, Boccellato F, Dobrindt U, Hermeking H, Sigal M, Meyer TF. Genomic aberrations after short-term exposure to colibactin-producing E. coli transform primary colon epithelial cells. Nat Commun. 2021;12:1003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 36. | Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2488] [Cited by in RCA: 3417] [Article Influence: 310.6] [Reference Citation Analysis (1)] |
| 37. | Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen ZS. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 1323] [Article Influence: 441.0] [Reference Citation Analysis (0)] |
| 38. | Garg M, Verma M, Khan AS, Yadav P, Rahman SS, Ali A, Kamthan M. Cadmium-induced augmentation of fungal translocation promotes systemic infection in mice via gut barrier disruption and immune dysfunction. Life Sci. 2025;362:123368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Alshehri D, Saadah O, Mosli M, Edris S, Alhindi R, Bahieldin A. Dysbiosis of gut microbiota in inflammatory bowel disease: Current therapies and potential for microbiota-modulating therapeutic approaches. Bosn J Basic Med Sci. 2021;21:270-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 2651] [Article Influence: 530.2] [Reference Citation Analysis (1)] |
| 41. | Belstrøm D. The salivary microbiota in health and disease. J Oral Microbiol. 2020;12:1723975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 42. | Bhat FA, Khan S, Khan AS, Haque SE, Akhtar M, Najmi AK. Cardio-oncological dialogue: Understanding the mechanistic correlation between heart failure and cancer. Life Sci. 2024;358:123170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203-209. [PubMed] |
| 44. | Chen Y, Xu J, Chen Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients. 2021;13:2099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 415] [Article Influence: 103.8] [Reference Citation Analysis (1)] |
| 45. | Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4469] [Cited by in RCA: 4046] [Article Influence: 289.0] [Reference Citation Analysis (0)] |
| 46. | Lupu VV, Adam Raileanu A, Mihai CM, Morariu ID, Lupu A, Starcea IM, Frasinariu OE, Mocanu A, Dragan F, Fotea S. The Implication of the Gut Microbiome in Heart Failure. Cells. 2023;12:1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 47. | Bruzzese E, Callegari ML, Raia V, Viscovo S, Scotto R, Ferrari S, Morelli L, Buccigrossi V, Lo Vecchio A, Ruberto E, Guarino A. Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with Lactobacillus GG: a randomised clinical trial. PLoS One. 2014;9:e87796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 48. | Ringseis R, Gessner DK, Eder K. The Gut-Liver Axis in the Control of Energy Metabolism and Food Intake in Animals. Annu Rev Anim Biosci. 2020;8:295-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 49. | Hartmann B, Longo M, Mathiesen DS, Hare KJ, Jørgensen NR, Esposito K, Deacon CF, Vilsbøll T, Holst JJ, Knop FK. Signs of a Glucose- and Insulin-Independent Gut-Bone Axis and Aberrant Bone Homeostasis in Type 1 Diabetes. J Clin Endocrinol Metab. 2023;109:e259-e265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Chew W, Lim YP, Lim WS, Chambers ES, Frost G, Wong SH, Ali Y. Gut-muscle crosstalk. A perspective on influence of microbes on muscle function. Front Med (Lausanne). 2022;9:1065365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 51. | De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms. 2021;9:353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 298] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 52. | Siddiqui R, Makhlouf Z, Alharbi AM, Alfahemi H, Khan NA. The Gut Microbiome and Female Health. Biology (Basel). 2022;11:1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 53. | Okamoto T, Hatakeyama S, Imai A, Yamamoto H, Yoneyama T, Mori K, Yoneyama T, Hashimoto Y, Nakaji S, Ohyama C. Altered gut microbiome associated with overactive bladder and daily urinary urgency. World J Urol. 2021;39:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Rutsch A, Kantsjö JB, Ronchi F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front Immunol. 2020;11:604179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 470] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 55. | Socała K, Doboszewska U, Szopa A, Serefko A, Włodarczyk M, Zielińska A, Poleszak E, Fichna J, Wlaź P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res. 2021;172:105840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 399] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 56. | Michel L, Prat A. One more role for the gut: microbiota and blood brain barrier. Ann Transl Med. 2016;4:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 57. | Desai D, Desai A, Jamil A, Csendes D, Gutlapalli SD, Prakash K, Swarnakari KM, Bai M, Manoharan MP, Raja R, Khan S. Re-defining the Gut Heart Axis: A Systematic Review of the Literature on the Role of Gut Microbial Dysbiosis in Patients With Heart Failure. Cureus. 2023;15:e34902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Bui TVA, Hwangbo H, Lai Y, Hong SB, Choi YJ, Park HJ, Ban K. The Gut-Heart Axis: Updated Review for The Roles of Microbiome in Cardiovascular Health. Korean Circ J. 2023;53:499-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 59. | Zhen J, Zhou Z, He M, Han HX, Lv EH, Wen PB, Liu X, Wang YT, Cai XC, Tian JQ, Zhang MY, Xiao L, Kang XX. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front Endocrinol (Lausanne). 2023;14:1085041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 60. | Jaworska K, Bielinska K, Gawrys-Kopczynska M, Ufnal M. TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts haemodynamic effects: implications for interpretation of cardiovascular actions of gut microbiome. Cardiovasc Res. 2019;115:1948-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 61. | Ali A, Zahra A, Yadav P, Kamthan M. Advancements in cardiac bioengineering: Bridging the gap between medicine and engineering. In: Futuristic Trends in Biotechnology Volume 3 Book 23. Chikkamagaluru: Iterative International Publishers, 2024: 18-34. [DOI] [Full Text] |
| 62. | Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, Delhaes L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front Cell Infect Microbiol. 2020;10:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 471] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 63. | Garcia-Nuñez M, Millares L, Pomares X, Ferrari R, Pérez-Brocal V, Gallego M, Espasa M, Moya A, Monsó E. Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J Clin Microbiol. 2014;52:4217-4223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 64. | Marsland BJ, Trompette A, Gollwitzer ES. The Gut-Lung Axis in Respiratory Disease. Ann Am Thorac Soc. 2015;12 Suppl 2:S150-S156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 374] [Article Influence: 41.6] [Reference Citation Analysis (1)] |
| 65. | Sze MA, Dimitriu PA, Hayashi S, Elliott WM, McDonough JE, Gosselink JV, Cooper J, Sin DD, Mohn WW, Hogg JC. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 410] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 66. | He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J. Gut-lung axis: The microbial contributions and clinical implications. Crit Rev Microbiol. 2017;43:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 67. | Luo M, Liu Y, Wu P, Luo DX, Sun Q, Zheng H, Hu R, Pandol SJ, Li QF, Han YP, Zeng Y. Alternation of Gut Microbiota in Patients with Pulmonary Tuberculosis. Front Physiol. 2017;8:822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 68. | Maji A, Misra R, Dhakan DB, Gupta V, Mahato NK, Saxena R, Mittal P, Thukral N, Sharma E, Singh A, Virmani R, Gaur M, Singh H, Hasija Y, Arora G, Agrawal A, Chaudhry A, Khurana JP, Sharma VK, Lal R, Singh Y. Gut microbiome contributes to impairment of immunity in pulmonary tuberculosis patients by alteration of butyrate and propionate producers. Environ Microbiol. 2018;20:402-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 69. | Wang L, Cai Y, Garssen J, Henricks PAJ, Folkerts G, Braber S. The Bidirectional Gut-Lung Axis in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2023;207:1145-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 79] [Reference Citation Analysis (0)] |
| 70. | Mehal WZ. The gut-liver axis: a busy two-way street. Hepatology. 2012;55:1647-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1254] [Article Influence: 250.8] [Reference Citation Analysis (1)] |
| 72. | Xie C, Pocha C. Crosstalk between Gut Microbiota and Hepatocellular Carcinoma. Gastrointest Disord. 2023;5:127-143. [DOI] [Full Text] |
| 73. | Simbrunner B, Caparrós E, Neuwirth T, Schwabl P, Königshofer P, Bauer D, Marculescu R, Trauner M, Scheiner B, Stary G, Mandorfer M, Reiberger T, Francés R. Bacterial translocation occurs early in cirrhosis and triggers a selective inflammatory response. Hepatol Int. 2023;17:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 74. | Yan F, Polk DB. Probiotics and Probiotic-Derived Functional Factors-Mechanistic Insights Into Applications for Intestinal Homeostasis. Front Immunol. 2020;11:1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 75. | Muscolino P, Granata B, Omero F, De Pasquale C, Campana S, Calabrò A, D'Anna F, Drommi F, Pezzino G, Cavaliere R, Ferlazzo G, Silvestris N, Speranza D. Potential predictive role of gut microbiota to immunotherapy in HCC patients: a brief review. Front Oncol. 2023;13:1247614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 76. | Svegliati-Baroni G, Patrício B, Lioci G, Macedo MP, Gastaldelli A. Gut-Pancreas-Liver Axis as a Target for Treatment of NAFLD/NASH. Int J Mol Sci. 2020;21:5820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 77. | Zhang Z, Tanaka I, Pan Z, Ernst PB, Kiyono H, Kurashima Y. Intestinal homeostasis and inflammation: Gut microbiota at the crossroads of pancreas-intestinal barrier axis. Eur J Immunol. 2022;52:1035-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 78. | Hu R, Liu Z, Geng Y, Huang Y, Li F, Dong H, Ma W, Song K, Zhang M, Song Y. Gut Microbiota and Critical Metabolites: Potential Target in Preventing Gestational Diabetes Mellitus? Microorganisms. 2023;11:1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 79. | Al-Ishaq RK, Samuel SM, Büsselberg D. The Influence of Gut Microbial Species on Diabetes Mellitus. Int J Mol Sci. 2023;24:8118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 80. | Crudele L, Gadaleta RM, Cariello M, Moschetta A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBioMedicine. 2023;97:104821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 95] [Reference Citation Analysis (1)] |
| 81. | Liu L, Tian F, Li GY, Xu W, Xia R. The effects and significance of gut microbiota and its metabolites on the regulation of osteoarthritis: Close coordination of gut-bone axis. Front Nutr. 2022;9:1012087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 82. | Wen K, Tao L, Tao Z, Meng Y, Zhou S, Chen J, Yang K, Da W, Zhu Y. Fecal and Serum Metabolomic Signatures and Microbial Community Profiling of Postmenopausal Osteoporosis Mice Model. Front Cell Infect Microbiol. 2020;10:535310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 83. | Novince CM, Whittow CR, Aartun JD, Hathaway JD, Poulides N, Chavez MB, Steinkamp HM, Kirkwood KA, Huang E, Westwater C, Kirkwood KL. Commensal Gut Microbiota Immunomodulatory Actions in Bone Marrow and Liver have Catabolic Effects on Skeletal Homeostasis in Health. Sci Rep. 2017;7:5747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 84. | Lyu Z, Hu Y, Guo Y, Liu D. Modulation of bone remodeling by the gut microbiota: a new therapy for osteoporosis. Bone Res. 2023;11:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 73] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 85. | Lahiri S, Kim H, Garcia-Perez I, Reza MM, Martin KA, Kundu P, Cox LM, Selkrig J, Posma JM, Zhang H, Padmanabhan P, Moret C, Gulyás B, Blaser MJ, Auwerx J, Holmes E, Nicholson J, Wahli W, Pettersson S. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019;11:eaan5662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 370] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 86. | Marullo AL, O'Halloran KD. Microbes, metabolites and muscle: Is the gut-muscle axis a plausible therapeutic target in Duchenne muscular dystrophy? Exp Physiol. 2023;108:1132-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 87. | Giron M, Thomas M, Dardevet D, Chassard C, Savary-Auzeloux I. Gut microbes and muscle function: can probiotics make our muscles stronger? J Cachexia Sarcopenia Muscle. 2022;13:1460-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 108] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 88. | Gao T, Wang X, Li Y, Ren F. The Role of Probiotics in Skin Health and Related Gut-Skin Axis: A Review. Nutrients. 2023;15:3123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 89. | Kunst C, Schmid S, Michalski M, Tümen D, Buttenschön J, Müller M, Gülow K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines. 2023;11:1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 86] [Reference Citation Analysis (0)] |
| 90. | Leelani N, Bajic P, Parekh N, Vij SC, Lundy SD. The emerging role of the gut-testis axis in male reproductive health and infertility. F S Rev. 2023;4:131-141. [DOI] [Full Text] |
| 91. | Yang P, Wang Z, Peng Q, Lian W, Chen D. Comparison of the Gut Microbiota in Patients with Benign and Malignant Breast Tumors: A Pilot Study. Evol Bioinform Online. 2021;17:11769343211057573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 92. | Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health. 2020;17:7618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 425] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 93. | Xu Q, Li D, Chen J, Yang J, Yan J, Xia Y, Zhang F, Wang X, Cao H. Crosstalk between the gut microbiota and postmenopausal osteoporosis: Mechanisms and applications. Int Immunopharmacol. 2022;110:108998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 94. | Yadav B, Prasad N, Saxena A. Gut microbiota dysbiosis and chronic kidney disease. J Renal Nutr Metab. 2020;6:70. [DOI] [Full Text] |
| 95. | Lohia S, Vlahou A, Zoidakis J. Microbiome in Chronic Kidney Disease (CKD): An Omics Perspective. Toxins (Basel). 2022;14:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 96. | Cao C, Zhu H, Yao Y, Zeng R. Gut Dysbiosis and Kidney Diseases. Front Med (Lausanne). 2022;9:829349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 97. | Xie A, Sheng J, Zheng F. Intestinal Microbiota and Kidney Diseases. Chin J Integr Med. 2018;24:406-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Jung J, Kim A, Yang SH. The Innovative Approach in Functional Bladder Disorders: The Communication Between Bladder and Brain-Gut Axis. Int Neurourol J. 2023;27:15-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 99. | Salazar AM, Neugent ML, De Nisco NJ, Mysorekar IU. Gut-bladder axis enters the stage: Implication for recurrent urinary tract infections. Cell Host Microbe. 2022;30:1066-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 100. | Li K, Chen C, Zeng J, Wen Y, Chen W, Zhao J, Wu P. Interplay between bladder microbiota and overactive bladder symptom severity: a cross-sectional study. BMC Urol. 2022;22:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 101. | Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 789] [Cited by in RCA: 2220] [Article Influence: 444.0] [Reference Citation Analysis (1)] |
| 102. | Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1071] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 103. | Ribet D, Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015;17:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 481] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 104. | Campbell C, Kandalgaonkar MR, Golonka RM, Yeoh BS, Vijay-Kumar M, Saha P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines. 2023;11:294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 105] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 105. | Ali A, AlHussaini KI. Pesticides: Unintended Impact on the Hidden World of Gut Microbiota. Metabolites. 2024;14:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 106. | Jiao Y, Wu L, Huntington ND, Zhang X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front Immunol. 2020;11:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 107. | Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI. Gut Microbiota and Immune System Interactions. Microorganisms. 2020;8:1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 108. | Miki T, Okada N, Hardt WD. Inflammatory bactericidal lectin RegIIIβ: Friend or foe for the host? Gut Microbes. 2018;9:179-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 109. | Doron I, Kusakabe T, Iliev ID. Immunoglobulins at the interface of the gut mycobiota and anti-fungal immunity. Semin Immunol. 2023;67:101757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 110. | Montgomery TL, Künstner A, Kennedy JJ, Fang Q, Asarian L, Culp-Hill R, D'Alessandro A, Teuscher C, Busch H, Krementsov DN. Interactions between host genetics and gut microbiota determine susceptibility to CNS autoimmunity. Proc Natl Acad Sci U S A. 2020;117:27516-27527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 111. | Valitutti F, Cucchiara S, Fasano A. Celiac Disease and the Microbiome. Nutrients. 2019;11:2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 112. | Zhang M, Sun K, Wu Y, Yang Y, Tso P, Wu Z. Interactions between Intestinal Microbiota and Host Immune Response in Inflammatory Bowel Disease. Front Immunol. 2017;8:942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 113. | Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 2018;33:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 937] [Article Influence: 133.9] [Reference Citation Analysis (0)] |
| 114. | Maeda Y, Takeda K. Host-microbiota interactions in rheumatoid arthritis. Exp Mol Med. 2019;51:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 115. | Verma M, Garg M, Khan AS, Yadav P, Rahman SS, Ali A, Kamthan M. Cadmium modulates intestinal Wnt/β-catenin signaling ensuing intestinal barrier disruption and systemic inflammation. Ecotoxicol Environ Saf. 2024;277:116337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 116. | Anwar H, Iftikhar A, Muzaffar H, Almatroudi A, Allemailem KS, Navaid S, Saleem S, Khurshid M. Biodiversity of Gut Microbiota: Impact of Various Host and Environmental Factors. Biomed Res Int. 2021;2021:5575245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 117. | Wang PX, Deng XR, Zhang CH, Yuan HJ. Gut microbiota and metabolic syndrome. Chin Med J (Engl). 2020;133:808-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 118. | Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, Billard E, Barnich N. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6:19032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 119. | Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 1070] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 120. | Karpiński TM. The Microbiota and Pancreatic Cancer. Gastroenterol Clin North Am. 2019;48:447-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 121. | Karpiński TM. Role of Oral Microbiota in Cancer Development. Microorganisms. 2019;7:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 122. | Venerito M, Vasapolli R, Rokkas T, Delchier JC, Malfertheiner P. Helicobacter pylori, gastric cancer and other gastrointestinal malignancies. Helicobacter. 2017;22:Suppl 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 123. | Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrède JP. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A. 2010;107:11537-11542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 612] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 124. | Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018;20:e12966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 125. | Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 648] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 126. | Schneider A, Zeiler M, Kopp K, Wagner G, Karwautz A. [The Therapeutic Potential of Prebiotics and Probiotics in Child and Adolescent Psychiatric Disorders]. Z Kinder Jugendpsychiatr Psychother. 2023;51:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 127. | Goldin BR, Gorbach SL. Effect of Lactobacillus acidophilus dietary supplements on 1,2-dimethylhydrazine dihydrochloride-induced intestinal cancer in rats. J Natl Cancer Inst. 1980;64:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 131] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 128. | Śliżewska K, Markowiak-Kopeć P, Śliżewska W. The Role of Probiotics in Cancer Prevention. Cancers (Basel). 2020;13:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 129. | Kotzampassi K, Stavrou G, Damoraki G, Georgitsi M, Basdanis G, Tsaousi G, Giamarellos-Bourboulis EJ. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. World J Surg. 2015;39:2776-2783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 130. | Liu Y, Tran DQ, Rhoads JM. Probiotics in Disease Prevention and Treatment. J Clin Pharmacol. 2018;58 Suppl 10:S164-S179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 131. | Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 472] [Article Influence: 39.3] [Reference Citation Analysis (1)] |
| 132. | van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2677] [Article Influence: 223.1] [Reference Citation Analysis (0)] |
| 133. | Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol. 2016;9:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 301] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 134. | Kim KO, Gluck M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin Endosc. 2019;52:137-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (1)] |
| 135. | Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, Klinder A, O'Riordan M, O'Sullivan GC, Pool-Zobel B, Rechkemmer G, Roller M, Rowland I, Salvadori M, Thijs H, Van Loo J, Watzl B, Collins JK. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 326] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 136. | Rasouli BS, Ghadimi-Darsajini A, Nekouian R, Iragian GR. In vitro activity of probiotic Lactobacillus reuteri against gastric cancer progression by downregulation of urokinase plasminogen activator/urokinase plasminogen activator receptor gene expression. J Cancer Res Ther. 2017;13:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 137. | Riaz Rajoka MS, Zhao H, Lu Y, Lian Z, Li N, Hussain N, Shao D, Jin M, Li Q, Shi J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018;9:2705-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 138. | Juul FE, Garborg K, Bretthauer M, Skudal H, Øines MN, Wiig H, Rose Ø, Seip B, Lamont JT, Midtvedt T, Valeur J, Kalager M, Holme Ø, Helsingen L, Løberg M, Adami HO. Fecal Microbiota Transplantation for Primary Clostridium difficile Infection. N Engl J Med. 2018;378:2535-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 139. | Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J, Campaniello MA, Mavrangelos C, Rosewarne CP, Bickley C, Peters C, Schoeman MN, Conlon MA, Roberts-Thomson IC, Andrews JM. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA. 2019;321:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 617] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 140. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 453] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 141. | DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis. 2016;22:1137-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 656] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 142. | Behrouzi A, Ashrafian F, Mazaheri H, Lari A, Nouri M, Riazi Rad F, Hoseini Tavassol Z, Siadat SD. The importance of interaction between MicroRNAs and gut microbiota in several pathways. Microb Pathog. 2020;144:104200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 143. | Kashyap PC, Chia N, Nelson H, Segal E, Elinav E. Microbiome at the Frontier of Personalized Medicine. Mayo Clin Proc. 2017;92:1855-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |