Published online Mar 30, 2020. doi: 10.5493/wjem.v10.i2.10
Peer-review started: October 20, 2019
First decision: December 17, 2019
Revised: February 14, 2020
Accepted: March 1, 2020
Article in press: March 1, 2020
Published online: March 30, 2020
Processing time: 158 Days and 19.6 Hours
Liver cancer is the 6th most common cancer in the world and the 4th most common death from cancer worldwide. Hepatic radioembolization is a minimally invasive treatment involving intraarterial administration of radioembolic microspheres.
To develop a neutron-activated, biodegradable and theranostics samarium-153 acetylacetonate (153SmAcAc)-poly-L-lactic acid (PLLA) microsphere for intraarterial radioembolization of hepatic tumors.
Microspheres with different concentrations of 152SmAcAc (i.e., 100%, 150%, 175% and 200% w/w) were prepared by solvent evaporation method. The microspheres were then activated using a nuclear reactor in a neutron flux of 2 × 1012 n/cm2/s1, converting 152Sm to Samarium-153 (153Sm) via 152Sm (n, γ) 153Sm reaction. The SmAcAc-PLLA microspheres before and after neutron activation were characterized using scanning electron microscope, energy dispersive X-ray spectroscopy, particle size analysis, Fourier transform infrared spectroscopy, thermo-gravimetric analysis and gamma spectroscopy. The in-vitro radiolabeling efficiency was also tested in both 0.9% sodium chloride solution and human blood plasma over a duration of 550 h.
The SmAcAc-PLLA microspheres with different SmAcAc contents remained spherical before and after neutron activation. The mean diameter of the microspheres was about 35 µm. Specific activity achieved for 153SmAcAc-PLLA microspheres with 100%, 150%, 175% and 200% (w/w) SmAcAc after 3 h neutron activation were 1.7 ± 0.05, 2.5 ± 0.05, 2.7 ± 0.07, and 2.8 ± 0.09 GBq/g, respectively. The activity of per microspheres were determined as 48.36 ± 1.33, 74.10 ± 1.65, 97.87 ± 2.48, and 109.83 ± 3.71 Bq for 153SmAcAc-PLLA microspheres with 100%, 150%, 175% and 200% (w/w) SmAcAc. The energy dispersive X-ray and gamma spectrometry showed that no elemental and radioactive impurities present in the microspheres after neutron activation. Retention efficiency of 153Sm in the SmAcAc-PLLA microspheres was excellent (approximately 99%) in both 0.9% sodium chloride solution and human blood plasma over a duration of 550 h.
The 153SmAcAc-PLLA microsphere is potentially useful for hepatic radioembolization due to their biodegradability, favorable physicochemical characteristics and excellent radiolabeling efficiency. The synthesis of the formulation does not involve ionizing radiation and hence reducing the complication and cost of production.
Core tip: We developed and tested a neutron-activated, biodegradable samarium-153 acetylacetonate-poly-L-lactic acid microspheres formulation for radioembolization of hepatic tumors. The formulation is potentially useful for intraarterial hepatic radioembolization as an alternative to yttrium-90 microspheres due to their biodegradability, favorable physicochemical characteristics and excellent radionuclide retention efficiency. The synthesis of the formulation does not involve ionizing radiation and hence reducing the complication and cost of production.
- Citation: Wong YH, Tan HY, Kasbollah A, Abdullah BJJ, Acharya RU, Yeong CH. Neutron-activated biodegradable samarium-153 acetylacetonate-poly-L-lactic acid microspheres for intraarterial radioembolization of hepatic tumors. World J Exp Med 2020; 10(2): 10-25
- URL: https://www.wjgnet.com/2220-315X/full/v10/i2/10.htm
- DOI: https://dx.doi.org/10.5493/wjem.v10.i2.10
Liver malignancy represent the 6th most common diagnosed cancer and 4th most common death from cancer worldwide[1]. There are more than 700000 people diagnosed with primary liver malignancy annually and about 50% of the colorectal carcinoma patients may develop liver metastasis[2,3]. Surgical resection or liver transplantation has been the preferred curative treatment for solid liver malignancy, unfortunately these treatments are only effective for early stage or localized liver tumors. The symptoms of liver cancer often appeared when cancer has progressed to the intermediate or advanced stage. In fact, more than 75% of the patients are diagnosed in intermediate or advanced stages where surgical resection or liver transplantation is no longer an option[4,5]. The prognosis of unresectable liver cancer was poor in the past and the management of the disease was limited to systemic pharmacotherapy, external beam radiotherapy or palliative treatments[6].
Radioembolization is an alternative minimally invasive treatment for intermediate and advanced stage liver cancer[7,8]. The treatment takes advantage of differential blood supply for normal liver parenchyma and liver tumors. Liver tumors are usually rich in vasculature and receive blood almost exclusively from hepatic artery while the normal liver parenchyma cells receive blood predominately (about 80%) from the portal vein[9-11]. During the treatment, microspheres within the size of 20-60 µm labelled with a radionuclide that emits short range beta radiation are intraarterially administered. The microspheres are preferentially lodged near the terminal arterioles of the tumors and induces both radiation and embolization effects. Since the beta radiations only travel a few millimeters in the tissues, the healthy liver tissues and surrounding organs receive minimal radiation, while the tumors are exposed to a tumoricidal radiation dose[12-14].
Currently, only the yttrium-90 (90Y) microspheres approved by the United States Food and Drug Administration for the treatment of hepatocellular carcinoma and metastatic colorectal carcinoma. The 90Y radioembolic agent are available in the forms of glass beads (TheraSphere®, BTG Interventional Medicine, United Kingdom) and resin microspheres (SIR-Spheres®, SIRTex Medical Limited, Australia)[15]. Nevertheless, there are several shortcomings that 90Y microspheres need to overcome. The glass- and resin-based microspheres are biocompatible but not biodegradable, therefore the microspheres will remain in the liver permanently after administration. In addition, the density of the glass-based microsphere (3.6 g/mL) is approximately three times higher than the blood plasma (1.05 g/mL), hence it’s settling velocity, injection and homogenous distribution of the glass microspheres within the tumor vascular are difficult to control[16]. 90Y is a pure beta emitter and does not carry any imaging properties, evaluation of extrahepatic activity and liver dosimetry post-administration is often performed on 90Y Bremsstrahlung single-photon emission computed tomography or positron emission tomography imaging that shows low quantitative accuracy or sensitivity[17]. Furthermore, 90Y is commonly produced using a high-cost strontium-90 generator associated with complicated chemical separation process[18].
Samarium-153 (153Sm) is a potential radionuclide that can be used for hepatic radioembolization. 153Sm can be produced from its non-radioactive isotope, 152Sm via neutron activation, i.e., 152Sm (n, γ) 153Sm. The 153Sm has been used for palliative pain treatment for bone metastases and radiosynovectomy in the past decades. The imaging properties of 153Sm have been demonstrated in some previous studies of pharmacoscintigraphy and gastrointestinal imaging research[19-21]. The simultaneous emission of beta and gamma radiations from 153Sm has rendered it a potential theranostic radionuclide for targeted cancer therapy.
This study aimed to develop a biodegradable radioembolic microspheres formulation loaded with 153Sm to overcome the limitations of 90Y microspheres for hepatic radioembolization. The physicochemical characteristics, radioactivity, radioimpurities as well as 153Sm retention efficiency of the developed microspheres were studied and discussed in the following sections.
Poly-L-lactic acid (PLLA) (viscosity = 0.8-1.2 dL/g), Samarium-152 acetylacetonate (152SmAcAc) (99% purity) and polyvinyl alcohol (PVA, average molecular weight = 67000) were procured from Sigma Aldrich (St. Louis, MI, United States). Analytical grade chemicals and deionized water were used in all the experiments unless stated otherwise.
152SmAcAc-PLLA microspheres with different concentrations of 152SmAcAc were prepared using oil-in-water solvent evaporation method[22]. Firstly, 20 mL of chloroform solution containing 1.0 g of PLLA and different amounts of 152SmAcAc (i.e., 1.0 g, 1.5 g, 1.75 g and 2.0 g) were added dropwise into 600 mL of 3% (w/v) PVA solution under overhead stirring at 950 rpm. The overhead stirring was continued for 12 h to allow evaporation of the chloroform. The resulting 152SmAcAc-PLLA microspheres were then collected through vacuum filtration using a filter paper of 15 µm pores. Zero point one mol/L hydrochloride acid (HCl) and distilled water were used to dissolve and wash off any unbound 152SmAcAc from the formulation. Subsequently, the microspheres were dried in a vacuum oven at room temperature for 48 h. Once the microspheres were completely dried, they were fractionated into 20-60 µm diameter using stainless steel sieves. Finally, the microspheres within 20-60 µm diameter were collected and stored in a sealed vial at –20 °C for further analysis.
The 152SmAcAc-PLLA microspheres were sent for neutron activation using a 750 kW pool-type research reactor (TRIGA Mark II, General Atomics, CA, United States). Prior to neutron activation, the samples were transferred into a polyethylene vial and sealed. The samples were neutron-activated using either the pneumatic transfer system (PTS) or rotary specimen rack (RR) method. The neutron activation protocols for both PTS and RR methods are given in Table 1. The radioactivity of the neutron-activated samples was measured using a calibrated dose calibrator (CRC 25R, Capintec, NJ, United States). The activity per microsphere was calculated by dividing the sample activity with the estimated number of microspheres present in the sample.
| Protocol | Pneumatic transfer system | Rotary specimen rack |
| Thermal neutron flux, θth (n/cm2/s) | 5.0 x 1012 | 2.0 x 1012 |
| Maximum duration of neutron activation | Up to 5 min | Up to 6 h |
| Location of neutron activation | Near to the core | Peripheral to the core |
| Sample delivery | Automatic | Manual |
Physicochemical characterization of the SmAcAc-PLLA microspheres before and after neutron activation was carried out using scanning electron microscopy (SEM), energy dispersive X-ray (EDX) spectroscopy, particle size analyzer, Fourier transform infrared spectroscopy (FTIR), thermo-gravimetric analysis (TGA) and wavelength dispersive X-ray fluorescence (WDXRF) spectrometry. The objectives and methods of each test were elaborated in the following sections.
SEM and EDX spectroscopy: Surface morphology of the SmAcAc-PLLA microspheres before and after neutron activation was examined using a SEM system (Quanta FEG 450, FEI, Oregon, United States). SEM images were obtained at 5 kV, 10 mm working distance and 2.0 spot size. Elemental compositions of the SmAcAc-PLLA microspheres before and after neutron activation were analyzed using EDX spectroscopy on the SEM system. The samples were mounted on aluminum stubs before performing the SEM and EDX analysis.
Laser scattering particle size analysis: The mean diameter and particle size distribution of the PLLA and SmAcAc-PLLA microspheres before and after neutron activation were determined using a laser scattering particle size distribution analyzer (Microtrac X100, Microtrac, United States). Microspheres samples suspended in distilled water were ultrasonicated before loading into the particle size analyzer for measurement.
FTIR spectroscopy: FTIR spectroscopy was performed to identify the chemical bonds of the microspheres. FTIR spectra within 600-4000 cm-1 of the PLLA, 152SmAcAc and 152SmAcAc-PLLA microspheres were obtained on a calibrated FTIR spectrometer (Spectrum 100, PerkinElmer Inc., MA, United States). Firstly, the samples were mixed with spectroscopy grade potassium bromide grain and dried in an oven to remove absorbed moisture. The potassium bromide-mixed samples were then pressed into pellets using designated tools provided by the manufacturer (PerkinElmer Inc., MA, United States) and subjected for FTIR analysis.
TGA: Thermal profiles of the 152SmAcAc-PLLA microspheres were studied using a TGA 8000™ analyzer (PerkinElmer Inc., MA, United States). The profiles of its raw materials, PLLA and 152SmAcAc, were also included for comparison. Approximately 5 mg of the sample was transferred into a ceramic sample pan and placed inside the TGA analyzer. The sample was then heated under nitrogen flow from 30–800 °C at a rate of 10 °C/min. TGA profiles of the samples were analyzed using the dedicated software (Pyris, PerkinElmer Inc., MA, United States).
WDXRF spectrometry: The amount of 152Sm presence in the 152SmAcAc-PLLA microspheres with different 152SmAcAc weight (1.0 g, 1.5 g, 1.75 g and 2.0 g) were determined using the semi-quantitative analysis of WDXRF spectrometry (Zetium, Malvern PANanalytical Ltd., Royston, United Kingdom).
Gamma spectroscopy was performed at 24 h after neutron activation using a coaxial, p-type hyperpure germanium detector (Canberra, Meriden, United States) to identify the radionuclides present in the formulation. The sample was placed at a calibrated distance in the hyperpure germanium detector and counted for 5 min live time. The gamma spectrum was obtained and analyzed using dedicated software (GenieTM 2000 Ver. 3.2, Canberra, United States).
The density of the 152SmAcAc-PLLA microspheres were determined based on Archimedes principle. The density was calculated based on the relation given in Equation (1). Mettler Toledo (ME 204) density meter (Ohio, United States) was used for this purpose.
(1) Density of Microspheres (ρs) = Mb × ρw / (Ma - Mw)
Where Mb is the mass of the sample on a balance, Ma is the mass of the sample in air, Mw is the mass of the sample in water and is the density of water (1 g/cm3).
The ρs was used to estimate the number of microspheres per gram of sample using Equation (2).
(2) Number of particles per gram = 6 × 1012 / (π × ρs × Dp3)
Where Dp is the mean diameter of the microspheres in µm and ρs is the density of the microspheres in g/cm3.
The viscosity, ηo of 153SmAcAc-PLLA microspheres [2.5% (w/v)] suspended in sodium chloride (NaCl) solution was measured at 37 °C using a rheometer (HAAKE MARS, Thermo Fisher Scientific, MA, United States). The reading was then incorporated into the Stokes’ Law to calculate the sedimentation rate (settling velocity)[13].
(3) Vsed = g × Dp2 × (ρs - ρf) / (18 × ηo)
Where Vsed is the sedimentation rate (cm/s), g is the gravitational acceleration constant (981 cm/s2) and ηo is the dynamic viscosity of the fluid (ρf = g/cm/s).
The radiolabeling efficiency of the neutron-activated 153SmAcAc-PLLA microspheres was tested in both 0.9% NaCl solution and human blood plasma over a period of 550 h. The microspheres were suspended in the test solutions and continuously mixed at 50 rpm for 1 h using a roller mixer. The samples were then centrifuged at 2000 rpm for 10 min. One milliliter of the supernatant was then collected and assayed using an automated gamma counter (2470 Wizard2, PerkinElmer, MA, United States). The procedures were repeated until 8 mL of assay samples were collected over a period of 550 h[22].
The 152SmAcAc-PLLA microspheres loaded with different amount of 152SmAcAc (1.0 g, 1.5 g, 1.75 g and 2.0 g) were successfully synthesized using the solvent evaporation method. The synthesis parameters such as stirring rate (950 rpm), PVA concentration (3% w/v) and polymer solution concentration were optimized in this study in order to produce microspheres with a mean diameter of approximately 35 µm and size distribution within 20-60 µm.
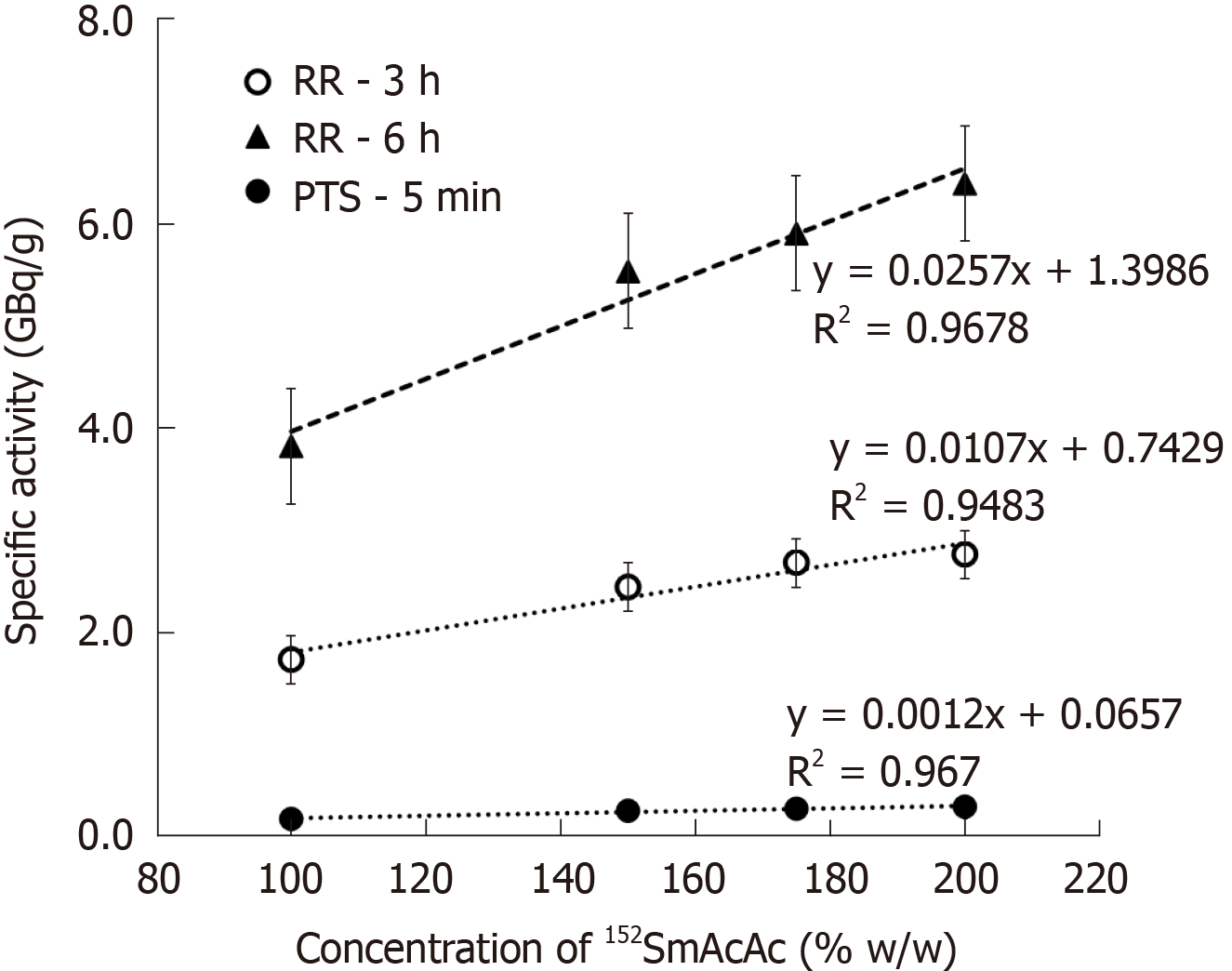
The 152SmAcAc-PLLA microspheres were neutron-activated using two different methods: (1) Automatic PTS control where the samples were sent near to the reactor core and neutron-activated for a maximum duration of 5 min; and (2) Manual loading RR method where the samples were neutron-activated for an extended duration of 3 h and 6 h at the peripheral location from the reactor core. Table 1 shows the neutron activation protocols for both PTS and RR methods. The specific activity (GBq/g) achieved for different formulations and neutron activation methods are given in Figure 1. The radioactivity increased linearly with the amount of 152SmAcAc in the formulations. However, the maximum amount of 152SmAcAc that can be loaded to the PLLA microsphere was 200% (w/w), and this achieved the maximum specific activity of 6.40 ± 0.07 GBq/g for a 6 h neutron activation using RR method. The radioactivity was proportional to the neutron activation time as theoretically known.
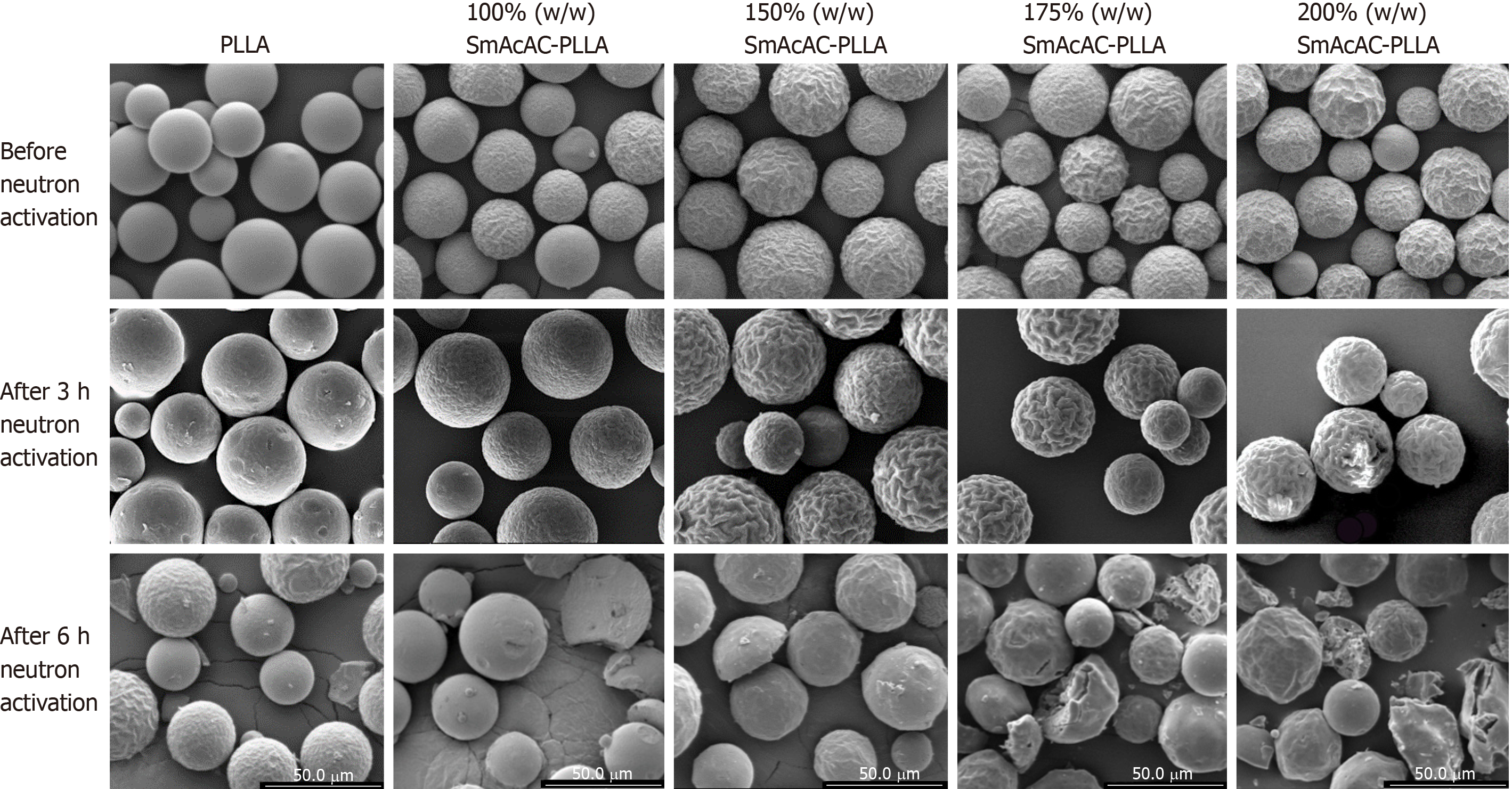
Figure 2 shows the SEM images of the PLLA and SmAcAc-PLLA microspheres before and after neutron activation. Spherical microspheres were successfully produced following oil-in-water solvent evaporation synthesis. There is no 152SmAcAc crystals were seen on the surface of the microspheres even at the highest concentration of 152SmAcAc. In addition, the washing process using diluted HCl did not cause any physical damage to the microspheres. A prolonged neutron activation time of 6 h in the thermal neutron flux of 2.0 × 1012 n/cm2/s1 has produced physical damage and fragmentation on the microspheres. The damage and fragmentation were more severe at higher concentration of 152SmAcAc. Nonetheless, neutron activation time of 3 h in the same neutron flux setting did not damage the physical structure of the microspheres. An extended part of the study has been carried out and found that neutron activation time longer than 4 h would cause physical damage and fragmentation of the PLLA microspheres (data were not included in this manuscript).
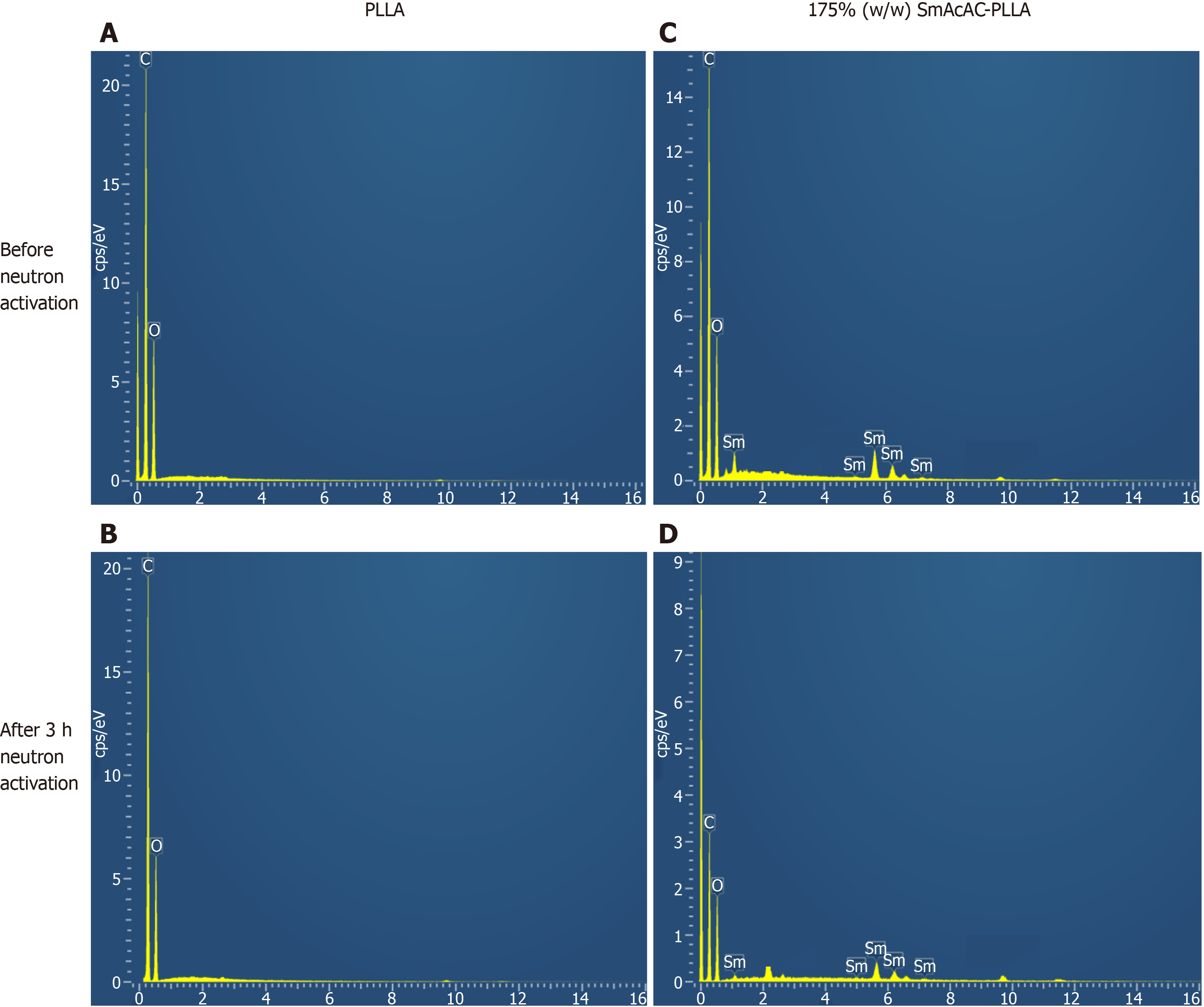
Figure 3 shows the EDX spectrum of the PLLA microspheres and SmAcAc-PLLA microspheres before and after 3 h neutron activation. The spectra show the presence of carbon (C) and oxygen (O) in the PLLA microspheres, and C, O and Sm in the SmAcAc-PLLA microspheres. No other element was detected after neutron activation for both microspheres. The hydrogen (H) presence in the microspheres could not be detected by EDX due to the limitation of EDX in detecting any chemical element lower than atomic number of 6. In addition, no constituent of chloride from the diluted HCl washing was detected on the EDX spectrum of the SmAcAc-PLLA microspheres.
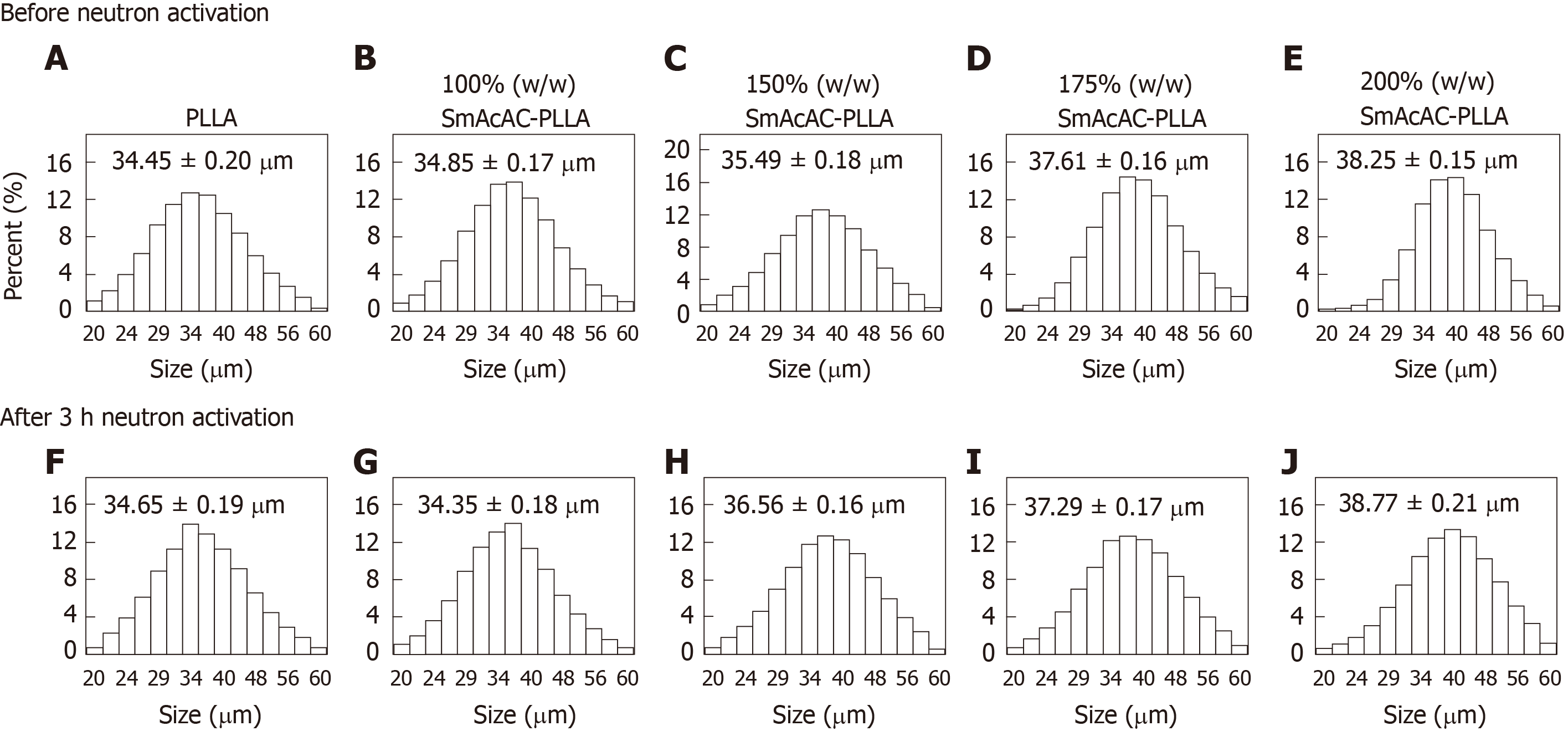
The mean diameter and size distribution of the PLLA and SmAcAc-PLLA microspheres before and after 3 h neutron activation are presented in Figure 4. The mean diameter of the PLLA microspheres was 34.45 ± 0.20 µm and the size distribution was within 20 µm and 60 µm. Different concentrations of 152SmAcAc in the PLLA microspheres did not change the size distribution significantly, however the mean diameter increased slightly with the amount of 152SmAcAc loaded to the polymer. For instance, the mean diameter increased from 34.85 ± 0.17 µm to 38.25 ± 0.15 µm when the amount of 152SmAcAc increased from 100% to 200% w/w. The mean diameter of the microspheres was not altered significantly after 3 h neutron activation.
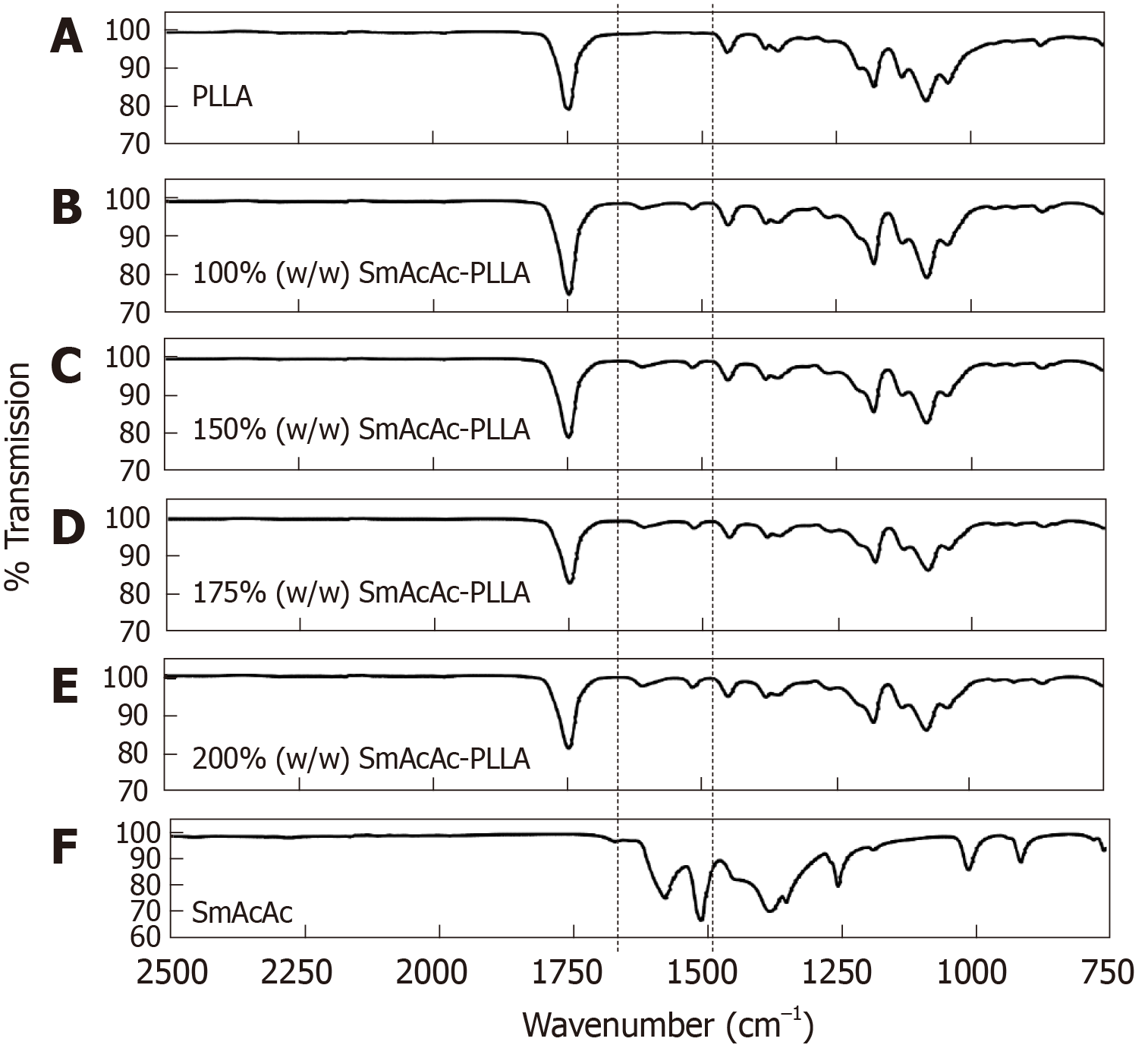
Figure 5 shows the FTIR spectrum of the PLLA (Figure 5A), 152SmAcAc-PLLA microspheres (Figure 5B-E) and 152SmAcAc crystal (Figure 5F). The FTIR spectrum of the 152SmAcAc crystal shows IR bands in the C=C and C=O regions (1700-1500 cm–1) (Figure 5F)[23-25]. The FTIR spectra of the 153SmAcAc-PLLA microspheres indicated that the C=C vibration of acetylacetonate was noticed at 1515 cm–1, similar to the wavelength for 152SmAcAc crystals. On the other hand, the C=O region of the acetylacetonate was shifted from 1580 cm–1 in the 152SmAcAc to 1610 cm–1 in the SmAcAc-PLLA microspheres (Figure 5B-E). This indicates that the PLLA carbonyl groups are interacting with the 152SmAcAc complex, resulting in the immobilization of the 152SmAcAc complex in the PLLA matrix. This in turn explains the low release characteristics of the 153SmAcAc-PLLA microspheres. There was no band signal observed at these wavelengths in the FTIR spectrum of the PLLA microspheres (Figure 5A). In addition, there was no noticeable change in the C=O band of the PLLA (1760 cm–1) since any shift would be masked by the strong absorption band of the remaining, non-coordinating C=O bonds.
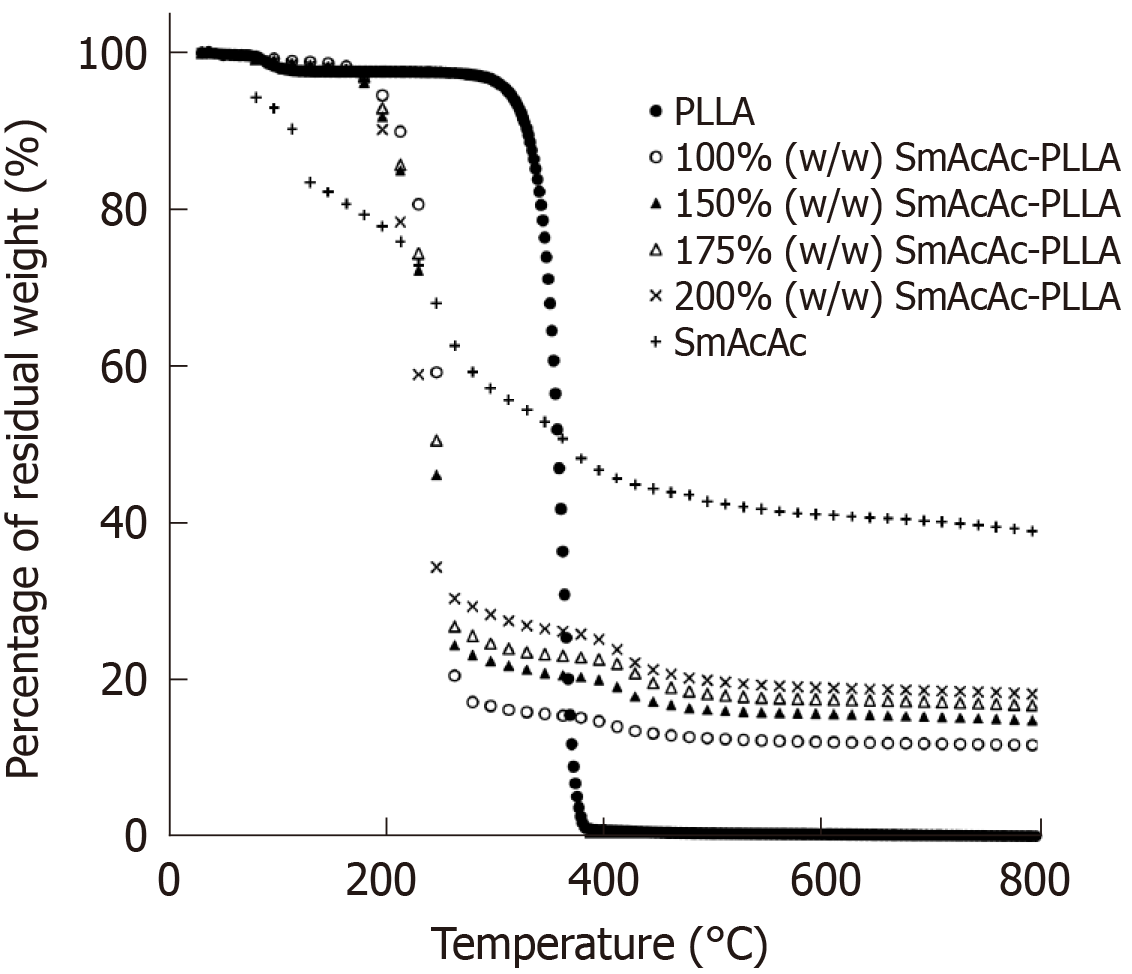
Figure 6 shows the TGA profile of the PLLA, 152SmAcAc-PLLA microspheres and 152SmAcAc crystal. The PLLA microspheres showed only one event of mass loss, which can be ascribed to the decomposition of polymer main chain starting from 250 °C. The TGA profile of the 152SmAcAc crystal shows multiple steps decomposition with the remaining residual weight indicating the presence of about 40% 152Sm in the crystal. On the other hand, the TGA profiles of the 152SmAcAc-PLLA microspheres show additional decomposition steps at above 200 °C, indicating decomposition of 152SmAcAc above this temperature. Loading of 152SmAcAc crystal to the PLLA polymer matrix has reduced the decomposition temperature from 250 °C to 200 °C. The residual weight presence in the 152SmAcAc-PLLA microspheres indicated the presence of 152Sm in the sample. The 152Sm content in the 152SmAcAc-PLLA microspheres was corresponded to the 152Sm content (between 12% and 18%) as determined by WDXRF (Table 2).
| Physicochemical properties | 100% (w/w) 153SmAcAc-PLLA | 150% (w/w) 153SmAcAc-PLLA | 175% (w/w) 153SmAcAc-PLLA | 200% (w/w) 153SmAcAc-PLLA |
| Mean diameter (µm) | 34.35 ± 0.18 | 36.56 ± 0.16 | 37.29 ± 0.17 | 38.77 ± 0.21 |
| Density (g/cm3) | 1.25 ± 0.030 | 1.29 ± 0.006 | 1.31 ± 0.047 | 1.35 ± 0.068 |
| Number of microspheres per g | 37 million | 30 million | 28 million | 24 million |
| Settling velocity (cm/s ) | 0.016 ± 0.0004 | 0.019 ± 0.0002 | 0.022 ± 0.0003 | 0.027 ± 0.0004 |
| Specific activity (GBq/g) | 1.74 ± 0.05 | 2.45 ± 0.05 | 2.69 ± 0.07 | 2.77 ± 0.09 |
| Activity per particle (Bq) | 46.31 ± 1.33 | 81.01 ± 1.65 | 95.40 ± 2.48 | 114.37 ± 3.72 |
| Samarium content (%) | 12.06 ± 0.69 | 14.56 ± 0.28 | 16.32 ± 0.34 | 17.28 ± 0.46 |
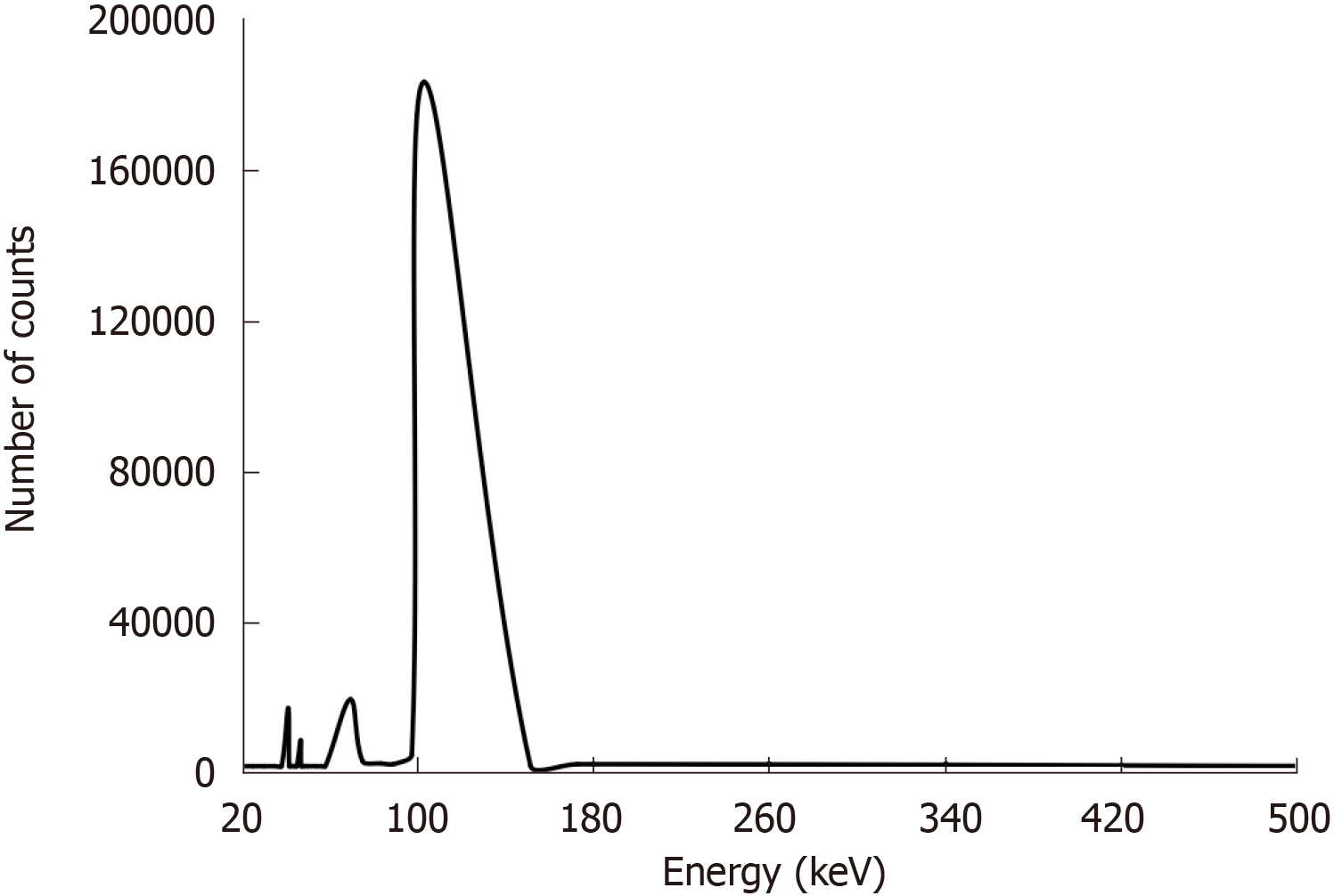
Figure 7 shows the gamma spectrum of the 153SmAcAc-PLLA microspheres acquired at 24 h after neutron activation. Several photopeaks were observed at 41 keV, 47 keV, 69 keV and 103 keV on the gamma spectrum of the 153SmAcAc-PLLA microspheres. The most dominant photopeak was found at 103 keV followed by a smaller photopeak at 69 keV. These energies corresponded to the principal gamma energies emitted by 153Sm. The other two photopeaks (41 keV and 47 keV) were the K-shell characteristic X-rays emitted following internal conversion of 153Sm. The gamma spectroscopy results were in accordance with the EDX spectroscopy results, showing no radionuclide impurities on the 152SmAcAc-PLLA microspheres after neutron activation
The density of the 152SmAcAc-PLLA microspheres ranged from 1.25 g/cm3 to 1.35 g/cm3 depending on the amount of the 152SmAcAc added in the formulation (Table 2). The density values thus obtained were used to determine the number of microspheres per gram using Equation (2) given in the Methods section and the results are presented in Table 2. The number of microspheres per gram is inversely proportional to the density and diameter of the microspheres. Since the density and diameter of the 153SmAcAc-PLLA microspheres increased when the amount of 152SmAcAc increased, this results in a reduction of the number of microspheres per gram for the formulation with increased 152SmAcAc concentration. For example, 37 million microspheres were estimated in a gram of 153SmAcAc-PLLA microspheres loaded with 100% (w/w) 152SmAcAc, while only 24 million microspheres were estimated in the 200% (w/w) SmAcAc. The activity per microsphere was estimated as 46.31 ± 1.39, 81.01 ± 1.52, 95.40 ± 2.59, and 114.37 ± 3.60 Bq for the formulations loaded with 100%, 150%, 175% and 200% (w/w) 152SmAcAc, respectively.
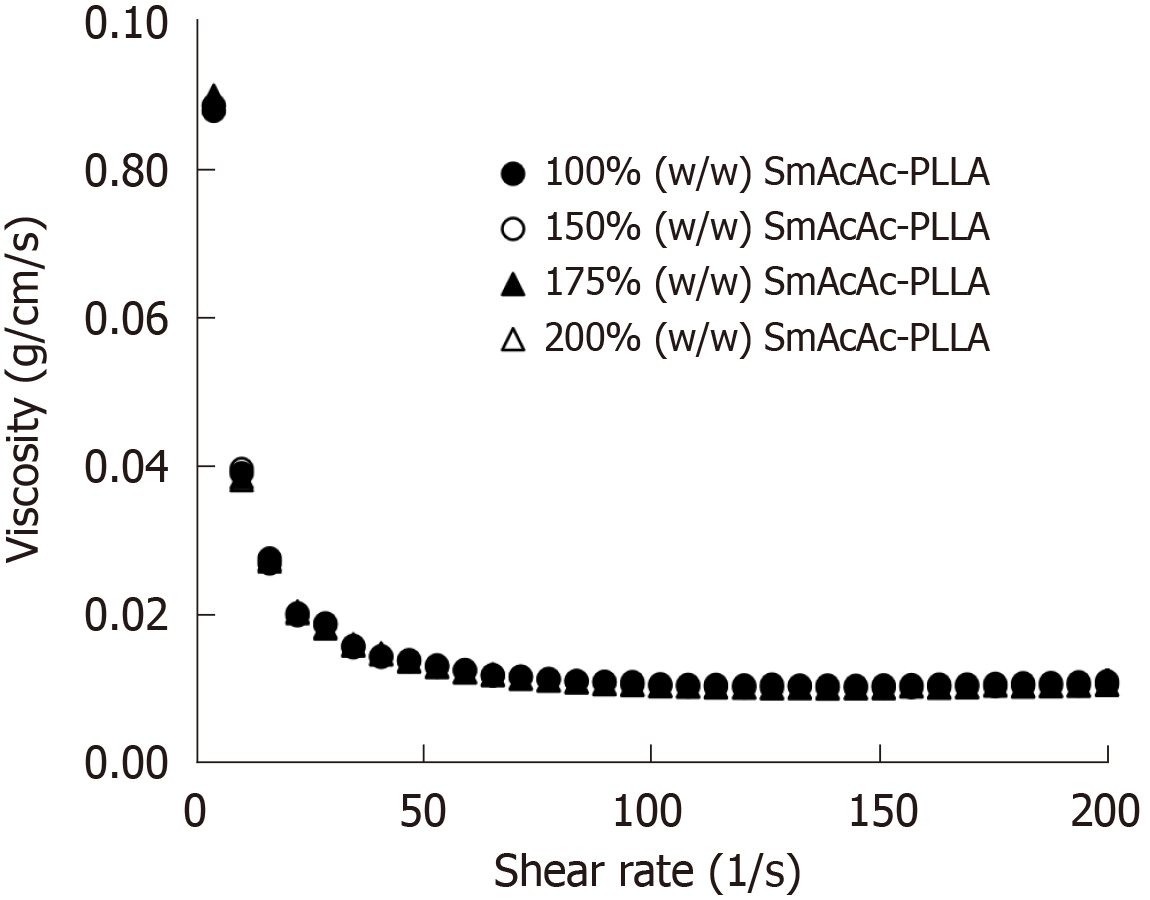
The viscosity of the microspheres suspension at 2.5% (w/v) concentration at 37 °C are shown in Figure 8. As demonstrated in the graph, the viscosity of the 152SmAcAc-PLLA suspension at different shear rates were substantially similar and hence the average viscosity value was used to represent the viscosity of the respected microspheres suspension (Figure 8). The settling velocity (cm/s) of the formulation is depending on the viscosity, density and size of the microspheres. The settling velocity of 153SmAcAc-PLLA are given in Table 2.
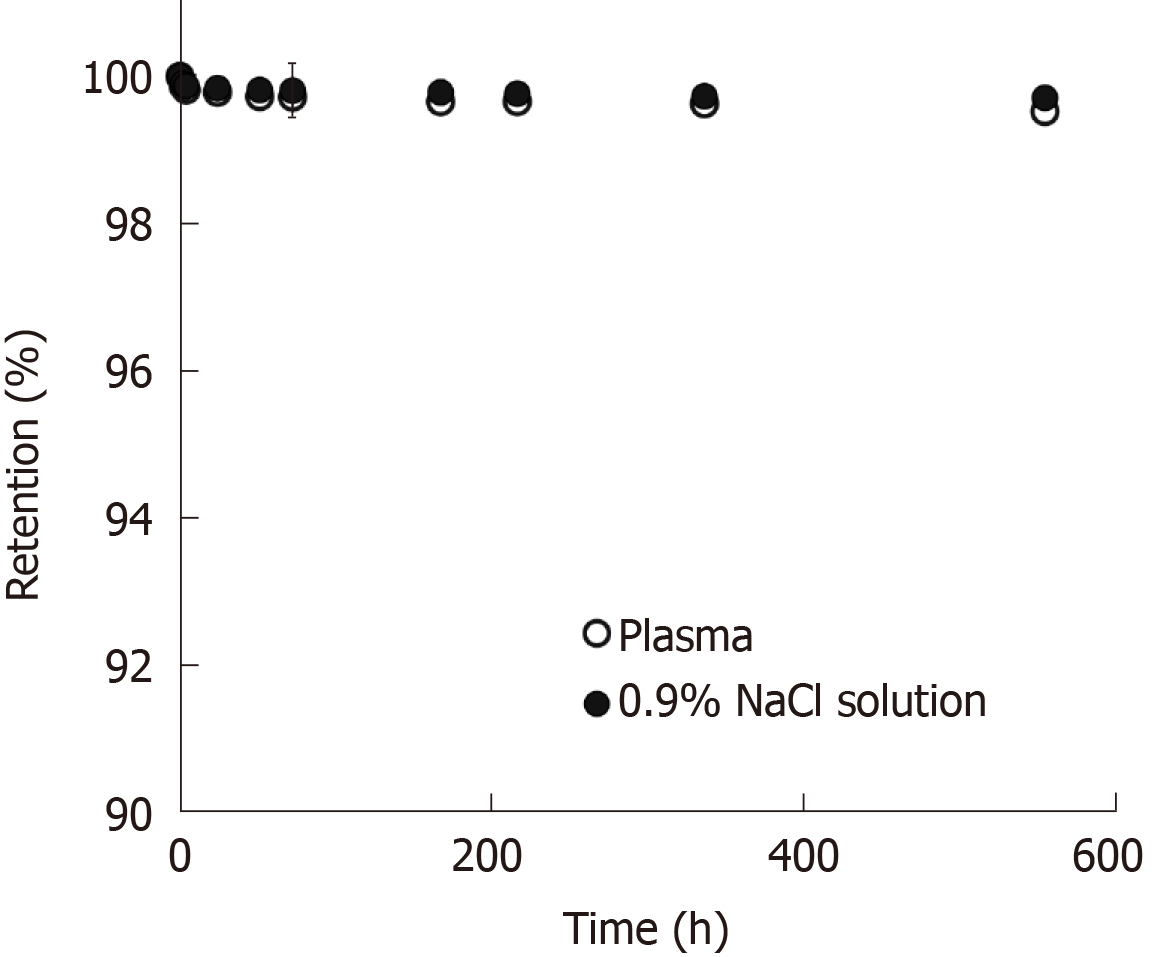
The in-vitro radiolabeling of 153SmAcAc-PLLA microspheres tested in 0.9% NaCl solution and human blood plasma is demonstrated in Figure 9. The formulation showed excellent 153Sm retention of more than 99% in 0.9% NaCl solution and human blood plasma over a test period of 550 h. A slightly lower 153Sm retention efficiency was observed in human blood plasma than in the 0.9% NaCl solution but the difference was negligible (less than 1%).
Radioembolization with the use of radiolabelled microspheres has several advantages over external beam radiotherapy for hepatic malignancies. Firstly, it treats liver tumors by dual-mechanism pathways: Embolization (blocking blood supply to the liver tumors) and internal radiation therapy using high energy short-range radiations such as beta[26]. Secondly, it is useful to treat diffuse hepatocellular carcinoma or liver metastasis when surgery or local treatment is not possible. Thirdly, it minimizes radiation damage to the surrounding healthy tissues[27]. Current formulations approved by the United States Food and Drug Administration for radioembolization of liver metastasis are resin-based (SIR-Sphere) and glass-based (TheraSphere) microspheres labelled with pure beta emitter, 90Y. Several researches have been conducted to explore alternative radionuclides and polymers to overcome some limitations of the 90Y microspheres[28-32]. A theranostic radionuclide that emits both beta and gamma radiations is preferred to facilitate treatment planning and dosimetry. Compared to the generator-based radionuclide production, neutron activation is a preferred method due to its relatively lower cost of production, simpler process, wider availability of nuclear reactors globally and reduces international shipping of radioactive products. Examples of neutron-activated radionuclides include 166Ho, 177Lu, 186Re and 188Re. Biodegradable polymer is also favored in order to minimize the risk of long-term complications such as hepatitis and cirrhosis.
This study aimed to develop a biodegradable PLLA microspheres loaded with stable 152SmAcAc. The microspheres were then activated using a nuclear reactor to produce radioactive 153SmAcAc-PLLA microspheres that emits both beta (Eβ-max = 810 keV) and gamma (103 keV) radiations, potentially useful for theranostic radioembolization of liver malignancies. PLLA is biocompatible for human body and its degradation reaction is mainly due to hydrolysis to lactic acid. The lactic acid produced during the biodegradation of PLLA can serve as an energy substrate for cells and has been shown to have antioxidant properties[33]. In addition, the PLLA has a near blood plasma density and thus could overcome the high density drawback from glass-based 90Y microspheres[28]. The developed 153SmAcAc-loaded PLLA microspheres formulation combines the benefits of being biocompatible, biodegradable, similar density to human blood plasma, and emitting both therapeutic and diagnostic radiations (theranostic). Moreover, the production cost is reduced due to the relatively easier and cheaper neutron activation method.
A simple one-step oil-in-water solvent evaporation technique was used in this study to incorporate 152SmAcAc crystals into the PLLA polymer. The 152Sm was complexed with the acetylacetonate ligand to enable its incorporation into PLLA via oil-in-water solvent evaporation[34]. The 152SmAcAc, ranging from 100 to 200% (w/w), was successfully encapsulated into the PLLA microspheres. Incorporation of 152SmAcAc beyond 200% (w/w) has resulted in irregular shape of the microspheres with excessive unbound 152SmAcAc crystals on the surface. The morphology and diameter of the microspheres were known to be affected by several synthesis parameters such as concentration of PVA and stirring rates, in which, a lower PVA concentration and stirring rate would produce microspheres with larger mean diameter and size distribution[35]. The optimum PVA concentration of 3% (w/w) and stirring rate of 950 rpm have resulted in spherical microspheres with a mean diameter of approximately 35 µm. The microspheres were sieved to obtain the size within 20-60 µm diameter, which is a strict requirement for intraarterial hepatic radioem-bolization[10].
The integrity of the 153SmAcAc-loaded PLLA microspheres after neutron activation is important to ensure its safe administration into human body. Several factors such as moisture content and neutron activation time are known to produce detrimental effects on the integrity of the PLLA microspheres[32]. In order to prevent deterioration during neutron activation, the formulations were dried in a vacuum oven for at least 48 h to remove any excessive moisture prior to neutron activation. The 153SmAcAc-PLLA microspheres remained intact and spherical with similar size distribution after 3 h neutron activation but was fragmented when the neutron activation time was prolonged to 6 h. The formulation with a higher loading of 152SmAcAc would experience a greater extent of fragmentation. The fragmentation maybe ascribed to the radiation damage to the PLLA microspheres. Similar fragmentation of PLLA microspheres after neutron activation has been observed in a previous study[32]. From the results of this study, the optimum neutron activation time for neutron activation of 152SmAcAc-PLLA microspheres under a thermal neutron flux of 2 × 1012 n/cm2/s1 was determined as 3 h.
In order to facilitate intraarterial injection via a microcatheter, the radioembolic microspheres are usually suspended in 0.9% NaCl solution or distilled water. The 153SmAcAc-PLLA microspheres suspension has a similar viscosity to the 0.9% NaCl solution (0.01 g/cm/s) but slightly lower than the viscosity of the human blood (0.03 g/cm/s)[36]. Owing to its lower density, the settling velocity of the 153SmAcAc-PLLA microspheres suspension was relatively lower compared to the glass- and resin-based microspheres[28,29,37]. A lower settling velocity could prevent the microspheres from settling in the microcatheter or blood vessels during administration and enable a more homogenous distribution of the microspheres within the tumor volume. The retention efficiency of radioactive 153Sm in the microspheres was excellent (> 99%) tested in both 0.9% NaCl solution and human blood plasma for an extended period of 550 h. High retention efficiency is required to minimize leakage of free 153Sm to other parts of the body. The excellent retention efficiency of the formulation can be ascribed to the interaction between the carbonyl groups of PLLA with the 153SmAcAc complex, which has resulted in the immobilization of the 153SmAcAc complex in the PLLA matrix.
152SmAcAc-PLLA microspheres with different concentrations of 152SmAcAc, ranging from 100%–200% w/w, were tested in this study. Higher concentration of 152SmAcAc is preferred to increase the specific activity of each microsphere. However, higher loading of 152SmAcAc would increase the density and mean diameter of the microsphere. In addition, it also increased the difficulties in removing the unbound 152SmAcAc. Therefore, it is important to find the optimum concentration of 152SmAcAc to be loaded to each PLLA microsphere to achieve the desired properties for radioembolization of liver tumors. According to the results of this study, 175% (w/w) 152SmAcAc is recommended as it produces microspheres with mean diameter of 37 µm and specific activity of 95 Bq per microsphere. This value is in between the protocols used in SIR-Sphere (40-70 Bq per microsphere) and TheraSphere (2500 Bq per microsphere). A further increase in the 152SmAcAc concentration (e.g., from 175% to 200%) produced a relatively small increment (about 3%) in the radioactivity but has further increased the mean diameter and density of the microspheres, resulting in reduced number of microspheres and increased settling velocity. In addition, higher amount of 152SmAcAc would also increase the difficulties in removing the unbound 152SmAcAc. Although a more concentrated HCl solution could be used during washing, this might cause structural damage to the PLLA. Therefore, the 175% (w/w) 152SmAcAc is recommended over the 200% (w/w) 152SmAcAc.
Although the specific activity (GBq/g) of the 153SmAcAc-PLLA microspheres was slightly lower than the target therapeutic dose of 3.0 GBq per vial in 90Y microspheres, administration of larger number of microspheres with medium specific activity has been suggested earlier[31] to produce better therapeutic efficacy than lower number of microspheres with higher specific activity. This is because a more even distribution of the radiation dose within the tumor volume is expected by a larger number of microspheres with lower specific activity while achieving the same total therapeutic dose prescribed for the treatment (total activity = number of microspheres x specific activity per microsphere). Nevertheless, a detailed dosimetry and treatment planning software are needed to determine the optimum dosage/approach for each individual patient. If necessary, the enriched form of 152Sm (about 99% 152Sm) can be used to replace the natural abundance 152Sm (about 27% 152Sm) if higher specific activity is desired. The radioactivity produced by the enriched 153Sm is expected to be around three to four times higher than the natural abundant 153Sm.
Production of radioactive formulations using neutron activation technique could possibly reduce the production cost and make the treatment more affordable across many nations. The commercially available 90Y is produced from strontium-90, a fission product of uranium in a nuclear reactor, by chemical high-purity separation. The elution and radiolabeling process involves multiple steps involving advanced technology and skills. It also increases radiological burden to the personnel as the whole production process is done under radioactive environment[18]. In comparison, the preparation of 152SmAcAc-PLLA microspheres is relatively easier, cheaper and does not involve ionizing radiation as non-radioactive form of 152Sm is used during the synthesis process. The formulation can be kept and only sent for neutron activation when needed. According to the latest International Atomic Energy Agency Research Reactor Database, there are 224 research reactors currently operates across 53 countries[38]. If the formulation can be activated locally, it would significantly reduce cross-borders radioactive shipments and lead time hence more radioembolization patients can be benefited. Some studies have suggested using neutron-activated 188Re and 166Ho as a substitute to 90Y, however these radionuclides have relatively short half-life, i.e., 17 h and 26 h, respectively. In our opinion, 153Sm has an ideal half-life of 46.3 h and it emits both therapeutic beta energies and diagnostic range gamma radiation, renders it a useful theranostics agent for liver radioembolization. Furthermore, it is readily available in a stable chemical form (152Sm, natural abundance 26%) and decays into a stable daughter (153Eu), hence minimizing the risk of radioactive waste.
In conclusion, a biocompatible, biodegradable 152SmAcAc-PLLA microspheres was successfully developed in this study. The microspheres have a mean diameter of approximately 37 µm and size distribution between 20 µm and 60 µm. The microspheres achieved a specific activity of 98 Bq per microsphere for the formulation loaded with 175% (w/w) 152SmAcAc. No significant radionuclide impurities were found and the 153Sm retention efficiency was above 99% when tested in 0.9% NaCl solution and human blood plasma for 550 h. Further studies are needed to complete the dosimetry and to compare the radioembolization efficiency with the commercially available formulations.
Liver cancer is the 6th most common cancer in the world and the 4th most common death from cancer worldwide. Hepatic radioembolization is a minimally invasive treatment involving intraarterial administration of radioembolic microspheres.
To develop a novel radioembolic microspheres formulation as an alternative to Yttrium-90 microspheres for intraarterial hepatic radioembolization.
To develop a neutron-activated, biodegradable and theranostics samarium-153 acetylacetonate (153SmAcAc)-poly-L-lactic acid (PLLA) microsphere for intraarterial radioembolization of hepatic tumors.
Microspheres with different concentrations of 152SmAcAc (i.e., 100%, 150%, 175% and 200% w/w) were prepared by solvent evaporation method. The microspheres were then activated using a nuclear reactor in a neutron flux of 2 × 1012 n/cm2/s1, converting 152Sm to Samarium-153 (153Sm) via 152Sm(n, γ) 153Sm reaction. The SmAcAc-PLLA microspheres before and after neutron activation were characterized using scanning electron microscope, energy dispersive X-ray spectroscopy, particle size analysis, Fourier transform infrared spectroscopy, thermo-gravimetric analysis and gamma spectroscopy. The in-vitro radiolabeling efficiency was also tested in both 0.9% sodium chloride solution and human blood plasma over a duration of 550 h.
The SmAcAc-PLLA microspheres with different SmAcAc contents remained spherical before and after neutron activation. The mean diameter of the microspheres was about 35 µm. Specific activity achieved for 153SmAcAc-PLLA microspheres with 100%, 150%, 175% and 200% (w/w) SmAcAc after 3 h neutron activation were 1.7 ± 0.05, 2.5 ± 0.05, 2.7 ± 0.07 and 2.8 ± 0.09 GBq/g, respectively. The activity of per microspheres were determined as 48.36 ± 1.33, 74.10 ± 1.65, 97.87 ± 2.48 and 109.83 ± 3.71 Bq for 153SmAcAc-PLLA microspheres with 100%, 150%, 175% and 200% (w/w) SmAcAc. The energy dispersive X-ray and gamma spectrometry showed that no elemental and radioactive impurities present in the microspheres after neutron activation. Retention efficiency of 153Sm in the SmAcAc-PLLA microspheres was excellent (about 99%) in both 0.9% sodium chloride solution and human blood plasma over a duration of 550 h.
The 153SmAcAc-PLLA microsphere is potentially useful for hepatic radioembolization due to their biodegradability, favorable physicochemical characteristics and excellent radiolabeling efficiency. The synthesis of the formulation does not involve ionizing radiation and hence reducing the complication and cost of production.
A neutron-activated, biodegradable 153SmAcAc-PLLA microspheres formulation has been developed and tested for radioembolization of hepatic tumors. Further studies are needed to complete the dosimetry and to compare the radioembolization efficiency with the commercially available formulations.
The neutron activation was supported by the Malaysian Nuclear Agency.
Manuscript source: Invited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: Malaysia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Bendary M, Mohamed SY S-Editor: Tang JZ L-Editor: A E-Editor: Qi LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55757] [Article Influence: 7965.3] [Reference Citation Analysis (132)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20500] [Article Influence: 2050.0] [Reference Citation Analysis (20)] |
| 3. | Kelly CM, Kemeny NE. Liver-directed therapy in metastatic colorectal cancer. Expert Rev Anticancer Ther. 2017;17:745-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4506] [Article Influence: 225.3] [Reference Citation Analysis (0)] |
| 5. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1274] [Article Influence: 141.6] [Reference Citation Analysis (2)] |
| 6. | Mathurin P, Rixe O, Carbonell N, Bernard B, Cluzel P, Bellin MF, Khayat D, Opolon P, Poynard T. Review article: Overview of medical treatments in unresectable hepatocellular carcinoma--an impossible meta-analysis? Aliment Pharmacol Ther. 1998;12:111-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Lambert B, Van de Wiele C. Treatment of hepatocellular carcinoma by means of radiopharmaceuticals. Eur J Nucl Med Mol Imaging. 2005;32:980-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Van Hazel G, Blackwell A, Anderson J, Price D, Moroz P, Bower G, Cardaci G, Gray B. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 308] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Burton MA, Gray BN, Klemp PF, Kelleher DK, Hardy N. Selective internal radiation therapy: distribution of radiation in the liver. Eur J Cancer Clin Oncol. 1989;25:1487-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Ackerman NB, Lien WM, Kondi ES, Silverman NA. The blood supply of experimental liver metastases. I. The distribution of hepatic artery and portal vein blood to "small" and "large" tumors. Surgery. 1969;66:1067-1072. [PubMed] |
| 11. | Hoefnagel CA. Radionuclide therapy revisited. Eur J Nucl Med. 1991;18:408-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Kennedy AS, McNeillie P, Dezarn WA, Nutting C, Sangro B, Wertman D, Garafalo M, Liu D, Coldwell D, Savin M, Jakobs T, Rose S, Warner R, Carter D, Sapareto S, Nag S, Gulec S, Calkins A, Gates VL, Salem R. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, Benson A, Espat J, Bilbao JI, Sharma RA, Thomas JP, Coldwell D. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 14. | Salem R, Lewandowski RJ. Chemoembolization and radioembolization for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2013;11:604-11; quiz e43-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Pasciak AS, Bourgeois AC, Bradley YC. A Microdosimetric Analysis of Absorbed Dose to Tumor as a Function of Number of Microspheres per Unit Volume in 90Y Radioembolization. J Nucl Med. 2016;57:1020-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Hossain KMZ, Patel U, Ahmed I. Development of microspheres for biomedical applications: a review. Prog Biomater. 2015;4:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Elschot M, Nijsen JF, Dam AJ, de Jong HW. Quantitative evaluation of scintillation camera imaging characteristics of isotopes used in liver radioembolization. PLoS One. 2011;6:e26174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Chakravarty R, Dash A, Pillai MR. Availability of yttrium-90 from strontium-90: a nuclear medicine perspective. Cancer Biother Radiopharm. 2012;27:621-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (2)] |
| 19. | Yeong CH, Abdullah BJ, Ng KH, Chung LY, Goh KL, Sarji SA, Perkins AC. Neutron-activated ¹⁵³Sm-ion-exchange resin as a tracer for gastrointestinal scintigraphy. Nucl Med Commun. 2011;32:1256-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Yeong CH, Abdullah BJ, Ng KH, Chung LY, Goh KL, Sarji SA, Perkins AC. Production and first use of 153SmCl3-ion exchange resin capsule formulation for assessing gastrointestinal motility. Appl Radiat Isot. 2012;70:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Yeong CH, Abdullah BJ, Ng KH, Chung LY, Goh KL, Perkins AC. Fusion of gamma scintigraphic and magnetic resonance images improves the anatomical delineation of radiotracer for the assessment of gastrointestinal transit. Nucl Med Commun. 2013;34:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Wong YH, Tan HY, Kasbollah A, Abdullah BJJ, Shah MNM, Yeong CH. Biodegradable Samarium-153–labelled microspheres for hepatic radioembolization: preparation, characterization and radiolabelling evaluation after neutron activation. Journal of Physics: Conference Series. 2019;1248:012066. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Babich IV, Plyuto YV, Van Langeveld AD, Moulijn JA. Role of the support nature in chemisorption of Ni(acac)2 on the surface of silica and alumina. Appl Surf Sci. 1997;3:267-272. [DOI] [Full Text] |
| 24. | Dunstan PO. Thermochemistry of adducts of nickel(II) acetylacetonate chelate with heterocyclic bases. Thermochim Acta. 1998;2:165-174. [DOI] [Full Text] |
| 25. | Dunstan PO. Thermochemistry of aniline-derivative adducts of nickel(II) acetylacetonate. Thermochim Acta. 1999;1:5-11. [DOI] [Full Text] |
| 26. | Kennedy A. Radioembolization of hepatic tumors. J Gastrointest Oncol. 2014;5:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 27. | Tsai CL, Chung HT, Chu W, Cheng JCH. Radiation therapy for primary and metastatic tumors of the liver. J Radiat Oncol. 2012;3:227-237. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Mumper RJ, Ryo UY, Jay M. Neutron-activated holmium-166-poly (L-lactic acid) microspheres: a potential agent for the internal radiation therapy of hepatic tumors. J Nucl Med. 1991;32:2139-2143. [PubMed] |
| 29. | Poorbaygi H, Reza Aghamiri SM, Sheibani S, Kamali-Asl A, Mohagheghpoor E. Production of glass microspheres comprising 90Y and (177)Lu for treating of hepatic tumors with SPECT imaging capabilities. Appl Radiat Isot. 2011;69:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Häfeli UO, Roberts WK, Pauer GJ, Kraeft SK, Macklis RM. Stability of biodegradable radioactive rhenium (Re-186 and Re-188) microspheres after neutron-activation. Appl Radiat Isot. 2001;54:869-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Hashikin NA, Yeong CH, Abdullah BJ, Ng KH, Chung LY, Dahalan R, Perkins AC. Neutron Activated Samarium-153 Microparticles for Transarterial Radioembolization of Liver Tumour with Post-Procedure Imaging Capabilities. PLoS One. 2015;10:e0138106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Nijsen JF, Zonnenberg BA, Woittiez JR, Rook DW, Swildens-van Woudenberg IA, van Rijk PP, van het Schip AD. Holmium-166 poly lactic acid microspheres applicable for intra-arterial radionuclide therapy of hepatic malignancies: effects of preparation and neutron activation techniques. Eur J Nucl Med. 1999;26:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Lampe KJ, Namba RM, Silverman TR, Bjugstad KB, Mahoney MJ. Impact of lactic acid on cell proliferation and free radical-induced cell death in monolayer cultures of neural precursor cells. Biotechnol Bioeng. 2009;103:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Mumper RJ, Jay M. Formation and stability of lanthanide complexes and their encapsulation into polymeric microspheres. J Phys Chem. 1992;21:8626-8631. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Subedi G, Shrestha AK, Shakya S. Study of effect of different factors in formulation of micro and nanospheres with solvent evaporation technique. Open Pharmaceutical Sciences Journal. 2016;3:182. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Dormandy JA. Clinical significance of blood viscosity. Ann R Coll Surg Engl. 1970;47:211-228. [PubMed] |
| 37. | Häfeli UO, Casillas S, Dietz DW, Pauer GJ, Rybicki LA, Conzone SD, Day DE. Hepatic tumor radioembolization in a rat model using radioactive rhenium (186Re/188Re) glass microspheres. Int J Radiat Oncol Biol Phys. 1999;44:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | International Atomic Energy Agency 2019. Research Reactor Database (RRDB) [Internet]. [cited 2019 Jul 28]. Available from: https://nucleus.iaea.org/RRDB/RR/ReactorSearch.aspx?rf=1. |