Published online Aug 4, 2017. doi: 10.5492/wjccm.v6.i3.164
Peer-review started: May 10, 2017
First decision: May 23, 2017
Revised: June 1, 2017
Accepted: June 30, 2017
Article in press: July 3, 2017
Published online: August 4, 2017
Processing time: 85 Days and 19.9 Hours
To determine the ability of intrapulmonary percussive ventilation (IPV) to promote airway clearance in spontaneously breathing patients and those on mechanical ventilation.
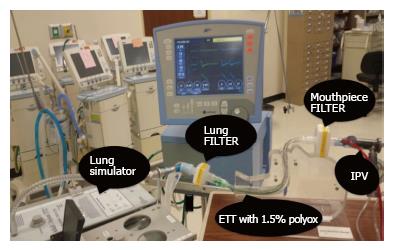
An artificial lung was used to simulate a spontaneously breathing patient (Group 1), and was then connected to a mechanical ventilator to simulate a patient on mechanical ventilation (Group 2). An 8.5 mm endotracheal tube (ETT) connected to the test lung, simulated the patient airway. Artificial mucus was instilled into the mid-portion of the ETT. A filter was attached at both ends of the ETT to collect the mucus displaced proximally (mouth-piece filter) and distally (lung filter). The IPV machine was attached to the proximal end of the ETT and was applied for 10-min each to Group 1 and 2. After each experiment, the weight of the various circuit components were determined and compared to their dry weights to calculate the weight of the displaced mucus.
In Group 1 (spontaneously breathing model), 26.8% ± 3.1% of the simulated mucus was displaced proximally, compared to 0% in Group 2 (the mechanically ventilated model) with a P-value of < 0.01. In fact, 17% ± 1.5% of the mucus in Group 2 remained in the mid-portion of the ETT where it was initially instilled and 80% ± 4.2% was displaced distally back towards the lung (P < 0.01). There was an overall statistically significant amount of mucus movement proximally towards the mouth-piece in the spontaneously breathing (SB) patient. There was also an overall statistically significant amount of mucus movement distally back towards the lung in the mechanically ventilated (MV) model. In the mechanically ventilated model, no mucus was observed to move towards the proximal/mouth piece section of the ETT.
This bench model suggests that IPV is associated with displacement of mucus towards the proximal mouthpiece in the SB patient, and distally in the MV model.
Core tip: Many respiratory conditions result in increased respiratory secretions and poor clearance, and are associated with poor patient outcomes. Intrapulmonary percussive ventilation (IPV) is an airway clearance technique that has become increasingly used over the last few years, however there is a paucity of data to support its efficacy. Using a simulated bench model, we found that IPV is associated with movement of mucus towards the mouth in the spontaneously breathing patient and thus supporting airway clearance. Interestingly, in patients on mechanical ventilation, IPV mainly displaced mucus distally into the lungs and thus may be harmful in this patient population.
- Citation: Fernandez-Restrepo L, Shaffer L, Amalakuhan B, Restrepo MI, Peters J, Restrepo R. Effects of intrapulmonary percussive ventilation on airway mucus clearance: A bench model. World J Crit Care Med 2017; 6(3): 164-171
- URL: https://www.wjgnet.com/2220-3141/full/v6/i3/164.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v6.i3.164
Many chronic conditions such as bronchiectasis, cystic fibrosis (CF), neuromuscular disease and chronic obstructive pulmonary disease (COPD) are associated with an increase in both the quantity and viscosity of respiratory secretions. Other conditions are associated with a decreased ability to clear secretions, such as those with impaired ciliary function or cough, with the latter being very common during mechanical ventilation, after strokes or surgical procedures, and in neuromuscular disorders[1]. Previous studies have shown that when these secretions are not adequately cleared, complications arise such as atelectasis, mucus plugging, and recurrent pneumonia[1]. Inadequate mucus clearance in patients in the intensive care unit (ICU) can lead to poor clinic outcomes such as prolonged time on mechanical ventilation, increase in need for tracheostomies, decreased quality of life, overall worsening lung function and an increase in mortality[2-6]. Administration of airway clearance therapies (ACTs) involve the use of manual techniques coupled with postural drainage, breathing exercises and mechanical devices to improve patient outcomes and optimize recovery after acute illnesses[7]. However, there are few studies on the optimal ACT and under which clinical settings they are most effective[8,9].
Intrapulmonary percussive ventilation (IPV) is one such ACT that has recently become increasingly utilized in hospitalized patients. During IPV treatments, the patient breathes through an accessory device called a Phasitron®, which delivers rapid, high flow, mini-bursts (percussions) of tidal volumes into the lungs while simultaneously delivering therapeutic aerosols. In the clinical setting, IPV can be administered by mouthpiece, mask or endotracheal tubes (ETTs). This technique is also thought to improve expiratory flow by opening collapsed airways, thus promoting mucus clearance.
Several reports have suggested that IPV facilitates airway clearance and improves ventilation in patients with conditions such as cystic fibrosis[10-14], neuromuscular disorders[15,16], atelectasis[17-19], inhalation injury[20-22], and COPD[23-26]. To our knowledge there has only been one study that has evaluated the efficacy of IPV as an ACT in spontaneously breathing patients, and it illustrated a positive benefit[27]. When oscillating devices such as IPV were compared to conventional physiotherapy for airway clearance in people with cystic fibrosis, the most recent Cochrane meta-analysis found little evidence to support the use of any particular oscillating device for airway clearance over any other ACT modality[28]. The handful of other studies that exist have compared IPV to conventional chest physical therapy and showed no additional benefit with IPV. These results raise the question of whether IPV indeed is able to act as an effective ACT or whether its benefit is simply theoretical[14,29]. Furthermore, to our knowledge only one study has evaluated the efficacy of IPV in mechanically ventilated patients, and although it showed some benefit, it was completed only in a specific population of eight patients with neuromuscular disease[15]. Despite IPV’s widespread use as an ACT across numerous clinical settings, there are a paucity of data to document its benefit.
The objective of this study was to evaluate the ability of IPV to promote airway clearance in both spontaneously breathing patients and those on mechanical ventilation using a controlled simulated bench model.
The artificial test lung was obtained from “Michigan Instruments” (Grand Rapids, MI) and utilized in both the spontaneously breathing (SB) group/model and the mechanically ventilated (MV) group/model. This artificial test lung has been validated and mimics the human lung in many ways. First, the artificial test lung has a total lung capacity that replicates a “normal” adult human lung, which is approximately 4-6 L. Thus the size of the artificial lung mimics that of a “normal” adult human lung. Second, the test lung also mimics the standard human lung’s residual volume (1.84 L). Third, we used a standard compliance of 30 mL/cmH2O in both groups which is equivalent to a patient with severe pneumonia, in order to mimic the actual scenario for which IPV would be utilized in clinical practice. Furthermore, we used a standard airway resistance of 5 cmH2O/L per second which mimics the normal airway resistance of an actual patient. Fourth, this artificial test lung has also been shown to mimic the pressure-to-flow and pressure-to-volume relationships in normal human lungs[30].
An artificial test lung was used to simulate a spontaneously breathing patient (Group 1 or SB), and then connected to a mechanical ventilator (Avea, BD; Yorba Linda, CA) to simulate a mechanically ventilated patient (Group 2 or MV). An 8.5 mm ETT connected to the test lung was used to simulate the patient airway. The ventilator parameters selected for the study were a tidal volume of 400 mL, a respiratory rate of 12 breaths/min, an inspiratory time of 1 s, and a PEEP of 5 cmH2O. These setting demonstrated little or no movement of mucus in the absence of IPV. Five milliliters of 1.5% of a water-soluble resin coagulant used as a mucus simulant (Polyox; Dow Chemical Company; Cary, NC, United States) were instilled into the mid-portion of the ETT. This percent viscosity is shown to be most consistent with that of mucus in a normal human airway[31]. An anesthesia filter was attached at both ends of the ETT to collect the artificial mucus displaced proximally (mouth-piece filter) and distally (lung filter) the proximal end was defined as the “Mouth Piece Filter”, which was the “goal exit site” of the displaced mucus. The distal end was defined as the “Lung Filter”, which was the site considered within the lungs. The experimental setup can be seen in Figures 1 and 2.
An Allosun portable oscillometer EM116 (Allosun, China) was used to document the rate on the IPV that generated a frequency of 240 cycles/min. This frequency was selected as it represents the highest frequency obtained by similar devices. An I/E ratio of 1:4 was selected and airway pressure was adjusted to 30 cmH2O prior to connecting each device to the inspiratory limb of the ventilator circuit.
In Group 1 (SB), 10 trials were performed to document variability between experiments. Since experimental variability was less than 5%, only 3 trials were completed for Group 2 (MV). Each experiment was run for 10 min since this is the typical time the treatment is administered in the clinical setting.
After each experiment, the weight of the following circuit components was determined and compared to their dry weights to calculate the weight of the displaced mucus: (1) “Mouth Piece Filter” (proximal filter); (2) Proximal ETT; (3) Mid-ETT (portion 23-27 cm); (4) Distal ETT; and (5) “Lung Filter” (distal filter).
The concept of fluid dynamics as it relates to the movement of mucus within the airway is also important, and it is worthwhile to acknowledge that variables such as temperature/humidity, the density/concentration of the mucus and flow conditions were controlled in this experiment. Each experiment was conducted in a lab room strictly controlled at 32 °C, which is the average temperature of the upper trachea in humans[32]. The humidity of the room was also strictly controlled at standard values (heated humidified air was not used). All experiments were completed with the same viscosity/density of artificial mucus which has been shown to be consistent with the mucus in a normal human airway as illustrated by Shah et al[31]. All experiments were conducted using the same flow as well. By keeping these variables constant, the effects of the intervention (use of IPV) in both groups could be evaluated within a constant/replicable environment/context.
Due to the limited sample size used in this study, the distribution of the data were not distributed normally, and for this reason only non-parametric tests were used[33]. All data are presented as medians with interquartile ranges (IQR), or means with standard deviations (SD) as appropriate. Paired nonparametric Mann-Whitney U test, Kruskal Wallis test or two-tailed Student’s t-test were used to compare data at different tube distances. All statistical calculations were performed by a trained and expert biostatistician, using Prism 5 software (GraphPad) and SPSS 21.0. P values < 0.05 were considered statistically significant.
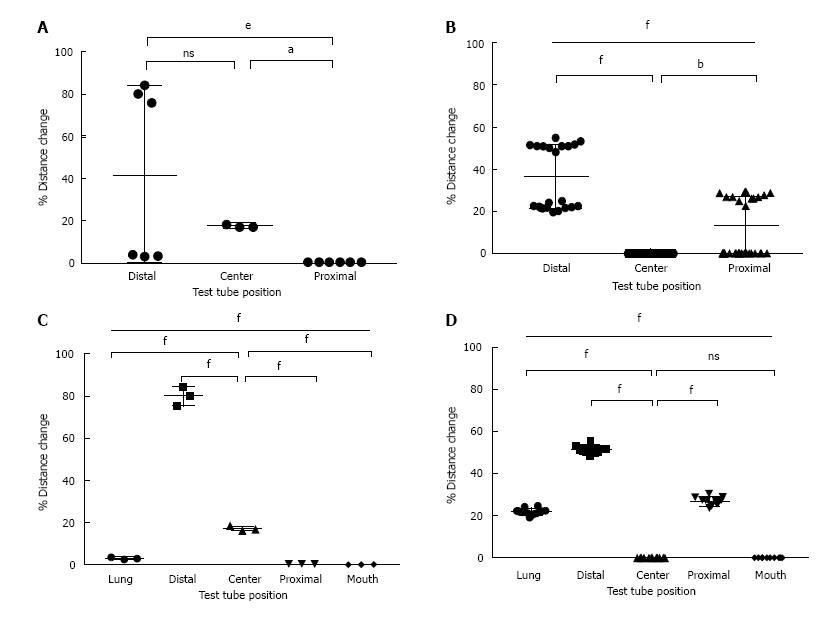
In Group 1 (spontaneously breathing model), 26.8% ± 3.1% of the simulated mucus was displaced proximally, compared to 0% in Group 2 (the mechanically ventilated model). In fact, 17% ± 1.5% of the mucus in Group 2 remained in the mid-portion of the ETT where it was initially instilled and 80% ± 4.2% was displaced distally back towards the lung. There was an overall statistically significant amount of mucus movement proximally towards the mouth-piece in the spontaneously breathing patient. There was also an overall statistically significant amount of mucus movement distally back towards the lung in the mechanically ventilated model. The amounts of mucus measured within each section of the circuit is shown in Table 1 and Figure 3.
| Location | Group 1 (%) | Group 2 (%) |
| Mouth piece/proximal filter | 0 | 0 |
| Proximal ETT | 26.8 ± 0.63 | 0 |
| Mid-ETT (portion 23-27cm) | 0 | 17 ± 1.04 |
| Distal ETT | 51.2 ± 1.75 | 80 ± 4.2 |
| Lung/distal filter | 22 ± 0.55 | 3 ± 1.04 |
The results from this study suggest that when IPV is used in a simulated model of a spontaneously breathing patient, it is associated with a statistically significant amount of mucus movement proximally, and thus supports airway clearance. In contrast, when IPV is used in the simulated model of a mechanically ventilated patient, it was found to be associated almost exclusively with the displacement of mucus distally back towards the lung, and thus did not support airway clearance.
IPV utilizes tidal volumes delivered at high oscillatory frequencies to loosen mucus and help with expectoration. Previous bench models have shown that high-frequency oscillations not only dislodge bronchial secretions from the walls of the airway but also reduce its actual viscosity making it easier to clear[34]. In a way these oscillations act like a “physical mucolytic”. One clinical study has shown that applying high-frequency oscillations directly to the airway opening of spontaneously breathing patients does indeed enhance secretion clearance compared to no therapeutic intervention[27]. Our study confirms the results from this study that IPV indeed improves mucus clearance in a simulated model of a spontaneously breathing patient. However, it is worth noting that when IPV has been compared to conventional chest physical therapy in spontaneously breathing patients, there appears to be no difference in efficacy of mucus clearance. In a study of 20 clinically stable CF patients, IPV was not associated with increased sputum clearance compared to conventional chest physical therapy[14]. Another study of 22 stable patients with bronchiectasis found similar results[29]. Taking the results from this current study and the study by George et al[27], IPV indeed improves sputum clearance; however, it may not provide added benefit compared to conventional chest physical therapy. However, the major value of IPV when compared to conventional chest physical therapy appears to be its increased tolerability, patient preference, replicability of its benefits and greater adherence to therapy, considering that conventional chest physical therapy is often uncomfortable and requires an experienced/trained individual to aid the patient for optimal results[29]. Since the benefit of chest physical therapy depends on the patient and the therapist, the benefits seen in the studies by Van Ginderdeuren et al[14] and Paneroni et al[29] may be difficult to replicate, making IPV more advantageous. Thus IPV may be a more effective clinical alternative for airway clearance in spontaneously breathing patients, although this requires further clinical studies to validate.
Similarly, there are few studies evaluating the efficacy of IPV as an ACT in mechanically ventilated patients. In one small observational study conducted in 8 patients with Duchenne Muscular Dystrophy who were ventilator dependent and had tracheostomies, IPV was shown to increase the quantity of mucus clearance[15]. Our study showed the opposite findings, and in fact showed that more than 80% of the mucus was displaced distally back towards the lung. The biologic plausibility of why IPV may be detrimental in patients on invasive MV is important to understand considering these negative consequences. It is possible that the interplay between the positive pressure from the MV and the percussive oscillatory pressure waves from the IPV machine created a flow that was directed distally rather than proximally towards the opening of the airway. While this finding was noted in our study, it is not clear if this occurs in vivo. But considering that the majority of the mucus (approximately 80%) was displaced distally in the MV group, and that our artificial test lung and bench model has been validated and extensively used by prior studies, it raises valid concerns about its safety that requires further testing[35-37]. IPV is currently being used in mechanically ventilated patients at variable driving pressures and oscillatory frequencies (our study chose the most common setting used clinically) and its use is growing exponentially. We hope the results of this bench study will result in additional future studies.
There are several limitations of this study. One of which is that in vitro models do not perfectly reflect the flow characteristics of a spontaneously breathing patient. Our in-vitro model did not measure the impact of the treatment effects within a chest cavity where recoil of the chest plays a significant role in increasing expiratory flows. If active expiration were to be simulated as happens in the spontaneously breathing patient, higher expiratory flows could have enhanced mucus transport to either the proximal end of the ETT or the filter representing the mouthpiece. However, there are many advantages to studying IPV in this simplified bench model that would be difficult to evaluate in an actual clinical setting. For example, using this simulated model, we can directly measure the amount of mucus in the airway and directly determine the amount displaced in either direction. In patients, we cannot control for the actual amount of mucus in the airway at time zero because this will vary from hour-to-hour and day-to-day. Furthermore, different patients will differ in their baseline amount of mucus production based on complex physiological mechanisms and differences in their disease processes. This simplified model allows control of many factors that cannot be controlled in an in-vivo model. One of the main reasons for performing this study is that many Health Care Professionals accept that IPV is beneficial in both spontaneously breathing and mechanically ventilated patients with almost no data to document or substantiate its actual benefit. Our goal was to raise awareness through a bench study and create interest in furthering clinical research in this area.
Another potential limitation worth mentioning is that our model of the human lung did not contain cilia. An important question is whether IPV may interact with human cilia and in a manner our model could not account for. However, we were unable to find any literature to support the concept that IPV may indeed promote or suppress ciliary function. While we are not capable of predicting the effects of IPV on ciliary motion, it is possible that IPV may reduce or enhance ciliary function. This is clearly an area of research that needs to be investigated. Additionally, many acute and chronic disease processes cause dysfunction of the mucociliary system[38]. For example many chronic pulmonary diseases such as primary ciliary dyskinesis, COPD, asthma and cystic fibrosis have abnormal functioning and dysplastic cilia when examined under electron microscopy[38]. Furthermore, cigarette smokers and those with acute pneumonia also have been shown to have significant ciliary dyskinesia from direct effects of bacterial and viral pathogens[38,39]. No bench model can attempt to reproduce the complexity or variability of ciliary function, but its plausible that the lack of cilia in our model mimics in some capacity the actual human lung during many disease states.
A third potential limitation in our study was that although we controlled for humidification by conducting our experiments in a tightly controlled environment within a room at standard humidity, we did not attach a humidifier to our experimental circuit separately. Dellamonica et al[40] found that the optimal way to effectively humidify this circuit was to attach a humidifier down stream from the IPV machine. Dellamonica et al[40] recognized that when IPV is combined with invasive mechanical ventilation, the production of high inspiratory flow rates and gas decompression prevented optimal humidification and warming of the inspired gas. This combination often results in the drying of mucus and the risk for airway obstruction. The question arises whether this may have caused the lack of proximal movement of the mucus in our MV model. Although this is plausible, if this was indeed the reason for the negative impact of IPV in our MV model, we would have expected the majority of the mucus to remain in the middle of the circuit where it was initially instilled, and not be displaced distally (> 80% of the mucus in fact moved distally). Furthermore, because each experiment was conducted for a very short period of time (approximately 10 min) the potential desiccating properties of the IPV machine should not likely have made a large impact. But regardless, further studies are needed to confirm or refute this hypothesis.
A fourth limitation is that our study used only fixed settings on the IPV. Although an I:E ratio of 1:4 is consistently selected by most users when administering IPV, it may have explained a lower mucus displacement towards the proximal filter than expected. Movement of mucus is dependent not only on viscosity/elasticity but also adhesivity. This model also did not utilize either artificial epithelial lining fluid or surfactant that might have better reflected the adhesive properties of mucus within human airways.
Overall, although IPV may indeed be a beneficial means to induce airway mucus clearance, this study highlights that that optimal clinical settings in both spontaneously breathing and mechanically ventilated patient need to be further elucidated to determine who will benefit from this mode of therapy and under which circumstances. It is also reasonable to infer that during mechanical ventilation, IPV may not be beneficial and could result in forward movement of secretions into the lung. Future studies on its use and optimal settings in both MV and SB patients are clearly warranted.
Many respiratory conditions are associated with an increase in respiratory secretions. Retention of these secretions is associated with poor patient outcomes. Intrapulmonary percussive ventilation (IPV) is a type of airway clearance technique that helps to remove airway mucus. Despite its widespread use across numerous clinical settings, there is a paucity of data to support its efficacy.
There is little research in the area of air way clearance therapies (ACT’s), and those that do exist have small sample sizes with a lack of effective control subjects. As the prevalence of cystic fibrosis increases due to patients living longer and the increased identification of previously undiagnosed patients with non-cystic fibrosis bronchiectasis, the need for more effective and validated airway clearance therapies is becoming more important.
There are few studies that have evaluated the effectiveness of IPV in both spontaneously breathing patients, and even fewer in those requiring mechanical ventilation. Furthermore, the study of ACTs in actual patients is complex due to patient-to-patient variabilities in their underlying disease states and variabilities in mucus production hour-to-hour and day-to-day. This study is one of the few bench studies that exist evaluating the effectiveness of IPV in two important clinical states, using an effective control group.
One of the main reasons for performing this study is that many Health Care Professionals accept that IPV is beneficial in both spontaneously breathing and mechanically ventilated patients with almost no data to document or substantiate its actual benefit. The results from this study suggest that when IPV is used in a simulated model of a spontaneously breathing patient, it is indeed associated with a statistically significant amount of mucus movement proximally, and thus supports airway clearance. In contrast, when IPV is used in the simulated model of a mechanically ventilated patient, it was found to be associated almost exclusively with the displacement of mucus distally back towards the lung, and thus did not support airway clearance. This study raises valid concerns about the safety of IPV in mechanically ventilated patients that requires further testing. Overall, although IPV may indeed be a beneficial means to induce airway mucus clearance, this study highlights that that optimal clinical settings in both spontaneously breathing and mechanically ventilated patient need to be further elucidated to determine who will benefit from this mode of therapy and under which circumstances.
IPV: This term stands for “intrapulmonary percussive ventilation”, which is a mechanical device that is widely used in the United States to help patients clear their secretions. It is especially used in those with bronchiectasis and those on mechanical ventilation who have particularly thick secretions and have poor cough reflexes due to sedations and acute-on-chronic disease states. This device delivers tidal volumes of air at varying frequencies into the airways of patients to help vibrate/percuss the airway and loosen impacted mucus.
This is an interesting and well-conducted bench study.
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Inchauspe AA, Shen HN S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Van der Schans CP. Bronchial mucus transport. Respir Care. 2007;52:1150-1156; discussion 1150-1156. [PubMed] |
| 2. | Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J. 1995;8:1333-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Kim V, Han MK, Vance GB, Make BJ, Newell JD, Hokanson JE, Hersh CP, Stinson D, Silverman EK, Criner GJ. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140:626-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1485] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 5. | Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153:1530-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 496] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 6. | Combes A, Costa MA, Trouillet JL, Baudot J, Mokhtari M, Gibert C, Chastre J. Morbidity, mortality, and quality-of-life outcomes of patients requiring & gt; or=14 days of mechanical ventilation. Crit Care Med. 2003;31:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Andrews J, Sathe NA, Krishnaswami S, McPheeters ML. Nonpharmacologic airway clearance techniques in hospitalized patients: a systematic review. Respir Care. 2013;58:2160-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Flume PA, Robinson KA, O’Sullivan BP, Finder JD, Vender RL, Willey-Courand DB, White TB, Marshall BC. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54:522-537. [PubMed] |
| 9. | Warnock L, Gates A. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database Syst Rev. 2015;12:CD001401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Natale JE, Pfeifle J, Homnick DN. Comparison of intrapulmonary percussive ventilation and chest physiotherapy. A pilot study in patients with cystic fibrosis. Chest. 1994;105:1789-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Homnick DN, White F, de Castro C. Comparison of effects of an intrapulmonary percussive ventilator to standard aerosol and chest physiotherapy in treatment of cystic fibrosis. Pediatr Pulmonol. 1995;20:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Newhouse PA, White F, Marks JH, Homnick DN. The intrapulmonary percussive ventilator and flutter device compared to standard chest physiotherapy in patients with cystic fibrosis. Clin Pediatr (Phila). 1998;37:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Varekojis SM, Douce FH, Flucke RL, Filbrun DA, Tice JS, McCoy KS, Castile RG. A comparison of the therapeutic effectiveness of and preference for postural drainage and percussion, intrapulmonary percussive ventilation, and high-frequency chest wall compression in hospitalized cystic fibrosis patients. Respir Care. 2003;48:24-28. [PubMed] |
| 14. | Van Ginderdeuren F, Verbanck S, Van Cauwelaert K, Vanlaethem S, Schuermans D, Vincken W, Malfroot A. Chest physiotherapy in cystic fibrosis: short-term effects of autogenic drainage preceded by wet inhalation of saline versus autogenic drainage preceded by intrapulmonary percussive ventilation with saline. Respiration. 2008;76:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Toussaint M, De Win H, Steens M, Soudon P. Effect of intrapulmonary percussive ventilation on mucus clearance in duchenne muscular dystrophy patients: a preliminary report. Respir Care. 2003;48:940-947. [PubMed] |
| 16. | Clini EM, Antoni FD, Vitacca M, Crisafulli E, Paneroni M, Chezzi-Silva S, Moretti M, Trianni L, Fabbri LM. Intrapulmonary percussive ventilation in tracheostomized patients: a randomized controlled trial. Intensive Care Med. 2006;32:1994-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Birnkrant DJ, Pope JF, Lewarski J, Stegmaier J, Besunder JB. Persistent pulmonary consolidation treated with intrapulmonary percussive ventilation: a preliminary report. Pediatr Pulmonol. 1996;21:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Deakins K, Chatburn RL. A comparison of intrapulmonary percussive ventilation and conventional chest physiotherapy for the treatment of atelectasis in the pediatric patient. Respir Care. 2002;47:1162-1167. [PubMed] |
| 19. | Yen Ha TK, Bui TD, Tran AT, Badin P, Toussaint M, Nguyen AT. Atelectatic children treated with intrapulmonary percussive ventilation via a face mask: clinical trial and literature overview. Pediatr Int. 2007;49:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Reper P, Wibaux O, Van Laeke P, Vandeenen D, Duinslaeger L, Vanderkelen A. High frequency percussive ventilation and conventional ventilation after smoke inhalation: a randomised study. Burns. 2002;28:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Hall JJ, Hunt JL, Arnoldo BD, Purdue GF. Use of high-frequency percussive ventilation in inhalation injuries. J Burn Care Res. 2007;28:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Chung KK, Wolf SE, Renz EM, Allan PF, Aden JK, Merrill GA, Shelhamer MC, King BT, White CE, Bell DG. High-frequency percussive ventilation and low tidal volume ventilation in burns: a randomized controlled trial. Crit Care Med. 2010;38:1970-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Nava S, Barbarito N, Piaggi G, De Mattia E, Cirio S. Physiological response to intrapulmonary percussive ventilation in stable COPD patients. Respir Med. 2006;100:1526-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Vargas F, Boyer A, Bui HN, Guenard H, Gruson D, Hilbert G. Effect of intrapulmonary percussive ventilation on expiratory flow limitation in chronic obstructive pulmonary disease patients. J Crit Care. 2009;24:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Vargas F, Bui HN, Boyer A, Salmi LR, Gbikpi-Benissan G, Guenard H, Gruson D, Hilbert G. Intrapulmonary percussive ventilation in acute exacerbations of COPD patients with mild respiratory acidosis: a randomized controlled trial [ISRCTN17802078]. Crit Care. 2005;9:R382-R389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Antonaglia V, Lucangelo U, Zin WA, Peratoner A, De Simoni L, Capitanio G, Pascotto S, Gullo A. Intrapulmonary percussive ventilation improves the outcome of patients with acute exacerbation of chronic obstructive pulmonary disease using a helmet. Crit Care Med. 2006;34:2940-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | George RJ, Johnson MA, Pavia D, Agnew JE, Clarke SW, Geddes DM. Increase in mucociliary clearance in normal man induced by oral high frequency oscillation. Thorax. 1985;40:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Morrison L, Agnew J. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database Syst Rev. 2009;CD006842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Paneroni M, Clini E, Simonelli C, Bianchi L, Degli Antoni F, Vitacca M. Safety and efficacy of short-term intrapulmonary percussive ventilation in patients with bronchiectasis. Respir Care. 2011;56:984-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Instruments M. Dual Adult TTL Training/Test Lung. User’s Manual. 2016;1:1-25. |
| 31. | Shah S, Fung K, Brim S, Rubin BK. An in vitro evaluation of the effectiveness of endotracheal suction catheters. Chest. 2005;128:3699-3704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | McFadden ER, Pichurko BM, Bowman HF, Ingenito E, Burns S, Dowling N, Solway J. Thermal mapping of the airways in humans. J Appl Physiol (1985). 1985;58:564-570. [PubMed] |
| 33. | Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. 2016;4:91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 34. | Tomkiewicz RP, Biviji A, King M. Effects of oscillating air flow on the rheological properties and clearability of mucous gel simulants. Biorheology. 1994;31:511-520. [PubMed] |
| 35. | Boukhettala N, Porée T, Diot P, Vecellio L. In vitro performance of spacers for aerosol delivery during adult mechanical ventilation. J Aerosol Med Pulm Drug Deliv. 2015;28:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Ari A, Areabi H, Fink JB. Evaluation of aerosol generator devices at 3 locations in humidified and non-humidified circuits during adult mechanical ventilation. Respir Care. 2010;55:837-844. [PubMed] |
| 37. | Berlinski A, Willis JR. Albuterol delivery by 4 different nebulizers placed in 4 different positions in a pediatric ventilator in vitro model. Respir Care. 2013;58:1124-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Tilley AE, Walters MS, Shaykhiev R, Crystal RG. Cilia dysfunction in lung disease. Annu Rev Physiol. 2015;77:379-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 39. | Konrad F, Schiener R, Marx T, Georgieff M. Ultrastructure and mucociliary transport of bronchial respiratory epithelium in intubated patients. Intensive Care Med. 1995;21:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Dellamonica J, Louis B, Lyazidi A, Vargas F, Brochard L. Intrapulmonary percussive ventilation superimposed on conventional ventilation: bench study of humidity and ventilator behaviour. Intensive Care Med. 2008;34:2035-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |