Published online Nov 4, 2015. doi: 10.5492/wjccm.v4.i4.287
Peer-review started: May 20, 2015
First decision: June 24, 2015
Revised: July 20, 2015
Accepted: August 20, 2015
Article in press: August 21, 2015
Published online: November 4, 2015
Processing time: 178 Days and 5 Hours
AIM: To determine the time course of intestinal permeability changes to proteolytically-derived bowel peptides in experimental hemorrhagic shock.
METHODS: We injected fluorescently-conjugated casein protein into the small bowel of anesthetized Wistar rats prior to induction of experimental hemorrhagic shock. These molecules, which fluoresce when proteolytically cleaved, were used as markers for the ability of proteolytically cleaved intestinal products to access the central circulation. Blood was serially sampled to quantify the relative change in concentration of proteolytically-cleaved particles in the systemic circulation. To provide spatial resolution of their location, particles in the mesenteric microvasculature were imaged using in vivo intravital fluorescent microscopy. The experiments were then repeated using an alternate measurement technique, fluorescein isothiocyanate (FITC)-labeled dextrans 20, to semi-quantitatively verify the ability of bowel-derived low-molecular weight molecules (< 20 kD) to access the central circulation.
RESULTS: Results demonstrate a significant increase in systemic permeability to gut-derived peptides within 20 min after induction of hemorrhage (1.11 ± 0.19 vs 0.86 ± 0.07, P < 0.05) compared to control animals. Reperfusion resulted in a second, sustained increase in systemic permeability to gut-derived peptides in hemorrhaged animals compared to controls (1.2 ± 0.18 vs 0.97 ± 0.1, P < 0.05). Intravital microscopy of the mesentery also showed marked accumulation of fluorescent particles in the microcirculation of hemorrhaged animals compared to controls. These results were replicated using FITC dextrans 20 [10.85 ± 6.52 vs 3.38 ± 1.11 fluorescent intensity units (× 105, P < 0.05, hemorrhagic shock vs controls)], confirming that small bowel ischemia in response to experimental hemorrhagic shock results in marked and early increases in gut membrane permeability.
CONCLUSION: Increased small bowel permeability in hemorrhagic shock may allow for systemic absorption of otherwise retained proteolytically-generated peptides, with consequent hemodynamic instability and remote organ failure.
Core tip: Although the concept of systemically circulating molecules from the bowel in response to shock is not new (e.g., bacterial “translocation”), the premise that small, proteolytically-derived molecules transit the bowel early in shock has not previously been examined. We offer evidence that proteolytically-derived peptides formed in the gut reach the systemic circulation in experimental hemorrhagic shock. The time-course and spatial disposition of low-molecular weight peptides in vivo was examined using real-time fluorescent intravital microscopy of the microcirculation and systemically as a first step towards demonstrating a pivotal role that these factors may play in affecting hemodynamic instability in early shock.
-
Citation: Alsaigh T, Chang M, Richter M, Mazor R, Kistler EB.
In vivo analysis of intestinal permeability following hemorrhagic shock. World J Crit Care Med 2015; 4(4): 287-295 - URL: https://www.wjgnet.com/2220-3141/full/v4/i4/287.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v4.i4.287
The small intestinal mucosa normally serves as a selective barrier to uncontrolled transport of large-molecular weight bowel contents into the systemic circulation, while simultaneously allowing the absorption of low-molecular weight nutrients necessary to sustain life. Severe hemorrhagic shock leads to decreased organ perfusion, especially to the bowel. Resultant ischemia to the gut adversely affects its function, and in particular the ability of the small bowel to act as a selective barrier to the uncontrolled egress of lumenal molecules into the systemic circulation. It has previously been shown that destruction of the small gut lumenal surface occurs very early in shock, and that this destruction appears to be mediated by enzymatic (proteolytic) activity at the bowel mucosa[1,2]. Under normal circumstances the bowel is protected from enzymatic degradation by a proteolytically-impermeant mucus layer. Maintenance of this layer requires ATP, and the ability of the protective mucus layer to prevent digestive enzyme attack of the mucosal wall is degraded with ischemia[3]. With the mucus layer compromised in shock, digestive enzymes in the bowel are able to destroy the underlying enterocyte layer, cell-cell junctions and the serosa, leading to increases in bowel permeability[1].
Enteral infusion of (serine) protease inhibitors into the small bowel lumen has been shown to be protective in multiple forms of experimental circulatory shock that result in gut ischemia, including hemorrhagic, endotoxic, and peritonitis shock[4]. Infusion of protease inhibitors enterally (but not systemically[5]) prevents or mitigates mortality in different species[6] after shock, including man[7], presumably by decreasing permeability of the small bowel to inflammatory mediators that otherwise cause systemic inflammation and multiple organ failure[8]. However, the mechanisms by which the mitigation of bowel injury improves outcomes after shock are largely unexplored.
Hemorrhagic shock has been reported to increase intestinal permeability, but this has largely been studied ex-vivo (e.g., Ussing chambers[9]) or using small markers such as radio-labeled sugars[10] or at single or later time points[11,12]. As such, the time-course and the extent of bowel permeability changes in this condition are largely unexplored. We hypothesized that proteolytically-derived peptides may be among the earliest mediators to cross the bowel mucosal barrier in shock and sought to determine their time-course in vivo, in order to further delineate the role and timing of bowel-mediated inflammation and remote organ injury in hemorrhagic shock.
The animal protocol was reviewed and approved by the University of California, San Diego Institutional Animal Care and Use Committee and conforms to the Guide for the Care and Use of Laboratory Animals by the United States National Institutes of Health (NIH Publication No. 85-23, 1996).
Eight-week-old non-fasted male Wistar rats (300-350 g, Charles River Breeding Laboratories, Wilmington, Mass) were randomly assigned to either hemorrhagic shock (n = 11) or sham shock control groups (n = 11). The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for over one week prior to experimentation. Animals were not fasted before experiments.
Animals were anesthetized (pentobarbital sodium, Abbott Laboratories, North Chicago, IL, 50 mg/kg, im) and the left femoral vein and artery were cannulated to facilitate continuous cardiovascular monitoring and blood withdrawal for the experimental procedure. Further pentobarbital was given intravenous (iv) as necessary to maintain adequate anesthetic plane. Heparin was given (10 U/mL iv) to facilitate exsanguination and prevent clotting in all animals. The animals breathed spontaneously without tracheotomy.
Hemorrhagic shock was induced by careful removal of blood via the femoral vein in 1 mL aliquots until a mean arterial pressure (MAP) of 35 mmHg was achieved. The MAP was maintained at 35 mmHg for 100 min, at which time shed blood was re-warmed to 37 °C and slowly reinfused in 1 mL aliquots, analogous to blood withdrawal, via the femoral vein. Animals were then observed for 100 min (Reperfusion) before termination of the experiment (Beuthanasia®, 0.22 mL/kg, iv). This model of hemorrhagic shock has previously been shown to result in ischemia-mediated damage to the small bowel[2,4]. Sham-shock animals were instrumented and manipulated as above without hemorrhage as the comparator group.
To determine the ability of bowel-generated proteolytically-cleaved peptides to diffuse into the central circulation, fluorescently-labeled casein (EnzChek Protease Assay kit, red-fluorescent BODIPY® TR-X, Invitrogen, Life Technologies, Grand Island, NY) was injected into the small bowel before the shock procedure (n = 6, both shock and sham shock control groups). The casein is labeled with multiple fluorophores and only fluoresces upon proteolytic cleavage. In confirmatory separate experiments, and because the molecular weight of the fluorescent casein-derived peptides was unknown, fluorescein isothiocyanate (FITC)-dextrans 20 (Sigma, St Louis), as a known molecular weight marker (20 kD MW) was substituted for the fluorescently-labeled casein (n = 5, both shock and sham shock control groups). Before induction of hemorrhagic shock (or analogous time period in the sham-shock control group) a midline incision was made and the small intestine was carefully exteriorized from the abdomen onto moist warmed (37 °C) gauze. Either one ml casein solution or FITC-dextrans 20 was injected sequentially along the length of the small bowel from the cecum proximally to the duodenum (10 mL of fluorescent solution total).
The rat mesentery was gently exposed and draped over a transparent pedestal on a heated animal stage (at 37 °C) as previously described[13]. The mesentery preparation was continuously superfused (2.0 mL/min) with Krebs-Henseleit solution (37 °C) containing a 95% N2-5% CO2 gas mixture, with care taken to maintain adequate fluid superfusion of the tissue. The mesenteric microcirculation was imaged using an intravital microscope [water immersion objective lens (× 25, numerical aperture = 0.60, Leitz; Wetzlar, Germany)] by a color charge-coupled device camera (DEI-470, Optronics Engineering; Goleta, CA; frame rate 1/125 s for bright field and 1/2 s for fluorescence light). All images were recorded (Model AG-a270P, Panasonic; Tokyo, Japan) and digitally stored for analysis. Fluorescent images were elicited using a 200-W mercury lamp. The light was passed through a quartz collector, heat filter (KG-2, Zeiss; Oberkochen, Germany), and fluorescent filter set (Excitation/Emission: 590/625 nm for casein peptide-derived fluorescence, Excitation/Emission: 485/535 nm for FITC dextrans-20 fluorescence (in separate experiments), L3 filter cube, Ploempak, Leitz). Single microscopic fields (approximately 300 μm × 350 μm) containing arterioles and venules were examined. Wright’s stain was used to identify the presence of inflammatory cell types. Briefly, mesentery sectors were excised, fixed in cold acetone (10 min) and subsequently stained with Wright’s stain (1 min). Slides were washed, dehydrated and cover-slipped for imaging.
After injection of fluorescent casein into the lumen of the small intestine, blood (50 μL) from the femoral artery was collected every 20 min for the duration of the experiment (200 min total) and plasma fluorescence was read immediately (SpectraMax Gemini XS, Molecular Devices, Sunnyvale, CA). In animals injected with FITC-dextrans 20, plasma was collected before the shock period and at reperfusion. At the end of the experiments heart, liver and lungs were collected, homogenized and fluorescence readings were performed to determine casein-derived peptide concentrations in these organs.
Results are presented as mean ± SD, where applicable. Unpaired comparisons of means between two groups in time were carried out using Repeated Measures ANOVA or two-tailed Student’s t-test where appropriate; comparisons between measurements in the same group were conducted using two-tailed Student’s paired t-test. Because of significant differences between pre-ischemic values in the (plasma) casein-derived peptide experiments data were normalized prior to conducting statistical comparisons. P < 0.05 was considered to be significant.
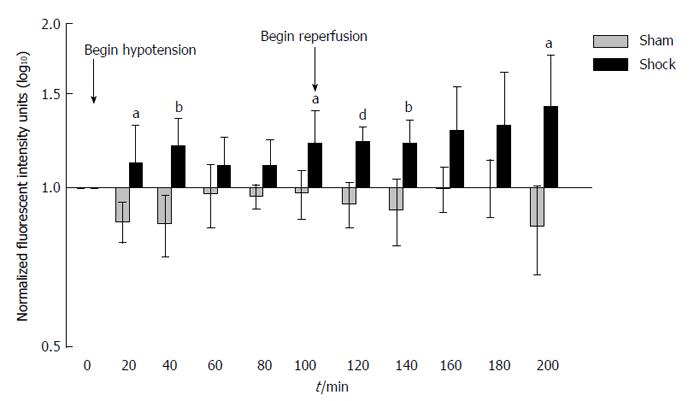
Hemorrhagic shock (average volume of blood withdrawn and subsequently reperfused: 6.6 ± 2.9 mL) led to time-dependent increases in small bowel permeability compared to that of sham-shock control animals as measured by increases in bowel-derived proteolytically-generated peptide fluorescence in the central circulation (Figure 1). A significant increase in small bowel permeability was apparent by as early as 20 min (P < 0.05 compared to the sham-shock control group), and was followed by a second and sustained increase in measured permeability upon reperfusion of shed blood (P < 0.05 and 0.01 compared to the sham-shock control group).
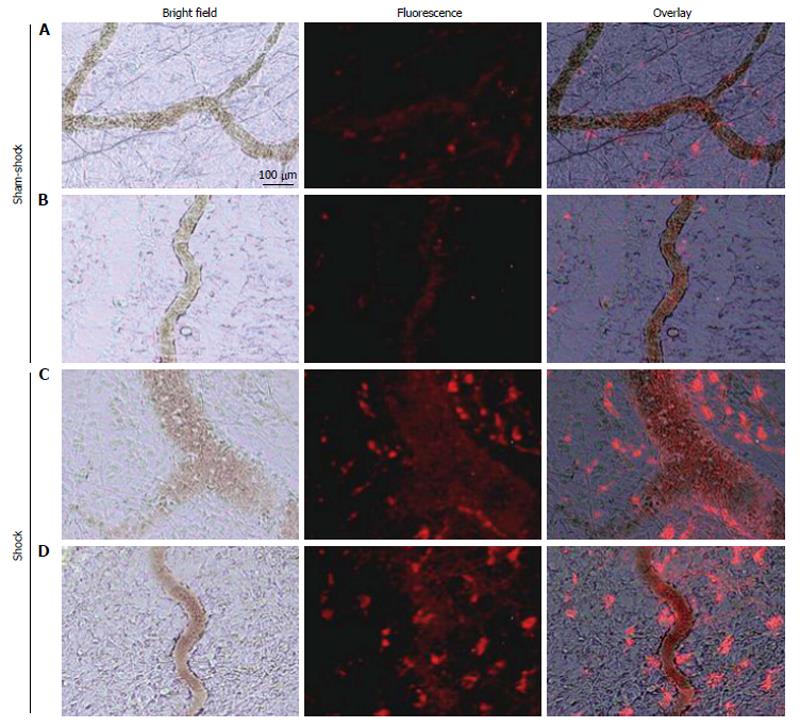
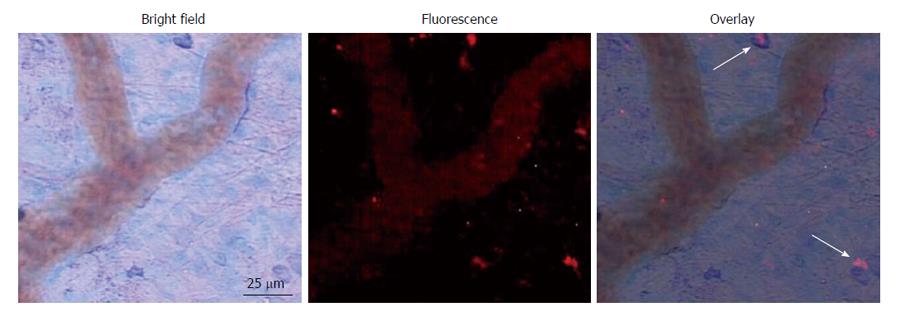
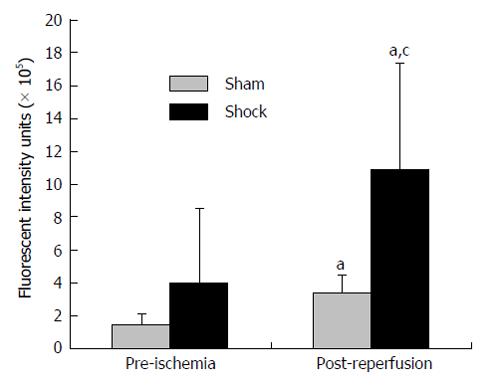
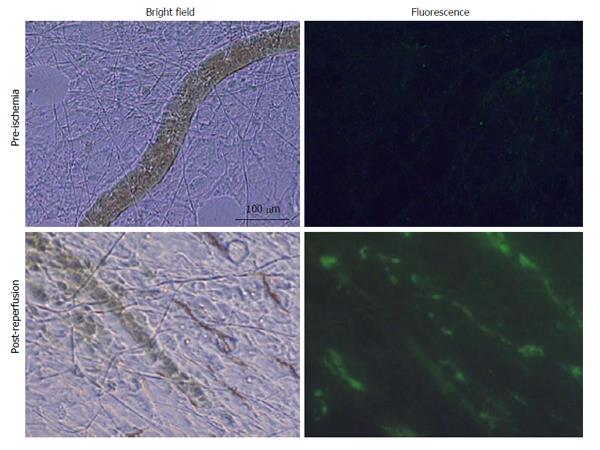
Co-incident with increased plasma concentrations in shock animals, the number of fluorescently-conjugated casein-derived peptides was substantially greater in the parenchyma and microvasculature of the mesentery of shocked animals (n = 6) compared to their non-shocked controls (n = 6) (Figure 2). Of note, there appeared to be extensive co-localization of casein-derived peptides with white blood cells in the microcirculation (Figure 3), suggesting that some of these casein-derived peptides may be inflammatory. In order to quantify the increases in bowel permeability to small molecular-weight molecules, in confirmatory experiments the larger FITC-dextrans 20 (molecular weight approximately 20 kD) tracer was injected into the small bowel instead of fluorescent casein at the initiation of the experimental procedures. These results demonstrate a significant increase in measured FITC fluorescence in the plasma of shocked animals (n = 5) compared to sham-shock controls (n = 5) after reperfusion [10.85 ± 6.52 vs 3.38 ± 1.11 fluorescent intensity units (× 105, P < 0.05)]. There were also significant increases in permeability-mediated FITC fluorescence after 100 min reperfusion compared to initial values in both shocked animals (10.85 ± 6.52 vs 3.97 ± 4.52, × 105, P < 0.05, n = 5) and controls (3.38 ± 1.11 vs 1.44 ± 0.64, × 105, P < 0.05, n = 5) (Figure 4). Intravital microscopy of the rat mesentery confirmed increases in microvascular permeability to FITC-dextrans 20 after hemorrhagic shock (Figure 5). However, less extravasation of FITC-dextrans 20 into the surrounding tissues was observed compared to that seen with casein-derived peptides, suggesting a possible differential increase in vascular permeability to the larger FITC-dextrans 20 molecule. Fluorescence of casein-derived peptides in remote tissues (n = 4 for both groups) displayed no significant differences in heart tissue (3210.7 ± 493.8 vs 2406.2 ± 841.8, P = 0.15) liver (4124.6 ± 1193.2 vs 6234.8 ± 1894.4, P = 0.11), and lung (2860.4 ± 1149.6 vs 3055.8 ± 1117.8, P = 0.81) in the shock group compared to sham-shock controls.
It has become increasingly apparent that fulminate circulatory shock, regardless of origin, results in small bowel ischemia[4]. Likewise, there is increasing evidence to suggest that prevention of bowel ischemia is beneficial to the organism, and preventing proteolytic digestion of the bowel mucosa, arising as a consequence of bowel ischemia, leads to improved outcomes in experimental shock[3,14,15]. The mechanisms by which bowel ischemia results in multiorgan failure and shock, are however, incompletely understood. It has long been hypothesized that “translocation” of bacterial product from the bowel to the systemic circulation contributes to deleterious outcomes in shock[16,17], but data supporting this theory are sparse and contradictory[10,18]. More importantly, there has been very little progress from a clinical perspective in attempting to modify or mitigate bacterial “translocation”, which lessens enthusiasm for this approach[19].
Alternatively, bowel ischemia, as we demonstrate here in response to experimental hemorrhagic shock of non-gastrointestinal origin, leads to very early increases in microvascular permeability to relatively low molecular weight products from the lumen of the bowel into the systemic circulation. Gut-derived peptides lie on a continuum from approximately 0.1-10 kD, several orders of magnitude smaller than bacteria and their generated inflammatory products[20], and can be found in a myriad number of forms and configurations. Therefore, it is reasonable to suggest that gut-derived peptides are among the first molecules from the bowel to enter the central circulation and subsequently reach remote organs. That many of these peptides have vasoactive and/or inflammatory potential is well-established, including peptides derived from casein[21,22].
We propose that some of the initial inflammatory mediators that circulate systemically in early experimental hemorrhagic shock are gut-derived proteolytically-generated peptides. Although increased bowel permeability in response to shock and other stressors has been well-documented as a general concept[23] and to fixed molecular weight tracers[24], we demonstrate here under real-time in vivo observation that in experimental hemorrhagic shock there is a significant increase in small bowel permeability compared to sham-shock control animals within 20 min of ischemia to proteolytically generated peptide products from the gut, implicating bowel compromise as an early and perhaps inciting event in this model. The rapid increase in measured bowel permeability during early ischemia occurs during a low-perfusion state with concomitant limited flux of fluorescently-labelled peptides, implying an underestimation of the permeability changes occurring at the bowel mucosa at this time. Conversely, sustained increases in small bowel permeability measured after blood reperfusion, where there is increased flux and possible “wash-out” of fluorescent tracer, may represent an over-estimation of increased bowel permeability rather than a second “reperfusion” injury.
An interesting observation from the study was the noticeable and frequent co-localization of casein-derived peptide fragments with possible inflammatory cells (mast cells vs macrophages, etc.). Although inflammatory activity of casein-derived peptides has previously been reported, this has not been directly confirmed in vivo[25-27]. Further investigation is necessary to confirm the robustness and clinical relevance of these findings, as fluorescently-labelled casein and FITC-dextrans 20 were used as markers of permeability and not for assessment of their intrinsic biologic activities. It is appreciated that there are limitations to the use of fluorescently-labelled markers when assessing permeability; it was for this reason that we chose to study small-molecule permeability using two different markers[28].
There are several limitations to this study. Among these includes our inability to categorize precisely the molecular weights of the fluorescently-bound casein-derived peptides secondary to the extreme heterogeneity of the cleavage products and the non-linear distribution of the fluorophores on the parent protein. However, it can be reasonably inferred that the MW’s of these fluorescent compounds are between 0.1-10 kD, (parent compound MW: 19-25 kD) and smaller than native casein[29]. Previous studies indicate that these peptides are produced in the bowel rather than proteolytically generated in the circulation after becoming systemic[3,30,31]. Although there is a lack of ancillary temporal data correlating in vivo effects with increases in permeability, confirmatory measurements using FITC-dextrans 20 support the assertion that in vivo permeability to larger molecular-weight particles also increases to some extent in shock[23,24]. Further studies using calibrated tracers at discrete time-points and anatomic locations are necessary to completely quantify these findings. Heparin given systemically before experimental hemorrhage is a possible confounder to our results when the coagulopathy of hemorrhage/trauma is considered. This is an unavoidable aspect of our hemorrhage model in which blood otherwise clots the catheters and while stored during the ischemic period. An unanticipated result was the heterogeneous accumulation of the fluorescently-labelled peptides in remote tissues. Because of the marked increase in fluorescently-labelled peptides in the mesentery and systemic circulation of animals exposed to experimental hemorrhagic shock compared to sham shock controls it was anticipated that remote tissues would demonstrate similar increases in gut-derived peptide concentrations after shock. The reasons for this lack of difference are unclear but could be due to preferential absorption in other non-measured tissues, heterogeneous accumulation in the selected organs, or simply minimal measured peptide uptake relative to organ tissue mass. Finally, it is acknowledged that the definition of “permeability” as used in these studies is semi-quantitative, in that what is measured is the resulting accumulation of tracer in the measured (vascular or tissue) compartment. As all variables except the changes in permeability are held constant between groups, the permeability results presented here are, strictly speaking, a ratio of permeability changes between the control and shock groups and as such are necessarily semi-quantitative[32].
In conclusion, this study demonstrates that early increases in small bowel permeability occur during experimental hemorrhagic shock and that proteolytically-derived peptides may be the defining molecules that instigate early inflammation and hemodynamic compromise. Further studies are needed to determine precisely the identity of these putative gut-derived inflammatory mediators and their origin, as well as strategies for preventing their egress into the systemic circulation.
Ischemia resulting from acute hemorrhage compromises the intestinal barrier, leading to increased permeability of the membrane to bowel-derived contents. Some of these molecules may be intrinsically inflammatory and may possibly contribute to the organ dysfunction and mortality seen in shock.
The ability of intestinal products, particularly those that are proteolytically generated, to escape into the central circulation following acute blood loss and their subsequent fate is not well understood. The authors were interested in determining in vivo the time course and the extent to which these particles access the central circulation following hemorrhagic shock.
The authors demonstrate in this manuscript that early increases in small bowel permeability occur very early during experimental hemorrhagic shock.
Proteolytically-derived peptides from the bowel enter the systemic circulation early in shock and may be defining molecules that instigate early inflammation and hemodynamic compromise in shock and associated poor bowel perfusion states.
This is a well written study investigating intestinal permeability after shock in a rodent model.
P- Reviewer: Esmat S, Rahbar E, Santos-Antunes J S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Chang M, Alsaigh T, Kistler EB, Schmid-Schönbein GW. Breakdown of mucin as barrier to digestive enzymes in the ischemic rat small intestine. PLoS One. 2012;7:e40087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Chang M, Kistler EB, Schmid-Schönbein GW. Disruption of the mucosal barrier during gut ischemia allows entry of digestive enzymes into the intestinal wall. Shock. 2012;37:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Kistler EB, Alsaigh T, Chang M, Schmid-Schönbein GW. Impaired small-bowel barrier integrity in the presence of lumenal pancreatic digestive enzymes leads to circulatory shock. Shock. 2012;38:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | DeLano FA, Hoyt DB, Schmid-Schönbein GW. Pancreatic digestive enzyme blockade in the intestine increases survival after experimental shock. Sci Transl Med. 2013;5:169ra11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Kistler EB, Lefer AM, Hugli TE, Schmid-Schönbein GW. Plasma activation during splanchnic arterial occlusion shock. Shock. 2000;14:30-34. [PubMed] |
| 6. | Kim HD, Malinoski DJ, Borazjani B, Patel MS, Chen J, Slone J, Nguyen XM, Steward E, Schmid-Schonbein GW, Hoyt DB. Inhibition of intraluminal pancreatic enzymes with nafamostat mesilate improves clinical outcomes after hemorrhagic shock in swine. J Trauma. 2010;68:1078-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Lee YT, Wei J, Chuang YC, Chang CY, Chen IC, Weng CF, Schmid-Schönbein GW. Successful treatment with continuous enteral protease inhibitor in a patient with severe septic shock. Transplant Proc. 2012;44:817-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Doucet JJ, Hoyt DB, Coimbra R, Schmid-Schönbein GW, Junger WG, Paul L W, Loomis WH, Hugli TE. Inhibition of enteral enzymes by enteroclysis with nafamostat mesilate reduces neutrophil activation and transfusion requirements after hemorrhagic shock. J Trauma. 2004;56:501-510; discussion 510-511. [PubMed] |
| 9. | Deitch EA, Senthil M, Brown M, Caputo F, Watkins A, Anjaria D, Badami C, Pisarenko V, Doucet D, Lu Q. Trauma-shock-induced gut injury and the production of biologically active intestinal lymph is abrogated by castration in a large animal porcine model. Shock. 2008;30:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Schlichting E, Grotmol T, Kähler H, Naess O, Steinbakk M, Lyberg T. Alterations in mucosal morphology and permeability, but no bacterial or endotoxin translocation takes place after intestinal ischemia and early reperfusion in pigs. Shock. 1995;3:116-124. [PubMed] |
| 11. | Du MH, Luo HM, Hu S, Lv Y, Lin ZL, Ma L. Electroacupuncture improves gut barrier dysfunction in prolonged hemorrhagic shock rats through vagus anti-inflammatory mechanism. World J Gastroenterol. 2013;19:5988-5999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Deitch EA, Morrison J, Berg R, Specian RD. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology, and intestinal permeability in conventional and antibiotic-decontaminated rats. Crit Care Med. 1990;18:529-536. [PubMed] |
| 13. | Kistler EB, Hugli TE, Schmid-Schönbein GW. The pancreas as a source of cardiovascular cell activating factors. Microcirculation. 2000;7:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Mitsuoka H, Kistler EB, Schmid-Schonbein GW. Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes. Proc Natl Acad Sci USA. 2000;97:1772-1777. [PubMed] |
| 15. | Mitsuoka H, Kistler EB, Schmid-Schönbein GW. Protease inhibition in the intestinal lumen: attenuation of systemic inflammation and early indicators of multiple organ failure in shock. Shock. 2002;17:205-209. [PubMed] |
| 16. | Leaphart CL, Tepas JJ. The gut is a motor of organ system dysfunction. Surgery. 2007;141:563-569. [PubMed] |
| 17. | Ravin HA, Rowley D, Jenkins C, Fine J. On the absorption of bacterial endotoxin from the gastro-intestinal tract of the normal and shocked animal. J Exp Med. 1960;112:783-792. [PubMed] |
| 18. | Yang R, Gallo DJ, Baust JJ, Watkins SK, Delude RL, Fink MP. Effect of hemorrhagic shock on gut barrier function and expression of stress-related genes in normal and gnotobiotic mice. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1263-R1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Bennett-Guerrero E, Grocott HP, Levy JH, Stierer KA, Hogue CW, Cheung AT, Newman MF, Carter AA, Rossignol DP, Collard CD. A phase II, double-blind, placebo-controlled, ascending-dose study of Eritoran (E5564), a lipid A antagonist, in patients undergoing cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2007;104:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Gillis M, De Ley J, De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970;12:143-153. [PubMed] |
| 21. | Kitazawa H, Yonezawa K, Tohno M, Shimosato T, Kawai Y, Saito T, Wang JM. Enzymatic digestion of the milk protein beta-casein releases potent chemotactic peptide(s) for monocytes and macrophages. Int Immunopharmacol. 2007;7:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Abdelhamid AE, Chuang SL, Hayes P, Fell JM. Evolution of in vitro cow’s milk protein-specific inflammatory and regulatory cytokine responses in preterm infants with necrotising enterocolitis. J Pediatr Gastroenterol Nutr. 2013;56:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Kuebler JF, Toth B, Rue LW, Bland KI, Chaudry IH. Differential alterations in intestinal permeability after trauma-hemorrhage. J Surg Res. 2003;112:198-204. [PubMed] |
| 24. | Russell DH, Barreto JC, Klemm K, Miller TA. Hemorrhagic shock increases gut macromolecular permeability in the rat. Shock. 1995;4:50-55. [PubMed] |
| 25. | Takano-Ishikawa Y, Goto M, Yamaki K. Analysis of leukocyte rolling and migration--using inhibitors in the undisturbed microcirculation of the rat mesentery--on inflammatory stimulation. Mediators Inflamm. 2004;13:33-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Kazlauskaite J, Biziulevicius GA, Zukaite V, Biziuleviciene G, Miliukiene V, Siaurys A. Oral tryptic casein hydrolysate enhances phagocytosis by mouse peritoneal and blood phagocytic cells but fails to prevent induced inflammation. Int Immunopharmacol. 2005;5:1936-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Rzodkiewicz P, Wojtecka-Lukasik E, Szukiewicz D, Schunack W, Maslinski S. Antihistaminic drugs modify casein-induced inflammation in the rat. Inflamm Res. 2010;59 Suppl 2:S187-S188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Rumbaut RE, Harris NR, Sial AJ, Huxley VH, Granger DN. Leakage responses to L-NAME differ with the fluorescent dye used to label albumin. Am J Physiol. 1999;276:H333-H339. [PubMed] |
| 29. | Fleminger G, Ragones H, Merin U, Silanikove N, Leitner G. Low molecular mass peptides generated by hydrolysis of casein impair rennet coagulation of milk. Int Dairy J. 2013;30:74-78. |
| 30. | Altshuler AE, Penn AH, Yang JA, Kim GR, Schmid-Schönbein GW. Protease activity increases in plasma, peritoneal fluid, and vital organs after hemorrhagic shock in rats. PLoS One. 2012;7:e32672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Altshuler AE, Richter MD, Modestino AE, Penn AH, Heller MJ, Schmid-Schönbein GW. Removal of luminal content protects the small intestine during hemorrhagic shock but is not sufficient to prevent lung injury. Physiol Rep. 2013;1:e00109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 449] [Article Influence: 26.4] [Reference Citation Analysis (0)] |