Published online Sep 9, 2025. doi: 10.5492/wjccm.v14.i3.102609
Revised: January 28, 2025
Accepted: February 21, 2025
Published online: September 9, 2025
Processing time: 268 Days and 9.5 Hours
Lung ultrasonography is being increasingly used in mechanically ventilated patients to evaluate the lung aeration during incremental positive end expiratory pressure (PEEP) adjustments and to evaluate the weaning process from mechanical ventilation. The effects of PEEP may vary across different lung path
To assess the role of lung ultrasonography in evaluating lung aeration during the application of PEEP in mechanically ventilated patients with various lung path
An observational study was conducted over 18 months in a tertiary care hospital. Patients of both genders, aged between 18-75 years, who had been admitted to the intensive care unit, and required mechanical ventilation, were studied. A standard ventilatory strategy was used and incremental levels of PEEP [5, 10, and 15 cm water (H2O)] were applied. Baseline characteristics, including oxygen saturation (SpO2), LUS, mean arterial pressure (MAP), heart rate (HR), and their changes with incremental PEEP levels, were recorded and analyzed.
In this study, 45.9% of patients required a PEEP of 5 cm H2O to achieve the endpoint of lung aeration (LUS of 0). In addition, 86.5% and 13.5% of patients reached the endpoint of lung aeration at PEEP levels of 10 and 15 cm H2O, respectively. The proportion of patients with higher lung scores decreased significantly with increasing PEEP levels (P < 0.001 for 5 and 10 cm H2O and P = 0.032 for 15 cm H2O). SpO2 increased significantly with higher PEEP levels
Increasing PEEP levels in mechanically ventilated patients improves lung aeration, which can be effectively assessed using bedside lung ultrasonography.
Core Tip: Increasing positive end expiratory pressure (PEEP) in titrated doses is a recruitment strategy aimed at improving oxygenation. The effectiveness of PEEP can vary across different lung pathologies and may not always correlate with changes in lung aeration as assessed by lung ultrasound scores (LUSs). In this study, the effect of incremental PEEP levels (5 cm H2O intervals) was evaluated in mechanically ventilated patients with various lung pathologies. Significant im
- Citation: Anegundi SS, Kurdi MS, Sutagatti JG, Theerth KA. Role of lung ultrasound in assessing positive end expiratory pressure induced lung recruitment in patients on mechanical ventilation. World J Crit Care Med 2025; 14(3): 102609
- URL: https://www.wjgnet.com/2220-3141/full/v14/i3/102609.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i3.102609
Mechanical ventilation in intensive care units (ICU) has undergone significant advancements in recent years. Since the early 1990s, ultrasound has been increasingly used to diagnose lung diseases in patients receiving mechanical ventilation[1]. Advances in the understanding of lung pathophysiology and ultrasonography principles have reshaped perspectives and ventilation strategies. Lung sonography has become a valuable tool for assessing lung aeration during recruitment maneuvers[2]. Transesophageal echocardiography focused on the left lower lobe, has demonstrated the potential to evaluate lung recruitment.
Incremental positive end expiratory pressure (PEEP) is a recruitment strategy used to enhance oxygenation, particularly in patients with acute respiratory distress syndrome (ARDS) requiring mechanical ventilation[3-5]. PEEP levels have to be appropriately titrated as higher PEEP levels can lead to hemodynamic compromise and potential worsening of ventilation perfusion mismatch. Despite its long-standing application in ARDS, the optimal approach for PEEP titration in different clinical scenarios remains a topic of debate.
Previous studies have examined the effect of PEEP-induced decreases in lung ultrasound scores (LUSs) in patients with ARDS. Based on these findings, this study was designed to determine whether lung ultrasonography can serve as a diagnostic tool for assessing PEEP-induced lung recruitment in mechanically ventilated patients with diverse lung pathologies. The study evaluated the role of lung ultrasonography in assessing lung aeration following PEEP application in patients with varying lung conditions. We hypothesized that changes in lung aeration with increasing PEEP would correlate with alterations in LUSs across different pathologies. The primary objective was to evaluate the relationship between increasing levels of PEEP and corresponding changes in LUS and peripheral oxygen saturation (SpO2). The secondary objective was to investigate the effect of PEEP on LUSs in various lung pathologies.
In this observational study, patients of both genders admitted in a tertiary care ICU were recruited over a period of 18 months, from March 2021 to August 2022, if their relatives provided consent. Included patients were those aged 18-75 years, who had been under mechanical ventilation and required PEEP. Patients were sedated with propofol, midazolam, or other sedative drugs. Exclusion criteria included any conditions that may have interfered with ultrasonography, such as subcutaneous emphysema of the chest, severe obesity, large thoracic surgical dressings, and pregnancy. Patients were also excluded if they lacked indications for PEEP changes or had contraindications, including peripheral pulmonary masses, absence of non-aerated lung areas, inability to maintain SpO2 above 85% during PEEP reduction, or large undrained pneumothoraces. The study was approved by the institutional ethics committee (KIMS: ETHICS COMM:412:2020-21, dated January 22, 2021) and was conducted in accordance with the principles of the Declaration of Helsinki (2013).
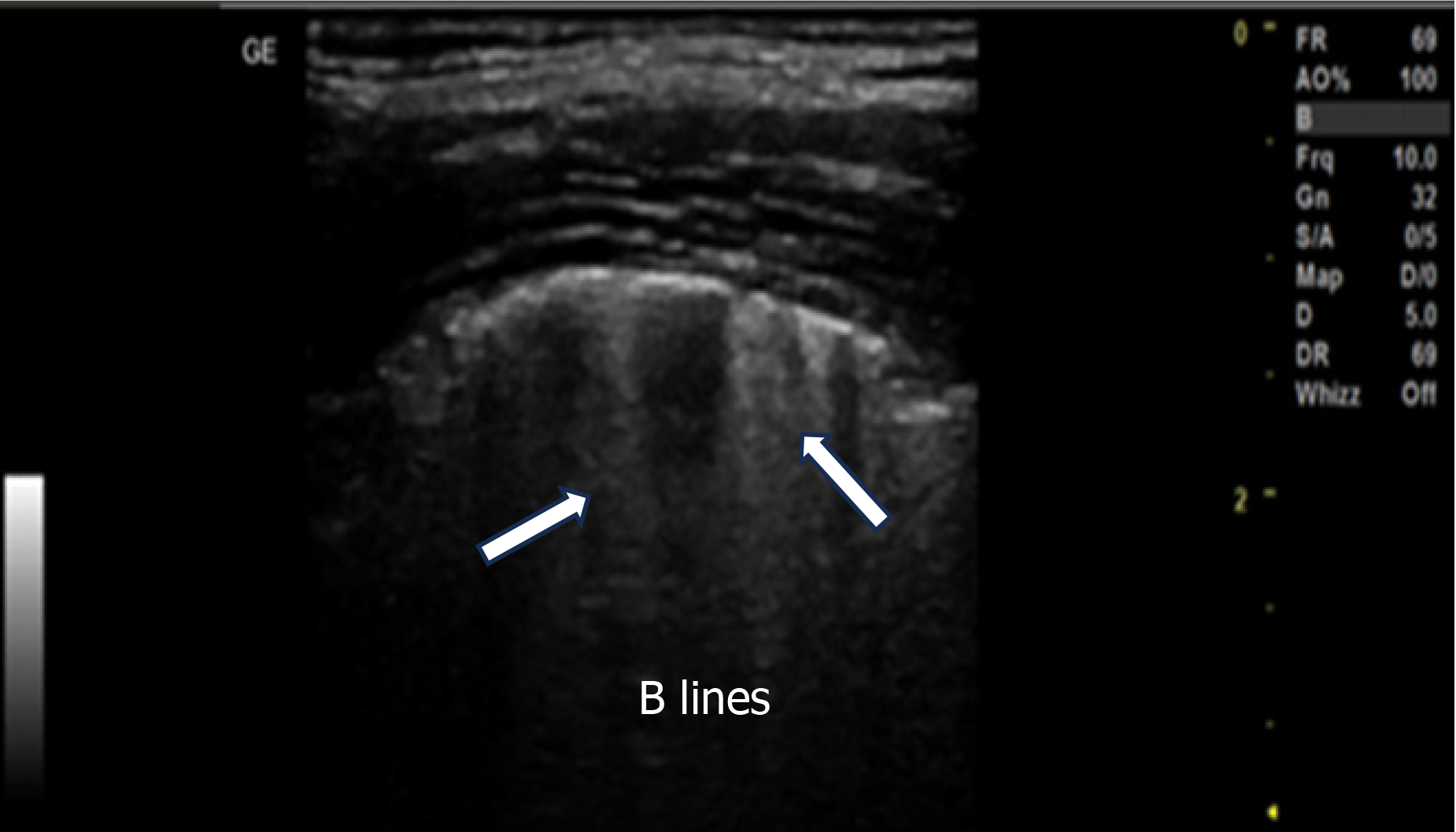
The baseline characteristics of the patients, including age, gender, prevalence of comorbidities and addictions, disease or indication for mechanical ventilation, heart rate (HR), mean arterial pressure (MAP), tidal volume, fraction of inspired oxygen (FiO2), and SpO2, were collected and recorded. All patients underwent mechanical ventilation in the volume assist-control mode, with tidal volume set at 6-8 mL/kg of body weight. The FiO2 level ranged from 0.6 to 1.0, ensuring arterial SpO2 of approximately 90% or higher. PEEP levels were applied by an intensivist in titrated doses of 5, 10, and 15 cm H2O, with each level maintained for at least 20 min. Ventilator settings, except for PEEP, and FiO2 levels remained unchanged throughout the study. Patients were positioned semi-recumbently with a 30° head-end elevation during the study. Lung ultrasonography was performed using an ultrasound machine (GE Versana Active™; Chicago, Illinois, United States) with curvilinear (4C-RS MHz) or linear (L6-12 RS) probes by an expert in lung ultrasound. The right diaphragm was first identified using sonography, and the probe was placed longitudinally along the posterior axillary line in the posteroinferior zone, perpendicular to the skin. LUSs were assessed along with MAP, HR, and SpO2 at the end of each PEEP setting (5, 10, and 15 cm H2O). The LUS for aeration was calculated based on the most severe ultrasound pattern observed in a given region of interest: Normal = 0, well-separated B-lines (> 3 mm inter-B-line distance) = 1, coalescent B-lines (< 3 mm inter-B-line distance) = 2, and consolidation = 3 (Figure 1). The scores ranged from 0 to 3, with higher scores indicating decreased aeration[6,7]. Lung ultrasonography during the study was conducted only on patients who required higher PEEP levels to reach the lung recruitment endpoint (defined as an LUS of 0). Stefanidis et al[2] found that the mean LUSs ± standard deviation (SD) of non-aerated areas in dependent lung regions significantly decreased as PEEP increased from 5 to 10 to 15 cm H2O (27 ± 31, 20 ± 24, and 11 ± 12 cm², respectively). To detect a minimal Pearson’s correlation coefficient of 0.5 with 80% power and a 95% confidence interval, a sample size of 37 was required[8]. Data were entered into Microsoft Excel and analyzed using International Business Machines (IBM corp, New York, USA) Statistical Package for the Social Sciences software, version 22.
Categorical data are presented as frequencies and proportions. The χ2 test was used to assess the significance of qualitative data. For qualitative data that did not meet the χ2 test criteria (limited to 2 × 2 tables), Fisher’s exact test was applied. Yates’s correction was implemented when 2 × 2 tables did not meet the assumptions of the χ2 test. McNemar’s test was used to compare results after the trials. The normality of continuous data was evaluated using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Continuous data are expressed as the mean and SD. Wilcoxon’s signed rank test was performed to compare results after the trials. A P value of < 0.05 was considered statistically significant, adhering to the assumptions of the respective statistical tests.
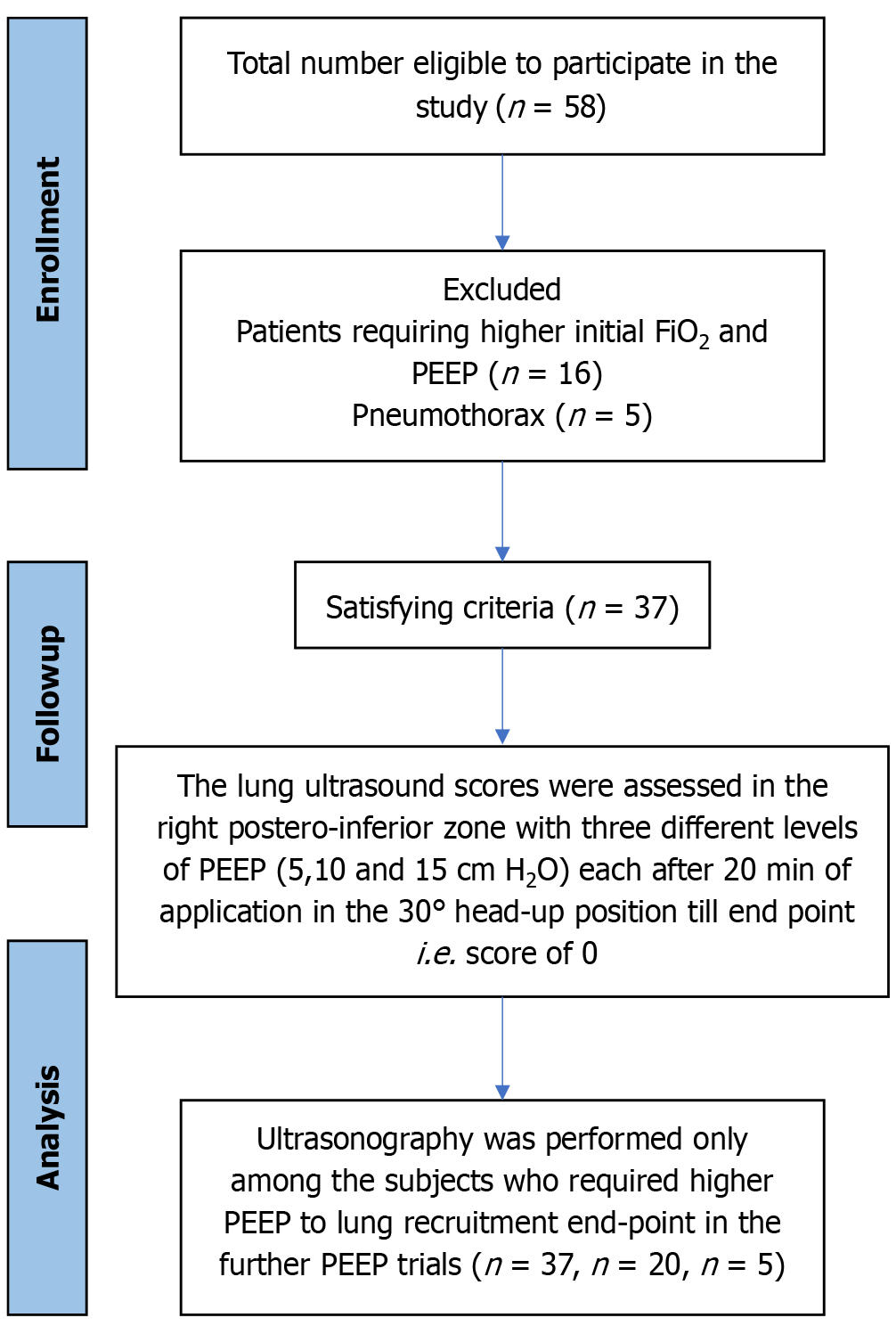
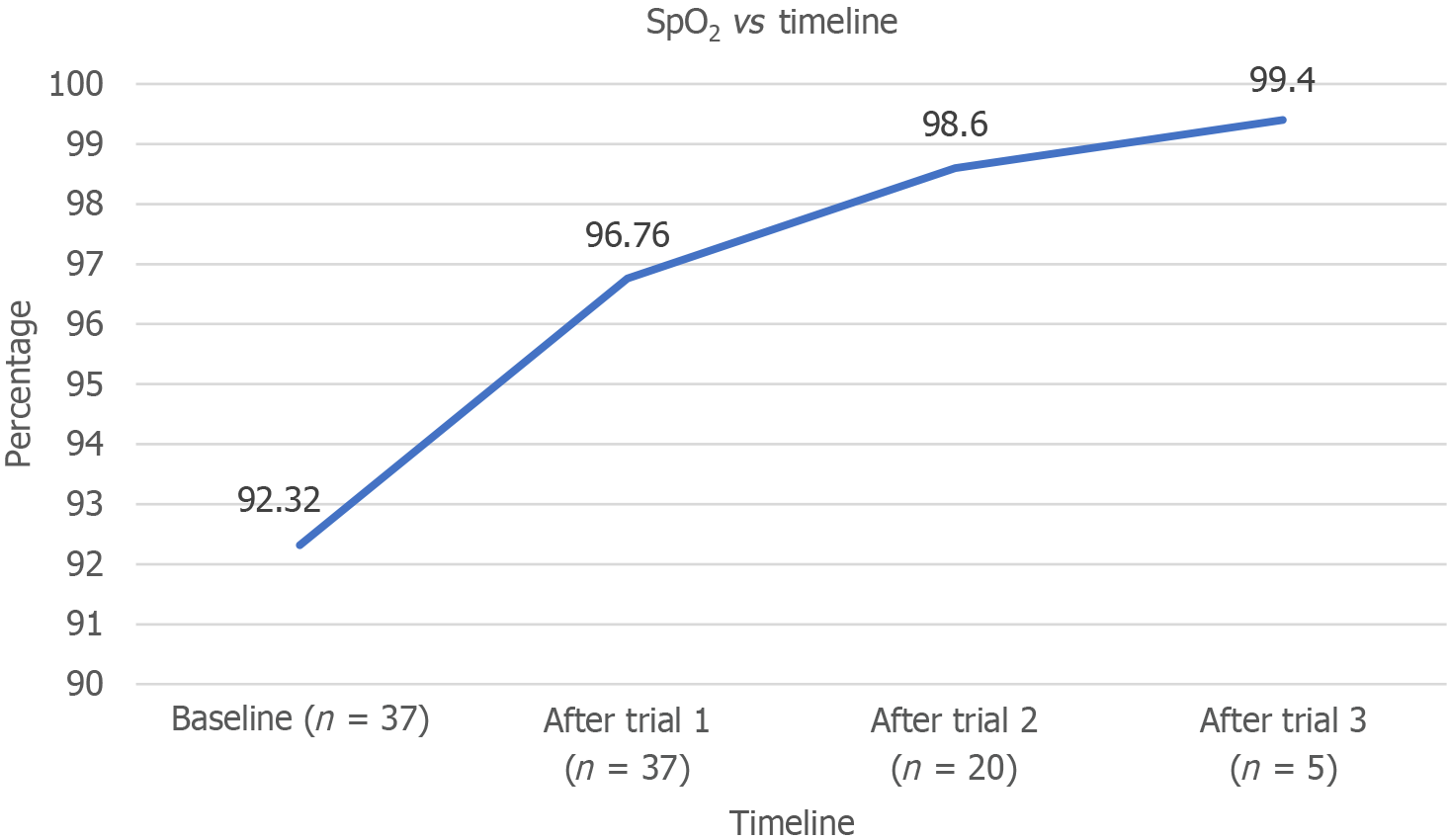
A total of 37 patients were analyzed (Figure 2). The majority of the participants were men (70.3%), with the remaining 29.7% being women. The mean ± SD age of the patients was 47.22 ± 15.72 years. Among the participants, 29% had diabetes or hypertension. Nearly half (45.9%) reported alcohol use, whereas 32.5% used smokeless tobacco; however, only 10.8% had a history of smoking. The most common indication for mechanical ventilation was airway protection (35.1%), followed by postanesthesia recovery (18.9%) and restricted ventilatory defect (13.5%; Table 1). Furthermore, 45.9% of the patients required a PEEP of 5 cm H2O to achieve the endpoint of lung aeration (LUS of 0). A total of 86.5% reached the endpoint at 10 cm H2O, whereas 13.5% required a PEEP of 15 cm H2O (Table 2). The mean baseline SpO2 was 92.32% ± 2.35%. SpO2 measurements were recorded only for patients requiring PEEP after each trial, with mean values of 96.76% ± 3.36%, 98.60% ± 2.16%, and 99.40% ± 1.34% after the first, second, and third trials, respectively. The increase in SpO2 over time was statistically significant (P < 0.001), confirming the effectiveness of PEEP in improving SpO2 (Figure 3).
| Indications | Frequency (n) | Percentage (%) |
| Airway protection | 13 | 35.1 |
| Alveolar filling defect | 4 | 10.8 |
| Impaired central drive | 4 | 10.8 |
| Post anesthesia recovery | 7 | 18.9 |
| Pulmonary vascular defect | 2 | 5.4 |
| Respiratory muscle weakness | 2 | 5.4 |
| Restrictive ventilatory defect | 5 | 13.5 |
| Positive end expiratory pressure | Frequency (n) | Percentage (%) | |
| 5 cm H2O (trial 1) | Yes | 37 | 100.0 |
| No | 0 | 0.0 | |
| 10 cm H2O (trial 2) | Yes | 20 | 54.1 |
| No | 17 | 45.9 | |
| 15 cm H2O (trial 3) | Yes | 5 | 13.5 |
| No | 32 | 86.5 | |
Baseline lung ultrasonography revealed that the majority of patients (43.2%) exhibited coalescent B-lines before the application of PEEP. Throughout the study, lung ultrasonography was performed exclusively on patients requiring higher PEEP levels to achieve the lung aeration endpoint. Most participants demonstrated progressive improvement in LUS, with better scores after each trial, and all patients ultimately achieved a score of 0 (Table 3). We noted a statistically significant reduction in LUSs over time, confirming the effectiveness of PEEP in improving lung aeration. The proportion of patients with higher LUS decreased significantly with increasing PEEP levels (P < 0.001 for 5 and 10 cm H2O; P = 0.032 for 15 cm H2O). Among lung disorders, the most common findings were pleural collections (63.6%) and pneumothorax (36.4%).
| Lung ultrasound score | Score 0 (normal) | Score 1 (well separated B-lines) | Score 2 (coalescent B-lines) | Score 3 (consolidation) | P value | ||||
| n | % | n | % | n | % | n | % | ||
| Baseline (n = 37) | 0 | 0.0 | 15 | 40.5 | 16 | 43.2 | 6 | 16.2% | - |
| After trial 1 (n = 37) | 17 | 45.9 | 14 | 37.8 | 6 | 16.2 | 0 | 0.0 | < 0.001 |
| After trial 2 (n = 20) | 15 | 75.0 | 4 | 20.0 | 1 | 5.0 | 0 | 0.0 | < 0.001 |
| After trial 3 (n = 5) | 5 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.032 |
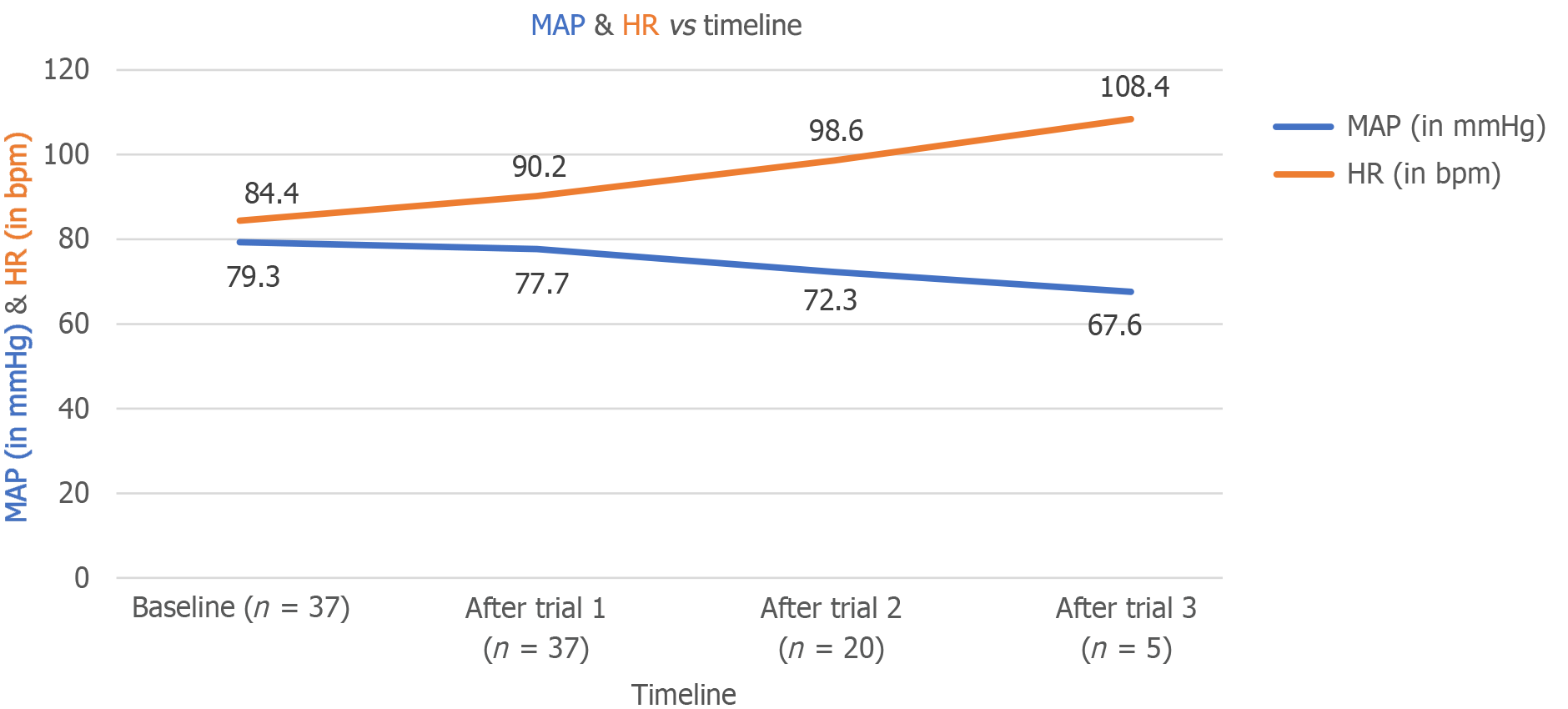
The mean ± SD baseline HR was 84.43 ± 10.74 beats per minute (bpm). HR was recorded only for patients requiring higher PEEP after each trial. The mean HR increased to 90.22 ± 10.00, 98.60 ± 9.52, and 108.40 ± 6.07 bpm after the first, second, and third trials, respectively (P < 0.001, P < 0.001, and P = 0.001, respectively). This significant increase in HR over time demonstrated the impact of PEEP on HR. The mean ± SD baseline MAP was 79.30 ± 6.83 mmHg. Similar to HR, MAP was recorded only for patients requiring higher PEEP after each trial. The mean MAP values decreased to 77.70 ± 6.59, 72.30 ± 6.24, and 67.60 ± 3.97 mmHg after the first, second, and third trials, respectively (P < 0.001, P < 0.001, and P = 0.003, respectively). This significant decline in MAP indicated the effect of PEEP on reducing arterial pressure (Figure 4). The validity of LUSs in assessing lung aeration was compared with actual SpO2 measurements. At the first trial (5 cm H2O PEEP), LUS and SpO2 showed almost perfect agreement, with sensitivity and specificity of 100.0% and 95.2%, respectively, and a diagnostic accuracy of 97.3%. At the second trial (10 cm H2O PEEP), substantial agreement was observed, with sensitivity and specificity of 100.0% and 62.5%, respectively, and a diagnostic accuracy of 85.0% (Table 4). However, no agreement was observed at the third trial (15 cm H2O PEEP) due to a specificity of 0.0%. Overall, sensitivity was 100.0%, with a diagnostic accuracy of 80.0% at 15 cm H2O. The baseline LUS scores showed no significant association with the characteristics of the study patients. However, nonaerated areas were more common in older adults and males, with aeration being more affected in individuals with a history of addictions. The analysis of mechanical ventilation indications and PEEP requirements for achieving 100.0% SpO2 revealed a significant association after the first PEEP trial. Patients with alveolar filling defects, respiratory muscle weakness, or restrictive ventilatory defects required 15 cm H2O to achieve 100.0% saturation (Table 5).
| Trial 1 (n = 37) | SpO2 | ||||||
| 100.0% | ≤ 99.0% | Total | |||||
| n | % | n | % | n | % | ||
| Lung ultrasound score | 100.0% | 16 | 43.2 | 1 | 2.7 | 17 | 45.9 |
| ≤ 99.0% | 0 | 0.0 | 20 | 54.1 | 20 | 54.1 | |
| Total | 16 | 43.2 | 21 | 56.8 | 37 | 100.0 | |
| Mechanical ventilation indications | After trial 1 (n = 37) | After trial 2 (n = 20) | After trial 3 (n = 5) | |||||||||
| Yes | No | Yes | No | Yes | No | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Airway protection | 10 | 76.9 | 3 | 23.1 | 2 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Alveolar filling defect | 0 | 0.0 | 4 | 100.0 | 1 | 25.0 | 3 | 75.0 | 2 | 100.0 | 0 | 0.0 |
| Impaired central drive | 3 | 75.0 | 1 | 25.0 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Post anesthesia recovery | 3 | 42.9 | 4 | 57.1 | 3 | 75.0 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 |
| Pulmonary vascular defect | 0 | 0.0 | 2 | 100.0 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 |
| Respiratory muscle weakness | 0 | 0.0 | 2 | 100.0 | 1 | 50.0 | 1 | 50.0 | 1 | 100.0 | 0 | 0.0 |
| Restrictive ventilatory defect | 0 | 0.0 | 5 | 100.0 | 3 | 60.0 | 2 | 40.0 | 1 | 50.0 | 1 | 50.0 |
| P value | 0.007 | 0.598 | 0.392 | |||||||||
This observational study evaluated lung aeration in response to changes in PEEP levels. Significant improvement in aeration was observed, as evidenced by decreasing LUS scores and increasing SpO2 levels.
This study included 37 patients aged 18 to 75 years, with a mean age of 47.2 years. The majority of patients were in the 46 to 60 years age group. No statistically significant correlation was observed between age and lung recruitment on lung ultrasound, likely due to the inclusion of diverse indications for mechanical ventilation, which were not specific to any particular age group. These findings are in agreement with those reported by Mojoli et al[9] and Chiumello et al[10]. Although the study sample included a higher proportion of male patients, primarily due to trauma cases, both sexes responded similarly to PEEP changes.
Among the 37 patients, 29% had diabetes mellitus and 29% had hypertension as comorbidities. Hypertensive individuals exhibited decreased arterial elasticity, making their hemodynamics more susceptible to PEEP, resulting in decreased blood pressure and increased HR, as reported by Zhou et al[11]. Smoking is associated with oxidative stress, protease-antiprotease imbalance, and inflammation, which ultimately lead to chronic obstructive pulmonary disease (COPD). Patients with COPD often have hyperinflated alveoli, resulting in auto-PEEP and a higher risk of PEEP-related complications[12]. However, in this study, comorbidities showed no statistically significant correlation with lung recruitment as assessed by lung ultrasound. In this study, 45.9% of patients required minimal PEEP (5 cm H2O) to achieve the lung recruitment endpoint. Airway protection and postanesthesia recovery accounted for approximately 55% of the patients, forming the majority who attained lung recruitment at lower PEEP levels. These individuals had physiologically normal lungs, where minimal PEEP associated with positive pressure ventilation was sufficient to normalize LUS scores. Patients with alveolar filling defects, respiratory muscle weakness, and restrictive ventilatory defects required higher PEEP levels. The majority of patients (86%) required a PEEP of 5-10 cm H2O to achieve the recruitment endpoint. Increasing PEEP elevates intrathoracic pressure, raising central venous pressure and reducing venous return. This reduction in venous return reduces cardiac output and lowers MAP. In addition, the associated decline in stroke volume increases HR. In this study, a statistically significant decrease in MAP and an increase in HR were observed with increasing PEEP levels, consistent with the findings of Zhou et al[11].
Positive pressure ventilation combined with sedation alters ventilation and perfusion, leading to a decrease in functional residual capacity. A minimum PEEP of 5 cm H2O is generally required to maintain adequate oxygenation at lower FiO2 levels. In this study, 43.2% of patients initially displayed coalescent B-lines in the right posteroinferior zone, with a baseline SpO2 of 96.76% ± 3.36%. To prevent hypoxia at a PEEP of 0, the initial FiO2 was set at 60%. Incremental increases in PEEP by 5 cm H2O significantly improved SpO2, with SpO2 values rising to 96.76% ± 3.36%, 98.60% ± 2.16%, and 99.40% ± 1.34% at PEEP levels of 5, 10, and 15 cm H2O, respectively. Nevertheless, it has been suggested that the application of PEEP can increase the aerated lung area by reducing pulmonary arteriovenous shunting[13]. All patients achieving 100% SpO2 also had an LUS score of 0 in the posterior-inferior zone, eliminating the need for additional PEEP trials. However, by the end of the three trials, all but one patient achieved 100% SpO2. This finding suggests that nonrecruited areas, contributing to pulmonary arteriovenous shunting, may persist even with an LUS of 0. Furthermore, increased aeration does not necessarily equate to recruitment because LUS scores are not definitive indicators of recruitment compared with computed tomography. These results are consistent with the findings of Chiumello et al[10] and Borges et al[14].
This study has several strengths. PEEP may not consistently result in improved lung aeration across different lung pathologies. However, the study validates the impact of PEEP-induced improvements in LUSs in various lung conditions, which have not been extensively studied outside of the context of ARDS. The inclusion of patients with diverse pathologies in the surgical ICU, such as postoperative cases and individuals with a history of chest trauma, enhances the generalizability of the findings. The results highlight the utility of LUS scores in determining appropriate PEEP levels while avoiding excessive PEEP and its associated complications. Furthermore, these findings pave the way for future research to explore the implications of improved oxygenation on outcomes such as weaning, ICU stay, and extubation.
The present study contains several potential limitations. The ventilatory strategy applied to all patients was standardized, with even those requiring airway protection but having normal lung function receiving an initial FiO2 of 60%. Future studies could explore the use of tailored ventilatory strategies. In addition, tidal volume was calculated based on the patient’s body weight rather than ideal body weight, which may affect results. Peripheral SpO2 was used to correlate with LUSs; however, arterial oxygenation, assessed via partial pressure of oxygen, could have provided a more accurate correlation. Lung recruitment is a gradual process that occurs over an extended duration. A longitudinal study design would have been better suited to monitor these changes over time. Interobserver variability in the assessment of LUS scores also poses a potential limitation because it may affect the consistency of results. In this study, the upper limit of PEEP was capped at 15 cm H2O to prevent complications such as hemodynamic instability and barotrauma. Higher PEEP levels could be explored in future studies with stringent patient monitoring. LUS scoring was limited to the right posterior-inferior zone due to difficulties in patient positioning, particularly in postoperative patients. However, this region is more susceptible to lung pathologies, including aspiration, and effectively reflects the utility of PEEP. Cases with extrapleural collections in this region were excluded from the analysis.
Increasing the PEEP levels in mechanically ventilated patients improves lung aeration, which can be effectively assessed using bedside lung ultrasonography. This tool serves as a valuable diagnostic method for tailoring PEEP administration and evaluating PEEP-induced lung recruitment in such patients.
| 1. | Lichtenstein D, Axler O. Intensive use of general ultrasound in the intensive care unit. Prospective study of 150 consecutive patients. Intensive Care Med. 1993;19:353-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Stefanidis K, Dimopoulos S, Tripodaki ES, Vitzilaios K, Politis P, Piperopoulos P, Nanas S. Lung sonography and recruitment in patients with early acute respiratory distress syndrome: a pilot study. Crit Care. 2011;15:R185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2410] [Cited by in RCA: 2241] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 4. | Tsubo T, Sakai I, Suzuki A, Okawa H, Ishihara H, Matsuki A. Density detection in dependent left lung region using transesophageal echocardiography. Anesthesiology. 2001;94:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Tsubo T, Yatsu Y, Suzuki A, Iwakawa T, Okawa H, Ishihara H, Matsuki A. Daily changes of the area of density in the dependent lung region - evaluation using transesophageal echocardiography. Intensive Care Med. 2001;27:1881-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, Rouby JJ; Lung Ultrasound Study Group. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit Care Med. 2012;40:2064-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 7. | Bouhemad B, Mongodi S, Via G, Rouquette I. Ultrasound for "lung monitoring" of ventilated patients. Anesthesiology. 2015;122:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Bujang MA, Baharum N. Sample Size Guideline for Correlation Analysis. World J Soc Sci Res. 2016;3:37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung Ultrasound for Critically Ill Patients. Am J Respir Crit Care Med. 2019;199:701-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 277] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 10. | Chiumello D, Mongodi S, Algieri I, Vergani GL, Orlando A, Via G, Crimella F, Cressoni M, Mojoli F. Assessment of Lung Aeration and Recruitment by CT Scan and Ultrasound in Acute Respiratory Distress Syndrome Patients. Crit Care Med. 2018;46:1761-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 11. | Zhou L, Cai G, Xu Z, Weng Q, Ye Q, Chen C. High positive end expiratory pressure levels affect hemodynamics in elderly patients with hypertension admitted to the intensive care unit: a prospective cohort study. BMC Pulm Med. 2019;19:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Munro N. Weaning smokers from mechanical ventilation. Crit Care Nurs Clin North Am. 2006;18:21-28, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Karbing DS, Panigada M, Bottino N, Spinelli E, Protti A, Rees SE, Gattinoni L. Changes in shunt, ventilation/perfusion mismatch, and lung aeration with PEEP in patients with ARDS: a prospective single-arm interventional study. Crit Care. 2020;24:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Borges JB, Okamoto VN, Matos GF, Caramez MP, Arantes PR, Barros F, Souza CE, Victorino JA, Kacmarek RM, Barbas CS, Carvalho CR, Amato MB. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;174:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 328] [Article Influence: 17.3] [Reference Citation Analysis (0)] |