Published online Nov 27, 2014. doi: 10.5411/wji.v4.i3.174
Revised: June 3, 2014
Accepted: July 25, 2014
Published online: November 27, 2014
Processing time: 256 Days and 4.5 Hours
Cluster of differentiation 74 (CD74) performs multiple roles in B cells, T cells, and antigen-presenting cells within the immune system; it also participates in major histocompatibility complex class II-restricted antigen presentation and inflammation. Recently, a role for CD74 in carcinogenesis has been described. CD74 promotes cell proliferation and motility and prevents cell death in a macrophage migration inhibitory factor-dependent manner. Its roles as an accessory signal receptor on the cell surface and the ability to interact with other signaling molecules make CD74 an attractive therapeutic target for the treatment of cancer. This review focuses on the original role of CD74 in the immune system and its emerging tumor-related functions. First, the structure of CD74 will be summarized. Second, the current understandings about the expression, cellular localization, molecular mechanisms and signaling pathways of CD74 in immunity and cancer will be reviewed. Third, the examples that suggest CD74 is a promising molecular therapeutic target are reviewed and discussed. Although the safety and efficacy of CD74-targeted strategies are under development, deeply understanding of the regulation of CD74 will hold promise for the use of CD74 as a therapeutic target and may develop the CD74-targeted therapeutic agents such as neutralized antibody and compounds.
Core tip: There are several structural and functional variants of cluster of differentiation 74 (CD74), each with their own expression pattern. Although this diversity may be required for normal homeostasis, it can lead to aberrant proliferation when dysregulated. This review focuses on the primary role of CD74 in the immune system and how the activity of this evolutionarily conserved molecule is subverted during tumorigenesis.
- Citation: Liu YH, Lin JY. Recent advances of cluster of differentiation 74 in cancer. World J Immunol 2014; 4(3): 174-184
- URL: https://www.wjgnet.com/2219-2824/full/v4/i3/174.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i3.174
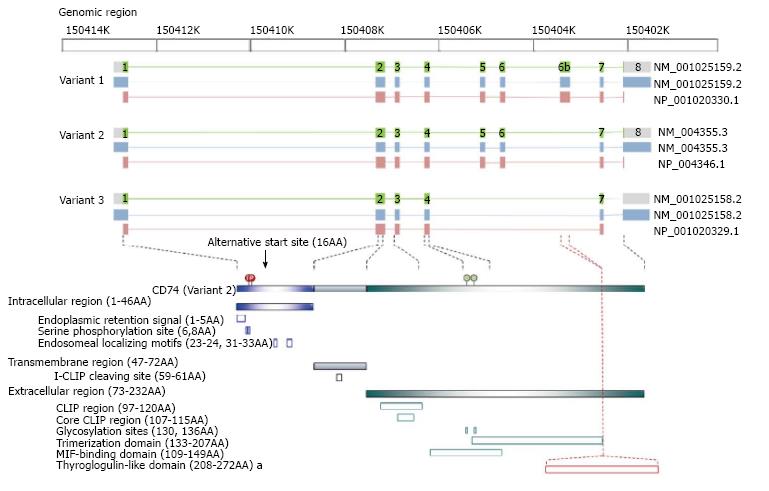
The cluster of differentiation 74 (CD74) gene, which is located on chromosome 5q32, encodes the type II integral membrane glycoprotein CD74; there are four major isoforms of this protein in humans[1]. This evolutionarily conserved molecule is the membrane form of the major histocompatibility complex class II (MHC class II) invariant chain (Ii) because none of the original isolates harbored polymorphisms[2]. The most common isoform of CD74 is the p33 isoform (with a molecular weight of 33 kDa), which has a 29-residue N-terminal intracellular region, a 26-residue hydrophobic transmembrane region, and a 160-residue C-terminal extracellular region containing two N-linked glycosylation sites[3,4]. The p35 isoform is also produced because of differential initiation of translation[5], whereas p41 and p43 isoforms arise because of alternative splicing of the exon 6b transcription products that encode a thyroglobulin type I cathepsin-binding domain[6-8]. Both the p33 and p35 isoforms regulate MHC class II antigen presentation through rapid internalization from the cell surface to endosomes (half-life under 10 min) when MHC class II-CD74 complexes are formed. However, approximately 2%-5% of these cell surface isoforms are not found in MHC class II complexes. Although the role(s) for the membrane-localized CD74 on some parenchymal epithelial cells remain largely unclear, the finding that it is involved in proliferative responses associated with intramembrane proteolysis (RIP)-processed led researchers to investigate its role in cancer[9]. Domains, motifs, and active residues as well as the corresponding functions within the intracellular[10-13], transmembrane[14], and extracellular region[7,9,15-21] of CD74 have been identified. Figure 1 illustrates the CD74 variants and their corresponding protein structures.
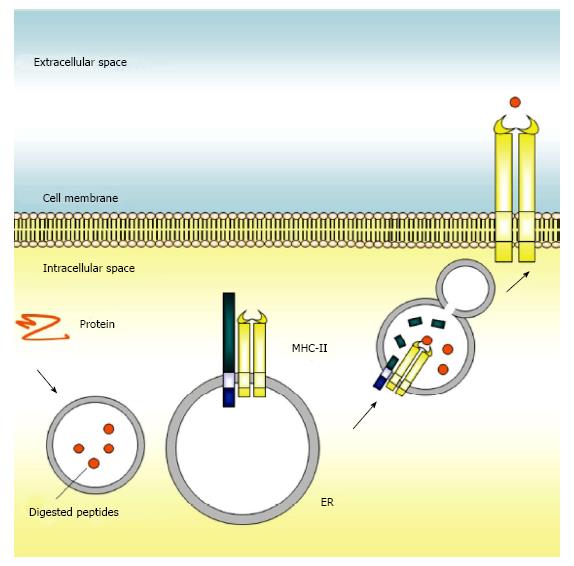
CD74 has several functions related to MHC class II- restricted antigen presentation, including the prevention of MHC class II to bind non-processed peptide and self-antigen[22]. CD74 was originally reported to be a molecular chaperone for regulating MHC class II folding in the rough endoplasmic reticulum (ER), where it was thought to play a major role in processing and transporting of MHC class II molecules in the immune system, and in particular in antigen-presenting cells. Once synthesized, CD74 self-assembles into a trimer and serves as a scaffold onto which nascent MHC class II molecules assemble. After trafficking to the late endosome, CD74 is cleaved by cathepsin S (cathepsin L in thymic epithelial cells), leaving a small peptide, CLIP, to block the peptide binding cleft of MHC class II and in turn to prevent premature binding of antigenic peptides to MHC class II. The CLIP-MHC class II complex will then transport through the endosomal pathway[5]. Upon binding of HLA-DM to MHC class II, CLIP is released, which allows the peptide-binding cleft of MHC class II to open and bind further antigenic peptides. The MHC class II molecules with bound antigenic peptides are then exported to the surface of the antigen-presenting cell for presentation of foreign peptides to CD4+ T cells[23,24]. Meanwhile, CLIP peptide is degraded by proteasomes, and newly synthesized CD74 is then generated.
Absence of CD74 results aberrant MHC class II-dependent antigen processing and perturbs host defenses. Deficiency of CD74 in mice is associated with aberrant MHC class II synthesis[25], delayed MHC class II presentation by antigen-presenting cells[26], and impaired maturation of CD4+ T cells[27]. However, knockout of CD74 in mice was able to mount an efficient response against viral infection[28]. Although how this efficient response for viral infection could work required further elucidated, an event that the function of CD4+ TH2 cells in CD74-null mice is compromised by CD4+ TH1 cells could in part explain the current observation[29]. In addition, this compromise emphasizes the crucial role of CD74 in immune regulation. An alternative strategy would be to specifically inhibit the antigen presentation mechanism and allow the pathogen to co-exist with the host during the initial phase of pathogen entry without promoting an immune reaction. This approach would be based on the observation that blockade of CD74 reduces migration inhibitory factor (MIF)-dependent monocyte arrest, chemokine expression, and neutrophil recruitment[30]. In other words, although initial inflammatory mediators are required for recruitment of neutrophils and to resolve infection-induced innate immunity, an over-robust response would generate excessive inflammatory mediators and trigger a hypersensitivity response, which could ultimately cause tissue damage and pathology. As further support of the hypothesis that attenuating CD74 function may be of benefit in some cases, mice that lack CD74 are known to be protected against bacterial infection[31]. However, a fine balance must be struck between modulating the host immune response and preventing the deleterious effects of pathogen exposure. All of these observations have generated considerable interest in CD74, as they suggest that the protein-binding ability of MHC class II molecules could be enhanced by modifying the expression and function of endoplasmic reticulum CD74. Figure 2 illustrates the canonical antigen presentation function of CD74 in the immune system.
New functions and novel interactions associated with the evolutionarily conserved CD74 protein are continually being revealed. Rare single-nucleotide polymorphisms (SNPs) in the CD74 gene have been reported, but SNPs in molecules that interact with CD74, such as MIF[9], CD44[32] and MHC class II[15] are more frequent and are associated with the development of cancer[33-37]. The imbalance in the regulation of inflammation that occurs in many cancers can induce cellular damage. This stimulates interaction between immune cells and the damaged cells, which then proliferate, invade, and subsequently develop into tumors[38]. Together with its role in several immunological processes, these findings indicate that CD74 is a potential therapeutic target.
A decade ago, CD74 was reported as an accessory signaling molecule in cancers because of its localization on the plasma membrane in certain cell types, and its role as a surface-binding receptor for MIF, a pro-inflammatory cytokine[9]. Indeed, it is now generally accepted that the oncogenic role of CD74 is MIF-dependent. In B cells, MIF induces NF-κB activation, cell proliferation, and survival[39]. MIF also induces upregulation of anti-apoptotic proteins Bcl-2 and Bcl-XL[14]. These findings suggest that CD74 stimulation initiates a pro-survival signal. Genomic and immunohistochemical studies have revealed upregulation of CD74 in various cancers, suggesting that it may have some relationship with tumorigenesis. Table 1 summarizes the current information regarding expression and clinical significance of CD74 in human cancers. One interpretation of these observations is that persistent overexpression of CD74 in the intracellular space and on the cell surface could impair MHC class II antigen presentation by tumor cells, thereby contributing to immune escape and facilitating tumor metastasis[40]. The underlying reasons for CD74 overexpression in cancer have remained largely unclear. However, the CD74 locus is a common insertion site for viruses in murine B lymphomas[1]; by inference, similar virus-mediated upregulation may occur in human tumors.
| Cancer type | Event | Method | Ref |
| Renal cell cancer | CD74 was detected in 53 of 60 (88.3%) renal cell cancer tissues | IHC | [90, 91] |
| CD74 is a useful diagnostic marker for distinguishing clear cell RCC from chromophobe and oncocytoma RCC | IHC | [92] | |
| CD74 was upregulated in 34 of 40 (85.0%) of clear cell RCC tissues compared with the corresponding normal kidney tissues, and the expression level was positively correlated with VEGF-D (Pearson’s correlation, r = 0.65, P < 0.001) | Quantitative real-time RT-PCR, IHC | [49] | |
| Malignant fibrous histiocytoma Thymic epithelial neoplasm | Differential expression of CD74 was found in atypical malignant fibrous histiocytoma (90% positive) and fibroxanthoma (10% positive), suggesting that CD74 may be a marker of tumor progression | IHC | [93] |
| CD74 was detected in 88% (15/17) of thymic carcinomas, 70% (14/20) of invasive thymomas, but only 33% (9/27) of benign thymomas (9/27), suggesting that CD74 is a useful marker for the classification of thymic epithelial neoplasms | IHC | [94] | |
| Colorectal cancer | A linear increase of CD74 expression was found in the progression from low- to high-grade invasive cancer tissues | IHC | [95] |
| High levels of CD74 were detected in 23 of 156 (15.0%) curatively resected colorectal cancer tissues | IHC | [96] | |
| CD74 was increased in dysplastic epithelial cells in 47 of 55 (85%) human colorectal adenomas, with CD74 and MIF protein levels together predicting increasing dysplasia in individual adenomas (P = 0.003) | IHC | [97] | |
| Gastric cancer | CD74 was detected in 48 of 126 (38.1%) gastric cancer tissues, and the expression was negatively correlated with the depth of invasion and HLA-DR expression. The patients with detectable CD74 show poor surgical outcomes (P < 0.05) CD74 was detected in 39 of 58 (67.2%) gastric carcinoma tissues, showing significant correlation with the differentiation of gastric carcinoma (P < 0.05) | IHC IHC | [98] [99] |
| Breast cancer | The expression of CD74 was significantly more abundant in invasive or metastatic tumors than in ductal carcinoma in situ (P = 0.02 and P = 0.05, respectively) | SAGE | [100] |
| CD74 was detected in 468 of 580 (80.7%) breast cancer tissues, and was related to lymph node metastasis and triple-negative breast cancer (P = 0.01 and 0.001). In addition, CD74 expression had a linear correlation with lymph node metastasis and triple-negative breast cancer (P = 0.02 and 0.001) | IHC | [101] | |
| Stat1 and CD74 overexpression is co-dependent and linked to increased invasion and lymph node metastasis in triple-negative breast cancer | LC-MS/MS, IHC | [102] | |
| CD74 expression was increased in high-grade, invasive urothelial carcinoma of the bladder | [103] | ||
| Multiple myeloma | CD74 was detected in 19 of 22 (86.4%) multiple myeloma tissues | IHC | [69] |
| Pancreatic cancer | CD74 was identified as an overexpressed gene when compared with two SAGE libraries (6 pancreatic cancers vs 11 non-neoplastic tissues), and the expression of CD74 was detected in 15 of 18 (83%) pancreatic ductal adenocarcinoma tissues | SAGE, IHC | [104] |
| CD74 was expressed in 52 of 67 (77.6%) pancreatic cancer tissues that was correlated with high perineural invasion (P < 0.008) | IHC | [105] | |
| Moreover, 47 of 68 (69.1%) and 21 of 68 (30.9%) pancreas tissues from patients receiving curative extended resection showed lower (< 70%) and higher (≥ 70%) CD74 expression, respectively. Patients with higher CD74 expression in pancreatic cancer tissues showed a higher rate of lymphatic permeation (P = 0.04), perineural invasion (P = 0.01), poor prognosis (P = 0.006), and poor survival (P = 0.003) compared with those with lower expression | IHC | [106] | |
| Fourteen of 46 (30.4%) and 32 of 46 (69.6%) pancreatic ductal adenocarcinoma tissues showed lower (< 25%) and higher (≥ 25%) CD74 expression, respectively. Patients with higher CD74 expression in pancreatic cancer tissues showed a higher rate of perineural invasion (P = 0.007) and poor 3- and 5-yr cumulative survival rates (41% and 62% vs 0% and 9%, P = 0.000) compared with those with lower expression | IHC | [107] | |
| Cervical squamous cell carcinoma | CD74 expression was significantly higher in CIN than in the normal samples and higher in SCC than in CIN | IHC | [108] |
| Urothelial carcinoma of the bladder | CD74 was detected in 192 of 342 (56.1%) urothelial carcinoma of the bladder tissues, which is associated with older age at diagnosis (≥ 68 yr, P = 0.048), high World Health Organization grade (P = 0.019), advanced stages (P = 0.001), non-papillary growth pattern (P = 0.040), the absence of tumor-infiltrating inflammatory cells (P < 0.001), and the presence of tumor-associated inflammatory cells (P = 0.017). However, CD74 expression was not related to recurrence-free and overall survivals in primary and subgroup analyses | IHC | [103] |
| Non-small cell lung cancer | A case report found a mutation in CD74-ROS1 that is associated with acquired resistance to crizotinib. | FISH, RT-PCR | [109] |
| CD74 was detected in 57 of 70 (81.4%) non-small cell lung cancer tissues | IHC | [110] | |
| CD74-ROS1 fusion transcript was detected in 5 of 1073 (0.5%) non-small cell lung cancer tissues | RT-PCR | [61] | |
| CD74-ROS1 fusion transcript was detected in 4 of 556 (0.7%) non–small cell lung cancer tissues | IHC | [111] | |
| CD74-ROS1 fusion transcript was detected in 1 of 114 (0.9%) non–small cell lung cancer tissues | RT-PCR | [56] | |
| CD74-ROS1 fusion transcript was detected in 2 of 208 (1.0%) never-smokers with lung adenocarcinoma tissues | RT-PCR | [112] | |
| CD74-ROS1 fusion transcript was detected in 2 of 447 (4.5%) never-smokers with lung adenocarcinoma tissues | Transcriptome sequencing | [113] | |
| Two CD74 polymorphisms, rs2748249 and rs1560661, are associated with hematologic toxicity in patients with non–small cell lung cancer after platinum-based chemotherapy | BeadChip | [114] |
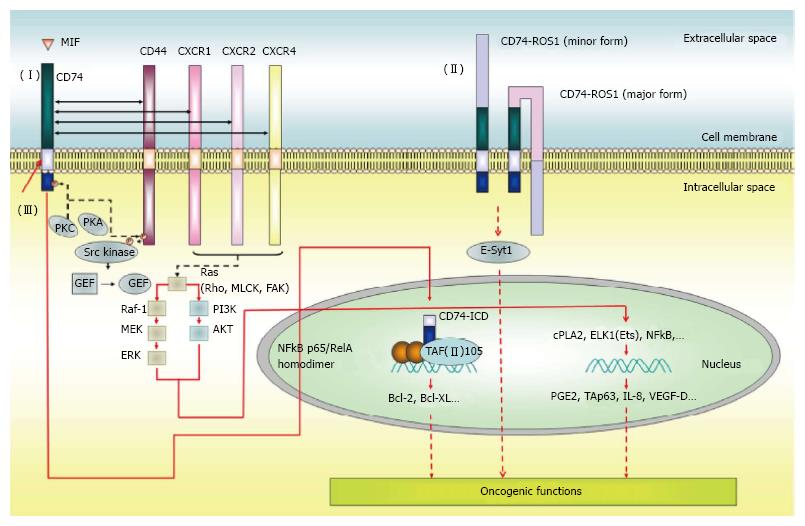
MIF is a multifunctional cytokine that is produced by several cell types, including epithelial cells and cells that participate in the innate and adaptive immune responses[41-43]. CD74 is a receptor for extracellular MIF that is expressed in human B cells[14], gastric epithelial cells[44] and type II alveolar epithelial cells[45]. Following MIF binding, CD74 is rapidly internalized, leading to downstream signaling cascades that trigger NF-κB activation[39], prostaglandin E2 production[9,46], TAp63 upregulation[47], and secretion of survival factors such as IL-8[48] and VEGF-D[49] via phosphorylation of ERK[9,50] and AKT[51]. The signaling cascades trigger cell proliferation and migration, and prevent apoptosis[14,49]. Overexpression of CD74 in HEK293 cells initials MIF-dependent MEK/ERK and PI3K/AKT activation. This is followed by NF-κB activation, which in turn triggers VEGF-D upregulation and VEGF-D-dependent cell proliferation and motility. The ultimate consequence is an increase in tumor mass, tumor-induced angiogenesis, and metastasis in xenograft-bearing mice[49].
However, unlike other ligand-receptor axes, such as EGF/EGFR[52] and VEGF-A/VEGFR2[53], CD74 lacks intracellular signaling motifs for transducing downstream signals. Therefore, it must recruit other molecules in order to transduce signals in response to MIF stimulation. Indeed, the intracytoplasmic signaling domain of CD44, a transmembrane protein with kinase-activating properties, can relay signaling downstream of the MIF-CD74 interaction[32]. CD74 forms a complex with CD44, which leads to PKA-dependent serine phosphorylation and Src activation; this eventually leads to p53 dephosphorylation, thereby stimulating cell proliferation and preventing apoptosis[32]. Another transduction mechanism involves the functional interaction between CD74 and CXCR chemokine receptors during CD74-dependent cancer cell proliferation and invasion[30,49,54]. There are also reports of fusions between CD74 and the oncogenic receptor tyrosine kinase, ROS1; the resultant fusion protein activates a novel invasiveness pathway through the phosphorylation of the extended synaptotagmin-like protein, E-Syt1, in non-small cell lung cancer[55-61]. Oncogenic CD74-ROS1 represents a tumor-specific target for drug therapy, against which next-generation kinase inhibitors can be developed. Whether CD74-ROS1 (or indeed, as yet unidentified CD74 fusion proteins) has additional substrates, and whether other coreceptors participate in CD74-dependent transformation, are important unresolved questions.
Most of the RIP-processed transcription factors are synthesized and maintained as inactive membrane-associated precursors that are activated after internal or environmental cues. Such stimuli include protease cleavage, which leads to release of intracellular fragments that translocate into the nucleus and drive transcription. This is exemplified by the functional interaction of CD74 with epithelial growth factor receptor (EGFR)[62]. The Leu-Leu-Leu intramembrane proteases (I-CLIPs) cleavage site within the transmembrane domain is essential for the cleavage of CD74, as mutation of these residues abolishes the release of intracellular domain (ICD) of CD74[14]. This cleavage occurs upon treatment with an activating anti-CD74 antibody, thereby liberating the CD74-ICD from the cell membrane into the cytoplasm. Following nuclear translocation, the CD74-ICD leads to the activation of the NF-κB p65/RelA homodimer and its coactivator, TAFII105, in CD74-overexpressed HEK293 cells and in mouse B lymphocytes[14,39,63-67]. Subsequently, the signaling cascade is attenuated by ubiquitin-dependent proteasomal degradation of CD74-ICD[67]. Figure 3 illustrates the function of CD74 in cancer development.
The high expression of CD74 in cancer cells in comparison with their normal counterparts provides a potential cancer-selective antitumor strategy. As mentioned above, oncogenic CD74-ROS1 represents a potential tumor-specific target against which next-generation kinase inhibitors might be developed. However, whether additional substrates co-exist with CD74-ROS1 or other unidentified CD74 fusion proteins, and whether other coreceptors participate in CD74-dependent transformation remains to be determined.
A monoclonal antibody, LL1, which binds to and rapidly internalizes cell surface CD74 into lysosomes[68], increases the survival of mice bearing xenografts[69]. Recent studies have also highlighted the efficacy of a humanized anti-CD74 monoclonal antibody derived from LL1, named milatuzumab, in the treatment of lymphoid malignancies[70,71], non-Hodgkin lymphoma[72], chronic lymphocytic leukemia[73], and mantle cell lymphoma[74]. A phase I multicenter, dose-escalation trial of monotherapy with milatuzumab in advanced multiple myeloma has been evaluated[75]. In addition, because the CD74 antibody enters lysosomes rapidly and at high concentration, it could be conjugated to a drug, and then used to target tumors expressing cell surface CD74. Successful preclinical examples include antibodies conjugated with radioisotopes[76,77] and doxorubicin[78,79], as well as combined therapy using milatuzumab and FTY720, a CD74 stimulator[80]. However, further selectivity must be developed, since such antibodies could potentially bind to all antigen-presenting cells.
Targeted therapy using small molecules is another developing field. Some small molecules have demonstrated activity against other proteins that associate with CD74, and can thus indirectly block CD74 function[30,81]. Examples are MIF activity modifiers, which prevent MIF binding to CD74. For instance, Ibudilast, a phosphodiesterase inhibitor, blocks MIF activity followed by inhibited chemotactic activity of peripheral blood mononuclear cells[82]. (S, R)-3(4-hydroxyphenyl)-4, 5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1) and 4-iodo-6-phenylpyrimidine (4-IPP) function as tautomerase inhibitors that also abolish MIF activity[83]. Ebselen disrupts the formation of MIF trimmers, thereby inactivating the complex[84]. A second set of examples is the cathepsin S inhibitors that prevent antigen presentation and disease progression through inhibiting CD74 degradation. The accumulated CD74 binds to MHC class II molecules within endocytic compartments, which are targets for treatment of autoimmune diseases using molecules such as Clik60[85], LHVS[86], and SB-331750[87], and RWJ-445380. Finally, there are CD74 expression modifiers such as Auraptene that suppresses CD74 expression and thus blocks Helicobacter pylori adhesion and pro-inflammatory mediator production in C57BL/6 mice[88,89]. However, whether these small molecules will have anti-cancer activity remains to be determined. More specific targeted approaches will emerge from the ongoing screening efforts to find compounds that directly target CD74. Combined with an effective method to deliver the targeting agents efficiently to the tumor, this would be a critical breakthrough for the field.
Recent advances in our knowledge of CD74 functions have emerged through discovery of its natural ligand, additional interacting proteins, and elucidation of molecular mechanisms associated with CD74 signaling in immunity and cancer. Normal expression in antigen-presenting cells maintain proper MHC class II-restricted antigen presentation and an appropriate immune regulation. However, aberrant expression of CD74 in cells leads to an unbalanced immune system, and possibly also oncogenesis, in a MIF-dependent manner. Despite the natural protective actions of CD74 in the immune system, functional studies from several CD74-focused experimental models show that CD74 inhibition will also likely halt cancer progression and improve patient prognosis. There are ongoing clinical studies into the role of CD74 in diverse diseases, including various types of cancer. Further research into CD74 and its effect on cellular processes, including the complex interactions between CD74 and its binding partners, will undoubtedly translate into clinical benefit for patients.
P- Reviewer: Chowdhury P, Shah A S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Pyrz M, Wang B, Wabl M, Pedersen FS. A retroviral mutagenesis screen identifies Cd74 as a common insertion site in murine B-lymphomas and reveals the existence of a novel IFNgamma-inducible Cd74 isoform. Mol Cancer. 2010;9:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Long EO, Strubin M, Wake CT, Gross N, Carrel S, Goodfellow P, Accolla RS, Mach B. Isolation of cDNA clones for the p33 invariant chain associated with HLA-DR antigens. Proc Natl Acad Sci USA. 1983;80:5714-5718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Claesson L, Larhammar D, Rask L, Peterson PA. cDNA clone for the human invariant gamma chain of class II histocompatibility antigens and its implications for the protein structure. Proc Natl Acad Sci USA. 1983;80:7395-7399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Strubin M, Mach B, Long EO. The complete sequence of the mRNA for the HLA-DR-associated invariant chain reveals a polypeptide with an unusual transmembrane polarity. EMBO J. 1984;3:869-872. [PubMed] |
| 5. | Arunachalam B, Lamb CA, Cresswell P. Transport properties of free and MHC class II-associated oligomers containing different isoforms of human invariant chain. Int Immunol. 1994;6:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Warmerdam PA, Long EO, Roche PA. Isoforms of the invariant chain regulate transport of MHC class II molecules to antigen processing compartments. J Cell Biol. 1996;133:281-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Koch N, Lauer W, Habicht J, Dobberstein B. Primary structure of the gene for the murine Ia antigen-associated invariant chains (Ii). An alternatively spliced exon encodes a cysteine-rich domain highly homologous to a repetitive sequence of thyroglobulin. EMBO J. 1987;6:1677-1683. [PubMed] |
| 8. | Fineschi B, Arneson LS, Naujokas MF, Miller J. Proteolysis of major histocompatibility complex class II-associated invariant chain is regulated by the alternatively spliced gene product, p41. Proc Natl Acad Sci USA. 1995;92:10257-10261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 769] [Cited by in RCA: 884] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 10. | Anderson HA, Roche PA. Phosphorylation regulates the delivery of MHC class II invariant chain complexes to antigen processing compartments. J Immunol. 1998;160:4850-4858. [PubMed] |
| 11. | Hofmann MW, Höning S, Rodionov D, Dobberstein B, von Figura K, Bakke O. The leucine-based sorting motifs in the cytoplasmic domain of the invariant chain are recognized by the clathrin adaptors AP1 and AP2 and their medium chains. J Biol Chem. 1999;274:36153-36158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Motta A, Bremnes B, Morelli MA, Frank RW, Saviano G, Bakke O. Structure-activity relationship of the leucine-based sorting motifs in the cytosolic tail of the major histocompatibility complex-associated invariant chain. J Biol Chem. 1995;270:27165-27171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Schutze MP, Peterson PA, Jackson MR. An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO J. 1994;13:1696-1705. [PubMed] |
| 14. | Starlets D, Gore Y, Binsky I, Haran M, Harpaz N, Shvidel L, Becker-Herman S, Berrebi A, Shachar I. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107:4807-4816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Ericson ML, Sundström M, Sansom DM, Charron DJ. Mutually exclusive binding of peptide and invariant chain to major histocompatibility complex class II antigens. J Biol Chem. 1994;269:26531-26538. [PubMed] |
| 16. | Roche PA, Cresswell P. Proteolysis of the class II-associated invariant chain generates a peptide binding site in intracellular HLA-DR molecules. Proc Natl Acad Sci USA. 1991;88:3150-3154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 161] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Ghosh P, Amaya M, Mellins E, Wiley DC. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 453] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 18. | Bijlmakers MJ, Benaroch P, Ploegh HL. Mapping functional regions in the lumenal domain of the class II-associated invariant chain. J Exp Med. 1994;180:623-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Ou WJ, Cameron PH, Thomas DY, Bergeron JJ. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993;364:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 437] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 20. | Sant AJ, Schwartz BD, Cullen SE. Cellular distribution of the Ia-associated chondroitin sulfate proteoglycan. J Immunol. 1985;135:408-415. [PubMed] |
| 21. | Sant AJ, Cullen SE, Giacoletto KS, Schwartz BD. Invariant chain is the core protein of the Ia-associated chondroitin sulfate proteoglycan. J Exp Med. 1985;162:1916-1934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Bertolino P, Rabourdin-Combe C. The MHC class II-associated invariant chain: a molecule with multiple roles in MHC class II biosynthesis and antigen presentation to CD4+ T cells. Crit Rev Immunol. 1996;16:359-379. [PubMed] |
| 23. | Jensen PE, Weber DA, Thayer WP, Westerman LE, Dao CT. Peptide exchange in MHC molecules. Immunol Rev. 1999;172:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Busch R, Rinderknecht CH, Roh S, Lee AW, Harding JJ, Burster T, Hornell TM, Mellins ED. Achieving stability through editing and chaperoning: regulation of MHC class II peptide binding and expression. Immunol Rev. 2005;207:242-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Bikoff EK, Huang LY, Episkopou V, van Meerwijk J, Germain RN, Robertson EJ. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med. 1993;177:1699-1712. [PubMed] |
| 26. | Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Mice lacking the MHC class II-associated invariant chain. Cell. 1993;72:635-648. [PubMed] |
| 27. | Wong P, Rudensky AY. Phenotype and function of CD4+ T cells in mice lacking invariant chain. J Immunol. 1996;156:2133-2142. [PubMed] |
| 28. | Battegay M, Bachmann MF, Burhkart C, Viville S, Benoist C, Mathis D, Hengartner H, Zinkernagel RM. Antiviral immune responses of mice lacking MHC class II or its associated invariant chain. Cell Immunol. 1996;167:115-121. [PubMed] |
| 29. | Topilski I, Harmelin A, Flavell RA, Levo Y, Shachar I. Preferential Th1 immune response in invariant chain-deficient mice. J Immunol. 2002;168:1610-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Schwartz V, Lue H, Kraemer S, Korbiel J, Krohn R, Ohl K, Bucala R, Weber C, Bernhagen J. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett. 2009;583:2749-2757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Zaidi T, Reidy T, D’Ortona S, Fichorova R, Pier G, Gadjeva M. CD74 deficiency ameliorates Pseudomonas aeruginosa-induced ocular infection. Sci Rep. 2011;1:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 523] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 33. | Yuan Q, Wang M, Wang M, Zhang Z, Zhang W. Macrophage migration inhibitory factor gene -173G& gt; C polymorphism and risk of bladder cancer in southeast China: a case-control analysis. Mol Biol Rep. 2012;39:3109-3115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Li H, Zang J, Wang P, Dai L, Zhang J, Wang K. Gastric cancer susceptibility in gastric cancer relatives: attributable risks of Macrophage migration inhibitory factor promoter polymorphism and Helicobacter pylori. Cytokine. 2012;60:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Wu S, Lian J, Tao H, Shang H, Zhang L. Correlation of macrophage migration inhibitory factor gene polymorphism with the risk of early-stage cervical cancer and lymphatic metastasis. Oncol Lett. 2011;2:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Vera PL, Meyer-Siegler KL. Association between macrophage migration inhibitory factor promoter region polymorphism (-173 G/C) and cancer: a meta-analysis. BMC Res Notes. 2011;4:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Jiang L, Deng J, Zhu X, Zheng J, You Y, Li N, Wu H, Lu J, Zhou Y. CD44 rs13347 C& gt; T polymorphism predicts breast cancer risk and prognosis in Chinese populations. Breast Cancer Res. 2012;14:R105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Cordon-Cardo CPrives C. At the crossroads of inflammation and tumorigenesis. J Exp Med. 1999;190:1367-1370. [PubMed] |
| 39. | Matza D, Wolstein O, Dikstein RShachar I. Invariant chain induces B cell maturation by activating a TAF(II)105-NF-kappaB-dependent transcription program. J Biol Chem. 2001;276:27203-27206. [RCA] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Xu M, Qiu G, Jiang Z, von Hofe E, Humphreys RE. Genetic modulation of tumor antigen presentation. Trends Biotechnol. 2000;18:167-172. [PubMed] [DOI] [Full Text] |
| 41. | Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1144] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 42. | Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895-1902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 788] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 43. | Bacher M, Meinhardt A, Lan HY, Mu W, Metz CN, Chesney JA, Calandra T, Gemsa D, Donnelly T, Atkins RC. Migration inhibitory factor expression in experimentally induced endotoxemia. Am J Pathol. 1997;150:235-246. [PubMed] |
| 44. | Beswick EJ, Pinchuk IV, Suarez G, Sierra JC, Reyes VE. Helicobacter pylori CagA-dependent macrophage migration inhibitory factor produced by gastric epithelial cells binds to CD74 and stimulates procarcinogenic events. J Immunol. 2006;176:6794-6801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Marsh LM, Cakarova L, Kwapiszewska G, von Wulffen W, Herold S, Seeger W, Lohmeyer J. Surface expression of CD74 by type II alveolar epithelial cells: a potential mechanism for macrophage migration inhibitory factor-induced epithelial repair. Am J Physiol Lung Cell Mol Physiol. 2009;296:L442-L452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA. 2002;99:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 482] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 47. | Lantner F, Starlets D, Gore Y, Flaishon L, Yamit-Hezi A, Dikstein R, Leng L, Bucala R, Machluf Y, Oren M. CD74 induces TAp63 expression leading to B-cell survival. Blood. 2007;110:4303-4311. [PubMed] [DOI] [Full Text] |
| 48. | Binsky I, Haran M, Starlets D, Gore Y, Lantner F, Harpaz N, Leng L, Goldenberg DM, Shvidel L, Berrebi A. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci USA. 2007;104:13408-13413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Liu YH, Lin CY, Lin WC, Tang SW, Lai MK, Lin JY. Up-regulation of vascular endothelial growth factor-D expression in clear cell renal cell carcinoma by CD74: a critical role in cancer cell tumorigenesis. J Immunol. 2008;181:6584-6594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Lue H, Kapurniotu A, Fingerle-Rowson G, Roger T, Leng L, Thiele M, Calandra T, Bucala R, Bernhagen J. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell Signal. 2006;18:688-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 51. | Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Lüscher B, Bernhagen J. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046-5059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 290] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 52. | Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1429] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 53. | Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 2039] [Article Influence: 135.9] [Reference Citation Analysis (1)] |
| 54. | Tarnowski M, Grymula K, Liu R, Tarnowska J, Drukala J, Ratajczak J, Mitchell RA, Ratajczak MZ, Kucia M. Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. Mol Cancer Res. 2010;8:1328-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 55. | Jun HJ, Johnson H, Bronson RT, de Feraudy S, White F, Charest A. The oncogenic lung cancer fusion kinase CD74-ROS activates a novel invasiveness pathway through E-Syt1 phosphorylation. Cancer Res. 2012;72:3764-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 56. | Matsuura S, Shinmura K, Kamo T, Igarashi H, Maruyama K, Tajima M, Ogawa H, Tanahashi M, Niwa H, Funai K. CD74-ROS1 fusion transcripts in resected non-small cell lung carcinoma. Oncol Rep. 2013;30:1675-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Kim MH, Shim HS, Kang DR, Jung JY, Lee CY, Kim DJ, Lee JG, Bae MK, Kim HR, Lim SM. Clinical and prognostic implications of ALK and ROS1 rearrangements in never-smokers with surgically resected lung adenocarcinoma. Lung Cancer. 2014;83:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 58. | Xu L, Zhao R, Dong Z, Zhu T. [Clinical significance of ROS1 rearrangements in non-small cell lung cancer]. Zhongguo Fei Ai Za Zhi. 2013;16:663-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Yoshida A, Tsuta K, Wakai S, Arai Y, Asamura H, Shibata T, Furuta K, Kohno T, Kushima R. Immunohistochemical detection of ROS1 is useful for identifying ROS1 rearrangements in lung cancers. Mod Pathol. 2014;27:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 60. | Stumpfova M, Jänne PA. Zeroing in on ROS1 rearrangements in non-small cell lung cancer. Clin Cancer Res. 2012;18:4222-4224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang R. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1220] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 62. | Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 818] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 63. | Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1183] [Cited by in RCA: 1381] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 64. | Matza D, Lantner F, Bogoch Y, Flaishon L, Hershkoviz R, Shachar I. Invariant chain induces B cell maturation in a process that is independent of its chaperonic activity. Proc Natl Acad Sci USA. 2002;99:3018-3023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Matza D, Kerem A, Medvedovsky H, Lantner F, Shachar I. Invariant chain-induced B cell differentiation requires intramembrane proteolytic release of the cytosolic domain. Immunity. 2002;17:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Matza D, Kerem A, Shachar I. Invariant chain, a chain of command. Trends Immunol. 2003;24:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Becker-Herman S, Arie G, Medvedovsky H, Kerem A, Shachar I. CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol Biol Cell. 2005;16:5061-5069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 68. | Hansen HJ, Ong GL, Diril H, Valdez A, Roche PA, Griffiths GL, Goldenberg DM, Mattes MJ. Internalization and catabolism of radiolabelled antibodies to the MHC class-II invariant chain by B-cell lymphomas. Biochem J. 1996;320:293-300. [PubMed] |
| 69. | Burton JD, Ely S, Reddy PK, Stein R, Gold DV, Cardillo TM, Goldenberg DM. CD74 is expressed by multiple myeloma and is a promising target for therapy. Clin Cancer Res. 2004;10:6606-6611. [PubMed] |
| 70. | Stein R, Mattes MJ, Cardillo TM, Hansen HJ, Chang CH, Burton J, Govindan S, Goldenberg DM. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res. 2007;13:5556s-5563s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 71. | Mark T, Martin P, Leonard JP, Niesvizky R. Milatuzumab: a promising new agent for the treatment of lymphoid malignancies. Expert Opin Investig Drugs. 2009;18:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Sharkey RM, Karacay H, Johnson CR, Litwin S, Rossi EA, McBride WJ, Chang CH, Goldenberg DM. Pretargeted versus directly targeted radioimmunotherapy combined with anti-CD20 antibody consolidation therapy of non-Hodgkin lymphoma. J Nucl Med. 2009;50:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Hertlein E, Triantafillou G, Sass EJ, Hessler JD, Zhang X, Jarjoura D, Lucas DM, Muthusamy N, Goldenberg DM, Lee RJ. Milatuzumab immunoliposomes induce cell death in CLL by promoting accumulation of CD74 on the surface of B cells. Blood. 2010;116:2554-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Alinari L, Yu B, Christian BA, Yan F, Shin J, Lapalombella R, Hertlein E, Lustberg ME, Quinion C, Zhang X. Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma. Blood. 2011;117:4530-4541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 75. | Kaufman JL, Niesvizky R, Stadtmauer EA, Chanan-Khan A, Siegel D, Horne H, Wegener WA, Goldenberg DM. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br J Haematol. 2013;163:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 76. | Ochakovskaya R, Osorio L, Goldenberg DM, Mattes MJ. Therapy of disseminated B-cell lymphoma xenografts in severe combined immunodeficient mice with an anti-CD74 antibody conjugated with (111)indium, (67)gallium, or (90)yttrium. Clin Cancer Res. 2001;7:1505-1510. [PubMed] |
| 77. | Michel RB, Rosario AV, Brechbiel MW, Jackson TJ, Goldenberg DM, Mattes MJ. Experimental therapy of disseminated B-Cell lymphoma xenografts with 213Bi-labeled anti-CD74. Nucl Med Biol. 2003;30:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 78. | Griffiths GL, Mattes MJ, Stein R, Govindan SV, Horak ID, Hansen HJ, Goldenberg DM. Cure of SCID mice bearing human B-lymphoma xenografts by an anti-CD74 antibody-anthracycline drug conjugate. Clin Cancer Res. 2003;9:6567-6571. [PubMed] |
| 79. | Sapra P, Stein R, Pickett J, Qu Z, Govindan SV, Cardillo TM, Hansen HJ, Horak ID, Griffiths GL, Goldenberg DM. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin Cancer Res. 2005;11:5257-5264. [PubMed] |
| 80. | Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, Mani R, Mao Y, Yu B, Quinion C. FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood. 2011;118:6893-6903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, Bucala R, Cappello M, Gross M, Gaeta F. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci USA. 2010;107:11313-11318. [PubMed] |
| 82. | Saito T, Tomita Y, Kimura M, Nishiyama T, Sato S. [Expression of HLA class II antigen-associated invariant chain on renal cell cancer]. Nihon Hinyokika Gakkai Zasshi. 1993;84:1036-1040. |
| 83. | Winner M, Meier J, Zierow S, Rendon BE, Crichlow GV, Riggs R, Bucala R, Leng L, Smith N, Lolis E. A novel, macrophage migration inhibitory factor suicide substrate inhibits motility and growth of lung cancer cells. Cancer Res. 2008;68:7253-7257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 84. | Ouertatani-Sakouhi H, El-Turk F, Fauvet B, Cho MK, Pinar Karpinar D, Le Roy D, Dewor M, Roger T, Bernhagen J, Calandra T. Identification and characterization of novel classes of macrophage migration inhibitory factor (MIF) inhibitors with distinct mechanisms of action. J Biol Chem. 2010;285:26581-26598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 85. | Saegusa K, Ishimaru N, Yanagi K, Arakaki R, Ogawa K, Saito I, Katunuma N, Hayashi Y. Cathepsin S inhibitor prevents autoantigen presentation and autoimmunity. J Clin Invest. 2002;110:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Riese RJ, Mitchell RN, Villadangos JA, Shi GP, Palmer JT, Karp ER, De Sanctis GT, Ploegh HL, Chapman HA. Cathepsin S activity regulates antigen presentation and immunity. J Clin Invest. 1998;101:2351-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 242] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 87. | Podolin PL, Bolognese BJ, Carpenter DC, Davis TG, Johanson RA, Fox JH, Long E, Dong X, Marquis RW, Locastro SM. Inhibition of invariant chain processing, antigen-induced proliferative responses, and the development of collagen-induced arthritis and experimental autoimmune encephalomyelitis by a small molecule cysteine protease inhibitor. J Immunol. 2008;180:7989-8003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Sekiguchi H, Takabayashi F, Irie K, Murakami A. Auraptene attenuates gastritis via reduction of Helicobacter pylori colonization and pro-inflammatory mediator production in C57BL/6 mice. J Med Food. 2012;15:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 89. | Sekiguchi H, Irie K, Murakami A. Suppression of CD74 expression and Helicobacter pylori adhesion by auraptene targeting serum starvation-activated ERK1/2 in NCI-N87 gastric carcinoma cells. Biosci Biotechnol Biochem. 2010;74:1018-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Saito T, Tomita Y, Kimura M, Nishiyama T, Sato S. [Expression of HLA class II antigen-associated invariant chain on renal cell cancer]. Nihon Hinyokika Gakkai Zasshi. 1993;84:1036-1040. [PubMed] |
| 91. | Saito T, Kimura M, Kawasaki T, Sato S, Tomita Y. MHC class II antigen-associated invariant chain on renal cell cancer may contribute to the anti-tumor immune response of the host. Cancer Lett. 1997;115:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 92. | Young AN, Amin MB, Moreno CS, Lim SD, Cohen C, Petros JA, Marshall FF, Neish AS. Expression profiling of renal epithelial neoplasms: a method for tumor classification and discovery of diagnostic molecular markers. Am J Pathol. 2001;158:1639-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 93. | Lazova R, Moynes R, May D, Scott G. LN-2 (CD74). A marker to distinguish atypical fibroxanthoma from malignant fibrous histiocytoma. Cancer. 1997;79:2115-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 94. | Datta MW, Shahsafaei A, Nadler LM, Freeman GJ, Dorfman DM. Expression of MHC class II-associated invariant chain (Ii; CD74) in thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol. 2000;8:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Jiang Z, Xu M, Savas L, LeClair P, Banner BF. Invariant chain expression in colon neoplasms. Virchows Arch. 1999;435:32-36. [PubMed] |
| 96. | Rossi HA, Liu Q, Banner B, Hsieh CC, Savas L, Savarese D. The prognostic value of invariant chain (Ii) and Her-2/neu expression in curatively resected colorectal cancer. Cancer J. 2002;8:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | Cuthbert RJ, Wilson JM, Scott N, Coletta PL, Hull MA. Differential CD74 (major histocompatibility complex Class II invariant chain) expression in mouse and human intestinal adenomas. Eur J Cancer. 2009;45:1654-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Iwashige H, Aridome K, Hokita S, Aikou T. Invariant chain expression in gastric cancer. Cancer Lett. 2001;168:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 99. | Tamori Y, Tan X, Nakagawa K, Takai E, Akagi J, Kageshita T, Egami H, Ogawa M. Clinical significance of MHC class II-associated invariant chain expression in human gastric carcinoma. Oncol Rep. 2005;14:873-877. [PubMed] |
| 100. | Porter D, Lahti-Domenici J, Keshaviah A, Bae YK, Argani P, Marks J, Richardson A, Cooper A, Strausberg R, Riggins GJ. Molecular markers in ductal carcinoma in situ of the breast. Mol Cancer Res. 2003;1:362-375. [PubMed] |
| 101. | Tian B, Zhang Y, Li N, Liu X, Dong J. CD74: a potential novel target for triple-negative breast cancer. Tumour Biol. 2012;33:2273-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Greenwood C, Metodieva G, Al-Janabi K, Lausen B, Alldridge L, Leng L, Bucala R, Fernandez N, Metodiev MV. Stat1 and CD74 overexpression is co-dependent and linked to increased invasion and lymph node metastasis in triple-negative breast cancer. J Proteomics. 2012;75:3031-3040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 103. | Choi JW, Kim Y, Lee JH, Kim YS. CD74 expression is increased in high-grade, invasive urothelial carcinoma of the bladder. Int J Urol. 2013;20:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Hustinx SR, Cao D, Maitra A, Sato N, Martin ST, Sudhir D, Iacobuzio-Donahue C, Cameron JL, Yeo CJ, Kern SE. Differentially expressed genes in pancreatic ductal adenocarcinomas identified through serial analysis of gene expression. Cancer Biol Ther. 2004;3:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 105. | Koide N, Yamada T, Shibata R, Mori T, Fukuma M, Yamazaki K, Aiura K, Shimazu M, Hirohashi S, Nimura Y. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clin Cancer Res. 2006;12:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 106. | Nagata S, Jin YF, Yoshizato K, Tomoeda M, Song M, Iizuka N, Kitamura M, Takahashi H, Eguchi H, Ohigashi H. CD74 is a novel prognostic factor for patients with pancreatic cancer receiving multimodal therapy. Ann Surg Oncol. 2009;16:2531-2538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Zhang JF, Hua R, Liu DJ, Liu W, Huo YM, Sun YW. Effect of CD74 on the prognosis of patients with resectable pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2014;13:81-86. [PubMed] |
| 108. | Cheng RJ, Deng WG, Niu CB, Li YY, Fu Y. Expression of macrophage migration inhibitory factor and CD74 in cervical squamous cell carcinoma. Int J Gynecol Cancer. 2011;21:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 109. | Awad MM, Katayama R, McTigue M, Liu W, Deng YL, Brooun A, Friboulet L, Huang D, Falk MD, Timofeevski S. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;368:2395-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 309] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 110. | McClelland M, Zhao L, Carskadon SArenberg D. Expression of CD74, the receptor for macrophage migration inhibitory factor, in non-small cell lung cancer. Am J Pathol. 2009;174:638-646. [PubMed] |
| 111. | Rimkunas VM, Crosby KE, Li D, Hu Y, Kelly ME, Gu TL, Mack JS, Silver MR, Zhou X, Haack H. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res. 2012;18:4449-4457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 112. | Kim HR, Lim SM, Kim HJ, Hwang SK, Park JK, Shin E, Bae MK, Ou SH, Wang J, Jewell SS. The frequency and impact of ROS1 rearrangement on clinical outcomes in never smokers with lung adenocarcinoma. Ann Oncol. 2013;24:2364-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 113. | Davies KD, Le AT, Theodoro MF, Skokan MC, Aisner DL, Berge EM, Terracciano LM, Cappuzzo F, Incarbone M, Roncalli M. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18:4570-4579. [PubMed] |
| 114. | Tan X, Wu Q, Cai Y, Zhao X, Wang S, Gao Z, Yang Y, Li X, Qian J, Wang J. Novel association between CD74 polymorphisms and hematologic toxicity in patients with NSCLC after platinum-based chemotherapy. Clin Lung Cancer. 2014;15:67-78.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |