Published online Sep 19, 2020. doi: 10.5409/wjcp.v9.i2.17
Peer-review started: April 30, 2020
First decision: May 24, 2020
Revised: June 7, 2020
Accepted: August 31, 2020
Article in press: August 31, 2020
Published online: September 19, 2020
Processing time: 140 Days and 5.8 Hours
Critically ill neonates and pediatric patients commonly require multiple low flow infusions. Volume limitations are imposed by small body habitus and co-morbidities like cardiopulmonary disease, renal failure, or fluid overload. Vascular access is limited by diminutive veins. Maintenance fluids or parenteral nutrition in conjunction with actively titrated infusions such as insulin, fentanyl, prostaglandins, inotropes and vasopressors may necessitate simultaneous infusions using a single lumen to maintain vascular catheter patency. This requirement for multiple titratable infusions requires concentrated medications at low flows, rather than more dilute drugs at higher flows that in combination may volume overload small infants.
To determine whether carrier fluid reduces variability that variability of low flow drug infusions is proportional to syringe size in pediatric critical care.
We assessed concentrations of orange “drug” in a 0.2 mL/h low flow clinical model with blue dyed carrier fluid at 5 mL/h, using 3-, 10-, or 60-mL syringes. A graduated volumetric pipette was used to measure total flow. Mean time to target concentration was 30, 21, and 46 min in 3-, 10-, and 60-mL syringes, respectively (P = 0.42). After achieving target concentration, more dilute drug was delivered by 60-mL (P < 0.001) and 10-mL syringes (P = 0.04) compared to 3-mL syringes. Drug overdoses were observed during the initial 45 min of infusion in 10-and 60-mL syringes. Total volumes infused after target concentration were less in the 60-mL condition compared to 3-mL (P < 0.01) and 10-mL (P < 0.001) syringes.
Linear mixed effects models demonstrated lesser delivered drug concentrations in the initial 30 min by 3-mL compared to 10-and 60-mL syringes (P = 0.005 and P < 0.001, respectively) but greater drug concentrations and total infused drug in the subsequent 30-60 and 60-90 min intervals with the 3- and 10-mL compared to 60-mL syringes.
With carrier fluid, larger syringes were associated with significantly less drug delivery, less total volume delivered, and other flow problems in our low flow drug model. Carrier fluid should not be used to compensate for inappropriately large syringes in critical low flow drug infusions.
Core Tip: Infusions of critical drugs in infants frequently require low flow rates. We previously observed errors in low flow infusions that were directly proportional to syringe size. Because low flow infusions in clinical practice are essentially always co-infused with a primary carrier fluid, we now use a similar model to test whether carrier fluid improves accuracy and flow continuity of low flow drug from large compared to smaller syringes. We report that despite carrier fluid, larger syringes were associated with less overall drug and fluid volumes delivered, worse flow continuity, and other flow problems in low flow infusions compared to smaller syringe sizes. Carrier fluid should not be used to compensate for errors introduced by syringe size in critical low flow drug infusions. Syringe size should be matched to the rate of infusion.
- Citation: Madson ZC, Vangala S, Sund GT, Lin JA. Does carrier fluid reduce low flow drug infusion error from syringe size? World J Clin Pediatr 2020; 9(2): 17-28
- URL: https://www.wjgnet.com/2219-2808/full/v9/i2/17.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v9.i2.17
Critically ill neonates and pediatric patients commonly require multiple low flow infusions. Volume limitations are imposed by small body habitus and co-morbidities like cardiopulmonary disease, renal failure, or fluid overload. Vascular access is limited by diminutive veins. Maintenance fluids or parenteral nutrition in conjunction with actively titrated infusions such as insulin, fentanyl, prostaglandins, inotropes and vasopressors may necessitate simultaneous infusions using a single lumen to maintain vascular catheter patency. This requirement for multiple titratable infusions requires concentrated medications at low flows, rather than more dilute drugs at higher flows that in combination may volume overload small infants.
Drug flow rates may commonly reach as low as 0.1-0.2 mL/h (100-200 microliters/h) in infants and small pediatric patients[1-3]. At low rates, flow variability is proportional to syringe size[1,3-5]. Larger syringes exhibit increased friction and variable compliance of the syringe plunger tip, hindering the necessary precision to displace their plungers in short increments[1,3-5]. However, competing safety considerations encourage pharmacy equipment standardization to the largest common syringe size within a hospital. Unit dosing, prefilled syringes, pre-programmed drug libraries, and pharmacy standardization[6-12] seek to minimize equipment options and, thus, avoid errors in calculations, drug preparation and dispensing, pump programming, and drug administration.
We previously demonstrated that syringe size is directly proportional to variability of low flow infusions[1]. However, as low flow drug infusions are generally found in clinical practice only with a primary infusion fluid, it is necessary to investigate the possible benefits or harms introduced by primary fluid combined with low flow infusions. To our knowledge, the influence of carrier fluid on low flow variability associated with syringe size has not been previously investigated. One might hypothesize that carrier fluid improves syringe-associated low flow drug variability by flushing drug from tubing dead space during start-up or drug interruptions[13] and diluting concentrated drug in dead space[14]. If verified, then use of carrier fluid would allow streamlining of options using larger common syringe sizes and simplified infusion pump libraries within institutions. In contrast, we hypothesized that carrier fluid might exacerbate low flow errors via perturbations attributable to the carrier fluid delivery system. We report here the results of our study of an in vitro low flow drug and carrier fluid model.
As this study did not require patient participation or patient data, the study was granted exemption from review by the Institutional Review Board of the University of California, Los Angeles.
All medical devices and equipment used in this study were standard equipment in our pediatrics care units. All infusions were performed with a Medfusion 4000 smart pump (Baxter; SIGMA, Medina, NY, United States). Disposable sterile BD syringes (Becton-Dickinson, Franklin Lakes, NJ, United States) sized 3-, 10-, and 60-mL were used. Blue (BL) food coloring (McCormick Culinary, Santa Rosa, CA, United States) in 0.9% normal saline was used as carrier fluid and diluent for orange (OR) (Chefmaster Liqua-Gel, Fullerton, CA, United States) low flow drug. For real time spectrophotometry, absorbances of colored fluids were measured directly through clear intravenous tubing (Intensive Care Unit Medical Extension Set 60 Inch Tubing 0.4 mL Priming Volume B2020) using a Public Lab Desktop Spectrometry Kit 3.0 (Publiclab.org).
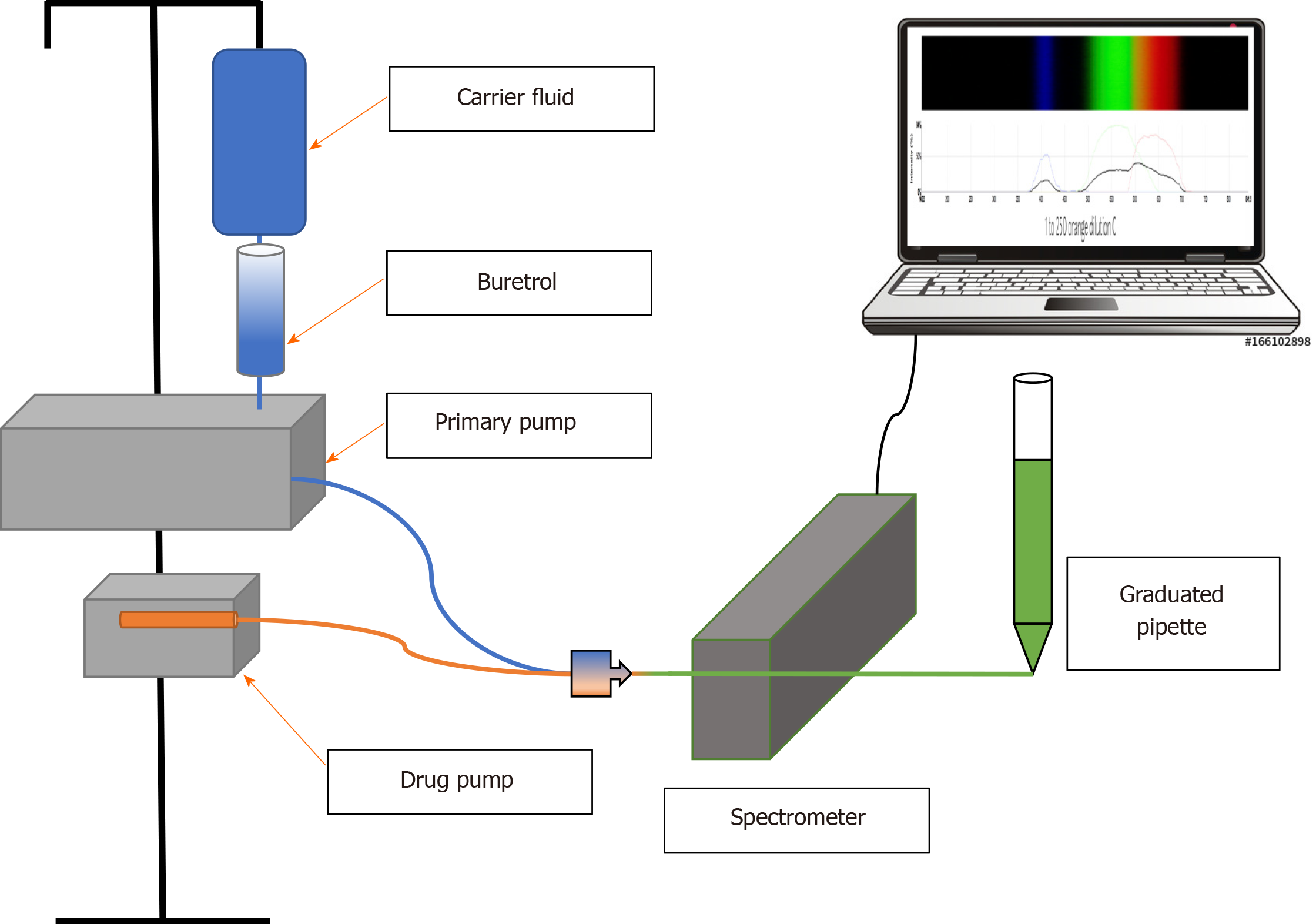
Carrier fluid was infused from a smart infusion pump (Baxter Sigma Spectrum Infusion Pump 35700BAX) via a valveless burette (Baxter Buretrol Clearlink System 2H8865) connected to an infusion tubing set with 2 Luer activated valves and a backcheck valve above the upper Y-site (Baxter Clearlink Continu-Flo UC8519). Per Food and Drug Administration recommendations[3], the lowest Y-site closest to the “patient” (in this case, the spectrometer) was used to connect a smart syringe pump (Medfusion Syringe Infusion Pump Model 4000) for simultaneous infusion using extension tubing. The length of IV tubing from the drug infusion pump to the spectrometer (Public Lab Desktop Spectrometry Kit 3.0) was set at 18.5 cm to allow complete mixing of drug and carrier fluids. Spectrometry was measured inline through the tubing. To simulate patient-side intravenous catheter resistance and perform volume measurements, the end of the tubing after the spectrometer was connected via 5 cm of extension tubing (cut from a Smiths Medical MX451FL extension set) to drain into the narrow end of a 1 mL glass pipette with 0.02 mL volumetric gradations.
Flow rate for the carrier fluid pump was set at 5 mL/h. This rate is commonly used in the neonatal clinical setting[5] and was used in our previous study[1]. Flow rate for the low flow drug was set at 0.2 mL/h for the same reasons. This rate was programmed into the smart pump as the drug model for all infusions: Epinephrine with standard neonatal drug concentration of 40 mcg/mL (our institutional practice) at a dose of 0.027 mcg/kg/min in a 5 kg infant, which yields a flow rate of 0.2 mL/h. For purposes of our experiments, we used OR food dye for “drug” diluted in BL dyed carrier fluid as our drug model. Changes in 433 nm BL λ peak transmittance were used to determine drug concentrations. Concentration curves were established in calibration studies by assessing 433 nm transmittance for 10 replicates at each concentration, averaging the results, and fitting to a power-law function. For each trial of 3-, 10-, and 60-mL syringe sizes (Becton-Dickinson Luer Lok), 5 spectrometry measurements per time point were recorded and the replicate determinations averaged. Spectrometer readings were recorded in 5 min intervals until either target concentration (determined by concentration curve studies of OR drug in BL carrier fluid) was reached and maintained or more than 90 min had elapsed. Total volume infused was measured using a 1-mL volumetric pipette with 0.02 mL gradations, which was connected directly to the end of the IV tubing for all experiments. A schematic of the experimental apparatus is diagrammed in Figure 1.
The same normal saline calibration was used for all calibrations and spectral analyses. Multiple factors that could affect spectral analysis, such as light source, ambient lighting, distance from the spectrometer, and alignment, were kept constant. Heights of the infusion pumps, spectrometer, infusion tubing, and volumetric collection pipette were kept constant.
Single factor ANOVA and Student’s t-test were used to compare continuous data where appropriate. In our initial analyses, start-up effects were censored by analyzing data after target concentration was achieved. Subsequently, we included start-up data in our linear mixed effects analysis of different time intervals during the infusions. To account for effects of different syringe sizes and time, we used linear mixed effects models of log-transformed dilution and amount of drug delivered to estimate percentage differences between syringe sizes at three 30 min time intervals, with the lesser dilution value as denominator. For infusion trials that reached and maintained target concentration before 90 min had elapsed, a series of the last recorded dilution values recorded at target concentration were repeated forward in order to make statistical comparisons with the infusions that required the full 90 min to reach target concentration. Models were also used to estimate within-infusion variances and compare these between syringe sizes. To account for excessive drug concentrations caused by flow variability of our syringe pump and carrier infusion pumps resulting in oversaturation of the spectrometer, we replaced oversaturated values with the highest detectable concentration of OR drug, which was 20-fold dilution based on calibration experiments. P values less than 0.05 were considered statistically significant.
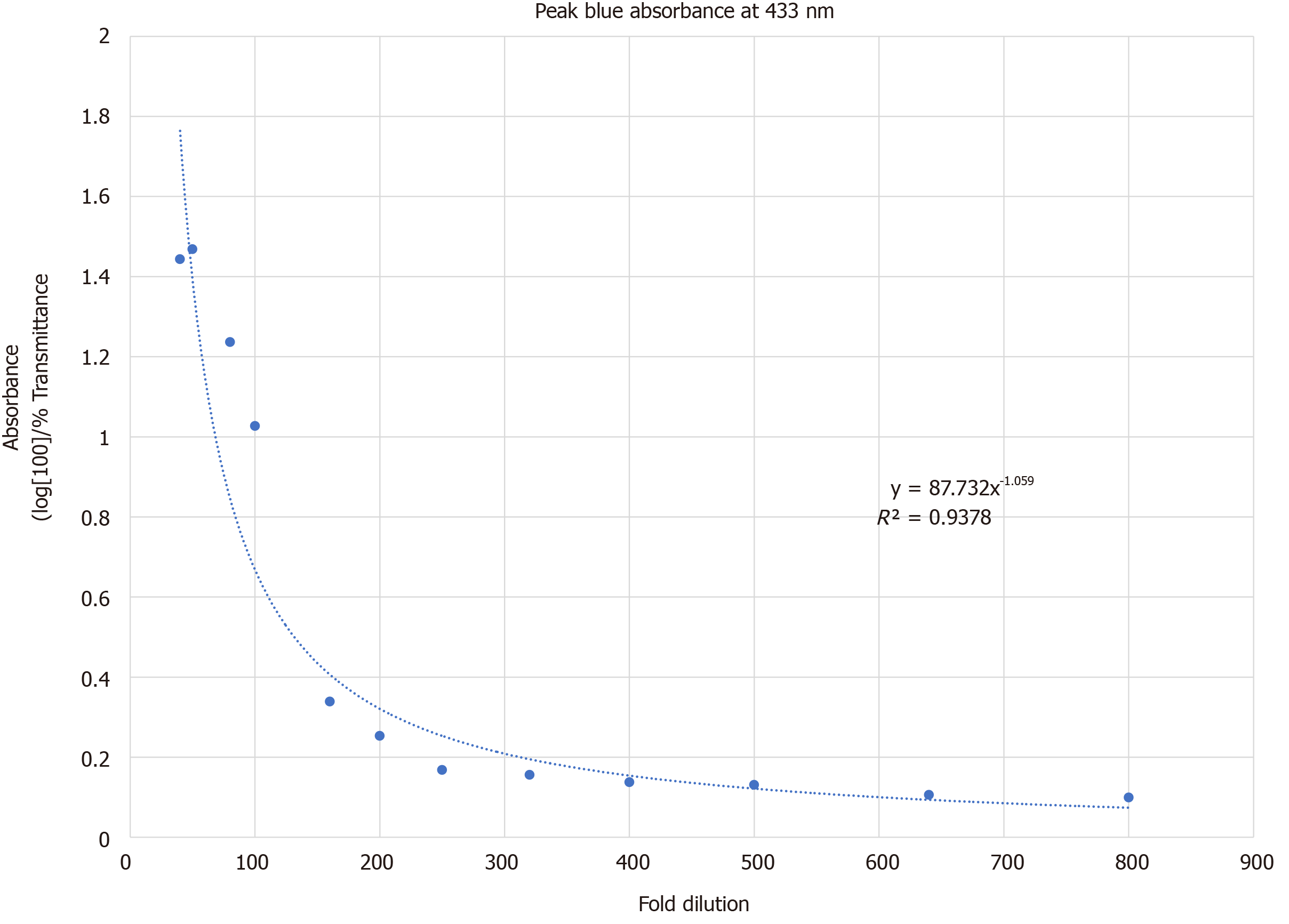
Absorbance at 433 nm was recorded at 12 different OR drug dilutions of 40-, 50-, 80-, 100-, 160-, 200-, 250-, 320-, 400-, 500-, 640-, and 800-fold. The curve was fitted to the power-law function, Absorbance433nm = 87.732 (fold-dilution)-1.059 and is demonstrated in Figure 2 (R2 = 0.9378). Based on this concentration curve and with a goal to maximize the range of measured absorbance vs fold-dilution in our experimental set-up, we set the target OR drug dilution at 100-fold in steady state flow. This translated to a flow of OR drug at 0.2 mL/h diluted by carrier flow at 5 mL/h.
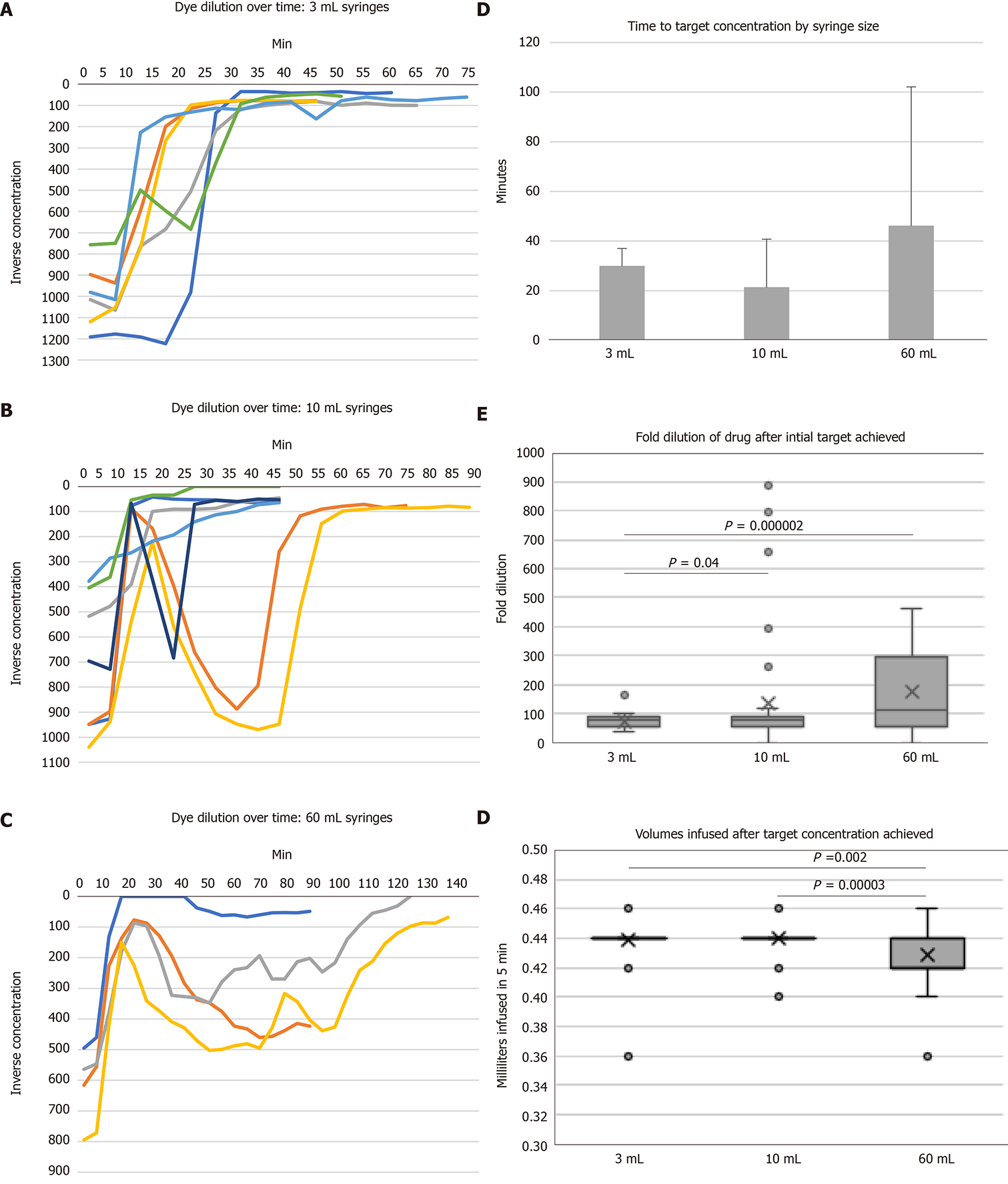
OR drug concentrations during 0.2 mL/h low flow infusions with different syringe sizes and a 5 mL/h carrier fluid are demonstrated in Figure 3A-C. One infusion failed to reach 1:100 target concentration by 90-min in the 60-mL syringe size (Figure 3C). No differences were observed in time to reach target drug concentration (Figure 3D). Times to target concentration were 30 ± 7, 21 ± 19, and 46 ± 55 min (mean ± SD, P = 0.42) for 3-, 10-, and 60-mL syringes, respectively. Only 3-mL syringes maintained target concentration after start-up (Figure 3A). Both the 10- and 60-mL syringe sizes (Figure 3B-C) were associated with under- and over-dosing after rapid achievement of 1:100 target concentration. In the 10-mL syringes, average OR drug dilutions after reaching target concentration were 73 ± 25, 137 ± 209, and 176 ± 146 for 3-, 10-, and 60-mL syringes, respectively (P < 0.001 for 3- vs 60-mL and P = 0.04 for 3- vs 10-mL syringes, Figure 3E).
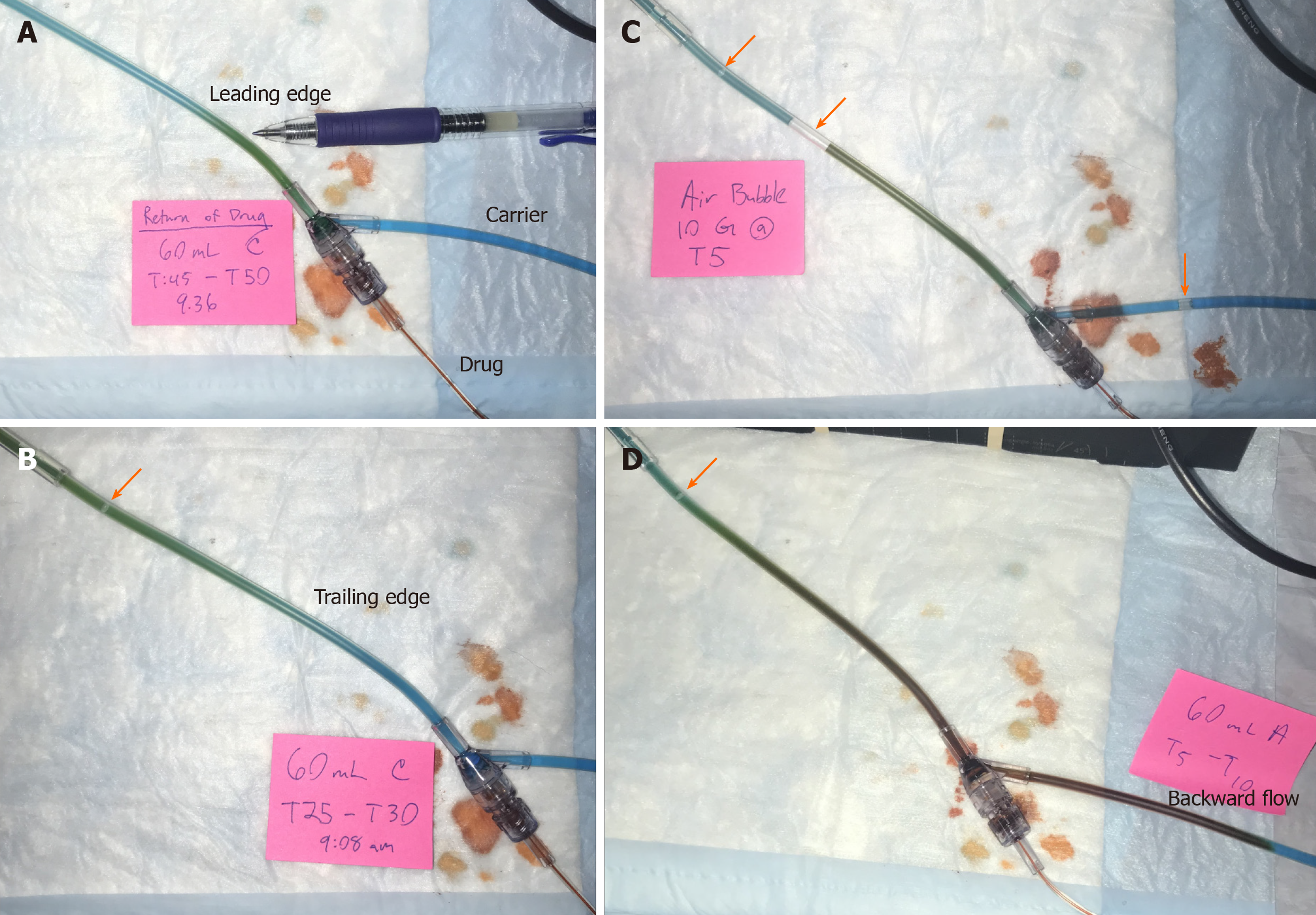
By design, a leading edge of visible color change in the infusion tubing was observed as OR drug traversed the tubing. OR drug mixed with BL carrier fluid into a green color (Figure 4A). This drug-containing green column ceased advancing shortly after achieving target concentration despite ongoing pump operation in three out of seven 10-mL syringes, three out of four 60-mL syringes, and none of the 3-mL syringes (Figure 4B). This interruption of steady state drug flow ranged from 15 to 40 min for trials of affected 10-mL syringes and at least 90 min for 60-mL syringe trials (Figure 4B and C). Despite careful and repeated priming of the drug infusion and carrier fluid lines using standard nursing practices, air bubbles were frequently observed entering the infusion system from the buretrol of the carrier infusion set (Figure 4B-D). Backward flow of OR drug into the proximal BL carrier fluid tubing was seen mainly in 60-mL syringes and to lesser degrees in smaller 3- and 10-mL syringes. In contrast to the green forward column of mixed OR and BL dyes, the fluid columns of backward flows maintained an OR color and persisted for unpredictable periods of time (Figure 4D).
Based on carrier flow of 5 mL/h and drug flow of 0.2 mL/h, expected volume of fluid per 5 min interval was 0.43 mL. To avoid errors introduced from start-up effects, we measured volumes after achieving 1:100 target concentration. Total infused volumes per 5 min period were 0.44 ± 0.02 mL, 0.44 ± 0.01 mL, and 0.43 ± 0.02 mL for 3, 10, and 60-mL syringes, respectively (P < 0.01 for 60- vs 10-mL and 60- vs 3-mL syringes).
Based on the experimental run curves in Figure 3A-C, we observed that most infusions reached 1:100 target concentration within 30 min and all the 10-mL syringes maintained 1:100 concentration by 60 min. Therefore, we analyzed the infusions in 30 min intervals up to 90 min in linear mixed effects models to compare overall drug delivery and variance, including the start-up periods. For total infused volumes, no mean differences were observed over each 30 min period.
Significant differences by syringe size were observed in OR drug concentration over time. In the initial 0-30 min period, 3-mL syringes delivered more dilute OR drug by 51% vs 10-mL (P = 0.005) and 83% vs 60-mL syringes (P < 0.001). Drug over-flows occurred in the 10- and 60-mL conditions, as noted above (Figure 3B-C). In subsequent time periods, no differences in concentration were observed between 3- and 10-mL syringes, but 60-mL syringes delivered more dilute OR drug in carrier fluid by more than 50% to a maximum of 106% greater dilution than 3- and 10-mL syringes in 30-60 and 60-90 min periods (P < 0.01 for each comparison). No differences in overall dilution variances were observed between syringe sizes.
Total drug delivered was calculated by multiplying volume times concentration (the inverse of dilution). Significant differences were observed only in comparisons of 60- vs 3- and 10-mL syringes. 60-mL syringes delivered less drug by 98% (P = 0.031) and 115% (P = 0.039) vs 3-mL syringes in the 30-60 min and 60-90 min time periods, respectively, and 111% (P = 0.012) greater dilution vs 10-mL syringes in the 60-90 min time period.
Carrier fluid, or primary infusion fluid, is a common pediatric intervention. Stable, infrequently titrated solutions for carrier flow include maintenance fluids or parenteral nutrition. These fluids maintain vascular access device patency[15], reduce drug delivery onset or offset times[13], and facilitate administration of multiple titratable drug infusions[16]. To our knowledge, our report is the first to demonstrate an interaction between carrier fluid and low flow infusion inaccuracies related to syringe size.
Ours and others’ previous work demonstrated significant variability of low flow infusions related to syringe size. Methods used in syringe-only studies include linear fluid displacement in our previous study [1] and gravimetry in others[17]. These methods are largely incompatible with carrier flow studies, in which spectrophotometry is most frequently used[15,18]. Hence, direct comparisons of low flow infusions from syringe pumps alone vs with added carrier fluid are not readily accomplished. However, by using our previously established experimental low flow syringe model[1] to investigate carrier fluid interactions, we revealed unanticipated issues. We previously found deviations from steady state of two-fold in 10-mL and six-fold in 60-mL syringes at 0.2 mL/h[1]. With the same drug infusion rate but added carrier flow, we observed similar six to nine-fold deviations in 10-mL syringe flow and up to five-fold deviations with no clear steady state pattern in 60-mL syringe flow up to 90 min after start-up.
Carrier flow comprised 96% of total flow in our model. We observed < 5% variability in total flow per 5 min period, consistent with stable carrier flow. A small but statistically significant difference of lesser total flow was observed in the 60- compared to 3- or 10-mL syringe conditions, which may be accounted for by syringe flow. We observed multiple infusion anomalies occurring in interactions between larger syringes and carrier flow. Problems included introduction of air bubbles, backward drug flow at the carrier fluid connector, and lack of mixing.
The buretrol was a frequent source of air bubbles, which in microfluidic systems contribute flow instability, increased compliance, and increased resistance[19]. While air introduction in carrier tubing is independent of drug syringe size, bubble effects may exacerbate syringe size-related anomalies. Flow variability resulting from stiction and compliance of a larger plunger[20] may add to flow inconsistencies caused by air bubbles in the infusion tubing. Because pressure drop across gas bubbles is inversely proportional to channel radius[19], smaller radius microbore tubing as recommended for low flow infusions[3] may exacerbate bubble effects.
Backwards flow at the carrier fluid connector occurred inconsistently at the start of infusions with larger syringes despite ongoing carrier flow and persisted for many minutes, leading to below target drug delivery. Rapid initiation of syringe flow is a feature of modern syringe pumps[21] and is used to overcome problems of mechanical slack[22] or “breakfree force” of the plunger[20], both of which are proportional to syringe size. While this rapid startup would generally exert minimal clinical effect if infused directly into the patient, addition of a connector to carrier fluid allows for backward flow and, thus, unpredictable drug delivery. We observed persistence of OR color (instead of green from the mixture of BL carrier and OR drug) and delayed clearance of drug from the proximal carrier tubing, both of which suggest lack of mixing. This offers indirect evidence of fluid layering and laminar flow, which contrasts with previously described Plug Flow and Well Mixed models of fluid flow[14,22]. In laminar flow conditions, the fluid edge may flow at a slower rate than the center and maintain distinct fluid compositions[13]. Hence, drug entering from the edge of tubing may travel slower than faster carrier fluid in the center. To our knowledge, this observation is previously undescribed in the clinical literature.
Limitations of our methods include an emphasis on readily available and low-cost experimental equipment to encourage reproducibility testing in other institutions. We found a nonlinear relationship between food dye concentration and absorbance, which may be due to additives. For future studies, we would use pure dyes that conform to the Beer-Lambert Law. Due to changes in our hospital equipment, the smart syringe pump in our current study was different from our previous publication[1]. We observed different syringe infusion characteristics, notably a more rapid start-up in the larger syringe sizes. This improved uniformity of time to target concentration, but in larger syringe sizes was associated with drug overdose, backward flow at the carrier fluid connector, and subsequent reductions in drug delivery. As backward flow was unanticipated and noted after trials of 3-mL syringes were completed, our study did not include spectral analysis proximal to the connector. We had no method to quantify air bubbles.
Despite the above limitations, our findings are qualitatively similar to previous publications on syringe size effects[1,3,4,17,23,24] while adding previously unreported problems of carrier fluid interactions with low flow infusions by syringe size. Importantly, our study provides no evidence to suggest that carrier fluid might reduce variability associated with low flows from larger syringes. This has important clinical implications for neonatal and small pediatric patients requiring critical short acting, high potency drug infusions such as epinephrine in settings where pharmacy standardization using prefilled or standardized syringes[10,12] may tend toward larger volume syringe sizes. Rather, we continue to recommend that syringe size be matched appropriately to the rate of infusion. In our health system, we now match syringe size to critical low flow pediatric infusions by using the smallest syringe capable of providing 12 h of infusion, or one nursing shift. Future studies will be needed to determine optimal carrier fluid to syringe flow ratios, the effects of tubing dead space on accuracy of low flow drug delivery with or without carrier fluid, and architecture of tubing connectors to reduce gas bubble introduction, improve mixing and reduce drug backflow.
Our study provides no evidence to suggest that carrier fluid might reduce variability associated with low flows from larger syringes. This has important clinical implications for neonatal and small pediatric patients requiring critical short acting, high potency drug infusions such as epinephrine in settings where pharmacy standardization using prefilled or standardized syringes[10,12] may tend toward larger volume syringe sizes.
Critically ill neonates and pediatric patients frequently require drug delivery via low flow infusions below 0.5 mL/h. The use of carrier fluid has become common in clinical practice to facilitate delivery of these low flow drug infusions.
Flow continuity problems of low flow infusions are known to be related to syringe size. However, competing safety considerations encourage pharmacy standardization to the largest common syringe size. As such, in clinical practice, carrier fluids are commonly used to reduce variability of drug delivery from larger syringe sizes.
To evaluate whether carrier fluid improves continuity in low flow drug delivery.
We simulated pediatric low flow infusions using dyed fluids in a drug infusion model. In-line spectrometry was used to measure drug concentrations. Administered fluid was determined volumetrically.
Low flow continuity errors were associated with larger syringe sizes and exacerbated by interactions with carrier fluid. Drug over- and underdosing, backward flow at the tubing connector, and frequent air bubbles from carrier fluid were observed.
Our study provides no evidence to suggest that carrier fluid might reduce variability associated with low flows from larger syringes.
Our study provides empiric data to suggest that continuity errors of low flow infusions are associated with larger syringes and not improved by carrier fluid. Syringe size should be matched to the rate of infusion. In our health system, we now match syringe size to critical low flow pediatric infusions by using the smallest syringe capable of providing 12 h of infusion.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Academy of Pediatrics, No. 906340; and Society of Critical Care Medicine, No. 32237.
Specialty type: Pediatrics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aseni P S-Editor: Zhang H L-Editor: A P-Editor: Li JH
| 1. | Neal D, Lin JA. The effect of syringe size on reliability and safety of low-flow infusions. Pediatr Crit Care Med. 2009;10:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Center for Devices and Radiological Health. Medical Product Safety Network (MedSun) Survey Final Report: Syringe Pump Survey. Silver Springs, MD: US Food and Drug Administration; 2016. Available from: https://wayback.archive-it.org/7993/20170112084319/http://www.fda.gov/downloads/MedicalDevices/Safety/MedSunMedicalProductSafetyNetwork/UCM526753.pdf. |
| 3. | US Food and Drug Administration. Syringe pump problems with fluid flow continuity at low infusion rates can result in serious clinical consequences: FDA safety communication. Silver Spring, MD; 2016. Available from: https://www.fdanews.com/ext/resources/files/2016/08/08-25-16-pumpsafetynotice.pdf?1480880246. |
| 4. | van der Eijk AC, van Rens RM, Dankelman J, Smit BJ. A literature review on flow-rate variability in neonatal IV therapy. Paediatr Anaesth. 2013;23:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Sherwin CM, Medlicott NJ, Reith DM, Broadbent RS. Intravenous drug delivery in neonates: lessons learnt. Arch Dis Child. 2014;99:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 6. | Association for the Advancement of Medical Instrumentation. Infusing Patients Safely: Priority Issues from the AAMI/FDA Infusion Device Summit. Arlington; 2010. Available from: https://s3.amazonaws.com/rdcmsaami/files/production/public/FileDownloads/Summits/AAMI_FDA_Summit_Report.pdf. |
| 7. | Institution for Safe Medication Practices. Proceedings from the ISMP Summit on the Use of Smart Infusion Pumps: Guidelines for Safe Implementation and Use. Horsham; 2009. Available from: https://www.ismp.org/resources/draft-guidelines-optimizing-safe-implementation-and-use-smart-infusion-pumps. |
| 8. | Stucky ER; American Academy of Pediatrics Committee on Drugs; American Academy of Pediatrics Committee on Hospital Care. Prevention of medication errors in the pediatric inpatient setting. Pediatrics. 2003;112:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Larsen GY, Parker HB, Cash J, O'Connell M, Grant MC. Standard drug concentrations and smart-pump technology reduce continuous-medication-infusion errors in pediatric patients. Pediatrics. 2005;116:e21-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Adapa RM, Mani V, Murray LJ, Degnan BA, Ercole A, Cadman B, Williams CE, Gupta AK, Wheeler DW. Errors during the preparation of drug infusions: a randomized controlled trial. Br J Anaesth. 2012;109:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Gorski LA. The 2016 Infusion Therapy Standards of Practice. Home Healthc Now. 2017;35:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 12. | Litman RS. How to prevent medication errors in the operating room? Take away the human factor. Br J Anaesth. 2018;120:438-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Peterfreund RA, Philip JH. Critical parameters in drug delivery by intravenous infusion. Expert Opin Drug Deliv. 2013;10:1095-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Lovich MA, Doles J, Peterfreund RA. The impact of carrier flow rate and infusion set dead-volume on the dynamics of intravenous drug delivery. Anesth Analg. 2005;100:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Bartels K, Moss DR, Peterfreund RA. An analysis of drug delivery dynamics via a pediatric central venous infusion system: quantification of delays in achieving intended doses. Anesth Analg. 2009;109:1156-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Turner MS, Hankins J, Alexander M, Corrigan A, Gorski L, Hankins J, Perucca R. Pharmacology. Alexander M, Corrigan A, Gorski L, Hankins J, Perucca R. Infusion Nursing: An Evidence-Based Approach. 3rd ed. St. Louis: Saunders Elsevier; 2009; 263. |
| 17. | Schmidt N, Saez C, Seri I, Maturana A. Impact of syringe size on the performance of infusion pumps at low flow rates. Pediatr Crit Care Med. 2010;11:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Tsao AC, Lovich MA, Parker MJ, Zheng H, Peterfreund RA. Delivery interaction between co-infused medications: an in vitro modeling study of microinfusion. Paediatr Anaesth. 2013;23:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Fu T, Yu, XY. Microfluidics in CO2 capture, sequestration, and applications. Yu , XY . Advances in Microfluidics - New Applications in Biology, Energy, and Material Sciences. Rijeka, Croatia: Books on Demand; 2016; 293. [DOI] [Full Text] |
| 20. | Dunster KR, Colditz PB. Flow continuity of infusion systems at low flow rates. Anaesth Intensive Care. 1995;23:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Medfusion: Medfusion Syringe Infusion Pump Model 4000 Operatorâs Manual. St. Paul: Smiths Medical ASD, Inc; 2010. [accessed 2020 May 26]. Available from: http://www.medfusionpump.com/assets/Literature/manuals/Operators_Manual_4000_40-5760-51A.pdf. |
| 22. | Lannoy D, Decaudin B, Simon N, Barthelemy C, Debaene B, Odou P. The impact on drug mass flow rate of interrupting and resuming carrier fluid flow: an in vitro study on a very low dead-space volume infusion set. Anesth Analg. 2012;114:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Neff SB, Neff TA, Gerber S, Weiss MM. Flow rate, syringe size and architecture are critical to start-up performance of syringe pumps. Eur J Anaesthesiol. 2007;24:602-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kim DW, Steward DJ. The effect of syringe size on the performance of an infusion pump. Paediatr Anaesth. 1999;9:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |