Published online Sep 9, 2025. doi: 10.5409/wjcp.v14.i3.104689
Revised: April 1, 2025
Accepted: April 9, 2025
Published online: September 9, 2025
Processing time: 170 Days and 3.4 Hours
Mucopolysaccharidosis type II (MPS II) is a chronic inherited disease with mul
To evaluate the predictors of fatal outcomes in MPS II patients.
In the retrospective cohort study, the clinical, laboratory data and enzyme re
Fatal outcomes occurred in 5%, 35%, 20%, and 40% of patients before 10, 10-14, 15-19, and ≥ 20 years. The most common causes of death were cardiovascular (29.4%), respiratory failure (17.6%), including pneumonia (17.6%), and their associations (17.6%) and MPS II progression (11.8%). Acute or chronic respiratory failure was in 53%. Died patients had higher birth weight, higher age of diagnosis, and start of ERT. Hydrocephalus, hydrocephalus bypass surgery, epilepsy, difficulty swallowing, and impaired movement after 12 years of age were significantly more common in the deceased patients. Cox regression analysis has revealed the following time-dependent covariates of the lethal outcome: 1st-year psychomotor development delay, delayed mental and speech develop
Increased birth weight, delayed diagnosis and the start of ERT, and development of neuronopathic form with impossible walking after 12 years were the main predictors of the fatal outcome.
Core Tip: Mucopolysaccharidosis type II is a rare lysosomal storage disease characterized by the progression of multiorgan dysfunction and increased mortality rates. The assessment of the death outcomes might improve the survival of these patients. High birth weight, delayed diagnosis, delayed enzyme replacement treatment, any signs of central nervous system involvement, and progressive impossibility of independent walking were the main predictors of the fatal outcome.
- Citation: Buchinskaya N, Vechkasova A, Vashakmadze N, Namazova-Baranova L, Ivanov D, Zakharova E, Kutsev S, Kostik M. Analysis of fatal outcomes of patients with mucopolysaccharidosis type II according to the Russian mucopolysaccharidosis registry. World J Clin Pediatr 2025; 14(3): 104689
- URL: https://www.wjgnet.com/2219-2808/full/v14/i3/104689.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i3.104689
Mucopolysaccharidosis type II (MPS II) or Hunter syndrome (OMIM 309900) is an X-linked, rare lysosomal storage disease. The disease is based on the accumulation of glycosaminoglycans (dermatan sulfate and heparan sulfate), which is caused by a deficiency in the lysosomal enzyme iduronate sulfatase[1,2]. IDS gene map at the chromosomal region Xq28 consists of nine exons. A continuous, progressive course characterizes the disease. However, the severity of the condition, the range of clinical symptoms, and the age at onset may vary among individuals[3]. The average frequency of incidents of MPS II in various countries of the world is 0.30–0.71 per 100000 live births[4] or 1:100000 to 1:170000 male births[5]. Clinically, MPS II can be divided into two forms: Neuronopathic and non-neuronopathic. In the neuronopathic forms of MPS, there is central nervous system damage, clinically expressed as delayed psychomotor and speech development, leading to varying degrees of intellectual disability, behavioral disorders, and epilepsy. Approximately 60% of cases of MPS II fall into the neuronopathic category[6,7]. The severe type is characterized by early onset, at around 2-4 years of age, and leads to death before 15 years of age, whereas the age of onset in attenuated form is late and greatly variable[2,8].
In the initial stages of severe forms of MPS II, individuals may experience recurrent infections in the upper respiratory tract and inguinal and umbilical hernias. Joint stiffness may also be present. As the disease progresses, patients may develop multiple dysostosis, spinal deformities, and joint contractures, which can lead to a shorter stature. The early development of coarse facial features, hepatosplenomegaly, hypertrophic cardiomyopathy, and myxomatous valvular degeneration characterize severe forms of MPS II. Additionally, patients may experience sensorineural hearing loss and symptoms affecting the central nervous system[8,9]. In less severe cases, the child's development remains unaffected, their height may reach average levels, the skeletal deformities are mild, the condition progresses slowly, and the fatal outcome typically occurs due to heart failure in the fourth to sixth decade of life[9,10].
The relationship between genotype and phenotype is often challenging to determine. Novel pathogenic variants and alterations in the IDS gene continue to be identified[11]. They include complete deletions, partial deletions, gene/pseudogene rearrangements, splicing mutations, and nonsense mutations linked to the neuronopathic forms of MPS II[6,9,10,12].
The retrospective study cohort extracted the clinical, epidemiological, instrumental, and laboratory data about 162 pa
In all cases, the clinical diagnosis of MPS II was confirmed by high urine excretion of glycosaminoglycans (dermatan sulfate and heparan sulfate) and enzymatic iduronate sulfatase deficiency in leukocytes, plasma or dried blood spots, except one patient for whom clinical data supporting the diagnosis of MPS II with only a high urinary excretion of glycosaminoglycans. Data on enzyme activity and molecular genetic studies in this patient were unavailable. Multiple possible sulphatase deficiency was excluded by measuring another sulphatase in lysosomal disease screening.
We extracted the following patients’ information for the assessment:Demographics: Age, sex, residence, family history of MPS II, age at first symptom(s), age at diagnosis, and time to diagnosis. Clinical features related to the disease: The main clinical symptoms were associated with the last data assessment, and some clinical signs were assessed twice in the first and last observations of the study. The neuronopathic form was determined if a patient has central nervous system involvement with at least one of the following symptoms: hydrocephalus, progressive intellectual disability, losing skills, and epilepsy). Laboratory data: The genetic analysis data (sequencing by Sanger) show significant changes in the IDS gene, including deletions, recombinant events involving IDS-IDS2, and missense mutations leading to a severe phenotype of MPS II, according to the previously published data and enzyme activity[5]. Treatment: The number of patients treated with enzyme replacement therapy (ERT), ERT onset age and duration, ERT delay. Outcomes: Alive or dead. The observation time was calculated using the date of death (for dead patients) or the data of the last available observation included in the registry.
We utilized the STATISTICA software package, version 10.0 (StatSoft Inc., St. Tulsa, OK, United States). Numerical indicators were presented with the median and interquartile range (IQR; 25th; 75th percentiles), and categorical variables were presented with absolute numbers and the parts (%). We used the Pearson criteria χ2 to compare the independent categorical variables and the Mann–Whitney criteria for independent numerical variables. Each predictor associated with the fatal outcome was assessed by analyzing the sensitivity and specificity. The cut-off values of the quantitative variables were calculated with area under the curve (AUC)-receiver operating characteristic (ROC) analysis with the determination of 95%CI, calculation of the odds ratio without taking into account the time of development of events of interest (fatal outcome) using 2 × 2 tables. Independent predictors of the fatal outcome were evaluated with multiple regression analysis. Survival analysis in each group was conducted utilizing the Kaplan-Meier method, with a fatal outcome as the event of interest. The log-rank test compared survival curves. Differences were considered statistically significant if the P value was less than 0.05.
Of the 160 patients enrolled in the study, 140 were still alive as of November, and 20 patients had passed away. The deceased patients had a significantly higher birth weight at 3.9 (3.4; 4.3) kg compared to the alive patients with a mean birth weight of 3.5 (3.2; 3.9) kg (P = 0.062) despite the similar gestation age. The height at birth did not differ significantly between the groups of survivors and non-survivors, with the mean height of the currently living patients being 53 (51; 54) centimeters and the mean height in the deceased group being 52 (51; 56) centimeters.
The age at diagnosis was also significantly different, with the deceased group having a mean age of 5.0 (4.0; 7.0) years compared to 3.0 (2.0; 5.0) years for the live group (P= 0.0006). Additionally, the ERT in the deceased patients occurred at an older age, with a mean onset of 7.5 (6.5, 11.5) years compared to a mean onset age of 5.0 (3.0; 9.0) years (P = 0.004).
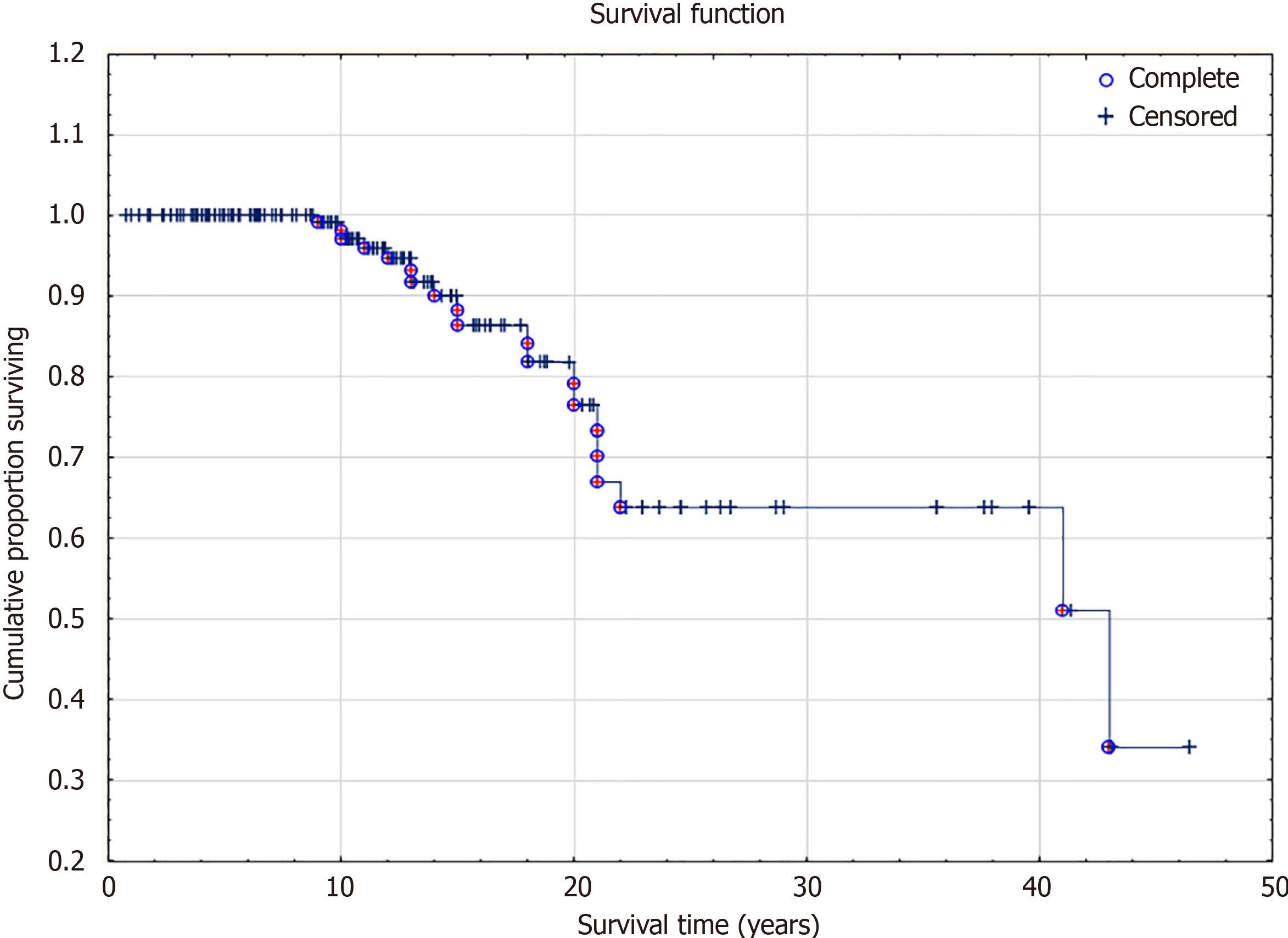
Fatal outcome before the age of 10 years – 1 patient (5%), aged 10-14 years old - 7 patients (35%), 15-19 age - 4 patients (20%), older than 20 years - 8 (40%) patients. The survival curve is in Figure 1. Among the deceased, 13 patients had a severe form of the disease, and 6 had an attenuated form. The severity of the course of MPS II could not be determined in one patient. The median age at onset of symptoms among patients with severe type MPS II was 1 year. Sixteen (84.2%) patients received ERT. ERT was administered to all 13 (100%) deceased patients with severe disease.
In the Russian registry of patients with MPS II, information on the causes of death is available for 17 out of 20 deceased individuals (Table 1). The most common causes of death included cardiovascular failure in 5 out of 17 cases (29.4%) and respiratory failure in 3 cases (17.6%). Pneumonia was reported as a cause of death in 3 out of 17 patients (17.6%), and an association between the cardiovascular and respiratory systems was observed in three patients (17.6%). The progression of the underlying disease (MPS II) was the cause of death for two patients (11.8%) and generalized viral infection in one case (6%). Acute or chronic respiratory failure, either alone or in combination with other causes, was present in 9 out of 17 patients (53%).
| ID | Height at birth, cm | 1st-year psychomotor delay | Delayed mental and speech | Intellectual disability: | Hydrocephalus | Epilepsy | Swallow disorders | Independent walking 5-12 / > 12 year | Hurler pheno-type | Cardio-myopathy | Myxomatous valve degeneration | Respiratory disorders | Tracheostomy | Carpal tunnel syndrome |
| 46 | 49 | No | Yes | No/no | Yes | No | No | Yes/yes | Yes | Yes | Yes | 0 | 0 | 0 |
| 58 | N/A | N/A | N/A | N/A/N/A | N/A | N/A | No | N/A/N/A | Yes | N/A | N/A | N/A | N/A | N/A |
| 65 | 52 | No | Yes | Mod/profound | Yes | Yes | Yes | Yes/no | Yes | Yes | Yes | Yes | 0 | Yes |
| 71 | 57 | Yes | No | No/no | N/A | N/A | N/A | Yes/yes | Yes | N/A | N/A | Yes | N/A | N/A |
| 72 | N/A | Yes | Yes | No/no | N/A | Yes | Yes | Yes/no | Yes | No | Yes | Yes | 0 | 0 |
| 92 | 52 | No | Yes | No/N/A | Yes | Yes | Yes | No/N/A | Yes | No | Yes | Yes | 0 | Yes |
| 93 | 51 | No | Yes | Profound/profound | No | Yes | Yes | No/no | Yes | No | Yes | Yes | Yes | Yes |
| 96 | 52 | No | Yes | Severe/severe | No | Yes | Yes | Yes/no | Yes | Yes | Yes | 0 | 0 | Yes |
| 104 | N/A | Yes | Yes | No/no | Yes | No | Yes | No/no | Yes | Yes | Yes | 1 | 0 | N/A |
| 106 | 50 | Yes | Yes | No/not significant | No | N/A | N/A | N/A/N/A | Yes | No | Yes | N/A | N/A | N/A |
| 109 | 57 | Yes | Yes | Moderate/severe | Yes | Yes | Yes | Yes/no | Yes | No | Yes | Yes | 0 | Yes |
| 110 | N/A | No | No | No/no | Yes | No | No | Yes/yes | Yes | No | Yes | Yes | 0 | Yes |
| 111 | 57 | Yes | Yes | Not significant/profound | Yes | Yes | Yes | Yes/no | Yes | Yes | Yes | Yes | 0 | Yes |
| 113 | 51 | N/A | N/A | N/A/N/A | N/A | N/A | N/A | N/A/N/A | Yes | N/A | N/A | N/A | N/A | N/A |
| 116 | 55 | Yes | Yes | Severe/N/A | Yes | N | Yes | N/A/N/A | Yes | Yes | Yes | 1 | 0 | 1 |
| 120 | N/A | No | No | No/no | N/A | N/A | N/A | Yes/no | N/A | N/A | N/A | N/A | N/A | N/A |
| 124 | 56 | Yes | Yes | No/severe | Yes | No | Yes | Yes/yes | Yes | Yes | Yes | Yes | 0 | 0 |
| 144 | N/A | No | Yes | No/N/A | Yes | No | No | Yes/N/A | Yes | N/A | N/A | Yes | 0 | Yes |
| 147 | 54 | No | No | No/no | No | No | No | Yes/yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 149 | 51 | Yes | Yes | Moderate/profound | Yes | Yes | Yes | Yes/no | Yes | Yes | Yes | Yes | Yes | Yes |
The clinical characteristics of patients with MPS II showed similarities and differences in their symptoms. The following symptoms were equally prevalent in the cohort of survivors and the cohort of those who had died: carpal tunnel syndrome, the Hurler phenotype, hepatosplenomegaly, and orthopedic abnormalities. The frequency of neuronopathic form was similar in both groups, amounting to 65% among the survivors/those who had died, respectively. Hydrocephalus (73.3% vs 41.4%, P = 0.019), bypass surgery for hydrocephalus (7.1% vs 0%, P = 0.003), epilepsy (53.3% vs 10.1%, P = 0.000009), difficulty swallowing (68.8% vs 23.0%, P = 0.0002), and impaired movement after the age of 12 (57.1% vs 22.1%, P = 0.002) were significantly more common in the group of deceased patients compared to the group of survivors. Hearing loss among deceased patients with MPS II occurred in 92.3% of cases, which was significantly higher than in the survivor group (P = 0.062). Respiratory disorders were present in 87.5% of patients, with a history of tracheotomy in 20% - significantly more frequent symptoms in the deceased MPS II group.
The pathology of the cardiovascular system was observed in 100% of cases in the form of cardiomyopathy (60%) and myxomatous valve changes. These conditions had a significantly higher prevalence in the group of deceased patients.
ERT received 134 out of 152 (88.2%) patients from the registry. Among those who received ERT, the median age of the first symptoms was 1.0 (1.0; 2.0) years in patients and 1.0 (0; 2.0) years in the group without ERT. The age of diagnosis did not differ between the treated and untreated groups and was 4.0 (2.0; 6.0). In the group that received ERT, 86 out of 134 (64.2%) had the neuronopathic form, compared to 9 out of 18 (50%) in the group that did not receive ERT.
Pathogenic and likely pathogenic variants in the IDS gene were found in two groups of patients with the same frequency. Variants of unknown significance in the IDS gene were not found in the group of deceased patients. Missense variants were the most common cause of MPS II in 71/118 (60.2%) patients with the known genotype. The main clinical characteristics and treatment are in Table 2.
| Parameter | Total n = 160 | Alive n = 140 | Median (IQR); min-max | Dead n = 20 | Median (IQR); min-max | P value | |
| General information | |||||||
| Age at last observation1 | 160 | 140 | 10.7 (5.9; 15.9); 0.7-46.4 | 20 | 16.5 (12.5; 21); 9.0-43 | 0.001 | |
| Birth weight, kg | 91 | 83 | 3.4 (3.2; 3.9); 1.5-4.7 | 8 | 3.9 (3.4; 4.3); 3.3-4.5 | 0.062 | |
| Height at birth, cm | 117 | 103 | 53 (51; 54); 40-60 | 14 | 52 (51; 56); 49-57 | 0.609 | |
| Age of first symptom, years | 128 | 112 | 1.0 (0; 2); 0-19.0 | 16 | 1.0 (0; 2); 0-8 | 0.982 | |
| Age at diagnosis, years | 145 | 125 | 3.0 (2; 5); 0-32 | 20 | 5.0 (4; 7); 2-38 | 0.0006 | |
| Ever received ERT | 152 | 133 | 118 (88.7) | 19 | 16 (84.2) | 0.569 | |
| Age of start ERT, years | 117 | 101 | 5.0 (3; 9); 0-43 | 16 | 7.5 (6.5; 11.5); 4-39 | 0.004 | |
| Pathogenic SNP | 118 | 102 | 59 (57.8) | 13 | 8 (61.5) | 0.532 | |
| Likely pathogenic SNP | 34 (33.3) | 5 (38.5) | |||||
| VUS SNP | 9 (8.8) | 0 (0) | |||||
| Neurology | |||||||
| Patients with a neuronopathic form | 98/148 | 130 | 85 (65.4) | 18 | 13 (72.2) | 0.565 | |
| Psychomotor development delay (up to 1 year) | 148 | 130 | 54 (41.5) | 18 | 9 (50.0) | 0.496 | |
| Delayed mental and speech development (1-3 years) | 147 | 129 | 85 (65.9) | 18 | 14 (77.8) | 0.314 | |
| Intellectual disability: From mild to profound (5-12 years) | 128 | 110 | 69 (62.7) | 18 | 13 (72.2) | 0.648 | |
| Intellectual disability: From mild to profound (after 12 years) | 84 | 69 | 40 (58.0) | 15 | 15 (66.7) | 0.546 | |
| Hydrocephalus | 131 | 116 | 18 (41.4) | 15 | 11 (73.3) | 0.019 | |
| Bypass surgery | 133 | 119 | 0 (0) | 14 | 1 (7.1) | 0.003 | |
| Epilepsy | 134 | 119 | 12 (10.1) | 15 | 8 (53.3) | 0.000009 | |
| Swallowing disorder | 129 | 113 | 26 (23.0) | 16 | 11 (68.8) | 0.0002 | |
| Impossible independent walking (5-12 years) | 136 | 120 | 12 (10.0) | 16 | 2 (12.5) | 0.757 | |
| Impossible independent walking (after 12 years) | 82 | 68 | 15 (22.1) | 14 | 8 (57.1) | 0.002 | |
| Carpal tunnel syndrome | 114 | 100 | 58 (58.0) | 14 | 11 (78.6) | 0.140 | |
| Somatic symptoms | |||||||
| Hurler phenotype | 142 | 123 | 118 (95.9) | 19 | 19 (100) | 0.371 | |
| Hepatosplenomegaly | 139 | 121 | 113 (93.4) | 18 | 18 (100) | 0.261 | |
| Hearing loss | 126 | 113 | 76 (67.3) | 13 | 12 (92.3) | 0.062 | |
| Respiratory disorders | 135 | 119 | 47 (39.5) | 16 | 14 (87.5) | 0.001 | |
| Tracheostomy | 136 | 121 | 2 (1.7) | 15 | 3 (20.0) | 0.0004 | |
| Any hernias | 136 | 118 | 98 (83.0) | 18 | 18 (100) | 0.059 | |
| Orthopedic problems | |||||||
| Any orthopedic features | 140 | 121 | 119 (98.4) | 19 | 19 (100) | 0.573 | |
| Spinal involvement | 132 | 117 | 76 (65.0) | 15 | 13 (86.7) | 0.091 | |
| Chest deformity | 139 | 123 | 67 (54.5) | 16 | 11 (68.8) | 0.279 | |
| Cardiac disorders | |||||||
| Cardiac disorders (cardiomyopathy + myxomatous valve disease) | 132 | 116 | 89 (76.7) | 16 | 16 (100) | 0.031 | |
| Cardiomyopathy | 131 | 115 | 34 (29.3) | 15 | 9 (60.0) | 0.017 | |
| Myxomatous valve disease | 130 | 115 | 78 (67.8) | 15 | 15 (100) | 0.009 | |
Tables 1 and 3 provide detailed epidemiologic, molecular, and clinical characteristics of the deceased patients. Missense variants were most common (eleven patients); two patients had nonsense, and four had large rearrangements in the IDS gene. Three patients had no data on molecular genetic testing.
| ID | Year of the death | Death age, year | Cause of death | SNP/Rearrangements | Protein | Exon/9 | Type of nucleotide change | Significance of variant | Normalized enzyme activity, % | Age of first symptoms, year | Age of diagnosis, year | ERT | Age of start ERT, year | MPS form, mild/severe |
| 46 | 2020 | 18 | CVF | c.253G > A | p.Ala85Thr | 3 | ms | Pathogenic | N/A | 2 | 8 | Yes | 10 | Mild |
| 58 | 2019 | 10 | Pneumonia | c.1295G > A | p.Cys432Tyr | 9 | ms | LP | N/A | 0 | 5 | Yes | 6 | N/A |
| 65 | 2020 | 21 | MPS | c.196C > T | p.Gln66Ter | 2 | ns | Pathogenic | 15 | 0 | 5 | Yes | 13 | Severe |
| 71 | 2016 | 21 | Acute CVF | c.257C > T | p.Pro86 Leu | 3 | ms | LP | 7.6 | N/A | 6 | No | N/A | Mild |
| 72 | 2020 | 14 | Acute CVF | c.1403G > A | p.Arg468Gln | 9 | ms | Pathogenic | N/A | N/A | 4 | Yes | 4 | Severe |
| 92 | 2021 | 11 | N/A | c.133G > T | p.Asp45Tyr | 2 | ms | LP | 3.8 | 1 | 5 | Yes | 7 | Severe |
| 93 | 2019 | 20 | RF, renal failure | c.395C > G | p.Ser132Trp | 3 | ms | LP | 0.1 | 2 | 4 | Yes | 11 | Severe |
| 96 | 2022 | 13 | CVF, dilated cardiomyopathy | c.262C > T | p.Arg88Cys | 3 | ms | Pathogenic | 0 | 0 | 3 | Yes | 5 | Severe |
| 104 | 2019 | 15 | Pneumonia | N/A | N/A | N/A | N/A | N/A | 0 | 3 | 5 | Yes | 7 | Severe |
| 106 | 2020 | 13 | N/A | Recombination between intron 7 of the IDS gene and the distal part of exon 3 of the IDSP1 pseudogene, with deletion of exons 1-3 of the IDS gene | N/A | N/A | N/A | Pathogenic | 0 | 2 | 2 | Yes | 7 | Severe |
| 109 | 2022 | 20 | CVF | Recombination between intron 7 of the IDS gene and the distal part of exon 3 of the IDSP1 pseudogene, without deletion | N/A | N/A | N/A | Pathogenic | 9.1 | 1 | 11 | Yes | 12 | Severe |
| 110 | 2018 | 43 | Chronic obstructive pulmonary disease. Pneumonia | c.1037C > T | p.Ala346Val | 8 | ms | LP | 0 | N/A | 20 | No | N/A | Mild |
| 111 | 2022 | 22 | MPS | c.133delG | p.Asp45Metfs*15 | 2 | ms | Pathogenic | NA | 1 | 5 | Yes | 11 | Severe |
| 113 | 2017 | 15 | CVF | Recombination between intron 7 of the IDS gene and the distal part of exon 3 of the IDSP1 pseudogene, without deletion | N/A | N/A | N/A | Pathogenic | 0.43 | 2 | 3 | N/A | N/A | N/A |
| 116 | 2018 | 10 | CVF | N/A | N/A | N/A | N/A | N/A | 0.01 | 0 | 6 | Yes | 6 | Severe |
| 120 | 2017 | 41 | CVF | N/A | N/A | N/A | N/A | N/A | 1.8 | 8 | 38 | Yes | 39 | Mild |
| 124 | 2022 | 18 | Viral infection | c.1340T > A | p.Leu447Term | 9 | ns | Pathogenic | 0 | 5 | 6 | Yes | 8 | Severe |
| 144 | 2018 | 9 | N/A | Del exon 1-7 | N/A | N/A | N/A | Pathogenic | 0.002 | 4 | No | Severe | ||
| 147 | 2016 | 21 | RF | c.1034G > C | p.Trp345Ser | 8 | ms | Pathogenic | 0.66 | 1 | 11 | Yes | 17 | Mild |
| 149 | 2017 | 12 | RF | c.1006G > C | p.Gly336Arg | 7 | ms | Pathogenic | 0.42 | 0 | 5 | Yes | 7 | Severe |
The univariant analysis has revealed the fourteen most significant predictors of the fatal outcome (Table 4). Delayed diagnosis, delayed treatment, and epilepsy and respiratory disturbances showed the highest predictive rate of fatal outcomes. AUC-ROC analysis allowed the detection of the cut-off age > 13 years as the predictor of fatal outcomes. In the neuronopathic forms of MPS II, the fatal outcome occurred at a much earlier age than in non-neuronopathic forms. The median age at death in mild cases was 18 years, and in severe cases, it was 16.5 years.
| MPS type II feature | Sensitivity | Specificity | OR (95%CI) | P value |
| Diagnosis age > 4 years | 70.0 | 72.8 | 6.3 (2.2; 17.6) | 0.0002 |
| ERT age > 5 years | 87.5 | 59.4 | 10.2 (2.2; 47.5) | 0.0005 |
| Birth weight > 3.88 kg | 62.5 | 72.3 | 4.4 (0.96; 19.7) | 0.042 |
| Age > 13 years | 75.0 | 65.0 | 5.6 (1.9; 16.2) | 0.0006 |
| Hydrocephalus | 73.3 | 58.6 | 3.9 (1.2; 13.0) | 0.019 |
| Epilepsia | 53.3 | 89.9 | 10.2 (3.1; 33.1) | 0.000009 |
| Swallowing disturbances | 68.8 | 77.9 | 7.4 (2.3; 23.1) | 0.0002 |
| Impossible to walk after 12 years | 61.5 | 77.9 | 5.7 (1.6; 19.9) | 0,002 |
| Heart disease | 100.0 | 23.3 | - | 0.031 |
| Cardiomyopathy | 60.0 | 70.7 | 3.6 (1.2; 11.0) | 0.017 |
| Myxomatous valve disease | 100.0 | 32.3 | - | 0.009 |
| Deafness | 92.3 | 32.7 | 5.8 (0.7; 46.7) | 0.062 |
| Respiratory disorders | 87.5 | 60.2 | 10.6 (2.3; 48.7) | 0.001 |
| Apnoe at the diagnosis | 54.5 | 73.6 | 3.4 (0.92; 12.3) | 0.057 |
| Apnoe at the last visit | 77.8 | 70.2 | 8.3 (1.6; 42.6) | 0.004 |
| Obstructive disease | 72.7 | 66.3 | 5.2 (1.3; 21.2) | 0.012 |
| Hernias | 100.0 | 16.9 | - | 0.059 |
In a post-hoc analysis of patients older than 13 years, only epilepsy (P = 0.039), swallowing disorders (P = 0.016), impossibility to walk (P = 0.006), myxomatous valve disease (P = 0.054), tracheostomy (P = 0.044) were associated with lethal outcome.
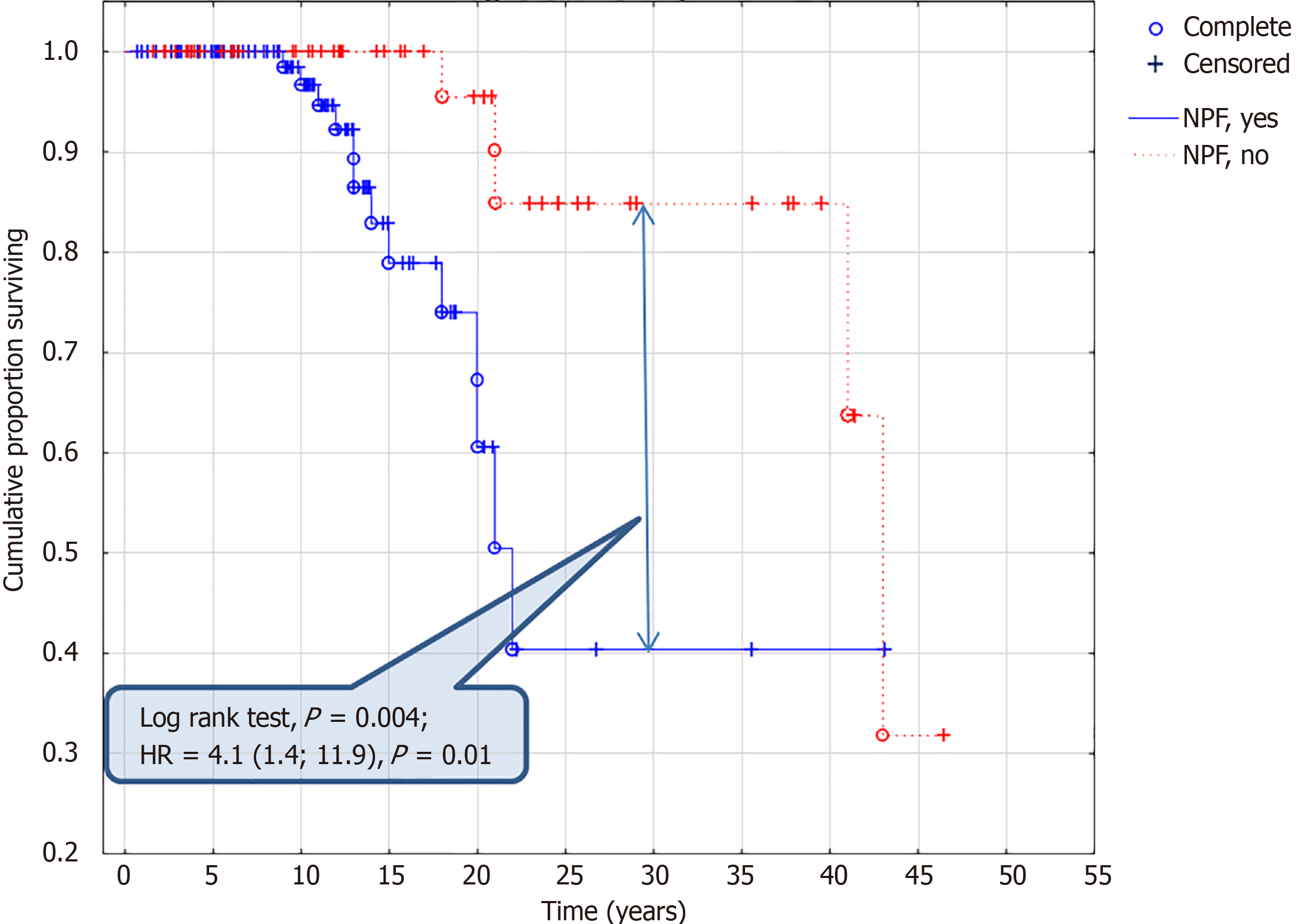
The analysis of the survival curve has revealed the following time-dependent predictors of the fatal outcome: Neuronopathic form (Figure 2) of the disease (P = 0.004), birthweight > 3.88 kg (P = 0.042), tracheostomy (P = 0.002), respiratory disorders (P = 0.009), myxomatous valve disease (P = 0.030), impossible walking after 12 years (P = 0.001), swallow disorders (P = 0.001), epilepsy (P = 0.0007), hydrocephalus (P = 0.030), intellectual disability (P = 0.0006), psychomotor delay (P = 0.031). In a post-hoc analysis in patients older than 13 years similar to univariate analysis, only epilepsy (P = 0.016), swallowing disorders (P = 0.004), impossibility to walk (P = 0.0005), myxomatous valve disease (P = 0.019), tracheostomy (P = 0.051) were associated with lethal outcome.
Cox regression analysis has revealed the following time-dependent covariates of the lethal outcome. Data are in Table 5. The absence of enzyme replacement therapy, delayed diagnosis, and treatment did not predict the deadly outcome in Cox regression models.
| Predictors | SE | HR (95%CI) | P value |
| Psychomotor development delay (up to 1 year) | 0.26 | 3.4 (1.2; 9.3) | 0.019 |
| Delayed mental and speech development (1-3 years) | 0.33 | 5.7 (1.8; 17.9) | 0.003 |
| Hydrocephalus | 0.30 | 3.3 (1.03; 10.4) | 0.044 |
| Epilepsia | 0.28 | 4.4 (1.5; 12.8) | 0.008 |
| Swallow disorders | 0.29 | 5.1 (1.6; 16.7) | 0.006 |
| Impossible walking at age < 12 years | 0.40 | 4.9 (1.01; 23.3) | 0.049 |
| Impossible walking at the age > 12 years | 0.31 | 9.4 (2.8; 31.3) | 0.0003 |
| Respiratory disorders | 0.38 | 5.7 (1.3; 25.6) | 0.023 |
| Tracheostomia | 0.33 | 4.7 (1.3; 16.7) | 0.022 |
| Neuronopathic form | 0.27 | 4.1 (1.4; 11.9) | 0.01 |
In our study, the predictors of lethal outcomes were identified. The majority of patients died after 20 years (40%). Died patients had a later start of ERT and the age of the diagnosis. Died patients had higher birth weights with similar gestation ages. Neuronopathic form, cardiovascular disease, and respiratory disorders were the main predictors of the fatal outcome. There were no associations of fatal outcomes with the severity of the IDS genetic variants.
Delayed treatment has interfered with delayed diagnosis and lack of timely access to treatment in the past. Now, the time from diagnosis to treatment in pediatric patients is less than one month due to a specific governmental program that has worked since 2015. All cases with delayed treatment were only before 2015. The delayed treatment showed the disease's natural course, the treatment's less clinical efficacy, and confirmation of the necessity of early access to the treatment.
The findings of the molecular-genetic study in the Russian population of patients with MPS II are consistent with those of other researchers: The prevalence of pathogenic and likely pathogenic variants among Russian patients was 93%, similar to the findings of other studies, such as those conducted in India (95.5%)[7].
According to the Hunter Outcome Survey (HOS) data, missense variants of the IDS gene (47/88, 53.4% of total variants) were the most frequent genetic variants in dead patients[10]. In our cohort, the frequency of the missense variants in deceased patients was also high (60.2%). Missense variants of the IDS gene were the most frequent genetic findings in previous studies in Russian MPS II patients[13].
Frequency of neuronopathic forms – in the Czech Republic, Slovaks, and Serbia accounted for 32/42 patients (76.2%); among the deceased patients, only 2 had intermediate forms[6]. In Japan, out of 28 patients - 8 (29%) had the attenuated and 20 (71%) had the severe form, and five patients (17.7%) died. Of these, two patients (40%) had attenuated, and three (60%) had severe phenotype MPS II[14]. In India, the prevalence of the attenuated phenotype was 32.6% (47 out of 144), while the prevalence of the severe phenotype was 63% (91 out of 144)[7]. The prevalence of neuronopathic forms in the Russian Federation is comparable to that in other countries - 98 out of 148 (66.2%). The majority of dead patients had neuronopathic forms of MPS (72.2%).
According to European authors, patients with a neuronopathic form of MPS II older than 10 years experience difficulties with independent movement, significantly reducing their daily activity and necessitating third-party assistance or the use of specialized devices to help[15]. According to our data, among children aged 5-12, 10% of living patients were unable to move independently, and 12.5% of deceased patients experienced this issue. However, in a group of patients older than 12, the difference in the frequency of impossibly independent moving between living and deceased patients was significant, with 22.1% and 57.1%, respectively. A substantial increase in the number of individuals unable to move independently as they age is an important predictor of mortality.
The age of death was roughly comparable among Russian patients with MPS II and patients from the HOS registry[16]. Differences in the proportion of patients who died between the Russian Registry and the HOS registry were those aged less than 10 years and older than 20 years. Under the age of 10 years, 16% died, according to the HOS registry, and 5%, according to the Russian Registry; at the age of 10-15 years, 38% and 35%; at the age of 15-20 years 22% and 20%; over the age of 20, 19% and 40%, respectively. The median age of death varies depending on the form of MPS II. Based on data from the HOS registry, in patients with cognitive impairment, the median age of death was 11.7 years, and in attenuated forms - 14.1 years[16]. In the Russian population, comparable data were obtained – 16.5 vs 18 years. Patients of the Russian population have a longer survival age compared to the data of the international registry of diseases despite the similar ratio of neuronopathic and non-neuronopathic forms among deceased patients with MPS II.
Chronic or acute respiratory failure (usually caused by pneumonia) was the most common cause of death in Russian patients with MPS II. Cardiovascular failure and isolated respiratory failure were the most common causes of death: 29.4% and 17.6% of cases. Our data on the most frequent causes of death is similar to that of other centers from the Czech Republic, Slovakia, and Serbia[6]. Compared to the HOS data, the leading cause of death was airway impairment at 46%, which exceeds the Russian data. Cardiac involvement accounted for 16% of the causes of death[16]. A higher rate of respiratory disorders and tracheostomy in the group of deceased patients characterizes a more severe course of MPS II.
According to the analysis of the HOS registry for 2017, among patients receiving ERT respiratory failure, the cause of death is indicated in 43% of cases, while among patients who did not receive ERT - 10%. Among the deceased Russian patients who received ERT, involvement of the respiratory system was indicated as the cause of death in 7/18 (39%) people with an established cause of death. Compared with HOS data, the age of first symptoms was lower in the Russian population, and the age of diagnosis was higher in these groups. There were no differences in the age of diagnosis in the group of patients who received and did not receive ERT: Median age at onset of symptoms of MPS for the treated and untreated groups was equal (1.6 and 1.5 years), as the median age at diagnosis (3.3 and 3.2 years)[17]. The median age of first MPS symptoms was 1 year in the group receiving and not receiving ERT; the median age of diagnosis also did not differ in these groups of patients, and it was 4.0 (2.0; 6.0) years. The frequency of severe forms in the group of treated/untreated patients differed significantly and amounted to 64.2% and 50%, respectively, which also differs from the HOS data, where the proportion of patients with cognitive impairment was 58.0% and 57.9%, respectively. ERT for MPS II has been used to treat this disorder in Japan since 2006[14], in Europe similarly[17], and in the Russian Federation since 2008. According to the HOS registry, patients with MPS II who received ERT have 10 years longer life expectancy than untreated patients – 33 years vs. 21.2 years. The Cox model adjusted for the condition at the time of treatment revealed a 54% lower mortality risk in the ERT-treated group than in the untreated group[17]. Our cohort Cox-regression analysis has not shown a statistical difference in the probability of fatal outcomes depending on ERT.
Possibly, the small sample size and a large proportion of young, alive patients led to the failure of this analysis, but dead patients had later started ERT - 7.5 (6.5; 11.5) years. It was late, compared to the age of the first symptoms of the disease, 1.0 (0; 2) years. We observe the long-term natural course of the disease, and delayed ERT doesn't influence the life duration due to multiple vital organ complications at the moment of the start of the ERT—any analysis of the associations between ERT and lethal outcome is required to note the age of ERT initiation. In our cohort, many patients had delayed ERT due to the absence of ERT before 2008 and lack of access to ERT before 2013.
The intrathecal administration of idursulfase for treating neuronopathic forms of MPS II in combination with intravenous administration of the enzyme has been introduced into therapy. The phase 2/3 trials of the drug (Intrathecal idursulfase-IT) were successfully evaluated, developed, and completed[18]. Neurocognitive development improved in children under 6 with missense mutations and a reduction in GAG levels in the cerebrospinal fluid across all age groups[18]. Sibling pairs demonstrate the effectiveness of early initiation of intrathecal ERT, which can stabilize or slow cognitive decline[19]. A 5-year follow-up study of patients with MPS II neuronopathic disease, who were treated with intracerebroventricular enzyme replacement therapy with idursulfase beta, showed the safety and efficacy of the treatment. The results indicated increased age at the onset of the disease (improved neurocognitive development) and reduced heparan sulfate excretion. The most significant benefit was observed in patients who started treatment before 3 years old[20-22]. The most promising approach to treating neuronopathic forms of MPS II is combination therapy, which can improve these patients' survival rates. This specific group of patients often experiences a more severe form of the disease, leading to early mortality.
In the Russian Federation, idursulfase beta for intracerebroventricular administration was registered in December 2024. Therefore, we do not have our own experience of treatment in this area up to now. The high prevalence of the neuronopathic forms makes the intracerebroventricular administration of the ERT very promising in our country. Now, the expert groups of specialists selecting the patients convenient for intracerebroventricular administration of the ERT were organized. These groups also validate the international questionnaires for neurocognitive development assessment and the Russian questionnaires created for conditions similar to those of MPS II populations. The absence of experience in the intracerebroventricular administration of the ERT deteriorates the outcomes. Currently, the following measures have been implemented in the Russian healthcare system to reduce delays in diagnosing MPS:
Education: Educational programs on the diagnosis of lysosomal storage diseases and inherited metabolic disorders have been introduced for senior medical students in the pediatric faculty of our university. Lectures on these topics are also provided for primary care physicians to raise awareness about rare diseases.
Diagnostics: Free diagnostic services are available for the diagnosis of mucopolysaccharidoses, accessible to all medical professionals in the Russian Federation. Enzyme activity is evaluated using filter-dried blood spot samples, allowing for easy referral for diagnosis from anywhere in the country. Further molecular genetic testing is also provided free of charge for patients.
The study's main limitations were the small sample size, retrospective type of study, missing data, and large proportion of young patients in the registry. The missing data about the genetic variants reduces the possibility of associating phenotype and outcomes. A small sample size increases the risk of type II errors, where actual effects may not be detected. Several external factors, e.g., the age of the diagnosis, access to fast diagnostics, and lack of treatment access before 2015, affected the study's results. The high prevalence of the neuronopathic forms and the absence of the intracerebroventricular administration of the ERT influenced the study outcomes. The small sample size (n = 20 deaths) limits the reliability of multivariate analyses, including Cox regression. The young age of the group of living patients creates additional limitations in the finding of the predictors of lethal outcomes. The study has some statistical limitations. Analyzing the multiple predictors without corrections on the co-linearity and multiple comparisons increased the risk of false-positive associations. All these abovementioned factors reduce the generalisability of the results on the more significant population of patients of different ages and severity and could skew the results and interpretations. A more comprehensive analysis that accounts for these confounders would provide more robust and reliable findings.
Increased birth weight, delayed diagnosis and start of ERT, and development of neuronopathic form with impossible walking at age > 12 years were the main predictors of the fatal outcome. Improvement of the diagnostics, early start of treatment with close monitoring of the development of neuronopathic form, correction of respiratory disturbances in MPS II patients with chronic lung disease, respiratory infectious profilaxis, and non-invasive ventilation as well as a combination with intrathecal ERT in neuronopathic patients might increase the life expectations.
The authors thank Aston Consulting for technical support of the study.
| 1. | Michaud M, Belmatoug N, Catros F, Ancellin S, Touati G, Levade T, Gaches F. [Mucopolysaccharidosis: A review]. Rev Med Interne. 2020;41:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L, Guffon N, Meldgaard Lund A, Malm G, Van der Ploeg AT, Zeman J. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr. 2008;167:267-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Zhang Z, Ma M, Zhang W, Zhou Y, Yao F, Zhu L, Wei M, Qiu Z. Phenotypic and genetic characteristics of 130 patients with mucopolysaccharidosis type II: A single-center retrospective study in China. Front Genet. 2023;14:1103620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Martin R, Beck M, Eng C, Giugliani R, Harmatz P, Muñoz V, Muenzer J. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome). Pediatrics. 2008;121:e377-e386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Baehner F, Schmiedeskamp C, Krummenauer F, Miebach E, Bajbouj M, Whybra C, Kohlschütter A, Kampmann C, Beck M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 275] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Dvorakova L, Vlaskova H, Sarajlija A, Ramadza DP, Poupetova H, Hruba E, Hlavata A, Bzduch V, Peskova K, Storkanova G, Kecman B, Djordjevic M, Baric I, Fumic K, Barisic I, Reboun M, Kulhanek J, Zeman J, Magner M. Genotype-phenotype correlation in 44 Czech, Slovak, Croatian and Serbian patients with mucopolysaccharidosis type II. Clin Genet. 2017;91:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Agrawal N, Verma G, Saxena D, Kabra M, Gupta N, Mandal K, Moirangthem A, Sheth J, Puri RD, Bijarnia-Mahay S, Kapoor S, Danda S, H SV, Datar CA, Ranganath P, Shukla A, Dalal A, Srivastava P, Devi RR, Phadke SR. Genotype-phenotype spectrum of 130 unrelated Indian families with Mucopolysaccharidosis type II. Eur J Med Genet. 2022;65:104447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Lin HY, Chuang CK, Huang YH, Tu RY, Lin FJ, Lin SJ, Chiu PC, Niu DM, Tsai FJ, Hwu WL, Chien YH, Lin JL, Chou YY, Tsai WH, Chang TM, Lin SP. Causes of death and clinical characteristics of 34 patients with Mucopolysaccharidosis II in Taiwan from 1995-2012. Orphanet J Rare Dis. 2016;11:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (3)] |
| 9. | Froissart R, Moreira da Silva I, Guffon N, Bozon D, Maire I. Mucopolysaccharidosis type II--genotype/phenotype aspects. Acta Paediatr Suppl. 2002;91:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yee KS, Alexanderian D, Merberg D, Natarajan M, Wang S, Wu Y, Whiteman DAH. Cognitive and adaptive behaviors associated with disease severity and genotype in patients with mucopolysaccharidosis II. Mol Genet Metab. 2023;140:107652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | D'Avanzo F, Rigon L, Zanetti A, Tomanin R. Mucopolysaccharidosis Type II: One Hundred Years of Research, Diagnosis, and Treatment. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Vollebregt AAM, Hoogeveen-Westerveld M, Kroos MA, Oussoren E, Plug I, Ruijter GJ, van der Ploeg AT, Pijnappel WWMP. Genotype-phenotype relationship in mucopolysaccharidosis II: predictive power of IDS variants for the neuronopathic phenotype. Dev Med Child Neurol. 2017;59:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 13. | Semyachkina AN, Voskoboeva EY, Nikolaeva EA, Zakharova EY. Analysis of long-term observations of the large group of Russian patients with Hunter syndrome (mucopolysaccharidosis type II). BMC Med Genomics. 2021;14:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Tomita K, Okamoto S, Seto T, Hamazaki T. Real world long-term outcomes in patients with mucopolysaccharidosis type II: A retrospective cohort study. Mol Genet Metab Rep. 2021;29:100816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Muenzer J, Giugliani R, Scarpa M, Tylki-Szymańska A, Jego V, Beck M. Clinical outcomes in idursulfase-treated patients with mucopolysaccharidosis type II: 3-year data from the hunter outcome survey (HOS). Orphanet J Rare Dis. 2017;12:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Jones SA, Almássy Z, Beck M, Burt K, Clarke JT, Giugliani R, Hendriksz C, Kroepfl T, Lavery L, Lin SP, Malm G, Ramaswami U, Tincheva R, Wraith JE; HOS Investigators. Mortality and cause of death in mucopolysaccharidosis type II-a historical review based on data from the Hunter Outcome Survey (HOS). J Inherit Metab Dis. 2009;32:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Burton BK, Jego V, Mikl J, Jones SA. Survival in idursulfase-treated and untreated patients with mucopolysaccharidosis type II: data from the Hunter Outcome Survey (HOS). J Inherit Metab Dis. 2017;40:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Muenzer J, Burton BK, Harmatz P, Gutiérrez-Solana LG, Ruiz-Garcia M, Jones SA, Guffon N, Inbar-Feigenberg M, Bratkovic D, Hale M, Wu Y, Yee KS, Whiteman DAH, Alexanderian D; HGT-HIT-094 Study Group. Intrathecal idursulfase-IT in patients with neuronopathic mucopolysaccharidosis II: Results from a phase 2/3 randomized study. Mol Genet Metab. 2022;137:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Muenzer J, Burton BK, Harmatz P, Gutiérrez-Solana LG, Ruiz-Garcia M, Jones SA, Guffon N, Inbar-Feigenberg M, Bratkovic D, Rust S, Hale M, Wu Y, Yee KS, Whiteman DAH, Alexanderian D. Evaluation of early treatment with intravenous idursulfase and intrathecal idursulfase-IT on cognitive function in siblings with neuronopathic mucopolysaccharidosis II. J Inherit Metab Dis. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Seo JH, Kosuga M, Hamazaki T, Shintaku H, Okuyama T. Impact of intracerebroventricular enzyme replacement therapy in patients with neuronopathic mucopolysaccharidosis type II. Mol Ther Methods Clin Dev. 2021;21:67-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Seo JH, Kosuga M, Hamazaki T, Shintaku H, Okuyama T. Intracerebroventricular enzyme replacement therapy in patients with neuronopathic mucopolysaccharidosis type II: Final report of 5-year results from a Japanese open-label phase 1/2 study. Mol Genet Metab. 2023;140:107709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Zanetti A, Tomanin R. Targeting Neurological Aspects of Mucopolysaccharidosis Type II: Enzyme Replacement Therapy and Beyond. BioDrugs. 2024;38:639-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |