Published online Sep 9, 2023. doi: 10.5409/wjcp.v12.i4.162
Peer-review started: July 9, 2023
First decision: July 19, 2023
Revised: August 9, 2023
Accepted: August 23, 2023
Article in press: August 23, 2023
Published online: September 9, 2023
Processing time: 58 Days and 18.3 Hours
Investigating gastrointestinal (GI) motility disorders relies on diagnostic tools to assess muscular contractions, peristalsis propagation and the integrity and coordination of various sphincters. Manometries are the gold standard to study the GI motor function but it is increasingly acknowledged that manometries do not provide a complete picture in relation to sphincters competencies and muscle fibrosis. Endolumenal functional lumen imaging probe (EndoFLIP) an emerging technology, uses impedance planimetry to measure hollow organs cross sectional area, distensibility and compliance. It has been successfully used as a complementary tool in the assessment of the upper and lower oesophageal sphincters, oesophageal body, the pylorus and the anal canal. In this article, we aim to review the uses of EndoFLIP as a tool to investigate GI motility disorders with a special focus on paediatric practice. The majority of EndoFLIP studies were conducted in adult patients but the uptake of the technology in paediatrics is increasing. EndoFLIP can provide a useful complementary data to the existing GI motility investigation in both children and adults.
Core Tip: Manometries are commonly used to investigate gastrointestinal (GI) motility disorders albeit with acknowledged limitation to their diagnostic yield. Endolumenal functional lumen imaging probe (EndoFLIP), an emerging technology uses impedance planimetry to provide cross sectional area, distensibility and diameter of a hollow organ. EndoFLIP is increasingly used as an adjunct diagnostic tool to provide diagnostic information and to guide therapy for many GI motility disorders.
- Citation: White E, Mutalib M. Use of endolumenal functional lumen imaging probe in investigating paediatric gastrointestinal motility disorders. World J Clin Pediatr 2023; 12(4): 162-170
- URL: https://www.wjgnet.com/2219-2808/full/v12/i4/162.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v12.i4.162
Investigating gastrointestinal (GI) motor function requires a careful assessment of muscle contractions and sphincters function[1]. Manometries can directly measure the lumen-generated pressure and are considered the gold standard to evaluate the strength and propagation of muscle contractions, peristalsis velocity and the integrity and coordination of different types of GI sphincters[2]. Sequential multi-channel impedances, in isolation or combined with manometry, will evaluate the directional flow and the bolus transit[3]. However, it has long been recognised that the competency of GI sphincters does not always relate to the tightness of their contractions[4-6]. Measuring the resistance to distension, also known as distensibility assessment, can provide an accurate geometric measurement of the sphincters and a plethora of diagnostic information[7].
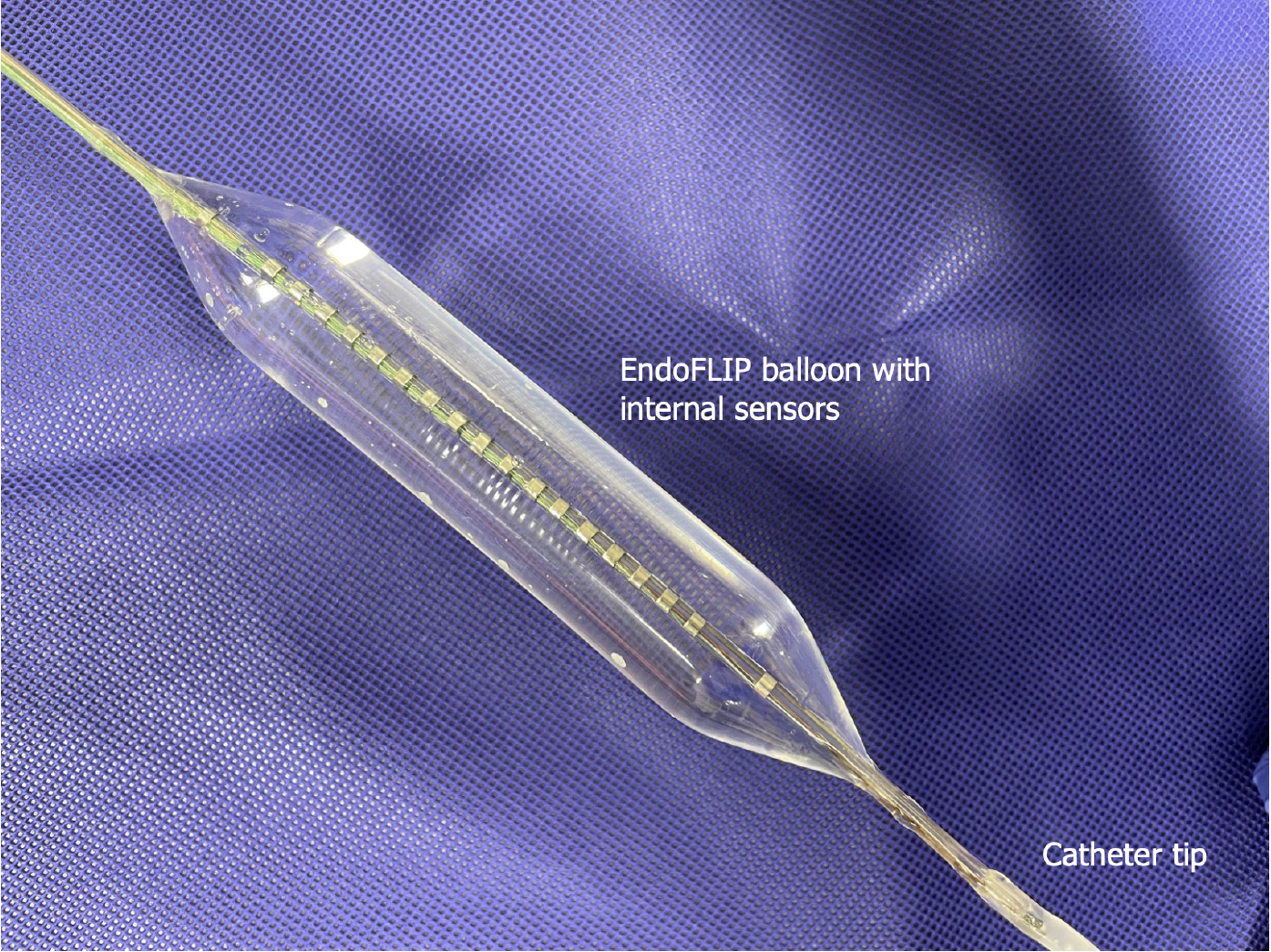
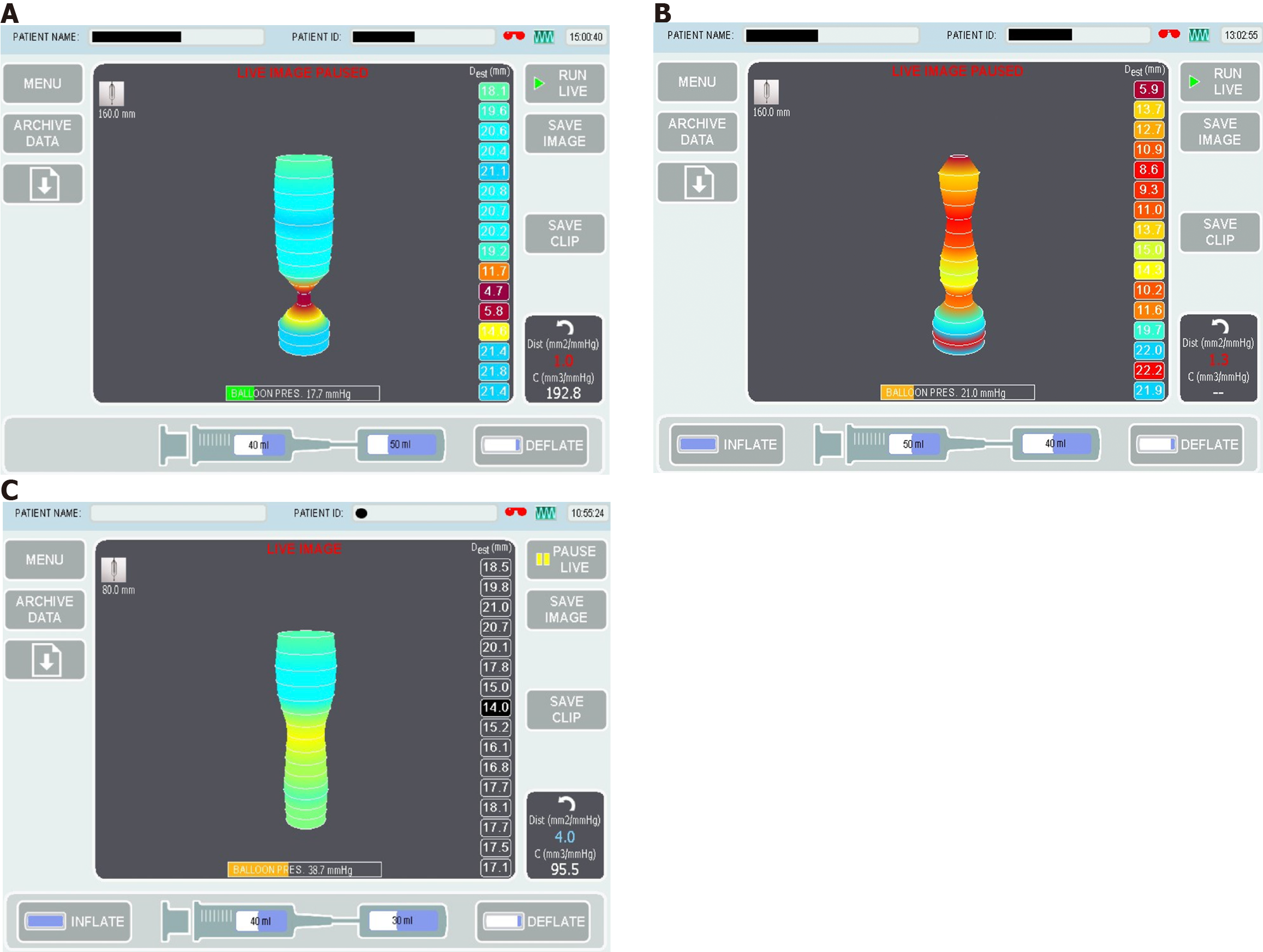
Endolumenal functional lumen imaging probe (EndoFLIP) uses the principles of impedance planimetry, a technical term that describes the measuring of the cross-sectional area (CSA) of a distending bag by using an internal electrical impedance measurement[8,9]. EndoFLIP consists of 16 sensors and a solid state pressure transducer placed inside a distensible and compliant balloon to provide a measurement length of 8 or 16 cm[10]. EndoFLIP is used during endoscopy under the same anaesthesia. To obtain the desired data, the balloon is filled with a conducting fluid (in a predetermined volume) (Figure 1), then the system will run a constant alternating current and the voltage difference between the excitation and the detection electrodes is measured[11]. The voltage is mathematically proportioned to the CSA of the balloon, from which, the balloon diameter can easily be calculated[12]. The EndoFLIP data will be presented as geometric plots and will provide the following values: Distensibility is the resistance of the luminal wall to a distending force, compliance is the change in volume in response to a change in pressure, the CSA, the internal diameter of the balloon and the intra balloon pressure[13,14] (Figure 2A).
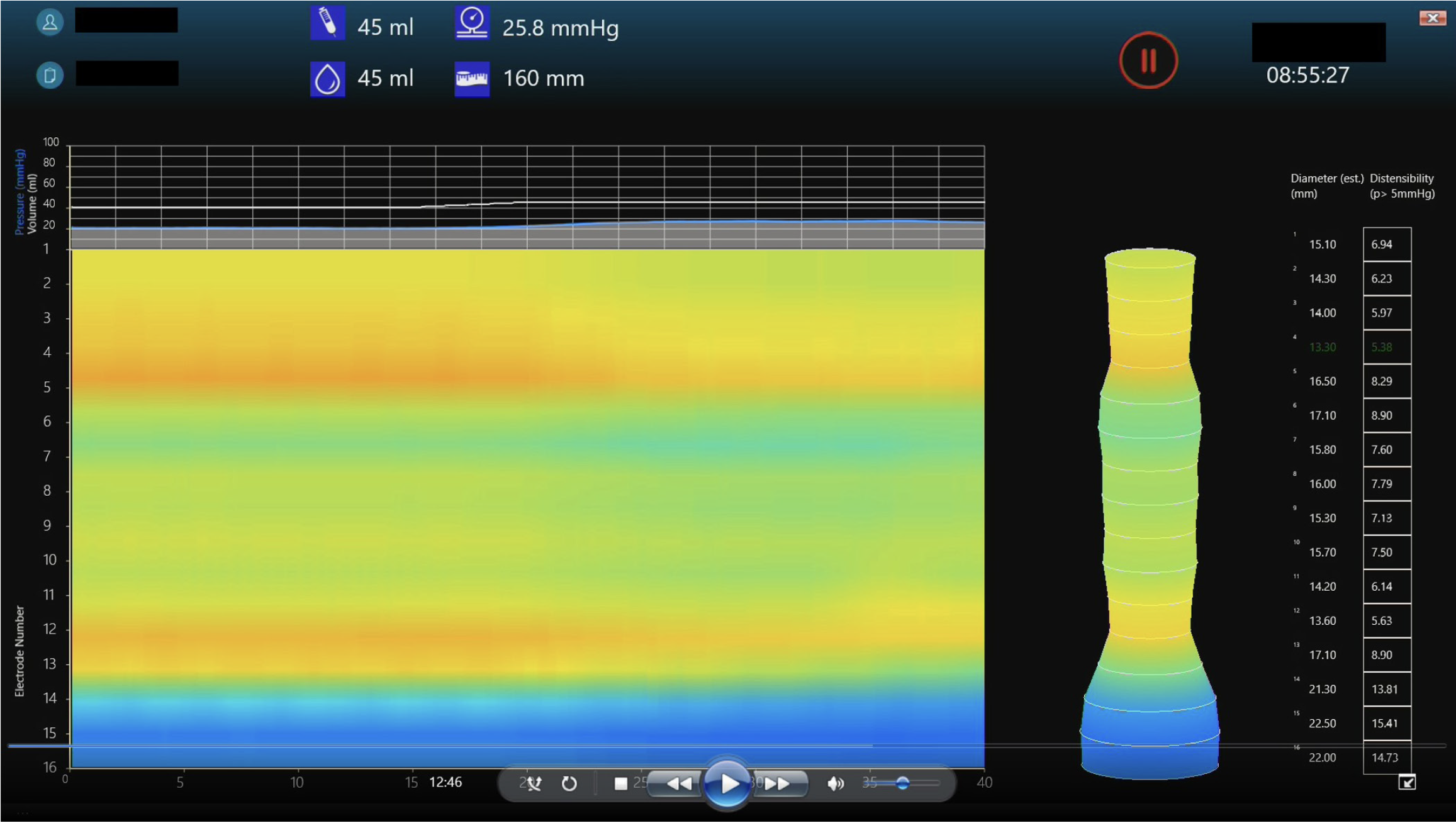
EndoFLIP has been increasingly used in clinical practice to study the lower oesophageal sphincter (LOS), the pylorus and the anal sphincters[11]. It has also been used to assess distensibility of the oesophageal body in conditions such as eosinophilic oesophagitis (EoE)[15] and to guide surgical interventions (e.g., during and post fundoplication)[16]. Recently, EndoFLIP topography was developed allowing real life display of the balloon diameter continuum during volumetric distension. Oesophageal body contraction can then be visualised and analysed[17,18] (Figure 3).
In this article, we aim to review the use of EndoFLIP as a tool to investigate GI motility disorders with a special focus on paediatric practice. We searched PubMed database for English Language literature on the following keywords: EndoFLIP; Gastrointestinal Disorders; GI disorders; oesophagus; pylorus; rectum; anorectum; paediatric; children using AND/OR combination as appropriate. The extracted articles were reviewed and their salient findings were summarised and presented in the headings below.
The oesophageal body, the LOS and the upper oesophageal sphincter were the most commonly studied part of the GI tract in regards to EndoFLIP measurement[19]. Normative values from healthy adults have been recently published in a meta-analysis[20].
Achalasia is an uncommon oesophageal motility disorder, characterised by impaired relaxation of the LOS and absent or spastic oesophageal body contractions[21]. It has three distinct types based on peristalsis patterns: Absent peristalsis in type I, pan-oesophageal body pressurisation in type II and an abnormal peristalsis with premature contractions in type III[22]. Impaired LOS relaxation is the hallmark of all three types. High resolution oesophageal manometry (HROM) is universally agreed on as the gold standard tool to diagnose and characterise achalasia[23].
In adults, EndoFLIP assessment of LOS can appropriately identify patients with achalasia who may have a normal integrated relaxation pressure but other features of achalasia[4,6]. EndoFLIP was able to bridge a gap in the understanding of the physiological behaviour of the LOS and the limitation of HROM as a sole diagnostic tool for major oesophageal motor disorders[24,25]. LOS distensibility index (DI) of < 2 mm2/mmHg is strongly suggestive of an obstructive process and is significantly lower in patients with achalasia compared to healthy volunteers[21,26,27]. LOS DI also appears to correlate well with achalasia symptoms prior to and after therapeutic interventions; achieving a definitive increase in DI after pneumatic dilatation was reported to be associated with improved clinical response[26,28]. Using DI, CSA and balloon pressure as complementary data can help predict response to therapeutic interventions [Heller’s myotomy and peroral endoscopic myotomy (POEM)] in patients with achalasia[29-31], however, the data on the use of EndoFLIP parameters as a predictor of pneumatic dilatation response is conflicting, Rohof et al[26] experience showed a positive outcome while Smeets et al[32] reports no added therapeutic benefits.
In paediatrics, EndoFLIP has been used intra-operatively during POEM and laparoscopic myotomy to guide and assess the immediate post-procedural DI[33,34]. The authors have reported a significant improvement in DI from baseline and a successful clinical outcome. Howk et al[33] have reported on the use of intra-operative EndoFLIP with the balloon inflated throughout the procedure to guide the myotomy length and provide a real time feedback on DI and CSA. Their use of EndoFLIP resulted in a shorter operative time. Figure 2A is showing EndoFLIP data from a child with type 1 achalasia showing reduced LOS DI and diameter.
EoE is a chronic immune mediated and eosinophil predominant inflammation characterised by symptoms related to oesophageal dysfunction[35]. EoE is associated with many types of oesophageal motility disorders and often thought to be secondary to oesophageal remodelling and fibrosis[36,37]. Oesophageal strictures and luminal narrowing are also common in patients with EoE[38,39]. Early identification of lamina propria fibrosis is crucial in guiding therapeutic strategies and endoscopic assessment but mucosal biopsies have a poor correlation with the degree of oesophageal fibrosis[40,41]. HROM findings are not specific or sensitive to EoE motility disorders and they do not describe a characteristic pattern for EoE symptoms[42,43].
In adults, EndoFLIP can provide an accurate information in relation to oesophageal distensibility and compliance. Reduced oesophageal distensibility is reported in both children and adults with EoE and is associated with increased risk of food bolus impaction and the need for oesophageal dilatation[40,44]. Distensibility is also significantly reduced in children with active EoE compared to an age matched control and the natural increase in oesophageal distensibility normally observed with age, appeared to plateau in children with EoE[44,45]. This may represent a fibrotic remodelling or a separate EoE phenotype[44]. Figure 2B is a child with EoE and reduced diameter/distensibility.
In paediatrics, a reduced oesophageal distensibility was reported in children with EoE and high endoscopic reference score part of (EREF)[44]. In EREF, rings and strictures are considered as features of fibrosis and remodelling changes, while oedema, furrows and exudates are considered as features of inflammatory changes[46]. It is increasingly recognised that inflammatory features can progress to fibrosis and remodelling in the context of EoE and although children can predominantly present with inflammatory EoE phenotype, early fibrosis in paediatric patients is commonly described[44,45]. Oesophageal DI, but not CSA or diameter, has a strong correlation with endoscopic fibrostenotic severity and history of food bolus impaction in paediatrics independent of age, height or oesophageal inflammation[44]. Oesophageal DI < 4.5 mm2/mmHg has been suggested as a predictor of the presence of grade II rings in children[44]. The association between EndoFLIP findings, particularly DI, and the degree of EoE inflammation in paediatric is conflicting and is in contrast to adult studies which showed no correlation between reduced DI and the degree of oesophageal inflammation[40]. This is likely to represent the small scales of paediatric studies and the difference in selection criteria.
Limited data is available on the use of EndoFLIP for the assessment of patient with gastrooesophageal reflux disease (GORD) and is mostly from adult studies. In adults, there were contradicting results regarding DI of the gastrooesophageal junction (GOJ) in the context of GORD[47,48]. In one study, GOJ DI was reported to be higher in patients with GORD compared to controls irrespective of the presence of distal oesophagitis, but the authors did not look for the presence of hiatal hernia[47]. Hiatal hernia is an important factor that may independently affect GOJ morphological studies, although in a separate study, Lottrup et al[49] have reported on the usefulness of EndoFLIP in identification and assessment of hiatal hernia. In another study, the authors did not find a statistically significant difference in the measurements of GOJ DI between patients with GORD and healthy volunteers but they did acknowledge the presence of many independent confounding factors (e.g., obesity)[48].
EndoFLIP can provide a useful tool in the assessment of fundoplication and can be used intraoperatively during anti-reflux surgery. Using FLIP intraoperatively can provide a real time measurement of GOJ DI and diameter, although direct clinical outcome data is lacking, proxy data appeared to support the clinical outcome associated with certain EndoFLIP values[16,50,51].
EndoFLIP offers a promising tool to study the oesophageal morphology post oesophageal atresia repair. One small paediatric study has reported on the use of EndoFLIP to assess the geometry of OGJ, oesophageal body distensibility and anastomotic strictures. They have reported a DI within published normal values for the oesophageal body and the OGJ, while their data on FLIP measurements of the anastomosis site were combined with other diagnostic modalities to help guide therapeutic and prognostic directions[52]. Figure 2C is a child with repaired oesophageal atresia showed a reduced diameter at the site of anastomotic scare and a dilated oesophageal segment above.
Another retrospective paediatric study has reported on the use of EndoFLIP and the FLIP dilatation catheter EsoFLIP in assessment and management of different types of oesophageal strictures in children. They reported a potential clinical benefit of reduction in procedural time and achieving a larger diameter changes post dilatation[53]. Both studies recruited a small number of children in a retrospective manner affecting their wider applicability. With such limited data, the use of EndoFLIP in the assessment of children with repaired oesophageal atresia and/or oesophageal strictures is to be explored.
In contrast to LOS, the pyloric sphincter did not receive the same level of diagnostic scrutiny. Although the pylorus plays a vital role in regulating gastric emptying (GE), the association between symptom severity and measured GE remains poor[54]. In part, this can be explained by the complex role of the pylorus sphincter compared to other GI sphincters. Detailed assessment of post prandial period reported that GE occurred during the relaxation of the pylorus and was driven primarily by the pressure generated by gastric tone and content (pressure pump) rather than the effect of antral contraction wave (peristalsis pump)[55]. On the other hand, contraction of the pylorus in the face of gastric peristalsis wave will lead to bolus retention and gastric content mixing. However, the pylorus pressure measurements did not differ in patients with normal or delayed GE[54,56].
In adults, geometric assessment of the pylorus by EndoFLIP has been widely studied in patients with gastroparesis or with symptoms suggestive of gastroparesis (such as nausea and vomiting)[57-59]. Pylorus DI has been found to correlate with GE and gastroparesis symptoms, a promising finding to identify a group of patients who may respond to targeted pylorus therapy[58,59].
EndoFLIP catheter is usually inserted per os under direct endoscopic vision and prior to endoscopic intubation of the pylorus. The effect of anaesthesia and sedation on the EndoFLIP findings are yet to be studied. Pylorus geometric measurements are often taken after the EndoFLIP balloon is filled with a predetermined volume of 20, 30, 40 and 50 mL. Pylorus DI was significantly lower in patients with gastroparesis compared to healthy volunteers, but there was no difference in DI between diabetic gastroparesis and other forms of the disorder[19]. Early satiety and postprandial fullness were inversely correlated to pylorus CSA and diameter, this has been the only clear association between a diagnostic investigation and symptoms of gastroparesis, in contrast to other investigative modalities such as GE scintigraphy and manometry[58]. Although the authors were unable to offer a concrete explanation of this association, and the absence of correlation with any EndoFLIP parameters and other gastroparesis symptoms, it is possible that the pylorus opening diameter rather than distensibility or compliance is the driving force behind these two symptoms but further research is needed to study this hypothesis.
EndoFLIP has also been used to predict clinical response to intra pylorus Botulinum Toxin injection[60]. Patients with gastroparesis and an abnormal pylorus EndoFLIP measurements appear to show sustained symptom improvement at 3 mo compared to gastroparesis patients with normal FLIP parameters[60]. A similar clinical response was also observed post pylorus dilatation and gastric POEM guided by EndoFLIP findings in patients with therapy refractory gastroparesis[59,61].
In paediatrics, Hirsch et al[62] have reported in a retrospective observational study in children with nausea and vomiting that the pylorus distensibility were lower in children with delayed GE compared to the normal GE group but the differences were not statistically significant. This is likely to be secondary to small sample size and patient selection. In the same study, EndoFLIP did not predict the symptomatic response to intra pylorus Botulinum Toxin injection[62]. A likely explanation is the selection criteria for included children as 44% had normal GE and previous adult studies did not show correlation between EndoFLIP and the reported symptoms of nausea and vomiting.
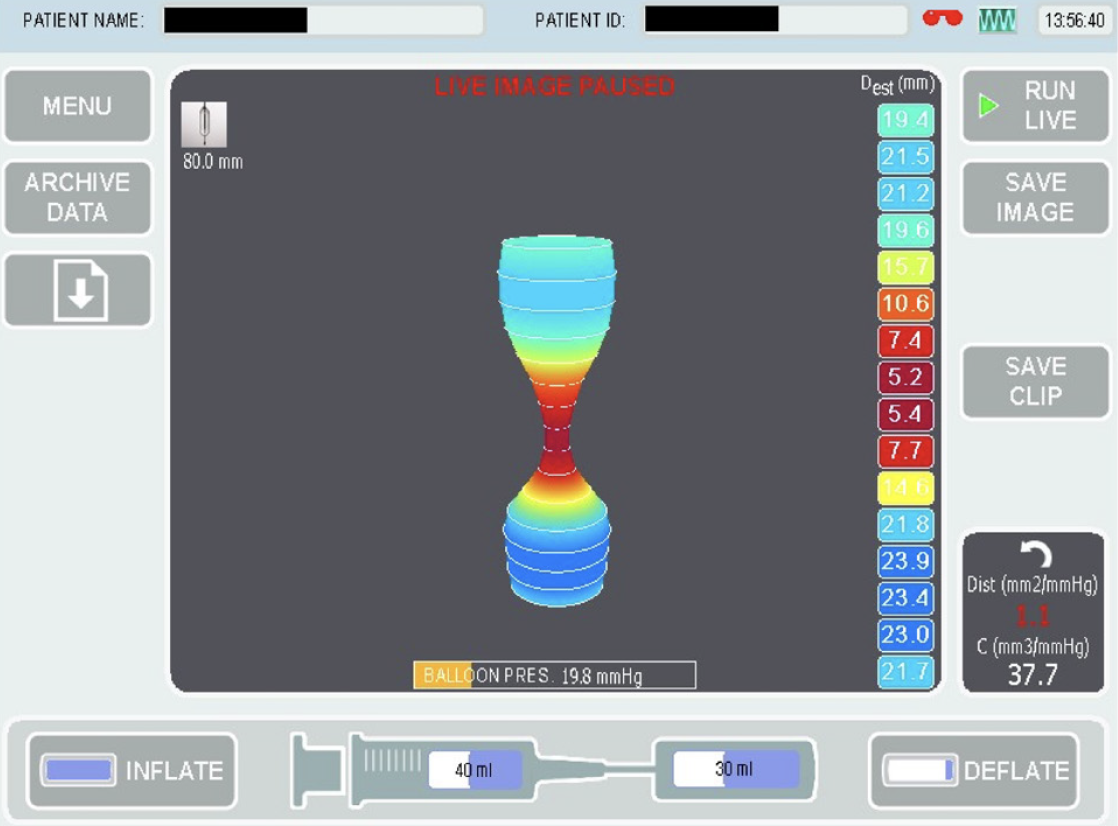
We have recently published our experience in using pylorus EndoFLIP in children with neuromuscular disorders and the response to Botulinum Toxin injection[63]. In our cohort, children with symptoms suggestive of gastroparesis had abnormal EndoFLIP measurements according to both adults and previous paediatric studies. We reported good symptoms improvement, weight gain and feed tolerance after Botulinum Toxin injections, a response that had been replicated after repeat injections 6-9 mo later. We did observe a trend of improvement in EndoFLIP measurements even though the FLIP remeasurements were obtained due to symptoms recurrence. Our sample size was small and we did not measure GE due to the comorbidities present in our studied group[63]. Figure 4 is from a child with gastroparesis showed reduced pylorus distensibility. EndoFLIP usage in the assessment of pylorus sphincter is increasing and appear to show promising diagnostic data, symptom correlation with GE and most importantly, it can identify a subset of patients who can response to targeted pylorus therapy.
There are very few studies reporting on the use of EndoFLIP in assessment of the geometry of the anal canal, all were from adult patient and healthy volunteers[64-66]. To date, there is no published paediatric study. Although there was a general harmony on the methods used in all published studies, there were no agreement on the relevant FLIP measurements or their applicability to clinical practice. All published studies have reported a higher anal distensibility in patients with faecal incontinence compared to healthy volunteers, however, the clinical utility of such a finding is yet to be explored.
EndoFLIP is a promising tool to further improve our ability to understand the motility function of the GI tract. By providing a detailed geometric assessment to the oesophageal lumen and various sphincters in the GI tract, EndoFLIP findings are complementary to the existing GI motility investigations, but the data does not support the use of EndoFLIP to replace standard GI motility investigations. EndoFLIP has been successfully used to aid diagnosis and to guide therapeutic interventions. EndoFLIP studies in paediatrics are lagging behind adult studies but they have replicated some of adult results. The absence of normative values for children may limit the wider uptake within paediatrics but with the increase in complexity and prevalence of paediatric GI motility disorders, EndoFLIP can provide a valuable diagnostic and prognostic data.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: British Society of Paediatric Gastroenterology, Hepatology and Nutrition; European Society of Paediatric Gastroenterology, Hepatology and Nutrition; European Society of Neurogastroenterology and Motility.
Specialty type: Pediatrics
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): E
P-Reviewer: Batta A, India; Lee ACW, Singapore S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Keller J, Bassotti G, Clarke J, Dinning P, Fox M, Grover M, Hellström PM, Ke M, Layer P, Malagelada C, Parkman HP, Scott SM, Tack J, Simren M, Törnblom H, Camilleri M; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol. 2018;15:291-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 2. | Rao SS, Camilleri M, Hasler WL, Maurer AH, Parkman HP, Saad R, Scott MS, Simren M, Soffer E, Szarka L. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23:8-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Bogte A, Bredenoord AJ, Oors J, Siersema PD, Smout AJ. Assessment of bolus transit with intraluminal impedance measurement in patients with esophageal motility disorders. Neurogastroenterol Motil. 2015;27:1446-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Ponds FA, Bredenoord AJ, Kessing BF, Smout AJ. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Carlson DA, Lin Z, Kahrilas PJ, Sternbach J, Hungness ES, Soper NJ, Balla M, Listernick Z, Tye M, Ritter K, Craft J, Ciolino JD, Pandolfino JE. High-Resolution Impedance Manometry Metrics of the Esophagogastric Junction for the Assessment of Treatment Response in Achalasia. Am J Gastroenterol. 2016;111:1702-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Triggs JR, Carlson DA, Beveridge C, Kou W, Kahrilas PJ, Pandolfino JE. Functional Luminal Imaging Probe Panometry Identifies Achalasia-Type Esophagogastric Junction Outflow Obstruction. Clin Gastroenterol Hepatol. 2020;18:2209-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Pandolfino JE, Shi G, Curry J, Joehl RJ, Brasseur JG, Kahrilas PJ. Esophagogastric junction distensibility: a factor contributing to sphincter incompetence. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1052-G1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Gyawali CP, Bredenoord AJ, Conklin JL, Fox M, Pandolfino JE, Peters JH, Roman S, Staiano A, Vaezi MF. Evaluation of esophageal motor function in clinical practice. Neurogastroenterol Motil. 2013;25:99-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | McMahon BP, Rao SS, Gregersen H, Kwiatek MA, Pandolfino JE, Drewes AM, Krarup AL, Lottrup C, Frøkjaer JB. Distensibility testing of the esophagus. Ann N Y Acad Sci. 2011;1232:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Savarino E, di Pietro M, Bredenoord AJ, Carlson DA, Clarke JO, Khan A, Vela MF, Yadlapati R, Pohl D, Pandolfino JE, Roman S, Gyawali CP. Use of the Functional Lumen Imaging Probe in Clinical Esophagology. Am J Gastroenterol. 2020;115:1786-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Hirano I, Pandolfino JE, Boeckxstaens GE. Functional Lumen Imaging Probe for the Management of Esophageal Disorders: Expert Review From the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15:325-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | McMahon BP, Frøkjaer JB, Liao D, Kunwald P, Drewes AM, Gregersen H. A new technique for evaluating sphincter function in visceral organs: application of the functional lumen imaging probe (FLIP) for the evaluation of the oesophago-gastric junction. Physiol Meas. 2005;26:823-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Donnan EN, Pandolfino JE. EndoFLIP in the Esophagus: Assessing Sphincter Function, Wall Stiffness, and Motility to Guide Treatment. Gastroenterol Clin North Am. 2020;49:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Carlson DA, Gyawali CP, Khan A, Yadlapati R, Chen J, Chokshi RV, Clarke JO, Garza JM, Jain AS, Katz P, Konda V, Lynch K, Schnoll-Sussman FH, Spechler SJ, Vela MF, Prescott JE, Baumann AJ, Donnan EN, Kou W, Kahrilas PJ, Pandolfino JE. Classifying Esophageal Motility by FLIP Panometry: A Study of 722 Subjects With Manometry. Am J Gastroenterol. 2021;116:2357-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 15. | Nicodème F, Hirano I, Chen J, Robinson K, Lin Z, Xiao Y, Gonsalves N, Kwasny MJ, Kahrilas PJ, Pandolfino JE. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11:1101-1107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 16. | Su B, Attaar M, Wong H, Callahan ZM, Kuchta K, Stearns S, Linn JG, Denham W, Haggerty SP, Ujiki MB. Using a standardized intra-operative endoflip protocol during fundoplication to identify factors that affect distensibility. Surg Endosc. 2021;35:5717-5723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Carlson DA, Lin Z, Kahrilas PJ, Sternbach J, Donnan EN, Friesen L, Listernick Z, Mogni B, Pandolfino JE. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology. 2015;149:1742-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Carlson DA, Kahrilas PJ, Ritter K, Lin Z, Pandolfino JE. Mechanisms of repetitive retrograde contractions in response to sustained esophageal distension: a study evaluating patients with postfundoplication dysphagia. Am J Physiol Gastrointest Liver Physiol. 2018;314:G334-G340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Desprez C, Roman S, Leroi AM, Gourcerol G. The use of impedance planimetry (Endoscopic Functional Lumen Imaging Probe, EndoFLIP(®) ) in the gastrointestinal tract: A systematic review. Neurogastroenterol Motil. 2020;32:e13980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Bredenoord AJ, Rancati F, Lin H, Schwartz N, Argov M. Normative values for esophageal functional lumen imaging probe measurements: A meta-analysis. Neurogastroenterol Motil. 2022;34:e14419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Savarino E, Bhatia S, Roman S, Sifrim D, Tack J, Thompson SK, Gyawali CP. Achalasia. Nat Rev Dis Primers. 2022;8:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 22. | Yadlapati R, Kahrilas PJ, Fox MR, Bredenoord AJ, Prakash Gyawali C, Roman S, Babaei A, Mittal RK, Rommel N, Savarino E, Sifrim D, Smout A, Vaezi MF, Zerbib F, Akiyama J, Bhatia S, Bor S, Carlson DA, Chen JW, Cisternas D, Cock C, Coss-Adame E, de Bortoli N, Defilippi C, Fass R, Ghoshal UC, Gonlachanvit S, Hani A, Hebbard GS, Wook Jung K, Katz P, Katzka DA, Khan A, Kohn GP, Lazarescu A, Lengliner J, Mittal SK, Omari T, Park MI, Penagini R, Pohl D, Richter JE, Serra J, Sweis R, Tack J, Tatum RP, Tutuian R, Vela MF, Wong RK, Wu JC, Xiao Y, Pandolfino JE. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0(©). Neurogastroenterol Motil. 2021;33:e14058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 585] [Article Influence: 146.3] [Reference Citation Analysis (1)] |
| 23. | Vaezi MF, Pandolfino JE, Yadlapati RH, Greer KB, Kavitt RT. ACG Clinical Guidelines: Diagnosis and Management of Achalasia. Am J Gastroenterol. 2020;115:1393-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 24. | Mearin F, Malagelada JR. Complete lower esophageal sphincter relaxation observed in some achalasia patients is functionally inadequate. Am J Physiol Gastrointest Liver Physiol. 2000;278:G376-G383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Ghosh SK, Kahrilas PJ, Lodhia N, Pandolfino JE. Utilizing intraluminal pressure differences to predict esophageal bolus flow dynamics. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1023-G1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Rohof WO, Hirsch DP, Kessing BF, Boeckxstaens GE. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 27. | Pandolfino JE, de Ruigh A, Nicodème F, Xiao Y, Boris L, Kahrilas PJ. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP™) in achalasia patients. Neurogastroenterol Motil. 2013;25:496-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 28. | Jain AS, Carlson DA, Triggs J, Tye M, Kou W, Campagna R, Hungness E, Kim D, Kahrilas PJ, Pandolfino JE. Esophagogastric Junction Distensibility on Functional Lumen Imaging Probe Topography Predicts Treatment Response in Achalasia-Anatomy Matters! Am J Gastroenterol. 2019;114:1455-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Teitelbaum EN, Soper NJ, Pandolfino JE, Kahrilas PJ, Hirano I, Boris L, Nicodème F, Lin Z, Hungness ES. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc. 2015;29:522-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 30. | Holmstrom AL, Campagna RJ, Carlson DA, Pandolfino JE, Soper NJ, Hungness ES, Teitelbaum EN. Comparison of preoperative, intraoperative, and follow-up functional luminal imaging probe measurements in patients undergoing myotomy for achalasia. Gastrointest Endosc. 2021;94:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Hsing LC, Choi K, Jung KW, Joo S, Kim N, Kim GH, Na HK, Ahn JY, Lee J, Kim DH, Choi KD, Song HJ, Lee GH, Jung HY. The Predictive Value of Intraoperative Esophageal Functional Luminal Imaging Probe Panometry in Patients With Achalasia Undergoing Peroral Endoscopic Myotomy: A Single-center Experience. J Neurogastroenterol Motil. 2022;28:474-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Smeets FG, Masclee AA, Keszthelyi D, Tjwa ET, Conchillo JM. Esophagogastric junction distensibility in the management of achalasia patients: relation to treatment outcome. Neurogastroenterol Motil. 2015;27:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Howk AA, Clifton MS, Garza JM, Durham MM. Impedance planimetry (EndoFLIP) assisted laparoscopic esophagomyotomy in pediatric population. J Pediatr Surg. 2022;57:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Yeung F, Wong IYH, Chung PHY, Wong KKY, Law SYK, Tam PKH. Peroral Endoscopic Myotomy with EndoFLIP and Double-Endoscope: Novel Techniques for Achalasia in Pediatric Population. J Laparoendosc Adv Surg Tech A. 2018;28:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Dhar A, Haboubi HN, Attwood SE, Auth MKH, Dunn JM, Sweis R, Morris D, Epstein J, Novelli MR, Hunter H, Cordell A, Hall S, Hayat JO, Kapur K, Moore AR, Read C, Sami SS, Turner PJ, Trudgill NJ. British Society of Gastroenterology (BSG) and British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut. 2022;71:1459-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 36. | Chai C, Krishnan U. Dysmotility in Eosinophilic Esophagitis. Front Pediatr. 2022;10:853754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 37. | Carlson DA, Shehata C, Gonsalves N, Hirano I, Peterson S, Prescott J, Farina DA, Schauer JM, Kou W, Kahrilas PJ, Pandolfino JE. Esophageal Dysmotility Is Associated With Disease Severity in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2022;20:1719-1728.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 38. | Hoofien A, Dias JA, Malamisura M, Rea F, Chong S, Oudshoorn J, Nijenhuis-Hendriks D, Otte S, Papadopoulou A, Romano C, Gottrand F, Miravet VV, Orel R, Oliva S, Junquera CG, Załęski A, Urbonas V, Garcia-Puig R, Gomez MJM, Dominguez-Ortega G, Auth MK, Kori M, Ben Tov A, Kalach N, Velde SV, Furman M, Miele E, Marderfeld L, Roma E, Zevit N. Pediatric Eosinophilic Esophagitis: Results of the European Retrospective Pediatric Eosinophilic Esophagitis Registry (RetroPEER). J Pediatr Gastroenterol Nutr. 2019;68:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1175-G1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 41. | Lin Z, Kahrilas PJ, Xiao Y, Nicodème F, Gonsalves N, Hirano I, Pandolfino JE. Functional luminal imaging probe topography: an improved method for characterizing esophageal distensibility in eosinophilic esophagitis. Therap Adv Gastroenterol. 2013;6:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Nennstiel S, Bajbouj M, Becker V, Slotta-Huspenina J, Wagenpfeil S, Schmid RM, Schlag C. High-resolution manometry in patients with eosinophilic esophagitis under topical steroid therapy-a prospective observational study (HIMEOS-study). Neurogastroenterol Motil. 2016;28:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Visaggi P, Ghisa M, Barberio B, Marabotto E, de Bortoli N, Savarino E. Systematic Review: esophageal motility patterns in patients with eosinophilic esophagitis. Dig Liver Dis. 2022;54:1143-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Hoffmann NV, Keeley K, Wechsler JB. Esophageal Distensibility Defines Fibrostenotic Severity in Pediatric Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2023;21:1188-1197.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 45. | Menard-Katcher C, Benitez AJ, Pan Z, Ahmed FN, Wilkins BJ, Capocelli KE, Liacouras CA, Verma R, Spergel JM, Furuta GT, Muir AB. Influence of Age and Eosinophilic Esophagitis on Esophageal Distensibility in a Pediatric Cohort. Am J Gastroenterol. 2017;112:1466-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 46. | Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 649] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 47. | Lee JM, Yoo IK, Kim E, Hong SP, Cho JY. The Usefulness of the Measurement of Esophagogastric Junction Distensibility by EndoFLIP in the Diagnosis of Gastroesophageal Reflux Disease. Gut Liver. 2021;15:546-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Tucker E, Sweis R, Anggiansah A, Wong T, Telakis E, Knowles K, Wright J, Fox M. Measurement of esophago-gastric junction cross-sectional area and distensibility by an endolumenal functional lumen imaging probe for the diagnosis of gastro-esophageal reflux disease. Neurogastroenterol Motil. 2013;25:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Lottrup C, McMahon BP, Ejstrud P, Ostapiuk MA, Funch-Jensen P, Drewes AM. Esophagogastric junction distensibility in hiatus hernia. Dis Esophagus. 2016;29:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Wu H, Attaar M, Wong HJ, Campbell M, Kuchta K, Denham EW 3rd, Linn J, Ujiki MB. Impedance Planimetry (Endoflip) and Ideal Distensibility Ranges for Optimal Outcomes after Nissen and Toupet Fundoplication. J Am Coll Surg. 2022;235:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 51. | Fanous M, Wei W. The Outcomes of Performing Partial Fundoplication Based on Endoflip Versus Manometric Findings. Am Surg. 2022;88:908-914. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Destro F, Costanzo S, Durante E, Carcassola MS, Meroni M, Brunero M, Riccio A, Calcaterra V, Pelizzo G. Utility of Functional Lumen Imaging Probe in Long-Term Follow-Up of Children with Esophageal Atresia: A Single-Center Retrospective Study. Children (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 53. | Ng K, Mogul D, Hollier J, Khashab MA. Utility of functional lumen imaging probe in esophageal measurements and dilations: a single pediatric center experience. Surg Endosc. 2020;34:1294-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Soliman H, Gourcerol G. Targeting the pylorus in gastroparesis: From physiology to endoscopic pyloromyotomy. Neurogastroenterol Motil. 2023;35:e14529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 55. | Wuestenberghs F, Gourcerol G. Pyloric distensibility in health and disease. Am J Physiol Gastrointest Liver Physiol. 2021;321:G133-G138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Sullivan A, Temperley L, Ruban A. Pathophysiology, Aetiology and Treatment of Gastroparesis. Dig Dis Sci. 2020;65:1615-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Snape WJ, Lin MS, Agarwal N, Shaw RE. Evaluation of the pylorus with concurrent intraluminal pressure and EndoFLIP in patients with nausea and vomiting. Neurogastroenterol Motil. 2016;28:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 58. | Malik Z, Sankineni A, Parkman HP. Assessing pyloric sphincter pathophysiology using EndoFLIP in patients with gastroparesis. Neurogastroenterol Motil. 2015;27:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 59. | Jehangir A, Malik Z, Petrov RV, Parkman HP. EndoFLIP and Pyloric Dilation for Gastroparesis Symptoms Refractory to Pyloromyotomy/Pyloroplasty. Dig Dis Sci. 2021;66:2682-2690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Desprez C, Melchior C, Wuestenberghs F, Zalar A, Jacques J, Leroi AM, Gourcerol G. Pyloric distensibility measurement predicts symptomatic response to intrapyloric botulinum toxin injection. Gastrointest Endosc. 2019;90:754-760.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 61. | Attaar M, Su B, Wong HJ, Kuchta K, Denham W, Haggerty S, Linn J, Ujiki MB. Significant changes in impedance planimetry (EndoFLIP™) measurements after peroral pyloromyotomy for delayed gastric emptying. Surg Endosc. 2022;36:1536-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Hirsch S, Nurko S, Liu E, Rosen R. A prospective study of intrapyloric botulinum toxin and EndoFLIP in children with nausea and vomiting. Neurogastroenterol Motil. 2022;34:e14428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Popescu M, White E, Mutalib M. EndoFLIP assessment of pyloric sphincter in children: a single-center experience. Transl Gastroenterol Hepatol. 2023;8:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 64. | Desprez C, Gourcerol G, Savoye-Collet C, Bridoux V, Duflot T, Leroi AM. Relationship between anal functional lumen imaging probe (EndoFLIP®) results and the clinical presentation of faecal incontinence. Colorectal Dis. 2022;24:1379-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 65. | Alqudah MM, Gregersen H, Drewes AM, McMahon BP. Evaluation of anal sphincter resistance and distensibility in healthy controls using EndoFLIP ©. Neurogastroenterol Motil. 2012;24:e591-e599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Zifan A, Sun C, Gourcerol G, Leroi AM, Mittal RK. Endoflip vs high-definition manometry in the assessment of fecal incontinence: A data-driven unsupervised comparison. Neurogastroenterol Motil. 2018;30:e13462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |