Published online Jan 9, 2022. doi: 10.5409/wjcp.v11.i1.71
Peer-review started: June 4, 2021
First decision: October 17, 2021
Revised: October 21, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 9, 2022
Processing time: 216 Days and 11.9 Hours
Right ventricular (RV) function is frequently overlooked during dilated cardiomyopathy (DCM) evaluation.
To evaluate RV function in children with idiopathic DCM using relatively recent echocardiographic modalities.
We prospectively studied the cardiac function in 50 children with idiopathic DCM and 50 healthy children as a control group, using four-dimensional echocardiography (4-DE), Tissue Doppler Imaging (TDI), and two-dimensional-speckles tracking echocardiography (2-D-STE). RV EF was measured by 4-DE.
The auto left (LV) ejection fractions (EF) measured by 2-D-STE were significantly lower in the patients' group than in the control. The sphericity index was also significantly lower in children with DCM than in the control. RV EF measured by 4-DE was significantly lower in the patient's group than the control. RV S wave, e´/a' ratio, myocardial performance index (MPI), and tricuspid annular plane systolic excursion (TAPSE) were significantly impaired in children with DCM than in control. Both LV and RV global longitudinal strains (GLS) were signi
There was impairment of the RV LGS and other systolic and diastolic parameters in children with DCM. STE and TDI can help to detect the early decline of RV function.
Core Tip: Cardiomyopathies are a group of cardiac muscle disorders characterized by mechanical and/or electrical impairment that give rise to dilated, hypertrophic or restrictive pathophysiology. In the current study, we prospectively studied the cardiac function in 50 children with idiopathic dilated cardiomyopathy (DCM) and 50 healthy children as a control group using Tissue Doppler Imaging (TDI) and two-dimensional-speckles tracking echocardiography. Right ventricular (RV) ejection fractions was measured by four-dimensional echocardiography. We found impairment of the RV LGS and other systolic and diastolic parameters in children with DCM. Speckles tracking echocardiography and TDI can help to detect the early decline of RV function.
- Citation: Al-Biltagi M, Elrazaky O, Mawlana W, Srour E, Shabana AH. Tissue Doppler, speckling tracking and four-dimensional echocardiographic assessment of right ventricular function in children with dilated cardiomyopathy. World J Clin Pediatr 2022; 11(1): 71-84
- URL: https://www.wjgnet.com/2219-2808/full/v11/i1/71.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v11.i1.71
Cardiomyopathies are a group of cardiac muscle disorders characterized by me
Clinical presentation of DCM mainly relates to the degree of LV or biventricular systolic dysfunction leading to pump failure. Heart failure signs and symptoms may be fulminant, acute, subacute, or chronic[1]. DCM diagnosis is primarily based on echocardiography that can readily identify the dilated chambers and the impaired function of left or both ventricles using M-mode, 2-dimensional echocardiography[3]. Tissue Doppler imaging (TDI) is a relatively new echocardiographic technique, useful to study the myocardial function of children with different pathologies[4]. Two-dimensional speckle tracking imaging is another relatively new echocardiographic modality that can provide non-Doppler, less angle-dependent, and objective quantification of myocardial deformation and left/right ventricular (LV/RV) systolic and diastolic dynamics by analyzing the motion of speckles identified on routine 2-dimensional sonograms. In addition, by tracking the displacement of the speckles during the cardiac cycle, strain and the strain rate can be rapidly measured offline after good image acquisition[5].
Most of the studies concerned with the diagnosis of dilated cardiomyopathies in children focus on LV function. As the disease usually starts in LV then RV, early detection of RV dysfunction could have a prognostic benefit. Unfortunately, few studies examined RV function[6-9]. So, this work aimed to evaluate the RV function and structure using tissue Doppler and speckling tracking echocardiography in children with primary dilated cardiomyopathy and correlate with other echocardiographic findings.
The research was a prospective cross-section study between April 2018 to June 2019. It involved 50 children with primary idiopathic dilated cardiomyopathy; aged between six months and eight years, selected in the order in which they were identified, with regular attendance to Pediatric Cardiology Unit in a Tertiary Care University Hospital. It also included o 50 healthy children of matching age and sex, coming for routine health check-up without any systemic disease that could affect the heart (control group). All parents, guardians, or next of kin signed informed consent for the minors to participate in this study. The Institutional Ethical and Research Review Board of Faculty of Medicine, Tanta University, approved the study.
The diagnosis of DCM based on the detection of dilatation of ventricles (LV) dilatation > 112% corrected to body surface area (BSA), age, and sex, or two standard deviations (SD) from the normal upper limit corrected to age, sex, and BSA plus (5%) and presence of systolic dysfunction [fractional shortening (FS) < 25% and/or left ventricle ejection fraction (LV EF) < 45%] detected in m-mode and 2-D echocardiography following the recommendation of the 2006 American Heart Association and 2017 British Society of Echocardiography[10]. Exclusion criteria included children with congenital or acquired heart diseases and children with dilated cardiomyopathy secondary to systemic diseases such as infections (ruled out by the history and laboratory tests for the previous infection with the common viral and bacterial causes), arrhythmias, endocrine diseases, neuromuscular diseases, rheumatologic and immunological diseases, nutritional deficiencies, conditions leading to ischemia, drug or toxins-induced, and systemic diseases.
All children had complete history taking (including personal, birth, developmental, feeding, and family history) and comprehensive clinical examination (including general, regional, and systemic examination). Cardiac examination aimed to detect cardiomegaly and evidence of the presence of a cardiac murmur. In addition, all children had echocardiographic examinations, including 2-D, M-mode, TDI, and 2D- speckles tracking echocardiography (STE).
Echocardiography was done using (Vingmed Vivid-7, General Electric Vingmed, and Milwaukee, Wisconsin, United States). The examination was performed using an S7 probe and V3 matrix real-time 3-dimensional probes at a depth of 16 cm in all the standard echocardiographic views following the American Society of Echocardiography recommendation[11]. All children were examined in the right anterior oblique position, when possible, while breathing room air or on oxygen supplement when required. Cardiovascular anomalies were carefully searched for and excluded by all standard views. LV EF, LV FS, end-diastolic and end-systolic volumes, systolic pulmonary artery pressure by using tricuspid regurgitation jet, and LV and RV diastolic function were measured according to the guidelines of the American Society of Echocardiography[12]. Tricuspid annular plane systolic excursion (TAPSE) was measured by 2-D echocardiography-guided M-mode from the apical 4-chamber view, with the cursor was placed at the free wall side of the tricuspid annulus. Care was taken to align the sample volume as vertically as possible concerning the cardiac apex. Maximal TAPSE was determined by the total excursion of the tricuspid annulus from its highest position after atrial ascent to the lowest point of descent during ventricular systole. The sphericity index (SI) was measured by calculating the ratio between the length (mitral annulus to apex in the apical view) and diameter (mid-cavity level in the short-axis view) of the LV. The smaller the SI is, the more globular the ventricle will be. It also predicts the functional capacity of patients with LV dysfunction[13]. Simultaneous ECG tracings obtained during M-mode recording were used to measure R-R intervals. Measurements were repeated on three occasions, and the average was obtained[14].
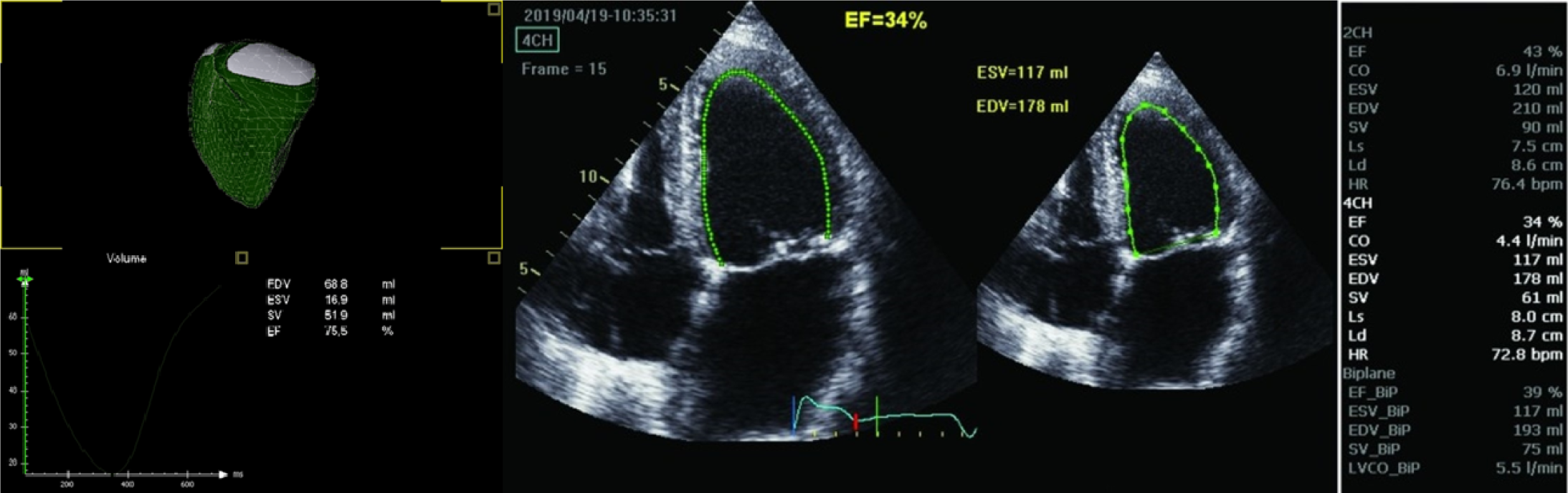
All children had three-dimensional (3-D)-echocardiography immediately after the 2-D-echocardiographic examination, using the same ultrasound machine equipped with a 4V probe. RV 3-D images were obtained in a full-volume dataset from the apical 4-chamber view, optimized for analysis of RV function. All the measurements of RV volumes and EF were made offline, using dedicated software. The semi-automatic analysis was performed using a manual tracing of the endocardial borders in end-systolic and end-diastolic frames in the sagittal, four-chamber, and coronal views. Besides, end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume, and EF were calculated using the software (Figure 1).
We used the same machine and probe to perform TDI at a depth of 16 cm in the parasternal and apical views (standard long axis and two- and four-chamber images). The baseline was adjusted to a low-velocity range (-20-20 cm/s) while using the pulsed-wave angle-corrected colour-coded TDI filters. The Doppler frame rates were varied between 80 and 115 frames/s depending on the sector width of the range of interest. We settled the setting to the minimal gain to reduce the background noise and get the highest quality images. The 2-millimeters sample volume was placed within the myocardium equidistant from the endocardial and epicardial borders.
The pulsed-wave TDI recorded the myocardial velocity curves of the septal mitral valve annulus, lateral mitral valve annulus, and lateral tricuspid valve annulus from the apical four-chamber planes. The timing of cardiac cycle events and their relations to respiratory events were defined by simultaneously tracing the electrocardiogram and respiration curve monitoring. The beginning of the QRS complex was the reference point. At least ten cardiac cycles were recorded at a speed of 100 mm/s. The images were stored electronically.
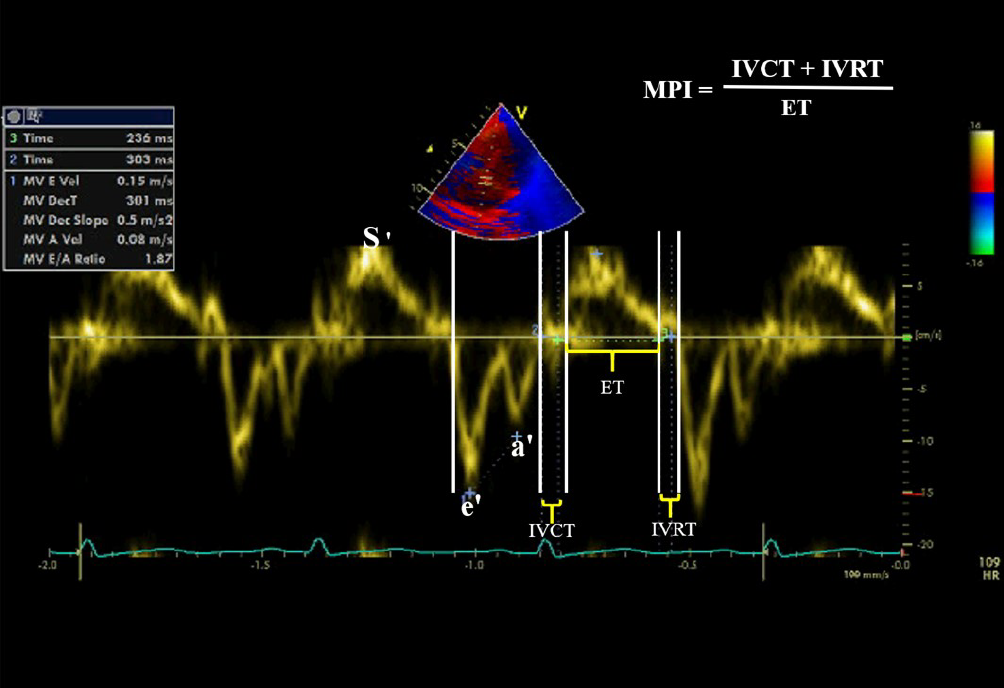
The systolic and diastolic mitral and tricuspid annulus velocities were determined by placing the PW-TDI sample volume at the level of the septal tricuspid annulus. In the spectral TDI display, the antegrade systolic wave S reflected the systolic function of either RV or LV, e´ retrograde wave reflected the early passive ventricular filling, while the retrograde a´ wave represented the atrial contraction. The early/atrial (e´/a´) ratio of tricuspid or mitral valve annulus reflected the diastolic function of the RV or LV (Figure 2). The isometric contraction time (ICT) (was defined as the time duration between the end of a´ wave to the beginning of S wave by TDI), the isometric relaxation time (IRT) (was defined as the interval between the end of S wave and the beginning of the early wave), and myocardial contraction time (CT) were measured by TDI[15]. Myocardial Performance or Tei index is a Doppler-derived time interval index that combines systolic and diastolic cardiac performance. It was calculated as previously described by Tei and colleagues, using the following formula: ICT + IRT/CT. Both ICT and IRT were corrected for heart rate[16-17]. We used the mean values for three heartbeats during expiration for the analysis, and all the measurements obtained by TDI were indexed for children’s body surface area.
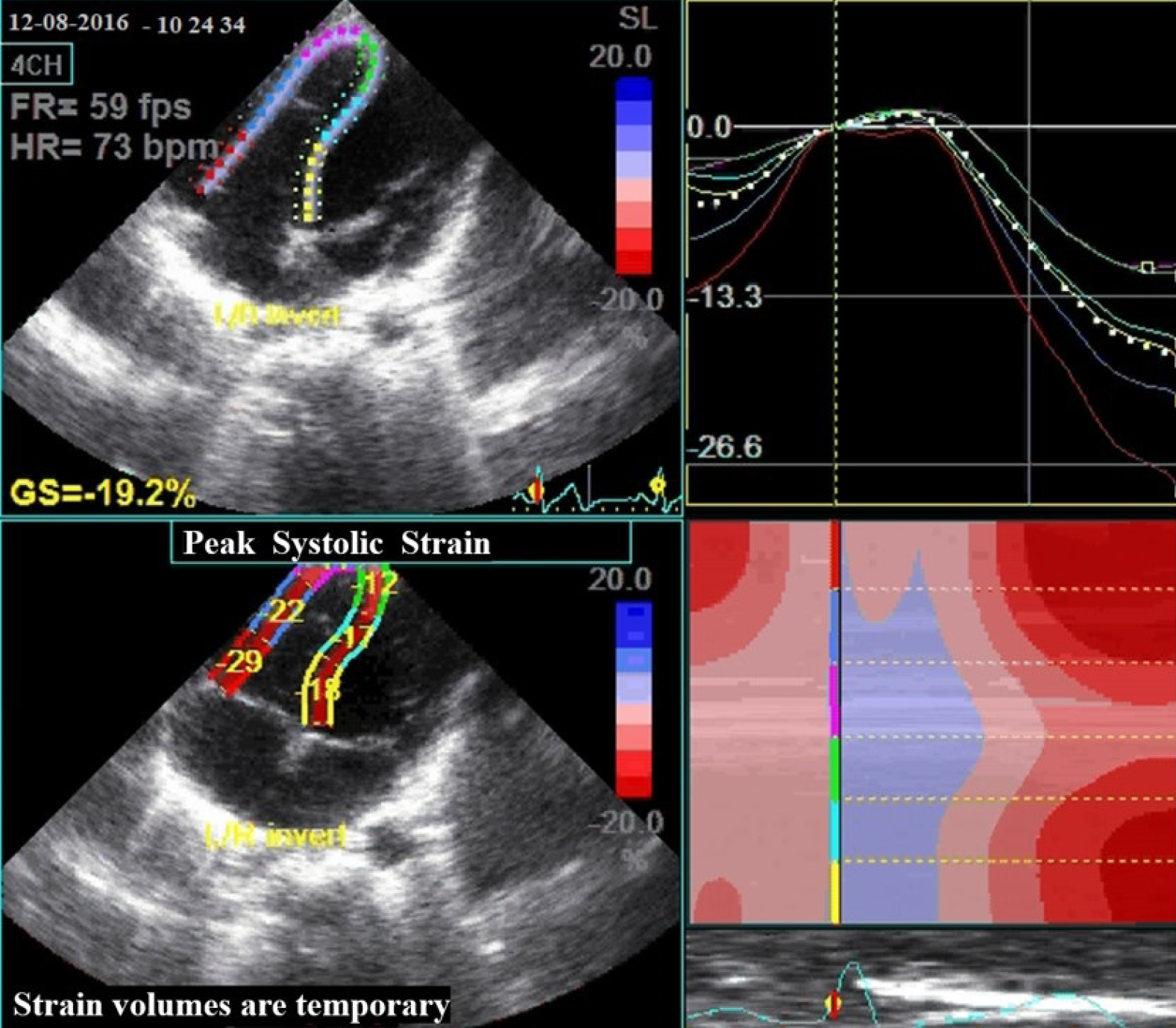
We used a 3.5-MHz transducer at a depth of 16 cm in the standard apical 4-chamber view (4-chamber image) to acquire an adequate image with novel speckle-tracking software (Figure 3). To prevent foreshortening, we visualized the apex of the left ventricle adequately. Then, 2-D speckle tracking strain imaging was used to study LV and RV deformation on standard grayscale images (frame rate, 55 ± 11 frames/s). It tracked the characteristic pattern of natural acoustic markers in the myocardial wall (“speckles”) from frame to frame throughout the cardiac cycle. Two-D longitudinal strain for all RV myocardial segments includes three segments from the lateral (anterior) wall (basal, mid, and apical) and three corresponding segments of the interventricular septum (because of the significant contribution of the interventricular septum to RV ejection). The myocardial strain was then calculated by the change in position of the speckle pattern to the initial position. Peak systolic longitudinal strain was calculated by averaging the peak systolic values of the six segments. Regional lengthening of myocardial strain was expressed as a positive value and thinning or shortening as a negative value[18].
We obtained RV 4D images in a full-volume dataset from the apical four-chamber view, optimized for analysis of RV function. In addition, we obtained multi-beat (3-6 beats) data on the multislice (short axis) visualization mode to ensure the full inclusion of the right ventricle in the dataset. We obtained all RV volumes and EF measurements offline, using dedicated software (Echo PAC PC, 113; GE, Horten, Norway). We conducted a semi-automated analysis, with a manual tracing of endocardial borders in end-systolic and end-diastolic frames in the sagittal, four-chamber, and coronal views, from the full-volume dataset. In addition, we calculated EDV, ESV, stroke volume, and EF using the software (Figure 1)[19].
Intra-observer variability for echocardiographic data was done for patients and controls, where the same sonographer repeated examinations twice on the same day of diagnosis. Intra-observer agreement in interpreting echocardiographic data was determined using Cohen kappa (κ) statistics.
We used Power and Precision V3 program to estimate the power level of the primary endpoint (e´/a´ ratio with a level of 1.2 ± 0.25) (http://www.Power-Analysis.com). It was more than 90% when using 50 patients for each group. We did the statistical analysis using SPSS version 22. We presented the data as mean ± SD and percentages as indicated. We checked the data for normal distribution (about 72% of the results were within one SD from the mean values, and about 96% were within two SD from the mean values). Categorical variables were evaluated using student t-test, Chi-square, and F-test (ANOVA). The Pearson correlation coefficient examined the correlation of different parameters. P value < 0.05 was considered statistically significant.
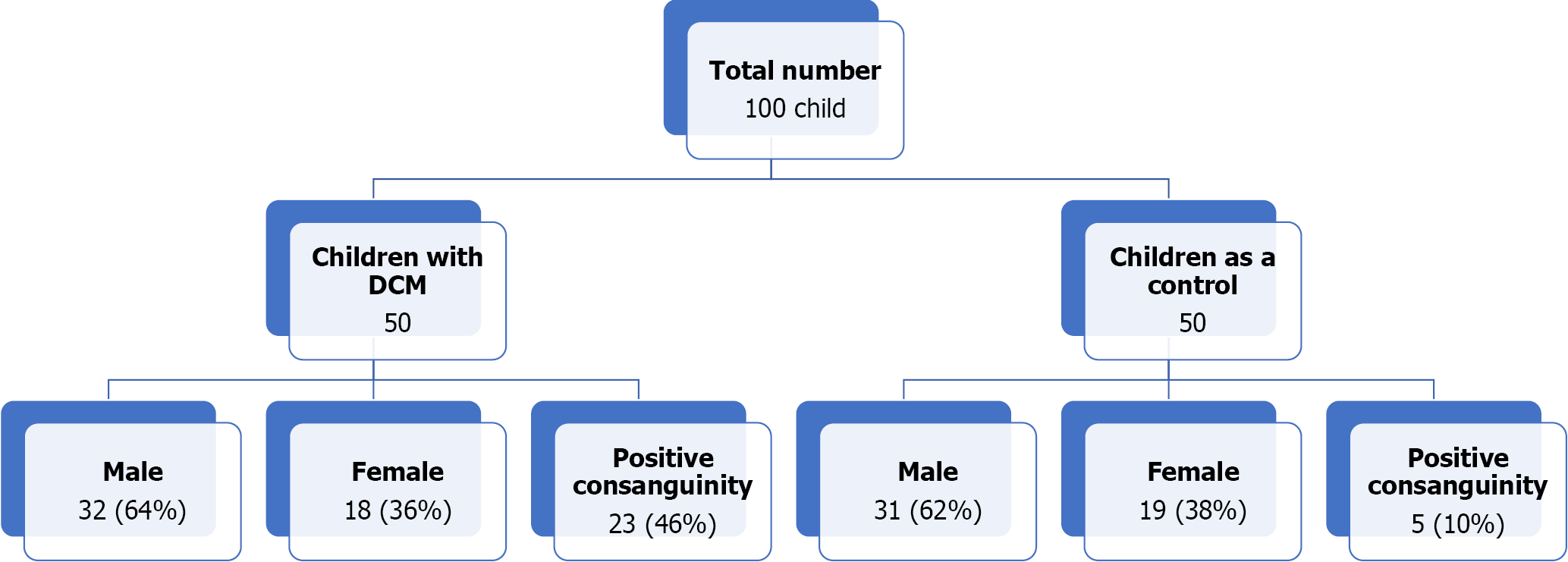
Figure 4 shows the flow chart of the study, which enrolled 50 children with idiopathic DCM and 50 clinically healthy children as a control group in the current study. Table 1 shows the demographics and the clinical data of the studied groups with no significant differences between the two groups regarding age and gender. However, DCM was more prevalent in males than in females (1.8:1). There was also more history of consanguinity and a more positive family history of DCM among children with DCM than in the control group. The table also showed that the patients’ group had significantly lower body weight, height, BMI, systolic and diastolic BP, and higher heart rate than the control group. About 58% of the DCM group showed systolic dysfunction when using RV EF measured by 4D echocardiography. The percentage of RV systolic dysfunction increased to 70% when using TAPSE. RV diastolic dysfunction by low RV e´/a´ ratio using tissue Doppler.
| Variables (mean ± SD) | Children with DCM (n = 50) | Controls (n = 50) | t | P value |
| Age | 4.9 ± 2.3 | 5.56 ± 2.4 | 0.18 | > 0.05 |
| Sex | ||||
| Male | 32 (64.0%) | 31 (62%) | ZS: 0.2 | > 0.05 |
| Female | 18 (36.0%) | 19 (38%) | ZS: 0.2 | > 0.05 |
| Consanguinity | 23 (46%) | 5 (10%) | -4 | 0.00011 |
| Family history of DCM, n (%) | 4 (8) | 0 | ||
| Weight | 16.92 ± 5.95 | 20.60 ± 5.97 | 3 | 0.0021 |
| Length (cm) | 98 ± 4.8 | 105 ± 4.4 | 7.6 | 0.00011 |
| BMI | 17.6 ± 2.1 | 18.7 ± 2.5 | 2.4 | 0.021 |
| Heart rate | 105 ± 12 | 91 ± 7 | 7.1 | 0.00011 |
| Systolic BP | 86 ± 8 | 98 ± 7 | 8 | 0/00011 |
| Diastolic BP | 63 ± 4 | 68 ± 5 | 5.5 | 0.00011 |
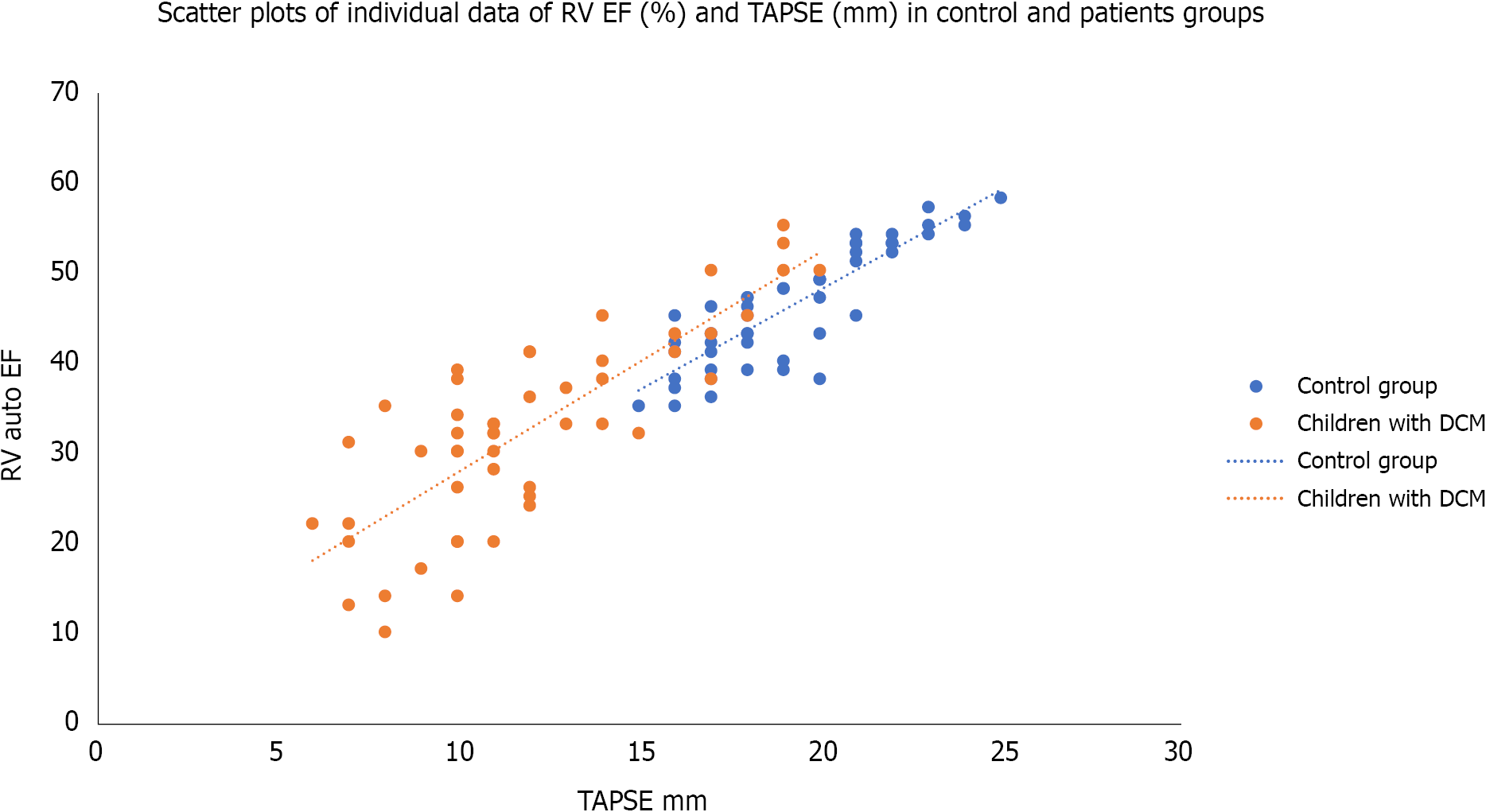
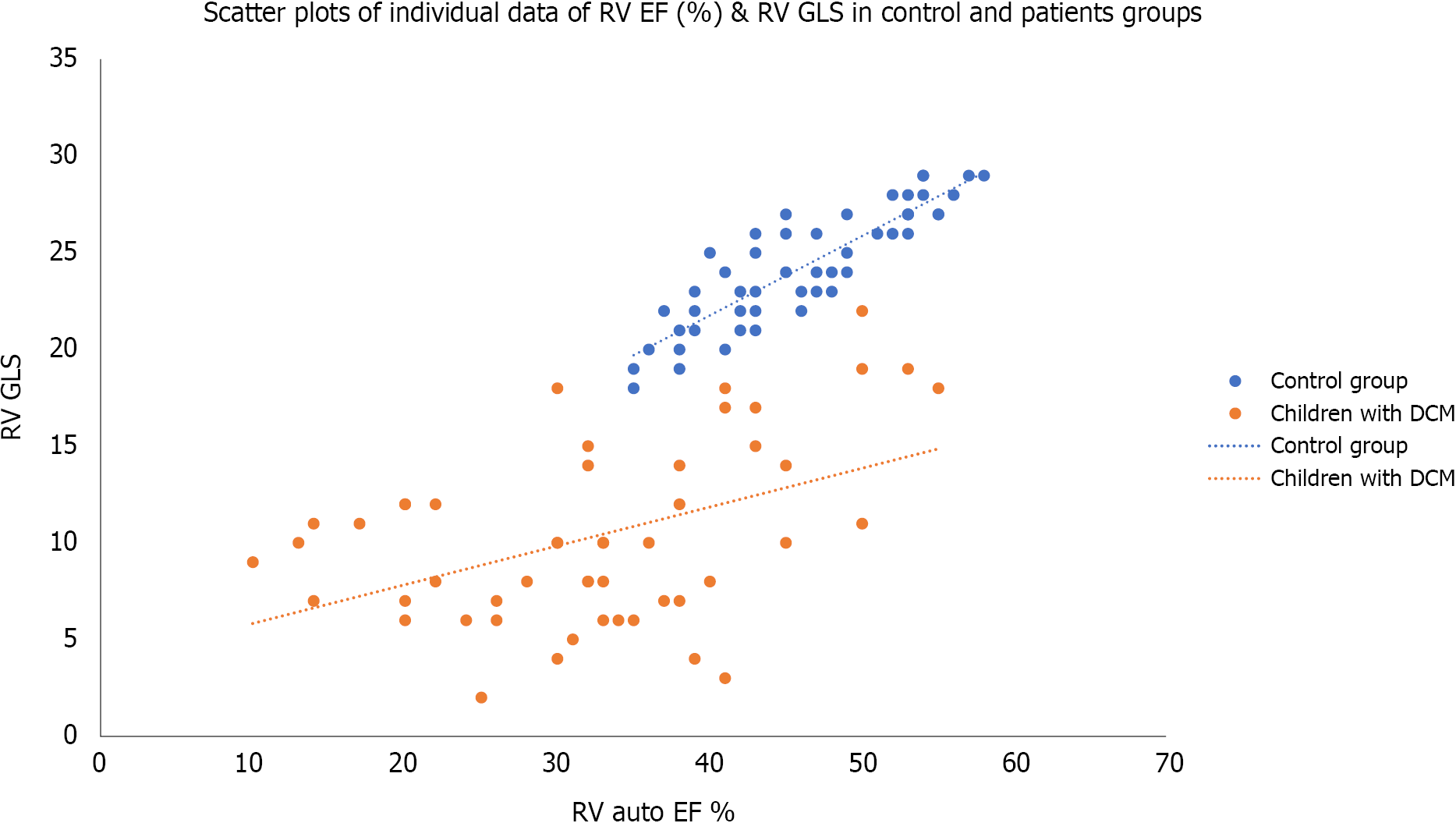
Table 2, Figures 5 and 6, show the echocardiographic parameters in both groups. The auto left ventricle ejection fraction measured by STE was significantly lower in the patients’ group than in the control. At the same time, the LV SI, which measures the ratio of the LV long axis to the short axis, was significantly lower in the children with DCM than in control (P < 0.0001). The tissue-Doppler derived mitral annulus S wave (which indicates LV systolic function), and e´/a´ ratio (which indicates LV diastolic function) were significantly lower in the patients’ group than in control (P < 0.0001). It also showed that LV myocardial performance index (MPI) (which reflects the global systolic and diastolic ventricular function) was more prolonged in the patients’ group than in the control (P < 0.0001). The speckle tracking echocardiography showed that the LV global longitudinal strains (GLS) was more significantly impaired in the patients’ group than in the control group (P < 0.0001). The table also showed RV echocardiographic parameters in both groups. TAPSE was significantly reduced in children with DCM than in the control. The auto RV EF measured by 4DE and the tissue Doppler-derived S wave and e´/a´ ratio were significantly lower, while RV MPI was more prolonged, and the systolic pulmonary pressure was significantly higher in the children with DCM than in control group. At the same time, the values of RV GLS, RV apical, mid, and basal strains were significantly lower in children with DMC than in the control group.
| Variables (mean ± SD) | Children with DCM (n = 50) | Controls (n = 50) | t | P value |
| LV echocardiographic parameters | ||||
| Auto LV EF (speckle tracking) | 43.4 ± 11.7 | 65.2 ± 7.6 | 11 | 0.00011 |
| Sphericity index | 1.2 ± 0.35 | 1.6 ± 0.3 | 6.2 | 0.00011 |
| Presence of mitral regurgitation, n (%) | 43 (86) | 1 (2) | ZS: 6.6 | 0.00011 |
| Mitral annulus systolic velocity (cm/sec) | 3.7 ± 1.1 | 6.933 ± 0.785 | 16.9 | 0.00011 |
| Mitral annulus e´/a´ ratio | 1.15 ± 0.4 | 1.540 ± 0.246 | 5.8 | 0.00011 |
| LV IVRT | 75.0 ± 18.3 | 64.0 ± 7.2 | 4 | 0.00011 |
| LV MPI | 1.9± 0.3 | 0.4 ± 0.08 | 423 | 0.00011 |
| LV GLS | -12.7 ± 4.9 | -24.4 ± 1.6 | 16 | 0.00011 |
| RV echocardiographic parameters | ||||
| 4D RV EF | 32.2 ± 10.5 | 46.2 ± 10.7 | 8.7 | 0.00011 |
| Tricuspid annulus S wave (cm/sec) | 4.42 ± 0.82 | 6.9 ± 0.8 | 15 | 0.00011 |
| Tricuspid annulus e´/a´ ratio | 1.17 ± 0.25 | 1.52 ± 0.3 | 6.34 | 0.00011 |
| RV MPI | 0.86 ± 0.16 | 0.40 ± 0.08 | 18.2 | 0.00011 |
| Mean pulmonary pressure (mmHg) | 28.5 ± 6 | 21 ± 4 | 7.4 | 0.00011 |
| TAPSE (mm) | 12.00 ± 3.56 | 19.30 ± 2.5 | 12.5 | 0.00011 |
| RV GLS | -10.34 ± 4.6 | -24.30 ± 2.9 | 18.5 | 0.00011 |
| RV apical strain | -13.3 ± 4.3 | -26.70 ± 1.3 | 21 | 0.00011 |
| RV mid strain | -12.4 ± 3.9 | -23.20 ± 1.7 | 17.9 | 0.00011 |
| RV basal strain | -13.7 ± 4.8 | -25.30 ± 1.5 | 16.3 | 0.00011 |
Table 3 shows the linear regression analysis of RV GLS with some clinical and echocardiographic parameters among the studied children with dilated cardiomyopathy. It was significantly associated with the duration since diagnosis, tricuspid annulus S wave, RV MPI, and TAPSE; while it did not show significant association with age or weight of the patients; RV e´/a´ ratio; or RV EF.
| Variables | RV LGS (%) among children with dilated cardiomyopathy (n = 50) | |
| β standardized coefficients | P value | |
| Age (yr) | -0.274 | 0.057 |
| Duration since diagnosis | 0.578 | 0.0011 |
| Weight (kg) | 0.273 | 0.058 |
| Tricuspid annulus (S) (cm/sec) | 0.384 | 0.0081 |
| RV e´/a´ ratio | 0.277 | 0.059 |
| RV MPI | -0.357 | 0.011 |
| RV EF (%) | 0.119 | 0.435 |
| TAPSE (mm) | 0.670 | 0.00011 |
Primary DCM is the presence of left or biventricular dilatation with severely impaired systolic function despite the absence of abnormal loading conditions. It is present in approximately 30%-40% of the cases. The pathological involvement is predominantly limited to the myocardium and is associated with a strong genetic inheritance in idiopathic cases[20]. It usually involves LV with some dysfunction of RV with a common clinical presentation of congestive cardiac failure. Recent studies showed the importance of RV dysfunction as a significant prognostic predictor of cardiac mortality[21]. Unfortunately, few studies are concerned with RV function in children with idiopathic DCM.
Assessment of cardiac size and function using various echocardiographic modes is an integral part of evaluating the child’s status. Using 4-D echocardiography allows us to have an external view of the heart with multiple internal perspectives[22]. As cardiac dilation precedes dysfunction in many cases of dilated cardiomyopathy, precise assessment of chamber dimensions, indexed according to body surface area, is essential for early diagnosis and the long-term follow-up of DCM[23]. Therefore, a comprehensive echocardiographic examination is indicated in cases with DCM, not only to assess LV size and function, but also to establish the diagnosis, identify the phenotype of DCM and the associated cardiac abnormalities such as valve disease, highlight the features requiring specific therapeutic management, and identify high-risk features associated with an adverse prognosis, including RV dysfunction[11].
Due to the complex three-dimensional geometry of RV wall motion (which affects the evaluation accuracy of the local dynamics derived indices), there is a need to quantify these factors accurately, which becomes possible by using 4-D echocardiography. The relatively newly developed real time 4-D echocardiography has the potential to circumvent the limitations induced by the RV complex anatomy as it does not rely on 2-D views[24]. In the current study, we assessed LV and RV functions using recent echocardiographic modalities (tissue Doppler, speckle tracking, and real-time 4-D echocardiography) in children with dilated cardiomyopathy. We found a marked reduction of LV systolic ejection fraction (EF) measured by real-time 3-Dimensional echocardiography compared to the control. Similar findings were reported by Gentile et al[25] which support that EF is an easy and sensitive tool for evaluating LV systolic function.
Tissue Doppler-derived mitral annulus systolic velocity (S wave) was significantly lower in our patients’ group than the control, matched with previous studies confirming usefulness for measuring the S as a tool for assessing systolic function. There was also a significant reduction of the mean value of LV (e´/a´ ratio) in our cases with DCM compared to control, clarifying the effect of LV systolic impairment on LV diastolic function[26,27]. These data confirm the presence of diastolic dysfunction in patients with dilated cardiomyopathy and impaired LV filling, which may even precede the presence of systolic dysfunction. These findings agreed with Friedberg et al[28] who found that diastolic dysfunction and mechanical desynchrony were more common in children with DCM than in the control. Like other previous reports[26,29], the tissue Doppler-derived MPI of LV in DCM cases was significantly prolonged compared to control, in the current study. This finding could be related to LV systolic and diastolic dysfunction reported in our patients, as the MPI reflects both the systolic and diastolic function of the ventricles.
There is a strong need to assess the SI in patients with DCM due to the presence of a broad spectrum of LV trabeculations, the dynamic interaction between subendocardial and subepicardial fiber helices in LV, and the increase in LV end-diastolic and end-systolic volumes. In addition, the presence of a broad spectrum of LV trabeculations among heart diseases, especially in DCM, could make differentiation of LV non-compaction cardiomyopathy from DCM difficult[30]. Meanwhile, the dynamic interaction between subendocardial and subepicardial fiber helices in LV causing twisting deformation plays a vital role in LV function[31].
The increase in LV end-diastolic and end-systolic volumes causes an increase in the myocardial mass and a change in the chamber geometry to a more spherical shape because of heart failure[30]. In the current study, there was a significant reduction in the SI of patients when compared with the controls. Previous studies supported that the LV SI was the strongest independent predictor of basal and apical LV peak systolic rotation and instantaneous LV peak systolic twist. So, LV apical rotation and twist are significantly influenced by LV configuration[13,32].
Ventricular strain can be determined by TDI, 2-D STE (which determines LV GLS %). Unlike the TDI-derived strain, which is angle-dependent, 2-D STE is less angle-dependent and can measure the strain by tracking the speckles, which are acoustic backscatter generated by ultrasound interactions with the myocardium[33]. Due to the complex orientation of the fibers in the intact LV wall, the myocardial deformation occurs in three dimensions and can be characterized not only in the longitudinal direction but also in the circumferential and radial directions. Sub-epicardial fibers play a significant role in radial and longitudinal strains, and sub-endocardial fibers play an essential role in circumferential strain. In the current study, LV GLS was significantly impaired in children with DCM compared to control. This finding agreed with several publications reporting different types of systolic strain impairment in patients with DCM[34].
Assessment of the RV function is paramount, especially in predicting the outcome, as it plays an important role in determining cardiac symptoms and exercise capacity in chronic heart failure. However, it is not easy to study the RV due to its complex anatomy and physiology. The current study showed a significant systolic dysfunction of the RV (as indicated by the reduced RV EF, TAPSE, and S′, and increased RV MPI) in children with DCM compared to the controls. RV dysfunction may be due to the close interaction between LV and RV function. The RV has mainly transverse muscle fibers in its free wall and shares oblique fibers in the IVS with the LV. Subsequently, its contraction augments RV contraction; a condition defined as systolic ventricular interaction[21].
The presence of MR further impairs the already compromised LV systolic and diastolic functions observed in children with DCM. The resulting dysfunction consequently led to pulmonary venous congestion, pulmonary hypertension, and increasing the RV afterload. Furthermore, these changes make the RV contraction more dependent on the oblique septal fibers, which are mechanically more efficient than the transverse fibers in the free wall. Besides, the marked impairment of the global LV deformation (including the IVS containing these oblique fibers) further reduces the systolic ventricular interaction and reduces the RV deformation. Moreover, as the LV acquires a more spherical shape, the septal fibers become less oblique, decreasing their mechanical efficiency with more and more deterioration of the RV deformation. This deterioration will further impair the RV GLS, as observed in our study. This finding also agreed with several publications reporting the decrease of the longitudinal systolic strain in cases of dilated cardiomyopathy[26,35].
The current study reported a significant RV GLS with tricuspid annulus S wave, RV MPI, and TABSE. However, the RV GLS was not associated with either the RV EF or RV e´/a´ ratio. Although RV MPI, RV S, and RV TABSE are tissue-dependent factors, reflecting the RV systolic function, they are more reliable than RV EF, which is load-dependent. This finding agreed with the work done by Agha et al[6] 2015, who found that TAPSE, S wave, and RV MPI were significantly correlated with the LV GLS. However, they also found a positive correlation of e´ wave and e´/a´ ratio with the LV GLS[6]. The differences between the current study and their study arise because they correlated the RV MPI, RV S wave, RV e´/a´ ratio, and RV TABSE with LV GLS and not RV GLS as observed in our study. Another difference was using the linear regression analysis in the current study to avoid any confounding factor. Seo et al[7] also found a significant association of the RV-Free wall LS with the prognosis in patients with DCM. Similarly, Zairi et al[8] found that TAPSE, S, Tei index, and strain of the lateral wall of the RV were independent predictors of major cardiovascular events in non-ischemic dilated cardiomyopathy. Tigen et al[9] also observed that the RV free wall basal segment longitudinal strain was sensitive to predict RV systolic dysfunction.
Early recognition of the RV dysfunction could help early detection of complications; and give us enough window to interfere with aggressive intervention, including cardiac transplantation, to avoid the increase in mortality rate. However, there are some limitations to the current study. The study was conducted as a cross-sectional study without doing serial echocardiographic examination and relating worsening cardiac function with the possibility of complications. The study also had relatively few numbers of patients.
There was impairment of the RV LGS and other systolic and diastolic parameters in children with DCM. Speckle tracking echocardiography and tissue Doppler can help detect the RV function's early decline, which serves as a good prognostic factor.
Dilated cardiomyopathy (DCM) is a clinical condition associated with left ventricular (LV) or biventricular dilation with an impaired contraction. Clinical presentation of DCM mainly relates to the degree of LV or biventricular systolic dysfunction leading to pump failure.
To diagnose early cardiac dysfunction in dilated cardiomyopathy, we need to perform a cardiac examination using a tool with high sensitivity. M-mode, 2-dimensional echocardiography, tissue Doppler imaging (TDI), and Two-dimensional speckle tracking imaging are commonly used echocardiographic modalities to provide accurate and early detection of cardiac dysfunction.
The study aimed to evaluate right ventricular (RV) function in children with idiopathic DCM using relatively recent echocardiographic modalities.
The study was a prospective case-control study, including 50 children with idiopathic DCM and 50 healthy children as a control group, to study RV function using four-dimensional echocardiography (4-DE), TDI, and two-dimensional-speckles tracking echocardiography (2-D-STE). RV ejection fractions (EF) was measured by 4-DE.
The auto left (LV) EF measured by 2-D-STE were significantly lower in the patients’ group than in the control. The sphericity index was also significantly lower in children with DCM than in the control. RV EF measured by 4-DE was significantly lower in the patient's group than the control. RV S wave, e´/a´ ratio, myocardial performance index (MPI), and tricuspid annular plane systolic excursion (TAPSE) were significantly impaired in children with DCM than in control. Both LV and RV global longitudinal strains (GLS) were significantly reduced in children with DCM than in control. RVGLS was significantly associated with the duration since diagnosis, tricuspid annulus S wave, RV MPI, and TAPSE, but not with the age of the patients, RV EF, or e´/a´ ratio.
Impairment of the RV LGS and other systolic and diastolic parameters in children with DCM using STE and TDI can help detect RV function's early decline.
We need to do a serial long-term echocardiographic study and relate worsening cardiac function to the possibility of complications.
We thank the anonymous referees for their useful suggestions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Tanta University; UMC/KAMC/AGU.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang DM S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, Liu PP, Matsumori A, Mazzanti A, McMurray J, Priori SG. Dilated cardiomyopathy. Nat Rev Dis Primers. 2019;5:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 431] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 2. | Lipshultz SE, Cochran TR, Briston DA, Brown SR, Sambatakos PJ, Miller TL, Carrillo AA, Corcia L, Sanchez JE, Diamond MB, Freundlich M, Harake D, Gayle T, Harmon WG, Rusconi PG, Sandhu SK, Wilkinson JD. Pediatric cardiomyopathies: causes, epidemiology, clinical course, preventive strategies and therapies. Future Cardiol. 2013;9:817-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331:1564-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 626] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 4. | Ho CY, Solomon SD. A clinician's guide to tissue Doppler imaging. Circulation. 2006;113:e396-e398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Mondillo S, Galderisi M, Mele D, Cameli M, Lomoriello VS, Zacà V, Ballo P, D'Andrea A, Muraru D, Losi M, Agricola E, D'Errico A, Buralli S, Sciomer S, Nistri S, Badano L; Echocardiography Study Group Of The Italian Society Of Cardiology (Rome, Italy). Speckle-tracking echocardiography: a new technique for assessing myocardial function. J Ultrasound Med. 2011;30:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 6. | Agha HM, Ibrahim H, El Satar IA, El Rahman NA, El Aziz DA, Salah Z, El Saeidi S, Mostafa F, Attia W, El Rahman MA, El Mohsen GA. Forgotten Right Ventricle in Pediatric Dilated Cardiomyopathy. Pediatr Cardiol. 2017;38:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Seo J, Jung IH, Park JH, Kim GS, Lee HY, Byun YS, Kim BO, Rhee KJ. The prognostic value of 2D strain in assessment of the right ventricle in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2019;20:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Zairi I, Mzoughi K, Jabeur M, Jnifene Z, Ben Moussa F, Kamoun S, Fennira S, Kraiem S. Right ventricular systolic echocardiographic parameters in dilated cardiomyopathy and prognosis. La Tunisie medicale. 2017;95:87-91. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Tigen K, Karaahmet T, Dundar C, Cincin A, Ozben B, Guler A, Gurel E, Sunbul M, Basaran Y. Right ventricular and atrial functions in patients with nonischemic dilated cardiomyopathy. Wien Klin Wochenschr. 2015;127:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2284] [Cited by in RCA: 2240] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 11. | Mathew T, Williams L, Navaratnam G, Rana B, Wheeler R, Collins K, Harkness A, Jones R, Knight D, O'Gallagher K, Oxborough D, Ring L, Sandoval J, Stout M, Sharma V, Steeds RP; British Society of Echocardiography Education Committee. Diagnosis and assessment of dilated cardiomyopathy: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2017;4:G1-G13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Picard MH, Adams D, Bierig SM, Dent JM, Douglas PS, Gillam LD, Keller AM, Malenka DJ, Masoudi FA, McCulloch M, Pellikka PA, Peters PJ, Stainback RF, Strachan GM, Zoghbi WA; American Society of Echocardiography. American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr. 2011;24:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 13. | Choi JH, Sung J. Left Ventricular Sphericity Index in Asymptomatic Population. J Cardiovasc Ultrasound. 2009;17:54-59. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Carluccio E, Biagioli P, Alunni G, Murrone A, Zuchi C, Coiro S, Riccini C, Mengoni A, D'Antonio A, Ambrosio G. Prognostic Value of Right Ventricular Dysfunction in Heart Failure With Reduced Ejection Fraction: Superiority of Longitudinal Strain Over Tricuspid Annular Plane Systolic Excursion. Circ Cardiovasc Imaging. 2018;11:e006894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 15. | Al-Biltagi M, Tolba OA, Rowisha MA, Mahfouz Ael-S, Elewa MA. Speckle tracking and myocardial tissue imaging in infant of diabetic mother with gestational and pregestational diabetes. Pediatr Cardiol. 2015;36:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Karatzis EN, Giannakopoulou AT, Papadakis JE, Karazachos AV, Nearchou NS. Myocardial performance index (Tei index): evaluating its application to myocardial infarction. Hellenic J Cardiol. 2009;50:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Ng AC, Thomas L, Leung DY. Tissue Doppler echocardiography. Minerva Cardioangiol. 2010;58:357-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Silverton N, Meineri M. Speckle Tracking Strain of the Right Ventricle: An Emerging Tool for Intraoperative Echocardiography. Anesth Analg. 2017;125:1475-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Felix A, Siciliano A, Belém L, de Azevedo F, Xavier S, De Lorenzo A, Filho C. Echocardiographic Assessment of Right Ventricular Function by Two-Dimensional Strain In Patients with Left-Sided Valvular Heart Disease: Comparison with Three-Dimensional Echocardiography. Int J Cardiovasc Sci. 2018;31:630-642. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Sanbe A. Dilated cardiomyopathy: a disease of the myocardium. Biol Pharm Bull. 2013;36:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Gulati A, Ismail TF, Jabbour A, Alpendurada F, Guha K, Ismail NA, Raza S, Khwaja J, Brown TD, Morarji K, Liodakis E, Roughton M, Wage R, Pakrashi TC, Sharma R, Carpenter JP, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation. 2013;128:1623-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 22. | Mele D, Bertini M, Malagù M, Nardozza M, Ferrari R. Current role of echocardiography in cardiac resynchronization therapy. Heart Fail Rev. 2017;22:699-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Egan M, Ionescu A. The pocket echocardiograph: a useful new tool? Eur J Echocardiogr. 2008;9:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Muraru D, Niero A, Rodriguez-Zanella H, Cherata D, Badano L. Three-dimensional speckle-tracking echocardiography: benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc Diagn Ther. 2018;8:101-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 25. | Gentile P, Merlo M, Cannatà A, Gobbo M, Artico J, Stolfo D, Gigli M, Ramani F, Barbati G, Pinamonti B, Sinagra G. Dilated Cardiomyopathy With Mid-Range Ejection Fraction at Diagnosis: Characterization and Natural History. J Am Heart Assoc. 2019;8:e010705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | McMahon CJ, Nagueh SF, Eapen RS, Dreyer WJ, Finkelshtyn I, Cao X, Eidem BW, Bezold LI, Denfield SW, Towbin JA, Pignatelli RH. Echocardiographic predictors of adverse clinical events in children with dilated cardiomyopathy: a prospective clinical study. Heart. 2004;90:908-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Mohammed A, Friedberg MK. Feasibility of a new tissue Doppler based method for comprehensive evaluation of left-ventricular intra-ventricular mechanical dyssynchrony in children with dilated cardiomyopathy. J Am Soc Echocardiogr. 2008;21:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Friedberg MK, Roche SL, Mohammed AF, Balasingam M, Atenafu EG, Kantor PF. Left ventricular diastolic mechanical dyssynchrony and associated clinical outcomes in children with dilated cardiomyopathy. Circ Cardiovasc Imaging. 2008;1:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Eto G, Ishii M, Tei C, Tsutsumi T, Akagi T, Kato H. Assessment of global left ventricular function in normal children and in children with dilated cardiomyopathy. J Am Soc Echocardiogr. 1999;12:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Marchal P, Lairez O, Cognet T, Chabbert V, Barrier P, Berry M, Méjean S, Roncalli J, Rousseau H, Carrié D, Galinier M. Relationship between left ventricular sphericity and trabeculation indexes in patients with dilated cardiomyopathy: a cardiac magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2013;14:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | van Dalen BM, Kauer F, Vletter WB, Soliman OI, van der Zwaan HB, Ten Cate FJ, Geleijnse ML. Influence of cardiac shape on left ventricular twist. J Appl Physiol (1985). 2010;108:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Stolfo D, Merlo M, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G. Evolution of left ventricular sphericity index in idiopathic dilated cardiomyopathy: clinical and prognostic implications. Eur Heart J. 2013;suppl 1:1196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Nesbitt GC, Mankad S. Strain and strain rate imaging in cardiomyopathy. Echocardiography. 2013;26:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Duan F, Xie M, Wang X, Li Y, He L, Jiang L, Fu Q. Preliminary clinical study of left ventricular myocardial strain in patients with non-ischemic dilated cardiomyopathy by three-dimensional speckle tracking imaging. Cardiovasc Ultrasound. 2012;10:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | den Boer SL, du Marchie Sarvaas GJ, Klitsie LM, van Iperen GG, Tanke RB, Helbing WA, Backx APCM, Rammeloo LAJ, Dalinghaus M, Ten Harkel ADJ. Distribution of strain patterns in children with dilated cardiomyopathy. Echocardiography. 2017;34:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |