Published online May 28, 2016. doi: 10.5319/wjo.v6.i2.33
Peer-review started: September 17, 2015
First decision: November 27, 2015
Revised: March 4, 2016
Accepted: March 17, 2016
Article in press: March 18, 2016
Published online: May 28, 2016
Processing time: 239 Days and 6.1 Hours
Aim was to gather relevant knowledge in evolution and development to find a rational explanation for the intricate and elaborate anatomy of the nose. According to classic embryology, the philtrum of the upper lip, nasal dorsum, septum and primary palate develop from the intermaxillary process, and the lateral walls of the nasal pyramid from the lateral nasal processes. The palatal shelves, which are outgrowths of the maxillary processes, form the secondary palate. The median nasal septum develops inferiorly from the roof of the nasal cavity. These valuable embryologic data do not explain the complex intricacy of the many anatomical structures comprising the nose. The evo-devo theory offers a rational explanation to this complex anatomy. Phylogenically, the nose develops as an olfactory organ in fish before becoming respiratory in tetrapods. During development, infolding of the olfactory placodes occurs, bringing the medial olfactory processes to form the septolateral cartilage while the lateral olfactory processes form the alar cartilages. The olfactory fascia units these cartilages to the olfactory mucosa, that stays separated from brain by the cartilaginous olfactory capsule (the ethmoid bone forerunner). Phylogenically, the respiratory nose develops between mouth and olfactory nose by rearrangement of the dermal bones of the secondary palate, which appears in early tetrapods. During development, the palatal shelves develop into the palatine processes of the maxillary bones, and with the vomer, palatine, pterygoid and inferior turbinate bones form the walls of the nasal cavity after regression of the transverse lamina. Applying the evolutionary developmental biology (evo-devo) discipline on our present knowledge of development, anatomy and physiology of the nose, significantly expands and places this knowledge in proper perspective. The clinicopathologies of nasal polyposis, for example, occurs specifically in the ethmoid labyrinth or, woodworker’s adenocarcinomas, occurring only in the olfactory cleft can now be explained by employing the evo-devo approach. A full understanding of the evo-devo discipline, as it pertains to head and neck anatomy, has profound implications to the otolaryngologist empowering his skills and abilities, and ultimately translating in improving surgical outcomes and maximizing patient care.
Core tip: The intricate and elaborate anatomy of the human nose can be best understood by gathering knowledge in evolution and development. Phylogenically and ontogenically, the nose results from two distinct entities: The olfactory and respiratory organ. In vertebrates, the olfactory placodes give rise to the fibrocartilaginous nose made of alar and septolateral cartilages, olfactory mucosa and the olfactory fascia; the respiratory nose develops by evolutionary remodeling of the palatal bones under the olfactory nose. In humans, the mammalian olfactory chambers are transformed into olfactory clefts and lateral masses of the ethmoid, and the transverse lamina separating the olfactory and respiratory noses has disappeared.
- Citation: Jankowski R, Márquez S. Embryology of the nose: The evo-devo concept. World J Otorhinolaryngol 2016; 6(2): 33-40
- URL: https://www.wjgnet.com/2218-6247/full/v6/i2/33.htm
- DOI: https://dx.doi.org/10.5319/wjo.v6.i2.33
Embryology of the nose is poorly described in classical textbooks, in which full of gaps and controversies are found about the different embryologic origins of the nasal bones, cartilages and soft tissue envelopes.
Embryology of the face is, in fact, a very difficult topic, which becomes more understandable in the evo-devo concept[1]. The evo-devo theory links the evolution from simple species to complex human development[2,3].
In classic embryology textbooks[4,5], the first 28 day of gestational life the face develops from five swellings: The paired maxillary and mandibular processes and the unpaired frontonasal process.
During the fifth week, the nasal placodes (i.e., nasal discs and nasal plates) develop as a result of ectodermal thickenings and can be observed on the frontonasal process.
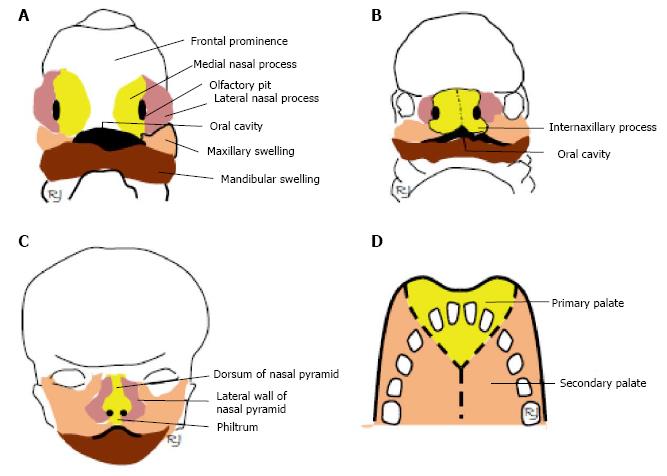
In the sixth week, infolding of the ectoderm at the epicenter of these nasal placodes initiates the formation of an oval pit (see below description of nasal pits) resulting in the division of the raised edge of each placode into medial and lateral nasal processes (Figure 1A).
During the sixth week, the medial nasal processes fuse to form the intermaxillary process (Figure 1B), which is the primordia of the septum and bridge of the nose (Figure 1C).
By the end of the seventh week, there is a lateral and inferior expansion of the medial nasal processes at their inferior tips before fusing to form the anterior roof of the oral cavity (Figure 1B). As the poles of the maxillary swellings continue to develop they come into contact with the intermaxillary process where they fuse with each other. On the superior labial region, the intermaxillary process develops into the philtrum (Figure 1C). Formation of the nasal passages result as the nasal pits deepen penetrating its underlying mesenchyme during week 6 of development. Mesenchyme is loosely organized cells derived from mesodermal embryonic tissue that develops into connective and skeletal tissues. An ectodermally enlarged nasal sac is formed during the last days of the sixth week by the fusion of the deep endings of the nasal pits, which are topographically located superoposterior to the intermaxillary process. During the last days of the sixth week and the first few days of the seventh week, a proliferation of cells occurs at the posterior wall and floor of the nasal sac forming a thickened plate-like fin of ectoderm origin essentially isolating the oral cavity from the nasal sac but still maintaining an epithelial continuity between the regions. This “keel” structure is now referred to as the nasal fin. The nasal sac enlarges as a result of vacuoles developing within the nasal fin and then it fuses with the sac. The nasal fin begins to attenuate to a thin membrane named the oronasal membrane, which demarcates the oral cavity from the nasal sac. Towards the end of the seventh week, the oronasal membrane obliterates creating the opening of the primitive choana. Formation of the nasal cavity floor, or primary palate, occurs by the backward growing of the intermaxillary process.
Throughout the eighth and ninth week, the development of the definitive and secondary palate occurs. The main portion of the definitive palate develops by two shelve-like outgrowths from the maxillary processes. These two thin medial extension outgrowths are called the palatine shelves, which appear during the sixth week of development. While these shelves are directed in a downward manner on either side of the tongue, it is during the ninth week where these shelves rotate and ascend rapidly attaining a horizontal position above the tongue. Fusion of the primary palate and the palatine shelves (along the midline) assists in the formation of the secondary palate (Figure 1D). The order of fusion first begins at the ventral region of the palatine shelves before proceeding dorsally.
Mesenchymal condensations occurs when previously dispersed mesenchymal cells come together to differentiate into a single tissue type and is considered the critical transitional stage that precedes cartilage formation during embryonic development[6]. When these mesenchymal cell condensations occur in the ventral region of the secondary palate endochondral ossification ensues to achieve the formation the hard palate. At the dorsal region of the secondary palate, myogenic mesenchymal cells come together to form the muscular layer of the soft palate.
During the formation of the secondary palate, there is a proliferation of cells from the mesoderm and ectoderm region of the medial nasal and frontonasal processes that help form the nasal septum along its midline. As a result, the two nasal passages of the nasal cavity have now been established, communicating with the pharynx located posterior to the secondary palate. This communicating portal is now termed the definitive choana.
According to classical concept, the philtrum of upper lip, the nasal dorsum, septum, and primary palate originate from the development of the intermaxillary process, whereas the lateral walls of the nasal pyramid develop from the lateral nasal processes (Figure 1A-C).
Two major questions which can be addressed to this classic description are why the nose is formed by such a complex intricacy of different anatomical structures, and why the origin and formation of these are not found in the classic embryological description. Examining the formation of the nose in the evolution of species may, actually, give clues to the answers[1].
The first vertebrates were jawless fish named agnathans who are classified in the phylum Chordata and subphylum Vertebrata and whose fossil ancestors can be traced back to the Cambrian period around 500 million years ago.
Living agnathans display a primitive or rudimentary olfactory organ. This organ consists of a median blind duct that communicates with the environment by means of an external nostril, but there is no posterior opening into the pharynx. The olfactory mucosa lies at the blind end in a chamber of the anterior braincase and is connected to the brain through olfactory filaments.
Lungfish played a critical role when organisms shifted from an aquatic mode of life to a terrestrial setting representing one of the most dynamic major adaptive shifts during the course of evolution. It was von Bischoff who first described the presence of choanae in lungfishes in 1840 as he considered these organisms excellent models for examining respiratory morphology of early tetrapods (i.e., a four-footed organism) as they appeared intermediate in morphology between amphibians and fishes[7].
The anatomy of lungfish shows that the olfactory passages open posteriorly in the oral region and into the respiratory portion of the organism. These posterior communicating pathways, however, were in all likelihood not used for the purposes of respiration but rather to increase the power of olfaction. The buccopharyngeal pump passes forceful currents of water between the nasal and oral regions as these fishes perform suction feeding and may perhaps serve as the mechanism by which they increase their olfactory sense.
When the first tetrapods arrived, which includes all vertebrates higher than fishes (e.g., amphibians, reptiles, etc.) one can appreciate the remarkable morphological diversity seen in body form of later tetrapods allowing them to utilized an equally broad array of terrestrial ecological niches.
In amphibians, their ability to smell is derived from the superficial oscillatory movements of their buccal floor in order to establish intimate contact to their immediate surroundings be it a terrestrial or aquatic medium. The nasal respiratory function, however, is secondary as skin respiration is predominant. Amphibians, as a result, recruited the olfactory organ, as an intermittent tool for its respiratory apparatus.
Thus, with the amphibians the primary olfactory nose has evolved towards a nose devoted to olfaction and respiration. The amphibian nose communicates externally via the external naris and connects with the oral cavity posteriorly to the primary palate by the internal naris. The nasal cavity of amphibians is lined by olfactory epithelium with the exception of its ventro-lateral wall region.
Rocek and Vesely[8] reported on the larval development of the South American toad (i.e., Pipa pipa) showing that the anterior skeletal portion of the amphibian snout is already formed by the following cartilaginous structures. First, a cartilaginous septolateral unit whose outgrowths occurs laterally from a “cartilago oblica” emanating from the “planum internasale”; and two, the distinct formation of a pair of “cartilago alaris”. The latter two assemblies appear to be persistent and continuous with the posterior end of the cartilaginous skeleton thereby essentially protecting its olfactory chambers.
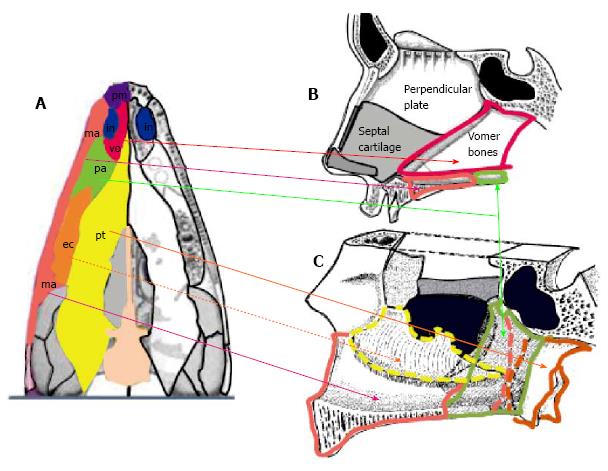
In the early fully terrestrial tetrapods, a transverse sheet of dermal bones has developed inferior to the braincase specifically in the roof of the mouth posterior to the internal naris and primary palate (Figure 2A). This dermal portion of the secondary palate consists of four paired bones: The vomer, palatine, ectopterygoid and pterygoid bones, which lie as a flat sheet between the two maxillary bones. Each internal nostril is bounded by the premaxilla of the primary palate anteriorly, the maxilla laterally, the vomer medially, and the palatine bone posteriorly[9]. This secondary hard palate configuration was probably the precursor in allowing permanent breathing to travel through the primary nose as inspiratory air would travel through a non-collapsible oral cavity before going to the trachea.
The reptilian vertebrate representative, the crocodilians, exhibit many of the above characteristics as does the mammalian condition, which also shares this bauplan suggesting convergent evolution taking place. Tracking the phylogenic history of the crocodilian, which spans over two-hundred million years, one can observe its akinetic skull features including the formation of its secondary nose.
Crocodilian evolution has been characterized by the gradual constitution of an akinetic skull and the formation of a secondary nose. Simultaneously with many modifications and reconfigurations of its palatal bones a secondary bony nasal passageway progressively develops, permitting inspiratory airflow to enter through its external naris and exit posteriorly through the internal naris or choana, which gradually shifted posteriorly until they were completely contained by the pterygoid bones.
Current hypotheses state that the evolution of feeding behaviors may have been the driver for the structural modifications of the crocodilian rostrum: The displacement and remodeling of the bones configuring the crocodilian secondary palate, which may initially have occurred reinforce the snout and skull instead of providing a physical bony partition between the oral and nasal cavities. As a result, the secondary nose could be regarded as an incidental byproduct of the masticatory mechanical forces between the dermal bones of the secondary palate and the skull base. The vacuities that were then occupied with air from the primary nose, were finally recruited to provide for the physiological function of breathing.
The fundamental configuration of the nasal fossa is a highly conserved region. As one tracks the evolution from crocodilian to the mammalian skull, very little change can be observed in this region with its persistent and constant morphology seen in a great majority of mammalian groups.
In mammalian groups, the palate separates the nasal and oral cavities: Primary, secondary, and soft palate. The primary internal naris remains as a vestigial opening, i.e., the anterior palatine canal, which is topographically positioned between primary and secondary palate.
The primary nose fully opens behind a virtual, coronal plane through the anterior palatine canal, into both the respiratory and olfactory noses. Respiratory and olfactory noses are separated from each other by the transverse lamina, a thin, bony axial structure. Thus, the respiratory nose appears as two paramedian, long axial channels walled in on the inferior, lateral and medial sides by the reconfiguration of the primary palatal bones (vomer, palatine, pterygoid, and inferior turbinate bones) between the two maxillary bones and their palatine processes, and partitioned from the olfactory nose through the transverse lamina. The olfactory nose is completely embedded in the anterior cranial base, that is, the ethmoid bone[10].
The living primates (which include humans) are taxonomically classified in two suborders: Strepsirrhini and Haplorhini, the latter group includes our human ancestors[11]. Through the course of primate evolution, profound changes in the nasal fossa allow one to differentiate the haplorhines from strepsirrhines and all other mammals.
The word haplorhine means “dry nose” whereas strepsirrhine means “wet nose”. As a result, strepsirrhine primates exhibit wet noses similarly to dogs and cats. Haplorhine primates have a fused frontal bone suture as well as a fused mandibular symphysis. While both haplorhine and strepsirrhine primates have a complete orbital ring of bone, only the haplorhine exhibit a complete bony enclosure posteriorly separating the periorbital contents from the temporalis muscle as it traverses through the infratemporal fossa on its way to attaching the coronoid process of the mandible[12]. The superior portion of the haplorhine nasal fossa is constricted by the orbital cones, which come about from the combined effect of orbital convergence and orbital frontation. Orbital convergence refers to the extent to which the orbital opening faces anteriorly improving stereoscopic vision that include the element of depth perception[13]. Orbital frontation refers to what extent the superior and inferior margins are to the plane of the orbital opening so that more “frontated” organisms tend to view from the orbital socket more horizontally rather than superiorly[13]. In most haplorhines, there is a considerable reduction of their snout length when compared to strepsirrhines. There is one more important anatomical distinction between these two subOrders of primates that resides within the nasal fossa and that is an absence of a transverse lamina in haplorhines, which translates in them not having a bony partition separating the respiratory and olfactory region within the nasal cavity proper.
Strepsirrhines, on the other hand, exhibit a partitioned respiratory and olfactory region within the nasal cavity by possessing a transverse lamina coupled by their complex ethmoturbinate system. But while the order of Primates is classified within the microsmatic group of mammals (this group shifted from an olfactory mode of existence to a visual reliance of subsistence), carnivores (classified as macrosmatic meaning their whole existence is based on smell) have the most complex and elaborate turbinate system in all of mammalia. Possession of four or more ethmoturbinates is found in strepsirrhines in contrast to the reported range of one to three pairs found in haplorhines. In addition, the haplorhine ethmoturbinates appear more reduced in size and are less intricately scrolled. Moreover, it appears that the tendency is toward a decrease and reconstitution of ethmoturbinate structural reorganization across the different haplorhine primate taxa.
While traditionally primates have been classified as microsmatic as mentioned above, other authors describe primates undergoing a reduction in their olfactory prowess. In 1970, Cartmill[14] proposed the visual predation hypothesis of primate origins, which may help to explain this reduction. The visual predation hypothesis explains the adaptive significance of a variety of skeletal features that characterize modern primates as they transitioned to an arboreal mode of life[14]. The change in orbital orientation enhanced stereoscopic vision, which was essential in the manually effective capture of food in a three dimensional setting of arboreal life but it may have initiated a cascade of morphological events to occur elsewhere in the craniofacial region particularly in the nasal area. As bony orbital modification and re-orientation occurred in these primates there was a concurrent reduction and re-arrangement of ethmoturbinate complexity to a more simple inferior-to-superior re-organization and, finally, a partial but not complete loss of olfactory mucosal area.
In humans, the evolutionary pattern of the nasal region as seen in the haplorhine non-human primates is continued in our species. The human nose appears as one organ with no morphological evidence distinguishing between the respiratory and olfactory noses. Studies of inspiratory airflow patterns in the nasal cavity, however, show the path of air flowing along the nasal floor and lower medial portion of the cavity (comparable to the inferior and middle meatus region) mimicking the respiratory pathway of an organism that possesses a transverse lamina[15].
The olfactory mucosa has been mapped to a small surface immediately inferior to the cribriform plate and to the upper portions of the nasal septum[16]. The reduction of the olfactory mucosa seen in humans is strongly associated with the adaptive shift of a quadropedal locomotion gait to bipedality. Homo erectus is considered the first committed biped in our evolutionary history, which required the repositioning of the foramen magnum (a more anterior inferior placement) in order to balance the skull over the vertebral column and accommodate erect posture. These morphological changes had the effect of changing the orientation of the cribifrom plate from a vertical to a more horizontal manner. This resulted in a conversion of the mammalian olfactory nose into the human ethmoid complex, partitioned on each side in two clinically relevant compartments: The olfactory cleft medially and the ethmoid labyrinth laterally (in which the olfactory mucosa has disappeared).
Despite the evolutionary trend towards regression in the sense of smell, the embryologic development of the human nose is best understood when considering its olfactory origin, the subsequent respiratory reorganization, and the constriction of the ethmoid bone imposed by the orbital cones.
The evo-devo approach, in comparison to the classical concept, explains why the nose is formed by a complex intricacy of different anatomical structures, and offers a rational explanation to this question[1] (the development of the paranasal sinuses, which occurs after birth, is not mentioned in this paper).
Phylogenically, the nose is exclusively an olfactory organ in fish, and the respiratory nose develops in crocodilians. Ontogenically, the growth and development of the olfactory nose precedes the development of the respiratory nose.
Development of the olfactory capsule: The first embryologic evidences of the nose appear during the fourth week under the mask of two olfactory placodes on the frontal process of the embryo. Simultaneously, the corresponding wall of the brain undergoes rapid mitotic activity with a small bulge becoming visible and demarcating the olfactory region. Histologically, the future olfactory bulb and structures called the amygdaloid body and hippocampal formation are found in the forebrain[17].
Approximately, at five weeks Carnegie stage (CS) 15, (Carnegie staging is a method for dating embryos), the appearance of an olfactory pit is observed. This occurs with the invagination of the central portion of the placode. The invagination is in the direction of the adjacent brain where an olfactory elevation appears. Crest cells begin to gather together forming cords or filaments that travel within the mesenchyme.
At CS 16, the future olfactory bulb and the olfactory tubercle appear as elevations along the olfactory area of the cerebral hemispheres, or telencephalon. Between these two telencephalic elevated regions and the olfactory pit there is a significant and concentrated area of mesenchyme through which crest cells and olfactory epithelium must penetrate as they migrate to their destinations.
At CS 17 (approximately six weeks), the olfactory pit gives rise to the olfactory sac along with the development of the olfactory fin. The olfactory fin is an important structure as it separates the primitive nasal and oral cavities.
At CS 18 (6 ½ wk), the formation of the superficial fiber layer of the olfactory bulb originates from the fiber contribution of the olfactory nerve. There is an increase in the separation between the floor of the nasal (olfactory) sac and the oral cavity along with the appearance of a primitive olfactory septum between the olfactory sacs. The primordia of the olfactory centers, which represent the highly complex group of neurons, will be located near the juncture of the temporal and parietal lobes where they will continue to develop in the brain.
At CS 19, as vacuoles cultivate within the nasal fin they fuse with the nasal sac resulting in the sac’s enlargement. The nasal sac’s enlargement thins the nasal fin to a slender membrane before rupturing and forming the primitive choanae.
At CS 20 and 21, the various olfactory centers maintain their development within the brain while the olfactory epithelial fibers penetrate the sea of mesenchyme to establish connections.
At CS 22, the lateral walls of the olfactory sacs begin to fold over to form furrows and ridges, that increase the surface of olfactory epithelium.
At CS 23 (around the eighth week), the mesenchymal olfactory septum between the olfactory sacs has become cartilaginous and is now part of the olfactory capsule, which in itself is cartilaginous based. The olfactory capsule presents with its typical “M” shape morphology enveloping and separating both olfactory conduits from the brain.
During human foetus development from the ninth to tenth weeks, six major furrows develop along with their corresponding ridges or folds called ethmoturbinals (i.e., turbinates arising from the ethmoid) arising from the lateral aspect of the cartilaginous olfactory capsule. This cartilaginous precursor will undergo mineralization forming the ethmoid bone.
Development of the olfactory conduits: The olfactory placodes are ectodermal thickenings. Infolding occurs at the epicenter of each olfactory placode and, as the placodes deepen, a raised rim on each placode results dividing the medial and lateral olfactory processes. The intermaxillary process is formed when the medial olfactory processes fuse at the midline.
In the images of the Wistar rat fetus[18,19], the primary nose becomes evident through the gap between the vertical palatal processes, after removal of the tongue. The histologic coronal sections of the snout of these rats clearly show that, the septum of the primary nose is already present and complete, before the flip up and development of the respiratory nose and secondary palate.
Additionally, from a phylogenic perspective, the cartilaginous skeleton of the amphibian snout is already comprised of a cartilaginous septolateral unit and an independent pair of “cartilago alaris”, and precedes the appearance of the secondary palate and respiratory nose in crocodilians.
From these observations, it seems logical to hypothesize the following: The invagination movement of the center of the olfactory placodes could also pull in the raised rim of each placodes resulting in bringing the medial olfactory processes to fuse at the midline. Formation of the septolateral cartilage ensues before bringing the lateral olfactory processes in front of the septolateral cartilage, giving origin to the alar cartilages. The quadrangular plate of the septolateral cartilage connects to the perpendicular plate arising from the cartilaginous nasal capsule (i.e., the forerunner of the ethmoid bone) to form the septum of the olfactory nose, which is visible in the Wistar rat fetus study[18,19].
Thus, the olfactory placodes and their derivative pits give rise by differentiation to the following: (1) The olfactory mucosa; (2) The septolateral and alar cartilages; and (3) The connecting tissues lying in between these structures, i.e., the olfactory fascia[20].
The different fibrous portion of the olfactory fascia may be described as ligaments that unit the nasal cartilages to each other and to the olfactory mucosa, and the fibrocartilaginous nose to the facial and skull base skeleton.
These elements form the olfactory nose (Figure 3), which in humans stay separated from the braincase by the ethmoid bone, and largely communicates with the respiratory nose at the expense of the disappearance of the transverse lamina, a bony plate phylogenically separating the olfactory and respiratory nose until the stage of early primates.
Phylogenically, the respiratory nose first appears in crocodilians. Based on paleontological data, the vomer bones are two plates of bone staying horizontal between the internal naris of early tetrapods which fused as a distinct plate located in the sagittal plane dividing the air passages of crocodilians[21].
Some evidence of a similar rearrangement in mammals has been published in a study of the Wistar rat secondary palate development[18,19]. The flip up of the palatal processes leads to their fusion behind the primary palate, leaving a gap between the upper surface of the secondary palate and the inferior border of the septum of the olfactory nose, which is progressively closed by a structure growing from the fused palatal shelves towards the septum of the olfactory nose. This growing structure, in the evo-devo concept, is believed to be the fused vomer bones[1].
The principle influence in palatal formation within crocodilians and other mammals appears to be in the significant development of the so-called palatine processes of the maxillae, the bones that give rise to the dentition, which push back the dermal palatal bones and is at the origin of their morphological changes and anatomical rearrangement. Applying the evo-devo perspective, the human respiratory nose appears as two paramedian, long axial conduits walled in on their inferior, lateral and medial sides by the rearranging of the dermal palatal bones (vomer, palatine, pterygoid, and inferior turbinate bones) between the two maxillary bones and their palatine processes (Figure 2). The transverse lamina, a bony structure which phylogenically was the floor of the ethmoidal chambers and the roof of the respiratory nose probably disappeared in the haplorhine ancestor of humans secondary to the constriction of the nasal fossae by the frontation and convergence of the orbital cones and the retraction of the snout.
The soft palate has evolved from the crocodilian basihyal valve, a significant gular fold that archers across the pterygoids immediately in front of the internal choana allowing crocodilians to have efficient respiratory function during submerged aquatic conditions. The basihyal valve consists of the following two flaps: The upper flap descends from the palate and gives rise to the mammalian soft palate, and the lower flap, located at the back of the tongue, is stringently reinforced by the hyoid cartilage (or the hyoid-epiglotic complex).
The development of the nose can be seen as the invagination of the olfactory organ between the two maxillae towards the anterior cranial base, with its floor being secondarily disturbed by the onset of nasal respiratory development at the expense of the oral cavity. Application of the evo-devo perspective provides new insight not only to the development of the nose, its complex nasal physiology and anatomy but more importantly, may explain the predisposition, direction and spread of various diseases in otorlaryngology. Armed with this knowledge, the otolaryngologist will better understand the clinical issues permitting modification of standard diagnostic, surgical and therapeutic management of the different diseases afflicting the craniofacial and neck regions. As a result, employing the evo-devo concept to our already pool of knowledge on Ear, Nose and Throat institutes will generate favorable surgical outcomes and, in effect, maximize patient care.
P- Reviewer: Zhou M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Jankowski R. The evo-devo origin of the nose, anterior skull base and midface. Paris, F: Springer 2013; . [DOI] [Full Text] |
| 2. | Raff RA. Evo-devo: the evolution of a new discipline. Nat Rev Genet. 2000;1:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Gould S. Ontogeny and Phylogeny. Cambridge, Massachusetts, USA: The Belknap Press of Harvard University Press 1977; . |
| 4. | Larsen W. Human embryology. 3rd ed. Philadelphia, Pennsylvania, USA: Churchill Livingstone 2001; . |
| 5. | Sadler T. Langman’s essential medical embryology. Baltimore, USA: Lippincott, Williams & Wilkins 2005; . |
| 6. | Mammoto T, Mammoto A, Torisawa YS, Tat T, Gibbs A, Derda R, Mannix R, de Bruijn M, Yung CW, Huh D. Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev Cell. 2011;21:758-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 7. | Márquez S, Pagano AS, Lawson W, Laitman JT. Evolution of the Human Nasal Respiratory Tract: Nose and Paranasal Sinuses. Sataloff’s Comprehensive Textbook of Otolaryngology Head and Neck Surgery. Philadelphia: Jaypee Medical Publishers 2015; 17-42. |
| 8. | Rocek Z, Vesely M. Development of the ethmoidal structures of the endocranium in the anuran Pipa Pipa. J Morphol. 1989;200:301-319. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Kimmel CB, Sidlauskas B, Clack JA. Linked morphological changes during palate evolution in early tetrapods. J Anat. 2009;215:91-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 10. | Jankowski R. Revisiting human nose anatomy: phylogenic and ontogenic perspectives. Laryngoscope. 2011;121:2461-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Fleagle JG. Primate Adaptation and Evolution. 3rd ed. San Diego, USA: Academic Press 2013; . |
| 12. | Ross C. Muscular and osseous anatomy of the primate anterior temporal fossa and the functions of the postorbital septum. Am J Phys Anthropol. 1995;98:275-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 13. | Ross C. Adaptive explanation for the Origins of the Anthropodea (Primates). Am J Primatol. 1996;40:205-230. [DOI] [Full Text] |
| 14. | Cartmill M. Morphology, function, and evolution of the anthropoid postorbital septum. Evolutionary Biology of New World Monkeys and Continental Drift. New York: Plenum Press 1980; 243-274. [DOI] [Full Text] |
| 15. | Kelly JT, Prasad AK, Wexler AS. Detailed flow patterns in the nasal cavity. J Appl Physiol (1985). 2000;89:323-337. [PubMed] |
| 16. | Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol. 2006;34:252-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 332] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 17. | Müller F, O’Rahilly R. Olfactory structures in staged human embryos. Cells Tissues Organs. 2004;178:93-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Ferguson MW. The mechanism of palatal shelf elevation and the pathogenesis of cleft palate. Virchows Arch A Pathol Anat Histol. 1977;375:97-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Ferguson MW. Palatal shelf elevation in the Wistar rat fetus. J Anat. 1978;125:555-577. [PubMed] |
| 20. | Jankowski R. Septoplastie et Septorhinoplastie par désarticulation: Histoire, Anatomie, Chirurgie et Architecture naturelles du nez. Paris, F: Elsevier Masson 2016; . |
| 21. | Moore W. The mammalian skull. Biological structure and function. Cambridge, UK: Cambridge University Press 1981; . |