Published online Aug 28, 2014. doi: 10.5319/wjo.v4.i3.12
Revised: June 10, 2014
Accepted: July 12, 2014
Published online: August 28, 2014
Processing time: 222 Days and 8.3 Hours
Advances in foetal imaging have increased our detection rate of craniofacial abnormalities in utero. Nasal glioma is a rare, benign, congenital facial defect. Once detected, further imaging is required to assess for intracranial communication, the presence of additional defects, determine the patency of the aerodigestive tract and decide on timing of delivery. The authors review the current literature on diagnosis and management of nasal glioma in this rapidly advancing field of craniofacial anomalies detected in utero. Literature search of EMBASE and MEDLINE databases yielded 594 articles, which were screened by 2 independent reviewers. A total of 7 papers were selected after exclusion. There have been seven cases of prenatally diagnosed nasal glioma. The earliest of these was detected at 20 wk gestation. The majority were investigated with foetal magnetic resonance imaging (MRI) to establish any intracranial communication or bony defects. Ultrasound monitoring, doppler waveform and 3D rendered images were utilised to delineate the lesion, monitor growth and differentiate potential diagnosis. Postnatal MRI is favoured by most to re-evaluate the lesion and aid surgical planning. Surgical resection was performed within the first few months of life. Diagnostic uncertainty was seen in all cases, until formal histology was obtained, emphasising the challenges, and need for early appropriate specialist input. Whilst the prenatal detection of craniofacial abnormalities increases, there remain diagnostic challenges in differentiating prenatal congenital midfacial defects in utero. These defects are best investigated and monitored using prenatal ultrasound and MRI, to narrow the differential diagnosis, guide timing of delivery and allow for appropriate surgical planning. Prenatally detected nasal glioma, may only be confirmed on histology and families must be counselled appropriately to prepare them for the possible alternative diagnoses. Early surgical resection was undertaken to achieve more favourable aesthetic outcomes, reduce complications of ocular development and provide definitive histological diagnosis.
Core tip: Advances in foetal imaging have increased our detection rate of craniofacial abnormalities in utero. This enables early surgical input providing differential diagnosis, surgical planning, timing of delivery and counselling for families. Seven cases of prenatally diagnosed nasal glioma have been reported. The authors advocate ultrasound and foetal magnetic resonance imaging (MRI) to delineate the lesion, exclude intracranial involvement and monitor size. Foetal MRI also provides accurate delineation of the upper aerodigestive tract, allowing clinicians to anticipate airway compromise, in this otherwise benign condition. Early surgical resection is advised, for better aesthetic outcomes and to ensure normal ocular development.
- Citation: Fox R, Okhovat S, Beegun I. Prenatal diagnosis and management of nasal glioma. World J Otorhinolaryngol 2014; 4(3): 12-16
- URL: https://www.wjgnet.com/2218-6247/full/v4/i3/12.htm
- DOI: https://dx.doi.org/10.5319/wjo.v4.i3.12
Advances in foetal imaging have improved our detection rate of craniofacial abnormalities in utero. These improvements allow for earlier diagnosis, which can be made as early as the 11th week of gestation[1]. As such, the Head and neck surgeon is thrust into a new role in foetal management as part of the multidisciplinary team, providing differential diagnosis, advising on timing of birth and postnatal surgical planning.
While craniofacial abnormalities are uncommon, the most frequently occurring include; encephaloceles, nasal gliomas and nasal dermal sinus cysts. The differential detectable on prenatal ultrasound also includes haemangiomas, dacryocystocele, teratoma and retinoblastoma[2]. These pathologies are of interest to maxillofacial, head and neck, ophthalmology and neurosurgeons alike and their role is integral in the psychosocial counselling of the parents, preparing them for delivery and discussion of treatment options and their timing. The authors’ review the reported cases of prenatal diagnosis and management of nasal glioma and review the literature on this rapidly advancing field of craniofacial anomalies detected in utero.
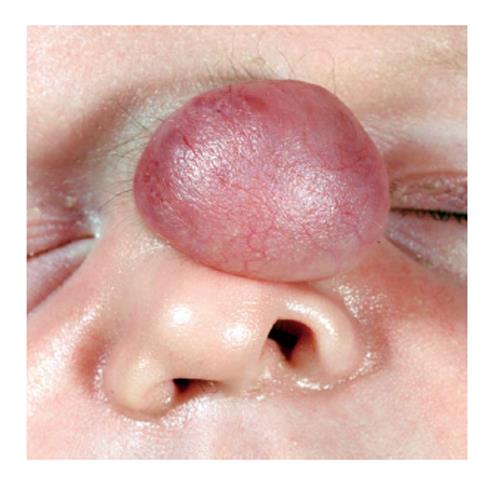
Nasal Glioma is a rare, benign, congenital facial lesion occurring in 1:20000-40000 live births[3]. They are comprised of heterotopic neuroglial tissue, arising in the midline and are most commonly extranasal (60%)[4] (Figure 1) but may be intranasal (30%) or display both extra and intranasal components (10%)[2]. It is important to differentiate nasal glioma from an anterior encephalocele, which involves a herniation of meninges through an incompletely closed fontanel, retaining intracranial communication, requiring neurosurgical assessment[5].
Prenatal ultrasound is typically used to confirm gestational age, foetal number, monitor foetal well being and detect gross abnormalities[6]. Modern ultrasound technology provides accurate multiplanar views of the foetal face via surface rendered images of 3D ultrasound, maintaining its prominent role in antenatal care[7,8]. Such advances have increased the detection rate of craniofacial abnormalities that once would not have been appreciated.
Complications of nasal glioma depend on its location and include nasal deformity, amblyopia, impaired visual field and nasal obstruction. As neonates are obligate nasal breathers this poses a threat to the foetal airway, requiring accurate delineation of the lesion and involvement of the appropriate specialists within the multidisciplinary team (MDT) that can address parental questions and anxiety and facilitate pre and postnatal planning.
Foetal magnetic resonance imaging (MRI) is favoured by most, to provide more accurate soft tissue imaging, confirm equivocal findings and identify intracranial involvement in utero[9]. It avoids unnecessary irradiation of mother and foetus and is favoured over computed tomography (CT). Is also provides synchronous identification of abnormalities of the upper aerodigestive tract, delineation of the foetal airway and ensures a well rehearsed MDT is prepared for definitive intra or postpartum airway interventions, should they be required[10]. Foetal MRI is not however considered an appropriate alternative to ultrasound, which can also provide doppler characteristics, and MRI should not be performed in isolation for foetal screening[6].
Should foetal MRI raise concerns of foetal airway compromise, intrapartum procedures can be performed to treat predicted complications of postpartum airway obstruction. The Ex-Utero Intrapartum Procedure utilises the utero-placental circulation, providing foetal oxygenation for up to 30-60 min[11]. Life saving airway interventions can be made on the partially delivered foetus whilst the mother is under general anaethesia. Management using this procedure requires detailed planning and a highly specialised, well-rehearsed MDT[11].
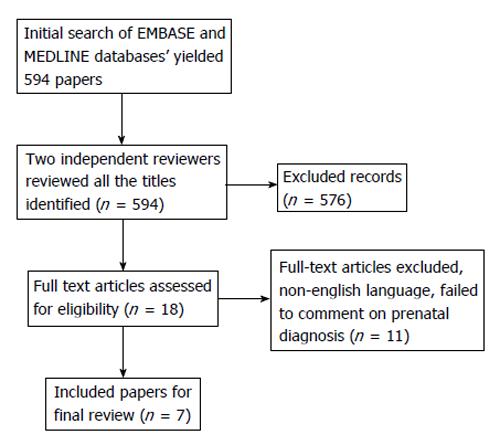
The literature search was conducted on two electronic databases, MEDLINE and EMBASE with titles including “Glioma”, “Prenatal”, “In Utero” and “Craniofacial” published from 1980 to present. Two independent reviewers screened 594 articles, and articles with repetition/duplication of original data, animal studies and studies written in non-english language were exclude. We were guided by the PRISMA checklist and flow diagram for article selection (Figure 2). A total of 7 papers were selected.
To date, very little has been published on prenatal management of nasal gliomas. There are seven cases reporting prenatally detected nasal glioma in the literature, summarised in Table 1. Five of these were successfully managed with good outcomes, one lead to termination of pregnancy and one resulted in death secondary to post-operative neonatal infection.
| Ref. | Gestational age at detection | Prenatal Ix | Postnatal Ix | Age at surgery | Outcome |
| Chmait et al[12], 2002 | 31 wk | Ultrasound + 3D image | MRI | 3 mo | Complete excision-forehead flap |
| Di Biasio et al[2], 2006 | 22 wk | Ultrasound (inc. Doppler) and MRI | MRI | 4 mo | Complete excision |
| Grzegorczyk et al[9], 2010 | 22 wk | Ultrasound (inc. Doppler) and MRI | MRI | 5 mo | Complete excision |
| Ajose-Popoola et al[5], 2011 | Second trimester | Ultrasound (inc. Doppler) and MRI | CT and MRI | 3 mo | Complete excision |
| Tonni et al[4], 2011 | Second trimester | Ultrasound (inc. Doppler) and Amniocentesis | N/A | N/A | Termination of pregnancy: elevated α-FP |
| Okumura et al[14], 2012 | 33 wk | Ultrasound (inc. Doppler) | CT and MRI | 8 d | Nasal and extranasal excision |
| Neonatal death secondary to LRTI | |||||
| Beegun et al[13], 2012 | 20 wk | Ultrasound (inc. Doppler) and MRI | MRI | 2 mo | Complete excision |
Chmait et al[12] (2002) were the first to report a prenatal diagnosis of nasal glioma in the literature. At 31 wk of gestation they identified a 19 mm × 15 mm left paraorbital cystic mass on 2D ultrasound scan. 3D ultrasound was performed and generated a surface rendered image of the foetal face, leading to a preliminary diagnosis of a dacryocystocele. No Doppler flow was present within the mass and no further prenatal imaging was undertaken.
The baby was delivered at term via uncomplicated spontaneous vaginal delivery, with a 20 mm firm, extranasal lesion in the left nasoglabellar region. The diagnosis was still inconclusive and postnatal MRI was performed, showing a distinct mass with no intercranial communication. The lesion was excised at 3 mo of age, using a forehead flap, and histological analysis confirmed a diagnosis of nasal glioma.
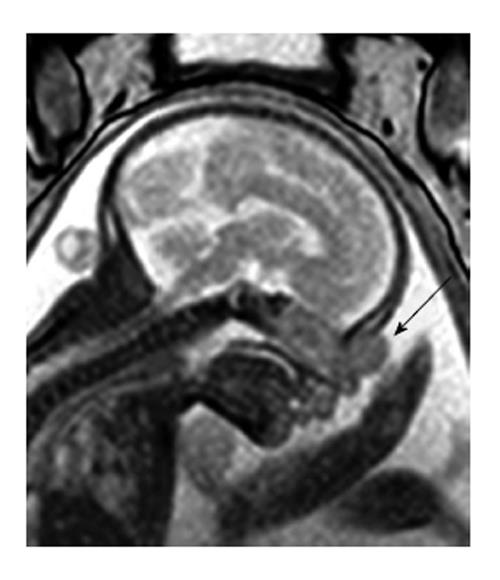
Beegun et al[13] (2012) reported the earliest prenatal detection of nasal glioma, at 20 wk gestation. A 10 mm × 10 mm × 9 mm left paraorbital soft tissue mass was reported on repeat ultrasound scan at 23 wk gestation. It demonstrated a single vessel supply but its origin and communication with the foetal brain could not be determined. Foetal MRI and repeat ultrasound were undertaken at 24 wk and showed the lesion had grown to 13 mm × 11 mm × 12 mm. The foetal brain appeared normal and was not in communication with the lesion. Repeat ultrasound scans were performed every two weeks to monitor the lesions size, which remained stable until 28 wk, where it increased to 16 mm × 12 mm. Repeat MRI at 28 wk and 36 wk did not show any associated bony erosion or deep extension. At this time the diagnosis was still in question.
The baby was delivered at 38 wk via uncomplicated spontaneous vaginal delivery, with a 20 mm × 15 mm soft cystic mass in the left nasoglabellar region, suspected to be a haemangioma. The lesion grew to 30 mm × 40 mm by 2 mo of age. A postnatal MRI excluded bony involvement however there were concerns that the lesion may obstruct the baby’s binocular vision and surgical excision with primary closure was undertaken at 2 mo of age and histology confirmed a diagnosis of nasal glioma.
Both De Biasio et al[2] (2006) and Grzegorczyk et al[9] (2010) report prenatal diagnosis of nasal glioma at 22 wk gestation. Basio detected a 7 mm lesion, with no doppler blood flow and undertook a foetal MRI that excluded intracranial communication and bone involvement, but no specific diagnosis could be made. Ultrasound monitoring showed the lesion increased in size to 20 mm at 32 wk gestation. The baby was delivered at 38 wk gestation via uncomplicated spontaneous vaginal delivery, with a 20 mm pink non-compressible mass medial to the left internal canthus. Postnatal MRI suggested a diagnosis of nasal glioma with partial intranasal extension. The lesion was excised at 4 mo of age and histology confirmed the diagnosis. Similarly, Grzegorczyk et al[9] identified a vascular hypoechoic mass on the left nasal bone on ultrasound scan. This was also investigated with foetal MRI that excluded intracranial communication and bony erosion. Following delivery, the lesion appeared as a reddish mass, suspected to be a haemangioma. Postnatal imaging further established low velocity flow consistent with a nasal glioma that was removed at 5 mo of age and the diagnosis was confirmed with histology.
Okumura et al[14] (2012) described a recent case of a craniofacial anomolie detected in the third trimester. A facial mass protruding from the left nostril with no doppler flow was identified at 33 wk gestation. No additional prenatal imaging was undertaken and the baby was delivered at 35 wk gestation via spontaneous vaginal delivery and immediately intubated. Postnatal CT scan was undertaken on day 8, and suggested an osseous defect the ethmoid bone with herniation of intracranial contents into the nasal cavity. A preliminary diagnosis of transethmoidal encephalocele was made. This was revised following postnatal MRI that demonstrated no intracranial involvement or bony defect, utilising better soft tissue delineation. An intranasal glioma was suspected and excised via endoscopic intranasal and oral routes, and the diagnosis was confirmed on histology. This case was complicated by the development of a lower respiratory tract infection and subsequent neonatal death.
Ajose-Popoola et al[5] (2011) described the management of a nasal glioma in a 3 mo old child, who had a 25 mm × 25 mm non-pulsatile solid nasoglabellar mass detected on prenatal ultrasound. It was subsequently investigated with foetal MRI that showed the mass was separated from brain parenchymal by a distinct cerebrospinal fluid plane. The baby was delivered without complication and postnatal MRI revealed a 28 mm × 18 mm × 18 mm mass in the midline, with possible intracranial communication through an anterior bony defect. Postnatal CT was undertaken at 3 mo of age, and showed the lesion had grown to a 28 mm × 20 mm × 20 mm mass that appeared to have intracranial connection via a 8 mm × 6 mm bony defect of the glabella and metopic suture. The lesion was excised via a midline nasal incision and no communication with the intracranial vault was seen. Histology confirmed a diagnosis of nasal glioma.
Tonni et al[4] (2011) described a second trimester detection of a midline craniofacial anomolie. Further examination via amniocentesis showed a 46,xx Karyotype with elevated α-FP levels. The parents declined further antenatal investigation and opted for legal termination of pregnancy in view of the severe psychophysical disturbances associated with the detection of the abnormality with life threatening risks to the mother. Tissue obtained by necropsy confirmed the diagnosis of nasal glioma.
Nasal glioma is a rare benign congenital midline facial defect that is being detected in the prenatal period with increasing frequency[2]. Advances in foetal imaging provide more accurate delineation of the foetal face. Ultrasound scanning is still the dominant antenatal imaging modality in modern obstetrics. The use of 3D rendered images along with doppler waveforms show characteristics that allows specialists to narrow their differential diagnosis[12].
An anterior encephalocele, appears as a midline cystic or solid mass emanating from a calvarial defect and may be accompanied by ventriculomegaly[2]. Haemangioma demonstrates a typical doppler blood flow pattern, high during arterial diastole, within a septate or solid mass protruding from the skull[2]. Nasal glioma are firm and nonpulsatile masses, most commonly originating from the nasoglabellar region, with low flow on doppler signal. Retinoblastoma appear as a heterogeneous mass arising directly from the orbit, with an irregular echogenic structure and covering membrane[2].
Beegun et al[13] emphasised the value of ultrasound in monitoring lesion size, using two-weekly ultrasound with repeated prenatal MRI to exclude bony erosion associated with lesion growth, in order to guide prenatal plans and timing of birth (Figure 3). Chmait et al[12], supports the use of 3D rendered ultrasound images but underscored the difficulty in achieving an accurate prenatal diagnosis. These cases all shared diagnostic uncertainty and each had an alternative working diagnosis, with diagnostic confirmation only being confirmed as nasal glioma with histological analysis.
Antenatal MRI is the modality of choice to investigate craniofacial abnormalities in utero and was performed in all except two cases; one was declined by parents who opted for termination of pregnancy, the other when the anomaly was detected late, in the third trimester. T1-weighted MRI demonstrates nasal glioma to be isointense to grey matter, with moderate contrast enhancement[4]. T2 weighted imaging will show hypointense mass similar to a congenital haemangioma, with low resistance arterial flow on doppler imaging[4]. It is important to identify any intracranial communication and exclude the presence of an anterior encephalocele. This distinction was unclear in two cases where a suggestion of intracranial communication was present even after postnatal imaging. Okumura et al[14] opted for initial postnatal CT to delineate the lesion. This suggested a small anterior calvarial bony defect; leading to a preliminary diagnosis of transethmoidal encephalocele that was revised once postnatal MRI was repeated. This emphasises the diagnostic difficulties inherent with these lesions. MRI is generally favoured over CT as it is at least as accurate as CT in detecting intracranial extension and avoids radiation to the head, neck and radiosensitive lens[15].
Grzegorczyk et al[9] recommends that pre and postnatal MRI should be performed, where available, in all cases where a craniofacial defect is detected on ultrasound. This can exclude intracranial extension, identify additional abnormalities, allow accurate planning of surgical approach, and reduce risks of incomplete resection.
Prenatal diagnosis of nasal glioma may be suggested as early as the second trimester, but diagnostic certainty is rarely achievable until postnatal imaging or histological examination. Investigations may suggest or exclude certain diagnosis but families must be counselled accordingly to ensure they are fully prepared for all possible diagnostic eventualities.
Doppler ultrasound provides important detection and monitoring facilities to guide pre and postnatal planning and direct the working diagnosis, differentiating glioma from haemangioma.
Prenatal MRI improves the diagnostic accuracy of ultrasound but should not be employed as an independent screening tool. Foetal MRI can identify associated intracranial communication, additional lesions, cerebral defects and delineate the upper aerodigestive tract of the neonate, predicting airway complications and allowing appropriate planning. Postnatal MRI imaging is essential to accurately identify the lesion, and is as good if not superior to CT in identifying intracranial extension, with the added benefit of avoiding neonatal exposure to ionising radiation[15]. It is also important to identify the glioma stalk, as full excision is required to reduce risk of recurrence, cerebrospinal fluid leak and meningitis.
When radiological investigations are combined with chorionic villous and or amniocentesis, the clinicians are provided with valuable diagnostic and prognostic information that may be used to empower families and inform a multidisciplinary discussion regarding genetic counselling, timing of delivery, postnatal treatment options and surgical planning[13]. The importance of this aspect of prenatal care cannot be underestimated, as the psychosocial impact of detecting these prenatal anomalies can be great.
Early surgical intervention is recommended, and is believed to correlate with more favourable aesthetic outcomes, reduce complications of ocular development and provide definitive histological diagnosis. Once a diagnosis of nasal glioma is confirmed, the overall prognosis is favourable, with low recurrence rate following complete excision.
P- Reviewer: Baglaj SM, Freiherr J, Rossi AC S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Ettema AM, Wenghoefer M, Hansmann M, Carels CE, Borstlap WA, Bergé SJ. Prenatal diagnosis of craniomaxillofacial malformations: a characterization of phenotypes in trisomies 13, 18, and 21 by ultrasound and pathology. Cleft Palate Craniofac J. 2010;47:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | De Biasio P, Scarso E, Prefumo F, Odella C, Rossi A, Venturini PL. Prenatal diagnosis of a nasal glioma in the mid trimester. Ultrasound Obstet Gynecol. 2006;27:571-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Strauss RB, Callicott JH, Hargett IR. Intranasal neuroglial heterotopia. So-called nasal glioma. Am J Dis Child. 1966;111:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Tonni G, Lituania M, Bonasoni MP, De Felice C. Prenatal ultrasound and histological diagnosis of fetal nasal glioma (heterotopic central nervous system tissue): report of a new case and review of the literature. Arch Gynecol Obstet. 2011;283 Suppl 1:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Ajose-Popoola O, Lin HW, Silvera VM, Teot LA, Madsen JR, Meara JG, Rahbar R. Nasal glioma: prenatal diagnosis and multidisciplinary surgical approach. Skull Base Rep. 2011;1:83-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Robson CD, Barnewolt CE. MR imaging of fetal head and neck anomalies. Neuroimaging Clin N Am. 2004;14:273-291, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Costello BJ, Edwards SP. Prenatal diagnosis and treatment of craniomaxillofacial anomalies. Oral Maxillofac Surg Clin North Am. 2010;22:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Pretorius DH, Nelson TR. Fetal face visualization using three-dimensional ultrasonography. J Ultrasound Med. 1995;14:349-356. [PubMed] |
| 9. | Grzegorczyk V, Brasseur-Daudruy M, Labadie G, Cellier C, Verspyck E. Prenatal diagnosis of a nasal glioma. Pediatr Radiol. 2010;40:1706-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Husein OF, Collins M, Kang DR. Neuroglial heterotopia causing neonatal airway obstruction: presentation, management, and literature review. Eur J Pediatr. 2008;167:1351-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Taghavi K, Beasley S. The ex utero intrapartum treatment (EXIT) procedure: application of a new therapeutic paradigm. J Paediatr Child Health. 2013;49:E420-E427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Chmait RH, Pretorius DH, Hull AD. Nasal glioma. Ultrasound Obstet Gynecol. 2002;20:417-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Beegun I, Dua R, Connor S, Bentley R. Prenatal diagnosis and management of a craniofacial glioma detected at 20 weeks’ gestation. Case report and review of the literature. Int J Oral Maxillofac Surg. 2012;41:200-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Okumura M, Francisco RP, Lucato LT, Zerbini MC, Zugaib M. Prenatal detection and postnatal management of an intranasal glioma. J Pediatr Surg. 2012;47:1951-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Huisman TA, Schneider JF, Kellenberger CJ, Martin-Fiori E, Willi UV, Holzmann D. Developmental nasal midline masses in children: neuroradiological evaluation. Eur Radiol. 2004;14:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |