Published online Aug 12, 2015. doi: 10.5318/wjo.v5.i3.110
Peer-review started: January 29, 2015
First decision: March 6, 2015
Revised: June 4, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: August 12, 2015
The renin-angiotensin system (RAS) regulates blood pressure (BP) homeostasis, systemic fluid volume and electrolyte balance. The RAS cascade includes over twenty peptidases, close to twenty angiotensin peptides and at least six receptors. Out of these, angiotensin II, angiotensin converting enzyme 1 and angiotensin II type 1 receptor (AngII-ACE1-AT1R) together with angiotensin (1-7), angiotensin converting enzyme 2 and Mas receptor (Ang(1-7)-ACE2-MasR) are regarded as the main components of RAS. In addition to circulating RAS, local RA-system exists in various organs. Local RA-systems are regarded as tissue-specific regulatory systems accounting for local effects and long term changes in different organs. Many of the central components such as the two main axes of RAS: AngII-ACE1-AT1R and Ang(1-7)-ACE2-MasR, have been identified in the human eye. Furthermore, it has been shown that systemic antihypertensive RAS- inhibiting medications lower intraocular pressure (IOP). These findings suggest the crucial role of RAS not only in the regulation of BP but also in the regulation of IOP, and RAS potentially plays a role in the development of glaucoma and antiglaucomatous drugs.
Core tip: Many of the central components of renin-angiotensin system (RAS) have been identified in different structures of the human eye. Recent findings suggest that local RAS accounts for long term changes in ocular tissue level. Antihypertensive drugs which inhibit RAS (Angiotensin converting enzyme or AT-receptor blockade) reduce intraocular pressure suggesting their possibility as anti-glaucomatous drugs in the future. Here we describe the local intraocular RAS especially in the anterior part of eye.
- Citation: Holappa M, Vapaatalo H, Vaajanen A. Ocular renin-angiotensin system with special reference in the anterior part of the eye. World J Ophthalmol 2015; 5(3): 110-124
- URL: https://www.wjgnet.com/2218-6239/full/v5/i3/110.htm
- DOI: https://dx.doi.org/10.5318/wjo.v5.i3.110
Glaucoma is after cataract the second leading cause of vision loss worldwide. In 2020, 79.6 million people are estimated to be diagnosed with glaucoma. The majority of these patients are estimated to have open angle glaucoma[1]. Glaucoma is a neurodegenerative disorder that leads to the loss of the axons of the optic nerve and to the death of retinal ganglion cells by non-apoptotic and apoptotic mechanisms all of which in the end cause visual field defects and irreversible vision loss[2-6]. Together with age and family history, increased intraocular pressure (IOP) is one of the known major risk factors for glaucoma[2,6,7]. In subjects with increased IOP, ocular hypotensive medication prevents or delays surgery of glaucoma[8]. A 30% reduction in IOP reduces disease progress 10%-35% in glaucoma patients[9,10]. Even though risk factors and possible outcomes of glaucoma are known, the exact mechanism behind development of glaucoma is still poorly known. Interestingly, imbalances in the local ocular renin-angiotensin system (RAS) cascade have been associated to glaucoma[3].
In addition to the circulating RAS that controls blood pressure (BP) homeostasis, electrolyte balance and systemic fluid volume, tissue-specific RAS, accounting for local effects and long-term changes in tissue level, have been described. Local RA-systems have been demonstrated in different organs studied[11,12], including the human eye[2,12-14]. Systemic antihypertensive drugs which inhibit RAS can reduce IOP. Certain ACE inhibitors[15] and AT1 receptor blockers[16] have been shown to reduce IOP in both non-glaucomatous and glaucomatous patients. In animal studies angiotensin converting enzyme (ACE) inhibitors[17,18], AT1 receptor blockers[19,20], and renin inhibitors[21] have been reported to lower IOP. These findings imply that RAS is not only important in the regulation of BP but that it is possibly also involved in the regulation of IOP[5,22]. However, the question of how RAS is involved in the regulation of IOP remains to be answered.
In this review we describe the tissue RAS cascade and concentrate on the anterior part of the eye. A survey of PubMed using the following keywords was performed to collect the literature on eye, IOP (38214, number of reports), RAS (26697), tissue RAS (4870), angiotensin (110705), angiotensin I (7879), angiotensin II (55855), angiotensin converting enzyme (45777), angiotensin (1-9) (28), angiotensin (1-7) (1043), Mas receptor (305), angiotensin receptor (16021), eye disease (4830), glaucoma (55288), diabetic retinopathy (DR) (25958), retinopathy of prematurity (ROP) (5710) and age-related macular degeneration (10875). Combining the used keywords allowed to narrow down the literature to 185 references which were used in this review. They were selected based on the abstracts.
The very first clue of the existence of RAS was found in 1898 when scientists Robert Tigerstedt and Per Bergman in Finland discovered that injecting renal homogenate from one rabbit to another causes an acute elevation of BP indicating that kidney secretes a vasopressor substance, named renin[23,24]. Due to the discovery of this hormone, RAS was first thought to be a hormone system through which the kidney influences systemic cardiovascular regulation[25]. Over 40 years later more RAS effectors were found. In 1940, groups working under Braun-Menéndez and Page reported that previously identified renin catalyzes the formation of pressor peptide, first named angiotonin or hypertensin, from a plasma protein substrate angiotensinogen[22,26,27]. Later angiotonin was renamed angiotensin[22].
In the early 1970s major components of the circulating RAS were found and its important role as a BP and fluid balance regulator was understood[23]. In addition, first antihypertensive medications were developed in the 1970s. First of these drugs was captopril, an ACE inhibitor that was designed to prevent the formation of vasoconstrictive peptide Angiotensin (Ang)II[22,23]. In 1988, AngII receptor type 1 blockers (ARBs) were invented which main goal was to prevent the direct effects of AngII mediated through angiotensin II type 1 receptor (AT1R)[12]. During past years many new peptides and a new angiotensin reseptor type (Mas receptor, MasR) have been identified. MasR is an important member of the RAS, and its actions are mainly opposite to those of AT1R. Mas-receptors play a role in cell proliferation and antifibrosis as well as vasodilatation and local fluid volume homeostasis. In fact, the potentials of MasR ligands, like Ang (1-7) and ACE2 in degrading vasoconstrictive Ang II to vasodilatory peptides are regarded as a present focus of cardiovascular drug development[28-30].
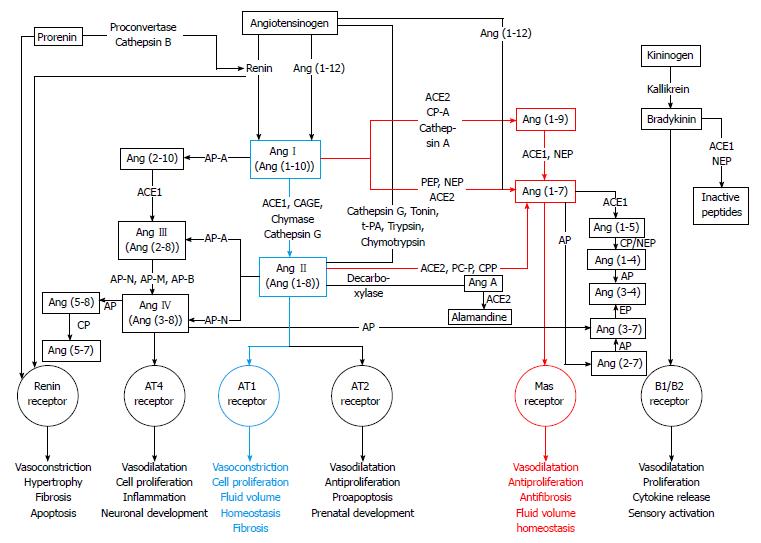
When RAS was first described, it was seen as a linear cascade consisting of only one substrate (angiotensinogen), two proteases (renin and ACE1), two peptides (AngI and AngII) and one receptor (AT1R). Today, RAS is known to consist of several enzyme pathways and to include over twenty peptidases, close to twenty angiotensin peptides and at least six receptors[31,32]. Thus, the classical linear cascade has evolved to a cascade with multiple mediators, multifunctional enzymes and multiple different receptors mediating the effects of angiotensin peptides[33-35]. The complexity of the RAS cascade known today is seen in Figure 1.
Angiotensinogen (AGT) is a 255 amino acids long α-glycoprotein that is synthesized in and released from liver. Renin catalyzes the reaction in which angiotensinogen is converted into AngI[22,36,37]. Mainly synthetized in the liver, angiotensinogen is also formed in heart, vessels, kidney and adipose tissue[38]. The synthesis of α-glycoprotein angiotensinogen is stimulated e.g. by inflammation, insulin and estrogens[36].
Angiotensin I (AngI), a weak active prohormone, also known as angiotensin (1-10), is a decapeptide generated from angiotensinogen by an enzyme renin[39]. AngI, a weak vasoconstrictor is further cleaved to an octapeptide AngII by ACE1 removing two amino acid residues (His-Leu) from the carboxy terminal of AngI[39,40]. AngII can also be generated by enzymes other than ACE1 such as chymase and cathepsin G.
Angiotensin II (AngII), also known as Ang(1-8), first isolated in 1940 and characterized as a potent vasoconstrictor that elevates BP[26,27]. Then, RAS was regarded as an endocrine system in which circulating AngII regulates electrolyte balance, vascular tone, thirst, water intake, aldosterone synthesis, sympathetic activity, sodium handling in the kidney, and antidiuretic vasopressin release from the posterior part of hypophysis[37]. In circulating RAS, renin formed in the kidney is the rate-limiting factor for AngII formation whereas in vascular tissue ACE1 and chymase are the main actors in AngII generation[41].
AngII exerts its main actions via two types of receptors, AT1R and AT2R[36,42]. AngII can be generated from AngI by three different categories of enzymes: ACE1, a metallo dipeptidyl carboxypeptidase, secondly aprotinin-sensitive serine proteases, such as trypsin, tonin, kallikrein and cathepsin G and thirdly a group of chymostatin-sensitive serine proteases, such as human chymase[43]. AngII, a potent vasoconstrictor stimulates the release of vasopressin and aldosterone and thus participates sodium and water retention all of which act in concert to raise BP[37]. ACE inhibitors as antihypertensive medication block the conversion of AngI to AngII by ACE1, thus antagonizing the harmful effects of AngII on AT1R[36].
Angiotensin III (AngIII), also known as Ang(2-8), is generated from AngII or from angiotensin (2-10) by aminopeptidase A and ACE1[22,23,36,37]. This heptapeptide was found in 1970s and it exerts its actions via AT1 and AT2 receptors. AngIII has higher affinity to AT2 receptors than to AT1 receptors[44]. AngIII induced vasoconstriction and release of aldosterone are close to those of AngII. AngIII has 40% of the vasoconstriction activity of AngII[22,23,37]. In some actions on AT1R the role of AngIII is at least equally important as that of AngII[23,37].
Angiotensin IV (AngIV), is generated from AngII by aminopeptidase N or from AngIII by several other aminopeptidases N, M and B[22,37]. This hexapeptide [Ang(3-8)] exerts its actions via angiotensin II type 4 receptor (AT4R) found in kidney, lung, brain and heart[23,45,46]. However, AngIV can also induce its effects such as renal vasodilatation, hypertrophy and regulation of cell growth in endothelial cells, cardiac fibroblasts and vascular smooth muscle cells by interacting with AT1R[47]. Furthermore, AngIV is thought to have an important regulatory role in cardiovascular damage, cognition and renal metabolism and it might be involved in the vascular inflammatory response[22,37].
Angiotensin (1-9) [Ang(1-9)] is formed by cleaving one amino acid residue from the carboxyl terminus of AngI by ACE2[48] and is metabolized by ACE1 and NEP to generate Ang(1-7)[49]. Ang(1-9) can also be generated from AngI through the activity of carboxypeptidase A or cathepsin A[50,51]. The formation of Ang(1-9) is dependent on ACE2 activity[49,52]. The biological function of Ang(1-9) is to increase nitric oxide formation and release of arachidonic acid, enhance bradykinin activity[50] and possibly be involved in the inhibition of platelet function[53]. Ang(1-9) may decrease BP and thus protect the heart and blood vessels and reduce hypertension[54]. Ang(1-9) could mediate its actions via the AT2 receptors[54,55].
Angiotensin (1-7) [Ang(1-7)] was originally believed to be an inactive component of RAS. In 1988 this heptapeptide was shown to have actions opposing those of AngII[37]. Ang(1-7) is generated from AngII by ACE2 or by other known peptidases such as prolylendopeptidase and prolyl-carboxipeptidase[23,37,42,56]. Ang(1-7) can also be synthesized directly from AngI by prolylendopeptidase and from Ang(1-9) or from prohormone Ang(1-12) bypassing the synthesis of AngII[37,56]. Furthermore, Ang(1-7) interacts with the kallikrein-kinin system, and can be converted into Ang(1-5) or into Ang (3-7)[22]. Ang(1-7) levels are elevated by ACE inhibitors that increase AngI concentration and on the other hand prevent Ang(1-7) degradation[37].
Ang(1-7) was thought to be devoid of biological functions[37]. Nowadays Ang(1-7) is seen as a protector peptide that counterbalances many functions of AngII by binding to MasR which mediates vasodilating and antiproliferative functions of Ang(1-7)[23,36,55,57]. Although MasR is the main receptor of Ang(1-7), some of the functions may still originate via AT1R and AT2R[54,55,57,58]. In addition to the inhibition of AngII-induced vasoconstriction by Ang(1-7), its antiarrhythmogenic, antithrombogenic and growth-inhibitory properties suggest that Ang(1-7) acts as a physiological counterregulator within the RAS, and that Ang(1-7) could be a potential target for drug development[33-35]. In fact, Ang(1-7) has been associated to pathophysiology of several diseases such as. hypertension[59-63], chronic renal diseases[61] and diabetic nephropathy[64,65].
In addition to previously described peptides, RAS cascade includes short peptides which functions and roles in this circulating and tissue-specific regulatory system are still poorly known.
Renin, ACE1 and ACE2 are seen as three key enzymes of the RAS. Renin, a specific enzyme having only one known substrate, is an aspartyl protease that cleaves its substrate angiotensinogen to form AngI. Renin cleaves the peptide bond between Leu10 and Val11 at the amino terminus of angiotensinogen. Renin is synthesized as a 406 amino acid residues long inactive prorenin in the juxtaglomerular apparatus of the kidney[22,36,37]. Upon demand synthesized prorenin is cleaved and activated by proconvertase or cathepsin B to generate 340 amino acid residues long catalytically active form of renin. Renin can also be synthesized in organs such as brain, heart, testis, pituitary and adrenal glands, arterial smooth muscle and eye[36]. Classically, renin is secreted by juxtaglomerular cells in response to three different stimuli: (1) decreased arterial BP; (2) decreased sodium levels in the macula densa ultrafiltrate; and (3) increased sympathetic nervous system activity[40,66,67]. Activation of prorenin can be either proteolytic or non-proteolytic. The proteolytic way is irreversible while the latter one is reversible[36].
ACE1 belongs to the M2 family of metallopeptidases containing zinc in its active site. ACE1 is a monomeric glycoprotein that has two different isoforms: somatic ACE1 (sACE1, 150-180 kDa) and germinal ACE1 (gACE1, 90-110 kDa)[36]. The somatic ACE1 is found in various epithelial and endothelial cells[68] whereas germinal ACE1 in germinal cells in the testis[36]. ACE1 is a type I integral membrane protein that consists of hydrophilic C-terminal cytoplasmic domain, hydrophobic transmembrane domain and a heavily glycosylated N-terminal ectodomain[36]. It is distributed in many tissues and is also found in biological fluids, e.g., in plasma and cerebrospinal fluid[69-71].
ACE1 has an activated water molecule complexed to Zn2+ in its active sites[72]. In addition, ACE1 activity depends on the presence of chloride that enhances the binding of different substrates[73]. As an exopeptidase ACE1 cleaves dipeptides from the free C-terminus of AngI and of the hypotensive peptide bradykinin[36,40]. ACE1 can also generate AngIII and Ang(1-7) and then further degrade Ang(1-7) to inactive Ang(1-5). Moreover, ACE1 acts in kallikrain-kinin system cleaving bradykinin to inactive compounds[36,40,57]. Because ACE1 participates in regulation of BP and in development of cardiovascular diseases, it is one major target for pharmacotherapy[36].
ACE2, the first known human homologue to ACE1 (42% sequence identity), was cloned in 2000[36,42,48,68,74]. ACE2 was first shown to convert AngI to Ang(1-9)[48]. Later, ACE2 was found to hydrolyze AngII into Ang(1-7) with much higher efficiency (approximately 400-fold) than the hydrolysis of AngI to Ang(1-9)[36,42,49,57,75]. ACE2 is a 805 amino acid residues long (120 kDa) type I transmembrane glycoprotein that has been found in organs such as kidney, heart, lungs, liver and brain. ACE2 has a conserved zinc metallopeptidase consensus sequence His-Glu-X-X-His, in wich X stands for any amino acid (HEXXH) in its active site and its activity is regulated by chloride ions[36]. Contrary to ACE1, primarily dipeptidylcarboxypeptidase, ACE2 functions as a monocarboxypeptidase cleaving a single amino acid residue (Phe) from AngII to generate Ang(1-7). Thus, it negatively regulates the activated RAS and ACE1 activity by degrading AngII and increasing Ang(1-7) formation[36,74]. ACE2 is not blocked by conventional ACE inhibitors[58].
ACE2 together with Ang(1-7) and MasR have become the focus of recent research regarding RAS[42,58]. ACE2 is seen as the key player maintaining the balance between the two main pathways of RAS: ACE1-AngII-AT1R and ACE2-Ang(1-7)-MasR[36]. Chronic and long lasting imbalance of these two enzymatic pathways may lead to pathophysiology of the renal, pulmonary, cardiovascular and central nervous system[76].
In addition to previously mentioned enzymes, there are several different peptidases and proteases that act on longer angiotensin peptides thus cleaving them into shorter peptides. For example, AngII can be generated from AngI by four different enzymes: ACE1, CAGE, chymase and cathepsin G[43]. Alternative enzymes acting on different angiotensin peptides are shown in Figure 1.
A number of studies have shown alternative pathways for AngII generation[77-79] being important in physiological and pathophysiological conditions[41,80]. AngII-forming enzymes can be divided into three categories: metallodipeptidyl carboxypeptidase known as ACE1, aprotinin-sensitive serine proteases such as tonin[81], cathepsin G[82], kallikrein[83], trypsin[84] and chymostatin-sensitive serine proteases such as human chymase[85,86] (Figure 1).
Human (pro)renin receptor [(P)RR] is a 350 amino acid residues long single transmembrane-domain protein containing unglycosylated N-terminal domain responsible for renin and prorenin binding and the short cytoplasmic tail that is involved in the intracellular signalling[36,87]. Compared to the binding of free renin, the binding of renin to (P)RR is 3- to 5-fold more catalytically efficient, thus cleaving AGT to AngI more effectively[36,37].
Four heptahelical G-protein-coupled receptors of RAS: AT1R, AT2R, AT4R and MasR, mediate the effects of angiotensins causing vasodilatation and vasoconstriction[55,88]. AT1 and AT2 receptors are mainly responsible for mediating the effects of AngII, whereas AT4 receptor is target of AngIV generated by degradation of AngII[23,37]. A break-down product of Ang(1-7), namely Ang(3-7), can also bind to AT4R. AT4 receptors are located in the brain, lungs, heart, kidneys and liver and they are related to cognitive functions and proliferative effects[43,45,46].
Although AT1 and AT2 subtypes bind Ang II in a similar manner, they differ in tissue-specific expression and genomic structure (only about 30% sequence homology) as well as in localization and regulation. AT1 receptors can be activated by Ang II but other peptides, such as Ang III, Ang IV and Ang(1-7), can also stimulate AT1R but with lower binding affinity[43]. AT1 and AT2 receptors mediate opposite effects of Ang II, the former having negative cardiovascular effects, such as vasoconstriction and aldosterone release, and the latter having positive cardiovascular effects[12]. Whereas the role and function of AT1R is quite well established, the function of AT2R is not as clearly defined[55]. AT2 receptors, which are activated by Ang II and also by Ang(1-7), may exert the antiproliferative, proapoptotic, vasodilatory and antihypertensive effects[43,89]. AT2 receptors are known to be involved in differentiation, regulation of growth and regeneration of neuronal tissue, and they are also known to play an important role in prenatal development. AT2 receptors can also inhibit AT1R signaling by directly binding into it. Thus they are considered to be cardiovascular protective receptors[12].
MasR was first discovered in year 1986 by Young et al[90] as proto-oncogene. Two years later high MasR levels were reported in the rat central nervous system by the same research group[91]. Later Kitaoka et al[92] described MasR expression in the eyes of rhesus macaque. It was early found in the mouse kidney and described as a factor involved in tumorigenesis[93]. Subsequently it is also found in other organs such as in heart, vessels, testis, kidney and brain[94] and very recently in the human eye[95]. MasR is a G protein coupled receptor that has seven transmembrane domains[93]. This receptor acts antagonistically to the AT1R, mediating number of positive cardiovascular effects, such as vasodilation and antiproliferative effects, of its ligand Ang(1-7)[43]. MasR is part of the counterregulatory arm of RAS (ACE2-Ang(1-7)-MasR) thus balancing the effects of ACE1-AngII-AT1R pathway[34,35].
In addition to circulatory RAS, various organs have their own local RA-systems accounting for long-term changes and local effects including proliferation, growth and protein synthesis at tissue level[12,23,41]. The first clues of the existence of local RA-systems came in 1971 when Ganten et al[96] demonstrated that RAS components could be produced locally in organs and tissues. This proves that RAS is not only a circulating hormonal system, as thought earlier, but also a tissue-specific regulatory system[23]. Heart, liver, brain, kidney, lungs, intestine and even the human eye have their own local RA-systems[2,12,37].
Local RAS includes all components necessary for independent production of different components of RAS, such as Ang II, angiotensinogen, ACE1, AT1R and AT2R[2,12,37]. Thus, RAS is not only an endocrine and circulating, but also a local paracrine and intracrine system regulating more functions than was previously thought[12,41]. Even though many of the local RA-systems operate independently from the circulatory RAS, in heart and kidney, tissue-RAS operates in close interaction with the systemic RAS thus complementing each other’s functions[37]. Based on the origin of Ang II, local RAS can be divided into extrinsic and intrinsic system, the former getting its Ang II from the circulation and the latter obtaining its Ang II through local biosynthesis[18].
Local RAS has also been identified in the human eye. Researchers have localized all of the central components of RAS, including its receptors, to the structures of the eye in variety of species[2,5]. Moreover, all components of the two main axes of RAS: Ang II-ACE1-AT1R and Ang(1-7)-ACE2-MasR have been identified in the ocular structures of different species. When human eye is considered, the components of the two main axes are found in retinal structures and in non-retinal structures of the human eye[2,95,97]. Our research group has very recently succeeded to determine Ang (1-7) and ACE2 in the human aqueous humor[97]. Tables 1 and 2 summarize the localization of RAS peptides and enzymes in non-retinal ocular structures of the human eye. Tables 3 and 4 summarize the localization of RAS receptors in non-retinal ocular structures of the human eye. Although, essential components of RAS haven been identified in the human eye, the importance and functions of intraocular RAS are still unknown. However, intraocular RAS has been the focus of growing interest in recent years due to its possible role in the regulation of IOP through its effects on aqueous humor formation and drainage[5,12]. Furthermore, intraocular RAS activity has been linked to the development of glaucoma through its effect on IOP[2].
| RAS | Tears | Bulbar | Cornea | Trabecular | Aqueous humor | Iris |
| component | lacrimal gland | conjunctiva | meshwork | |||
| Prorenin | White et al[98] | White et al[98] | Danser et al[99] | White et al[98] | ||
| Renin | White et al[98] | White et al[98] | White et al[98] | |||
| AGT | White et al[98] | White et al[98] | Chowdhury et al[100] | White et al[98] | ||
| ACE1 | Vita et al[101] | Savaskan et al[13] | Savaskan et al[13] | Savaskan et al[13] | Vita et al[101] | Ferrari-Dileo et al[106] |
| Sharma et al[102] | Weinreb et al[104] | |||||
| Immonen et al[103] | White et al[98] | White et al[98] | Aydin et al[105] | White et al[98] | ||
| Holappa et al[97] | ||||||
| ACE2 | Holappa et al[97] | |||||
| Ang I | Danser et al[14] | Danser et al[14] | ||||
| Osusky et al[107] | ||||||
| Ang II | Savaskan et al[13] | Savaskan et al[13] | Osusky et al[107] | Danser et al[14] | Danser et al[14] | |
| Savaskan et al[13] | Osusky et al[107] | Senanayake et al[108] | ||||
| Ang(1–7) | Vaajanen et al[95] | Holappa et al[97] |
| RAS component | Ciliary body/non-pigmented ciliary epithelium | Lens | Vitreous | Optic nerve head | Sclera | |
| Prorenin | Sramek et al[109] | White et al[98] | Danser et al[99] | White et al[98] | ||
| Danser et al[99] | Wallow et al[110] | |||||
| Wallow et al[110] | ||||||
| Berka et al[111] | ||||||
| Renin | Berka et al[111] | White et al[98] | White et al[98] | |||
| AGT | Sramek et al[112] | Sramek et al[112] | ||||
| ACE1 | Igic et al[113] | Savaskan et al[13] | Ferrari-Dileo et al[106] | Ferrari-Dileo et al[106] | White et al[98] | |
| Ferrari-Dileo et al[106] | White et al[98] | Nakanishi et al[114] | ||||
| Sramek et al[112] | Ishizaki et al[115] | |||||
| Aydin et al[105] | ||||||
| ACE2 | ||||||
| Ang I | Danser et al[14] | |||||
| Ang II | Danser et al[14] | Senanayake et al[108] | Senanayake et al[108] | Savaskan et al[13] | ||
| Savaskan et al[13] | ||||||
| Ang(1–7) | Vaajanen et al[95] | Vaajanen et al[95] | ||||
| RAS component | Ciliary body/non-pigmented ciliary epithelium | Lens | Vitreous | Optic nerve head | Sclera | |
| (P)RR | White et al[98] | White et al[98] | ||||
| AT, unknown subtype | Lograno et al[117] | |||||
| Lin et al[116] | ||||||
| AT1R | Cullinane et al[118] | Senanayake et al[108] | Senanayake et al[108] | |||
| AT2R | Senanayake et al[108] | Senanayake et al[108] | ||||
| AT4R | ||||||
| MasR | Vaajanen et al[95] | |||||
Concerning intraocular local RAS, there has been debate whether intraocular angiotensins originate from local production or from the blood compartment[14]. It has been shown that neither Ang I, Ang II nor angiotensinogen are able to pass the blood-brain barrier which is similar to blood-retina barrier in the eye[14,119,120]. Circulating angiotensins cannot reach the vitreous fluid when blood-retina barrier is intact[14]. However, if disrupted their entering the eye through blood-retina barrier becomes possible[99]. In porcine ocular tissues Ang I and Ang II levels are 5 to 100-fold over those found from admixture with blood or diffusion from blood[14]. In rabbit and pig ACE1 activity has been shown to be higher in ocular tissues than in plasma[121,122]. The local intraocular RAS is estimated to have a role in the regulation of IOP affecting the formation of aqueous humor and the drainage. It has been shown that systemic antihypertensive RAS-inhibiting medications lower IOP. Certain ACE inhibitors[15] and AT1 receptor blockers[16] have proved to lower IOP in both non-glaucomatous and glaucomatous patients. In animal studies, ACE inhibitors[17,18], AT1 receptor blockers[19,20] and renin inhibitors[21] have been reported to reduce IOP. It has also been suggested that Ang II can increase aqueous humor secretion via AT1 receptor[118].
Aqueous humor formation: Intraocular pressure (IOP) can be described as a net sum of homeostatic balance between aqueous humor formation and outflow[123,124]. In the healthy human eye, the flow of aqueous humor against the resistance generates an IOP of about 15 mmHg[125]. Maintaining the optimal physiological IOP is fundamental to keep the optical and refractive properties of the eye, including the right shape of the eye[124,126]. The circulating fluid nourishes unvascularized eye structures such as the cornea and the lens. The normal aqueous humor formation rate is 2.5-2.8 μL/min and the entire volume is replaced every 100 min[5]. This is reduced during sleep, with ageing, and in some systemic diseases like diabetes[127]. Currently IOP is the main risk factor for glaucoma that is amenable to treatment[128].
The ciliary body epithelial is responsible for the production of aqueous humor[123] which is secreted mainly by active ionic transport across the epithelium against a concentration gradient[129]. Active secretion requires energy, produced in hydrolysis of adenosine triphosphate (ATP) by Na+/K+ ATPase. Active transport of Na+ into the posterior chamber by the non-pigmented ciliary epithelial cells induces also water movement from the stromal pool into the posterior chamber. Active transport of Cl- and HCO3- occurs to a lesser extent[130]. In addition to the active secretion two other physiological processes exist in the fluid formation: diffusion from the blood compartment and ultrafiltration. They are passive and require no cellular activity[131]. The whole ciliary body system and its aqueous humor formation should be regarded as a multifunctional and interactive process. Aqueous humor is a mixture of organic solutes, electrolytes, growth factors, cytokines and proteins[132-136]. After the production it is secreted into the posterior chamber from where it flows between the lens and iris into the anterior chamber[132,137,138].
Aqueous humor outflow:Via anterior chamber and through the trabecular meshwork and the canal of Schlemm, aqueous humor escapes the eye into the venous blood system[123]. It can leave the eye through three different main routes: the trabecular, the uveoscleral or the uveolymphatic pathways[128]. Trabecular outflow is the main route of drainage accounting for 90% of all aqueous humor outflow, and it is pressure-dependent[5,128,139]. The fluid outflow through the trabecular meshwork is affected by adhesions of trabecular meshwork cells and by the state of the actin cytoskeleton[140].
Outflow, where aqueous humor drains through the ciliary muscle and exits through the supraciliary space and across the anterior or posterior sclera into choroidal vessels, is called the uveoscleral outflow[141] which is independent of IOP and particularly impacted by age[139]. A third outflow route is suggested to exist: channels in the stroma of the ciliary body and interstitial spaces between ciliary muscle bundles. It may function as a backup outflow system[142]. The relevance of this pathway remains to be determined. The other alternative, minor outflow pathways are via iris vessels, corneal endothelium, or anterior vitreous body[143].
Pharmacological treatment of glaucoma reduces IOP by decreasing the rate of aqueous humor formation or by increasing the rate of aqueous humor outflow[144].
It is well-known that defects in the RAS cascade are involved in several cardiovascular and renal diseases, including heart failure, hypertension, ventricular hypertrophy, cardiac remodelling, and chronic renal failure[145-147], but interestingly, imbalances in the RAS cascade are also involved in glaucoma[3], which is a neurodegenerative disorder that leads to the loss of the axons populating the optic nerve and to the death of retinal ganglion cells by non-apoptotic and apoptotic mechanisms[2,3,6]. Together with age and family history, increased IOP is one of the known major risk factors for glaucoma[2,6,7]. Diabetes, migraine/vasospasms and vascular dysfunction are also considered as risk factors for glaucoma development[5,6,128].
Ocular hypotensive medications, laser procedures and surgical means are currently the major therapeutic tools to treat glaucoma[2,6,22]. They all act by lowering IOP thus affecting the onset of the disease[5]. Interestingly, antihypertensive medications acting on RAS have been shown to lower also IOP, suggesting that compounds blocking RAS might be potential anti-glaucomatous drugs in the future[22]. ACE inhibitors can decrease AngII levels in aqueous humor[107]. By reducing blood flow in the ciliary body ACE inhibitors could also decrease aqueous humour production[148]. Furthermore, by preventing the breakdown of bradykinin ACE inhibitors are able to promote synthesis of endogenous prostaglandins, which, as shown with marketed prostaglandin analogues, could increase the uveoscleral outflow thus lowering IOP[149,150]. Biosynthesis of certain matrix metalloproteinases is thought to be associated with increased uveoscleral outflow which leads to relaxation of the ciliary muscle and reduction and compaction of extracellular matrix components within the ciliary muscle, the sclera, the iris and within tissues of the uveoscleral outflow route, all of which might lower IOP by facilitating aqueous humor outflow[151]. ACE-inhibitors activate also the nitric oxide pathway by preventing bradykinin breakdown which increases endothelial nitric oxide formation and causes vasodilatation. Bradykinin stimulates the synthesis of prostaglandins and nitric oxide which also antagonize the vasoconstrictive effects of endothelin-1 and inhibit the overall production of endothelin-1 by endothelial cells. Endothelin 1 is a vasoconstrictive peptide that promotes contraction in the human ophthalmic artery and in the porcine ophthalmic and ciliary arteries[152-154].
Moreover, RAS activity has been described in cultured non-pigmented human ciliary epithelial cells which participate in aqueous humor formation and many of the central components of RAS have been identified in eye structures responsible for aqueous humor formation such as ciliary body[2,116,118]. AngII can activate Ca2+ signalling system that increases potassium ion channel activity[155]. Together with cell volume loss, these effects suggest that AngII acts as a operated secretagogue in the non-pigmented ciliary cells[118]. In addition, Ang II activates Na+/H+ exchange which leads to an increase in cytoplasmic sodium concentration[129]. In ciliary and renal tubular epithelium sodium handling related mechanisms are common pathogenetic factors. This might explain the coexistence of glaucoma and systemic hypertension[156]. Other explanations have also been suggested for the relationship between hypertension and glaucoma development. Hypertension is shown to cause impairment in autoregulation of the posterior ciliary circulation[157] and suggested to induce microvascular damage thus worsening blood flow to the optic nerve[158]. Furthermore, antihypertensive therapy has been described to cause hypotensive episodes that can injure the optic nerve[159].
In addition to possible role of RAS in the aqueous humor formation, RAS is suggested to act in aqueous humor outflow. AngII is able to promote cell proliferation in bovine trabecular meshwork cells and increase synthesis of collagen in vitro. Moreover, intracamerally administered AngII reduces uveoscleral outflow[160]. Paradoxically, natural and synthetic AngII, when administered intravenously, lowered IOP in anaesthetized cats[161].
In addition to glaucoma, local intraocular RAS has been associated with other severe eye diseases that can lead to permanent vision loss, such as age-related macular degeneration (AMD), ROP and DR. Dysregulation of RAS cascade participate in the development of these severe eye diseases.
In elderly people, AMD is one of the leading causes of visual impairment. Both dry and wet forms of the disease are associated with vision loss. Dry forms of the disease accounting for 90% of the cases lead to the significant decline of photoreceptors which ultimately causes central vision loss. On the contrary, wet form of AMD is characterized with pathological growth of cloroidal blood vessels that will eventually populate retina after breaking through the underlying Bruch’s membrane. In addition to old age, environmental factors, smoking, genetic susceptibility and systemic hypertension are regarded as risk factors for developing AMD. Interestingly dysregulation of the RAS cascade is suggested to play a role in the development of AMD[2,162,163].
Three key observations are held as evidence showing the possible involvement of RAS in the development of AMD. Firstly, systemic hypertension is a risk for the development of AMD. Secondly, dysregulation of RAS may have an impact on retinal pigment epithelium function and photoreceptor viability due to the observations that AngII can modulate retinal pigment epithelium. Thirdly, AngII is involved in retinal angiogenesis thus it might have a role in choroidal neovascularisation[2,162]. Animal studies have proven that administered AT1R antagonist (losartan)[164] and other AT1 receptor blockers[165] and (pro)renin receptor inhibitor[166] can reduce choroidal neovascularization thus having a positive effect on AMD.
ROP is a neovascular disease affecting premature newborns. ROP is associated with pathological retinal neovascularisation that causes complications such as tractional retinal detachment, macula dragging and vitreal haemorrhage, all of which can lead to vision loss[162]. The main risk factors for the disease are low birth weight and lower gestational age, both of which correlate with immaturity of retina at birth. In fact, in industrialized countries, approximately two-thirds of infants with birth weight less than 1.25 kg manifest some degree of retinopathy[167]. The cause of ROP is thought to be the retinal blood vessels expanding from the optic nerve which growth halts when a premature neonate is brought into a high oxygen environment. When the newborn is brought back to normal conditions, the inner vasculature in retina fails to regain normal vessel growth thus creating an avascular area and causing neovascularisation and epiretinal angiogenesis that can lead to vision loss[168].
Studies using animal models have suggested that RAS is involved in the development of ROP. Infants that are diagnosed with ROP have had elevated serum prorenin levels[169], ocular renin levels[170,171] and increased AT1R and AT2R expression[170]. Treating oxygen induced retinopathy in animal models with ACE inhibitors and AT1R antagonists during the normal air conditions reduces pathological angiogenesis on the surface of the retina[170,172-174]. On the contrary, the role of AT2R in retinal vascular pathology and the effects of the use of AT2R antagonists on retinal angiogenesis are still debatable[171,173,175,176].
The development of progressive vascular pathology within the inner retina characterizes DR which is among of the leading causes of blindness worldwide[163,177]. Alterations in the blood-retinal barrier, ischemia, dilated capillaries associated with poor retinal perfusion, retinal microaneurysms, loss of pericytes leading to changes in vascular permeability and the release of growth factors which may induce neovascularisation are all implications of DR[178]. DR can occur as non-proliferative DR (NPDR), which corresponds to the early state of the disease, or as more advanced form of the disease: proliferative DR (PDR). In NPDR the breakdown of the blood-retinal barrier and weakened retinal blood vessels lead to the formation of microaneurysms that can leak fluid into retina causing swelling of the macula. In PDR blood vessels can grow into the vitreous and on the surface of the retina[177,179]. Blocking the RAS cascade seems to reduce the incidence and progression of DR suggesting that RAS may be implicated in the pathogenesis of the disease[180-182]. However, more research is required to understand the complex interplay between RAS cascade and DR.
Systemic RAS regulates BP homeostasis, body fluid volume and electrolyte balance. An interesting new observation is intraocular, local RAS, especially existed in the eye structures which are involved in aqueous humor dynamics. Human and animal studies have both shown that antihypertensive drugs blocking RAS at any level can reduce IOP suggesting that these kind of compounds may be potential anti-glaucomatous drugs in the future. Furthermore, compounds elevating Ang(1-7) formation, activating Mas receptors and positively affecting ACE2 activity offer new intriguing opportunities for ocular pharmacology in the future. Although IOP represents the major risk factor in glaucoma, reduction of IOP does not always prevent the progression of disease like in low-tension glaucoma, indicating that factors other than elevated IOP are involved in glaucoma progression. Apoptosis of retinal ganglion cells may be the main possible unsolved reason. ACE inhibitors[183], ARBs[184] and Mas-receptor ligands[185] have showed some potential neuroprotective effects, which will stimulate research activity in the future.
The authors wish to thank the Päivikki and Sakari Sohlberg Foundation, the Eye Foundation, the Glaucoma Research Foundation Lux, the Competitive Research Funding of Tampere University Hospital (Grant 9S072) and the Foundation for Clinical Chemistry Research. Under preparation of this manuscript a review related to our topic was published Sharif NA. Novel Potential Treatment Modalities for Ocular Hypertension: Focus on Angiotensin and Bradykinin System Axes. J Ocul Pharmacol Ther 2015; 31(3): 131-145.
P- Reviewer: Chaudhry IA, Hong YJ S- Editor: Song XX L- Editor: A E- Editor: Jiao XK
| 1. | Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4855] [Cited by in F6Publishing: 4624] [Article Influence: 256.9] [Reference Citation Analysis (0)] |
| 2. | Giese MJ, Speth RC. The ocular renin-angiotensin system: a therapeutic target for the treatment of ocular disease. Pharmacol Ther. 2014;142:11-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Foureaux G, Nogueira JC, Nogueira BS, Fulgêncio GO, Menezes GB, Fernandes SO, Cardoso VN, Fernandes RS, Oliveira GP, Franca JR. Antiglaucomatous effects of the activation of intrinsic Angiotensin-converting enzyme 2. Invest Ophthalmol Vis Sci. 2013;54:4296-4306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Liu T, Xie L, Ye J, Liu Y, He X. Screening of candidate genes for primary open angle glaucoma. Mol Vis. 2012;18:2119-2126. [PubMed] [Cited in This Article: ] |
| 5. | Vaajanen A, Vapaatalo H. Local ocular renin-angiotensin system - a target for glaucoma therapy? Basic Clin Pharmacol Toxicol. 2011;109:217-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1247] [Cited by in F6Publishing: 1316] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 7. | Tuulonen A, Forsman E, Hagman J, Harju M, Kari O, Lumme P, Luodonpää M, Määttä M, Saarela V, Vaajanen A. 2014. Available from: http://www.kaypahoito.fi/web/kh/suositukset/suositus?id=hoi37030. [Cited in This Article: ] |
| 8. | Hirooka K, Baba T, Fujimura T, Shiraga F. Prevention of visual field defect progression with angiotensin-converting enzyme inhibitor in eyes with normal-tension glaucoma. Am J Ophthalmol. 2006;142:523-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Collaborative normal-tension glaucoma study group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:487-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1035] [Cited by in F6Publishing: 1058] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 10. | Collaborative normal-tension glaucoma study group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:498-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 846] [Cited by in F6Publishing: 899] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 11. | Kramkowski K, Mogielnicki A, Buczko W. The physiological significance of the alternative pathways of angiotensin II production. J Physiol Pharmacol. 2006;57:529-539. [PubMed] [Cited in This Article: ] |
| 12. | Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1160] [Cited by in F6Publishing: 1189] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 13. | Savaskan E, Löffler KU, Meier F, Müller-Spahn F, Flammer J, Meyer P. Immunohistochemical localization of angiotensin-converting enzyme, angiotensin II and AT1 receptor in human ocular tissues. Ophthalmic Res. 2004;36:312-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Danser AH, Derkx FH, Admiraal PJ, Deinum J, de Jong PT, Schalekamp MA. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci. 1994;35:1008-1018. [PubMed] [Cited in This Article: ] |
| 15. | Costagliola C, Di Benedetto R, De Caprio L, Verde R, Mastropasqua L. Effect of oral captopril (SQ 14225) on intraocular pressure in man. Eur J Ophthalmol. 1994;5:19-25. [PubMed] [Cited in This Article: ] |
| 16. | Costagliola C, Verolino M, De Rosa ML, Iaccarino G, Ciancaglini M, Mastropasqua L. Effect of oral losartan potassium administration on intraocular pressure in normotensive and glaucomatous human subjects. Exp Eye Res. 2000;71:167-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Shah GB, Sharma S, Mehta AA, Goyal RK. Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma. J Cardiovasc Pharmacol. 2000;36:169-175. [PubMed] [Cited in This Article: ] |
| 18. | Watkins RW, Baum T, Cedeno K, Smith EM, Yuen PH, Ahn HS, Barnett A. Topical ocular hypotensive effects of the novel angiotensin converting enzyme inhibitor SCH 33861 in conscious rabbits. J Ocul Pharmacol. 1987;3:295-307. [PubMed] [Cited in This Article: ] |
| 19. | Wang RF, Podos SM, Mittag TW, Yokoyoma T. Effect of CS-088, an angiotensin AT1 receptor antagonist, on intraocular pressure in glaucomatous monkey eyes. Exp Eye Res. 2005;80:629-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Inoue T, Yokoyoma T, Mori Y, Sasaki Y, Hosokawa T, Yanagisawa H, Koike H. The effect of topical CS-088, an angiotensin AT1 receptor antagonist, on intraocular pressure and aqueous humor dynamics in rabbits. Curr Eye Res. 2001;23:133-138. [PubMed] [Cited in This Article: ] |
| 21. | Giardina WJ, Kleinert HD, Ebert DM, Wismer CT, Chekal MA, Stein HH. Intraocular pressure lowering effects of the renin inhibitor ABBOTT-64662 diacetate in animals. J Ocul Pharmacol. 1990;6:75-83. [PubMed] [Cited in This Article: ] |
| 22. | Vaajanen A, Luhtala S, Oksala O, Vapaatalo H. Does the renin-angiotensin system also regulate intra-ocular pressure? Ann Med. 2008;40:418-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med. 2008;264:224-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 390] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 24. | Tigerstedt R, Bergman P. Niere und Kreislauf. Scand Arch Physiol. 1898;8:223-271. [DOI] [Cited in This Article: ] [Cited by in Crossref: 678] [Cited by in F6Publishing: 678] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 25. | Bader M, Ganten D. Update on tissue renin-angiotensin systems. J Mol Med (Berl). 2008;86:615-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Braun-Menendez E, Fasciolo JC, Leloir LF, Muñoz JM. The substance causing renal hypertension. J Physiol. 1940;98:283-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 27. | Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. J Exp Med. 1940;71:29-42. [PubMed] [Cited in This Article: ] |
| 28. | Paulis L, Unger T. Novel therapeutic targets for hypertension. Nat Rev Cardiol. 2010;7:431-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Ferrario CM. ACE2: more of Ang-(1-7) or less Ang II? Curr Opin Nephrol Hypertens. 2011;20:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Ferreira AJ, Murça TM, Fraga-Silva RA, Castro CH, Raizada MK, Santos RA. New cardiovascular and pulmonary therapeutic strategies based on the Angiotensin-converting enzyme 2/angiotensin-(1-7)/mas receptor axis. Int J Hypertens. 2012;2012:147825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Lazartigues E. A map and new directions for the (pro)renin receptor in the brain: focus on “A role of the (pro)renin receptor in neuronal cell differentiation”. Am J Physiol Regul Integr Comp Physiol. 2009;297:R248-R249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Santos RA, Ferreira AJ, Simões E Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol. 2008;93:519-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 33. | Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: more than regulation of blood pressure? Hypertension. 2007;50:596-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Santos RA, Ferreira AJ. Angiotensin-(1-7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2007;16:122-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Simões e Silva AC, Pinheiro SV, Pereira RM, Ferreira AJ, Santos RA. The therapeutic potential of Angiotensin-(1-7) as a novel Renin-Angiotensin System mediator. Mini Rev Med Chem. 2006;6:603-609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Guang C, Phillips RD, Jiang B, Milani F. Three key proteases--angiotensin-I-converting enzyme (ACE), ACE2 and renin--within and beyond the renin-angiotensin system. Arch Cardiovasc Dis. 2012;105:373-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Ribeiro-Oliveira A, Nogueira AI, Pereira RM, Boas WW, Dos Santos RA, Simões e Silva AC. The renin-angiotensin system and diabetes: an update. Vasc Health Risk Manag. 2008;4:787-803. [PubMed] [Cited in This Article: ] |
| 38. | Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689-1697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 543] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 39. | Hildebrand D, Merkel P, Eggers LF, Schlüter H. Proteolytic processing of angiotensin-I in human blood plasma. PLoS One. 2013;8:e64027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Masuyer G, Yates CJ, Sturrock ED, Acharya KR. Angiotensin-I converting enzyme (ACE): structure, biological roles, and molecular basis for chloride ion dependence. Biol Chem. 2014;395:1135-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Miyazaki M, Takai S. Tissue angiotensin II generating system by angiotensin-converting enzyme and chymase. J Pharmacol Sci. 2006;100:391-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12:170-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Becari C, Oliveira EB, Salgado MC. Alternative pathways for angiotensin II generation in the cardiovascular system. Braz J Med Biol Res. 2011;44:914-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Padia SH, Kemp BA, Howell NL, Siragy HM, Fournie-Zaluski MC, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type 1 receptor-blocked rats. Hypertension. 2007;49:625-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Albiston AL, Peck GR, Yeatman HR, Fernando R, Ye S, Chai SY. Therapeutic targeting of insulin-regulated aminopeptidase: heads and tails? Pharmacol Ther. 2007;116:417-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Chai SY, Fernando R, Peck G, Ye SY, Mendelsohn FA, Jenkins TA, Albiston AL. The angiotensin IV/AT4 receptor. Cell Mol Life Sci. 2004;61:2728-2737. [PubMed] [Cited in This Article: ] |
| 47. | Li XC, Campbell DJ, Ohishi M, Yuan S, Zhuo JL. AT1 receptor-activated signaling mediates angiotensin IV-induced renal cortical vasoconstriction in rats. Am J Physiol Renal Physiol. 2006;290:F1024-F1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1-E9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2045] [Cited by in F6Publishing: 2084] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 49. | Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 446] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 50. | Jackman HL, Massad MG, Sekosan M, Tan F, Brovkovych V, Marcic BM, Erdös EG. Angiotensin 1-9 and 1-7 release in human heart: role of cathepsin A. Hypertension. 2002;39:976-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Kokkonen JO, Saarinen J, Kovanen PT. Regulation of local angiotensin II formation in the human heart in the presence of interstitial fluid. Inhibition of chymase by protease inhibitors of interstitial fluid and of angiotensin-converting enzyme by Ang-(1-9) formed by heart carboxypeptidase A-like activity. Circulation. 1997;95:1455-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Garabelli PJ, Modrall JG, Penninger JM, Ferrario CM, Chappell MC. Distinct roles for angiotensin-converting enzyme 2 and carboxypeptidase A in the processing of angiotensins within the murine heart. Exp Physiol. 2008;93:613-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Mogielnicki A, Kramkowski K, Chabielska E, Buczko W. Angiotensin 1-9 influences hemodynamics and hemostatics parameters in rats. Pol J Pharmacol. 2003;55:503-504. [Cited in This Article: ] |
| 54. | Ocaranza MP, Michea L, Chiong M, Lagos CF, Lavandero S, Jalil JE. Recent insights and therapeutic perspectives of angiotensin-(1-9) in the cardiovascular system. Clin Sci (Lond). 2014;127:549-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | McKinney CA, Fattah C, Loughrey CM, Milligan G, Nicklin SA. Angiotensin-(1-7) and angiotensin-(1-9): function in cardiac and vascular remodelling. Clin Sci (Lond). 2014;126:815-827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 56. | Varagic J, Trask AJ, Jessup JA, Chappell MC, Ferrario CM. New angiotensins. J Mol Med (Berl). 2008;86:663-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216:R1-R17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 360] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 58. | Dilauro M, Burns KD. Angiotensin-(1-7) and its effects in the kidney. ScientificWorldJournal. 2009;9:522-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 59. | Ferrario CM, Martell N, Yunis C, Flack JM, Chappell MC, Brosnihan KB, Dean RH, Fernandez A, Novikov SV, Pinillas C. Characterization of angiotensin-(1-7) in the urine of normal and essential hypertensive subjects. Am J Hypertens. 1998;11:137-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. J Hypertens. 1996;14:799-805. [PubMed] [Cited in This Article: ] |
| 61. | Simões e Silva AC, Diniz JS, Pereira RM, Pinheiro SV, Santos RA. Circulating renin Angiotensin system in childhood chronic renal failure: marked increase of Angiotensin-(1-7) in end-stage renal disease. Pediatr Res. 2006;60:734-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Simões e Silva AC. Pathophysiology of arterial hypertension: Insights from pediatric studies. Curr Pediatr Rev. 2006;2:209-223. [Cited in This Article: ] |
| 63. | Simões E Silva AC, Diniz JS, Regueira Filho A, Santos RA. The renin angiotensin system in childhood hypertension: selective increase of angiotensin-(1-7) in essential hypertension. J Pediatr. 2004;145:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 65. | Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J, Cooper ME. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 2003;41:392-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 269] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 66. | Ferrão FM, Lara LS, Lowe J. Renin-angiotensin system in the kidney: What is new? World J Nephrol. 2014;3:64-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 52] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Persson PB. Renin: origin, secretion and synthesis. J Physiol. 2003;552:667-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238-33243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1545] [Cited by in F6Publishing: 1545] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 69. | Parkin ET, Turner AJ, Hooper NM. Secretase-mediated cell surface shedding of the angiotensin-converting enzyme. Protein Pept Lett. 2004;11:423-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Hooper NM, Turner AJ. An ACE structure. Nat Struct Biol. 2003;10:155-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Balyasnikova IV, Karran EH, Albrecht RF, Danilov SM. Epitope-specific antibody-induced cleavage of angiotensin-converting enzyme from the cell surface. Biochem J. 2002;362:585-595. [PubMed] [Cited in This Article: ] |
| 72. | Lew RA. The zinc metallopeptidase family: new faces, new functions. Protein Pept Lett. 2004;11:407-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 73. | Riordan JF. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003;4:225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 74. | Guy JL, Lambert DW, Warner FJ, Hooper NM, Turner AJ. Membrane-associated zinc peptidase families: comparing ACE and ACE2. Biochim Biophys Acta. 2005;1751:2-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838-14843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1073] [Cited by in F6Publishing: 1062] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 76. | Ferreira AJ, Santos RA, Bradford CN, Mecca AP, Sumners C, Katovich MJ, Raizada MK. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 77. | Campbell DJ. Circulating and tissue angiotensin systems. J Clin Invest. 1987;79:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 424] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 78. | Balcells E, Meng QC, Johnson WH, Oparil S, Dell’Italia LJ. Angiotensin II formation from ACE and chymase in human and animal hearts: methods and species considerations. Am J Physiol. 1997;273:H1769-H1774. [PubMed] [Cited in This Article: ] |
| 79. | Takai S, Sakaguchi M, Jin D, Yamada M, Kirimura K, Miyazaki M. Different angiotensin II-forming pathways in human and rat vascular tissues. Clin Chim Acta. 2001;305:191-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Resende MM, Mill JG. Alternate angiotensin II-forming pathways and their importance in physiological or physiopathological conditions. Arq Bras Cardiol. 2002;78:425-438. [PubMed] [Cited in This Article: ] |
| 81. | Boucher R, Demassieux S, Garcia R, Genest J. Tonin, angiotensin II system. A review. Circ Res. 1977;41:26-29. [PubMed] [Cited in This Article: ] |
| 82. | Tonnesen MG, Klempner MS, Austen KF, Wintroub BU. Identification of a human neutrophil angiotension II-generating protease as cathepsin G. J Clin Invest. 1982;69:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Maruta H, Arakawa K. Confirmation of direct angiotensin formation by kallikrein. Biochem J. 1983;213:193-200. [PubMed] [Cited in This Article: ] |
| 84. | Arakawa K. Serine protease angiotensin II systems. J Hypertens Suppl. 1996;14:S3-S7. [PubMed] [Cited in This Article: ] |
| 85. | Urata H, Healy B, Stewart RW, Bumpus FM, Husain A. Angiotensin II-forming pathways in normal and failing human hearts. Circ Res. 1990;66:883-890. [PubMed] [Cited in This Article: ] |
| 86. | Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348-22357. [PubMed] [Cited in This Article: ] |
| 87. | Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 432] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 88. | Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11:165-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 89. | de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415-472. [PubMed] [Cited in This Article: ] |
| 90. | Young D, Waitches G, Birchmeier C, Fasano O, Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986;45:711-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 348] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 91. | Young D, O’Neill K, Jessell T, Wigler M. Characterization of the rat mas oncogene and its high-level expression in the hippocampus and cerebral cortex of rat brain. Proc Natl Acad Sci USA. 1988;85:5339-5342. [PubMed] [Cited in This Article: ] |
| 92. | Kitaoka T, Sharif M, Hanley MR, Hjelmeland LM. Expression of the MAS proto-oncogene in the retinal pigment epithelium of the rhesus macaque. Curr Eye Res. 1994;13:345-351. [PubMed] [Cited in This Article: ] |
| 93. | Bader M. ACE2, angiotensin-(1-7), and Mas: the other side of the coin. Pflugers Arch. 2013;465:79-85. [PubMed] [Cited in This Article: ] |
| 94. | Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX, Greenberg BH. Angiotensin-(1-7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol. 2005;289:H2356-H2363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 95. | Vaajanen A, Kalesnykas G, Vapaatalo H, Uusitalo H. The expression of Mas-receptor of the renin-angiotensin system in the human eye. Graefes Arch Clin Exp Ophthalmol. 2015;253:1053-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 96. | Ganten D, Marquez-Julio A, Granger P, Hayduk K, Karsunky KP, Boucher R, Genest J. Renin in dog brain. Am J Physiol. 1971;221:1733-1737. [PubMed] [Cited in This Article: ] |
| 97. | Holappa M, Valjakka J, Vaajanen A. Angiotensin(1-7) and ACE2, “The Hot Spots” of Renin-Angiotensin System, Detected in the Human Aqueous Humor. Open Ophthalmol J. 2015;9:28-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 98. | White AJ, Cheruvu SC, Sarris M, Liyanage SS, Lumbers E, Chui J, Wakefield D, McCluskey PJ. Expression of classical components of the renin-angiotensin system in the human eye. J Renin Angiotensin Aldosterone Syst. 2015;16:59-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Danser AH, van den Dorpel MA, Deinum J, Derkx FH, Franken AA, Peperkamp E, de Jong PT, Schalekamp MA. Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. J Clin Endocrinol Metab. 1989;68:160-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 200] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 100. | Chowdhury UR, Madden BJ, Charlesworth MC, Fautsch MP. Proteome analysis of human aqueous humor. Invest Ophthalmol Vis Sci. 2010;51:4921-4931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 101. | Vita JB, Anderson JA, Hulem CD, Leopold IH. Angiotensin-converting enzyme activity in ocular fluids. Invest Ophthalmol Vis Sci. 1981;20:255-257. [PubMed] [Cited in This Article: ] |
| 102. | Sharma OP, Vita JB. Determination of angiotensin-converting enzyme activity in tears. A noninvasive test for evaluation of ocular sarcoidosis. Arch Ophthalmol. 1983;101:559-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 103. | Immonen I, Friberg K, Sorsila R, Fyhrquist F. Concentration of angiotensin-converting enzyme in tears of patients with sarcoidosis. Acta Ophthalmol (Copenh). 1987;65:27-29. [PubMed] [Cited in This Article: ] |
| 104. | Weinreb RN, Polansky JR, Kramer SG, Baxter JD. Acute effects of dexamethasone on intraocular pressure in glaucoma. Invest Ophthalmol Vis Sci. 1985;26:170-175. [PubMed] [Cited in This Article: ] |
| 105. | Aydin E, Demir HD, Sahin S. Plasma and aqueous humor angiotensin-converting enzyme levels in patients with diabetic retinopathy. Curr Eye Res. 2010;35:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Ferrari-Dileo G, Ryan JW, Rockwood EJ, Davis EB, Anderson DR. Angiotensin-converting enzyme in bovine, feline, and human ocular tissues. Invest Ophthalmol Vis Sci. 1988;29:876-881. [PubMed] [Cited in This Article: ] |
| 107. | Osusky R, Nussberger J, Amstutz C, Flammer J, Brunner HR. Individual measurements of angiotensin II concentrations in aqueous humor of the eye. Eur J Ophthalmol. 1994;4:228-233. [PubMed] [Cited in This Article: ] |
| 108. | Senanayake Pd, Drazba J, Shadrach K, Milsted A, Rungger-Brandle E, Nishiyama K, Miura S, Karnik S, Sears JE, Hollyfield JG. Angiotensin II and its receptor subtypes in the human retina. Invest Ophthalmol Vis Sci. 2007;48:3301-3311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 109. | Sramek SJ, Wallow IH, Day RP, Ehrlich EN. Ocular renin-angiotensin: immunohistochemical evidence for the presence of prorenin in eye tissue. Invest Ophthalmol Vis Sci. 1988;29:1749-1752. [PubMed] [Cited in This Article: ] |
| 110. | Wallow IH, Sramek SJ, Bindley CD, Darjatmoko SR, Gange SJ. Ocular renin angiotensin: EM immunocytochemical localization of prorenin. Curr Eye Res. 1993;12:945-950. [PubMed] [Cited in This Article: ] |
| 111. | Berka JL, Stubbs AJ, Wang DZ, DiNicolantonio R, Alcorn D, Campbell DJ, Skinner SL. Renin-containing Müller cells of the retina display endocrine features. Invest Ophthalmol Vis Sci. 1995;36:1450-1458. [PubMed] [Cited in This Article: ] |
| 112. | Sramek SJ, Wallow IH, Tewksbury DA, Brandt CR, Poulsen GL. An ocular renin-angiotensin system. Immunohistochemistry of angiotensinogen. Invest Ophthalmol Vis Sci. 1992;33:1627-1632. [PubMed] [Cited in This Article: ] |
| 113. | Igić R, Kojović V. Angiotensin I converting enzyme (kininase II) in ocular tissues. Exp Eye Res. 1980;30:299-303. [PubMed] [Cited in This Article: ] |
| 114. | Nakanishi T, Koyama R, Ikeda T, Shimizu A. Catalogue of soluble proteins in the human vitreous humor: comparison between diabetic retinopathy and macular hole. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;776:89-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Ishizaki E, Takai S, Ueki M, Maeno T, Maruichi M, Sugiyama T, Oku H, Ikeda T, Miyazaki M. Correlation between angiotensin-converting enzyme, vascular endothelial growth factor, and matrix metalloproteinase-9 in the vitreous of eyes with diabetic retinopathy. Am J Ophthalmol. 2006;141:129-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Lin C, Stone RA, Wax MB. Angiotensin binding sites in rabbit anterior uvea and human ciliary epithelial cells. Invest Ophthalmol Vis Sci. 1990;31:147-152. [PubMed] [Cited in This Article: ] |
| 117. | Lograno MD, Reibaldi A. Receptor-responses in fresh human ciliary muscle. Br J Pharmacol. 1986;87:379-385. [PubMed] [Cited in This Article: ] |
| 118. | Cullinane AB, Leung PS, Ortego J, Coca-Prados M, Harvey BJ. Renin-angiotensin system expression and secretory function in cultured human ciliary body non-pigmented epithelium. Br J Ophthalmol. 2002;86:676-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 119. | Schelling P, Ganten U, Sponer G, Unger T, Ganten D. Components of the renin-angiotensin system in the cerebrospinal fluid of rats and dogs with special consideration of the origin and the fate of angiotensin II. Neuroendocrinology. 1980;31:297-308. [PubMed] [Cited in This Article: ] |
| 120. | Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23:279-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 230] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 121. | Ramirez M, Davidson EA, Luttenauer L, Elena PP, Cumin F, Mathis GA, De Gasparo M. The renin-angiotensin system in the rabbit eye. J Ocul Pharmacol Ther. 1996;12:299-312. [PubMed] [Cited in This Article: ] |
| 122. | Geng L, Persson K, Nilsson SF. Angiotensin converting anzyme (ACE) activity in porcine ocular tissue: effects of diet and ACE inhibitors. J Ocul Pharmacol Ther. 2003;19:589-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 123. | Janssen SF, Gorgels TG, van der Spek PJ, Jansonius NM, Bergen AA. In silico analysis of the molecular machinery underlying aqueous humor production: potential implications for glaucoma. J Clin Bioinforma. 2013;3:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Civan MM, Macknight AD. The ins and outs of aqueous humour secretion. Exp Eye Res. 2004;78:625-631. [PubMed] [Cited in This Article: ] |
| 125. | Brubaker RF. The flow of aqueous humor in the human eye. Trans Am Ophthalmol Soc. 1982;80:391-474. [PubMed] [Cited in This Article: ] |
| 126. | Mark HH. Aqueous humor dynamics in historical perspective. Surv Ophthalmol. 2010;55:89-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Brubaker RF, Nagataki S, Townsend DJ, Burns RR, Higgins RG, Wentworth W. The effect of age on aqueous humor formation in man. Ophthalmology. 1981;88:283-288. [PubMed] [Cited in This Article: ] |
| 128. | Buys ES, Potter LR, Pasquale LR, Ksander BR. Regulation of intraocular pressure by soluble and membrane guanylate cyclases and their role in glaucoma. Front Mol Neurosci. 2014;7:38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 129. | Hou Y, Delamere NA. Influence of ANG II on cytoplasmic sodium in cultured rabbit nonpigmented ciliary epithelium. Am J Physiol Cell Physiol. 2002;283:C552-C559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 130. | Caprioli J. The ciliary epithelia and aqueous humor. Adler’s Physiology of the eye. 9th ed. St Louis: Mosby-Year book Inc 1992; 228-247. [Cited in This Article: ] |
| 131. | Millar JC, True Gablet B, Kaufman PL. Aqueous humor dynamics. In: Eds Tasman W, Jaeger EA. Duane’s Ophthalmology, CD-ROM edition. Lippincott Williams and Wilkins 2006; . [Cited in This Article: ] |
| 132. | To CH, Kong CW, Chan CY, Shahidullah M, Do CW. The mechanism of aqueous humour formation. Clin Exp Optom. 2002;85:335-349. [PubMed] [Cited in This Article: ] |
| 133. | Freddo TF. The Glenn A. Fry Award Lecture 1992: aqueous humor proteins: a key for unlocking glaucoma? Optom Vis Sci. 1993;70:263-270. [PubMed] [Cited in This Article: ] |
| 134. | McLaren JW, Ziai N, Brubaker RF. A simple three-compartment model of anterior segment kinetics. Exp Eye Res. 1993;56:355-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 135. | Barsotti MF, Bartels SP, Freddo TF, Kamm RD. The source of protein in the aqueous humor of the normal monkey eye. Invest Ophthalmol Vis Sci. 1992;33:581-595. [PubMed] [Cited in This Article: ] |
| 136. | Freddo TF, Bartels SP, Barsotti MF, Kamm RD. The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci. 1990;31:125-137. [PubMed] [Cited in This Article: ] |
| 137. | Fitt AD, Gonzalez G. Fluid mechanics of the human eye: aqueous humour flow in the anterior chamber. Bull Math Biol. 2006;68:53-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 138. | Murray DL, Bartels SP. The relationship between aqueous humor flow and anterior chamber protein concentration in rabbits. Invest Ophthalmol Vis Sci. 1993;34:370-376. [PubMed] [Cited in This Article: ] |
| 139. | Lütjen-Drecoll E, Gabelt BT, Tian B, Kaufman PL. Outflow of aqueous humor. J Glaucoma. 2001;10:S42-S44. [PubMed] [Cited in This Article: ] |
| 140. | Tan JC, Peters DM, Kaufman PL. Recent developments in understanding the pathophysiology of elevated intraocular pressure. Curr Opin Ophthalmol. 2006;17:168-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 319] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 141. | Fautsch MP, Johnson DH. Aqueous humor outflow: what do we know? Where will it lead us? Invest Ophthalmol Vis Sci. 2006;47:4181-4187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 142. | Yücel YH, Johnston MG, Ly T, Patel M, Drake B, Gümüş E, Fraenkl SA, Moore S, Tobbia D, Armstrong D. Identification of lymphatics in the ciliary body of the human eye: a novel “uveolymphatic” outflow pathway. Exp Eye Res. 2009;89:810-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 143. | Weinreb RN. Uveoscleral outflow: the other outflow pathway. J Glaucoma. 2000;9:343-345. [PubMed] [Cited in This Article: ] |
| 144. | Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov. 2012;11:541-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 145. | Ferreira AJ, Bader M, Santos RA. Therapeutic targeting of the angiotensin-converting enzyme 2/Angiotensin-(1-7)/Mas cascade in the renin-angiotensin system: a patent review. Expert Opin Ther Pat. 2012;22:567-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 146. | Luo BP, Brown GC. Update on the ocular manifestations of systemic arterial hypertension. Curr Opin Ophthalmol. 2004;15:203-210. [PubMed] [Cited in This Article: ] |
| 147. | Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107:1287-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 457] [Cited by in F6Publishing: 460] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 148. | Reitsamer HA, Kiel JW. Relationship between ciliary blood flow and aqueous production in rabbits. Invest Ophthalmol Vis Sci. 2003;44:3967-3971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Lotti VJ, Pawlowski N. Prostaglandins mediate the ocular hypotensive action of the angiotensin converting enzyme inhibitor MK-422 (enalaprilat) in African green monkeys. J Ocul Pharmacol. 1990;6:1-7. [PubMed] [Cited in This Article: ] |
| 150. | Nilsson SF, Samuelsson M, Bill A, Stjernschantz J. Increased uveoscleral outflow as a possible mechanism of ocular hypotension caused by prostaglandin F2 alpha-1-isopropylester in the cynomolgus monkey. Exp Eye Res. 1989;48:707-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 188] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 151. | Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002;47 Suppl 1:S53-S64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 152. | Momose N, Fukuo K, Morimoto S, Ogihara T. Captopril inhibits endothelin-1 secretion from endothelial cells through bradykinin. Hypertension. 1993;21:921-924. [PubMed] [Cited in This Article: ] |
| 153. | Haefliger IO, Flammer J, Lüscher TF. Nitric oxide and endothelin-1 are important regulators of human ophthalmic artery. Invest Ophthalmol Vis Sci. 1992;33:2340-2343. [PubMed] [Cited in This Article: ] |
| 154. | Yao K, Tschudi M, Flammer J, Lüscher TF. Endothelium-dependent regulation of vascular tone of the porcine ophthalmic artery. Invest Ophthalmol Vis Sci. 1991;32:1791-1798. [PubMed] [Cited in This Article: ] |
| 155. | Capponi AM, Lew PD, Jornot L, Vallotton MB. Correlation between cytosolic free Ca2+ and aldosterone production in bovine adrenal glomerulosa cells. Evidence for a difference in the mode of action of angiotensin II and potassium. J Biol Chem. 1984;259:8863-8869. [PubMed] [Cited in This Article: ] |
| 156. | Langman MJ, Lancashire RJ, Cheng KK, Stewart PM. Systemic hypertension and glaucoma: mechanisms in common and co-occurrence. Br J Ophthalmol. 2005;89:960-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 157. | Grunwald JE, Riva CE, Stone RA, Keates EU, Petrig BL. Retinal autoregulation in open-angle glaucoma. Ophthalmology. 1984;91:1690-1694. [PubMed] [Cited in This Article: ] |
| 158. | Piltz-seymour JR, Grunwald JE, Hariprasad SM, Dupont J. Optic nerve blood flow is diminished in eyes of primary open-angle glaucoma suspects. Am J Ophthalmol. 2001;132:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 159. | Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117:603-624. [PubMed] [Cited in This Article: ] |
| 160. | Vaajanen A, Vapaatalo H, Kautiainen H, Oksala O. Angiotensin (1-7) reduces intraocular pressure in the normotensive rabbit eye. Invest Ophthalmol Vis Sci. 2008;49:2557-2562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 161. | Macri FJ. The action of angiotensin on intraocular pressure. Arch Ophthalmol. 1965;73:528-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 162. | Fletcher EL, Phipps JA, Ward MM, Vessey KA, Wilkinson-Berka JL. The renin-angiotensin system in retinal health and disease: Its influence on neurons, glia and the vasculature. Prog Retin Eye Res. 2010;29:284-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 163. | Marin Garcia PJ, Marin-Castaño ME. Angiotensin II-related hypertension and eye diseases. World J Cardiol. 2014;6:968-984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 164. | Hikichi T, Mori F, Takamiya A, Sasaki M, Horikawa Y, Takeda M, Yoshida A. Inhibitory effect of losartan on laser-induced choroidal neovascularization in rats. Am J Ophthalmol. 2001;132:587-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 165. | Nagai N, Oike Y, Izumi-Nagai K, Urano T, Kubota Y, Noda K, Ozawa Y, Inoue M, Tsubota K, Suda T. Angiotensin II type 1 receptor-mediated inflammation is required for choroidal neovascularization. Arterioscler Thromb Vasc Biol. 2006;26:2252-2259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 166. | Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, Tsubota K, Itoh H, Oike Y, Ishida S. (Pro)renin receptor promotes choroidal neovascularization by activating its signal transduction and tissue renin-angiotensin system. Am J Pathol. 2008;173:1911-1918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 167. | Cryotherapy for Retinopathy of Prematurity Group. Multicenter Trial of Cryotherapy for Retinopathy of Prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001;119:1110-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 219] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 168. | Lutty GA, Chan-Ling T, Phelps DL, Adamis AP, Berns KI, Chan CK, Cole CH, D’Amore PA, Das A, Deng WT. Proceedings of the Third International Symposium on Retinopathy of Prematurity: an update on ROP from the lab to the nursery (November 2003, Anaheim, California). Mol Vis. 2006;12:532-580. [PubMed] [Cited in This Article: ] |
| 169. | Yokota H, Nagaoka T, Mori F, Hikichi T, Hosokawa H, Tanaka H, Ishida Y, Suzuki F, Yoshida A. Prorenin levels in retinopathy of prematurity. Am J Ophthalmol. 2007;143:531-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 170. | Moravski CJ, Kelly DJ, Cooper ME, Gilbert RE, Bertram JF, Shahinfar S, Skinner SL, Wilkinson-Berka JL. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000;36:1099-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 149] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 171. | Sarlos S, Rizkalla B, Moravski CJ, Cao Z, Cooper ME, Wilkinson-Berka JL. Retinal angiogenesis is mediated by an interaction between the angiotensin type 2 receptor, VEGF, and angiopoietin. Am J Pathol. 2003;163:879-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 172. | Downie LE, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. AT1 receptor inhibition prevents astrocyte degeneration and restores vascular growth in oxygen-induced retinopathy. Glia. 2008;56:1076-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 173. | Lonchampt M, Pennel L, Duhault J. Hyperoxia/normoxia-driven retinal angiogenesis in mice: a role for angiotensin II. Invest Ophthalmol Vis Sci. 2001;42:429-432. [PubMed] [Cited in This Article: ] |
| 174. | Tadesse M, Yan Y, Yossuck P, Higgins RD. Captopril improves retinal neovascularization via endothelin-1. Invest Ophthalmol Vis Sci. 2001;42:1867-1872. [PubMed] [Cited in This Article: ] |
| 175. | Otani A, Takagi H, Suzuma K, Honda Y. Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res. 1998;82:619-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 176. | Otani A, Takagi H, Oh H, Suzuma K, Matsumura M, Ikeda E, Honda Y. Angiotensin II-stimulated vascular endothelial growth factor expression in bovine retinal pericytes. Invest Ophthalmol Vis Sci. 2000;41:1192-1199. [PubMed] [Cited in This Article: ] |
| 177. | Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 754] [Cited by in F6Publishing: 741] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 178. | Ciulla TA, Harris A, Kagemann L, Danis RP, Pratt LM, Chung HS, Weinberger D, Garzozi HJ. Choroidal perfusion perturbations in non-neovascular age related macular degeneration. Br J Ophthalmol. 2002;86:209-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 179. | Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677-1682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1980] [Cited by in F6Publishing: 2033] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 180. | Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338:645-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 782] [Cited by in F6Publishing: 694] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 181. | Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3760] [Cited by in F6Publishing: 3464] [Article Influence: 111.7] [Reference Citation Analysis (1)] |
| 182. | Jonas JB, Hayreh SS, Martus P. Influence of arterial hypertension and diet-induced atherosclerosis on macular drusen. Graefes Arch Clin Exp Ophthalmol. 2003;241:125-134. [PubMed] [Cited in This Article: ] |
| 183. | Hirooka K, Shiraga F. Potential role for angiotensin-converting enzyme inhibitors in the treatment of glaucoma. Clin Ophthalmol. 2007;1:217-223. [PubMed] [Cited in This Article: ] |
| 184. | White AJ, Heller JP, Leung J, Tassoni A, Martin KR. Retinal ganglion cell neuroprotection by an angiotensin II blocker in an ex vivo retinal explant model. J Renin Angiotensin Aldosterone Syst. 2015;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 185. | Vaajanen A, Puranen J, Kalesnykas G, Vapaatalo H, Uusitalo H. Neuroprotective effects of mas-receptor ligands in an experimental rat glaucoma. J Pharm Pharmacol. 2014;2:114-122. [Cited in This Article: ] |