Published online Nov 12, 2014. doi: 10.5318/wjo.v4.i4.113
Revised: July 5, 2014
Accepted: September 4, 2014
Published online: November 12, 2014
Processing time: 169 Days and 10.9 Hours
Central serous chorioretinopathy (CSCR) is considered a benign, self-limiting disease. However, as many as third of the patients have recurrent episodes or chronic disease that may cause significant functional impairment. New diagnostic tools and new treatment modalities are emerging in order to improve the functional outcomes of these patients. Spectral domain optical coherence tomography (SD-OCT) has the ability to image individual layers of the retina and choroid. SD-OCT images in CSCR patients have demonstrated increased subfoveal thickness measurements, high reflective deposits in areas of subretinal precipitates and changes in the Retinal pigment epithelium layers of the asymptomatic eyes of patients with supposedly unilateral CSCR. A positive correlation was found between the level of distribution to the layer of inner segment/outer segment junction of the photoreceptors and the visual impairment. Fundus autoflouresence images show a wide variety during different stages of the disease in CSCR patients. Minimal abnormalities during the early stages are followed by hyperautofluoresence in the detached area in later stages, often in a manner of inferior gravitation and at the borders of the detachments. The chronic phase is characterized by varying degrees of atrophy and areas of decreased autofluorescence surrounding areas of chronic leaks. These changes help differentiate an active disease from an inactive state. Multifocal electroretinography (mfERG) has the ability to demonstrate a persistent depression despite the resolution of subretinal detachments. It is therefore being investigated as a follow up tool for patients with chronic CSCR. An excellent correlation was found between changes in mfERG and visual function. Macular microperimetry, measuring retinal sensitivity within the central visual field, is intended to compensate for the underestimation of visual impairment in patients with macular diseases. Reduced retinal sensitivity was found in areas of previous subretinal fluids in CSCR patients. The device can also serve as a follow up tool in these patients. Regarding treatment in CSCR patients, focal argon laser photocoagulation treatment may be applied to small extrafoveal leaks. However, the main purpose of this treatment is to shorten disease duration, with no advantage over observation regarding final visual outcome, rate of progression to chronic CSCR or number of recurrences. Photodynamic therapy (PDT) with verteporfin has been shown to completely resolve serous detachment in 60%-80% of patients and to have a partial affect in the remaining patients. Reduced-fluence treatment is replacing full-fluence therapy in order to minimize side effects with no accompanying reduced effectiveness. Visual acuity is also improved following reduced-fluence PDT compared to placebo. It has also been found that patients with intense hyperfluorescence are more likely to show resolution of accumulating fluid compared to patients with mild or no leakage observed on indocyanine-green angiography prior to treatment. Regarding newer treatment modalities, intravitreal injections of anti-vascular endothelial growth factor agents have a limited effect in patients with CSCR. Recent reports have not demonstrated an advantage for this treatment in regards to anatomic and functional outcome. Micropulse diode laser was not proven to be safer or more effective than argon laser or PDT. Corticosteroid antagonists, not tested in controlled trials, may have a beneficial effect in patients with CSCR. Aspirin may also play a role in treating these patients, with rapid recovery of visual acuity and reduced number of recurrences observed. In conclusion, imaging is evolving rapidly while the clinical implications of these new imaging modalities are less clear. Large randomized trials investigating different treatment modalities are still lacking.
Core tip: (1) New diagnostic tools and therapies may improve the prognosis of patients with chronic or recurrent central serous chorioretinopathy; (2) Changes in fundus autoflouresence images help differentiate an active disease from an inactive state; (3) Multifocal electroretinography and macular microperimetry may serve as follow up tools due to their ability to measure macular visual function; (4) Focal argon laser photocoagulation shortens disease duration but does not affect final prognosis; (5) Reduced-fluence photodynamic therapy improves visual acuity and resolves serous detachments; and (6) The role of anti-vascular endothelial growth factor agents, micropulse diode laser, corticosteroid antagonists, aspirin, anti-viral or Helicobacter pylori treatment is still being investigated.
- Citation: Schaap-Fogler M, Ehrlich R. What is new in central serous chorioretinopathy? World J Ophthalmol 2014; 4(4): 113-123
- URL: https://www.wjgnet.com/2218-6239/full/v4/i4/113.htm
- DOI: https://dx.doi.org/10.5318/wjo.v4.i4.113
Central serous chorioretinopathy (CSCR) was first described by Albrecht von Graefe in 1866 as a relapsing central luetic retinitis[1]. The various terms later used to describe the disease, including the current acceptable term CSCR first used by Gass[2] in 1967, have omitted the relapsing characteristic from the term. However, relapsing serous detachments of the neurosensory retina are known to occur in as many as third of the patients[3]. Moreover, the recurrent and chronic nature of CSCR may result in severe and irreversible visual loss in these patients[4]. Therefore, in order to improve patients outcome, there is an ongoing search for new diagnostic tools, shedding more light on disease pathophysiology, and for new treatments and different treatment protocols.
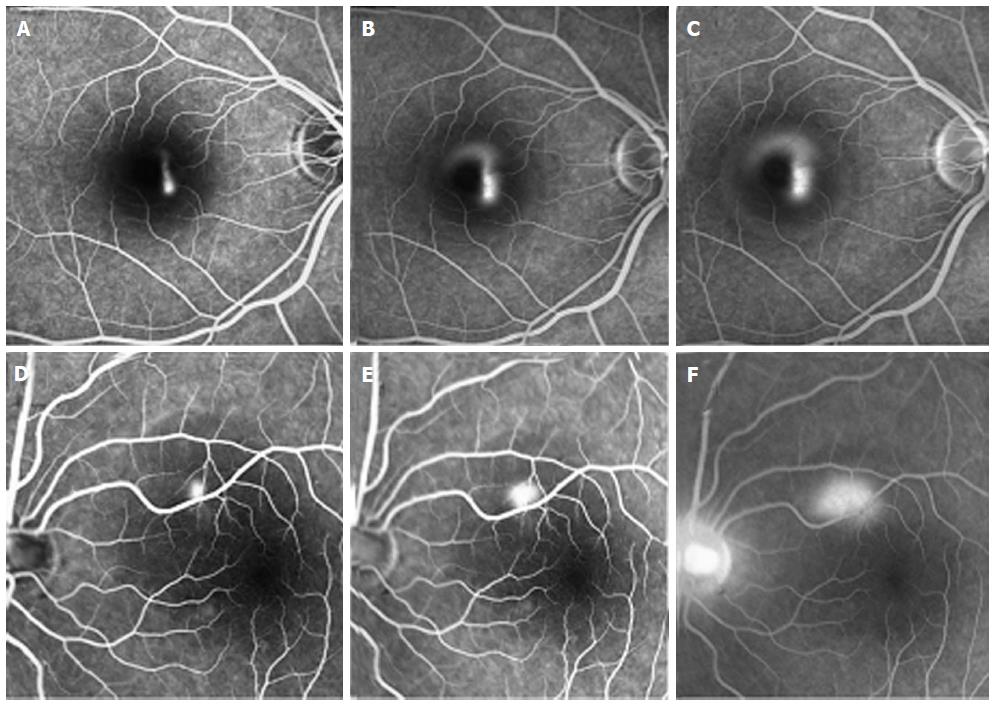
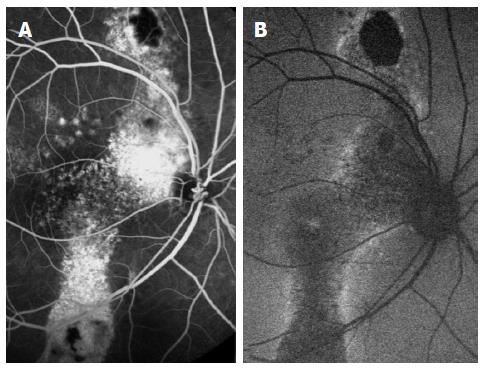
The typical presentation of CSCR is serous detachment of the neurosensory retina, but the source of the accumulating subretinal fluid is still not completely understood[2-13]. Retinal pigment epithelium (RPE) dysfunction has been hypothesized as the primary pathologic mechanism in CSCR, in part due to images obtained using fluorescein angiography (FA)[4-6]. These images show characteristic single or multiple leaks from the RPE, implicating the RPE as a major factor in the pathophysiology, as can be seen in Figure 1. However, different investigative tools led investigators to challenge this hypothesis. According to some reports, the choroid seems to be primarily affected, with the retinal changes seen with FA representing a later stage in the mechanism of the disease progression[7].
In order to further understand the role of the choroid in the disease, indocyanine-green angiography (ICGA) images were investigated. The congestion and dilatation of choroidal capillaries and veins, the choroidal staining and the leakage into the interstitial space all prove the choroid plays a major role in the accumulation of fluid in CSCR[12,13]. These changes in ICGA images are demonstrated in Figure 2. However, ICGA was found to have some limitations as a tool for diagnosis and follow up of patients. Previous studies on cross-sectional optical coherence tomography (OCT) images of eyes with chronic CSCR reported increased choroidal thickness observed, with no corresponding hyperflouresence observed on ICGA[14]. Moreover, ICGA gives a 2-dimensional scans, which means that all choroidal layers overlap in the angiogram.
The introduction of spectral domain optical coherence tomography (SD-OCT) as a more accurate imaging tool, with its ability to characterize individual layers of the retina and choroid and its noninvasive characteristic, has led to important observations regarding CSCR. The subfoveal thickness was found to be increased by 50%-80% in CSCR patients compared to normal eyes in different reports, when measured by enhanced depth imaging OCT[14-17].
Another measurement that can be performed with SD-OCT is the thickness of the outer nuclear layer (ONL). In one study, ONL thickness was found to be correlated with visual acuity in patients with resolving CSCR[18]. In that study, the mean ONL thickness measured in patients with resolved CSC was 74.6 μm in patients with visual acuity worse than 20/20 compared to 103 μm in patients with visual acuity of 20/20 or better[18]. That same group of researchers also showed elongation of photoreceptors outer segments and decreased thickness of the outer nuclear layer in CSCR, as a possible sign for photoreceptors apoptosis[19].
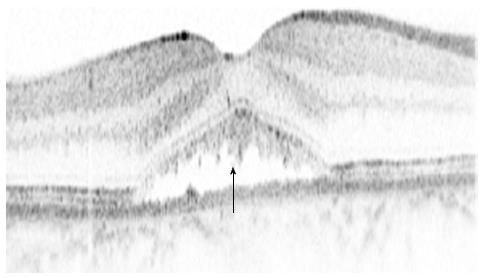
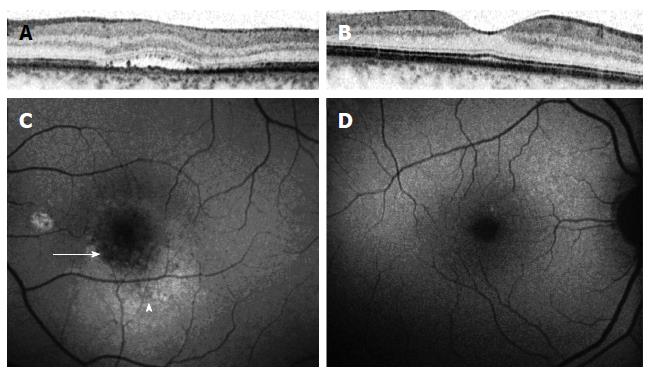
SD-OCT has also shed some light on the multiple, dot-like, yellow precipitates and subretinal yellow material within the area of a serous retinal detachment in patients with CSCR. These deposits correlate with high reflective deposits on SD-OCT[20,21] (Figure 3). Different hypothesis regarding these substances has been proposed, including the accumulation of shed photoreceptor outer segments, fibrin or lipids, or macrophages clearing the subretinal space. However, the exact nature of these deposits and their origin is yet to be determined[21].
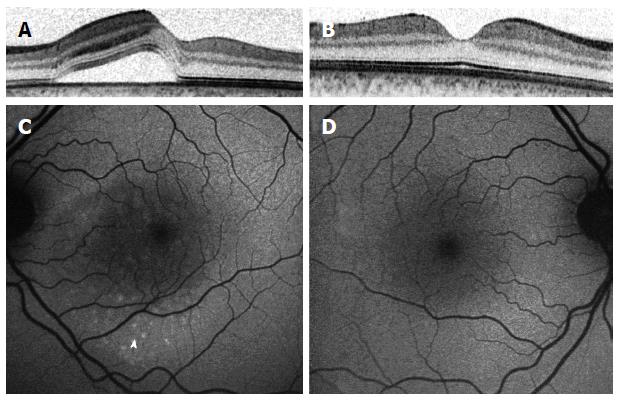
The bilateral nature of CSCR was also demonstrated by SD-OCT, even in eyes with supposedly unilateral disease[22]. Changes in the RPE cells layer has been previously shown in patients with CSCR, specifically around areas of a demonstrated leakage on FA[23]. This study investigated these RPE changes in the asymptomatic eyes of patients with CSCR in the other eye, using 3 dimensional single-layer RPE analyses. Presence of RPE bumps was observed in 94% of eyes and pigment epithelium detachment (PED) in 11.8% of eyes, compared to 8% of eyes with RPE bumps and no PED observed in normal control eyes[23].
Special attention has been addressed to the layer of inner segment/outer segment (IS/OS) junction in different retinal disorders. The level of disruption to this layer in different retinal disorders has an excellent correlation with visual acuity[24-27]. That correlation is also maintained in patients with CSCR[18,28-30]. The length of IS/OS disruption, loss of foveal IS/OS and the level of integrity of the external limiting membrane layer were also found to be significantly correlated with visual acuity[31].
A newer generation of SD-OCT, swept-source OCT (SS-OCT), has also been investigated in patients with CSCR[32,33]. Ferrara et al[32] investigated the images of 15 eyes with chronic CSCR using SS-OCT. They documented PEDs in all eyes, as well as morphologic changes in the choroid underneath observed RPE changes and beyond these changes. They also observed focal and diffuse vascular dilation at the level of the choriocapillaris in half of the enrolled eyes, and at the level of Sattler’s and Haller’s layers in all eyes[32].
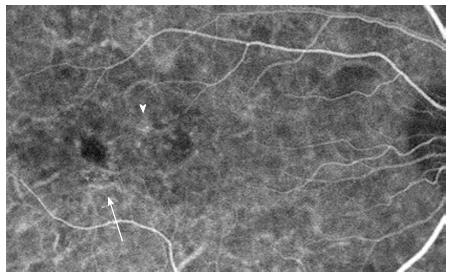
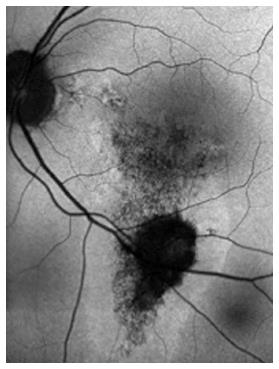
Fundus autoflouresence (FAF) images were also shown to have an added value in the understanding of CSCR pathophysiology. Images of patients through different stages of the disease show a variety of autoflouresence phenomena, implying an ongoing damage to the RPE and photoreceptors. While Patients imaged within the first month following diagnosis have minimal abnormalities seen in their FAF photography (Figure 4), the next months are characterized by an increased hyperautofluorescent in the detached area of retina[34-36]. Some hyperautoflouresence appears as a granulated process, in correspondence to pinpoint subretinal precipitates[37]. The material often gravitates inferiorly or is shown to be collected in deposits at the border of the detachment[34]. These patterns are demonstrated in Figure 5. After resolution of subretinal fluid, the hyperautofluorescence of the fundus abates, suggesting that the accumulated material could be cleared with time. The chronic phase of the disease is characterized by varying degrees of atrophy, including areas of geographic atrophy and areas within fluid tracts descending inferiorly[34] (Figure 6). Areas of chronic leaks can have decreased autofluorescence surrounding them. These areas of hypoautofluorescence appear to expand in size with increasing chronicity of the disease[38]. In chronic CSCR eyes, inactive disease can be differentiated from an active disease by the lack of hyperautoflouresence, with only the atrophic component remaining as areas of hypo-autofluorescence[34] (Figure 7).
The main advantage of multifocal electroretinography (mfERG) is the ability to demonstrate a persistent depression despite the resolution of the accumulating subretinal fluids. Hence, it has been investigated as a follow up tool for patients with chronic or recurrent CSCR as well as for examination of the seemingly healthy fellow eye[39]. It has been argued whether the pathologic findings in mfERG correspond with and are limited to the clinically observed areas of detachments or extend beyond these areas[39-41]. Excellent correlation was observed between changes in mfERG and function[42]. In a cross-sectional observational study by Lai and colleagues on 45 eyes with acute CSCR, it has been demonstrated that despite the fact that the outer retinal dysfunction is mostly localized to the central macula, a more widespread impairment in the more peripheral macula exists in the inner layers of the retina[43].
Visual function evaluated only by the measurement of visual acuity may underestimate the level of impairment in patients with macular diseases[44-49]. Macular microperimetry was designed to detect more subtle defects in visual function in these patients by measuring retinal sensitivity within the central visual field[50,51]. The device can also serve as a follow up tool due to its image-registration facility. Reduced retinal sensitivity is observed in areas with previous subretinal fluids[28]. This reduced sensitivity also corresponds with RPE irregularities found on OCT[52].
Acute CSCR is a self-limited disease in the majority of cases, with good final visual outcomes. The common management of acute CSCR still remains observation and risk modification, with the exception of certain indications prompting immediate medical management. The common indications for the initiation of treatment are non-resolved subretinal fluid for 3 mo, decreased visual acuity from CSCR in the fellow eye or the need for immediate visual acuity rehabilitation. However, chronic CSCR as well as frequent recurrences of serous detachments may lead to RPE atrophy and other changes in the neurosensory retina leaving the patient with impairment of visual function[53]. Therefore, earlier treatment in selected patients may improve the final outcome and even prevent further damage.
Treatment with argon laser may be applied to small extrafoveal leaks on FA, mainly to shorten disease duration. Long term follow up results for argon laser treatment demonstrate no advantage over observation regarding final visual outcome, rate of progression to chronic CSCR or number of recurrences[54-56]. The main disadvantages of this treatment are the limited ability to affect final prognosis, the need for specific extrafoveal lesions to perform the procedure and possible side effects including the growth of new choroidal neovascularization (CNV)[56].
Standard dose photodynamic therapy (PDT) with injection of verteporfin has been shown to completely resolve serous detachment in 60%-80% of patients and to have a partial affect in the remaining patients[57-60]. However, serious side effects such as sudden visual loss, new CNV and atrophy of the RPE, had led to reduced-fluence treatment development[61,62].
Randomized and non-randomized trials on reduced-fluence PDT have found this treatment to be as effective as full-fluence therapy in regards to fluid resolution and functional outcome. Chan and colleagues performed a double masked randomized controlled trial on 63 eyes with acute CSCR treated with either half-dose PDT or placebo[63]. One year following treatment, approximately 95% of eyes in the PDT group compared to 58% in the placebo group had no subretinal fluid on OCT. Visual acuity at one year follow up was improved or stabilized in all patients in the PDT group compared to approximately 79% of patients in the placebo group[63]. Wu and colleagues observed an improvement in mfERG in 24 eyes with acute CSCR, compared with 10 eyes in placebo group[64]. New imaging modalities, such as microperimetry, demonstrated the efficacy of PDT, beyond improvement in visual acuity[65-67].
There is still an ongoing search for the best way to reduce PDT dose, either by decreasing the laser therapy time, lowering the laser energy, altering the time interval between injections of verteporfin or lowering the dose of verteporfin. Zhao et al[68] conducted a research testing different doses of verteporfin for CSCR patients. Their conclusion was that 30% of the standard dose was optimal both for achieving fluid resolution and for avoiding adverse events[68].
In order to compare half-dose PDT to argon laser, Lim and colleagues prospectively assessed 26 eyes with CSCR[69]. Their results showed an earlier anatomic and functional resolution after treatment with half-dose PDT compared to laser. These differences, however, were no longer noted 3 mo following treatment[69].
Clinical response for this treatment has been linked to the level of hyperfluorescence observed on ICGA[70]. Patients with intense hyperfluorescence were more likely to show resolution of the serous detachment compared to patients with mild or no leaks observed on ICGA prior to treatment[70]. A recent report by Kim et al[71] evaluated the efficacy of half-dose PDT targeting the focal leakage point on FA for acute CSCR. In this retrospective trial, all 10 eyes treated in this manner had complete resolution of subretinal fluid compared to 27.3% of eyes receiving no treatment. These differences were minimized at 12 mo follow up; with 90% of PDT group and 63.6% of observation group showing no subretinal fluid. No differences were noted in final visual acuity or recurrence rates between the two groups[71]. Therefore, this treatment protocol may serve as a substitute for focal argon laser treatment for hastening absorption of subretinal fluid.
Anti-vascular endothelial growth factor agents: Intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents have dramatically changed the anatomical and functional prognosis of patients with retinal and choroidal diseases[72-74]. However, in patients with CSCR, improvement in prognosis following injections is more questionable, and anti-VEGF agents are not considered first line treatment. Despite the fact that VEGF levels were not found to be elevated in the aqueous humor of eyes with CSCR, many uncontrolled studies reported favorable results for anti-VEGF agents[75-84]. The largest series to date published by Lim et al[75] included 40 eyes in a prospective interventional case series. In their study, following one or two injections, 82.5% of patients achieved resolution of subretinal fluid at 4 mo follow up. However, they only included patients with acute CSCR, known to have a better prognosis; with no comparison arm for this study, and a relatively short follow up period[75].
A recent prospective, randomized study by Bae and colleagues compared ranibizumab injections to low-fluence PDT in 16 eyes with chronic CSCR[85]. Their conclusion was that the effect of ranibizumab was not promising compared with that of low-fluence PDT, in terms of anatomic outcomes. An important observation was that 50% of eyes in the ranibizumab group accomplished complete resolution only after they underwent additional low-fluence PDT[85].
A meta-analysis, conducted by Chung et al[86], identified four clinical controlled studies evaluating the effects of intravitreal bevacizumab injection in CSCR. In their data analysis, no significant differences in BCVA or central macular thickness (CMT) were found at 6 mo after injection between the bevacizumab group and the observation group. Another important issue they raised was that no report assessed severe complications or side effects of these intravitreal injections in patients with CSCR[86].
The main advantage of diode laser over argon laser is deeper penetration, reaching the choroid, mainly implicated as the pathologic origin of the subretinal fluid[87]. That sets the theoretical basis for the trials investigating the role of this laser in CSCR, as an attractive replacement for focal argon laser treatment. Verma and colleagues conducted a small randomized trial comparing the results of these two types of lasers in patients with acute CSCR[88]. Despite the fact that visual acuity was better in the diode laser treatment group 4 wk following the procedure, this difference was no longer observed 4 wk later[88]. Micropulse diode laser is considered less damaging to the RPE and photoreceptors, by applying short multiple pulses of energy instead of continuous energy. However, unlike the argon laser, the micropulse diode laser is less widely available and does not cause retinal bleaching guiding the operator when to stop laser application. In addition, micropulse diode laser was not proven to be safer or more effective than argon laser or PDT, and still requires a focal leak as seen on FA to guide treatment[89]. Therefore, the role of this laser as a substitute for conventional laser is still questionable.
The basis for corticosteroid antagonists administration for CSCR is the association found between the development of the disease and endogenous hypercortisolism (Cushing’s syndrome)[90]. The hypothesis is that if this association exists with other hypercortisolemic states, than blocking the effect of corticosteroids may play a role in treating CSCR[91]. That hypothesis is further supported by the elevated serum cortisol levels commonly found in patients with CSCR[92-94]. The proposed medications, including ketoconazole, mifepristone (RU486), finasteride, rifampin, and anti-adrenergics, have not been tested in randomized, controlled trials.
Ketoconazole, an adrenocorticoid agent, has been investigated by two groups for the treatment of CSCR. In a prospective, case control study, Golshahi and colleagues treated 15 patients with new onset subretinal fluid with 200 mg of ketoconazole per day for 4 wk[95]. No statistically significant benefit was found for that dosage[95]. An increased dose of 600 mg per day for 4 wk was later adminstered by Meyerle et al[96]. The results of this prospective, uncontrolled pilot study on 5 patients with chronic CSCR showed reduced serum cortisol levels, stable visual acuity, and anatomic improvement at 8 wk. They suggested larger, controlled trials to test the efficiency of ketoconazole in CSC patients[96].
Nielsen and colleagues treated 16 chronic CSCR patients with mifepristone (RU486), an active anti-glucocorticosteroid and anti-progesterone agent[97]. Favorable response was seen, with seven subjects gaining five or more letters of vision and seven subjects with improved OCT findings. Despite the fact that treatment was well tolerated without serious adverse effects in these patients, main obstetric concerns regarding this drug still limit its use[97].
Anti-adrenic agents, proposed to cause reduction of the adrenergic drive induced by stress, were also investigated for the treatment of CSCR. In his monkey model for experimental CSCR, Yoshioka suggested that inhibition of adrenergic receptors, particularly alpha receptors, may be beneficial[98]. Later studies investigating beta-blocking agents have shown partial improvement in CSCR patients, with no difference found between selective and non-selective agents[99-102]. However, none of the studies were controlled or randomized, and significant systemic side effects further limit the use of these agents.
The hypothesis that hypercoagulability plays a role in the pathogenesis of CSCR was based on a previous work showing increased levels of plasminogen activator inhibitor in patients with CSCR, compared to controls[103,104]. Caccavale and colleagues treated 107 CSCR patients with 100 mg acetyl salicylic acid (aspirin) once daily for one month and then every other day for five months[105]. A rapid recovery of visual acuity was observed after the first week of therapy, with low recurrence rate[105].
The hypothesis that an inflammatory damaging process has a role in the pathogenesis of CSCR is based on the characteristic of the disease. The proceeding stress as well the recurrent episodes of the disease have led investigators to consider a viral or a bacterial etiology.
The most investigated infectious association to CSCR is between the disease and Helicobacter pylori (H. pylori) infection[106-110]. Some investigators have noted a beneficial effect for H. pylori treatment in patients with CSCR[111,112]. In a randomized, controlled trial, twenty-five H. pylori- infected acute CSCR patients were treated with an anti-H. pylori treatment; another twenty-five patients with the same clinical presentations served as the control[112]. Subretinal fluid reabsorption time was significantly reduced in the treatment group, with no beneficial effect observed for final visual acuity[112]. Larger studies to confirm the association between H. pylori and CSCR are warranted.
Regarding a viral etiology, no large studies to establish the association between CSCR and any virus were published. Rathschuler et al[113] reported two cases of acute CSCR immediately started with an antiviral therapy (Acycloguanosine), with immediate regression of symptoms accompanied by an anatomic resolution of the leakage and the detachment. Larger studies to confirm the association between H. pylori or a viral etiology and CSCR are warranted.
CSCR is a common cause of visual impairment, especially in the middle aged population. Despite the fact that most patients will have spontaneous recovery, those with recurrent episodes or chronic disease may remain with significant functional impairment. The exact pathophysiology leading to subretinal fluid accumulation remains undetermined, but it is probably a combination of choroidal and RPE pathology. While imaging is evolving rapidly, the clinical implications of all these new imaging modalities are less clear. Treatment is still a subject of dispute, regarding indications, proper initiation time and type of treatment for both acute and choronic CSCR. That is mainly due to the fact that large randomized trials are still lacking.
P- Reviewer: Davey PG, Koleva-Georgieva DN S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Von Graefe A. Kurzere Abhandlungen. Notizen und casaistische Mitheilungen vermischten Inhalts: VI (Ueber zentrale recidivirende Retinitis). Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1866;12:211-215. |
| 2. | Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63:Suppl: 1-139. [PubMed] |
| 3. | Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 251] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Spitznas M. Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol. 1986;224:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Negi A, Marmor MF. Experimental serous retinal detachment and focal pigment epithelial damage. Arch Ophthalmol. 1984;102:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Marmor MF. New hypotheses on the pathogenesis and treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 1988;226:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Gass JDM. Specific diseases causing disciform macular detachment. Stereoscopic Atlas of Macular Diseases. 1997;1:52-70. |
| 8. | Gass JDM. Pathogenesis of disciform detachment of the Neuroepithelium: I. General concepts and classifications. Am J Ophthalmol. 1967;63:573-585. [PubMed] |
| 9. | Gass JDM. Pathogenesis of disciform detachment of the neuroepithelium: IV. Fluorescein angiographic study of senile disciform macular degeneration. Am J Ophthalmol. 1967;63:645-659. |
| 10. | Gass JDM. Pathogenesis of disciform detachment of the neuroepithelium: V. Disciform macular detachment secondary to focal choroiditis. Am J Ophthalmol. 1967;63:661-687. |
| 11. | Ryan SJ. Central serous chorioretinopathy. Retina 3rd ed. St Louis: Mosby 2001; 1153-1181. |
| 12. | Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 476] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 13. | Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26-34. [PubMed] |
| 14. | Regatieri CV, Branchini L, Fujimoto JG, Duker JS. Choroidal imaging using spectral-domain optical coherence tomography. Retina. 2012;32:865-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 700] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 16. | Kim SW, Oh J, Kwon SS, Yoo J, Huh K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011;31:1904-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | Kim YT, Kang SW, Bai KH. Choroidal thickness in both eyes of patients with unilaterally active central serous chorioretinopathy. Eye (Lond). 2011;25:1635-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Matsumoto H, Sato T, Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am J Ophthalmol. 2009;148:105-110.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Matsumoto H, Kishi S, Otani T, Sato T. Elongation of photoreceptor outer segment in central serous chorioretinopathy. Am J Ophthalmol. 2008;145:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Kon Y, Iida T, Maruko I, Saito M. The optical coherence tomography-ophthalmoscope for examination of central serous chorioretinopathy with precipitates. Retina. 2008;28:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Maruko I, Iida T, Ojima A, Sekiryu T. Subretinal dot-like precipitates and yellow material in central serous chorioretinopathy. Retina. 2011;31:759-765. [PubMed] |
| 22. | Brandl C, Helbig H, Gamulescu MA. Choroidal thickness measurements during central serous chorioretinopathy treatment. Int Ophthalmol. 2014;34:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Gupta P, Gupta V, Dogra MR, Singh R, Gupta A. Morphological changes in the retinal pigment epithelium on spectral-domain OCT in the unaffected eyes with idiopathic central serous chorioretinopathy. Int Ophthalmol. 2010;30:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Oh J, Smiddy WE, Flynn HW, Gregori G, Lujan B. Photoreceptor inner/outer segment defect imaging by spectral domain OCT and visual prognosis after macular hole surgery. Invest Ophthalmol Vis Sci. 2010;51:1651-1658. [PubMed] |
| 25. | Oishi A, Otani A, Sasahara M, Kojima H, Nakamura H, Kurimoto M, Yoshimura N. Photoreceptor integrity and visual acuity in cystoid macular oedema associated with retinitis pigmentosa. Eye (Lond). 2009;23:1411-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Kim HJ, Kang JW, Chung H, Kim HC. Correlation of foveal photoreceptor integrity with visual outcome in idiopathic epiretinal membrane. Curr Eye Res. 2014;39:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Reznicek L, Cserhati S, Seidensticker F, Liegl R, Kampik A, Ulbig M, Neubauer AS, Kernt M. Functional and morphological changes in diabetic macular edema over the course of anti-vascular endothelial growth factor treatment. Acta Ophthalmol. 2013;91:e529-e536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Kim SW, Oh J, Huh K. Correlations among various functional and morphological tests in resolved central serous chorioretinopathy. Br J Ophthalmol. 2012;96:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Ojima Y, Hangai M, Sasahara M, Gotoh N, Inoue R, Yasuno Y, Makita S, Yatagai T, Tsujikawa A, Yoshimura N. Three-dimensional imaging of the foveal photoreceptor layer in central serous chorioretinopathy using high-speed optical coherence tomography. Ophthalmology. 2007;114:2197-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Piccolino FC, de la Longrais RR, Ravera G, Eandi CM, Ventre L, Abdollahi A, Manea M. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139:87-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Yalcinbayir O, Gelisken O, Akova-Budak B, Ozkaya G, Gorkem Cevik S, Yucel AA. Correlation of spectral domain optical coherence tomography findings and visual acuity in central serous chorioretinopathy. Retina. 2014;34:705-712. [PubMed] |
| 32. | Ferrara D, Mohler KJ, Waheed N, Adhi M, Liu JJ, Grulkowski I, Kraus MF, Baumal C, Hornegger J, Fujimoto JG. En face enhanced-depth swept-source optical coherence tomography features of chronic central serous chorioretinopathy. Ophthalmology. 2014;121:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Jirarattanasopa P, Ooto S, Tsujikawa A, Yamashiro K, Hangai M, Hirata M, Matsumoto A, Yoshimura N. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology. 2012;119:1666-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 34. | Spaide R. Autofluorescence from the outer retina and subretinal space: hypothesis and review. Retina. 2008;28:5-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Spaide RF, Klancnik JM. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology. 2005;112:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | von Rückmann A, Fitzke FW, Fan J, Halfyard A, Bird AC. Abnormalities of fundus autofluorescence in central serous retinopathy. Am J Ophthalmol. 2002;133:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Matsumoto H, Kishi S, Sato T, Mukai R. Fundus autofluorescence of elongated photoreceptor outer segments in central serous chorioretinopathy. Am J Ophthalmol. 2011;151:617-623.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Imamura Y, Fujiwara T, Spaide RF. Fundus autofluorescence and visual acuity in central serous chorioretinopathy. Ophthalmology. 2011;118:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Marmor MF, Tan F. Central serous chorioretinopathy: bilateral multifocal electroretinographic abnormalities. Arch Ophthalmol. 1999;117:184-188. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Moschos M, Brouzas D, Koutsandrea C, Stefanos B, Loukianou H, Papantonis F, Moschos M. Assessment of central serous chorioretinopathy by optical coherence tomography and multifocal electroretinography. Ophthalmologica. 2007;221:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Vajaranant TS, Szlyk JP, Fishman GA, Gieser JP, Seiple W. Localized retinal dysfunction in central serous chorioretinopathy as measured using the multifocal electroretinogram. Ophthalmology. 2002;109:1243-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Yip YW, Ngai JW, Fok AC, Lai RY, Li H, Lam DS, Lai TY. Correlation between functional and anatomical assessments by multifocal electroretinography and optical coherence tomography in central serous chorioretinopathy. Doc Ophthalmol. 2010;120:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Lai TY, Lai RY, Ngai JW, Chan WM, Li H, Lam DS. First and second-order kernel multifocal electroretinography abnormalities in acute central serous chorioretinopathy. Doc Ophthalmol. 2008;116:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | McClure ME, Hart PM, Jackson AJ, Stevenson MR, Chakravarthy U. Macular degeneration: do conventional measurements of impaired visual function equate with visual disability? Br J Ophthalmol. 2000;84:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | West SK, Munoz B, Rubin GS, Schein OD, Bandeen-Roche K, Zeger S, German S, Fried LP. Function and visual impairment in a population-based study of older adults. The SEE project. Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1997;38:72-82. [PubMed] |
| 46. | Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM. Influence of age-related maculopathy on visual functioning and health-related quality of life. Am J Ophthalmol. 1999;128:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Scott IU, Schein OD, West S, Bandeen-Roche K, Enger C, Folstein MF. Functional status and quality of life measurement among ophthalmic patients. Arch Ophthalmol. 1994;112:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 168] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Remky A, Lichtenberg K, Elsner AE, Arend O. Short wavelength automated perimetry in age related maculopathy. Br J Ophthalmol. 2001;85:1432-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Hazel CA, Petre KL, Armstrong RA, Benson MT, Frost NA. Visual function and subjective quality of life compared in subjects with acquired macular disease. Invest Ophthalmol Vis Sci. 2000;41:1309-1315. [PubMed] |
| 50. | Roisman L, Ribeiro JC, Fechine FV, Lavinsky D, Moraes N, Campos M, Farah ME. Does microperimetry have a prognostic value in central serous chorioretinopathy? Retina. 2014;34:713-718. [PubMed] |
| 51. | Oh J, Kim SW, Kwon SS, Oh IK, Huh K. Correlation of fundus autofluorescence gray values with vision and microperimetry in resolved central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2012;53:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Ojima Y, Tsujikawa A, Hangai M, Nakanishi H, Inoue R, Sakamoto A, Yoshimura N. Retinal sensitivity measured with the micro perimeter 1 after resolution of central serous chorioretinopathy. Am J Ophthalmol. 2008;146:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58:103-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 447] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 54. | Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol. 1983;95:457-466. [PubMed] |
| 55. | Leaver P, Williams C. Argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1979;63:674-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Ficker L, Vafidis G, While A, Leaver P. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988;72:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 181] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Yannuzzi LA, Slakter JS, Gross NE, Spaide RF, Costa D, Huang SJ, Klancnik JM, Aizman A. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2003;23:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 320] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 58. | Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003;23:752-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 276] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 59. | Chan W-M, Lam DSC, Lai TYY, Tam BSM, Liu DTL, Chan CKM. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87:1453-1458. [RCA] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 346] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 60. | Taban M, Boyer DS, Thomas EL, Taban M. Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol. 2004;137:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 61. | Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond). 2010;24:1743-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 62. | Lee TG, Kim JE. Photodynamic therapy for steroid-associated central serous chorioretinopathy. Br J Ophthalmol. 2011;95:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008;115:1756-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 64. | Wu ZHY, Lai RYK, Yip YWY, Chan WM, Lam DSC, Lai TYY. Improvement in multifocal electroretinography after half-dose verteporfin photodynamic therapy for central serous chorioretinopathy: a randomized placebo-controlled trial. Retina. 2011;31:1378-1386. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Ehrlich R, Mawer NP, Mody CH, Brand CS, Squirrell D. Visual function following photodynamic therapy for central serous chorioretinopathy: a comparison of automated macular microperimetry versus best-corrected visual acuity. Clin Experiment Ophthalmol. 2012;40:e32-e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Fujita K, Yuzawa M, Mori R. Retinal sensitivity after photodynamic therapy with half-dose verteporfin for chronic central serous chorioretinopathy: short-term results. Retina. 2011;31:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 67. | Senturk F, Karacorlu M, Ozdemir H, Karacorlu SA, Uysal O. Microperimetric changes after photodynamic therapy for central serous chorioretinopathy. Am J Ophthalmol. 2011;151:303-9.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Zhao MW, Zhou P, Xiao HX, Lv YS, Li CA, Liu GD, Li XX. Photodynamic therapy for acute central serous chorioretinopathy: the safe effective lowest dose of verteporfin. Retina. 2009;29:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Lim JW, Kang SW, Kim YT, Chung SE, Lee SW. Comparative study of patients with central serous chorioretinopathy undergoing focal laser photocoagulation or photodynamic therapy. Br J Ophthalmol. 2011;95:514-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 70. | Inoue R, Sawa M, Tsujikawa M, Gomi F. Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am J Ophthalmol. 2010;149:441-6.e1-441-6.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Kim KS, Lee WK, Lee SB. Half-dose photodynamic therapy targeting the leakage point on the fluorescein angiography in acute central serous chorioretinopathy: a pilot study. Am J Ophthalmol. 2014;157:366-373.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2456] [Cited by in RCA: 2606] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 73. | Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, Klesert TR. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 2007;125:1460-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 74. | Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, Friedman SM, Glassman AR, Miller KM. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1061] [Cited by in RCA: 1027] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 75. | Lim JW, Kim MU. The efficacy of intravitreal bevacizumab for idiopathic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Artunay O, Yuzbasioglu E, Rasier R, Sengul A, Bahcecioglu H. Intravitreal bevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: a prospective, controlled clinical study. Curr Eye Res. 2010;35:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 77. | Chhablani JK, Narayanan R. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010;30:1323-1324; author reply 1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Inoue M, Kadonosono K, Watanabe Y, Kobayashi S, Yamane S, Arakawa A. Results of one-year follow-up examinations after intravitreal bevacizumab administration for chronic central serous chorioretinopathy. Ophthalmologica. 2011;225:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Lee ST, Adelman RA. The treatment of recurrent central serous chorioretinopathy with intravitreal bevacizumab. J Ocul Pharmacol Ther. 2011;27:611-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Alomran MS. Intravitreal Bevacizumab for the Treatment of Central Serous Chorioretinopathy. Ophthalmic Surg Lasers Imaging. 2010;Apr 2; Epub ahead of print. [PubMed] |
| 81. | Lim SJ, Roh MI, Kwon OW. Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina. 2010;30:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Schaal KB, Hoeh AE, Scheuerle A, Schuett F, Dithmar S. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009;19:613-617. [PubMed] |
| 83. | Huang WC, Chen WL, Tsai YY, Chiang CC, Lin JM. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eye (Lond). 2009;23:488-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 84. | Torres-Soriano ME, García-Aguirre G, Kon-Jara V, Ustariz-Gonzáles O, Abraham-Marín M, Ober MD, Quiroz-Mercado H. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol. 2008;246:1235-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 85. | Bae SH, Heo JW, Kim C, Kim TW, Lee JY, Song SJ, Park TK, Moon SW, Chung H. A randomized pilot study of low-fluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. Am J Ophthalmol. 2011;152:784-792.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Chung YR, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review. Eye (Lond). 2013;27:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | Brancato R, Scialdone A, Pece A, Coscas G, Binaghi M. Eight-year follow-up of central serous chorioretinopathy with and without laser treatment. Graefes Arch Clin Exp Ophthalmol. 1987;225:166-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Verma L, Sinha R, Venkatesh P, Tewari HK. Comparative evaluation of diode laser versus argon laser photocoagulation in patients with central serous retinopathy: a pilot, randomized controlled trial [ISRCTN84128484]. BMC Ophthalmol. 2004;4:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Chen SN, Hwang JF, Tseng LF, Lin CJ. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology. 2008;115:2229-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 90. | Bouzas EA, Scott MH, Mastorakos G, Chrousos GP, Kaiser-Kupfer MI. Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol. 1993;111:1229-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 165] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 91. | Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology. 2002;109:1765-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81:962-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 93. | Haimovici R, Rumelt S, Melby J. Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology. 2003;110:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 94. | Zakir SM, Shukla M, Simi ZU, Ahmad J, Sajid M. Serum cortisol and testosterone levels in idiopathic central serous chorioretinopathy. Indian J Ophthalmol. 2009;57:419-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Golshahi A, Klingmüller D, Holz FG, Eter N. Ketoconazole in the treatment of central serous chorioretinopathy: a pilot study. Acta Ophthalmol. 2010;88:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Meyerle CB, Freund KB, Bhatnagar P, Shah V, Yannuzzi LA. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina. 2007;27:943-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Nielsen JS, Jampol LM. Oral mifepristone for chronic central serous chorioretinopathy. Retina. 2011;31:1928-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Yoshioka H. The etiology of central serous chorioretinopathy. Nihon Ganka Gakkai Zasshi. 1991;95:1181-1195. [PubMed] |
| 99. | Browning DJ. Nadolol in the treatment of central serous retinopathy. Am J Ophthalmol. 1993;116:770-771. [PubMed] |
| 100. | Tatham A, Macfarlane A. The use of propranolol to treat central serous chorioretinopathy: an evaluation by serial OCT. J Ocul Pharmacol Ther. 2006;22:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Chrapek O, Spacková K, Rehák J. Treatment of central serous chorioretinopathy with beta blockers. Cesk Slov Oftalmol. 2002;58:382-386. [PubMed] |
| 102. | Fabianová J, Porubská M, Cepilová Z. Central serous chorioretinopathy--treatment with beta blockers. Cesk Slov Oftalmol. 1998;54:401-404. [PubMed] |
| 103. | Iijima H, Iida T, Murayama K, Imai M, Gohdo T. Plasminogen activator inhibitor 1 in central serous chorioretinopathy. Am J Ophthalmol. 1999;127:477-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Yamada R, Yamada S, Ishii A, Tane S. Evaluation of tissue plasminogen activator and plasminogen activator inhibitor-1 in blood obtained from patients of idiopathic central serous chorioretinopathy. Nihon Ganka Gakkai Zasshi. 1993;97:955-960. [PubMed] |
| 105. | Caccavale A, Imparato M, Romanazzi F, Negri A, Porta A, Ferentini F. A new strategy of treatment with low-dosage acetyl salicylic acid in patients affected by central serous chorioretinopathy. Med Hypotheses. 2009;73:435-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Mauget-Faÿsse M, Kodjikian L, Quaranta M, Ben Ezra D, Trepsat C, Mion F, Mégraud F. Helicobacter pylori in central serous chorioretinopathy and diffuse retinal epitheliopathy. Results of the first prospective pilot study. J Fr Ophtalmol. 2002;25:1021-1025. [PubMed] |
| 107. | Ahnoux-Zabsonre A, Quaranta M, Mauget-Faÿsse M. Prevalence of Helicobacter pylori in central serous chorioretinopathy and diffuse retinal epitheliopathy: a complementary study. J Fr Ophtalmol. 2004;27:1129-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 108. | Cotticelli L, Borrelli M, D’Alessio AC, Menzione M, Villani A, Piccolo G, Montella F, Iovene MR, Romano M. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol. 2006;16:274-278. [PubMed] |
| 109. | Asensio-Sánchez VM, Rodríguez-Delgado B, García-Herrero E, Cabo-Vaquera V, García-Loygorri C. Central serous chorioretinopathy as an extradigestive manifestation of Helicobacter pylori gastric infection. Arch Soc Esp Oftalmol. 2008;83:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Misiuk-Hojło M, Michałowska M, Turno-Krecicka A. Helicobacter pylori--a risk factor for the developement of the central serous chorioretinopathy. Klin Oczna. 2009;111:30-32. [PubMed] |
| 111. | Giusti C. Central serous chorioretinopathy: a new extragastric manifestation of Helicobacter pylori?: Analysis of a clinical case. Clin Ter. 2001;152:393-397. [PubMed] |
| 112. | Rahbani-Nobar MB, Javadzadeh A, Ghojazadeh L, Rafeey M, Ghorbanihaghjo A. The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy. Mol Vis. 2011;17:99-103. [PubMed] |
| 113. | Rathschuler F, Lai S, Ghiglione D, Rossi P, Ciurlo G. A new therapeutical approach to central serous retinopathy, a hypothesis. Int Ophthalmol. 1990;14:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |