Peer-review started: September 29, 2021
First decision: December 2, 2021
Revised: December 9, 2021
Accepted: February 12, 2022
Article in press: February 12, 2022
Published online: February 25, 2022
Processing time: 146 Days and 23 Hours
Hemophagocytic lymphohistiocytosis (HLH) is a severe and potentially deadly condition associated with extensive inflammation and immune activation. Cytokine adsorption may serve as a supportive treatment that can stabilize organ function in affected patients by reducing their circulating cytokines levels. To date, no descriptions of clinical experiences associated with the use of HA330-II column hemoadsorption for the treatment of children affected by HLH have been published.
We describe the case of an 11-year-old child with Epstein-Barr virus-associated HLH complicated by liver failure. She underwent HA330-II column hemoadsorption and chemotherapy and exhibited reductions in levels of inflammatory cytokines, including interleukin (IL), IL-6, IL-8, IL-10, and interferon-γ. The patient’s condition and laboratory parameters gradually improved with treatment.
Hemoadsorption may play an important role in cytokine storm elimination in children with HLH combined with liver failure and consequent multiple organ failure.
Core Tip: Hemophagocytic hymphohistiocytosis (HLH) is an often fatal disease. We report an 11-year-old female who was diagnosed with Epstein-Barr virus-HLH and presented with coagulation disorders, liver damage, and respiratory insufficiency. In the present case, initially elevated interleukin (IL)-6, IL-8, IL-10, and interferon-γ levels were reduced to within normal ranges following hemoadsorption with HA330-II, and the patient’s condition gradually improved. HA330-II hemoadsorption has the ability to bridge the patient until chemotherapy can contribute to reduced HLH disease activity.
- Citation: Gao Q, Xin XW, Zhao C, Wang YJ, Wang W, Yin Y, Wang XR, Jin YP. Efficacy of HA330-II column hemoadsorption in Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis combined with liver failure: A case report. World J Hematol 2022; 9(1): 6-12
- URL: https://www.wjgnet.com/2218-6204/full/v9/i1/6.htm
- DOI: https://dx.doi.org/10.5315/wjh.v9.i1.6
Hemophagocytic lymphohistiocytosis (HLH) is a severe and potentially lethal disorder associated with excessive inflammation and unrestrained immune activation[1]. Patients with HLH exhibit dramatically elevated levels of cytokines, including interleukin (IL)-1, IL-2, IL-6, IL-18, tumor necrosis factor-α, and interferon (IFN)-γ[2]. HLH is classified into two groups: Primary and acquired. Primary HLH primarily develops as a consequence of genetic defects during infancy, while acquired HLH occurs in the context of auto-inflammatory/autoimmune diseases, lymphoma, or certain viral infections, with Epstein-Barr virus (EBV) being a common cause. Animal studies[3,4] and case series have demonstrated that a reduction in circulating cytokine levels achieved via hemoadsorption can be effective as a treatment for HLH[5,6]. HA330-II perfusion columns have been reported to be capable of absorbing multiple inflammatory factors and have been successfully used as a component of a double plasma molecular adsorption system to treat patients suffering from liver failure[7,8]. In the present article, we describe one case of a child diagnosed with EBV-associated HLH combined with liver failure who successfully underwent HA330-II column hemoadsorption and chemotherapy treatment. Through these treatments, the patient’s condition improved and her recovery was satisfactory.
An 11-year-old female was admitted to our hospital with a 5 d history of high fever with chills and a headache as well as damaged liver function. She did not exhibit a cough, abdominal pain, or vomiting.
Before this visit, she had been given a penicillinase antibiotic for 3 d and azithromycin for 1 d. Her clinical state rapidly deteriorated, and she developed respiratory failure, capillary leak syndrome, and hypotension. As such, she was admitted to the pediatric intensive care unit.
The patient had a history of encephalitis 3 years ago, and she had recovered after treatment for 2 wk.
This patient had no specific personal or family history.
On initial examination, the patient had a fever with a maximal temperature of 40.0 °C, a respiratory rate of 30 breaths per min, a heart rate of 122 bpm, and a blood pressure of 82/41 mmHg at admission. She was in a poor mental state and presented with jaundice and hepatosplenomegaly. Other physical examinations were normal.
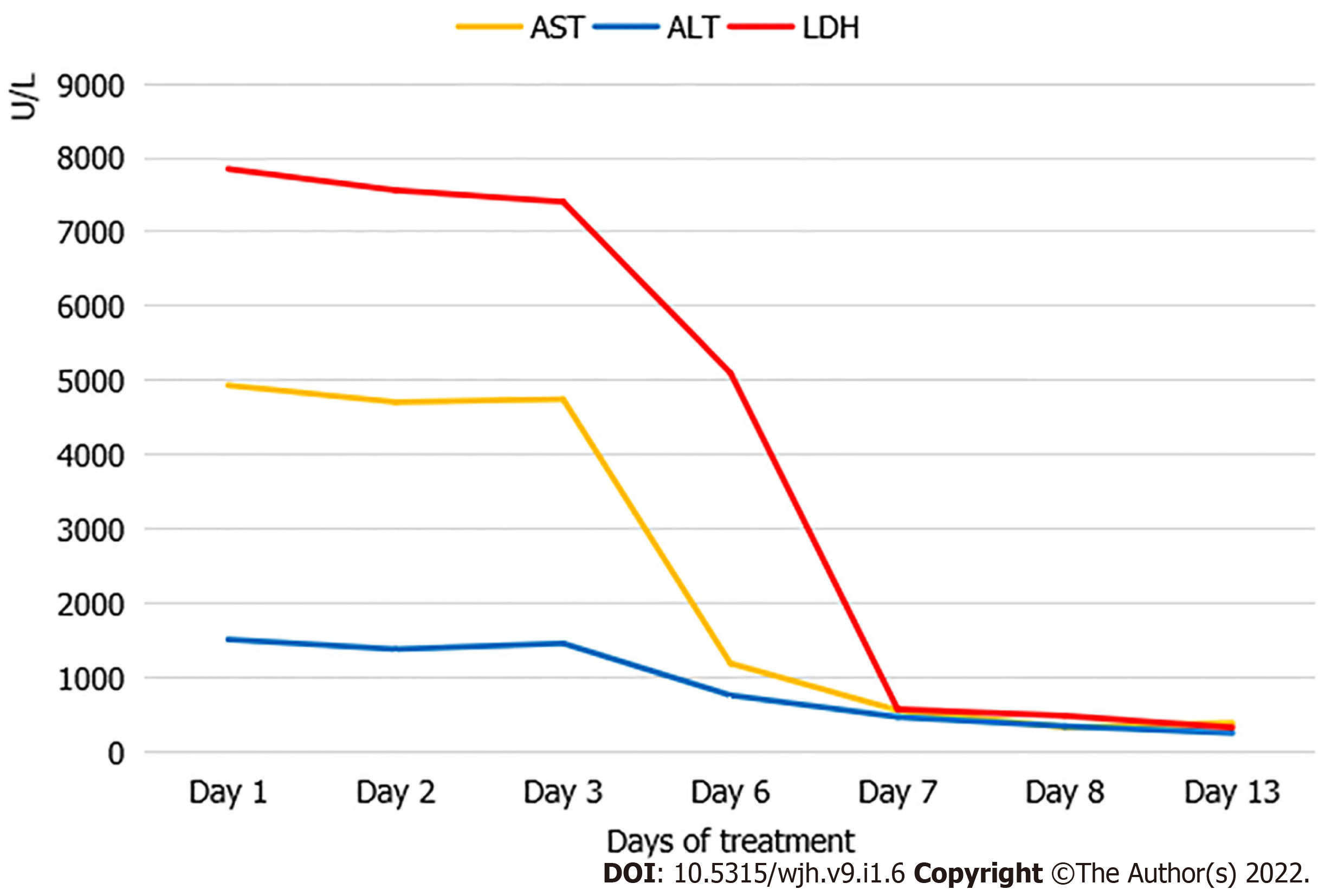
Initial laboratory studies in the pediatric intensive care unit revealed excessive hyperferritinemia (58360 ng/mL, reference range: 11.0-306.8 ng/mL), low natural killer cell activity (0.32%, reference range: 5%-26%), hypofibrinogenemia (0.84 g/L, reference range: 1.50-4.35 g/L), leukopenia (2.07 × 109/L, reference range: 3.5-9.5 × 109/L), neutropenia (0.92 × 109/L, reference range: 1.8-6.3 × 109/L) elevated international normalized ratio values (1.92, reference range: 0.8-1.2), thrombocytopenia (42 × 109/L, reference range: 125-350 × 109/L), elevated alanine transaminase (ALT) levels (921 U/L, reference range: 7-40 U/L), aspartate aminotransferase (AST) levels (2223 U/L, reference range: 13-35 U/L), and elevated total bilirubin (TBIL) levels (108.7 mmol/L, reference range: 3.5-23.5 mmol/L). The patient also exhibited high levels of C reactive protein (76.40 mg/L, reference range: 0-8 mg/L), procalcitonin (2.73 ng/mL, reference range: 0-0.05 ng/mL), IL-6 (154.06 pg/mL, reference range: 0-5.4 pg/mL), IL-8 (32.67 pg/mL, reference range: 0-20.6 pg/mL), IL-10 (169.81 pg/mL, reference range: 0-12.9 pg/mL), and IFN-γ (4387.41 pg/mL, reference range: 0-23.1 pg/mL). EBV-DNA loads were also found to be significantly elevated (3.82 × 106 copies/mL). Multiple blood and sputum cultures as well as the other viral polymerase chain reaction tests for common respiratory viruses and cytomegalovirus were all negative. A bone marrow biopsy revealed the presence of hemophagocytosis.
A thoracic-abdominal computer tomography analysis revealed pulmonary inflammation and no evidence of tumors.
She was diagnosed with EBV-associated hemophagocytic syndrome combined with liver failure in accordance with the HLH-2004 guidelines[9].
Treatment with meropenem, norepinephrine, intravenous immunoglobulin, ganciclovir, and dexamethasone as well as high-flow nasal cannula placement were initiated. However, these approaches were ineffective as evidenced by sustained fever, hypoxemia, and hypotension. Chemotherapy was recommended for the patient, but her parents refused and asked for other therapeutic options. In light of the refractory state of her HLH and her poor general condition, we next sought to achieve the immediate suppression of hypercytokinemia. Accordingly, we initiated blood purification. Plasma exchange was initially considered but could not be performed owing to reduced plasma separator access due to the coronavirus disease 2019 pandemic. As such, we tried to perform hemoadsorption (HA330-II perfusion column, Zhuhai Health Sails Biotechnology Co., Ltd., Zhuhai, China) in this patient. Informed written consent was obtained from the patient’s parent. Heparin sodium was employed for anticoagulation, and the patient was infused with platelets and fibrinogen prothrombin complex concentrate.
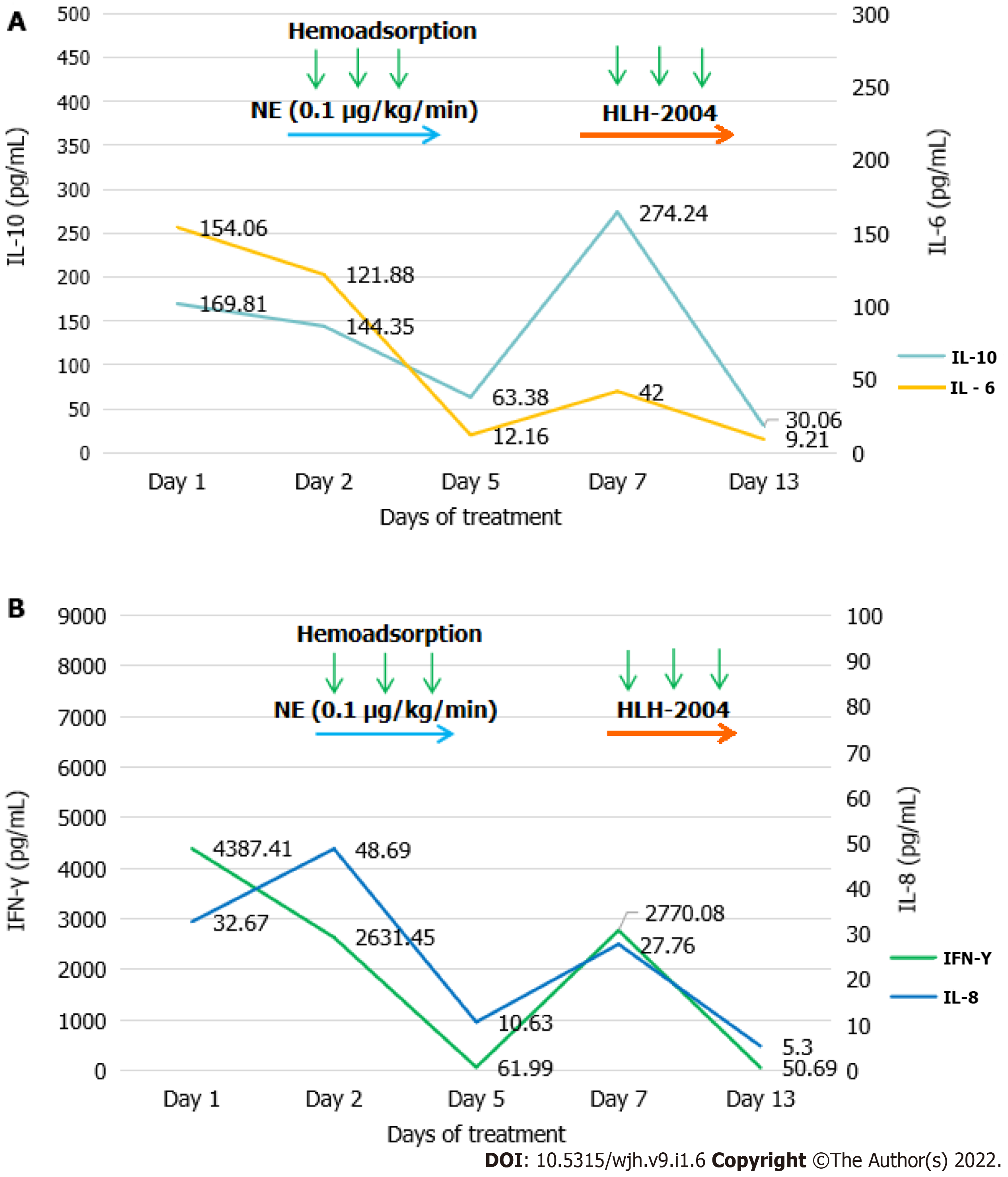
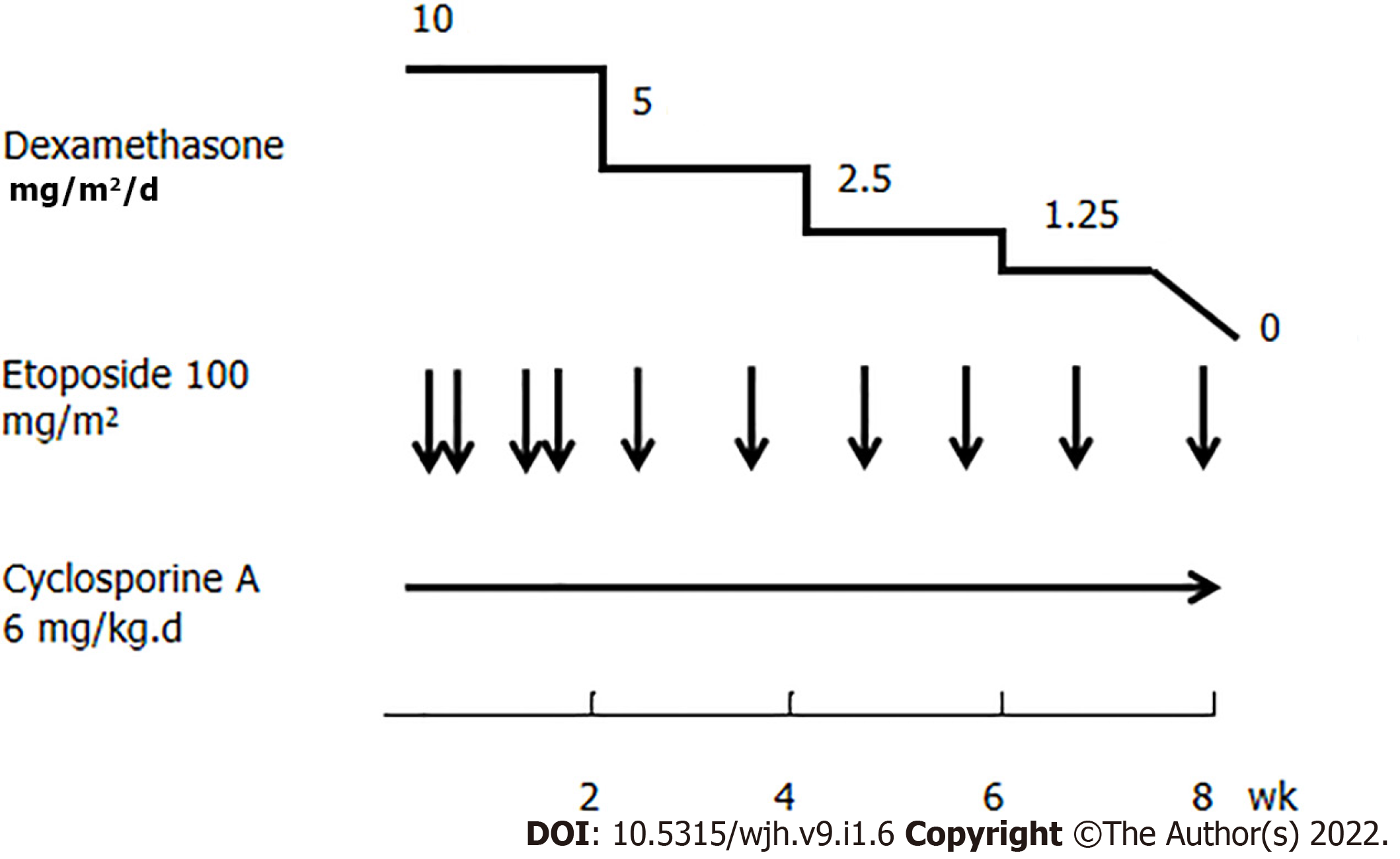
On the 1st day of hospitalization, she was in poor condition with respect to her clinical symptoms and biochemical parameters. She also needed a high dose of norepinephrine to maintain appropriate cardiovascular function. On the 2nd day of hospitalization, one round of the above mentioned hemoadsorption strategy was implemented. This hemoadsorption approach was implemented two more times over a 3-d period. On the 5th day of hospitalization, the patient exhibited significantly decreased levels of IL-6 (12.16 pg/mL), IL-8 (10.63 pg/mL), IL-10 (63.38 pg/mL), and IFN-γ (61.99 pg/mL) (Figure 1). She was gradually weaned off norepinephrine treatment, and her fever disappeared while her total leukocyte and neutrophil counts increased. However, no significant improvement in liver function was observed (ALT 766 U/L, AST 1196 U/L, TBIL 170.54 mmol/L, Fib 1.23 g/L), and inflammatory markers rebounded after hemoadsorption had been discontinued for 2 d, at which time the patient again developed a fever that reached as high as 40.0 C. At that time, her parents provided consent for chemotherapy (HLH-2004) treatment (Figure 2), which was initiated in combination with hemoadsorption.
The hemoadsorption approach was implemented an additional three times over a 5-d period, and the patient’s condition gradually improved. On the 13th day of hospitalization, decreased levels of IL-10 (30.06 pg/mL) and IFN-γ (50.69 pg/mL) (Figure 1), improved liver function (ALT 257 U/L, AST 393 U/L, TBIL 79.91 mmol/L, Fib 1.45 g/L) (Figure 3), and increased platelet counts were all evident. She was discharged on the 40th day after admission owing to her good recovery status. The patient underwent an additional 30 d of chemotherapeutic treatment without significant adverse events. Furthermore, no disease recurrence was evident as of 8 mo post-discharge.
HLH is an often fatal disease, and affected patients typically present with high-grade fever, progressive hypocytosis, liver dysfunction, and coagulopathy[9]. In our case, the patient presented with a persistent fever that had been present for more than 1 wk, hypocytosis, hypofibrinogenemia, splenomegaly, hyperferritinemia, and lymphohistiocytic accumulation in the bone marrow. HLH was thus diagnosed in this child. The most common treatment for HLH at present is chemotherapy[9]. However, when patients also present with serious organ damage, chemotherapy may not work, as the existence of severe multiorgan failure at presentation can lead to a high mortality rate[10]. At admission, our patient was in poor condition and presented with coagulation disorders, liver damage, and respiratory insufficiency. In such a context, cytokine adsorption has the potential to bridge the patient until chemotherapy can contribute to reduced HLH disease activity[5].
The primary pathophysiological characteristics of HLH include excessive cytotoxic T cell and macrophage activation and expansion. These activated immune cells, in turn, produce excessively high levels of inflammatory cytokines, including IL-1, IL-2, IL-6, IL-10, TNF-α, and IFN-γ, which can promote further cytotoxic T cell and macrophage activation and expansion, thereby exacerbating the ongoing cytokine storm and driving consequent multiple organ failure[2]. On admission, patients present with increased circulating levels of certain inflammatory cytokines, such as IL-6, IL-8, IL-10, and IFN-γ, suggesting an ongoing systemic inflammatory reaction. HA330-II is a neutral microporous resin column with abundant micropores and a high specific surface area[11]. HA330-II cartridges have the ability to adsorb medium and large-sized inflammatory cytokines, including those of the IL and TNF families. In the present case, initially elevated IL-6, IL-8, IL-10, and IFN-γ levels were reduced to within normal ranges following hemoadsorption with HA330-II. This indicated that this column was able to reduce effectively inflammatory cytokines levels in our treated patient. However, this approach was unable to remediate effectively HLH-related liver failure in this patient, whereas hemoadsorption combined with chemotherapy was found to be more effective than hemoadsorption alone.
With respect to hemoadsorption, the CytoSorbTM column or endotoxin-binding polymyxin B-immobilized fiber column hemoadsorption approaches have also been reported for the treatment of HLH[12,13]. Cytokine adsorption associated with the CytoSorbTM column results in an improvement in the condition of treated patients, thus aiding in achieving symptom relief in affected individuals. Polymyxin B-immobilized fiber columns have also contributed to the recovery of circulatory dynamics associated with HLH. Several recent studies have demonstrated that HA330-II hemoadsorption plays a critical part in the elimination of inflammatory cytokines[14,15]. To the best of our knowledge, this is the first report to describe the clinical application of an HA330-II perfusion column in children suffering from HLH. However, as this is a report of outcomes for a single patient, large-scale trials will be necessary to investigate the clinical indications for such hemoadsorption treatment.
In summary, for EBV-associated HLH, HA330-II column-mediated hemoadsorption can safely reduce the levels of circulating inflammatory cytokines, serving as a beneficial and essential supplement to chemotherapy.
We are grateful to the reviewers and editor for their constructive comments.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bendari M, Navarrete Arellano M S-Editor: Guo XR L-Editor: Filipodia P-Editor: Cai YX
| 1. | Skinner J, Yankey B, Shelton BK. Hemophagocytic Lymphohistiocytosis. AACN Adv Crit Care. 2019;30:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med. 2015;66:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 3. | Peng ZY, Carter MJ, Kellum JA. Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Crit Care Med. 2008;36:1573-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Xu X, Jia C, Luo S, Li Y, Xiao F, Dai H, Wang C. Effect of HA330 resin-directed hemoadsorption on a porcine acute respiratory distress syndrome model. Ann Intensive Care. 2017;7:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Rademacher JG, Wulf G, Koziolek MJ, Zeisberg M, Wallbach M. Cytokine adsorption therapy in lymphoma-associated hemophagocytic lymphohistiocytosis and allogeneic stem cell transplantation. J Artif Organs. 2021;24:402-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Ishikawa Y, Nishizawa H, Kasuya T, Fujiwara M, Ono F, Kimura Y, Nanao T, Aoki M, Fujimoto J, Kita Y. Successful treatment of fatal macrophage activation syndrome and haemophagocytic lymphohistiocytosis by combination therapy including continuous haemodiafiltration with a cytokine-adsorbing haemofilter (AN69ST) in a patient with systemic lupus erythematosus. Mod Rheumatol Case Rep 2017; 2: 25-29. [DOI] [Full Text] |
| 7. | Zhong S, Wang N, Zhao J, Zhang L, Luo L, Zeng WQ, Shi XF, Wang ZY, Cai DC, Zhang DZ, Zhou Z, Hu P. [Plasma exchange combined with double plasma absorption therapy improve the prognosis of acute-on-chronic liver failure]. Zhonghua Gan Zang Bing Za Zhi. 2018;26:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Yang CF, Zhang Z, Zhang XY, Li YM. Artificial liver support system in pediatric acute liver failure due to mushroom poisoning: Case series. Ann Hepatol. 2021;23:100290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3599] [Article Influence: 199.9] [Reference Citation Analysis (1)] |
| 10. | Johnson B, Giri S, Nunnery SE, Wiedower E, Jamy O, Yaghmour G, Chandler JC, Martin MG. Comorbidities Drive Outcomes for Both Malignancy-Associated and Non-Malignancy-Associated Hemophagocytic Syndrome. Clin Lymphoma Myeloma Leuk. 2016;16:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Chen J, Han W, Chen J, Zong W, Wang W, Wang Y, Cheng G, Li C, Ou L, Yu Y. High performance of a unique mesoporous polystyrene-based adsorbent for blood purification. Regen Biomater. 2017;4:31-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Greil C, Roether F, La Rosée P, Grimbacher B, Duerschmied D, Warnatz K. Rescue of Cytokine Storm Due to HLH by Hemoadsorption in a CTLA4-Deficient Patient. J Clin Immunol 2017; 37: 273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Tatara R, Sato M, Fujiwara S, Oh I, Muroi K, Ozawa K, Nagai T. Hemoperfusion for Hodgkin lymphoma-associated hemophagocytic lymphohistiocytosis. Intern Med. 2014;53:2365-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Chen G, Wu M, Wu B, Liu F, Liu J, Liu L. Effects of dual plasma molecular adsorption system on liver function, electrolytes, inflammation, and immunity in patients with chronic severe hepatitis. J Clin Lab Anal. 2019;33:e22926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Wan YM, Li YH, Xu ZY, Yang J, Yang LH, Xu Y, Yang JH. Therapeutic plasma exchange versus double plasma molecular absorption system in hepatitis B virus-infected acute-on-chronic liver failure treated by entercavir: A prospective study. J Clin Apher. 2017;32:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |