Peer-review started: September 1, 2016
First decision: October 26, 2016
Revised: November 18, 2016
Accepted: December 16, 2016
Article in press: December 19, 2016
Published online: February 6, 2017
Processing time: 142 Days and 23.6 Hours
Disseminated intravascular coagulation (DIC) is a syndrome characterized by the systemic activation of blood clotting, which generates large amount of intravascular thrombin and fibrin. Various diseases may cause acceleration of the clotting cascade, inactivate the endogenous anticoagulants and modify fibrinolysis, having thus the formation of micro thrombi in the systemic circulation. The abnormalities in the hemostatic system in patients with DIC result from the sum of pathways that generate both hypercoagulability and augmented fibrinolysis. When the hypercoagulability state prevails, the main manifestation is organic failure. This subtype of DIC is often referred as “organ impairment” type, frequently seen in patients suffering from severe sepsis. To identify the underlying infection, early initiation of culture-based antimicrobial treatment, and to resolve any infection source promptly are keystone actions of DIC related to sepsis prevention and treatment. These should be combined with specific treatment related to each DIC subtype. In the context of septic shock, DIC is associated to increased severity, greater number and seriousness of organ failures, more frequent side-effects from treatment itself, and worse outcomes. Therefore, we ought to review the information available in the literature about approach and management of DIC in severe sepsis.
Core tip: Disseminated intravascular coagulation (DIC) is a syndrome characterized by the systemic activation of blood clotting, which generates large amount of intravascular thrombin and fibrin. In the context of severe sepsis and septic shock, DIC is related to increased severity, greater number and seriousness of organ failures, more frequent side-effects from treatment itself, and worse outcomes. We ought to review the most important and updated information available in the literature about DIC in severe sepsis and septic shock.
- Citation: Zaragoza JJ, Espinoza-Villafuerte MV. Current approach to disseminated intravascular coagulation related to sepsis - organ failure type. World J Hematol 2017; 6(1): 11-16
- URL: https://www.wjgnet.com/2218-6204/full/v6/i1/11.htm
- DOI: https://dx.doi.org/10.5315/wjh.v6.i1.11
Disseminated intravascular coagulation (DIC) is a syndrome characterized by the systemic activation of blood clotting that generates a large amount of intravascular thrombin and fibrin. This process results in small and medium vessel thrombosis and, eventually, organ failure and severe hemorrhage[1,2]. DIC could be the consequence of infections, hematologic malignancy, obstetric complications, trauma, aneurisms or hepatopathy. Each etiology signifies individual hazards related to the underlying disorder. Therefore, the diagnosis and treatment should be dictated by the disease[3,4].
In the context of septic shock, DIC is related to increased severity, number and seriousness of organ failures, more frequent side-effects from treatment itself, and worse outcomes, including death[5,6]. Therefore, we ought to review the most important and updated information available in the literature about DIC in severe sepsis and septic shock setting.
Hemostasis is an organized process that aids to maintain vascular integrity. In the presence of endovascular damage, thrombin generation with simultaneous negative feedbacks and coordination of fibrinolysis occur, to avoid massive hemorrhage or excessive thrombosis. The first step in hemostasis is the formation of a platelet plug over the damaged zone[7]. On the surface of platelets, Integrins interact with each other and with endothelial cells surface through the von Willebrand factor and fibrinogen. Nevertheless, the formation of a platelet plug is not enough to achieve stable hemostasis, given that the contribution of a fibrin mesh to stabilize the structure of the clot is needed.
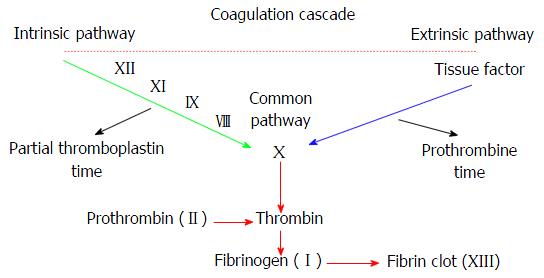
Physiologic clotting initiates with tissue factor (TF) and activated Factor VII (FVII) complexes that cleave Factor X (FX) into activated FX. This initial step has a short duration, due to quick inhibition of TF-aFVVII complexes by the tissue factor inhibitor. The second pathway starts with Factor IX (FIX) that cracks into activated FIXand joins activated FVIII to transform FX into activated FX. Activated FX forms a complex with activated Factor V (FV), with both phospholipids on platelet surface and calcium to turn prothrombin into thrombin[8]. Subsequently, thrombin turns fibrinogen into fibrin. At that time, activated Factor XIII (FXIII) forms crossbred fibrin connections inside the clot, which serve as an additional support. Finally, fibrin clots are degraded by a protease called plasmin (Figure 1)[8].
Any alteration in hemostasis balance could generate hemorrhage or thrombosis[8]. In critically ill patients, this alteration is usually associated with sepsis, malignancy, and multiple trauma. These diseases usually accelerate the clotting cascade, inactivate endogenous anticoagulants, and modify fibrinolysis, resulting in micro thrombi formation in the systemic circulation[3].
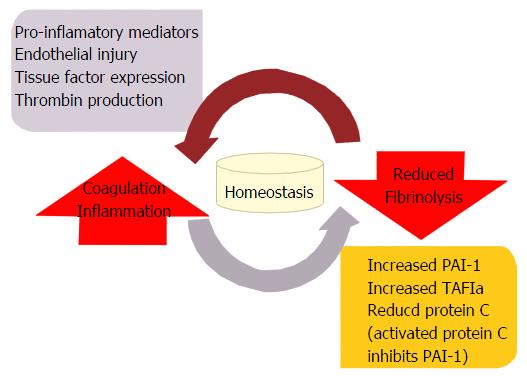
The abnormalities in the hemostatic system in patients with DIC result from either hypercoagulability or hyper-fibrinolysis[8] (Figure 2). When hypercoagulability prevails, the main clinical manifestation is organ failure. This type of DIC is referred as organ impairment type (both hypercoagulability and/or hypo-fibrinolysis exist)[9]. Organ impairment or organ failure DIC subtype is often seen in patients with severe sepsis. The activation of the coagulation cascade is an important part of the defense mechanisms to prevent infection dissemination. The increase in serum plasminogen activator inhibitor type 1 (PAI-1) caused by high levels of cytokines and lipopolysaccharides (LPS) in the blood of septic patients has been identified as one of the causes of hypo-fibrinolysis. Moreover, activated neutrophils in patients with sepsis liberate histones, neutrophil elastase and Catepsin G as a defense mechanism against pathogens[10]. Histones promote endothelial cell apoptosis, and platelet aggregation; meanwhile, neutrophil elastase inhibit Antithrombin (AT) and the Catepsin G decrease levels of the tissue factor pathway inhibitor (TFPI) promoting thrombus formation[10,11].
Endotoxin LPS are a component of the external membrane of gram negative bacteria, responsible of many of the cases of sepsis[12]. The entrance of endotoxin into systemic circulation causes the production of pro inflammatory cytokines. The consequent tissue damage is aggravated through free radicals generated by activated leucocytes. This causes an imbalance in normal hemostasis with the ulterior formation of thrombi in small and medium blood vessels that promote loss of vascular tone. All of this mechanisms contribute to the development of multiple organ failure[11-13].
Tumor necrosis factor: Tumor necrosis factor alpha (TNF-α) is synthesized in macrophages, and it is amongst the first cytokines to appear when endotoxin reaches blood circulation. It grasps it maximum concentration at 90 min from stimuli; then, it gradually disappears despite if the toxic stimulus remains. TNF-αhas an important role initiating the inflammatory cytokine cascade and tissue damage. It has effects over monocytes, neutrophils, and vascular endothelium causing the production of other pro inflammatory interleukins (1b, 6 or 8). Furthermore, it stimulates the production of adhesion molecules such as Intercellular Adhesion Molecule-1, vascular cell adhesion molecule-1 or E-Selectin.
Interleukin 1b: When the LPS enter the bloodstream, one can detect interleukin 1b (IL-1b) in plasma, and its presence serves as a severity marker. Patients with septic shock have high levels of IL-1b. It has been shown that the administration of this protein in primates induces a reduced fibrinolytic response equivalent to the one obtained with LPS or TNF-α. This suggests that IL-1b contributes to hypo-fibrinolysis mediated by PAI-1 in the presence of endo-toxemia[14,15].
IL-6: Endothelial cells synthesize IL-6 in presence of LPS. It also appears in the general circulation just after TNA-α shows up. IL-6 has a pathophysiologic role during sepsis as a clotting activator, and its concentration correlates with the disease severity[14,15].
Other cytokines: Other molecules participate in the inflammatory process in presence of the LPS: IL-12, IL-8, and interferon-γ. Nevertheless, their role in DIC is not yet well defined[15].
At the bedside, is necessary to consider the clinical conditions that could alter the commonly used laboratory tests to diagnose DIC. Ergo, the diagnosis requires clinical expertise along biochemical workshop. The recurrently used test that might be affected include platelet count, prothrombin time (PT), fibrinogen, and fibrin degradation products (FDP), among others. Some clinical guidelines issued recommendations regarding this aspect[1,16,17]. In 2013 the International Society of Thrombosis and Hemostasis published recommendations for diagnosis and treatment of disseminated intravascular coagulation[18]. This guidance was based on a previous consensus by the British Committee for Standards in Hematology, the Japanese Society of Thrombosis and Hemostasis, and the Italian Society for Hemostasis and Thrombosis (Società Italiana per lo Studio dell’Emostasi e della Trombosi - SISET). They stated that in sepsis related DIC the major variation is either hyper-coagulation or hypo-fibrinolysis. As mentioned above, the main clinical manifestation is organ failure, so several validated score systems to recognize DIC have been distributed using platelet count, prothrombin time, and anti-thrombin. The Japanese Association of Acute Medicine (JAAM) published a score system to detect sepsis related DIC, with a sensitivity and specificity of 100% and 65.0% respectively[5,19,20] (Table 1). Recently, Iba et al[21] proposed a modified version of the JAAM-DIC diagnostic criteria. They suggest to replace Systemic Inflammatory Response Syndrome (SIRS) by antithrombin activity, since SIRS is no longer used for the diagnosis of Sepsis. The new criteria could diagnose the same number of patients with comparable severity (mortality, 34.6% vs 34.8%). Also, mortality increased as the baseline antithrombin activity decreased (patients with a baseline antithrombin activity ≥ 70% had a mortality of 26.5% vs 35.5% for those with an antithrombin activity < 70%). Despite this promising results, future studies to examine the worth of the modified scoring system in different populations are warranted[21].
| Parameter | Points |
| SIRS criteria | |
| 3 or more | 1 |
| 2-0 | 0 |
| Platelet count (× 103/µL) | |
| < 80 or a reduction of > 50% in 24 h | 3 |
| 80-120 or a reduction of > 30% in 24 h | 1 |
| > 120 | 0 |
| Prothrombin time | |
| 1.2 times over control or higher | 1 |
| < 1.2 times over control | 0 |
| Fibrin degradation products/fibrinogen (mg/L) | |
| 25 or more | 3 |
| 10 to 24 | 1 |
| < 10 | 0 |
| Diagnosis DIC: 4 or more points |
A complete coagulation examination, including prothrombin time and platelet count is essential[4]. In some types of DIC (bleeding, massive hemorrhage, and asymptomatic) identifying the elevation of fibrin-associated biomarkers (D dimer, FDP, and soluble Fibrin) is useful to establish diagnosis[9]. Table 2 highlights the laboratory tests useful to diagnose DIC in a septic patient. It is important to consider that a coagulation disorder has around 35%-40% chance to be related to any other cause beside sepsis. A positive result does not guarantee the diagnosis. Delabranche et al[22] in 2016 published a multicenter, prospective observational study completed in 4 intensive care units in France. They used de JAAM score, sequential organ failure assessment score, and the acute physiology and chronic health evaluation II to identify patients with DIC at early stage. They concluded that a combination of PT, endothelium-derived CD105+-microparticles, and platelet count at admission could predict the absence of disseminated intravascular coagulation[22].
| Test | Alteration | Other causes |
| Platelet count | Reduction | Bone marrow abnormalities |
| Anti-thrombin/C protein | Reduction | Hepatic failure, capillary leakage syndrome |
| Prothrombin time | Extended | Hepatic failure, vitamin K deficiency |
| Soluble fibrin/thrombin | Increased | VTD, surgery |
| vWF-PP/PAI-1 | Increased | Organic failure |
| aPTT | Bifasic wave | Infection |
| ADAMTS-13 | Reduction | Hepatic failure, thrombotic microangiopathy |
| FDP/DD | Increased | VTD, surgery |
Liu et al[23] found four thrombin derived biomarkers that were triggered before PT, activated partial thromboplastin time (aPTT), or platelet count became altered. These markers include fibrinopeptide type A, soluble fibrin monomer complex, prothrombin fragment 1 + 2 (F1 + 2), and the thrombin-antithrombin complex. The F1 + 2 represents the total amount of fibrin produced, while the other three markers only show it partially. F1 + 2 is considered the most sensitive marker of thrombin production.
In the last few years the identification of endothelial damage markers and inflammatory cascade activators have made possible to find coincidences between the inflammation trigger mechanisms and coagulation. This extend the possibilities for future treatment targets[11].
To identify the underlying infection, early initiation of culture-based antimicrobial treatment, and to resolve any infection source promptly are keystone actions of DIC related to sepsis prevention and treatment. Table 3 lists key recommendations for the treatment of different types of CID.
| Dysfunction | Recommended treatment |
| Pre-DIC | Treat cause and |
| UFH 70 IU/kg per day or | |
| LWMH anti-Xa target: 0.8-1.2 | |
| Multiple organ failure | Treat cause and |
| AT 30 IU/kg per day of 3 d | |
| Hemorrhagic | Treat cause and |
| Hemo-transfusion | |
| Anti-fibrinolytics | |
| Protease synthetic inhibitor | |
| Massive hemorrhage | Treat cause and |
| Hemo-transfusion | |
| Anti-fibrinolytics | |
| Protease synthetic inhibitor |
The Surviving Sepsis Campaign guidelines[24], do not recommend treatment of any associated coagulopathy as for the lack of evidence to support it. Recently, Umemura et al[25] reported a meta-analysis of anticoagulation therapy in three different types of patients: (1) septic patients without coagulopathy; (2) patients with sepsis induced coagulopathy; and (3) patients with induced sepsis DIC. They identified that only septic induced DIC patients had a reduced mortality with no difference in the prevalence of hemorrhagic complications[25]. In septic patients, biomarkers of the homeostasis loss, such as histones (H3, H4), the TFPI, and the neutrophil extracellular traps are useful to determine whether to start treatment[26].
AT has proven to be effective to revert sepsis induced DIC. As mentioned above, when germs disseminate throughout the organism, a diffuse coagulopathy that results in massive thrombi formation in small and medium blood vessels occur[13]. The KybertSept trial[27] was the first to evaluate the effectiveness of AT substitution in patients with severe sepsis and septic shock. The results showed an increase in the incidence of bleeding complications related to AT use. It is important to reflect that some of their patients used heparin as deep vein thrombosis prophylaxis. A sub-analysis of patients without heparin prophylaxis showed a reduction of adverse effects in AT group[27]. Later on, Gando et al[28] showed that in patients with activated-AT levels of 50%-80%, the administration of AT at a dose of 30 UI/kg perday during 3 d improved platelet counts, and reduced the score punctuation for sepsis associated DIC without increasing bleeding events[28].
Antithrombin-III (AT-III) inactivates thrombin and other proteases, including FXa[29]. Heparin attaches to a AT-III producing a conformational change that increases AT-III activity. The unfractionated heparin (UFH) dose in Pre-DIC is 70 UI/kg per hour in continuous infusion for 5-7 d[23]. There are few randomized controlled trials evaluating the utility of heparin in DIC. Liu et al[23] shown that low molecular weight heparin was superior to UFH due to a higher inhibition of FXa[29]. The utility of other compounds like Fondaparinux and Danaparoid sodium is restricted to asymptomatic DIC for risk reduction of thrombotic events[9].
Because of coagulation factors (specially fibrinogen) and platelet consumption, most clinical guidelines[1,16,17] recommend blood components administration only in hemorrhagic and massive hemorrhage DIC. The recommended platelet goal count has been established at 50 × 103/μL if active bleeding or 20 × 103/μL along high risk of hemorrhage. If PT or aPTT are 1.5 times over the standard, or fibrinogen is below 1.5 g/dL, fresh frozen plasma (15 mL/kg) is indicated. If volume restriction is intended, a concentrate of prothrombin complex, cryoprecipitates, or purify fibrinogen concentrates are preferred[1,16,17].
Thrombomodulin may reduce massive thrombotic events caused by the expression of extracellular histones observed in sepsis DIC[26]. In the double blind controlled study, Vincent et al[30] administered human recombinant thrombomodulin to patients with sepsis induced DIC that developed one or more organ failures and an international normalized ratio > 1.4. The dose of 0.06 mg/kg per day for 6 d along with conventional treatment reduced the severity of hematologic failure and reduced DIC incidence. Further trials are needed to safely recommend the therapy.
In critically ill patients, the early diagnosis of coagulopathy is essential to reduce morbidity and mortality. Identification of sepsis related DIC is difficult, especially when precise laboratory tests are not available. Clinicians should suspect the diagnosis in every severe sepsis or septic shock patient, and use whatever tools accessible to investigate it. It is important to treat promptly even subtle changes linked to coagulopathy, to diminish the extent of DIC.
Manuscript source: Invited manuscript
Specialty type: Hematology
Country of origin: Mexico
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Donadello K, Martin-Barrasa JL, Stover CM S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Wada H, Asakura H, Okamoto K, Iba T, Uchiyama T, Kawasugi K, Koga S, Mayumi T, Koike K, Gando S. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb Res. 2010;125:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Dhainaut JF, Yan SB, Joyce DE, Pettilä V, Basson B, Brandt JT, Sundin DP, Levi M. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004;2:1924-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 287] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Hook KM, Abrams CS. The loss of homeostasis in hemostasis: new approaches in treating and understanding acute disseminated intravascular coagulation in critically ill patients. Clin Transl Sci. 2012;5:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Ishikura H, Nishida T, Murai A, Nakamura Y, Irie Y, Tanaka J, Umemura T. New diagnostic strategy for sepsis-induced disseminated intravascular coagulation: a prospective single-center observational study. Crit Care. 2014;18:R19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Gando S, Saitoh D, Ogura H, Fujishima S, Mayumi T, Araki T, Ikeda H, Kotani J, Kushimoto S, Miki Y. A multicenter, prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit Care. 2013;17:R111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 6. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17211] [Article Influence: 1912.3] [Reference Citation Analysis (2)] |
| 7. | Smith SA, Travers RJ, Morrissey JH. How it all starts: Initiation of the clotting cascade. Crit Rev Biochem Mol Biol. 2015;50:326-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 294] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 8. | Davizon P, Munday AD, López JA. Tissue factor, lipid rafts, and microparticles. Semin Thromb Hemost. 2010;36:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Levi M. Diagnosis and treatment of disseminated intravascular coagulation. Int J Lab Hematol. 2014;36:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 987] [Cited by in RCA: 1191] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 11. | Zeerleder S, Hack CE, Wuillemin WA. Disseminated intravascular coagulation in sepsis. Chest. 2005;128:2864-2875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Sun H. The interaction between pathogens and the host coagulation system. Physiology (Bethesda). 2006;21:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Lehr HA, Bittinger F, Kirkpatrick CJ. Microcirculatory dysfunction in sepsis: a pathogenetic basis for therapy? J Pathol. 2000;190:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Kinasewitz GT, Yan SB, Basson B, Comp P, Russell JA, Cariou A, Um SL, Utterback B, Laterre PF, Dhainaut JF. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569]. Crit Care. 2004;8:R82-R90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 231] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Iba T, Ito T, Maruyama I, Jilma B, Brenner T, Müller MC, Juffermans NP, Thachil J. Potential diagnostic markers for disseminated intravascular coagulation of sepsis. Blood Rev. 2016;30:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 690] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 17. | Di Nisio M, Baudo F, Cosmi B, D’Angelo A, De Gasperi A, Malato A, Schiavoni M, Squizzato A. Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res. 2012;129:e177-e184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Wada H, Thachil J, Di Nisio M, Mathew P, Kurosawa S, Gando S, Kim HK, Nielsen JD, Dempfle CE, Levi M. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 19. | Gando S, Saitoh D, Ogura H, Mayumi T, Koseki K, Ikeda T, Ishikura H, Iba T, Ueyama M, Eguchi Y. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Sawamura A, Hayakawa M, Gando S, Kubota N, Sugano M, Wada T, Katabami K. Application of the Japanese Association for Acute Medicine disseminated intravascular coagulation diagnostic criteria for patients at an early phase of trauma. Thromb Res. 2009;124:706-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Iba T, Di Nisio M, Thachil J, Wada H, Asakura H, Sato K, Kitamura N, Saitoh D. Revision of the Japanese Association for Acute Medicine (JAAM) disseminated intravascular coagulation (DIC) diagnostic criteria using antithrombin activity. Crit Care. 2016;20:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Delabranche X, Quenot JP, Lavigne T, Mercier E, François B, Severac F, Grunebaum L, Mehdi M, Zobairi F, Toti F. Early Detection of Disseminated Intravascular Coagulation During Septic Shock: A Multicenter Prospective Study. Crit Care Med. 2016;44:e930-e939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Liu XL, Wang XZ, Liu XX, Hao D, Jaladat Y, Lu F, Sun T, Lv CJ. Low-dose heparin as treatment for early disseminated intravascular coagulation during sepsis: A prospective clinical study. Exp Ther Med. 2014;7:604-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3146] [Cited by in RCA: 3171] [Article Influence: 264.3] [Reference Citation Analysis (0)] |
| 25. | Umemura Y, Yamakawa K, Ogura H, Yuhara H, Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2016;14:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 26. | Iba T, Gando S, Thachil J. Anticoagulant therapy for sepsis-associated disseminated intravascular coagulation: the view from Japan. J Thromb Haemost. 2014;12:1010-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, Chalupa P, Atherstone A, Pénzes I, Kübler A. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869-1878. [PubMed] |
| 28. | Gando S, Saitoh D, Ishikura H, Ueyama M, Otomo Y, Oda S, Kushimoto S, Tanjoh K, Mayumi T, Ikeda T. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care. 2013;17:R297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 29. | Robertson MS. Heparin: the cheap alternative for immunomodulation in sepsis? Crit Care Resusc. 2006;8:235-238. [PubMed] |
| 30. | Vincent JL, Ramesh MK, Ernest D, LaRosa SP, Pachl J, Aikawa N, Hoste E, Levy H, Hirman J, Levi M. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med. 2013;41:2069-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |