Revised: May 9, 2014
Accepted: June 18, 2014
Published online: August 6, 2014
Processing time: 225 Days and 0.3 Hours
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory syndrome that develops as a primary (familial/hereditary) or secondary (non-familial/hereditary) disease characterized in the majority of the cases by hereditary or acquired impaired cytotoxic T-cell (CTL) and natural killer responses. The molecular mechanisms underlying impaired immune homeostasis have been clarified, particularly for primary diseases. Familial HLH (familial hemophagocytic lymphohistiocytosis type 2-5, Chediak-Higashi syndrome, Griscelli syndrome type 2, Hermansky-Pudlak syndrome type 2) develops due to a defect in lytic granule exocytosis, impairment of (signaling lymphocytic activation molecule)-associated protein, which plays a key role in CTL activity [e.g., X-linked lymphoproliferative syndrome (XLP) 1], or impairment of X-linked inhibitor of apoptosis, a potent regulator of lymphocyte homeostasis (e.g., XLP2). The development of primary HLH is often triggered by infections, but not in all. Secondary HLH develops in association with infection, autoimmune diseases/rheumatological conditions and malignancy. The molecular mechanisms involved in secondary HLH cases remain unknown and the pathophysiology is not the same as primary HLH. For either primary or secondary HLH cases, immunosuppressive therapy should be given to control the hypercytokinemia with steroids, cyclosporine A, or intravenous immune globulin, and if primary HLH is diagnosed, immunochemotherapy with a regimen containing etoposide or anti-thymocyte globulin should be started. Thereafter, allogeneic hematopoietic stem-cell transplantation is recommended for primary HLH or secondary refractory disease (especially EBV-HLH).
Core tip: This review discusses the diagnostic criteria for hemophagocytic lymphohistiocytosis (HLH), the algorithms used to identify the underlying immune defects at the molecular level, and the optimal therapeutic approaches. For any HLH cases, a screening for primary HLH should be made following the diagnostic algorithm. During the process, immunosuppressive therapy should be started to control the hypercytokinemia with steroids, cyclosporine A, or intravenous immune globulin, and if primary HLH is confirmed, immunochemotherapy with a regimen containing etoposide or anti-thymocyte globulin should be given. Supportive measures to control hemorrhage/organ dysfunction are also required. In cases of primary HLH or secondary/refractory HLH, timely allogeneic hematopoietic stem cell transplantation is recommended.
- Citation: Imashuku S. Hemophagocytic lymphohistiocytosis: Recent progress in the pathogenesis, diagnosis and treatment. World J Hematol 2014; 3(3): 71-84
- URL: https://www.wjgnet.com/2218-6204/full/v3/i3/71.htm
- DOI: https://dx.doi.org/10.5315/wjh.v3.i3.71
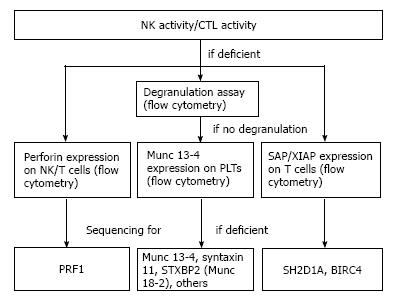
Hemophagocytic lymphohistiocytosis (HLH) is a primary (familial/hereditary) or secondary (non-familial/hereditary) hyperinflammatory and hypercytokinemic syndrome[1,2]. Immune homeostasis is maintained by regulating the proliferation and apoptosis of activated lymphocytes and their associated granule-dependent cytotoxic activity, which plays a critical role in defense against tumor cells and cells infected with viruses[3]. The hypercytokinemia conditions associated with HLH are caused by cytokine releases from activated T cells and macrophages as a result of impaired activation-induced cell death due to uncontrolled immune responses resulting from the impaired ability of cytotoxic T-cell (CTL) and natural killer (NK) cells to kill their target cells[4-6]. Thus, marked activation of macrophages occurs, which results in hypercytokinemia. Previous reviews of primary HLH identified a hereditary impairment in the molecules involved in the multistep processes of cytolysis (from cell activation to the release of perforin and granzymes)[7,8]. Indeed, primary HLH is caused by loss-of-function mutations in the genes encoding perforin and various molecules involved in the transport, fusion and exocytosis of secretory vesicles[4-8]. In other forms of HLH, particularly Epstein-Barr virus (EBV)-driven primary HLH, deficiency in SAP (which is important for CTL function) is responsible for the marked reduction in CTL and NK cell activity; however, in XIAP (which is important for apoptosis), CTL and NK cell activity are not altered[9-11]. Secondary HLH develops in apparently immunocompetent subjects; however, some of these subjects show acquired functional reductions in CTL and NK cell activity, which are associated with viral, bacterial or parasite infections, metabolic diseases, autoimmune diseases/rheumatological conditions (termed as macrophage activation syndrome; MAS), or malignancy[12-19]. Initial diagnostic work-up in the diagnosis of HLH includes the detection of the expansion of CD8+ T cell subset in the peripheral blood[20,21] and the identification of the factors that trigger the development of HLH, particularly infectious agents[22,23]. In addition, for all HLH cases, molecular screening must be performed to determine whether the disease is primary or secondary[6,24,25]. The assay of NK or CTL activity[26,27] and flow cytometric analysis of molecules, such as perforin, Munc 13-4, SAP/XIAP, is essential for rapid diagnosis[6,24,25,28] (Figure 1). Age-related factors were emphasized in the past because primary HLH usually develops during the first 3 years of life; however, more recently, late-onset cases have been identified[29-33]. The diversity of clinical features associated with primary HLH has been examined using detailed genotype-phenotype analysis methods. Among infectious agents, EBV plays a major role in cases of infection-associated primary or secondary HLH. Thus, quantification of cell-free or peripheral mononuclear cell EBV-DNA levels is extremely useful for the diagnosis of EBV-HLH and chronic active EBV infection (CAEBV)-related HLH[34-36]. The HLH-94 or HLH-2004 regimens employed in many centers has led to a significant improvement in the therapeutic results due to the efficacy of a combination of immunochemotherapy and allogeneic HSCT for treating primary and secondary HLH, especially for refractory EBV-HLH[37-39]; however, the diversity of the clinical features associated with primary HLH raises questions regarding the appropriate timing of HSCT[40-48].
The initial symptoms of HLH include persistent fever, hepatic and/or renal dysfunction, splenomegaly, hemorrhagic diathesis, neurological symptoms, and other features, caused by hyperinflammatory conditions[1,2,38,49-52]. The clinical features of primary and secondary cases are not significantly different; however, some types of primary HLH are associated with hypogammaglobulinemia-related symptoms (e.g., FHL5 and XLP1)[45,53], enteropathy and renal tubular dysfunction due to the epithelial abnormalities in FHL5[54] and oculocutaneous albinism in patients with Griscelli syndrome type 2 (GS-2), Chediak-Higashi syndrome (CHS), and Hermansky-Pudlak syndrome type 2 (HPS-II)[55-59], although occurrence of HLH in HPS-II deficiency is limited to a single case[58]. The most ominous findings in cases of HLH are central nervous system (CNS) disease[60-62] or an association with primary or therapy-related hematological malignancies[63-65]. The HLH conditions do not show the same severity, which is determined by the type of NK deficiency[26,27] in association with the type of genetic mutations in the primary HLH[42-48], or the degree of lymphoproliferation as represented by serum soluble IL-2R levels in the secondary HLH[66]. HLH occurs in all age groups, from premature infants and neonates to the elderly, but the majority of primary HLH cases occur in early infancy. For cases occurring during the fetal and neonatal periods[67,68], pre- or post-natal molecular diagnosis is essential[69]. Primary HLH can also develop in adolescents and adults[29-33]. Thus, especially in this older age group, a molecular diagnosis is recommended to enable a definite diagnosis of primary or secondary HLH.
The most common “trigger” for HLH is infectious disease. Viral and other types of infection cause secondary HLH[13,14,22,23,49,50]. Among them, EBV-HLH and CAEBV-related HLH, which are defined by the specific diagnostic criteria[34-36], are the most common form of secondary HLH; however, infection-induced HLH also occurs in individuals with primary HLH, MAS, and malignancy. Post-organ transplant-HLH, or post-HSCT-HLH, is a distinct subtype of secondary HLH that was described recently[70-72]. Among the various malignancies, lymphoma-associated HLH (LAHS) is the most common[73-75]. Progress in molecular diagnostic techniques has led to the identification of molecular abnormality of primary HLH in cases of secondary HLH[76,77] as well as in hematological malignancies[63,64,78-80]. In addition, genotype-phenotype correlations have been identified in patients with primary HLH, particularly those associated with FHL2, FHL3, and FHL5[42-46] and with XLP[53,81-83]. It is these types of studies that identified the existence of atypical late-developing primary HLH cases in adolescents and adults[29-33], and the identification of which raises questions about how promptly HSCT should be introduced.
The cardinal laboratory features associated with HLH include bicytopenia, high levels of serum ferritin, triglyceride, transaminases, lactate dehydrogenase and soluble IL-2R. Serum creatinine and BUN levels are often elevated, while plasma fibrinogen is decreased. Deficient NK activity has generally been noted[12,14-17,26,27]. Hemophagocytosis is observed on bone marrow smears or in lymph node or liver biopsies; however, the detection of hemophagocytes is not mandatory for the diagnosis. Although detection of abnormal karyotypes in bone marrow cells is rare in patients with HLH, they are occasionally detectable in cases of EBV-HLH, correlated with CAEBV[84]. Immunopathological features in HLH are characterized by uncontrolled activation of T cells, especially a significant increase in the subpopulation of CD8+ T cells with clonal expansion[20,21] and macrophages in association with overproduction of various cytokines[85,86].
The molecular defects associated with primary HLH are listed in Table 1. Molecular abnormalities have been identified in the perforin-granzyme cytotoxic molecule pathway (in FHL type 2-5, GS-2, CHS, HPS-II), T-cell activation pathway (in XLP1), the apoptotic pathway (in XLP2), and the inducible T-cell kinase pathway (in ITK deficiency)[11,87-100]. More recently, CD27 deficiency[101,102] and magnesium transporter 1 (MAGT1) deficiency, also termed as XMEN (X-linked immunodeficiency with Mg2+ defect, EBV infection and neoplasm)[103], were identified. These EBV-driven ITK, or CD27 deficiency and XMEN give rise to EBV- associated lymphoproliferative disease (LPD), but does not primarily predispose to HLH; although hemophagocytosis was described in some of the cases[100,102]. These novel discoveries are expected to help elucidate the molecular mechanisms causing the inherited forms of EBV-LPD and HLH.
| Disease | Molecular abnormalities(chromosome location) |
| CTL molecule dysfunction | |
| Pore formation | |
| FHL2 | Perforin (10q21-2) |
| Vesicle priming fusion | |
| FHL3 | Munc13-4/Unc 13D (17q25) |
| FHL4 | Syntaxin 11 (6q24) |
| FHL5 | STXBP2/Munc18-2 (19p13) |
| Vesicle docking/trafficking | |
| Chediak-Higashi syndrome | LYST (1q42.1-42.2) |
| Griscelli syndrome, type 2 | Rab27a (15q21) |
| Hermansky-Pudlak syndrome II | AP-3 (3q24) |
| EBV-driven | |
| XLP1 | SAP/SH2D1A (Xq25) |
| XLP2 (XIAP) | BIRC4 (Xq24-25) |
| ITK deficiency1 | ITK (5q34) |
| CD27 deficiency1 | CD27 (12p13) |
| XMEN1 | MAGT1 (Xq21.1) |
Achieving the definitive diagnosis of HLH is often challenging[104,105]. Currently, HLH is diagnosed according to globally accepted diagnostic criteria shown in Table 2[38]. Differential diagnoses include fulminant hepatitis or acute hepatic failure[106], severe sepsis, systemic inflammatory response syndrome, and other hyperinflammatory conditions[107]. In the differentiation of primary and secondary HLH, screening measures are employed, which include NK and CTL activity determination, degranulation assays as well as flow cytometric assay of the expression of perforin and other molecules (Figure 1)[6,24-27]. More recently, Western blot analysis was found to be useful to screen for primary HLH by detecting FHL-related proteins in platelets[28]. An accurate diagnosis is made by performing mutation analysis of the genes responsible for these hereditary diseases. EBV-HLH is diagnosed using a combination of HLH diagnostic criteria and EBV-specific data (i.e., the number of EBV-DNA copies and antibody expression patterns in the serum)[34-36]. Although the majority of EBV-HLH cases in Asia are thought to be secondary HLH, molecular and genetic analyses need to be performed to determine whether they are in fact primary HLH, particularly in patients with refractory EBV-HLH[6,44,81-83,99-103]. In Europe, some patients with FHL3 or FHL5 presented with clinical features suggestive of CAEBV-related HLH[44]. Also, since the risk of malignancy is high in the condition of CTL dysfunction, patients presenting with hematological malignancies could be searched for primary HLH-related gene mutations[78-80].
| The diagnosis of HLH can be established if one of either (1) or (2) below is fulfilled | |
| (1) A molecular diagnosis consistent with HLH | |
| (2) Clinical diagnostic criteria fulfilled for 5 out of the 8 criteria below | |
| Clinical criteria | 1 fever |
| 2 splenomegaly | |
| Routine laboratory criteria | 3 bicytopenia (Hb < 90 g/L, |
| platelets < 100 × 109/L, neutrophils < 1.0 × 109/L) | |
| 4 Hypertriglyceridemia (> 3.0 mmol/L) | |
| and/or hypofibrinogenemia (< 1.5 g/L) | |
| Specific histopathological/marker criteria | 5 hemophagocytosis |
| 6 low or absent NK cell activity | |
| 7 hyperferritinemia (> 500 μg/L) | |
| 8 hyper-sIL-2R-nemia (> 2400 U/mL) | |
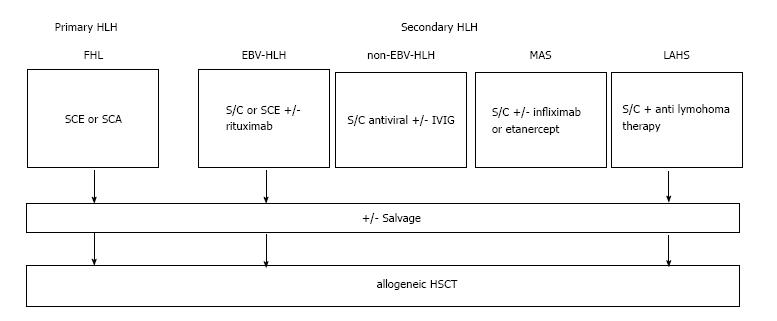
The ultimate treatment goal of HLH is to have disease-free survival without CNS sequelae and treatment-related acute myeloid leukemia (t-AML). The outcome of HLH depends on the severity of clinical features at the onset and types of HLH (primary or secondary). In particular, primary HLH, refractory EBV-HLH and LAHS without treatment have a poor outcome. In principle, primary HLH cases are fatal if HSCT is not performed[108]. In refractory secondary HLH, immunotherapy may not be curative, when the patients require both salvage chemotherapy and HSCT (Figure 2). Preferably, it is essential to perform HSCT before the development of CNS disease or of t-AML. In the prognostic analysis of HLH, it was found that after initial treatment, death during the acute phase occurs in 10%-15% of patients, usually due to life-threatening infections, hemorrhage, and/or irreversible organ dysfunction[37,109,110]. Death at the later stages of treatment is often due to reactivation of the disease and adverse effects associated with HSCT[109,110]. These data indicate the requirement of improved outcome of HSCT in the treatment of HLH. Although late onset cases of primary HLH are believed to carry a better prognosis, there is a report that adolescents and young adults with HLH who undergo allogeneic HSCT are at increased risk of mortality compared to younger patients[111]. The factors suggestive of a poor prognosis for those with EBV-HLH are summarized in Table 3.
| Persistent increase of cell-free EBV genome copies |
| Chromosome abnormality |
| Correlation with chronic active EBV infection (CAEBV)1 |
| In association with primary HLH |
| Severe organ dysfunction, such as renal failure, CNS hemorrhage |
| Choice of treatment, such as timing of etoposide use, HSCT |
Any patients with HLH can be treated first with immunosuppressive regimens designed to control the hypercytokinemia and hyperinflammation. Such treatments include steroids (prednisolone or dexamethasone), cyclosporine A (CSA), or intravenous immune globulin (IVIG). During the initial period of therapy, finding out the triggering factors and underlying diseases as well as molecular diagnostic analyses are recommended to determine familial or non-familial diseases (Figure 1). If confirmed, primary HLH is similarly treatable with HLH-directed immunochemotherapy[6,37-41,112-116]. On the other hand, if apparent infection-triggered HLH is confirmed, rigorous treatment of any identified infectious agents is important. For any secondary HLH, application of treatment should aim to target the underlying diseases. Patients with very severe cases of HLH requiring hemodynamic and respiratory support are treated in the intensive care unit. Inotropic agents are life-saving for those that are hemodynamically unstable[117,118]. Antibacterial or antifungal agents are also required to treat opportunistic infections due to HLH-related neutropenia. Because severe thrombocytopenia and coagulopathy are both life-threatening conditions, the patient may require infusions of concentrated platelets, fresh frozen plasma, fibrinogen, and recombinant thrombomodulin[49,51,119]. Although there is no definite consensus on its benefit, plasma exchange or exchange transfusion may be used to treat the hypercytokinemia and reduce the hemorrhagic tendency during the initial treatment phase[120,121]. The addition of acyclovir to the therapeutic regimen for those with EBV-HLH is not thought to be beneficial because there is no objective evidence showing a clinical improvement using this drug[122]. However, acyclovir is useful for treating neonatal herpes simplex virus (HSV)-HLH in infancy[49,123,124]. Indeed, a combination of high-dose acyclovir, steroid pulse therapy, IVIG, and blood transfusion has proved successful for treating neonatal HSV-HLH[125]. In patients with mycobacterium tuberculosis-associated HLH, early diagnostic confirmation and the timely administration of antituberculous medication is crucial for an improved outcome[126]. For patients with leishmania-related HLH, amphotericin B was shown to be effective[127]; however, for those with human immunodeficiency virus-related HLH, outcome remains poor even in the era of highly active antiretroviral therapy[128].
The efficacy of intrathecal chemotherapy for treating CNS disease in HLH patients has not been sufficiently evaluated. At present, the outcome for HLH patients with CNS disease is poor, even when treated with a combination of systemic immunochemotherapy and intrathecal chemotherapy[60-62]. The HLH-94 study tested the ability of a high systemic dose of dexamethasone to prevent the development of CNS disease. In addition, the study examined the use of intrathecal methotrexate in patients showing neurological symptoms at the time of disease onset; however, neither treatment appeared to prevent the exacerbation of CNS disease[37,62]. Data show that at the time of HLH diagnosis, neurological symptoms were already present in 37% of patients, and abnormal findings regarding the CSF were made in 52%; in all, 63% of patients had either neurological symptoms or abnormal CSF findings. CNS sequelae were more common in the latter group and, consistent with this, a substantial proportion of HLH survivors suffer neurological sequelae. Thus, early diagnosis of HLH and an evaluation of the CNS status including the CSF, coupled with early systemic HLH therapy, is crucial; in addition, the timely use of HSCT should be considered if reactivation of HLH with or without CNS disease is suspected to develop or to be exacerbated during the treatment[37,62,129]. However, since childhood survivors of HLH even after HSCT are shown to be at risk of long-term cognitive and psychosocial difficulties[130], prospective and systematic long-term follow-up of neurological function in these post-HSCT patients is essential.
In the past, IVIG was used to treat various types of HLH[49,131,132]; however, this form of treatment seems best suited to enterovirus-, hepatitis-, cytomegalovirus-, or bacteria-associated HLH[133-137]. Combined treatment with antibiotics and IVIG resulted in the full recovery of a patient with Group G streptococcal endocarditis-associated HLH[138]. Treatment with steroids alone can be effective for some HLH cases[139]. CSA quickly and efficiently suppresses the cytokines secreted by dysregulated T-cells and activated macrophages; indeed, CSA is able to control various cytokine-related pathological conditions[140,141]. Currently, the majority of HLH cases are treated first with a combination of steroids and CSA[49,140-142]. The prompt and continuous infusion of CSA (1-3 mg/kg per day over several days) is required to alleviate the cytokine “storm” as quickly as possible in patients with severe HLH, but without renal failure[49]. In addition, CSA treatment effectively supports neutrophil recovery especially in severely neutropenic Asian EBV-HLH patients during the acute phase[143].
The etoposide/steroid/CSA triple combination was used in the HLH-94 or HLH-2004 regimen, consisting of 8 wk of initial therapy followed by continuation therapy and allogeneic HSCT if required. This regimen is now used in many centers to treat HLH, which comprises of dexamethasone (starting dose, 10 mg/d, IV or PO, followed by tapering), CSA (dose adjusted to obtain trough levels of 200 μg/L, PO, daily), and etoposide (150 mg/m2, IV; a total of 10 doses during the initial 8 wk). This regimen has considerably improved the outcome for HLH patients: in the initial analysis comprising 113 patients, the 3-year survival rate was 45% (± 10%)[37]. The CNS outcomes in the patients treated with this regimen were published in 2008[62]. Long-term follow-up results for 227 patients were published in 2011, where the estimated 5-year survival rate was 54% ± 6%, and the 5-year survival rate for 124 patients who received HSCT was 66% ± 8%[109]. The same group also suggested that this regimen should be revised as HLH-2004[38]; however, the therapeutic results of HLH-2004 have not yet been published. The HLH-94 regimen was also found to be effective when used to treat secondary EBV-HLH[144-146]. On the other hand, along the usage of HLH-94-type HLH treatment, several cases of t-AML were reported[65]. A French group used an ATG/steroid/CSA combination to treat patients with primary HLH[116,147,148], and similar to the etoposide/steroid/CSA regimen, this combination was also followed by HSCT. The effectiveness of this treatment was first described in 1993[147], where the regimen comprised steroids (2-5 mg/kg per day methylprednisolone, IV, followed by tapering), rabbit ATG (5-10 mg/kg per day for 5 d), and CSA (4-6 mg/kg per day, PO, daily). The study results were published in 2007[148]. In 38 consecutive patients with use of 45 courses of ATG, this regimen resulted in a rapid and complete response in 73%, a partial response in 24%, and no response in only one patient. Subsequent HSCT, when performed early after a complete or partial response, led to a high cure rate of 16 out of 19 cases. Overall, 21 of the 38 patients survived and there were four toxicity-related deaths. The same group also published the HSCT results for 48 patients in 2006[116]. Unfortunately, no direct comparison is possible between the therapeutic results performed after the ATG-regimen or after the HLH-94-type regimen. A regimen comprising HIT (hybrid immunotherapy)-HLH, which uses a combination of ATG/etoposide in the initial treatment phase, is currently being tested (unpublished; Jordan M, Histiocyte Society Clinical Studies 2013).
Rituximab is an effective treatment for some cases of EBV-HLH and has been used as a form of pre-emptive B-cell-directed therapy in patients with XLP1-related EBV-HLH or other severe forms of EBV-HLH in which EBV resides within B cells[149-152]. More recently, Chellapandian et al[153] examined 42 EBV-HLH cases and found that a combination of rituximab and conventional immunochemotherapy improved patient symptoms and reduced both the viral load and the level of inflammation. In the past, rituximab was thought to be unsuitable as a treatment for Asian patients with EBV-HLH in which EBV resides in T-cells or NK cells; however, the inclusion of rituximab in the initial treatment regimen may be useful in such cases[154]. R-CHOP (a combination of rituximab, doxorubicin, vincristine, cyclophosphamide and prednisolone) as well as R-etoposide are an effective combination for treating EBV-LPD-associated HLH[152]. The combination of rituximab and CSA induced remission in one patient with EBV-HLH occurring in association with CHS[57]. In addition, intrathecal rituximab is an effective treatment for post-transplant EBV-positive CNS lesions[155,156]. Alemtuzumab is effective as a bridge to allogeneic HSCT in primary HLH patients undergoing salvage treatment[157]. Marsh et al[158] reported that of 22 patients who received alemtuzumab (median dose, 1 mg/kg; range, 0.1-8.9 mg/kg) over a median of 4 d (range, 2-10), 64% experienced a partial response within 2 weeks. Indeed, 77% survived and underwent allogeneic HSCT, where the adverse events, including cytomegalovirus and adenovirus viremia, were reported to be “acceptable”. Alemtuzumab has also been used to treat refractory MAS[159]. As other biological and experimental agents, the anti-CD25 antibody (daclizumab) was successfully used in a single adult patient with HLH[160] and the anti-TNF-α antibody (infliximab/etanercept) is an effective treatment for MAS[161-165]. Because IFN-γ plays a major role in the pathogenesis of HLH, a humanized anti-IFN-γ antibody, NI-0501(NovImmune), is currently being tested as a future treatment for the disease (unpublished; Arico M, Histiocyte Society Clinical Studies 2013), based on the murine model studies[166,167]. A study in XMEN patients showed that magnesium supplementation is an effective treatment because magnesium restores decreased intracellular free Mg2+ levels and corrects defective expression of NK activating receptor (NKG2D), while concurrently reducing the number of EBV-infected cells in vivo [103].
Patients with primary HLH and those with refractory secondary HLH are candidates for allogeneic HSCT[37-41,112-116,168]. Primary HLH cases with nonsense (disruptive) gene mutations such as premature stop codon, or sequence frameshift generally develop symptoms in early infancy, thus require early introduction of HSCT. In these cases, delayed HSCT may have a risk of reactivation of HLH, development of CNS disease or hematological malignancies. Those with missense (hypomorphic) mutations may often wait for transplantation until adolescence or young adulthood. In secondary HLH, HSCT is planned whenever the disease becomes refractory to immunochemotherapy. For HSCT, reduced intensity conditioning (RIC) rather than myeloablative conditioning (MAC) is the preferred regimen because it results in better patient survival; however, the RIC regimen may result in mixed donor chimerism during the post-transplant period[41]. Landman-Parker et al[169] showed that partial engraftment of donor bone marrow cells after HSCT is sufficient to obtain long-term remission in patients with primary HLH. Experimental transplantation of perforin-deficient mice showed that 10%-20% perforin-expressing cells, with either mixed hematopoietic or CD8 (+) T-cell chimerism, are sufficient to re-establish immune regulation[170]. These data suggest that stable levels of donor chimerism (> 10%) could maintain remission in the HLH patients after HSCT. Of the 40 HLH patients who underwent allogeneic HSCT between 2003 and 2009 in Cincinnati, 14 received MAC comprising busulfan, cyclophosphamide, and ATG plus or minus etoposide, while 26 patients received RIC comprising fludarabine, melphalan, and alemtuzumab. All patients engrafted successfully, and the overall estimated 3-year survival after HSCT was 43% for those receiving MAC and 92% for those receiving RIC (P = 0.0001)[41]. In Japan, 57 patients (43 with primary HLH and 14 with EBV-HLH) underwent HSCT between 1995 and 2005. Data show that EBV-HLH patients had a better prognosis after HSCT than primary HLH patients, also demonstrating that the RIC-conditioning regimen significantly improves the outcome of patients undergoing allogeneic HSCT[40].
Recent progress has been reviewed on how to understand the pathogenesis, how to diagnose and how to make treatment decisions in patients with HLH. Although the outcomes have significantly improved over the past decade, further refinement of treatment is required at the initial phase of the disease as well as pre- and post HSCT periods with special care for CNS disease in order to promise a cure with excellent quality of life in these patients with HLH.
P- Reviewer: Lozano ML S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med. 2012;63:233-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 402] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 2. | Chandrakasan S, Filipovich AH. Hemophagocytic lymphohistiocytosis: advances in pathophysiology, diagnosis, and treatment. J Pediatr. 2013;163:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Pachlopnik Schmid J, Côte M, Ménager MM, Burgess A, Nehme N, Ménasché G, Fischer A, de Saint Basile G. Inherited defects in lymphocyte cytotoxic activity. Immunol Rev. 2010;235:10-23. [PubMed] |
| 4. | Cetica V, Pende D, Griffiths GM, Aricò M. Molecular basis of familial hemophagocytic lymphohistiocytosis. Haematologica. 2010;95:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Gholam C, Grigoriadou S, Gilmour KC, Gaspar HB. Familial haemophagocytic lymphohistiocytosis: advances in the genetic basis, diagnosis and management. Clin Exp Immunol. 2011;163:271-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041-4052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 795] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 7. | de Saint Basile G, Ménasché G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol. 2010;10:568-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 330] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | de Saint Basile G, Ménasché G, Latour S. Inherited defects causing hemophagocytic lymphohistiocytic syndrome. Ann N Y Acad Sci. 2011;1246:64-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Dupré L, Andolfi G, Tangye SG, Clementi R, Locatelli F, Aricò M, Aiuti A, Roncarolo MG. SAP controls the cytolytic activity of CD8+ T cells against EBV-infected cells. Blood. 2005;105:4383-4389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Snow AL, Marsh RA, Krummey SM, Roehrs P, Young LR, Zhang K, van Hoff J, Dhar D, Nichols KE, Filipovich AH. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J Clin Invest. 2009;119:2976-2989. [PubMed] |
| 11. | Rigaud S, Fondanèche MC, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 527] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 12. | Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer cell activity in families of patients with systemic lupus erythematosus: demonstration of a killing defect in patients. Clin Exp Immunol. 2005;141:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Rajagopala S, Dutta U, Chandra KS, Bhatia P, Varma N, Kochhar R. Visceral leishmaniasis associated hemophagocytic lymphohistiocytosis--case report and systematic review. J Infect. 2008;56:381-388. [PubMed] |
| 14. | Bogdan C. Natural killer cells in experimental and human leishmaniasis. Front Cell Infect Microbiol. 2012;2:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Lee SJ, Cho YN, Kim TJ, Park SC, Park DJ, Jin HM, Lee SS, Kee SJ, Kim N, Yoo DH. Natural killer T cell deficiency in active adult-onset Still’s Disease: correlation of deficiency of natural killer T cells with dysfunction of natural killer cells. Arthritis Rheum. 2012;64:2868-2877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Villanueva J, Lee S, Giannini EH, Graham TB, Passo MH, Filipovich A, Grom AA. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther. 2005;7:R30-R37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Yoshida Y, Machigashira K, Suehara M, Arimura H, Moritoyo T, Nagamatsu K, Osame M. Immunological abnormality in patients with lysinuric protein intolerance. J Neurol Sci. 1995;134:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Georgeson GD, Szony BJ, Streitman K, Kovács A, Kovács L, László A. Natural killer cell cytotoxicity is deficient in newborns with sepsis and recurrent infections. Eur J Pediatr. 2001;160:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Mazodier K, Marin V, Novick D, Farnarier C, Robitail S, Schleinitz N, Veit V, Paul P, Rubinstein M, Dinarello CA. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106:3483-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Kasahara Y, Yachie A. Cell type specific infection of Epstein-Barr virus (EBV) in EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Crit Rev Oncol Hematol. 2002;44:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Wada T, Sakakibara Y, Nishimura R, Toma T, Ueno Y, Horita S, Tanaka T, Nishi M, Kato K, Yasumi T. Down-regulation of CD5 expression on activated CD8+ T cells in familial hemophagocytic lymphohistiocytosis with perforin gene mutations. Hum Immunol. 2013;74:1579-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Imashuku S. Differential diagnosis of hemophagocytic syndrome: underlying disorders and selection of the most effective treatment. Int J Hematol. 1997;66:135-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Ansuini V, Rigante D, Esposito S. Debate around infection-dependent hemophagocytic syndrome in paediatrics. BMC Infect Dis. 2013;13:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Aricó M, Moretta L, Pende D. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108:2316-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Bryceson YT, Pende D, Maul-Pavicic A, Gilmour KC, Ufheil H, Vraetz T, Chiang SC, Marcenaro S, Meazza R, Bondzio I. A prospective evaluation of degranulation assays in the rapid diagnosis of familial hemophagocytic syndromes. Blood. 2012;119:2754-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 26. | Schneider EM, Lorenz I, Müller-Rosenberger M, Steinbach G, Kron M, Janka-Schaub GE. Hemophagocytic lymphohistiocytosis is associated with deficiencies of cellular cytolysis but normal expression of transcripts relevant to killer-cell-induced apoptosis. Blood. 2002;100:2891-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Horne A, Zheng C, Lorenz I, Löfstedt M, Montgomery SM, Janka G, Henter JI, Marion Schneider E. Subtyping of natural killer cell cytotoxicity deficiencies in haemophagocytic lymphohistocytosis provides therapeutic guidance. Br J Haematol. 2005;129:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Murata Y, Yasumi T, Shirakawa R, Izawa K, Sakai H, Abe J, Tanaka N, Kawai T, Oshima K, Saito M. Rapid diagnosis of FHL3 by flow cytometric detection of intraplatelet Munc13-4 protein. Blood. 2011;118:1225-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Ueda I, Kurokawa Y, Koike K, Ito S, Sakata A, Matsumora T, Fukushima T, Morimoto A, Ishii E, Imashuku S. Late-onset cases of familial hemophagocytic lymphohistiocytosis with missense perforin gene mutations. Am J Hematol. 2007;82:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Nagafuji K, Nonami A, Kumano T, Kikushige Y, Yoshimoto G, Takenaka K, Shimoda K, Ohga S, Yasukawa M, Horiuchi H. Perforin gene mutations in adult-onset hemophagocytic lymphohistiocytosis. Haematologica. 2007;92:978-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Beaty AD, Weller C, Levy B, Vogler C, Ferguson WS, Bicknese A, Knutsen AP. A teenage boy with late onset hemophagocytic lymphohistiocytosis with predominant neurologic disease and perforin deficiency. Pediatr Blood Cancer. 2008;50:1070-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Sieni E, Cetica V, Piccin A, Gherlinzoni F, Sasso FC, Rabusin M, Attard L, Bosi A, Pende D, Moretta L. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7:e44649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Weisfeld-Adams JD, Mehta L, Rucker JC, Dembitzer FR, Szporn A, Lublin FD, Introne WJ, Bhambhani V, Chicka MC, Cho C. Atypical Chédiak-Higashi syndrome with attenuated phenotype: three adult siblings homozygous for a novel LYST deletion and with neurodegenerative disease. Orphanet J Rare Dis. 2013;8:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Imashuku S. Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol. 2002;44:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Kimura H, Morita M, Yabuta Y, Kuzushima K, Kato K, Kojima S, Matsuyama T, Morishima T. Quantitative analysis of Epstein-Barr virus load by using a real-time PCR assay. J Clin Microbiol. 1999;37:132-136. [PubMed] |
| 36. | Okano M, Kawa K, Kimura H, Yachie A, Wakiguchi H, Maeda A, Imai S, Ohga S, Kanegane H, Tsuchiya S. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Henter JI, Samuelsson-Horne A, Aricò M, Egeler RM, Elinder G, Filipovich AH, Gadner H, Imashuku S, Komp D, Ladisch S. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100:2367-2373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 618] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 38. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3593] [Article Influence: 199.6] [Reference Citation Analysis (1)] |
| 39. | Horne A, Janka G, Maarten Egeler R, Gadner H, Imashuku S, Ladisch S, Locatelli F, Montgomery SM, Webb D, Winiarski J. Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis. Br J Haematol. 2005;129:622-630. [PubMed] |
| 40. | Ohga S, Kudo K, Ishii E, Honjo S, Morimoto A, Osugi Y, Sawada A, Inoue M, Tabuchi K, Suzuki N. Hematopoietic stem cell transplantation for familial hemophagocytic lymphohistiocytosis and Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in Japan. Pediatr Blood Cancer. 2010;54:299-306. [PubMed] |
| 41. | Marsh RA, Vaughn G, Kim MO, Li D, Jodele S, Joshi S, Mehta PA, Davies SM, Jordan MB, Bleesing JJ. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood. 2010;116:5824-5831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 42. | Trizzino A, zur Stadt U, Ueda I, Risma K, Janka G, Ishii E, Beutel K, Sumegi J, Cannella S, Pende D. Genotype-phenotype study of familial haemophagocytic lymphohistiocytosis due to perforin mutations. J Med Genet. 2008;45:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Sieni E, Cetica V, Santoro A, Beutel K, Mastrodicasa E, Meeths M, Ciambotti B, Brugnolo F, zur Stadt U, Pende D. Genotype-phenotype study of familial haemophagocytic lymphohistiocytosis type 3. J Med Genet. 2011;48:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Rohr J, Beutel K, Maul-Pavicic A, Vraetz T, Thiel J, Warnatz K, Bondzio I, Gross-Wieltsch U, Schündeln M, Schütz B. Atypical familial hemophagocytic lymphohistiocytosis due to mutations in UNC13D and STXBP2 overlaps with primary immunodeficiency diseases. Haematologica. 2010;95:2080-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Pagel J, Beutel K, Lehmberg K, Koch F, Maul-Pavicic A, Rohlfs AK, Al-Jefri A, Beier R, Bomme Ousager L, Ehlert K. Distinct mutations in STXBP2 are associated with variable clinical presentations in patients with familial hemophagocytic lymphohistiocytosis type 5 (FHL5). Blood. 2012;119:6016-6024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 46. | Meeths M, Entesarian M, Al-Herz W, Chiang SC, Wood SM, Al-Ateeqi W, Almazan F, Boelens JJ, Hasle H, Ifversen M. Spectrum of clinical presentations in familial hemophagocytic lymphohistiocytosis type 5 patients with mutations in STXBP2. Blood. 2010;116:2635-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 47. | Zhang K, Jordan MB, Marsh RA, Johnson JA, Kissell D, Meller J, Villanueva J, Risma KA, Wei Q, Klein PS. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood. 2011;118:5794-5798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 48. | Sepulveda FE, Debeurme F, Ménasché G, Kurowska M, Côte M, Pachlopnik Schmid J, Fischer A, de Saint Basile G. Distinct severity of HLH in both human and murine mutants with complete loss of cytotoxic effector PRF1, RAB27A, and STX11. Blood. 2013;121:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Imashuku S. Advances in the management of hemophagocytic lymphohistiocytosis. Int J Hematol. 2000;72:1-11. [PubMed] |
| 50. | Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, Yamamoto K, Horiuchi H, Takada K, Ohshima K. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 51. | Baars JW, de Boer JP, Wagstaff J, Roem D, Eerenberg-Belmer AJ, Nauta J, Pinedo HM, Hack CE. Interleukin-2 induces activation of coagulation and fibrinolysis: resemblance to the changes seen during experimental endotoxaemia. Br J Haematol. 1992;82:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Nawathe PA, Ravindranath TM, Satwani P, Baird JS. Severe hemorrhagic coagulopathy with hemophagocytic lymphohistiocytosis secondary to Epstein-Barr virus-associated T-cell lymphoproliferative disorder. Pediatr Crit Care Med. 2013;14:e176-e181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Pachlopnik Schmid J, Canioni D, Moshous D, Touzot F, Mahlaoui N, Hauck F, Kanegane H, Lopez-Granados E, Mejstrikova E, Pellier I. Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency). Blood. 2011;117:1522-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 54. | Stepensky P, Bartram J, Barth TF, Lehmberg K, Walther P, Amann K, Philips AD, Beringer O, Zur Stadt U, Schulz A. Persistent defective membrane trafficking in epithelial cells of patients with familial hemophagocytic lymphohistiocytosis type 5 due to STXBP2/MUNC18-2 mutations. Pediatr Blood Cancer. 2013;60:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Meeths M, Bryceson YT, Rudd E, Zheng C, Wood SM, Ramme K, Beutel K, Hasle H, Heilmann C, Hultenby K. Clinical presentation of Griscelli syndrome type 2 and spectrum of RAB27A mutations. Pediatr Blood Cancer. 2010;54:563-572. [PubMed] |
| 56. | Trottestam H, Beutel K, Meeths M, Carlsen N, Heilmann C, Pasić S, Webb D, Hasle H, Henter JI. Treatment of the X-linked lymphoproliferative, Griscelli and Chédiak-Higashi syndromes by HLH directed therapy. Pediatr Blood Cancer. 2009;52:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Ogimi C, Tanaka R, Arai T, Kikuchi A, Hanada R, Oh-Ishi T. Rituximab and cyclosporine therapy for accelerated phase Chediak-Higashi syndrome. Pediatr Blood Cancer. 2011;57:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Enders A, Zieger B, Schwarz K, Yoshimi A, Speckmann C, Knoepfle EM, Kontny U, Müller C, Nurden A, Rohr J. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood. 2006;108:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 59. | Jessen B, Bode SF, Ammann S, Chakravorty S, Davies G, Diestelhorst J, Frei-Jones M, Gahl WA, Gochuico BR, Griese M. The risk of hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type 2. Blood. 2013;121:2943-2951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Haddad E, Sulis ML, Jabado N, Blanche S, Fischer A, Tardieu M. Frequency and severity of central nervous system lesions in hemophagocytic lymphohistiocytosis. Blood. 1997;89:794-800. [PubMed] |
| 61. | Imashuku S, Hyakuna N, Funabiki T, Ikuta K, Sako M, Iwai A, Fukushima T, Kataoka S, Yabe M, Muramatsu K. Low natural killer activity and central nervous system disease as a high-risk prognostic indicator in young patients with hemophagocytic lymphohistiocytosis. Cancer. 2002;94:3023-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Horne A, Trottestam H, Aricò M, Egeler RM, Filipovich AH, Gadner H, Imashuku S, Ladisch S, Webb D, Janka G. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol. 2008;140:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 63. | Clementi R, Locatelli F, Dupré L, Garaventa A, Emmi L, Bregni M, Cefalo G, Moretta A, Danesino C, Comis M. A proportion of patients with lymphoma may harbor mutations of the perforin gene. Blood. 2005;105:4424-4428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 64. | Trapani JA, Thia KY, Andrews M, Davis ID, Gedye C, Parente P, Svobodova S, Chia J, Browne K, Campbell IG. Human perforin mutations and susceptibility to multiple primary cancers. Oncoimmunology. 2013;2:e24185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Imashuku S. Etoposide-related secondary acute myeloid leukemia (t-AML) in hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Imashuku S, Hibi S, Sako M, Ishida Y, Mugishima H, Chen J, Tsunematsu Y. Soluble interleukin-2 receptor: a useful prognostic factor for patients with hemophagocytic lymphohistiocytosis. Blood. 1995;86:4706-4707. [PubMed] |
| 67. | Woods CW, Bradshaw WT, Woods AG. Hemophagocytic lymphohistiocytosis in the premature neonate. Adv Neonatal Care. 2009;9:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Bechara E, Dijoud F, de Saint Basile G, Bertrand Y, Pondarré C. Hemophagocytic lymphohistiocytosis with Munc13-4 mutation: a cause of recurrent fatal hydrops fetalis. Pediatrics. 2011;128:e251-e254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | zur Stadt U, Pruggmayer M, Jung H, Henter JI, Schneider M, Kabisch H, Janka G. Prenatal diagnosis of perforin gene mutations in familial hemophagocytic lymphohistiocytosis (FHLH). Prenat Diagn. 2002;22:80-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Soyama A, Eguchi S, Takatsuki M, Hidaka M, Tomonaga T, Yamanouchi K, Miyazaki K, Inokuma T, Tajima Y, Kanematsu T. Hemophagocytic syndrome after liver transplantation: report of two cases. Surg Today. 2011;41:1524-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Takagi S, Masuoka K, Uchida N, Ishiwata K, Araoka H, Tsuji M, Yamamoto H, Kato D, Matsuhashi Y, Kusumi E. High incidence of haemophagocytic syndrome following umbilical cord blood transplantation for adults. Br J Haematol. 2009;147:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Koyama M, Sawada A, Yasui M, Inoue M, Kawa K. Encouraging results of low-dose etoposide in the treatment of early-onset hemophagocytic syndrome following allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2007;86:466-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Shimazaki C, Inaba T, Okano A, Hatsuse M, Takahashi R, Hirai H, Sudo Y, Ashihara E, Adachi Y, Murakami S. Clinical characteristics of B-cell lymphoma-associated hemophagocytic syndrome (B-LAHS): comparison of CD5+ with CD5- B-LAHS. Intern Med. 2001;40:878-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Takahashi N, Miura I, Chubachi A, Miura AB, Nakamura S. A clinicopathological study of 20 patients with T/natural killer (NK)-cell lymphoma-associated hemophagocytic syndrome with special reference to nasal and nasal-type NK/T-cell lymphoma. Int J Hematol. 2001;74:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Fung KM, Chakrabarty JH, Kern WF, Magharyous H, Gehrs BC, Li S. Intravascular large B-cell lymphoma with hemophagocytic syndrome (Asian variant) in a Caucasian patient. Int J Clin Exp Pathol. 2012;5:448-454. [PubMed] |
| 76. | Balta G, Azik FM, Gurgey A. Defective UNC13D gene-associated familial hemophagocytic lymphohistiocytosis triggered by visceral leishmaniasis: a diagnostic challenge. J Pediatr Hematol Oncol. 2014;36:e42-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Hazen MM, Woodward AL, Hofmann I, Degar BA, Grom A, Filipovich AH, Binstadt BA. Mutations of the hemophagocytic lymphohistiocytosis-associated gene UNC13D in a patient with systemic juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:567-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 78. | Clementi R, Dagna L, Dianzani U, Dupré L, Dianzani I, Ponzoni M, Cometa A, Chiocchetti A, Sabbadini MG, Rugarli C. Inherited perforin and Fas mutations in a patient with autoimmune lymphoproliferative syndrome and lymphoma. N Engl J Med. 2004;351:1419-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Chang TY, Jaffray J, Woda B, Newburger PE, Usmani GN. Hemophagocytic lymphohistiocytosis with MUNC13-4 gene mutation or reduced natural killer cell function prior to onset of childhood leukemia. Pediatr Blood Cancer. 2011;56:856-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Machaczka M, Klimkowska M, Chiang SC, Meeths M, Müller ML, Gustafsson B, Henter JI, Bryceson YT. Development of classical Hodgkin’s lymphoma in an adult with biallelic STXBP2 mutations. Haematologica. 2013;98:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Booth C, Gilmour KC, Veys P, Gennery AR, Slatter MA, Chapel H, Heath PT, Steward CG, Smith O, O’Meara A. X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: a multicenter study on the manifestations, management and outcome of the disease. Blood. 2011;117:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 82. | Marsh RA, Madden L, Kitchen BJ, Mody R, McClimon B, Jordan MB, Bleesing JJ, Zhang K, Filipovich AH. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116:1079-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 83. | Filipovich AH, Zhang K, Snow AL, Marsh RA. X-linked lymphoproliferative syndromes: brothers or distant cousins? Blood. 2010;116:3398-3408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 84. | Imashuku S, Hibi S, Tabata Y, Itoh E, Hashida T, Tsunamoto K, Ishimoto K, Kawano F. Outcome of clonal hemophagocytic lymphohistiocytosis: analysis of 32 cases. Leuk Lymphoma. 2000;37:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Osugi Y, Hara J, Tagawa S, Takai K, Hosoi G, Matsuda Y, Ohta H, Fujisaki H, Kobayashi M, Sakata N. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89:4100-4103. [PubMed] |
| 86. | Takada H, Ohga S, Mizuno Y, Suminoe A, Matsuzaki A, Ihara K, Kinukawa N, Ohshima K, Kohno K, Kurimoto M. Oversecretion of IL-18 in haemophagocytic lymphohistiocytosis: a novel marker of disease activity. Br J Haematol. 1999;106:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint Basile G. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 858] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 88. | Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachée-Chardin M, Chedeville G, Tamary H. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. 2003;115:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 655] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 89. | zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler AS, Henter JI, Kabisch H, Schneppenheim R, Nürnberg P, Janka G. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 396] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 90. | zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, Strauss J, Kasper B, Nürnberg G, Becker C. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85:482-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 91. | Côte M, Ménager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, Al-Manjomi F, Al-Harbi M, Alangari A, Le Deist F. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119:3765-3773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 92. | Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 689] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 93. | Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 554] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 94. | Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci USA. 1998;95:13765-13770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 387] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 95. | Karim MA, Nagle DL, Kandil HH, Bürger J, Moore KJ, Spritz RA. Mutations in the Chediak-Higashi syndrome gene (CHS1) indicate requirement for the complete 3801 amino acid CHS protein. Hum Mol Genet. 1997;6:1087-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 96. | Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 729] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 97. | Huizing M, Scher CD, Strovel E, Fitzpatrick DL, Hartnell LM, Anikster Y, Gahl WA. Nonsense mutations in ADTB3A cause complete deficiency of the beta3A subunit of adaptor complex-3 and severe Hermansky-Pudlak syndrome type 2. Pediatr Res. 2002;51:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 98. | Jones ML, Murden SL, Brooks C, Maloney V, Manning RA, Gilmour KC, Bharadwaj V, de la Fuente J, Chakravorty S, Mumford AD. Disruption of AP3B1 by a chromosome 5 inversion: a new disease mechanism in Hermansky-Pudlak syndrome type 2. BMC Med Genet. 2013;14:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Huck K, Feyen O, Niehues T, Rüschendorf F, Hübner N, Laws HJ, Telieps T, Knapp S, Wacker HH, Meindl A. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009;119:1350-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 100. | Stepensky P, Weintraub M, Yanir A, Revel-Vilk S, Krux F, Huck K, Linka RM, Shaag A, Elpeleg O, Borkhardt A. IL-2-inducible T-cell kinase deficiency: clinical presentation and therapeutic approach. Haematologica. 2011;96:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 101. | van Montfrans JM, Hoepelman AI, Otto S, van Gijn M, van de Corput L, de Weger RA, Monaco-Shawver L, Banerjee PP, Sanders EA, Jol-van der Zijde CM. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J Allergy Clin Immunol. 2012;129:787-793.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 102. | Salzer E, Daschkey S, Choo S, Gombert M, Santos-Valente E, Ginzel S, Schwendinger M, Haas OA, Fritsch G, Pickl WF. Combined immunodeficiency with life-threatening EBV-associated lymphoproliferative disorder in patients lacking functional CD27. Haematologica. 2013;98:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 103. | Chaigne-Delalande B, Li FY, O’Connor GM, Lukacs MJ, Jiang P, Zheng L, Shatzer A, Biancalana M, Pittaluga S, Matthews HF. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 104. | Rosado FG, Kim AS. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139:713-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 105. | Lehmberg K, Ehl S. Diagnostic evaluation of patients with suspected haemophagocytic lymphohistiocytosis. Br J Haematol. 2013;160:275-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 106. | Parizhskaya M, Reyes J, Jaffe R. Hemophagocytic syndrome presenting as acute hepatic failure in two infants: clinical overlap with neonatal hemochromatosis. Pediatr Dev Pathol. 1999;2:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 107. | Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/ systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 108. | Aricò M, Janka G, Fischer A, Henter JI, Blanche S, Elinder G, Martinetti M, Rusca MP. Hemophagocytic lymphohistiocytosis. Report of 122 children from the International Registry. FHL Study Group of the Histiocyte Society. Leukemia. 1996;10:197-203. [PubMed] |
| 109. | Trottestam H, Horne A, Aricò M, Egeler RM, Filipovich AH, Gadner H, Imashuku S, Ladisch S, Webb D, Janka G. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118:4577-4584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 463] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 110. | Imashuku S, Teramura T, Tauchi H, Ishida Y, Otoh Y, Sawada M, Tanaka H, Watanabe A, Tabata Y, Morimoto A. Longitudinal follow-up of patients with Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Haematologica. 2004;89:183-188. [PubMed] |
| 111. | Chandrakasan S, Marsh RA, Bellman D. Adolescents and young adults with HLH who undergo allogeneic HCT are at increased risk of mortality compared to younger patients. Program of the Histiocyte Society Annual Meeting. 2013;27. |
| 112. | Gross TG, Filipovich AH, Conley ME, Pracher E, Schmiegelow K, Verdirame JD, Vowels M, Williams LL, Seemayer TA. Cure of X-linked lymphoproliferative disease (XLP) with allogeneic hematopoietic stem cell transplantation (HSCT): report from the XLP registry. Bone Marrow Transplant. 1996;17:741-744. [PubMed] |
| 113. | Marsh RA, Rao K, Satwani P, Lehmberg K, Müller I, Li D, Kim MO, Fischer A, Latour S, Sedlacek P. Allogeneic hematopoietic cell transplantation for XIAP deficiency: an international survey reveals poor outcomes. Blood. 2013;121:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 114. | Hamidieh AA, Pourpak Z, Yari K, Fazlollahi MR, Hashemi S, Behfar M, Moin M, Ghavamzadeh A. Hematopoietic stem cell transplantation with a reduced-intensity conditioning regimen in pediatric patients with Griscelli syndrome type 2. Pediatr Transplant. 2013;17:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | Rihani R, Barbar M, Faqih N, Halalsheh H, Hussein AA, Al-Zaben AH, Rahman FA, Sarhan M. Unrelated cord blood transplantation can restore hematologic and immunologic functions in patients with Chediak-Higashi syndrome. Pediatr Transplant. 2012;16:E99-E105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Ouachée-Chardin M, Elie C, de Saint Basile G, Le Deist F, Mahlaoui N, Picard C, Neven B, Casanova JL, Tardieu M, Cavazzana-Calvo M. Hematopoietic stem cell transplantation in hemophagocytic lymphohistiocytosis: a single-center report of 48 patients. Pediatrics. 2006;117:e743-e750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 117. | Mischler M, Fleming GM, Shanley TP, Madden L, Levine J, Castle V, Filipovich AH, Cornell TT. Epstein-Barr virus-induced hemophagocytic lymphohistiocytosis and X-linked lymphoproliferative disease: a mimicker of sepsis in the pediatric intensive care unit. Pediatrics. 2007;119:e1212-e1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 118. | Letsas KP, Filippatos GS, Delimpasi S, Spanakis N, Kounas SP, Efremidis M, Tsakris A, Kardaras F. Enterovirus-induced fulminant myocarditis and hemophagocytic syndrome. J Infect. 2007;54:e75-e77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 119. | Uni M, Yoshimi A, Maki H, Maeda D, Nakazaki K, Nakamura F, Fukayama M, Kurokawa M. Successful treatment with recombinant thrombomodulin for B-cell lymphoma-associated hemophagocytic syndrome complicated by disseminated intravascular coagulation. Int J Clin Exp Pathol. 2013;6:1190-1194. [PubMed] |
| 120. | Kizaki Z, Fukumochi H, Sawai T, Ishimura K, Esumi N, Nagai T, Todo S, Imashuku S. [Exchange transfusion and combination chemotherapy in the treatment of 4 cases of childhood malignant histiocytosis]. Rinsho Ketsueki. 1987;28:845-851. [PubMed] |
| 121. | Matsumoto Y, Naniwa D, Banno S, Sugiura Y. The efficacy of therapeutic plasmapheresis for the treatment of fatal hemophagocytic syndrome: two case reports. Ther Apher. 1998;2:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 122. | Sullivan JL, Byron KS, Brewster FE, Baker SM, Ochs HD. X-linked lymphoproliferative syndrome. Natural history of the immunodeficiency. J Clin Invest. 1983;71:1765-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 123. | Suzuki N, Morimoto A, Ohga S, Kudo K, Ishida Y, Ishii E. Characteristics of hemophagocytic lymphohistiocytosis in neonates: a nationwide survey in Japan. J Pediatr. 2009;155:235-238.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 124. | Imashuku S, Tanaka T, Togari H. Detection of hemophagocytes in blood smears in fatal disseminated neonatal herpes simplex virus infection. Int J Pediatr Hematol/Oncol. 2000;6:425-428. |
| 125. | Yamada K, Yamamoto Y, Uchiyama A, Ito R, Aoki Y, Uchida Y, Nagasawa H, Kimura H, Ichiyama T, Fukao T. Successful treatment of neonatal herpes simplex-type 1 infection complicated by hemophagocytic lymphohistiocytosis and acute liver failure. Tohoku J Exp Med. 2008;214:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 126. | Brastianos PK, Swanson JW, Torbenson M, Sperati J, Karakousis PC. Tuberculosis-associated haemophagocytic syndrome. Lancet Infect Dis. 2006;6:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 127. | Cançado GG, Freitas GG, Faria FH, de Macedo AV, Nobre V. Hemophagocytic lymphohistiocytosis associated with visceral leishmaniasis in late adulthood. Am J Trop Med Hyg. 2013;88:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 128. | Fardet L, Lambotte O, Meynard JL, Kamouh W, Galicier L, Marzac C, de Labarthe A, Cabane J, Lebbe C, Coppo P. Reactive haemophagocytic syndrome in 58 HIV-1-infected patients: clinical features, underlying diseases and prognosis. AIDS. 2010;24:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 129. | Shuper A, Attias D, Kornreich L, Zaizov R, Yaniv I. Familial hemophagocytic lymphohistiocytosis: improved neurodevelopmental outcome after bone marrow transplantation. J Pediatr. 1998;133:126-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 130. | Jackson J, Titman P, Butler S, Bond K, Rao A, Veys P, Chiesa R, Leiper A, Riley L, Gilmour K. Cognitive and psychosocial function post hematopoietic stem cell transplantation in children with hemophagocytic lymphohistiocytosis. J Allergy Clin Immunol. 2013;132:889-95.e1-3. [PubMed] |
| 131. | Larroche C, Bruneel F, André MH, Bader-Meunier B, Baruchel A, Tribout B, Genereau T, Zunic P. [Intravenously administered gamma-globulins in reactive hemaphagocytic syndrome. Multicenter study to assess their importance, by the immunoglobulins group of experts of CEDIT of the AP-HP]. Ann Med Interne (Paris). 2000;151:533-539. [PubMed] |
| 132. | Emmenegger U, Spaeth PJ, Neftel KA. Intravenous immunoglobulin for hemophagocytic lymphohistiocytosis? J Clin Oncol. 2002;20:599-601. [PubMed] |
| 133. | Lindamood KE, Fleck P, Narla A, Vergilio JA, Degar BA, Baldwin M, Wintermark P. Neonatal enteroviral sepsis/meningoencephalitis and hemophagocytic lymphohistiocytosis: diagnostic challenges. Am J Perinatol. 2011;28:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 134. | Fukazawa M, Hoshina T, Nanishi E, Nishio H, Doi T, Ohga S, Hara T. Neonatal hemophagocytic lymphohistiocytosis associated with a vertical transmission of coxsackievirus B1. J Infect Chemother. 2013;19:1210-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 135. | Katsibardi K, Moschovi MA, Theodoridou M, Spanakis N, Kalabalikis P, Tsakris A, Tzortzatou-Stathopoulou F. Enterovirus-associated hemophagocytic syndrome in children with malignancy: report of three cases and review of the literature. Eur J Pediatr. 2008;167:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Hot A, Madoux MH, Viard JP, Coppéré B, Ninet J. Successful treatment of cytomegalovirus-associated hemophagocytic syndrome by intravenous immunoglobulins. Am J Hematol. 2008;83:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Tai CM, Liu CJ, Yao M. Successful treatment of acute hepatitis A-associated hemophagocytic syndrome by intravenous immunoglobulin. J Formos Med Assoc. 2005;104:507-510. [PubMed] |
| 138. | Naffaa M, Awad J, Oren I, Braun E, Lavi N. Group G streptococcal endocarditis-associated hemophagocytic syndrome. Int J Infect Dis. 2013;17:e1237-e1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 139. | Shiraishi A, Ohga S, Doi T, Ishimura M, Takimoto T, Takada H, Miyamoto T, Abe Y, Hara T. Treatment choice of immunotherapy or further chemotherapy for Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2012;59:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 140. | Abella EM, Artrip J, Schultz K, Ravindranath Y. Treatment of familial erythrophagocytic lymphohistiocytosis with cyclosporine A. J Pediatr. 1997;130:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 141. | Mouy R, Stephan JL, Pillet P, Haddad E, Hubert P, Prieur AM. Efficacy of cyclosporine A in the treatment of macrophage activation syndrome in juvenile arthritis: report of five cases. J Pediatr. 1996;129:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 154] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 142. | Tsuda H. The use of cyclosporin-A in the treatment of virus-associated hemophagocytic syndrome in adults. Leuk Lymphoma. 1997;28:73-82. [PubMed] |
| 143. | Imashuku S, Hibi S, Kuriyama K, Tabata Y, Hashida T, Iwai A, Kato M, Yamashita N, Oda MUchida M, Kinugawa N. Management of severe neutropenia with cyclosporin during initial treatment of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis. Leuk Lymphoma. 2000;36:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 144. | Imashuku S, Hibi S, Ohara T, Iwai A, Sako M, Kato M, Arakawa H, Sotomatsu M, Kataoka S, Asami K. Effective control of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis with immunochemotherapy. Histiocyte Society. Blood. 1999;93:1869-1874. [PubMed] |
| 145. | Imashuku S, Kuriyama K, Teramura T, Ishii E, Kinugawa N, Kato M, Sako M, Hibi S. Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. J Clin Oncol. 2001;19:2665-2673. [PubMed] |
| 146. | Lee JS, Kang JH, Lee GK, Park HJ. Successful treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis with HLH-94 protocol. J Korean Med Sci. 2005;20:209-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 147. | Stéphan JL, Donadieu J, Ledeist F, Blanche S, Griscelli C, Fischer A. Treatment of familial hemophagocytic lymphohistiocytosis with antithymocyte globulins, steroids, and cyclosporin A. Blood. 1993;82:2319-2323. [PubMed] |
| 148. | Mahlaoui N, Ouachée-Chardin M, de Saint Basile G, Neven B, Picard C, Blanche S, Fischer A. Immunotherapy of familial hemophagocytic lymphohistiocytosis with antithymocyte globulins: a single-center retrospective report of 38 patients. Pediatrics. 2007;120:e622-e628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 149. | Milone MC, Tsai DE, Hodinka RL, Silverman LB, Malbran A, Wasik MA, Nichols KE. Treatment of primary Epstein-Barr virus infection in patients with X-linked lymphoproliferative disease using B-cell-directed therapy. Blood. 2005;105:994-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 150. | Bond J, Shahdadpuri R, Mc Mahon C, O’marcaigh A, Cotter M, Smith O. Successful treatment of acute Epstein-Barr virus infection associated with X-linked lymphoproliferative disorder with rituximab. Pediatr Blood Cancer. 2007;49:761-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 151. | Balamuth NJ, Nichols KE, Paessler M, Teachey DT. Use of rituximab in conjunction with immunosuppressive chemotherapy as a novel therapy for Epstein Barr virus-associated hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol. 2007;29:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 152. | Bosman G, Langemeijer SM, Hebeda KM, Raemaekers JM, Pickkers P, van der Velden WJ. The role of rituximab in a case of EBV-related lymphoproliferative disease presenting with haemophagocytosis. Neth J Med. 2009;67:364-365. [PubMed] |
| 153. | Chellapandian D, Das R, Zelley K, Wiener SJ, Zhao H, Teachey DT, Nichols KE. Treatment of Epstein Barr virus-induced haemophagocytic lymphohistiocytosis with rituximab-containing chemo-immunotherapeutic regimens. Br J Haematol. 2013;162:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 154. | Imashuku S, Kudo N, Kubo K, Yachie A. Are regimens containing rituximab effective in the initial treatment of Epstein-Barr virus-positive natural killer cell lymphoproliferative disease-associated hemophagocytic lymphohistiocytosis? Int J Hematol. 2013;98:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 155. | Bonney DK, Htwe EE, Turner A, Kelsey A, Shabani A, Hughes S, Hughes I, Wynn RF. Sustained response to intrathecal rituximab in EBV associated Post-transplant lymphoproliferative disease confined to the central nervous system following haematopoietic stem cell transplant. Pediatr Blood Cancer. 2012;58:459-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 156. | Czyzewski K, Styczynski J, Krenska A, Debski R, Zajac-Spychala O, Wachowiak J, Wysocki M. Intrathecal therapy with rituximab in central nervous system involvement of post-transplant lymphoproliferative disorder. Leuk Lymphoma. 2013;54:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 157. | Strout MP, Seropian S, Berliner N. Alemtuzumab as a bridge to allogeneic SCT in atypical hemophagocytic lymphohistiocytosis. Nat Rev Clin Oncol. 2010;7:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 158. | Marsh RA, Allen CE, McClain KL, Weinstein JL, Kanter J, Skiles J, Lee ND, Khan SP, Lawrence J, Mo JQ. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer. 2013;60:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 159. | Keith MP, Pitchford C, Bernstein WB. Treatment of hemophagocytic lymphohistiocytosis with alemtuzumab in systemic lupus erythematosus. J Clin Rheumatol. 2012;18:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 160. | Olin RL, Nichols KE, Naghashpour M, Wasik M, Shelly B, Stadtmauer EA, Vogl DT. Successful use of the anti-CD25 antibody daclizumab in an adult patient with hemophagocytic lymphohistiocytosis. Am J Hematol. 2008;83:747-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 161. | Henzan T, Nagafuji K, Tsukamoto H, Miyamoto T, Gondo H, Imashuku S, Harada M. Success with infliximab in treating refractory hemophagocytic lymphohistiocytosis. Am J Hematol. 2006;81:59-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 162. | Oda Y, Urushidani Y, Ooi S, Endoh A, Nakamura R, Adachi K, Fukushima H. Hemophagocytic lymphohistiocytosis in a rheumatoid arthritis patient treated with infliximab. Intern Med. 2012;51:655-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 163. | Ideguchi H, Ohno S, Takase K, Hattori H, Kirino Y, Takeno M, Ishigatsubo Y. Successful treatment of refractory lupus-associated haemophagocytic lymphohistiocytosis with infliximab. Rheumatology (Oxford). 2007;46:1621-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 164. | Takahashi N, Naniwa T, Banno S. Successful use of etanercept in the treatment of acute lupus hemophagocytic syndrome. Mod Rheumatol. 2008;18:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 165. | Kikuchi H, Yamamoto T, Asako K, Takayama M, Shirasaki R, Ono Y. Etanercept for the treatment of intractable hemophagocytic syndrome with systemic lupus erythematosus. Mod Rheumatol. 2012;22:308-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 166. | Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 552] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 167. | Pachlopnik Schmid J, Ho CH, Chrétien F, Lefebvre JM, Pivert G, Kosco-Vilbois M, Ferlin W, Geissmann F, Fischer A, de Saint Basile G. Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Mol Med. 2009;1:112-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 168. | Sato E, Ohga S, Kuroda H, Yoshiba F, Nishimura M, Nagasawa M, Inoue M, Kawa K. Allogeneic hematopoietic stem cell transplantation for Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disease in Japan. Am J Hematol. 2008;83:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 169. | Landman-Parker J, Le Deist F, Blaise A, Brison O, Fischer A. Partial engraftment of donor bone marrow cells associated with long-term remission of haemophagocytic lymphohistiocytosis. Br J Haematol. 1993;85:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 170. | Terrell CE, Jordan MB. Mixed hematopoietic or T-cell chimerism above a minimal threshold restores perforin-dependent immune regulation in perforin-deficient mice. Blood. 2013;122:2618-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |