Peer-review started: August 31, 2015
First decision: September 28, 2015
Revised: October 19, 2015
Accepted: December 13, 2015
Article in press: December 14, 2015
Published online: February 2, 2016
Processing time: 154 Days and 23 Hours
Infantile hemangioma (IH) is the most common benign tumor seen in infancy. This review provides up-to-date information on the pathophysiology, variations in clinical presentation, and natural history of IH, elaborating on associated anomalies, such as PHACE(S) syndrome and LUMBAR syndrome. Because of the benign and self-limiting characteristics seen in more than 90% of cases of IH, a conservative approach is usually chosen. However, some circumstances, such as ulceration, vision loss, breathing difficulties, or potential disfigurement, will require treatment during the proliferative phase. For decades, treatment of IH has primarily consisted of corticosteroids or surgery. Since 2008, propranolol has become the treatment of first choice. In this article, we bring to light the crucial changes in the treatment of IH over the past years. To date, there is still a lack of data on the possible long-term effects of propranolol treatment in young infants. A theoretical probability of the central nervous system being affected (that is, impairment of short- and long-term memory, psychomotor function, sleep quality, and mood) has recently been suggested. This review highlights research topics concerning these long-term adverse effects. Finally, information is provided on the potential instruments to measure IH severity and activity in clinical trials and/or in clinical practice and the recently developed and first-validated IH-specific quality-of-life questionnaire.
Core tip: The discovery that propranolol is efficacious in the treatment of infantile hemangioma (IH) has led to an upsurge in publications, increasing our knowledge of this subject. In this review, we provide the most up-to-date information on the pathophysiology, variations in clinical presentation, and natural history of IH. We look at possible working mechanisms of several treatments and the current concerns regarding the treatment of first choice, propranolol. Finally, we provide an overview of instruments, measuring IH severity and/or activity and IH-related quality of life.
- Citation: Moyakine AV, Vleuten CJVD. Propranolol for infantile hemangioma: Current state of affairs. World J Dermatol 2016; 5(1): 4-16
- URL: https://www.wjgnet.com/2218-6190/full/v5/i1/4.htm
- DOI: https://dx.doi.org/10.5314/wjd.v5.i1.4
Infantile hemangioma (IH) is a benign vascular tumor caused by endothelial cell proliferation. With a prevalence of about 4%-10% in the first year of life, it is the most common benign tumor of infancy[1-4]. IHs may be located in any region of the body, including the internal organs, but are mostly (60%) located in the skin of the head and neck region[5,6]. The liver is the most common extracutaneous site of IHs. Hepatic IHs, which can be focal, multifocal, or diffuse, are the most common benign liver tumors of infancy[7]. IHs are seen 3-5 times more often in females than in males. Other risk factors for developing an IH [including their crude odds ratios (OR)] are: Caucasian race, low birth weight (OR = 1.8), prematurity (OR = 1.8), family history of IH (OR = 2.5), and being born from a multiple birth (OR = 2.2)[8-10]. Because of their benign and self-limiting character, no intervention is needed in more than 90% of cases. However, there are circumstances that will require treatment during the proliferative phase. These concern infants with IHs with a substantial morbidity, such as ulceration, vision loss, breathing difficulties, or potential disfigurement because of the tumor location. Until 2008, the treatment of IHs consisted of systemic or intralesional corticosteroids or surgery[11,12]. In 2008, treatment of IH with propranolol was reported for the first time[13]. After that, multiple publications followed, and the approach to IHs dramatically changed. This shift in the management of cutaneous IHs has also influenced the treatment of hepatic IHs[7,14,15]. Propranolol is currently considered to be the treatment of first choice for IHs.
Propranolol has been used for several decades to treat cardiovascular diseases, such as hypertension, ischemic heart disease, and arrhythmias in adults and children. Although there is an abundance of experience with propranolol in infants, responses to propranolol have been far better studied in adults than in children[16]. Propranolol has its side effects, although these are mild compared with previous IH treatments. The short-term side effects consist of hypotension, bradycardia, respiratory symptoms, hypoglycemia, gastrointestinal complaints, and cold extremities. The lipophilic nature of propranolol facilitates the crossing of the blood-brain barrier, causing adverse effects such as a sleepy and drowsy feeling during the day and restlessness at night[17]. Based on studies in adult volunteers and animals, it has been postulated that there may be long-term side effects of this drug, affecting the developing central nervous system, when given to infants[18].
Our review summarizes the discoveries that have been made since 2008 regarding the treatment of IHs with propranolol. It also highlights the most important areas that still remain unknown.
Despite its high incidence, the pathophysiology of IH is still unclear. There is no universally accepted theory, and no single hypothesis is sufficient to describe and explain all of its features. The three most common hypotheses that partially explain development of IH are listed below.
IH endothelial cells share immunohistochemical markers with the placental microvasculature. Both possess glucose transporter protein type 1 (GLUT-1), Lewis Y antigen, merosin, laminin, chemokine receptor 6, CD15, insulin-like growth factor 2 (IGF-2), and indoleamine 2,3-dioxygenase. This immunohistochemical profile differentiates IHs from other vascular birthmarks or tumors[19-22]. In addition, there is a high level of genetic similarity between the placenta and IH[23]. Therefore, it was hypothesized that embolization of placental endothelial cells to the fetus could play a role in the pathogenesis of IH. This hypothesis was strengthened by findings that transcervical chorionic villus sampling is associated with a threefold increased incidence of IH and that placental abnormalities, such as abnormal placentation, are associated with a higher incidence of IH[24-27]. However, the latter may also be explained by the hypoxia hypothesis. In contrast to the placental embolization theory are the failed attempts to detect the presence of maternal–fetal chimerism in IH tissue[28].
Both angiogenesis (growth of new blood vessels from pre-existing vessels) and vasculogenesis (de novo formation of blood vessels from stem cells) are hypothesized to contribute to IH formation. IHs may result from somatic mutations in a gene mediating endothelial cell proliferation (growth regulatory pathways)[29]. Such mutations may alternate the vascular endothelial growth factor (VEGF) signaling pathway by reducing the expression of VEGF receptor 1 (VEGFR-1), which causes hyperactivity of VEGFR-2 and may induce IH formation through angiogenesis[30]. IGF-2 and basic fibroblast growth factor also stimulate angiogenesis and are upregulated in proliferating IHs[31,32]. Endothelial progenitor cells (EPCs), stem cells of vascular origin that are capable of differentiating into endothelial cells, seem to play a role in the development of IH through vasculogenesis[33]. EPCs possess the surface markers (CD34+ and CD133+) that are also found in endothelial cells of growing IHs, suggesting that these bone-marrow-derived progenitor cells may play a key role in the pathogenesis of IHs by inducing postnatal formation of vascular tissue[34,35]. In 2008, Khan et al[36] injected immune-deficient mice with CD133+ EPCs, which resulted in the development of GLUT-1-positive vascular tumors in these mice. These findings greatly supported the angiogenesis theory.
Hypoxia, either local or systemic, seems to be the most influential inducer of IH development. Hypoxia stimulates the proliferation of EPCs[24,37-41]. Transcription factor hypoxia-inducible factor 1α (HIF-1α) plays a key role in the tissue hypoxia theory. A hypoxic environment triggers the production of HIF-1α. HIF-1α in turn stimulates transcription of target genes, such as GLUT-1, VEGF and IGF-2[42-45]. These stimulations may take place either directly by HIF-1a signaling or by hypoxia-induced regulation of mammalian target of rapamycin (mTOR) complex 1 signaling. Deregulation of the mTOR pathway may lead to disorganized growth[46,47]. Overexpression of VEGF may also take place via the activation of the HIF-2α pathway as a response to the pathologic signal of a “dangerous hypoxic situation”[48]. It has also been demonstrated that the combination of hypoxia and an estrogenic environment has a synergic effect on IH endothelial cell proliferation, which may explain the greater incidence of IHs in girls[48].
As stated above, none of these three theories explains the pathogenesis of IH completely. Given the great variability of clinical presentations of IH, the uneven distribution of IHs over the body, the increased prevalence of IHs in Caucasians, and its familial occurrence, it is most likely that IH pathogenesis is not restricted to one factor, but to a combination of genetic predisposition and various environmental factors[48,49].
IHs develop in the first days, weeks, or months of life. They are not to be confused with congenital hemangiomas, which are fully developed at birth and either rapidly involute during the first year of life (rapidly involuting congenital hemangiomas) or do not involute at all (non-involuting congenital hemangiomas)[50,51]. Many children who develop an IH are born with a visible precursor lesion, such as a pale macule with telangiectasia or mottled vascular stain, at the future IH location[52]. Fully developed, an IH feels elastic and frequently warm. The tumor is not pulsating and is painless, except in the case of ulceration[48]. There is a great variation in size, but in most cases (80%), IHs are not greater than 3 cm in diameter[8]. Recognized risk factors for developing an IH include female sex, prematurity, multiple gestation, and low birth weight. Caucasians are at greater risk of developing an IH compared with individuals of Hispanic or African origin[5,6,53].
In the classification of the International Society for the Study of Vascular Anomalies (ISSVA), four different patterns of IH are described[54]. According to their pattern, IHs can be grouped into focal, multifocal, segmental (plaque-like, covering an embryologic segment), and intermediate/indeterminate[48,50]. Intermediate/indeterminate IHs show characteristics of both focal and segmental IHs. They do not entirely encompass an accepted embryologic segment nor do they arise from a single focus[48,51]. Segmental IHs have a higher complication rate and are associated abnormalities[55]. Apart from the pattern, the ISSVA classification makes a distinction between four different types of IHs, according to their clinical appearance: (1) superficial (50%-60%); (2) deep (15%); (3) mixed (25%-35%), which are distinguished by the layer(s) of the skin affected[55]; and (4) reticular/abortive/minimal growth, which is distinguished by its typical growth pattern[56,57].
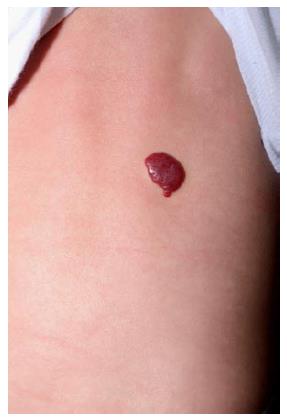
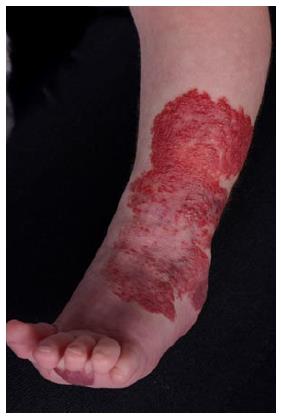
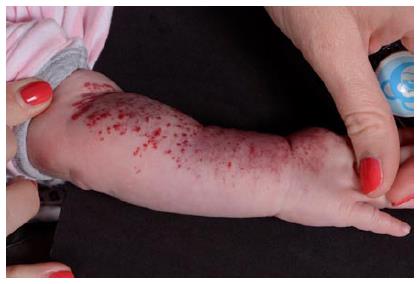
Superficial IHs are the most common type of IHs. They involve the papillary dermis and appear as bright red “strawberry” lesions in the case of a localized superficial IH (Figure 1) or as a plaque-like red lesion in the case of a segmental superficial IH (Figure 2). Segmental IHs are more often associated with complications, such as ulceration and associated anomalies, and more often require therapy[8,48].
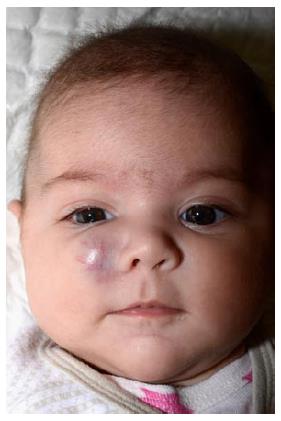
Deep IHs involve the deep, reticular dermis and subcutis, resulting in a tumor with a bluish shine or (when deeper) normal skin color (Figure 3). Because of these characteristics, deep IHs may easily be misdiagnosed at first[55]. Deep IHs appear later than superficial IHs; typically around the age of 2 mo, and may have a longer proliferative phase compared with the superficial types[17,51,52].
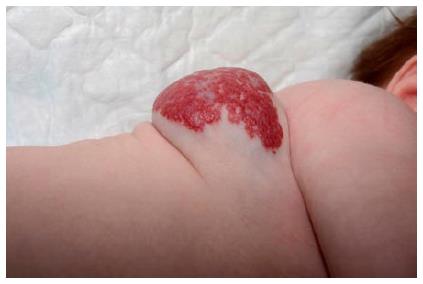
Mixed IHs have both superficial and deep components (Figure 4). The proliferative phase of the deep component in mixed IHs also stops later than in superficial IHs[17,48].
A minority of IHs have arrested or minimal growth beyond the stage resembling the precursor lesions. Although their natural course is different from that of the other three types, these lesions do express GLUT-1 proteins and have similar other immunohistochemical characteristics (Figure 5)[56,57]. Several terms have been used to describe these in the literature. The most commonly used terms are reticular, abortive, or minimal growth IH. IHs of this type seem to have a predilection for the lower body[57]. The exact incidence of this type of IH is unknown, but it is believed to be relatively rare. However, a recent study by Munden et al[27] in which 578 pregnant women were prospectively enrolled and their infants followed up for 9 mo after birth, reports that of the infants with an IH, 20% had a reticular, abortive, or minimal growth IH.
Despite several hypotheses, the pathogenesis of segmental vs focal and superficial vs deep IHs remains unclear[19].
IHs have a unique pattern of evolution. As stated above, IHs are not fully developed at birth, but start to grow shortly after birth (usually within a few days or weeks) from normal appearing skin or a precursor lesion[51]. This typical delay serves as a diagnostic tool, especially in deep IHs where the skin color may be bluish or even normal[48]. After a relatively short proliferative phase in the first 3-9 mo of life, the slow involution phase takes place between the median age of 2-4 years[8,48,58,59]. However, the proliferative phase may extend until 12 mo after birth, and in some cases, up to 24 mo after birth[48,60]. Approximately 25%-69% patients with IH may develop a residual lesion after complete involution of the IH. Residual lesions may consist of skin atrophy, skin surplus, telangiectasias, pigmentation, scarification after ulceration and/or fibrofatty tissue[3,58,61]. Epidermal invasion of an IH in combination with a deep component in the IH is most prone to residual lesions[58]. The difference in reported incidence of residual lesions in several studies may be explained by usage of different populations (e.g., secondary/tertiary referral vs primary referral).
There are two types of IHs that may be predictive of an underlying anomaly. These are (1) large, flat, segmental IHs of the face, which are associated with PHACE(S) syndrome and (2) IHs in the lumbosacral or perineal region, which may be predictive of LUMBAR syndrome [also known as Perineal hemangioma, External genitalia malformations, Lipomyelomeningocele, Vesicorenal abnormalities, Imperforate anus, and Skin tag (PELVIS) or Spinal dysraphism, Anogenital, Cutaneous, Renal and urologic anomalies, associated with an Angioma of Lumbosacral localization (SACRAL) syndrome].
The term PHACE was introduced in 1996 by Frieden et al[62], describing a combination of five anomalies: (1) posterior fossa abnormalities; (2) hemangioma of the face (segmental); (3) arterial abnormalities (intra- and extracranial); (4) cardiac and aortic defects; and (5) eye anomalies. A sixth anomaly: Sternal cleft or supraumbilical raphe was added later[48]. PHACES syndrome is a spectrum of anomalies, because most affected children (70%) have only one extracutaneous manifestation[63]. The so-called “Dandy-Walker syndrome” is the most common brain involvement, followed by cerebellar hypoplasia or dysgenesia as a result of posterior fossa abnormalities[48,63]. Until 2009, a diagnosis of PHACES syndrome required the presence of a segmental, flat IH of the face in addition to one or more of the five anomalies described above[62,64]. In 2009, a consensus was reached defining PHACES as the presence of a characteristic segmental hemangioma or hemangioma greater than 5 cm in diameter of the face or scalp plus one major criterion or two minor criteria[65]. The exact incidence of PHACES is unknown. It has been postulated that in 20%-31% of children with segmental facial IHs, there is an association with PHACES[64,66]. A full workup for PHACES syndrome is suggested in every infant with a large (> 5 cm), segmental, facial hemangioma. This includes a complete physical examination as well as careful cardiac (including echocardiogram), ophthalmologic and neurologic (including MRI of the head and MRA of the entire head and neck area) assessments[67].
IHs in the lumbosacral area or perineum are also associated with underlying structural anomalies. These IHs are also most commonly, but not exclusively, segmental[68]. A tethered cord in the context of spina bifida occulta should be considered, although more extensive associated morbidity may be the case. For these conditions, different acronyms have been suggested, such as SACRAL[69] and PELVIS[70]. The most recently proposed acronym, LUMBAR is preferred; it refers to the association of lower body hemangioma and other cutaneous defects, urogenital anomalies, ulceration, myelopathy, bony deformities, anorectal malformations, arterial anomalies, and renal anomalies[68]. There is no diagnostic consensus for LUMBAR, SACRAL, or PELVIS, such as for PHACES. Screening with ultrasound scanning of the spine, abdomen, and pelvis is suggested for all patients with a segmental IH greater than 2.5 cm in diameter of any lumbosacral or perineal region who are younger than 3 mo. For children older than 3 mo, MRI is indicated[68,71].
The management of IHs has been changed drastically since the discovery of the efficacy of propranolol treatment for this indication in 2008[13]. Although there are no uniform international guidelines available for the treatment of IHs, propranolol is now considered to be the treatment of first choice. Before that, a whole range of treatments had been applied. Some of these treatments are rarely or no longer used (e.g., X-irradiation therapy) because of their side effects and/or low efficacy.
X-irradiation: Although there was already evidence that IHs involute spontaneously, X-irradiation has been widely used for two decades between 1930 and 1950, resulting in (unnecessary) radiation exposure and post-radiation skin atrophy, pigmentation, telangiectasia, contractures, and risk of skin cancer[72-74].
Vincristine: Vincristine is a vinca alkaloid that is widely used in cancer chemotherapy. Treatment of IHs with vincristine was first described in 1993[75]. This chemotherapeutic drug inhibits microtubule formation, causing arrest of mitosis and subsequent apoptosis[76]. Additionally, vincristine seems to affect angiogenesis[77]. Nowadays, it may only be indicated for severe IHs that are resistant to other therapies. The use of vincristine requires a central venous catheter for chronic administration. Furthermore, it has potential severe side effects, such as peripheral mixed sensorimotor neurotoxicity[78]. Other, less severe, side effects include rash, alopecia, and local reactions, such as phlebitis and necrosis[74].
Interferon: The use of subcutaneous interferon a-2a and -2b for the treatment of IHs was first described in 1989[79]. Its therapeutic effectiveness has been attributed to its anti-angiogenic properties. Interferon a induces apoptosis of endothelial cells, which might also explain the clinically and histologically observed involution without any sign of inflammation or necrosis[80]. Despite its high success rates, the use of interferon in the treatment of complicated IHs has been abandoned, because of its major side effects, such as spastic diplegia and blood abnormalities[81,82].
Topical corticosteroids: Potent topical steroids have been described for small, superficial, localized IHs[83]. Side effects include acne, perioral dermatitis, hypertrichosis, cutaneous atrophy, striae, hypopigmentation, and subcutaneous fat atrophy. Since the availability of topical b-blockers, with fewer side effects, topical steroids are less often prescribed in current practice[76].
Topical imiquimod: Imiquimod is an immune modulator. In 2002, the potential of imiquimod to shorten the involution phase of IH was first reported[84]. Due to its anti-angiogenic and apoptotic effects, imiquimod contributes to the regression of IH[85,86]. Its efficacy is equivalent to the efficacy of the topical b-blocker timolol (0.5% ophthalmic solution), which was first described a few years after the discovery of propranolol treatment for IHs[87,88]. However, timolol is more effective than imiquimod in terms of color involution and onset time[89]. Furthermore, imiquimod has a less favorable adverse-reaction profile and has never really become a very common treatment for IHs that are suitable for topical therapy[88].
Watchful waiting: Knowing IH’s natural history, it is justified to be restrictive in actively treating this self-limiting condition. Starting in the 1950s, physicians began to prefer this approach over the invasive X-irradiation and/or surgical removal[73]. At the present time, watchful waiting is still considered to be the best approach for the vast majority of patients with IH.
Systemic propranolol (first choice): In 2008, after the report of the very successful therapeutic effect of propranolol, IH treatment changed drastically[13]. Currently, propranolol has become the treatment of first choice for IHs. It seems that propranolol stops growth and induces an IH regression that is much better and safer than previous therapies[90]. Recently, Léauté-Labrèze et al[91], published a large-scale randomized placebo-controlled trial showing that propranolol is effective at a dose of 3 mg/kg per day for 6 mo in the treatment of IHs. This treatment resulted in a significantly higher success rate compared with placebo (60% vs 4%). These outcomes are in line with the results of the RCT conducted by Hogeling et al[92] in 2011. Earlier, Malik et al[93] had shown in their RCT that propranolol had a consistent, rapid therapeutic effect with a lower number of complications compared with prednisolone. They also demonstrated that a combination of both propranolol and prednisolone was not superior to propranolol alone[93]. An RCT carried out by Zaher et al[94] proved the superiority of oral admission of propranolol compared with topical and intralesional application. While the general mechanism of action of propranolol is well established as an antagonist of both b1- and b2-adrenergic receptors, the precise mechanism of action on IHs remains uncertain[19]. It is known that propranolol is effective in IH through vasoconstriction, inhibition of angiogenesis, induction of apoptosis, or dysregulation of the renin–angiotensin system (RAS)[95,96].
The most common serious adverse effects of propranolol are bradycardia, hypoglycemia, and hypotension. Other reported adverse side effects in adults and children include bronchospasms, congestive heart failure, hypothermia, somnolence, sleep disturbance, nightmares, depression, nausea, vomiting, diarrhea, hyperkalemia, gastro-esophageal reflux, psoriatic drug rash, and respiratory symptoms[92]. Because of the lipophilic nature of propranolol and the potential to penetrate the blood-brain barrier, the probability of affecting the developing central nervous system of infants with IH was postulated in a report in 2013[97]. This information was further elaborated by Langley et al[18] in 2015. In 2014, Gonski et al[98] showed no gross motor development problems in propranolol-treated children with IH. Recently, our group confirmed these findings. We not only looked for problems with gross motor development, but also included the fine motor/adaptation/personal social functioning and communication in our study[99-101], using the “van Wiechen scheme”, a Dutch screening instrument based on the developmental model of an American developmental psychologist and pediatrician (A. Gesell). No signs of psychomotor developmental problems were found[101]. Despite these promising findings, it is still unclear what effects, either subtle or not, propranolol has on the developing brain. Future prospective studies on later age, using universal screening tools or more advanced neuropsychologic tests are needed to support these findings. Until then, propranolol should only be prescribed for children with IHs with current or impending complications.
Topical b-blockers (first choice): As an alternative to oral b-blockers, topical b-blockers have been used for superficial IHs. There are different forms of topical b-blockers, but timolol (0.5% ophthalmic solution or 0.1% gel), a non-selective b-blocker, is most widely used[76]. In 2013, a double-blind placebo-controlled RCT was published, comparing topical timolol 0.5% solution with placebo for superficial IHs. Timolol was shown to be safe and effective[102]. Recently, timolol 0.5% ophthalmic solution was compared with laser treatment, where timolol proved to be a safe, effective, and painless alternative to lasers for the treatment of superficial IHs. In mixed IHs, laser treatment provided better results than timolol, because of its deeper penetration[103]. Comparison between timolol 0.5% ophthalmic solution and 5% imiquimod cream in 54 patients with IH (half of the IH was treated with timolol and other half with imiquimod) showed similar efficacy, but fewer side effects were seen in the timolol group[89].
Systemic corticosteroids (second choice): In the 1960s, systemic corticosteroids were found to be an effective treatment for IHs[104,105]. The mechanism of action is still not completely understood, but the main theory is that corticosteroids suppress the VEGF-A expression and therefore inhibit angiogenesis and/or vasculogenesis[106]. The usually recommended dose is 2-3 mg/kg per day, which is most effective in the early proliferating phase[107,108]. With a treatment response of 84%-90% and an overall rebound rate of 36%, this therapy became the first-choice therapy for severe IHs, requiring intervention[73,107,109]. The most common side effects of systemic corticosteroids are cushingoid facies (71%), personality changes (29%), gastric irritation (21%), fungal infection (6%), and diminished weight gain (42%) and height (35%)[110]. Other possible side effects were systemic infection, hypertension, increased appetite, aseptic necrosis of bones and cardiomyopathy[20]. Currently, systemic corticosteroids have become a little-used second-line option, because of the lower efficacy and less favorable side-effect profile compared with propranolol[76].
Intralesional corticosteroids (in specified indications): Intralesional corticosteroids (mostly triamcinolone 10 mg/mL) offer an alternative to systemic therapy for small IHs[76]. This therapy was initially used by ophthalmologists for periorbital IHs. Because of the risk of retinal artery damage and blindness, intralesional corticosteroids are no longer used for periorbital IHs[111-113]. The common side effects may include subcutaneous atrophy and hypopigmentation[76].
Surgery (in specified indications): Surgical treatment of IH is suitable in some specific cases. It is indicated in well-circumscribed, pedunculated, or ulcerated lesions that have failed to respond to medical treatment, grow rapidly, or cause significant deformity[114]. Although propranolol treatment has been a breakthrough in the management of IHs, many children still require plastic surgery after the involution phase. At the present time, most surgical interventions in IHs are used to treat those involuted IHs that have left residual lesions, such as skin surplus, scarification after ulceration and/or fibrofatty tissue[115,116].
Laser therapy (in specified indications): Pulsed dye laser (PDL) is the most commonly used laser treatment for superficial and ulcerating IHs and for residual lesions. The literature on the effectiveness of PDL in IHs is somewhat controversial. Some earlier studies suggest that early treatment of IHs with PDL prevents further growth, induces tumor regression, and improves cosmetic outcome, while an randomized controlled trial of 121 infants showed no significant difference in complete clearance or minimum residual signs between the PDL-treated group and the observational group[117-120]. Conventional PDL is ineffective in the treatment of deep IHs. Its penetration depth is limited due to the optical absorption and scattering in the epidermis and dermis[121]. Introduction of a long-pulse PDL in combination with an epidermal cooling system made a greater depth of vascular injury possible[120,122]. Additionally, the use of long-pulse PDL with an epidermal cooling system decreases the risk of scarring and induction of ulceration[122]. These types of laser treatment are not painless and may require anesthesia in infants.
The larger, deep IHs may also be effectively treated using the neodymium-doped yttrium aluminum garnet (ND:YAG) laser. However, due to greater risk of scarring or hypo- or hyperpigmentation, this therapy should be preserved for difficult, recalcitrant cases[121,123,124].
Therapy with the fractionated CO2 laser is reserved for involuted IHs with residual fibrofatty tissue, atrophic plaques, or other textural changes[125].
Other systemicβ-blockers: Propranolol is a non-selective, lipophilic, b-adrenergic receptor antagonist, which binds to b1- and b2-adrenergic receptors[126]. The potential side effects of propranolol made physicians and researchers search for an alternative b-blocker that is as effective as propranolol, but with fewer side effects. It was suggested that a hydrophilic, selective b1-blocker, atenolol, which occurs at lower concentrations in the brain, may have these characteristics[127,128]. A small randomized controlled trial showed no significant difference in effectiveness between atenolol and propranolol. However, no difference in adverse effects was demonstrated either[129]. In 2009, oral nadolol, a non-selective b-blocker, which is significantly less lipophilic than propranolol, was found to have a significant effect on IH growth, with a rapid reduction in size[130,131]. Recently, a small retrospective study of 48 participants showed effects of nadolol similar to those of propranolol. Although serious adverse effects were rare, side effects such as sleep disturbance, behavior problems, gastrointestinal symptoms, and cold extremities were still frequently seen[132]. In 2010, a case report suggested the use of acebutolol for the treatment of infantile subglottic hemangioma, because of fewer side effects on resting heart rate than propranolol, metoprolol, and atenolol[133].
In general, b-blocker lipophility and/or selectivity are factors that determine the efficacy and side-effect profile. It is unclear whether a degree of lipophilicity may be required for tissue penetration and efficacy of IH treatment. It is also unclear whether b1- or b2-blockade or a combination of the two is needed to achieve a therapeutic effect. In conclusion, the search for a b-blocker with the best effectiveness and the most favorable side-effects profile, is still ongoing.
Rapamycin: Rapamycin, also known as sirolimus, is a bacterial macrolide that also has antifungal effects. Since rapamycin is an mTOR inhibitor, it inhibits mTOR signaling, an important regulator of growth and proliferation. By inhibiting the mTOR signaling pathway, rapamycin decreases the elevated VEGF and HIF-1 levels produced by endothelial cells, and reduces IH proliferation[134-136]. Rapamycin not only negatively affects cell proliferation, but also metabolism, as well as angiogenesis. Additionally, rapamycin seems to limit stem cell replicative capabilities, affecting vasculogenesis[137]. At this time, rapamycin treatment use is restricted to clinical trials until better safety data are available[20,76].
Angiotensin-converting enzyme inhibitors: With the expanding knowledge on IH pathogenesis as a result of the discovery of the efficacy of b-blockers for this indication, the regulation of hemogenic endothelium regulated by the RAS in IHs became a point of interest with possible therapeutic consequences[138]. A year later, expression of components of the RAS by the endothelium of proliferating IHs was shown[139]. The role of the RAS in IH is supported by the clinical observation of a higher incidence of IHs in premature infants, females, and Caucasians, since these groups have a higher renin level or activity than full-term infants, males, and black infants, respectively[139-142]. In connection with these findings, a clinical trial of eight patients with IH conducted in 2012 reported promising results for captopril treatment[143]. Shortly after that, it was contradicted by a small retrospective review from Australia, assessing patients with IH who had to discontinue treatment with prednisolone because of steroid-induced hypertension. Of the patients who received captopril after discontinuing prednisolone, 33% demonstrated no changes in IH and 58% demonstrated a worsening[144]. More prospective randomized studies are needed to confirm or disprove these findings.
Oral itraconazole: Recently, efficacy of oral itraconazole was reported in six infants with IH. An obvious clinical improvement was noted in all cases during a 3-mo period, with an improvement of 80%-100%. Side effects were mild and limited[145]. The exact mechanism of itraconazole effectiveness is not yet fully understood, but it seems that itraconazole has an anti-angiogenic effect by inhibiting the VEGRF-2[146]. The future will teach us what itraconazole adds to the therapeutic arsenal for IHs.
The number of prospective studies of IH and its treatment has increased rapidly. Especially since the discovery of propranolol for this indication, the need for validated and reliable instruments to measure IH severity and activity in clinical trials has become an important issue. In 2011, the Hemangioma Activity Score (HAS) was developed, which provided a total activity score by measuring the swelling, color, and ulceration of IH. HAS seems to be suitable for evaluating IH activity and response to treatment over time[147,148]. In 2012, the Hemangioma Investigator Group Research Core developed another scoring system, the Hemangioma Severity Scale (HSS)[149]. The HSS not only takes the objective items, such as size, location, and complications into account, but it also assesses the subjective items, such as pain and risk of disfigurement[149]. Recently, a group of Bulgarian dermatologists presented the Hemangioma Activity and Severity Index[150].
Time will tell which scoring system has the best qualities to be implemented in clinical practice and used for research purposes.
It is well known that visible abnormalities, such as IH, may affect the quality of life (QoL) of children or their parents/caregivers. Several studies have tried to measure the impact of IH on children and their parents. Until recently, either validated non-IH-specific or non-validated but IH-specific questionnaires have been used, providing controversial information[151-153]. This controversy may be explained by the absence of attention to impact of IH-specific factors (e.g., localization, size, and complications) in non-IH-specific questionnaires or by use of non-validated IH-specific questionnaires. Most of them measure the overall psychosocial well-being instead of measuring a specific IH-related psychosocial impact[151]. In February 2015, Chamlin et al[154] presented a validated IH-specific QoL questionnaire. It is only matter of time before the first reports of the impact of IHs on the QoL of children and their parents will appear using this validated, IH-specific questionnaire, giving more reliable information. These reports will be followed by studies on the effects of different treatments on QoL. This information will provide us with the tools to optimally deploy the therapeutic arsenal for IHs.
The discovery that propranolol is efficacious in the treatment of IH has led to an upsurge in publications, increasing our knowledge of this subject. In this review, we provided the most up-to-date information about the pathophysiology, variations in clinical presentation, and natural history of IHs. We looked at possible working mechanisms of several treatments and current worries regarding the treatment of first choice, propranolol. Finally, we provided an overview of the instruments measuring IH severity and/or activity and IH-related QoL.
P- Reviewer: Fernandez-Pineda I, Ji Y S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Kanada KN, Merin MR, Munden A, Friedlander SF. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Hoornweg MJ, Smeulders MJ, Ubbink DT, van der Horst CM. The prevalence and risk factors of infantile haemangiomas: a case-control study in the Dutch population. Paediatr Perinat Epidemiol. 2012;26:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol. 2008;25:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 357] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 4. | Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218-222. [PubMed] |
| 5. | Metry D. Update on hemangiomas of infancy. Curr Opin Pediatr. 2004;16:373-377. [PubMed] |
| 6. | Metry DW, Hawrot A, Altman C, Frieden IJ. Association of solitary, segmental hemangiomas of the skin with visceral hemangiomatosis. Arch Dermatol. 2004;140:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Hsi Dickie B, Fishman SJ, Azizkhan RG. Hepatic vascular tumors. Semin Pediatr Surg. 2014;23:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 363] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 9. | Drolet BA, Swanson EA, Frieden IJ. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr. 2008;153:712-75, 715.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Rasul S. Clinical characteristics and risk factors for infantile hemangioma--a case control study. Eur J Pediatr Surg. 2014;24:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Maguiness SM, Frieden IJ. Current management of infantile hemangiomas. Semin Cutan Med Surg. 2010;29:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Maguiness SM, Frieden IJ. Management of difficult infantile haemangiomas. Arch Dis Child. 2012;97:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1472] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 14. | Kuroda T, Hoshino K, Nosaka S, Shiota Y, Nakazawa A, Takimoto T. Critical hepatic hemangioma in infants: recent nationwide survey in Japan. Pediatr Int. 2014;56:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Bosemani T, Puttgen KB, Huisman TA, Tekes A. Multifocal infantile hepatic hemangiomas--imaging strategy and response to treatment after propranolol and steroids including review of the literature. Eur J Pediatr. 2012;171:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Lawley LP, Siegfried E, Todd JL. Propranolol treatment for hemangioma of infancy: risks and recommendations. Pediatr Dermatol. 2009;26:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Hermans DJ, Bauland CG, Zweegers J, van Beynum IM, van der Vleuten CJ. Propranolol in a case series of 174 patients with complicated infantile haemangioma: indications, safety and future directions. Br J Dermatol. 2013;168:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Langley A, Pope E. Propranolol and central nervous system function: potential implications for paediatric patients with infantile haemangiomas. Br J Dermatol. 2015;172:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Lee KC, Bercovitch L. Update on infantile hemangiomas. Semin Perinatol. 2013;37:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | North PE, Waner M, Mizeracki A, Mrak RE, Nicholas R, Kincannon J, Suen JY, Mihm MC. A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol. 2001;137:559-570. [PubMed] |
| 22. | Phung TL, Hochman M. Pathogenesis of infantile hemangioma. Facial Plast Surg. 2012;28:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Barnés CM, Huang S, Kaipainen A, Sanoudou D, Chen EJ, Eichler GS, Guo Y, Yu Y, Ingber DE, Mulliken JB. Evidence by molecular profiling for a placental origin of infantile hemangioma. Proc Natl Acad Sci USA. 2005;102:19097-19102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Bauland CG, van Steensel MA, Steijlen PM, Rieu PN, Spauwen PH. The pathogenesis of hemangiomas: a review. Plast Reconstr Surg. 2006;117:29e-35e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Burton BK, Schulz CJ, Angle B, Burd LI. An increased incidence of haemangiomas in infants born following chorionic villus sampling (CVS). Prenat Diagn. 1995;15:209-214. [PubMed] |
| 26. | López Gutiérrez JC, Avila LF, Sosa G, Patron M. Placental anomalies in children with infantile hemangioma. Pediatr Dermatol. 2007;24:353-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Munden A, Butschek R, Tom WL, Marshall JS, Poeltler DM, Krohne SE, Alió AB, Ritter M, Friedlander DF, Catanzarite V. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 28. | Pittman KM, Losken HW, Kleinman ME, Marcus JR, Blei F, Gurtner GC, Marchuk DA. No evidence for maternal-fetal microchimerism in infantile hemangioma: a molecular genetic investigation. J Invest Dermatol. 2006;126:2533-2538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Walter JW, North PE, Waner M, Mizeracki A, Blei F, Walker JW, Reinisch JF, Marchuk DA. Somatic mutation of vascular endothelial growth factor receptors in juvenile hemangioma. Genes Chromosomes Cancer. 2002;33:295-303. [PubMed] |
| 30. | Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 268] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 31. | Ritter MR, Dorrell MI, Edmonds J, Friedlander SF, Friedlander M. Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proc Natl Acad Sci USA. 2002;99:7455-7460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Chang J, Most D, Bresnick S, Mehrara B, Steinbrech DS, Reinisch J, Longaker MT, Turk AE. Proliferative hemangiomas: analysis of cytokine gene expression and angiogenesis. Plast Reconstr Surg. 1999;103:1-9; discussion 10. [PubMed] |
| 33. | Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952-958. [PubMed] |
| 34. | Yu Y, Flint AF, Mulliken JB, Wu JK, Bischoff J. Endothelial progenitor cells in infantile hemangioma. Blood. 2004;103:1373-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Boscolo E, Bischoff J. Vasculogenesis in infantile hemangioma. Angiogenesis. 2009;12:197-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 36. | Khan ZA, Boscolo E, Picard A, Psutka S, Melero-Martin JM, Bartch TC, Mulliken JB, Bischoff J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592-2599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Mihm MC, Nelson JS. Hypothesis: the metastatic niche theory can elucidate infantile hemangioma development. J Cutan Pathol. 2010;37 Suppl 1:83-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Chang EI, Chang EI, Thangarajah H, Hamou C, Gurtner GC. Hypoxia, hormones, and endothelial progenitor cells in hemangioma. Lymphat Res Biol. 2007;5:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Kleinman ME, Greives MR, Churgin SS, Blechman KM, Chang EI, Ceradini DJ, Tepper OM, Gurtner GC. Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma. Arterioscler Thromb Vasc Biol. 2007;27:2664-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Drolet BA, Frieden IJ. Characteristics of infantile hemangiomas as clues to pathogenesis: does hypoxia connect the dots? Arch Dermatol. 2010;146:1295-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Janmohamed SR, Brinkhuizen T, den Hollander JC, Madern GC, de Laat PC, van Steensel MA, Oranje AP. Support for the hypoxia theory in the pathogenesis of infantile haemangioma. Clin Exp Dermatol. 2015;40:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. [PubMed] |
| 43. | Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1738] [Cited by in RCA: 1787] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 44. | Kimura S, Kitadai Y, Tanaka S, Kuwai T, Hihara J, Yoshida K, Toge T, Chayama K. Expression of hypoxia-inducible factor (HIF)-1alpha is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur J Cancer. 2004;40:1904-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Rathmell WK, Acs G, Simon MC, Vaughn DJ. HIF transcription factor expression and induction of hypoxic response genes in a retroperitoneal angiosarcoma. Anticancer Res. 2004;24:167-169. [PubMed] |
| 46. | Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655-29660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 368] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 47. | Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 687] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 48. | Léauté-Labrèze C, Prey S, Ezzedine K. Infantile haemangioma: part I. Pathophysiology, epidemiology, clinical features, life cycle and associated structural abnormalities. J Eur Acad Dermatol Venereol. 2011;25:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Hoeger PH. Infantile haemangioma: new aspects on the pathogenesis of the most common skin tumour in children. Br J Dermatol. 2011;164:234-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18:S117-S120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Restrepo R, Palani R, Cervantes LF, Duarte AM, Amjad I, Altman NR. Hemangiomas revisited: the useful, the unusual and the new. Part 1: overview and clinical and imaging characteristics. Pediatr Radiol. 2011;41:895-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin Pediatr Surg. 2014;23:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW, Newell B. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr. 2007;150:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 281] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 54. | Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, Burrows P, Frieden IJ, Garzon MC, Lopez-Gutierrez JC. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136:e203-e214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 807] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 55. | Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 263] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 56. | Corella F, Garcia-Navarro X, Ribe A, Alomar A, Baselga E. Abortive or minimal-growth hemangiomas: Immunohistochemical evidence that they represent true infantile hemangiomas. J Am Acad Dermatol. 2008;58:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Suh KY, Frieden IJ. Infantile hemangiomas with minimal or arrested growth: a retrospective case series. Arch Dermatol. 2010;146:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Bauland CG, Lüning TH, Smit JM, Zeebregts CJ, Spauwen PH. Untreated hemangiomas: growth pattern and residual lesions. Plast Reconstr Surg. 2011;127:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 59. | Chang LC, Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 428] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 60. | Brandling-Bennett HA, Metry DW, Baselga E, Lucky AW, Adams DM, Cordisco MR, Frieden IJ. Infantile hemangiomas with unusually prolonged growth phase: a case series. Arch Dermatol. 2008;144:1632-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Enjolras O, Gelbert F. Superficial hemangiomas: associations and management. Pediatr Dermatol. 1997;14:173-179. [PubMed] |
| 62. | Frieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol. 1996;132:307-311. [PubMed] |
| 63. | Metry DW, Dowd CF, Barkovich AJ, Frieden IJ. The many faces of PHACE syndrome. J Pediatr. 2001;139:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 199] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 64. | Metry DW, Haggstrom AN, Drolet BA, Baselga E, Chamlin S, Garzon M, Horii K, Lucky A, Mancini AJ, Newell B. A prospective study of PHACE syndrome in infantile hemangiomas: demographic features, clinical findings, and complications. Am J Med Genet A. 2006;140:975-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 65. | Metry D, Heyer G, Hess C, Garzon M, Haggstrom A, Frommelt P, Adams D, Siegel D, Hall K, Powell J. Consensus Statement on Diagnostic Criteria for PHACE Syndrome. Pediatrics. 2009;124:1447-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 66. | Haggstrom AN, Garzon MC, Baselga E, Chamlin SL, Frieden IJ, Holland K, Maguiness S, Mancini AJ, McCuaig C, Metry DW. Risk for PHACE syndrome in infants with large facial hemangiomas. Pediatrics. 2010;126:e418-e426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 67. | Hartemink DA, Chiu YE, Drolet BA, Kerschner JE. PHACES syndrome: a review. Int J Pediatr Otorhinolaryngol. 2009;73:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Iacobas I, Burrows PE, Frieden IJ, Liang MG, Mulliken JB, Mancini AJ, Kramer D, Paller AS, Silverman R, Wagner AM. LUMBAR: association between cutaneous infantile hemangiomas of the lower body and regional congenital anomalies. J Pediatr. 2010;157:795-801.e1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 69. | Stockman A, Boralevi F, Taïeb A, Léauté-Labrèze C. SACRAL syndrome: spinal dysraphism, anogenital, cutaneous, renal and urologic anomalies, associated with an angioma of lumbosacral localization. Dermatology. 2007;214:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 70. | Girard C, Bigorre M, Guillot B, Bessis D. PELVIS Syndrome. Arch Dermatol. 2006;142:884-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 71. | Drolet BA, Chamlin SL, Garzon MC, Adams D, Baselga E, Haggstrom AN, Holland KE, Horii KA, Juern A, Lucky AW. Prospective study of spinal anomalies in children with infantile hemangiomas of the lumbosacral skin. J Pediatr. 2010;157:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Mulliken JB, Young AE. Treatment of hemangiomas. Mcallister L (editor). Vascular Birthmarks: Hemangiomas and Malformations WB Saunders. Philadelphia, PA 1988; 77-103. |
| 73. | Frieden IJ. Infantile hemangioma research: looking backward and forward. J Invest Dermatol. 2011;131:2345-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Janmohamed SR, Madern GC, de Laat PC, Oranje AP. Educational paper: therapy of infantile haemangioma--history and current state (part II). Eur J Pediatr. 2015;174:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Boehm DK, Kobrinsky NL. Treatment of cavernous hemangioma with vincristine. Ann Pharmacother. 1993;27:981. [PubMed] |
| 76. | Ames JA, Sykes JM. Current trends in medical management of infantile hemangioma. Curr Opin Otolaryngol Head Neck Surg. 2015;23:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Schirner M, Hoffmann J, Menrad A, Schneider MR. Antiangiogenic chemotherapeutic agents: characterization in comparison to their tumor growth inhibition in human renal cell carcinoma models. Clin Cancer Res. 1998;4:1331-1336. [PubMed] |
| 78. | Pérez-Valle S, Peinador M, Herraiz P, Saénz P, Montoliu G, Vento M. Vincristine, an efficacious alternative for diffuse neonatal haemangiomatosis. Acta Paediatr. 2010;99:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Orchard PJ, Smith CM, Woods WG, Day DL, Dehner LP, Shapiro R. Treatment of haemangioendotheliomas with alpha interferon. Lancet. 1989;2:565-567. [PubMed] |
| 80. | Sgonc R, Fuerhapter C, Boeck G, Swerlick R, Fritsch P, Sepp N. Induction of apoptosis in human dermal microvascular endothelial cells and infantile hemangiomas by interferon-alpha. Int Arch Allergy Immunol. 1998;117:209-214. [PubMed] |
| 81. | Mabeta P, Pepper MS. Hemangiomas - current therapeutic strategies. Int J Dev Biol. 2011;55:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Dubois J, Hershon L, Carmant L, Bélanger S, Leclerc JM, David M. Toxicity profile of interferon alfa-2b in children: A prospective evaluation. J Pediatr. 1999;135:782-785. [PubMed] |
| 83. | Garzon MC, Lucky AW, Hawrot A, Frieden IJ. Ultrapotent topical corticosteroid treatment of hemangiomas of infancy. J Am Acad Dermatol. 2005;52:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Martinez MI, Sanchez-Carpintero I, North PE, Mihm MC. Infantile hemangioma: clinical resolution with 5% imiquimod cream. Arch Dermatol. 2002;138:881-884; discussion 884. [PubMed] |
| 85. | Sidbury R, Neuschler N, Neuschler E, Sun P, Wang XQ, Miller R, Tomai M, Puscasiu E, Gugneja S, Paller AS. Topically applied imiquimod inhibits vascular tumor growth in vivo. J Invest Dermatol. 2003;121:1205-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Hazen PG, Carney JF, Engstrom CW, Turgeon KL, Reep MD, Tanphaichitr A. Proliferating hemangioma of infancy: successful treatment with topical 5% imiquimod cream. Pediatr Dermatol. 2005;22:254-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 88. | Qiu Y, Ma G, Yang J, Hu X, Chen H, Jin Y, Lin X. Imiquimod 5% cream versus timolol 0.5% ophthalmic solution for treating superficial proliferating infantile haemangiomas: a retrospective study. Clin Exp Dermatol. 2013;38:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 89. | Hu L, Huang HZ, Li X, Lin XX, Li W. Open-label nonrandomized left-right comparison of imiquimod 5% ointment and timolol maleate 0.5% eye drops in the treatment of proliferating superficial infantile hemangioma. Dermatology. 2015;230:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, Chun RH, Garzon MC, Holland KE, Liberman L. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 91. | Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, Phillips RJ, Caceres H, Lopez Gutierrez JC, Ballona R. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 499] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 92. | Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:e259-e266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 93. | Malik MA, Menon P, Rao KL, Samujh R. Effect of propranolol vs prednisolone vs propranolol with prednisolone in the management of infantile hemangioma: a randomized controlled study. J Pediatr Surg. 2013;48:2453-2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 94. | Zaher H, Rasheed H, Esmat S, Hegazy RA, Gawdat HI, Hegazy RA, El-Komy M, Abdelhalim DM. Propranolol and infantile hemangiomas: different routes of administration, a randomized clinical trial. Eur J Dermatol. 2013;23:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 95. | Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 342] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 96. | Ji Y, Chen S, Xu C, Li L, Xiang B. The use of propranolol in the treatment of infantile haemangiomas: an update on potential mechanisms of action. Br J Dermatol. 2015;172:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 97. | Bryan BA. Reconsidering the Use of Propranolol in the Treatment of Cosmetic Infantile Hemangiomas. Angiol. 2013;1:e101. [DOI] [Full Text] |
| 98. | Gonski K, Wargon O. Retrospective follow up of gross motor development in children using propranolol for treatment of infantile haemangioma at Sydney Children’s Hospital. Australas J Dermatol. 2014;55:209-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Gesell A. The mental growth of the pre-school child: A psychological outline of normal development from birth to the sixth year, including a system of developmental diagnosis. New York: MacMillan Co 1925; . |
| 100. | Gesell A, Amatruda CS. Developmental diagnosis; normal and abnormal child development. Oxford: Hoeber 1941; . |
| 101. | Moyakine AV, Hermans DJ, Fuijkschot J, van der Vleuten CJ. Propranolol treatment of infantile hemangiomas does not negatively affect psychomotor development. J Am Acad Dermatol. 2015;73:341-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Chan H, McKay C, Adams S, Wargon O. RCT of timolol maleate gel for superficial infantile hemangiomas in 5- to 24-week-olds. Pediatrics. 2013;131:e1739-e1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 103. | Tawfik AA, Alsharnoubi J. Topical timolol solution versus laser in treatment of infantile hemangioma: a comparative study. Pediatr Dermatol. 2015;32:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Fost NC, Esterly NB. Successful treatment of juvenile hemangiomas with prednisone. J Pediatr. 1968;72:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 164] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 105. | Zarem HA, Edgerton MT. Induced resolution of cavernous hemangiomas following prednisolone therapy. Plast Reconstr Surg. 1967;39:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 200] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 106. | Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J. Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med. 2010;362:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 107. | Bennett ML, Fleischer AB, Chamlin SL, Frieden IJ. Oral corticosteroid use is effective for cutaneous hemangiomas: an evidence-based evaluation. Arch Dermatol. 2001;137:1208-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 108. | Xu SQ, Jia RB, Zhang W, Zhu H, Ge SF, Fan XQ. Beta-blockers versus corticosteroids in the treatment of infantile hemangioma: an evidence-based systematic review. World J Pediatr. 2013;9:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | Grover C, Kedar A, Arora P, Lal B. Efficacy of oral prednisolone use in the treatment of infantile hemangiomas in Indian children. Pediatr Dermatol. 2011;28:502-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Boon LM, MacDonald DM, Mulliken JB. Complications of systemic corticosteroid therapy for problematic hemangioma. Plast Reconstr Surg. 1999;104:1616-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 166] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 111. | Brown BZ, Huffaker G. Local injection of steroids for juvenile hemangiomas which disturb the visual axis. Ophthalmic Surg. 1982;13:630-633. [PubMed] |
| 112. | Egbert JE, Schwartz GS, Walsh AW. Diagnosis and treatment of an ophthalmic artery occlusion during an intralesional injection of corticosteroid into an eyelid capillary hemangioma. Am J Ophthalmol. 1996;121:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Shorr N, Seiff SR. Central retinal artery occlusion associated with periocular corticosteroid injection for juvenile hemangioma. Ophthalmic Surg. 1986;17:229-231. [PubMed] |
| 114. | Leone F, Benanti E, Marchesi A, Marcelli S, Gazzola R, Vaienti L. Surgical excision of Infantile Haemangiomas: a technical refinement to prevent bleeding complications. Pediatr Med Chir. 2014;36:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 115. | Nomura T, Osaki T, Ishinagi H, Ejiri H, Terashi H. Simple and easy surgical technique for infantile hemangiomas: intralesional excision and primary closure. Eplasty. 2015;15:e3. [PubMed] |
| 116. | Mulliken JB, Rogers GF, Marler JJ. Circular excision of hemangioma and purse-string closure: the smallest possible scar. Plast Reconstr Surg. 2002;109:1544-1554; discussion 1555. [PubMed] |
| 117. | Landthaler M, Hohenleutner U, el-Raheem TA. Laser therapy of childhood haemangiomas. Br J Dermatol. 1995;133:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 118. | Hohenleutner S, Badur-Ganter E, Landthaler M, Hohenleutner U. Long-term results in the treatment of childhood hemangioma with the flashlamp-pumped pulsed dye laser: an evaluation of 617 cases. Lasers Surg Med. 2001;28:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 119. | Batta K, Goodyear HM, Moss C, Williams HC, Hiller L, Waters R. Randomised controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangiomas: results of a 1-year analysis. Lancet. 2002;360:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 120. | Kwon SH, Choi JW, Byun SY, Kim BR, Park KC, Youn SW, Huh CH, Na JI. Effect of early long-pulse pulsed dye laser treatment in infantile hemangiomas. Dermatol Surg. 2014;40:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 121. | Zhong SX, Tao YC, Zhou JF, Liu YY, Yao L, Li SS. Infantile Hemangioma: Clinical Characteristics and Efficacy of Treatment with the Long-Pulsed 1,064-nm Neodymium-Doped Yttrium Aluminum Garnet Laser in 794 Chinese Patients. Pediatr Dermatol. 2015;32:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 122. | Kono T, Sakurai H, Groff WF, Chan HH, Takeuchi M, Yamaki T, Soejima K, Nozaki M. Comparison study of a traditional pulsed dye laser versus a long-pulsed dye laser in the treatment of early childhood hemangiomas. Lasers Surg Med. 2006;38:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 123. | Ulrich H, Bäumler W, Hohenleutner U, Landthaler M. Neodymium-YAG Laser for hemangiomas and vascular malformations -- long term results. J Dtsch Dermatol Ges. 2005;3:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 124. | Pancar GS, Aydin F, Senturk N, Bek Y, Canturk MT, Turanli AY. Comparison of the 532-nm KTP and 1064-nm Nd: YAG lasers for the treatment of cherry angiomas. J Cosmet Laser Ther. 2011;13:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 125. | Brauer JA, Geronemus RG. Laser treatment in the management of infantile hemangiomas and capillary vascular malformations. Tech Vasc Interv Radiol. 2013;16:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 126. | Filippi L, Dal Monte M, Casini G, Daniotti M, Sereni F, Bagnoli P. Infantile hemangiomas, retinopathy of prematurity and cancer: a common pathogenetic role of the β-adrenergic system. Med Res Rev. 2015;35:619-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 127. | Doshan HD, Rosenthal RR, Brown R, Slutsky A, Applin WJ, Caruso FS. Celiprolol, atenolol and propranolol: a comparison of pulmonary effects in asthmatic patients. J Cardiovasc Pharmacol. 1986;8 Suppl 4:S105-S108. [PubMed] |
| 128. | Raphaël MF, de Graaf M, Breugem CC, Pasmans SG, Breur JM. Atenolol: a promising alternative to propranolol for the treatment of hemangiomas. J Am Acad Dermatol. 2011;65:420-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 129. | Ábarzúa-Araya A, Navarrete-Dechent CP, Heusser F, Retamal J, Zegpi-Trueba MS. Atenolol versus propranolol for the treatment of infantile hemangiomas: a randomized controlled study. J Am Acad Dermatol. 2014;70:1045-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 130. | Pope E, Chakkittakandiyil A, Lara-Corrales I, Maki E, Weinstein M. Expanding the therapeutic repertoire of infantile haemangiomas: cohort-blinded study of oral nadolol compared with propranolol. Br J Dermatol. 2013;168:222-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 131. | Woods PB, Robinson ML. An investigation of the comparative liposolubilities of beta-adrenoceptor blocking agents. J Pharm Pharmacol. 1981;33:172-173. [PubMed] |
| 132. | Randhawa HK, Sibbald C, Garcia Romero MT, Pope E. Oral Nadolol for the Treatment of Infantile Hemangiomas: A Single-Institution Retrospective Cohort Study. Pediatr Dermatol. 2015;32:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 133. | Blanchet C, Nicollas R, Bigorre M, Amedro P, Mondain M. Management of infantile subglottic hemangioma: acebutolol or propranolol? Int J Pediatr Otorhinolaryngol. 2010;74:959-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 134. | Hammill AM, Wentzel M, Gupta A, Nelson S, Lucky A, Elluru R, Dasgupta R, Azizkhan RG, Adams DM. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57:1018-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 417] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 135. | Kaylani S, Theos AJ, Pressey JG. Treatment of infantile hemangiomas with sirolimus in a patient with PHACE syndrome. Pediatr Dermatol. 2013;30:e194-e197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 136. | Medici D, Olsen BR. Rapamycin inhibits proliferation of hemangioma endothelial cells by reducing HIF-1-dependent expression of VEGF. PLoS One. 2012;7:e42913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 137. | Greenberger S, Yuan S, Walsh LA, Boscolo E, Kang KT, Matthews B, Mulliken JB, Bischoff J. Rapamycin suppresses self-renewal and vasculogenic potential of stem cells isolated from infantile hemangioma. J Invest Dermatol. 2011;131:2467-2476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 138. | Itinteang T, Tan ST, Brasch H, Day DJ. Haemogenic endothelium in infantile haemangioma. J Clin Pathol. 2010;63:982-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 139. | Itinteang T, Brasch HD, Tan ST, Day DJ. Expression of components of the renin-angiotensin system in proliferating infantile haemangioma may account for the propranolol-induced accelerated involution. J Plast Reconstr Aesthet Surg. 2011;64:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 140. | Stephenson TJ, Broughton Pipkin F, Elias-Jones AC. Factors influencing plasma renin and renin substrate in premature infants. Arch Dis Child. 1991;66:1150-1154. [PubMed] |
| 141. | Youmbissi TJ, Tedong F, Fairbank ST, Blackett-Ngu K, Mbede J. Plasma renin activity studies in a group of African neonates and children. J Trop Pediatr. 1990;36:128-130. [PubMed] |
| 142. | Broughton Pipkin F, Smales OR, O’Callaghan M. Renin and angiotensin levels in children. Arch Dis Child. 1981;56:298-302. [PubMed] |
| 143. | Tan ST, Itinteang T, Day DJ, O’Donnell C, Mathy JA, Leadbitter P. Treatment of infantile haemangioma with captopril. Br J Dermatol. 2012;167:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 144. | Christou EM, Wargon O. Effect of captopril on infantile haemangiomas: a retrospective case series. Australas J Dermatol. 2012;53:216-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 145. | Ran Y, Chen S, Dai Y, Kang D, Lama J, Ran X, Zhuang K. Successful treatment of oral itraconazole for infantile hemangiomas: a case series. J Dermatol. 2015;42:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 146. | Nacev BA, Grassi P, Dell A, Haslam SM, Liu JO. The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells. J Biol Chem. 2011;286:44045-44056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 147. | Janmohamed SR, de Waard-van der Spek FB, Madern GC, de Laat PC, Hop WC, Oranje AP. Scoring the proliferative activity of haemangioma of infancy: the Haemangioma Activity Score (HAS). Clin Exp Dermatol. 2011;36:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 148. | Janmohamed SR, van Oosterhout M, de Laat PC, van Rosmalen J, Madern GC, Oranje AP. Scoring the therapeutic effects of oral propranolol for infantile hemangioma: A prospective study comparing the Hemangioma Activity Score (HAS) with the Hemangioma Severity Scale (HSS). J Am Acad Dermatol. 2015;73:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 149. | Haggstrom AN, Beaumont JL, Lai JS, Adams DM, Drolet BA, Frieden IJ, Garzon MC, Holland KE, Horii KA, Lucky AW. Measuring the severity of infantile hemangiomas: instrument development and reliability. Arch Dermatol. 2012;148:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 150. | Semkova K, Kazandjieva J, Kadurina M, Tsankov N. Hemangioma Activity and Severity Index (HASI), an instrument for evaluating infantile hemangioma: development and preliminary validation. Int J Dermatol. 2015;54:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 151. | Zweegers J, van der Vleuten CJ. The psychosocial impact of an infantile haemangioma on children and their parents. Arch Dis Child. 2012;97:922-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 152. | Cohen-Barak E, Rozenman D, Shani Adir A. Infantile haemangiomas and quality of life. Arch Dis Child. 2013;98:676-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Hoornweg MJ, Grootenhuis MA, van der Horst CM. Health-related quality of life and impact of haemangiomas on children and their parents. J Plast Reconstr Aesthet Surg. 2009;62:1265-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 154. | Chamlin SL, Mancini AJ, Lai JS, Beaumont JL, Cella D, Adams D, Drolet B, Baselga E, Frieden IJ, Garzon M. Development and Validation of a Quality-of-Life Instrument for Infantile Hemangiomas. J Invest Dermatol. 2015;135:1533-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |