Peer-review started: August 15, 2014
First decision: October 17, 2014
Revised: November 14, 2014
Accepted: November 27, 2014
Article in press: December 31, 2014
Published online: February 2, 2015
Processing time: 158 Days and 19.7 Hours
During the past few decades, the investigative tech-nologies of molecular biology - especially sequencing - underwent huge advances, leading to the sequencing of the entire human genome, as well as the identification of several candidate genes and the causative genetic variations that are responsible for monogenic skin diseases. These advances provided a solid basis for subsequent studies elucidating mechanisms of monogenic skin diseases and improving our understanding of common skin diseases. Furthermore, these discoveries also contributed to the development of novel therapeutic modalities for monogenic skin diseases. In this review, we have used the disease spectrum caused by mutations in the CYLD gene - Brooke-Spiegler syndrome, familial cylindromatosis and multiple familial trichoepithelioma type 1 - as a model for demonstrating the knowledge explosion for this group of diseases.
Core tip: Although dermatology is a morphology-orientated specialty, genetic investigation can help understand the events taking place in the skin of the affected patients. Genetic investigation of Brooke-Spiegler syndrome, familial cylindromatosis and multiple familial trichoepithelioma type 1 further supported the clinical hypothesis that these monogenic skin diseases are not different entities, but rather clinical variants of a disease spectrum caused by mutations in the cylindromatosis (CYLD) gene. In addition to understanding the underlying mechanisms of these allelic variants, genetic investigation can also accelerate the development of novel therapeutic modalities, such as therapy using tropomyosin-receptor-kinase specific lestaurtinib for patients with germline CYLD mutations.
- Citation: Nagy N, Farkas K, Kemény L, Széll M. Knowledge explosion for monogenic skin diseases. World J Dermatol 2015; 4(1): 44-49
- URL: https://www.wjgnet.com/2218-6190/full/v4/i1/44.htm
- DOI: https://dx.doi.org/10.5314/wjd.v4.i1.44
From ancient times to the present, the basic approach for diagnosing skin diseases has been to classify the diseases according to their visible signs and symptoms. This approach highlights that dermatology is still a highly morphology-orientated specialty. The end of the 18th century saw great breakthroughs in dermatology: the first comprehensive textbook of modern dermatology was published in 1799 by Francesco Bianchi[1] and the first great school of dermatology was established in Paris in 1801[2]. Since that time, the desire to understand the nature of observed skin lesions constantly drives the development of dermatology and the incorporation of novel investigative methods into its everyday practice.
Among these methods, dermatohistopathology has had the highest impact on the diagnosis of skin diseases. Although the microscope was invented by Anton van Leeuwenhoek as early as 1673, the first standardized and classified nomenclature of dyes and stains was prepared only in 1924[3,4]. Since that time, enzyme histochemistry, electron microscopy, polarizing microscopy, immune-histochemistry and in vivo confocal microscopy have all become diagnostic tools in dermatohistopathology and have been integrated into everyday dermatology practices[3,4]. In recent decades, developments in the investigative fields of clinical genetics and genomics have further accelerated our knowledge about skin diseases.
Breeding agricultural plants and animals characterized the pre-Mendel era of genetics[5,6]. After Gregor Mendel established the basic rules of heredity in the nineteenth century[7], several major discoveries, such as the identification of DNA as the material encoding inheritable information, of the genetic code and of the mechanisms of gene expression, have initiated the era of molecular genetics[8,9]. Very recently, the enormous technical development of sequencing methods and platforms has resulted in large-scale genomic projects, which produce amounts of data that were unimaginable a few decades ago[10,11].
These discoveries and techniques have been used to identify several normal genetic variations, as well as candidate genes and their disease-causing mutations, accelerating the elucidation of the genetic background of several monogenic skin diseases. In this review, we present the knowledge explosion for monogenic skin diseases, using as an example the disease spectrum caused by mutations in the CYLD gene, which involves Brooke-Spiegler syndrome (BSS) (OMIM 605041), familial cylindromatosis (FC) (OMIM 132700) and multiple familial trichoepithelioma type 1 (MFT1) (OMIM 601606) (Table 1).
| Name of clinical variant | Familial cylindromatosis | Brooke-Spiegler syndrome | Multiple familial trichoepithelioma type 1 |
| Clinical symptoms | Cylindromas | Cylindromas Trichoepitheliomas Spiradenomas | Trichoepitheliomas |
| Genetic background | Any type of mutation | Any type of mutation | Any type, but mainly missense mutations |
BSS is a rare monogenic skin disease characterized by the development of a wide variety of benign skin appendageal tumors, such as cylindromas, trichoepitheliomas and/or spiradenomas[12,13]. BSS was named after the two physicians who first reported these neoplasms in 1892 and 1899: Henry G Brooke and Eduard Spiegler, respectively[14,15]. FC, which was originally considered a separate rare disease, is characterized by the development of cylindromas[16]. FC was first reported in 1842 and 1899 by Henry Ancell and Eduard Spiegler, respectively[15,17]. MFT1, which was also reported as another rare entity, is characterized by the development of trichoepitheliomas[16] and was first reported in 1892 by Brooke[14] and Fordyce[18].
Comparing the clinical features of these tumors, cylindromas are benign, skin-colored tumors usually present as multiple turban-like protrusions on the scalp, trichoepitheliomas are small, benign, skin-colored tumors, typically located at the center of the face, and spiradenomas are purple, benign, nodular tumors, usually located on the trunk or limbs[19]. The histological characteristics of cylindromas are dermal nodules of epithelial cells lined by membrane-like basement material and arranged in a “jigsaw puzzle” pattern, of trichoepitheliomas are dermal nodules of basaloid cells with peripheral palisades arranged in nests or cribriform patterns and of spiradenomas are dermal nodules comprised of large light-colored epithelial cells with abundant cytoplasm at the center and small darker epithelial cells at the periphery[20-22]. Hybrid tumors can also occur, such as spiradenocylindromas, which exhibit the characteristics of both cylindromas and spiradenomas[23].
The candidate gene for BSS was first mapped to chromosome 16q12-q13 in 2000[24], and the causative CYLD gene and its first pathogenic mutation was identified in an affected German pedigree in 2002[25]. The candidate gene for FC was first mapped to chromosome 16q12-q13 in 1995[26]; however, the causative CYLD gene and the first 21 pathogenic mutations were identified as late as 2000[27]. It was first suggested in 1995 that MFT1 and FC may be caused by the dysfunction of the same gene, since both type of tumors can occur in the same patient or in different patients within a single family[28]. The causative gene for MFT1 was identified as CYLD, and the first pathogenic mutation was detected in an affected Turkish family in 2003[29].
These clinical variants - BSS, FC and MFT1 - were originally described as distinct clinical entities. However, due to their overlapping clinical symptoms and their manifestation within the same family, they are currently considered as part of a phenotypic spectrum of the same entity[30-32]. This hypothesis is supported by genetic evidence: several mutations - the c.1112C/A p.S371X, the c.2272C/T p.R758X and the c.2806C/T p.R936X nonsense mutations - lead to the development of all three clinical variants (Table 2)[33-42].
| CYLD cDNA | CYLDprotein | Detected in patients with | Nationality | Ref. |
| c.1112C > A | p.S371X | BSS, FC, MFT1 | American, African American, Irish, Dutch, Austrian, Czech, Slovak, Chinese | Bignell et al[27], 2000; Bowen et al[30], 2005; Saggar et al[32], 2008; Linos et al[40], 2011; Kazakov et al[39], 2011; Grossmann et al[33], 2013; Kacerovska et al[51], 2013; Lv et al[41], 2013; Van den Ouweland et al[36], 2011 |
| c.2272C > T | p.R758X | BSS, FC, MFT1 | American, South African, Austrian, Czech, Dutch, Chinese, Japanese | Bignell et al[27], 2000; Kazakov et al[38], 2009; Kazakov et al[39], 2011; Grossmann et al[33], 2013; Oiso et al[42], 2004; Zhang et al[37], 2006; van den Ouweland et al[36], 2011 |
| c.2806C > T | p.R936X | BSS, FC, MFT1 | American, Canadian, Anglo-Saxon, Czech, Hungarian, Chinese | Bignell et al[27], 2000; Bowen et al[30], 2005; Saggar et al[32], 2008; Kazakov et al[38], 2009; Grossmann et al[33], 2013; Young et al[31], 2006; Nagy et al[35], 2013 |
Presumably, this is due to the fact that the nonsense mutations of the CYLD gene are in general recurrent ones and develop due to de novo events indicating mutational hotspots on the gene[35]. Patients carrying the same nonsense mutation from different mutational events often exhibit extreme phenotypic differences, which might be the consequences of yet unknown genetic factors that modify the development of the phenotype.
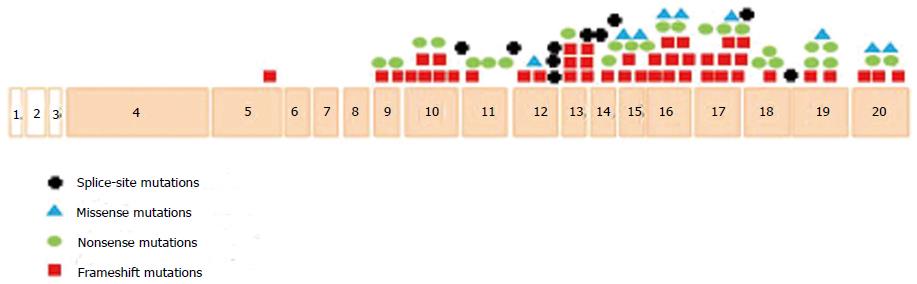
To date, a total of 99 disease-causing CYLD mutations have been reported worldwide (Figure 1)[43-46]. The majority (82%) of CYLD mutations identified to date are located between exon 12 and 20. This finding has a significant diagnostic relevance, as mutation screening of the affected individuals should begin with examination of the exon 12-20 region. Within this region, exons 16 and 17 contain the highest number of mutations (16%). Now that, because the causative mutation can be identified prenatally as well as preimplantation, diagnosis can be offered to affected families. This information can have a huge impact on family planning, since the symptoms of all clinical variants can be very stigmatizing[35].
Several functional studies have been performed to elucidate the underlying mechanism of the CYLD-mutation disease spectrum. The CYLD gene encodes an enzyme with deubiquitinase activity, which is involved in the post-translational modification of its target proteins by removing Lys63-linked ubiquitin chains[47]. CYLD interacts with several members of the NF-ĸB signaling pathway, including the TRAF2, TRAF6, NEMO and BCL3 proteins, acting as a negative regulator[48]. Mutations of the CYLD gene, in general, result in decreased activity of the CYLD enzyme. The reduced activity leads to the hyperubiquitination of interaction partners and influences several signaling pathways, such as the NF-κB pathway, as well as affects several biological processes, such as the development of the skin appendages and tumor formation[34].
It is interesting to note that, although the CYLD protein is expressed in a wide range of human tissues, the reason why dysfunction manifests only in skin symptoms is still unclear[49-51]. Moreover, patients carrying the same mutation from different mutational events often exhibit extreme differences in their clinical and histological manifestations[35]. These differences might be the consequences of yet unknown genetic, environmental and/or lifestyle factors that modify the development of the phenotype. Further studies are needed to elucidate the putative factors that are responsible for the observed late onset of the symptoms, for the development of only skin manifestations and for the great variation in phenotypes and histological findings.
To date, no causative therapy is available for BSS. However, recent gene expression studies demonstrated that tumors with somatic CYLD mutation have impaired TRK signaling and treatment with a small TRK-inhibiting molecule, lestaurtinib, can reduce colony formation and proliferation of tumor cells with somatic CYLD mutation[52]. These data may have huge clinical significance, since lestaurtinib treatment might be a novel therapeutic modality for patients suffering from symptoms caused by germline CYLD mutations.
Although dermatology and genetics are considered separate disciplines, the combination of these two fields has already resulted in enormous improvement in the understanding of monogenic skin diseases, such as the skin-disease spectrum caused by mutations in the CYLD gene. Genetic studies have proved that BSS, FC and MFT1, which were originally considered different entities, result from mutations of the same gene. Moreover, mutations of the CYLD gene have been reported in patients presenting all clinical variants. Genetic screening and the identification of the disease-causing mutation have already been of great significance for family planning in prenatal and preimplantation diagnosis. Furthermore, molecular biological investigation demonstrated that all known CYLD mutations lead to decreased activity of the encoded CYLD deubiquitinase enzyme and, thus, influence several signal transduction pathways. Currently, only symptomatic surgical treatment is available for patients with BSS, FC or MFT1. Gene expression studies of solid tumors carrying the CYLD mutation identified modifications in the TRK signaling pathway and raised the possibility that treatment with lestaurtinib could potentially be a novel therapeutic modality for patients with germline CYLD mutation. Future genetic studies could also provide a solid basis for the development of novel causative therapies that will be more specific and effective than the symptomatic treatments currently available for patients with the FC, BSS and MFT1 variants.
P- Reviewer: Deng H, Garcia-Elorriaga G S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Shelley WB. Major contributors to American dematology - 1876 to 1926. Arch Dermatol. 1976;112 Spec no:1642-1646. [PubMed] |
| 2. | Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI. Fitzpatrick’s Dermatology in General Medicine. 6th ed. New York: McGraw-Hill Professional 2003; 3. |
| 3. | Campbell GA, Sauber L. Getting the most from dermato-pathology. Vet Clin North Am Small Anim Pract. 2007;37:393-402, viii. [PubMed] |
| 4. | Bhawan J. The evolution of dermatopathology -- the American experience. Am J Dermatopathol. 2006;28:67-71. [PubMed] |
| 6. | Hansen MM, Limborg MT, Ferchaud AL, Pujolar JM. The effects of Medieval dams on genetic divergence and demographic history in brown trout populations. BMC Evol Biol. 2014;14:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Mendel G, Corcos AF, Monaghan FV, Weber MC. Gregor Mendel’s Experiments on Plant Hybrids: A Guided Study. New Brunswick, New Jersey: Rutgers University Press 1993; . |
| 8. | Watson JD, CRICK FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737-738. [PubMed] |
| 9. | Min Jou W, Haegeman G, Ysebaert M, Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972;237:82-88. [PubMed] |
| 10. | Sanger F, Air GM, Barrell BG, Brown NL, Coulson AR, Fiddes CA, Hutchison CA, Slocombe PM, Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977;265:687-695. [PubMed] |
| 11. | Stoneking M, Krause J. Learning about human population history from ancient and modern genomes. Nat Rev Genet. 2011;12:603-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Weyers W, Nilles M, Eckert F, Schill WB. Spiradenomas in Brooke-Spiegler syndrome. Am J Dermatopathol. 1993;15:156-161. [PubMed] |
| 13. | Blake PW, Toro JR. Update of cylindromatosis gene (CYLD) mutations in Brooke-Spiegler syndrome: novel insights into the role of deubiquitination in cell signaling. Hum Mutat. 2009;30:1025-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Jungehülsing M, Wagner M, Damm M. Turban tumour with involvement of the parotid gland. J Laryngol Otol. 1999;113:779-783. [PubMed] |
| 15. | Lavorato FG, Miller MD, Obadia DL, Nery NS, Silva RS. Syndrome in question. Brooke-Spiegler syndrome. An Bras Dermatol. 2014;89:175-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Lee DA, Grossman ME, Schneiderman P, Celebi JT. Genetics of skin appendage neoplasms and related syndromes. J Med Genet. 2005;42:811-819. [PubMed] |
| 17. | Ancell H. History of a remarkable case of tumours, developed on the head and face; accompanied with a similar disease in the abdomen. Med Chir Trans. 1942;25:227-306.11. [PubMed] |
| 18. | Centurión SA, Schwartz RA, Lambert WC. Trichoepithelioma papulosum multiplex. J Dermatol. 2000;27:137-143. [PubMed] |
| 19. | Uede K, Yamamoto Y, Furukawa F. Brooke-Spiegler syndrome associated with cylindroma, trichoepithelioma, spiradenoma, and syringoma. J Dermatol. 2004;31:32-38. [PubMed] |
| 20. | Lian F, Cockerell CJ. Cutaneous appendage tumors: familial cylindromatosis and associated tumors update. Adv Dermatol. 2005;21:217-234. [PubMed] |
| 21. | Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms--part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol. 2007;60:129-144. [PubMed] |
| 22. | Obaidat NA, Alsaad KO, Ghazarian D. Skin adnexal neoplasms--part 2: an approach to tumours of cutaneous sweat glands. J Clin Pathol. 2007;60:145-159. [PubMed] |
| 23. | Pizinger K, Michal M. Malignant cylindroma in Brooke-Spiegler syndrome. Dermatology. 2000;201:255-257. [PubMed] |
| 24. | Fenske C, Banerjee P, Holden C, Carter N. Brooke-Spiegler syndrome locus assigned to 16q12-q13. J Invest Dermatol. 2000;114:1057-1058. [PubMed] |
| 25. | Poblete Gutiérrez P, Eggermann T, Höller D, Jugert FK, Beermann T, Grussendorf-Conen EI, Zerres K, Merk HF, Frank J. Phenotype diversity in familial cylindromatosis: a frameshift mutation in the tumor suppressor gene CYLD underlies different tumors of skin appendages. J Invest Dermatol. 2002;119:527-531. [PubMed] |
| 26. | Biggs PJ, Wooster R, Ford D, Chapman P, Mangion J, Quirk Y, Easton DF, Burn J, Stratton MR. Familial cylindromatosis (turban tumour syndrome) gene localised to chromosome 16q12-q13: evidence for its role as a tumour suppressor gene. Nat Genet. 1995;11:441-443. [PubMed] |
| 27. | Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160-165. [PubMed] |
| 28. | Gerretsen AL, Beemer FA, Deenstra W, Hennekam FA, van Vloten WA. Familial cutaneous cylindromas: investigations in five generations of a family. J Am Acad Dermatol. 1995;33:199-206. [PubMed] |
| 29. | Hu G, Onder M, Gill M, Aksakal B, Oztas M, Gürer MA, Celebi JT. A novel missense mutation in CYLD in a family with Brooke-Spiegler syndrome. J Invest Dermatol. 2003;121:732-734. [PubMed] |
| 30. | Bowen S, Gill M, Lee DA, Fisher G, Geronemus RG, Vazquez ME, Celebi JT. Mutations in the CYLD gene in Brooke-Spiegler syndrome, familial cylindromatosis, and multiple familial trichoepithelioma: lack of genotype-phenotype correlation. J Invest Dermatol. 2005;124:919-920. [PubMed] |
| 31. | Young AL, Kellermayer R, Szigeti R, Tészás A, Azmi S, Celebi JT. CYLD mutations underlie Brooke-Spiegler, familial cylindromatosis, and multiple familial trichoepithelioma syndromes. Clin Genet. 2006;70:246-249. [PubMed] |
| 32. | Saggar S, Chernoff KA, Lodha S, Horev L, Kohl S, Honjo RS, Brandt HR, Hartmann K, Celebi JT. CYLD mutations in familial skin appendage tumours. J Med Genet. 2008;45:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Grossmann P, Vanecek T, Steiner P, Kacerovska D, Spagnolo DV, Cribier B, Rose C, Vazmitel M, Carlson JA, Emberger M. Novel and recurrent germline and somatic mutations in a cohort of 67 patients from 48 families with Brooke-Spiegler syndrome including the phenotypic variant of multiple familial trichoepitheliomas and correlation with the histopathologic findings in 379 biopsy specimens. Am J Dermatopathol. 2013;35:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Nagy N, Farkas K, Kinyo A, Nemeth IB, Kis E, Varga J, Bata-Csorgo Z, Kemeny L, Szell M. A novel missense mutation of the CYLD gene identified in a Hungarian family with Brooke-Spiegler syndrome. Exp Dermatol. 2012;21:967-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Nagy N, Rajan N, Farkas K, Kinyó A, Kemény L, Széll M. A mutational hotspot in CYLD causing cylindromas: a comparison of phenotypes arising in different genetic backgrounds. Acta Derm Venereol. 2013;93:743-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | van den Ouweland AM, Elfferich P, Lamping R, van de Graaf R, van Veghel-Plandsoen MM, Franken SM, Houweling AC. Identification of a large rearrangement in CYLD as a cause of familial cylindromatosis. Fam Cancer. 2011;10:127-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Zhang G, Huang Y, Yan K, Li W, Fan X, Liang Y, Sun L, Li H, Zhang S, Gao M. Diverse phenotype of Brooke-Spiegler syndrome associated with a nonsense mutation in the CYLD tumor suppressor gene. Exp Dermatol. 2006;15:966-970. [PubMed] |
| 38. | Kazakov DV, Zelger B, Rütten A, Vazmitel M, Spagnolo DV, Kacerovska D, Vanecek T, Grossmann P, Sima R, Grayson W. Morphologic diversity of malignant neoplasms arising in preexisting spiradenoma, cylindroma, and spiradenocylindroma based on the study of 24 cases, sporadic or occurring in the setting of Brooke-Spiegler syndrome. Am J Surg Pathol. 2009;33:705-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Kazakov DV, Vanecek T, Zelger B, Carlson JA, Spagnolo DV, Schaller J, Nemcova J, Kacerovska D, Vazmitel M, Sangüeza M. Multiple (familial) trichoepitheliomas: a clinicopathological and molecular biological study, including CYLD and PTCH gene analysis, of a series of 16 patients. Am J Dermatopathol. 2011;33:251-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Linos K, Schwartz J, Kazakov DV, Vanecek T, Carlson JA. Recurrent CYLD nonsense mutation associated with a severe, disfiguring phenotype in an African American family with multiple familial trichoepithelioma. Am J Dermatopathol. 2011;33:640-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Lv HL, Huang YJ, Zhou D, Du YF, Zhao XY, Liang YH, Quan C, Zhang H, Zhou FS, Gao M. A novel missense mutation of CYLD gene in a Chinese family with multiple familial trichoepithelioma. J Dermatol Sci. 2008;50:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Oiso N, Mizuno N, Fukai K, Nakagawa K, Ishii M. Mild phenotype of familial cylindromatosis associated with an R758X nonsense mutation in the CYLD tumour suppressor gene. Br J Dermatol. 2004;151:1084-1086. [PubMed] |
| 43. | Vanecek T, Halbhuber Z, Kacerovska D, Martinek P, Sedivcova M, Carr RA, Slouka D, Michal M, Kazakov DV. Large germline deletions of the CYLD gene in patients with Brooke-Spiegler syndrome and multiple familial trichoepithelioma. Am J Dermatopathol. 2014;36:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Wu JW, Xiao SX, Huo J, An JG, Ren JW. A novel frameshift mutation in the cylindromatosis (CYLD) gene in a Chinese family with multiple familial trichoepithelioma. Arch Dermatol Res. 2014;306:857-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Guardoli D, Argenziano G, Ponti G, Nasti S, Zalaudek I, Moscarella E, Lallas A, Piana S, Specchio F, Martinuzzi C. A novel CYLD germline mutation in Brooke-Spiegler syndrome. J Eur Acad Dermatol Venereol. 2014;Jul 30; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Qian F, Zhai Y, Yuan X, Li P, Wang W, Ding Y, Wang J, Wu B, Cheng H, Sun L. A novel mutation of CYLD gene in a Chinese family with multiple familial trichoepithelioma. Australas J Dermatol. 2014;55:232-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Komander D, Lord CJ, Scheel H, Swift S, Hofmann K, Ashworth A, Barford D. The structure of the CYLD USP domain explains its specificity for Lys63-linked polyubiquitin and reveals a B box module. Mol Cell. 2008;29:451-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 48. | Reiley WW, Zhang M, Jin W, Losiewicz M, Donohue KB, Norbury CC, Sun SC. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7:411-417. [PubMed] |
| 49. | Nasti S, Pastorino L, Bruno W, Gargiulo S, Battistuzzi L, Zavattaro E, Leigheb G, De Francesco V, Tulli A, Mari F. Five novel germline function-impairing mutations of CYLD in Italian patients with multiple cylindromas. Clin Genet. 2009;76:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Chen M, Liu H, Fu X, Yu Y, Yu G, Liu H, Tian H, Zhou G, Zhang D, Wang G. Mutation analysis of the CYLD gene in two Chinese families with multiple familial Trichoepithelioma. Australas J Dermatol. 2011;52:146-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Kacerovská D, Szép Z, Kolláriková L, Vaneček T, Michal M, Daniš D, Kazakov D. A novel germline mutation in the CYLD gene in a Slovak patient with Brooke-Spiegler syndrome. Cesk Patol. 2013;49:89-92. [PubMed] |
| 52. | Rajan N, Elliott R, Clewes O, Mackay A, Reis-Filho JS, Burn J, Langtry J, Sieber-Blum M, Lord CJ, Ashworth A. Dysregulated TRK signalling is a therapeutic target in CYLD defective tumours. Oncogene. 2011;30:4243-4260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |